Abstract
Introduction
Urologists have been criticized for over-treating men with low-risk prostate cancer, and for passively observing older men with higher-risk disease. Proponents of active surveillance (AS) for low-risk disease and critics of watchful waiting (WW) for higher-risk disease have advocated for more judicious use of observation. Thus, we compared two population-based cohorts to determine how expectant management has evolved over the past two decades.
Methods
5,871 men with localized prostate cancer were enrolled in the Prostate Cancer Outcomes Study (PCOS: 1994 to 1995) or the Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR: 2011 to 2012) study. We compared use of definitive treatment vs. expectant management (WW/AS) across cohorts, focusing on the influence of disease risk, age and comorbidities.
Results
Use of WW/AS was similar in PCOS and CEASAR (14% in each). Compared to PCOS, more men in CEASAR with low-risk disease selected WW/AS (25% vs. 15%, respectively), whereas fewer men with intermediate- (7% vs. 14%) and high-risk (3% vs. 10%) disease chose WW/AS (p<0.001 for each). The association of disease risk with WW/AS was significantly larger in CEASAR than PCOS (OR, 7.3; 95% confidence interval [CI], 3.4 to 15.7). Older age was associated with WW/AS in both cohorts, but there was no association between comorbidity and WW/AS in CEASAR.
Conclusions
Use of WW/AS was more aligned with disease risk in CEASAR compared PCOS, suggesting there has been a pivot from WW to AS. While older men were more likely to be observed, comorbidity had little, if any, influence.
Introduction
Though prostate cancer (PCa) remains the second-leading cause of cancer death in men,1 the natural history of localized PCa varies from indolent to aggressive.2 Deciding whether to treat or observe localized PCa depends on accurate estimation of both prostate cancer-specific mortality (PCSM) and other-cause mortality.3
Failure to balance these competing risks has resulted in widespread overtreatment of low-risk disease and undertreatment of high-risk disease among elderly men.4–6 Over the past decade, however, some thought leaders have promoted active surveillance (AS) as a means to reduce harms associated with overtreatment of low-risk disease, and urologic guidelines panels have adjusted their recommendations accordingly.7 Similarly, recent attention has focused on under-treatment of older men with higher-risk disease, who may be placed on watchful waiting (WW) rather than being offered curative treatment.6
In this study, we sought to determine the extent to which evidence based recommendations regarding more judicious use of expectant management have been implemented at the population level. We compared the use of treatment and observation in two large, population based studies, the Comparative Effectiveness Analysis of Surgery and Radiation (CEASAR) study, accrued in 2011–12, and the Prostate Cancer Outcome Study (PCOS), accrued in 1994–95. Our aim was to determine how the use of observation has changed with respect to disease risk, age and comorbidity, and to determine whether there is evidence of a pivot from the WW modality toward AS in this interval.
Methods
Patients
The analytic cohort was drawn from PCOS and CEASAR, two population-based PCa cohorts. PCOS enrolled 5,424 patients from six participating Surveillance Epidemiology and End Results (SEER) sites (Connecticut, Utah, New Mexico, as well as the metropolitan areas of Atlanta, Georgia, Los Angeles, California, and Seattle-Puget Sound, Washington) in 1994–95.8
CEASAR used similar accrual mechanisms to enroll 3,691 men in 2011–12 from 5 SEER registries (Atlanta, Los Angeles, Louisiana, New Jersey, and Utah) as well as newly-accrued CaPSURE study participants.9
Inclusion in CEASAR was restricted to men under 80 years old, with clinically localized disease (cT1 or T2) and PSA below 50 ng/ml, whereas PCOS was less restricted. Therefore, in order to create a homogenous analytic cohort of men who might be eligible for treatment or observation, the current study included the 2,625 men from PCOS and 3,246 men from CEASAR who were between 40 and 75 years old at enrollment, had a baseline survey, either a 6-month or 12-month survey, PSA less than 50, non-missing Gleason score, clinical stage T1 or T2, and sufficient treatment information.
Data collection
At baseline, demographic and clinical information were collected. Functional status was assessed the UCLA Prostate Cancer Index (UCLA-PCI) in PCOS and the Expanded Prostate Cancer Index Composite (EPIC)-26 in CEASAR. The two questionnaires asked similar, but slightly different questions regarding sexual, urinary, and bowel function. Thus, we normalized functional domain scores to the cohort mean and standard deviation.
Twelve months after diagnosis, data were abstracted from medical records.10 Treatment choice was determined by the most reliable source of information available in the following order: medical chart abstraction, patient reported treatment selection, SEER registry data. WW/AS was defined as no record of definitive treatment within one year after diagnosis, or documentation of WW/AS in the medical chart, or patient-reported WW/AS in the absence of treatment. Patients who underwent androgen deprivation monotherapy (ADT) were initially grouped with those undergoing definitive treatment and were omitted in subsequent sensitivity analyses.
Statistical analysis
Patient and disease characteristics were compared across cohorts. Rates of definitive treatment vs. WW/AS were compared by cohort across recurrence risk strata.
The odds of choosing WW/AS in CEASAR and PCOS were compared using a logistic regression model. Covariates of interest included age, number of comorbidities, and modified D’Amico risk score. The comorbidities assessed common to both cohorts were heart failure, angina, stroke, heart attack, hypertension, diabetes, colitis, and lung disease. The number of comorbidities was grouped as 0, 1, or 2 or more. In CEASAR, the low modified D’Amico risk score was defined as PSA less than 10, the Gleason sum less than 7, and T1 clinical stage. In PCOS, men were considered low-risk using the same PSA and Gleason sum cutoffs, but with clinical stage of T1 or T2 to account for the fact that some in PCOS were coded as “T1/T2”. The model included two-way interaction terms between the risk factors (age, comorbidities, and modified D’Amico risk group) and cohort (PCOS vs. CEASAR) and adjusted for race, functional status, study site, and socioeconomic factors (marital status, education, inflation-adjusted income, insurance, and employment status).
Sensitivity analysis was performed for patients managed at sites common to both PCOS and CEASAR. In a separate sensitivity analysis, patients who only received ADT were excluded.
To determine the intensity of post-diagnostic surveillance, we tallied post-diagnosis PSA testing and repeat biopsies in CEASAR patients. These data were not available for PCOS patients.
As a secondary analysis, we computed 15-year overall survival probability and PCa-specific mortality for patients in PCOS. These data are not available for CEASAR patients.
All p values were two-sided, with p < 0.05 considered statistically significant. R software v3.1.0 (R Foundation, Vienna, Austria) was used for all statistical analysis. Estimates are reported with 95% confidence interval.
Results
The 2,625 PCOS subjects and 3,246 CEASAR subjects were demographically similar (Table 1). Regarding disease characteristics (Table 2), more men in CEASAR had non-palpable disease than those in PCOS (76% vs. 32%, respectively, p < 0.001), but fewer men in CEASAR harbored Gleason 6 or lower tumors (51% vs. 66%, respectively, p < 0.001). Of note, even if the 24% of men with uncertain clinical T-stage in PCOS were classified as T1, the percentage of men with non-palpable disease would still be lower than in CEASAR. The differences in T-stage and Gleason sum at presentation likely reflect the stage migration during the interval between studies,11 as well as changes in the Gleason classification system, which was updated in 2005.12
Table 1.
Demographic characteristics of study cohort
| CEASAR | PCOS | p-value | |
|---|---|---|---|
| Age, yrs | |||
| Median (Q1, Q3) | 64 (59, 70) | 66 (59, 71) | <0.001 |
| Race, % (n) | |||
| White | 73 (2343) | 70 (1849) | <0.001 |
| Black | 15 (487) | 16 (425) | |
| Hispanic | 7 (225) | 13 (351) | |
| Other | 4 (139) | 0 (0) | |
| Number of comorbidities, % (n) | |||
| 0 | 34 (891) | 50 (1311) | <0.001 |
| 1 | 41 (1088) | 30 (799) | |
| 2 or more | 25 (664) | 19 (515) | |
| Income, % (n) | |||
| $30,000 or less | 22 (591) | 24 (582) | <0.001 |
| $30,000–100,000 | 51 (1370) | 58 (1378) | |
| More than $100,000 | 27 (730) | 18 (420) | |
| Education, % (n) | |||
| Less than grade school | 5 (135) | 9 (232) | <0.001 |
| Less than high school | 6 (158) | 11 (297) | |
| High school graduate | 21 (604) | 20 (527) | |
| Some college | 22 (635) | 24 (627) | |
| College graduate | 23 (652) | 15 (387) | |
| Advanced degree | 24 (675) | 20 (520) | |
| Employment, % (n) | |||
| Full time | 41 (1306) | 28 (726) | <0.001 |
| Part time | 8 (249) | 9 (245) | |
| Retired | 46 (1484) | 59 (1526) | |
| Other | 5 (163) | 4 (97) | |
| Marital status, % (n) | |||
| Married | 80 (2275) | 81 (1929) | 0.164 |
| Not married | 20 (575) | 19 (442) | |
| Insurance, % (n) | |||
| Private or HMO | 47 (1489) | 49 (1187) | <0.001 |
| Medicare | 48 (1514) | 45 (1075) | |
| VA or Military | 1 (39) | 4 (97) | |
| Medicaid | 2 (53) | 1 (15) | |
| Other | 1 (45) | 1 (26) | |
| No insurance | 1 (46) | 0 (12) | |
| Self-Reported Overall Health, % (n) | |||
| Excellent | 19 (614) | 19 (449) | 0.256 |
| Very good | 38 (1239) | 37 (866) | |
| Good | 31 (1002) | 31 (737) | |
| Fair | 9 (306) | 11 (257) | |
| Poor | 2 (78) | 2 (57) | |
CEASAR - Comparative Effectiveness Analysis of Surgery and Radiation; HMO - health maintenance organization; PCOS - Prostate Cancer Outcomes Study; VA - US Department of Veterans Affairs
Table 2.
Clinical characteristics by cohort
| CEASAR | PCOS | p-value | |
|---|---|---|---|
| PSA, ng/mL | |||
| Median (Q1, Q3) | 5.5 (4.3, 7.6) | 7.5 (5.2, 11.9) | <0.001 |
| Clinical T-stage, % (n) | |||
| T1 | 76 (2453) | 32 (831) | <0.001 |
| T2 | 24 (783) | 44 (1164) | |
| T1 or T2 | 0 (0) | 24 (630) | |
| Biopsy Gleason score, % (n) | |||
| 6 or less | 51 (1658) | 66 (1734) | <0.001 |
| 7 | 38 (1236) | 25 (669) | |
| 8–10 | 11 (352) | 8 (222) | |
| Risk of recurrence, % (n) | |||
| Low | 44 (1426) | 49 (1277) | 0.004 |
| Intermediate | 39 (1254) | 35 (907) | |
| High | 17 (558) | 17 (441) | |
CEASAR - Comparative Effectiveness Analysis of Surgery and Radiation; PCOS - Prostate Cancer Outcomes Study; PSA - prostate specific antigen
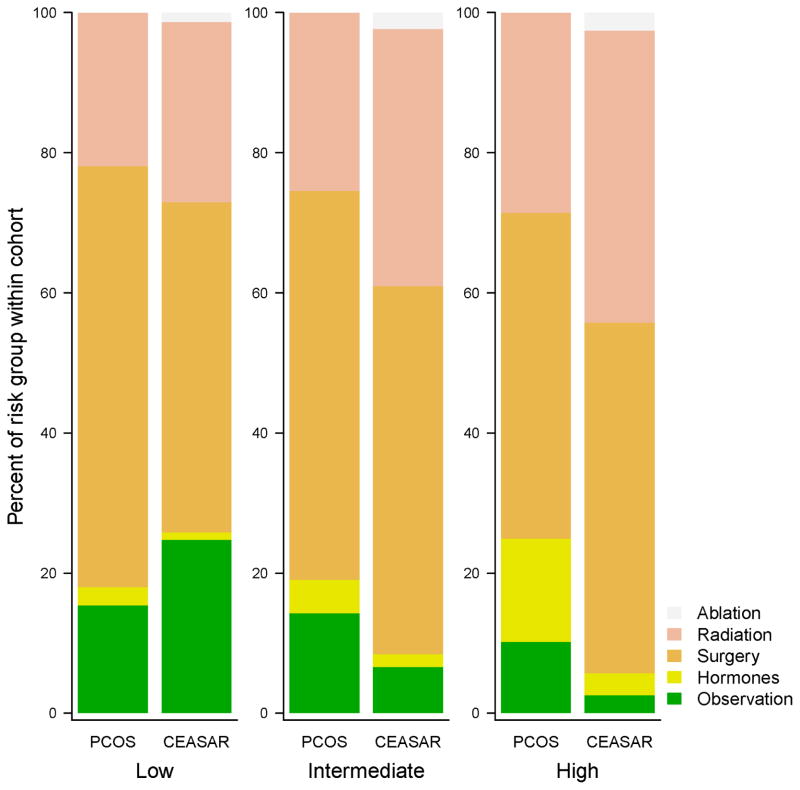
Overall rates of WW/AS were similar between cohorts (14% vs. 14%, p=0.85). However, its use across disease risk strata changed markedly (Figure 1). Men with low-risk disease were far more likely to undergo WW/AS in CEASAR than in PCOS (25% vs. 15%, p < 0.001), whereas a lower percentage of men with intermediate or high-risk disease underwent WW/AS in CEASAR (7% vs. 14% for intermediate risk disease, p < 0.001, and 3% vs. 10% for high-risk disease, p < 0.001). Grouping primary ADT and WW/AS together demonstrated that non-definitive management for high-risk disease declined from 25% in PCOS to 6% in CEASAR (p < 0.001).
Figure 1.
Percentage of men selecting a given treatment by risk classification and cohort in unadjusted analysis.
In the multivariable model, men in CEASAR were significantly more likely to select WW/AS compared to those in PCOS (p<0.0001). Disease risk was strongly associated with selecting WW/AS in CEASAR (OR for low-risk vs high-risk 17.5 [9.3, 32.9]. While having low-risk disease was also a strong predictor of WW/AS in PCOS (OR 2.4 [1.6, 3.7], it was much more important in CEASAR (ratio of OR in CEASAR to OR in PCOS 7.3 [3.4, 15.7] (Table 3). This interaction between disease risk and cohort indicates that risk is used much differently in CEASAR compared to PCOS (p-interaction <0.001).
Table 3.
Factors associated with an observational strategy in a multivariable model
| CEASAR | PCOS | Difference in effect between cohorts | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| OR | 95% CI | p-value | OR | 95% CI | p-value | OR/OR+ | 95% CI | P-value | |
|
|
|
|
|||||||
| Age | 0.008 | ||||||||
| 70 vs. 60 years | 2.66 | (2.10, 3.38) | <0.001 | 3.98 | (3.08, 5.14) | <0.001 | 0.67 | [0.50, 0.90] | |
| No. of comorbidities | 0.16 | ||||||||
| 1 vs. 0 | 0.78 | (0.56, 1.03) | 0.103 | 1.09 | (0.78, 1.52) | 0.604 | 0.71 | [0.46, 1.11] | |
| 2+ vs. 0 | 1 | (0.71, 1.43) | 0.979 | 1.54 | (1.08, 2.22) | 0.019 | 0.65 | [0.4, 1.06] | |
| Disease risk | <0.001 | ||||||||
| Int vs. Low | 0.18 | (0.14, 0.25) | <0.001 | 0.65 | (0.48, 0.89) | 0.007 | 0.28 | [0.18, 0.44] | |
| High vs. Low | 0.06 | (0.03, 0.11) | <0.001 | 0.42 | (0.27, 0.64) | <0.001 | 0.14 | [0.06, 0.29] | |
| Disease risk (other pairwise comparisons) | |||||||||
| Low vs. High | 17.48 | (9.29, 32.88) | <0.001 | 2.39 | (1.55, 3.68) | <0.001 | 7.32 | (3.41, 15.71) | <0.001 |
| Intermediate vs. High | 3.23 | (1.67, 6.23) | <0.001 | 1.55 | (0.99, 2.43) | 0.054 | 2.08 | (0.94, 4.60) | |
CEASAR - Comparative Effectiveness Analysis of Surgery and Radiation; PCOS - Prostate Cancer Outcomes Study; OR -odds ratio; CI - confidence interval. Adjusted for site, race, income, marital status, education, employment, sexual and urinary domain scores, insurance status, overall health.
Ratio of effect of risk factor for CEASAR compared with PCOS
P-value for anova test of overall interaction effect
The association between advanced age (>70 years) and WW/AS was stronger in PCOS than in CEASAR (p-interaction = 0.008, Table 3). The corresponding cohort-specific results for the number comorbidities were closer to the null value, and to each other (p-interaction =0.16). There was a significant association for 0 vs. 2 comorbid conditions, but only in the PCOS data.
In sensitivity analyses, no difference in outcomes was observed after excluding patients receiving ADT. The findings were also similar when restricting the analysis to participants from SEER sites common to both PCOS and CEASAR.
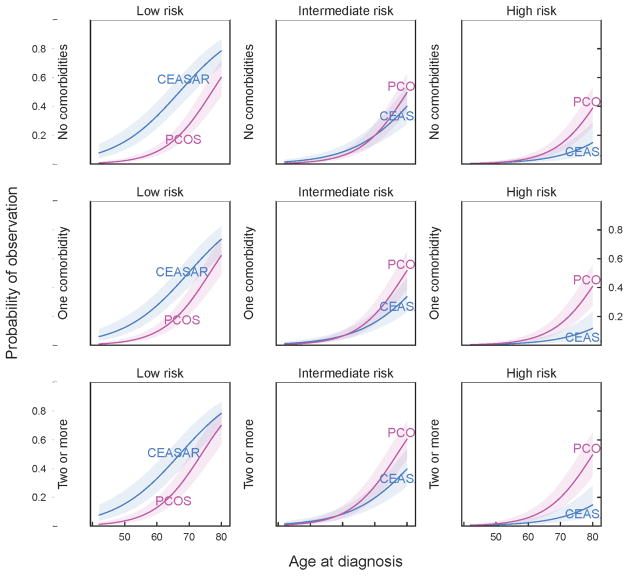
The model enables us to compare estimates of the likelihood of WW/AS across cohorts, based on disease risk, age and comorbidity (Figure 2). For example, a 60-year old man with no comorbidities and low-risk disease had a 33% (25, 43) chance of WW/AS in CEASAR vs. 9% (6, 13) in PCOS. On the other end of the life expectancy spectrum, a 75-year old man with 2 or more comorbidities, with low-risk disease had a 69% (57, 78) chance of WW/AS in CEASAR vs. 53% (41, 66) in PCOS, while a similar patient with high-risk disease had a 11% (6, 21) chance of WW/AS in CEASAR vs. 32% (21, 46) in PCOS.
Figure 2.
Cohort differences in effects of age, comorbidity, and recurrence risk on probability of choosing WW/AS strategy for localized prostate cancer. Estimates are based on multivariable logistic regression and calculated for men with median values of demographic and health characteristics.
After a median follow-up of 13 months, 90% of men in the CEASAR cohort had at least one subsequent PSA measurement, and 35% underwent prostate biopsy after diagnosis, suggesting most of these men were on AS rather than WW.
The median overall survival in the PCOS observation group was 11.5 years (95% CI 9.6–12.6 years). At 15 years after enrollment, overall mortality was 68.8% (62.5–75.0) and PCSM was 16.6% (12.0–22.6%). (see Supplemental figures).
Discussion
In this study, we found that the use of observation changed substantially between the PCOS era (1994–95) and the CEASAR era (2011–12). While observation was used in a comparable proportion in each cohort (14%), it was used much more frequently for low-risk disease in CEASAR and much less frequently for intermediate and high-risk disease. The high rates of subsequent testing in the CEASAR cohort suggests that the majority of observation patients diagnosed in 2011–12 were indeed on AS, while the high PCSM in PCOS suggests that WW was applied to men with a high risk of progression in the 1990s. Taken together, these findings suggest that the modality of observation evolved during this interval from WW to AS, in accord with the available evidence and guidelines.
We also found that older age was associated with likelihood of being observed in both eras, but its effect was smaller in the CEASAR study. This may suggest that physicians and patients have become more comfortable with the concept of AS in younger men, rather than reserving observation as a palliative strategy for those at high risk of other-cause mortality (WW). Comorbidity did not have a significant effect on decisions to treat vs. observe in CEASAR, and its effect did not change significantly from PCOS.
Concerns regarding over-detection and overtreatment of low-risk PCa developed following the spike in incidence of PCa due to the adoption of PSA screening in the early 1990’s.13 Over the past two decades, evidence has accrued demonstrating the harms of aggressive treatment, the safety of AS for low-risk disease, and the absence of a survival benefit for treatment of low-risk disease.14–18 These factors have prompted guideline panels to expand the indications for expectant management to include AS for management of low-risk PCa, even among men with a long life expectancy.7
Yet population-based studies suggest that, until recently, definitive treatment for low-risk disease was the norm.4, 19 More recent evidence suggests the use of WW/AS for low-risk disease is rising in some scenarios. For example, use of WW/AS is increasing among the Medicare population and among practices engaged in a quality collaborative in Michigan.20, 21 In CaPSURE, a PCa registry which collects data primarily from community practices, the rate of WW/AS among men with low-risk disease grew from 6.7–14.3% in 1990–2009 to 40.4% in 2010–13.22 The current study of two large population-based cohorts with carefully curated datasets confirms that the use of WW/AS for men with low-risk disease has expanded significantly between the early PSA era and the contemporary era (15% in PCOS vs. 25% in CEASAR), and demonstrates that observation is rising nationally, not just in academic centers, among the elderly, and in quality collaboratives. Furthermore, it demonstrates a transition in the mode of observation from WW to AS. Finally, it demonstrates that, to some degree, disease risk taking precedence over age and comorbidities in treatment vs. observation decisions.
While overtreatment of low-risk disease is widely publicized, that older men with high-risk disease are often undertreated is less commonly acknowledged. However, data suggest that roughly half of men with high-risk disease are undertreated, with 60–67% of men over the age of 75 with high-risk disease receiving no therapy or ADT alone to their apparent detriment.4, 5, 23–25 Our data indicate an encouraging trend away from WW/AS among men with intermediate or high-risk disease.
A rational approach to treatment selection for localized PCa would take into account age and comorbidity status to determine other-cause mortality risk in addition to PCSM risk.3 However, treatment decisions often fail to account adequately for comorbidity, and it is not clear how best to improve the attention to comorbidity.26 Our data indicate that more needs to be done to incorporate comorbidity and life expectancy into treatment decisions.
The study results must be interpreted in the context of the study design and available data. There are some important differences between the two cohorts that are worth considering. First, due to stage migration, changes in biopsy techniques (i.e., more thorough sampling) and changes in the Gleason scoring system,11, 12 the distribution of men classified as low-risk in PCOS contains some men considered intermediate-risk in CEASAR, and the Will Rogers phenomenon may explain a portion of the difference in utilization of WW/AS in men with low-risk disease.27 Second, unmeasured confounders may have differed between cohorts and modulated the relationship between cohort and selection of WW/AS. For instance, biopsy findings such as the number of positive cores or length of cancer within a core are inclusion criteria for some AS programs and could confound the association between cohort and utilization of AS. Finally, we could not reliably distinguish between AS and WW in either dataset, though in CEASAR the use of PSA measurements and prostate biopsies after diagnosis suggest the majority of men were on AS and the high rate of PCSM in PCOS suggests many of those men were on WW. Nonetheless, the similarity in data collection strategies and richness of the datasets enabled us to expand upon the findings of prior studies that have used only administrative and registry data, and those based on selective registries.
Conclusions
We found that men diagnosed with localized PCa in the contemporary era experience more judicious use of expectant management compared to men diagnosed in the mid-1990s. The use of WW/AS was more aligned with disease risk in the contemporary cohort, such that men with low-risk disease were more likely to undergo WW/AS, while those with intermediate- and high-risk disease were more likely to undergo treatment compared to the cohort accrued in the 1990s. In addition, we found evidence of a pivot from WW to AS. While older age was a strong predictor of WW/AS in both cohorts, its use was different in CEASAR compared to PCOS. Comorbidity status remains underutilized in deciding whom to observe. Overall, these findings suggest a growing acceptance of WW/AS for men with low-risk disease and definitive therapy for intermediate or high-risk disease, and a pivot from WW to AS over this interval, which may be a prerequisite for realizing the potential advantages of prostate cancer screening. While our results are encouraging, there remains substantial room for improvement in continuing to increase use of WW/AS in low-risk disease and integrating comorbidity information into decision-making.
Supplementary Material
Acknowledgments
Funding: This study was supported by then National Cancer Institute, National Institutes of Health (grant 1R03CA173812 and R01-CA114524), the US Agency for Healthcare Research and Quality (grants 1R01HS019356 and 1R01HS022640-01), and the following contracts to each of the participating institutions: N01-67007, N01-PC-67009, N01-PC-67010, N01-PC-67006, N01-PC-67005, and N01-PC-67000, and through a contract from the Patient-Centered Outcomes Research Institute.
We acknowledge the following contributors, who were investigators in the Comparative Effectiveness Analysis of Surgery And Radiation (CEASAR) study and Prostate Cancer Outcomes Study (PCOS): Peter C. Albertsen, Matthew R. Cooperberg, Michael Goodman, Sheldon Greenfield, Ann Hamilton, Richard M. Hoffman, Sherrie H. Kaplan, Lisa Paddock, Janet L. Stanford, Antoinette M. Stroup, Xiao-Cheng Wu.
Key to Abbreviations
- AS
Active surveillance
- PCOS
Prostate Cancer Outcomes Study
- CEASAR
Comparative Effectiveness Analysis of Surgery and Radiation
- WW/AS
Watchful waiting or active surveillance
- PCSM
Prostate cancer-specific mortality
- PSA
Prostate specific antigen
- SEER
Surveillance Epidemiology and End Results
- CaPSURE
Cancer of the prostate strategic urologic research endeavor
- UCLA-PCI
University of California, Los Angeles Prostate Cancer Index
- EPIC
Expanded prostate cancer index composite
- OR
Odds Ratio
- ADT
Androgen deprivation therapy
- PCa
Prostate cancer
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
BIBLIOGRAPHY
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Albertsen PC, Hanley JA, Fine J. 20-year outcomes following conservative management of clinically localized prostate cancer. JAMA. 2005;293:2095. doi: 10.1001/jama.293.17.2095. [DOI] [PubMed] [Google Scholar]
- 3.Daskivich TJ, Litwin MS, Penson DF. Effect of age, tumor risk, and comorbidity in a u.s. Population-based cohort of men with prostate cancer. Ann Intern Med. 2013;159:370. doi: 10.7326/0003-4819-159-5-201309030-00018. [DOI] [PubMed] [Google Scholar]
- 4.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 2010;28:1117. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamilton AS, Albertsen PC, Johnson TK, et al. Trends in the treatment of localized prostate cancer using supplemented cancer registry data. BJU Int. 2011;107:576. doi: 10.1111/j.1464-410X.2010.09514.x. [DOI] [PubMed] [Google Scholar]
- 6.Daskivich TJ, Lai J, Dick AW, et al. Questioning the 10-year Life Expectancy Rule for High-grade Prostate Cancer: Comparative Effectiveness of Aggressive vs Nonaggressive Treatment of High-grade Disease in Older Men With Differing Comorbid Disease Burdens. Urology. 2016 doi: 10.1016/j.urology.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 7.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate Cancer, Version 1.2016. J Natl Compr Canc Netw. 2016;14:19. doi: 10.6004/jnccn.2016.0004. [DOI] [PubMed] [Google Scholar]
- 8.Potosky AL, Harlan LC, Stanford JL, et al. Prostate cancer practice patterns and quality of life: the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 1999;91:1719. doi: 10.1093/jnci/91.20.1719. [DOI] [PubMed] [Google Scholar]
- 9.Cooperberg MR, Broering JM, Litwin MS, et al. The contemporary management of prostate cancer in the United States: lessons from the cancer of the prostate strategic urologic research endeavor (CapSURE), a national disease registry. J Urol. 2004;171:1393. doi: 10.1097/01.ju.0000107247.81471.06. [DOI] [PubMed] [Google Scholar]
- 10.Barocas DA, Chen V, Cooperberg M, et al. Using a population-based observational cohort study to address difficult comparative effectiveness research questions: the CEASAR study. J Comp Eff Res. 2013;2:445. doi: 10.2217/cer.13.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eifler JB, Feng Z, Lin BM, et al. An updated prostate cancer staging nomogram (Partin tables) based on cases from 2006 to 2011. BJU Int. 2012;111:22. doi: 10.1111/j.1464-410X.2012.11324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epstein JI, Allsbrook WC, Jr, Amin MB, et al. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29:1228. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 13.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst. 2009;101:1325. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klotz L, Vesprini D, Sethukavalan P, et al. Long-term follow-up of a large active surveillance cohort of patients with prostate cancer. J Clin Oncol. 2015;33:272. doi: 10.1200/JCO.2014.55.1192. [DOI] [PubMed] [Google Scholar]
- 15.Resnick MJ, Koyama T, Fan KH, et al. Long-term functional outcomes after treatment for localized prostate cancer. N Engl J Med. 2013;368:436. doi: 10.1056/NEJMoa1209978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilt TJ, Brawer MK, Jones KM, et al. Radical prostatectomy versus observation for localized prostate cancer. N Engl J Med. 2012;367:203. doi: 10.1056/NEJMoa1113162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370:932. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tosoian JJ, Trock BJ, Landis P, et al. Active surveillance program for prostate cancer: an update of the Johns Hopkins experience. J Clin Oncol. 2011;29:2185. doi: 10.1200/JCO.2010.32.8112. [DOI] [PubMed] [Google Scholar]
- 19.Barocas DA, Cowan JE, Smith JA, Jr, et al. J Urol. 2008;180:1330. doi: 10.1016/j.juro.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Ritch CR, Graves AJ, Keegan KA, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015;193:801. doi: 10.1016/j.juro.2014.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2014;67:44. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Cooperberg MR, Carroll PR. Trends in Management for Patients With Localized Prostate Cancer, 1990–2013. JAMA. 2015;314:80. doi: 10.1001/jama.2015.6036. [DOI] [PubMed] [Google Scholar]
- 23.Hamilton AS, Fleming ST, Wang D, et al. Clinical and Demographic Factors Associated With Receipt of Non Guideline-concordant Initial Therapy for Nonmetastatic Prostate Cancer. Am J Clin Oncol. 2014 doi: 10.1097/COC.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bekelman JE, Mitra N, Handorf EA, et al. Effectiveness of androgen-deprivation therapy and radiotherapy for older men with locally advanced prostate cancer. J Clin Oncol. 2015;33:716. doi: 10.1200/JCO.2014.57.2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daskivich TJ, Lai J, Dick AW, et al. Comparative effectiveness of aggressive versus nonaggressive treatment among men with early-stage prostate cancer and differing comorbid disease burdens at diagnosis. Cancer. 2014;120:2432. doi: 10.1002/cncr.28757. [DOI] [PubMed] [Google Scholar]
- 26.Kutikov A, Cooperberg MR, Paciorek AT, et al. Evaluating prostate cancer mortality and competing risks of death in patients with localized prostate cancer using a comprehensive nomogram. Prostate Cancer Prostatic Dis. 2012;15:374. doi: 10.1038/pcan.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Albertsen PC, Hanley JA, Barrows GH, et al. Prostate cancer and the Will Rogers phenomenon. J Natl Cancer Inst. 2005;97:1248. doi: 10.1093/jnci/dji248. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.