Abstract
The Rbfox genes encode an ancient family of sequence-specific RNA binding protein (RBP) that are critical developmental regulators in multiple tissues including skeletal muscle, cardiac muscle, and brain. The hallmark of Rbfox proteins is a single high-affinity RRM domain, highly conserved from insects to humans, that binds preferentially to UGCAUG motifs at diverse regulatory sites in pre-mRNA introns, mRNA 3′UTRs, and pre-miRNAs hairpin structures. Versatile regulatory circuits operate on Rbfox1 pre-mRNA and mRNA to ensure proper expression of Rbfox1 protein isoforms, which then act on the broader transcriptome to regulate alternative splicing networks, mRNA stability and translation, and microRNA processing. Complex Rbfox1 expression is encoded in a large gene encompassing multiple promoters and alternative splicing options that govern spatiotemporal expression of structurally distinct and tissue-specific protein isoforms with different classes of RNA targets. Nuclear Rbfox1 is a candidate master regulator that binds intronic UGCAUG elements to impact splicing efficiency of target alternative exons, many in transcripts for other splicing regulators. Tissue-specificity of Rbfox-mediated alternative splicing is executed by combinatorial regulation through the integrated activity of Rbfox proteins and synergistic or antagonistic splicing factors. Studies in animal models show that Rbfox1-related genes are critical for diverse developmental processes including germ cell differentiation and memory in Drosophila, neuronal migration and function in mouse brain, myoblast fusion and skeletal muscle function, and normal heart function. Finally, genetic and biochemical evidence suggest that aberrations in Rbfox-regulated circuitry are risk factors for multiple human disorders, especially neurodevelopmental disorders including epilepsy and autism, and cardiac hypertrophy.
Graphical Abstract
INTRODUCTION
Tissue-specific regulation of alternative pre-mRNA splicing diversifies a gene’s functional output by facilitating expression of distinct protein isoforms in cell type- and developmental-specific patterns. Transcriptome characterization via RNA-seq analysis has revealed an enormous capacity for cells to alter splicing patterns as they specialize through normal differentiation or respond to signals in the environment. Much has been learned about the trans-acting RNA binding proteins (RBPs) and the cis-acting regulatory motifs that govern RNA splicing events, leading to improved models of the splicing regulatory code 1, 2. Based on these foundational studies of basic RBP mechanisms, current efforts focus on exploring the physiological function of RBP-regulated splicing networks during normal differentiation and development. Correlating specific physiological deficits that occur upon RBP knockdown or knockout, with specific alternative splicing defects induced in the relevant tissue, provides new insights into normal developmental processes. Moreover, since aberrant splicing networks are increasingly appreciated as major contributors to human disease, mechanistic studies may ultimately provide therapeutic avenues for new classes of disease.
The Rbfox protein family represents an ancient group of RNA binding proteins that exert a powerful impact on a variety of RNA metabolic processes. Rbfox1 encodes highly versatile RNA binding proteins that play integral roles in RNA metabolism during development of organisms from nematodes and insects to mammals. In humans, Rbfox1 and the closely related Rbfox2 and Rbfox3 genes specify proteins that bind with high affinity and specificity to (U)GCAUG motifs located at strategic regulatory points in the transcriptome: introns that flank tissue-specific alternative exons, 3′UTR sequences in mRNAs, pre-microRNA hairpins, and likely others. Moreover, their function can be expanded by participation in a larger assemblage of splicing regulators (LASR) that can bind at additional sites 3. Versatility is encoded in the Rbfox gene architecture: via alternative promoter choice and alternative pre-mRNA splicing, the genes encode multiple protein isoforms with differential subcellular localization and differential interaction with regulatory co-factors. New data support the hypothesis that Rbfox1 functions in a complex cellular circuitry that (1) integrates inputs from multiple physiological and developmental cues to modulate the output of structurally and functionally distinct protein Rbfox1 isoforms in the appropriate spatiotemporal patterns; and (2) utilizes this complement of Rbfox1 isoforms to execute powerful controls on RNA processing events that specify the cell-type specific proteome. The latter includes not only regulation of alternative splicing, its most well studied function, but also mRNA stability and translation efficiency, and microRNA processing. This review will focus on the complex expression of Rbfox genes themselves, and summarize recent studies investigating the importance of Rbfox-regulated networks to normal development. These principles will be examined mainly in regard to Rbfox1 and Rbfox2 function in brain and muscle, due to increasing evidence for their developmental functions in these tissues in animal models and human disease states. Functions of Rbfox2 in other tissues, and functions of Rbfox3 in brain, are considered briefly where relevant; future studies will surely provide new insights into these functions for reporting at a later date.
RBFOX1 GENES AND PROTEINS
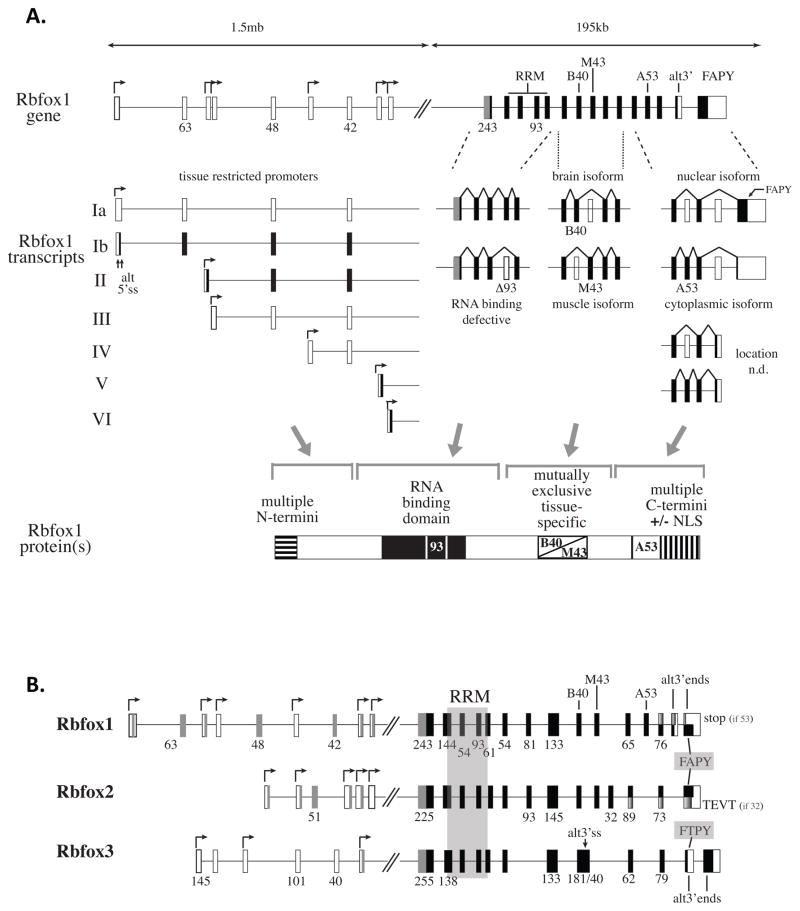
Understanding the range of Rbfox1 developmental functions requires appreciation of its complex gene structure and regulation. Rbfox1 is encoded by an extremely large gene of ~1.7mb having multiple transcription start sites at alternative first exons spanning ~1.3mb 4 (Figure 1A). Even this representation may understate the transcription potential, because it does not include a few ‘singleton’ genome browser annotations representing additional candidate start sites. This long expanse of sequence is often described as the “5′ untranslated region”; however, it contains exons of 63, 48, and 42nt that are highly conserved from reptiles to man, and which maintain an open reading frame that can in principle be translated to generate Rbfox1 proteins with alternative N-terminal domains (transcript classes Ia and II). In the downstream coding region of the gene, alternative splicing produces diversity by modulating inclusion of exons that generate active vs inactive RRM (RNA recognition motif) domains, exons that encode brain- and muscle-specific protein isoforms that may have different target specificities 5, and exons that change the C-terminal reading frame to control expression of a nuclear localization signal 6. Finally, a little-noticed alternative 3′ end, also highly conserved among mammals, can encode two additional C-termini. Notably, Rbfox gene structure in Drosophila already possessed two important features of the mammalian genes: an RRM coding domain, the core of which is contained within two exons identical in size and similar in sequence to human Rbfox genes; and the capability to generate cytoplasmic and nuclear isoforms via alternative splicing of 3′ exons 7.
Figure 1.
Rbfox1 gene architecture encodes diverse protein isoforms. A. In the long 5′ region, multiple promoters operating a distinct first exons provide potential for independent regulation by various physiological signals. In the RRM region, exons of 54 and 93 nucleotide are conserved from Drosophila to humans; the 93nt exon is deleted in some isoforms to generate isoforms with non-functional RRMs. Mutually exclusive exons B40 (brain-expressed 40nt exon) and M43 (muscle-expressed 43nt exon) are differentially spliced to generate tissue-specific protein isoforms 5. In the 3′ region, inclusion of exon A53 (alternative 53nt exon 5), also designated as exon 19 19, shifts the translational reading frame of the downstream exons and results in loss of the NLS. White box, untranslated sequence; black boxes, translated sequence; gray boxes, alternative N-terminal sequences predicted by reading frame analysis; gradient-shaded boxes, alternative C-terminal reading frames generated by inclusion or exclusion of A53 (Rbfox1) or 32nt exon (Rbfox2). n.d., not determined. B. Organization of Rbfox family genes. Rbfox2 and Rbfox3 are also encoded by large complex genes with many of the same features as Rbfox1 4, 84. Excluding genome browser annotations only represented once in the database, Rbfox2 and Rbfox3 have 5 and 3 promoters, respectively, spanning ~200–300kb of 5′ sequence. In the core coding region between alternative N- and C-terminal domains, paralogous exons of the three genes are aligned (intron lengths not to scale). Highly conserved RRM domains and RF(A/T)PY nuclear localization signals are indicated by shaded regions.
Together these transcriptional and post-transcriptional processes specify a family of Rbfox1 protein isoforms summarized in Figure 1A. The single RRM has been highly conserved through evolution, being identical in human Rbfox1 and Rbfox2, almost identical (97%) in Rbfox3 and only slightly diverged from the evolutionarily distant Drosophila (94% identity) and C. elegans (77% identity) proteins. In binary binding assays Rbfox proteins exhibit remarkable binding specificity and high affinity for the (U)GCAUG motif 8–11, properties conferred by extensive RNA-protein contacts evident in the solution structure of UGCAUGU-bound human Rbfox1 12. Even Drosophila and C. elegans Rbfox1 orthologs associate with UGCAUG elements 7,13. Regarding other domains, at least some of the alternative N- and C-terminal domains are required for splicing activity 5, 8, 14, and may facilitate interactions with other RBPs 14, 15 and assembly into the LASR complex 3.
Analogous to Rbfox1, the Rbfox2 and Rbfox3 genes exhibit features that facilitate expression of diverse isoforms (Figure 1B), including: multiple transcriptional promoters distributed over hundreds of kilobases of 5′ sequence; a highly conserved RRM domain that can be spliced to generate active or inactive forms through alternative splicing of a 93nt exon; and alternative splicing events that generate alternative C-terminal domains. All three genes express isoforms that either contain or lack the RF(A/T)PY nuclear localization signal 6. All of these features likely contribute to the increasingly wide array of RNA metabolism functions associated with various Rbfox proteins.
REGULATION OF RBFOX EXPRESSION
Precise control of splicing factor expression is critical for normal cell identity, and conversely, aberrant expression is a frequent cause of disease including cancer. Increasing evidence suggests that proper spatiotemporal expression of Rbfox proteins is dictated by continuous integration of inputs from various developmental and environmental signals that control Rbfox transcription, alternative splicing, and mRNA stability.
Rbfox1 transcription is regulated during development and differentiation of skeletal and cardiac muscle, as well as being differentially regulated in various neuronal regions. Some of the promoters are tissue-specific 4, 5, but little is known their regulation. Presumably the long 5′ region is also populated with multiple enhancers sensitive to developmental and environmental input.
Alternative splicing decisions within Rbfox pre-mRNAs are in some cases subject to auto-regulation and/or cross-regulation. In Rbfox1 and Rbfox2, a 93nt coding exon in the RRM domain possesses UGCAUG motifs in the immediate flanking introns, two of which are conserved to Drosophila 4, 5, 16. All three mammalian Rbfox paralogs can bind to these sites17 and induce exon skipping, which leads to synthesis of a dominant negative isoform that cannot bind RNA but antagonizes splicing enhancer activity of the full length protein 4, 5, 16. In the Rbfox2 gene, inclusion of two poison exons that induce nonsense mediated decay (NMD) 18 can be regulated via Rbfox binding to highly conserved UGCAUG motifs in the flanking introns. Finally, the alternative 3′ terminal exon in Rbfox1 possesses a highly conserved upstream UGCAUG and thus is a candidate for auto-regulation.
Interestingly, an environmentally-responsive splicing switch involving the A53 exon can modulate the relative output of nuclear vs cytoplasmic isoforms of Rbfox1 to alter splicing outcomes 19. Neuronal depolarization induces numerous splicing switches, among which is repression of Rbfox1 exon A53 splicing. Rbfox1 transcripts lacking A53 encode nuclear isoforms of the protein, effectively increasing nuclear Rbfox1 and rescuing some depolarization-induced splicing changes as part of an adaptive response.
Rbfox1 mRNA stability and/or translation represent additional ‘post-splicing’ steps at which the cellular content of available Rbfox protein can be modulated, e.g., by interaction with other RBPs 20 or with microRNAs 21. In humans, FRG1 is an RBP that binds Rbfox1 mRNA, decreasing its half-life, reducing expression of Rbfox1 protein, and disturbing Rbfox1 splicing networks 20. In Drosophila, A2bp1/Rbfox1 acts as a memory-promoting gene of potential relevance to human patients with autism. Behavioral, molecular, cellular, and genetic data suggest that miR-980 represses Rbfox1 expression via interaction with 3′UTR motifs, and may trigger downstream changes in Rbfox1-regulated splicing networks to impact specific memory phenotypes 21.
Additional regulatory effects on Rbfox1 mRNA stability and translation, on protein activity or availability, almost certainly remain to be discovered. Unexplained discordance between changes in Rbfox1 mRNA levels and protein levels have been observed during skeletal muscle differentiation in vitro 22, suggesting the possibility of translational regulation. Finally, the availability of Rbfox proteins for splicing regulation may also be regulated by expression of nuclear lncRNAs that harbor multiple Rbfox binding motifs and are proposed to function as molecular sinks for Rbfox proteins 23.
Distribution of Rbfox binding sites in the genome
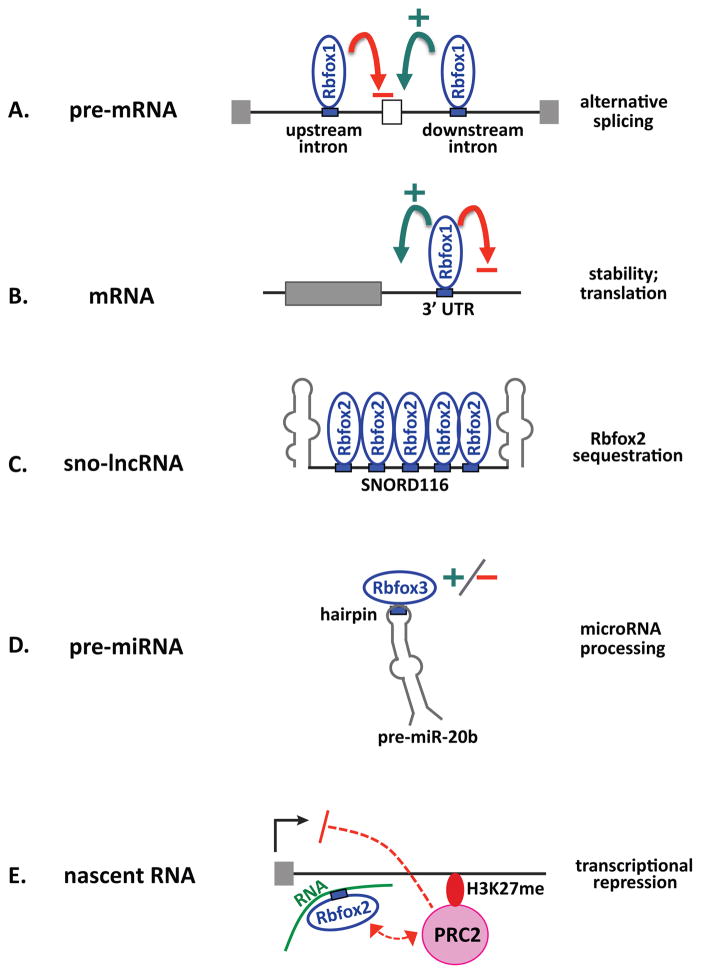
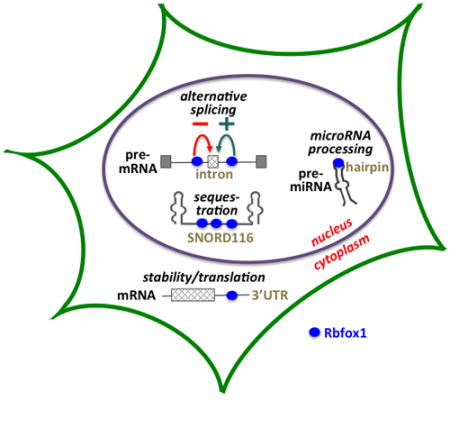
Genome-wide surveys of conserved UGCAUG motifs and unbiased CLIP-seq analyses of actual Rbfox binding sites indicate that major sites for Rbfox proteins include introns in pre-mRNA, 3′UTRs in mRNA, and intergenic sites (Figure 2). In mouse brain most of the Rbfox CLIP tags (~70%) reside in introns, with ~15% located in 3′UTRs 17, 24 and ~13% in intergenic regions 17; fewer sites map to coding exons and 5′ UTRs. Subcellular fractionation studies show that chromatin-associated nascent transcripts are enriched for intronic CLIP tags, while cytoplasmic RNA primarily binds Rbfox at 3′UTRs 25. Rbfox binding has also been documented in hairpins of pre-miRNAs 26, 27, in a subset of sno-lncRNAs 23, and at promoter-proximal sequences in nascent RNAs that can influence transcription 28. Rbfox binding can positively or negatively impact RNA processing activities as indicated in the figure. The obvious implication is that Rbfox plays major regulatory roles in pre-mRNA alternative splicing, potentially alternative polyadenylation, mRNA stability and translation, miRNA processing, and even transcription.
Figure 2.
Sites of Rbfox function in the transcriptome. A. Binding of Rbfox proteins upstream inhibits, while binding downstream enhances, inclusion of the adjacent alternative exon. B. Binding of Rbfox proteins to 3′UTR elements can enhance or inhibit RNA stability and/or translation. C. Binding to a specific subset of sno-lncRNAs can reduce the amount of Rbfox2 protein available for other nuclear functions. D. Binding of Rbfox3 to the hairpin loop of pre-miRNAs can promote or repress processing. E. Binding of Rbfox2 to nascent promoter-proximal transcripts can recruit PRC2 complexes that repress transcription 28. Black arrow indicates transcription start site; curved green arrow indicates enhancer activity; curved red arrow represents silencer activity.
Many Rbfox CLIP-seq tags lack the prototypical UGCAUG motif, due in part to direct binding with reduced affinity at related motifs such as GCACG 10. Additionally, Rbfox proteins may be recruited to cross-linking sites indirectly as part of the LASR complex via direct binding of hnRNP M 3, or by virtue of RNA secondary structure that juxtaposes distal UGCAUG motifs with other sequences 24. A very recent observation at least partially solves this mystery by showing that cellular Rbfox proteins can reside in a bigger complex termed LASR (large assembly of splicing regulators) 3. In this complex Rbfox can be recruited to RNA indirectly, e.g., through the binding of other components including hnRNP M protein, allowing it to crosslink to RNA and exhibit splicing enhancer activity in the absence of UGCAUG motifs 3.
ALTERNATIVE SPLICING REGULATION BY RBFOX1
UGCAUG motifs are greatly enriched and evolutionarily conserved in proximal introns within 200–300 nt of tissue-specific alternative exons, but are less common and less conserved near non-tissue-specific alternative exons 29 and constitutive exons. The degree of exon inclusion correlates with the number of UGCAUG motifs, at least for muscle-specific exons 30. UGCAUG motifs are enriched adjacent to tissue-specific alternative exons in brain, muscle, selected epithelial and mesenchymal cells, and certain breast cancers 31–34; microexons in brain 35; and alternative “poison” exons in mouse embryonic stem cells that contain premature termination codons 36; and are even enriched and conserved in more distal intronic regions >500nt from neighboring alternative exons 24, 37, 38. Genome-wide mapping of Rbfox binding sites in the brain transcriptome have revealed ~1,059 direct Rbfox target alternative splicing events 17. Over half of these targets show dynamic changes during brain development, suggesting a major role for the Rbfox splicing program.
Rbfox motifs in the downstream intron generally function to enhance splicing, while upstream motifs tend to inhibit splicing, of a neighboring alternative exon. This conclusion is supported by bioinformatic 1, 17, 24, 39–42 and experimental analyses (8 and others) that correlate splicing behavior and motif location. The molecular mechanisms for position-dependent effects on exon splicing are not well understood. In some cases, Rbfox binding may inhibit splicing by competing with constitutive splicing machinery at the branchpoint and/or 3′ splice site 43, 44. Alternatively, Rbfox binding might positively influence splicing by recruiting U1 snRNP to the 5′ splice site via interaction with the U1-C polypeptide 45, 46.
Cooperative regulation of splicing networks by Rbfox1 and other RBPs
Given that 105 to 106 UGCAUG hexamers likely reside in the human transcriptome, what distinguishes functional vs non-functional sites, and what determines tissue-specificity of functional sites? Functional sites tend to reside in highly conserved regions 24, 29, 41 having specific sequence 3 and secondary structure biases 47, indicating that the neighborhood matters. Presumably adjacent sequences are relatively deficient in splicing silencer elements, except when required for regulated antagonism, and enriched in motifs that favor binding of co-regulatory factors that confer tissue-specificity.
A well documented example of coordinate Rbfox splicing regulation occurs in the fibroblast growth factor receptor pre-mRNA in C. elegans muscle 13. Genetic and biochemical evidence show that SUP-12 and the Rbfox-related protein ASD-1 bind cooperatively and stably to adjacent motifs upstream of exon 5B to inhibit its splicing and allow activation of the downstream exon 5A in muscle. Loss of either interaction results in weaker binding in vitro and weaker repression of exon 5B in vivo. This study concluded that co-regulation of tissue-specific splicing, through cooperative binding of multiple factors having partially overlapping expression patterns, provides greater regulatory flexibility than would a simple binary interaction of Rbfox with its motifs 13.
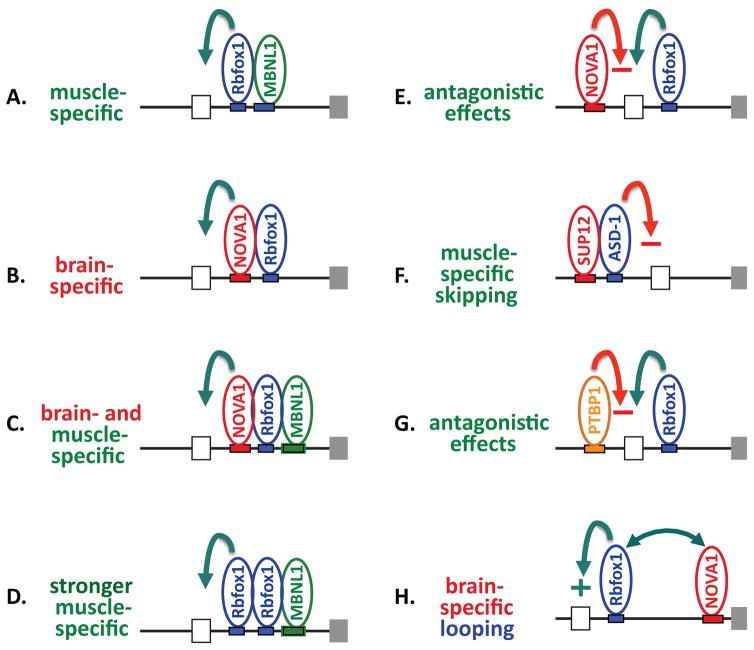
In fact, several co-regulatory networks involving Rbfox proteins have been at least partially characterized in mammalian tissues, likely providing considerable flexibility in regulating sub-networks of tissue-restricted Rbfox target exons (Figure 3). In mouse brain, about 15% of the exons in the NOVA-regulated splicing network have UGCAUG motifs in the flanking intron 31, and experimental analysis confirmed that NOVA and Rbfox synergistically regulate Gabgr2 exon 9. In the human embryonic muscle cell line HFN, a co-regulatory network of splicing events under combinatorial control by Rbfox1 and Mbnl1 was demonstrated 33. In embryonic stem cells, Rbfox2 and MBNL1 cooperate in regulating exons important for pluripotent stem cell differentiation 32, and similar cooperation between Rbfox2 and Mbnl1 can occur in mesenchymal cells 48. In epithelial cells coordinated networks of Rbfox2/ESRP-regulated exons have been reported 49, 50. Finally, co-regulation of splicing events has also been reported between Rbfox and PTBP proteins 35, 51 and between Rbfox and HNRNPH proteins 14, 15. For most of these examples, it appears that Rbfox can either synergize with or antagonize activity of the co-regulators, providing considerable flexibility to the networks.
Figure 3.
Coordinate determination of lineage-specific alternative splicing by Rbfox and other RBPs. A. Model based on co-regulation by Rbfox1 and Mbnl1 in a muscle cell line 33. B. Model based on the common overlap between Rbfox- and Nova-regulated exons in brain 31. C. Model of dual specificity by intersection of muscle- and brain-specific regulatory patterns. D. Model based on co-regulation by Rbfox1 and Mbnl1 in a muscle cell line 33 and correlation of exon inclusion with number of UGCAUG motifs 30. E. Demonstrated antagonistic effects on splicing for an alternative exon in Arhgef12 31. F. Coordinated repression of exon 5B in the egl-15 gene in C. elegans 13. G. Antagonistic effects on splicing of brain microexons H. Brain-specific enhancer activity by looping across the intron, proposed for Gabrg2 exon 9 31. Green arrows indicate enhancer activity; red arrows indicate silencer activity.
Regulating alternative splicing of noncoding exons down-regulates gene expression via AS-NMD
Rbfox proteins can indirectly effect changes in gene expression, when they mediate alternative splicing coupled to nonsense-mediated decay (AS-NMD), due to the introduction of premature termination codons in specific transcript isoforms. This mechanism was demonstrated for Rbfox2-mediated regulation of numerous AS-NMD events in embryonic stem cells 36, but similar outcomes likely could be effected by Rbfox1 or Rbfox3 in other tissues. Changes in expression of Rbfox that increase or decrease relative expression of exons containing premature termination codons will modulate the proportion of transcripts subject to nonsense-mediated decay, thus altering gene expression. Some of the affected transcripts encode splicing factors, and could provide a mechanism for escaping the self-limiting window of expression enforced by negative auto-regulation of such transcripts 36.
NON-SPLICING FUNCTIONS OF RBFOX1
Pre-mRNA splicing is but one of many RNA metabolic processes that are regulated by RBPs in the nucleus, and in the cytoplasm, of higher eukaryotes. This section briefly reviews evidence of Rbfox regulation of microRNA processing in the nucleus, and of mRNA stability and translation efficiency in the cytoplasm. The discovery of cytoplasmic Rbfox1 isoforms 5, 18, and the strong enrichment of UGCAUG motifs in 3′ UTRs 11, 41, clearly indicate that this versatile RBP must have non-splicing functions. Interestingly, both negative and positive effects on mRNA abundance have been demonstrated experimentally in contexts as diverse such as mouse hippocampal neurons 25 and Drosophila germline 7, suggesting that the direction and degree of cytoplasmic Rbfox-dependent gene regulation might depend on combinatorial interactions with other regulators.
Rbfox regulation of microRNA processing
Two groups have reported that Rbfox proteins can regulate processing of microRNA precursors 26, 27. One study used a CLIP-seq approach to identify pri-miRNAs that bind Rbfox3 both in neuronally-differentiated P19 cells and in the mouse central nervous system. Rbfox3 was shown to positively regulate processing of individual pri-miRNAs to pre-miRNAs (e.g., pre-miR-15a), while negatively regulating other pre-miRNA processing events (e.g. pre-miR-485) 26. Selected isoforms of Rbfox1 and Rbfox2 exhibited similar regulation of these two representative pre-miRNAs when exogenously expressed in P19 cells, albeit with weaker activity than Rbfox3. Surprisingly, most of the miRNAs regulated by Rbfox3 in this system do not contain the typical (U)GCAUG sequence motif, suggesting that Rbfox functions in combination with other factors to mediate recruitment of the microprocessor complex to pri-miRNAs. The second study focused on two miRNAs (miR-20b and miR-107) that do contain essential GCAUG motifs in the terminal loops of their primary transcripts 27. Binding of these transcripts to Rbfox1 and Rbfox2 inhibits processing of the pri-microRNAs to pre-microRNAs, reduces expression of the mature microRNAs, and increases expression of targets normally down-regulated by these microRNAs. Together these studies show that Rbfox family members can exert physiological effects in yet another aspect of RNA metabolism, i.e., regulation of miRNA biogenesis.
Cytoplasmic Rbfox1 regulatory networks impact mRNA stability of developmentally important genes from Drosophila to man
The Drosophila A2BP1/Rbfox1 gene encodes a cytoplasmic isoform that affects development through its effects on mRNA stability and translation during germ cell differentiation 7. In this context, cytoplasmic Rbfox1 represses expression of target transcript(s). Isoform-specific knockdown and rescue experiments show that mRNAs with 3′UTR GCAUG motifs, including transcripts encoding the RBP Pumilio, are targets of repression by cytoplasmic but not nuclear Rbfox1 protein. Here Rbfox1 functions in circuitry that titrates Pumilio as needed for germ line maintenance: knockdown of cytoplasmic Rbfox increases Pumilio expression and inhibits germ cell differentiation, while over-expression represses Pumilio to cause precocious germ cell differentiation. Of note, the authors point out that Rbfox1, Rbfox2, and Rbfox3 physically associate with Pumilio1 and Pumilio2 mRNA in the mouse nervous system 7, raising the possibility that critical functions for cytoplasmic Rbfox have been conserved to man.
In fact, functional studies of individual Rbfox1 isoforms in mouse hippocampal neurons had already implicated cytoplasmic Rbfox1 in regulating expression of synaptic and autism-related genes through effects on mRNA abundance 25. Primary hippocampal neurons express abundant cytoplasmic Rbfox that can be depleted by knockdown of Rbfox1 and Rbfox3 transcripts, the two paralogs known to encode cytoplasmic isoforms. Transcriptome analyses of these cells, along with binding assays and genetic rescue experiments, all support the hypothesis that cytoplasmic Rbfox binds to, and positively regulates abundance of, cytoplasmic mRNAs naturally tagged with the UGCAUG motif. Cytoplasmic Rbfox1-regulated transcripts are enriched for genes involved in cortical development and autism, implying that cytoplasmic Rbfox impacts an essential component of the neural development program with disease relevance 25. In the same cells, Rbfox knockdown elicited changes in alternative splicing that could be rescued by expression of nuclear Rbfox1, independent of the abundance effects of cytoplasmic Rbfox.
That Rbfox1-mediated effects on mRNA stability are relevant to human disease is further suggested by bioinformatics analyses that correlate 3′UTR motifs with RBP expression 11. Abundance of transcripts with UGCAUG motifs in the 3′UTR correlates positively with Rbfox1 expression across many tissues, and knockdown of Rbfox1 decreases their abundance, supporting the hypothesis that Rbfox1 enhances mRNA stability. As predicted by this model, Rbfox1-deficient autistic brains exhibit reduced abundance of mRNAs having 3′ Rbfox motifs 11. Since these transcripts are enriched for voltage-gated ion channels, Rbfox1-mediated regulation of transcript stability may affect nervous-system-specific processes.
Rbfox2 interaction with PRC2 can impact transcription networks
Finally, Rbfox2 can impact transcript abundance via a novel mechanism involving interaction with Polycomb repressive complex 2 (PRC2) 28, which methylates H3K27 and is associated with gene silencing activity. Rbfox2 was shown to interact with chromatin-associated RNA, often near gene promoters, and also to bind directly to purified PRC2 complex. An array of bioinformatics and biochemical studies was employed to develop an intriguing model that Rbfox2 interacts with nascent RNA and recruits PRC2 to target genes so as to repress expression. Functional interplay between Rbfox2 and PRC2 may regulate homeostatic gene expression in a nascent RNA abundance-dependent manner to increase or decrease PRC2 recruitment 28. This activity might be restricted to Rbfox2, since it depends on a unique C-terminal domain not found in other Rbfox paralogs.
DEVELOPMENTAL FUNCTIONS OF RBFOX PROTEINS IN ANIMAL MODELS
Rbox function in brain
A brain function for human Rbfox1 was initially proposed on the basis of its interaction with ataxin2, the gene affected in patients with spinocerebellar ataxia type 2 52, and on the abundance of UGCAUG motifs near brain-specific alternative exons. Subsequent exploration of Rbfox protein expression and function in the brain has focused on the mouse cerebellum utilizing Rbfox1loxP/loxP and Rbfox2loxP/loxP strains generated in the Black lab to engineer conditional knockouts 53, 54. Mice with central nervous system (CNS) knockout of either Rbfox1 or Rbfox2 have distinct neurological phenotypes, consistent with their different pattern of expression in the normal cerebellum 53, 54. Rbfox1 knockout mice experience spontaneous seizures and greatly exaggerated responses to systemic administration of kainic acid, a neuroexcitatory agent used in epilepsy studies. Rbfox2 knockout mice have small cerebellums, exhibit progressive difficulty in movement, abnormalities in Purkinje cell function, and not infrequently develop hydrocephalus at a young age. In the search for Rbfox-mediated splicing switches that might explain these phenotypes, normal Rbfox1−/− and Rbfox2−/− brains were compared using Affymetrix exon-junction (MJAY) microarrays. Modest numbers of significant splicing differences were detected for alternative exons in the knockout brains, most of which had adjacent intronic UGCAUG motifs and are likely to be direct targets. Correlating specific splicing changes with specific defects in these pleiotropic phenotypes is challenging, probably due to compensatory changes in Rbfox expression when one of the genes is inactivated. Nevertheless, splicing changes in the Rbfox1 knockout were observed in a few ion channels and neurotransmitters that are involved in synaptic transmission and membrane excitation, and might contribute to the seizure phenotype. The authors cite previous reports showing that two genes with disturbed splicing previously have been implicated in patients with epilepsy (Gabrg2a), or have been shown to alter seizure susceptibility in mice (Grin1).
Knockout of Rbfox gene(s) specifically in post-natal Purkinje cells resulted in aberrant alternative splicing in the Scn8a gene for sodium channel Nav1.6, likely relevant to the observed electrophysiological abnormalities in firing of pacemaking action potentials 54. Importantly, Purkinje cell-specific knockout of the same sodium channel gene was shown by others to generate a pacemaking defect similar to the Rbfox deficiency.
Together these studies have begun to reveal critical functions for both Rbfox1 and Rbfox2 not only during brain development, but also for maintenance of functioning circuits in the adult brain. However, there are inherent difficulties in trying to deconvolute relevant splicing targets and physiological functions across complex brain regions for individual Rbfox proteins, especially in the face of compensatory regulation by other Rbfox paralogs when one is knocked out. This might explain why the number of observed splicing changes is much fewer than expected given the large number of targets predicted by CLIP-seq experiments 17, and why changes in gene expression expected due to loss of cytoplasmic Rbfox1 25 were not observed.
Interestingly, Hamada et al. employed in utero electroporation of RNAi vectors to knock down expression of cytoplasmic 55 or nuclear 56 isoforms of Rbfox1 in ventricular zone progenitor cells of D14.5 mouse embryos. Using a co-transfected GFP expression vector to facilitate imaging of newly generated excitatory neurons during corticogenesis, they demonstrated similar but not identical phenotypes, with defects in neuronal migration and axon growth being more severe under conditions of nuclear Rbfox1 deficiency. Importantly, developmental defects could be rescued by expression of the relevant RNAi-resistant Rbfox1 isoforms. These studies concluded that both cytoplasmic and nuclear isoforms of Rbfox1 are critical for establishing the architecture of the developing cerebral cortex, but detailed transcriptome analyses to identify alterations in splicing or transcript stability have not yet been reported.
Rbfox function in muscle
An important role for Rbfox proteins in vertebrate muscle was suggested by the abundance of intronic UGCAUG motifs flanking muscle-specific alternative exons 30, 41, 57, especially those undergoing developmentally-regulated splicing transitions during maturation of embryonic to adult heart 58. Mutation of the fox-1 and asd-1 paralogs in C. elegans caused aberrant splicing in body wall muscles 59, and knockdown of two muscle-expressed Rbfox genes in zebrafish resulted in abnormal splicing of muscle-specific alternative exons having intronic UGCAUG motifs 60. Rbfox-deficient zebrafish exhibit severe functional defects in both cardiac and skeletal muscle, including reduced heart rate and disorganized skeletal muscle myofibrils leading to loss of embryo motility. Because muscle-specific splicing events are often highly conserved from zebrafish and chickens to mammals 57, 58, 60, it is not surprising that Rbfox knockdowns described in the next section were shown to disturb myoblast differentiation, mature skeletal muscle function, and cardiac function in mice. Importantly, the latter have potential relevance to dilated cardiomyopathy (DCM) in humans.
Conditional knockout in skeletal muscle
In the C2C12 mouse myoblast differentiation model, siRNA-mediated Rbfox1 knockdown had only modest effects, which was unexpected because substantial up-regulation of Rbfox1 accompanies induction of differentiation in these cells. Instead, it was knockdown of Rbfox2 that strongly impaired myoblast fusion 61. Two Rbfox2-regulated alternative exons were identified as critical splicing targets: one that generates an alternative isoform of Mef2d transcription factor, and another that ultimately reduces ROCK2 kinase expression by introducing a premature stop codon that induces NMD. Remarkably, the fusion defect in Rbfox2-deficient myoblasts could be substantially rescued by forced expression of the Rbfox-dependent Mef2d isoform and knockdown of ROCK2 expression.
In normally-developed mature skeletal muscle, Rbfox1-deficiency was engineered in adult mice by doxycycline-induced expression of muscle actin promoter-driven Cre recombinase 22 using the Rbfox1loxP/loxP model. Phenotypic effects included progressive loss of performance in force generation assays, structural irregularities in sarcomeres, and functionally aberrant calcium handling. RNA-seq analysis detected many changes in alternative splicing, enriched in genes functioning in calcium signaling, cytoskeleton organization, and myofibrillar structure. The authors concluded that the phenotype of Rbfox1-deficient muscle probably results from combinatorial effects on splicing of multiple genes necessary for maintenance of muscle structural and functional integrity.
These studies show that Rbfox1 and Rbfox2 regulate distinct sets of splicing events in proliferating myoblasts and differentiated myotubes. To what extent Rbfox effects on mRNA stability and translation, or on miRNA processing might contribute to the phenotype is unknown. Future studies will be needed to integrate the Rbfox-dependent component of muscle differentiation with the overall splicing program, since differentiating muscle expresses many Rbfox-independent splicing transitions.
Conditional knockouts in heart
Rbfox1 protein levels increase post-natally while Rbfox2 expression is higher in the neonatal heart but reduced in the mature adult mouse heart 58, 62, suggesting both overlapping and unique functions during cardiac maturation. Many splicing transitions are dynamically regulated during normal heart development, and the strong enrichment of UGCAUG flanking many of the switched exons further supports a critical role for Rbfox splicing networks 58.
Two recent papers explored the biomedical importance of cardiac Rbfox regulation, using mechanical and genetic models of heart failure in the mouse 62, 63. Both Rbfox1 and Rbfox2 were specifically down-regulated in pressure-overload induced heart failure, in contrast to most other splicing factors, leading to reversion of many developmentally regulated Rbfox target exons to a more fetal-like splicing pattern. Importantly, re-expression of an Rbfox1 transgene not only ameliorates the pathological hypertrophy in these animals, but also rescues some of the aberrant splicing events. In the genetic model, mice with conditional knockout of cardiac Rbfox1 were engineered using the Rbfox1loxP/loxP system. These mice exhibit decreased cardiac function at 6 months, greatly accelerated adverse reactions to pressure overload induced by transaortic constriction, and substantial overlap in aberrant splicing events compared with mechanical heart failure.
An important Rbfox1 regulatory target that likely contributes to the aberrant phenotype in failing hearts is the MEF2 family of transcription factors. Splicing of a key Rbfox- and UGCAUG-regulated alternative exon in MEF2a is perturbed in both heart failure models, but rescued by expression of transgenic Rbfox1 in mice, or by expression of the correct MEF2a isoform in zebrafish. Remarkably, relevance of the RBFox1/Mef2 regulatory circuit to human disease is supported by the finding of decreased Rbfox1 expression and the same aberrant splicing event in dilated cardiomyopathy (DCM) human heart samples 62.
Mice with conditional knockout of cardiac Rbfox2 at the start of cardiogenesis are born normally, with hearts of grossly normal morphology, but they experience progressive heart failure involving a progressive dilated cardiomyopathy phenotype with severe contraction defects 63. Precise comparison with the Rbfox1 knockout is challenging because the two models were generated using different transcriptional promoters to drive expression of Cre recombinase, but both models clearly generate serious cardiac defects, and both exhibit aberrant splicing in genes implicated in heart function and disease.
DISRUPTION OF RBFOX FUNCTION IS ASSOCIATED WITH HUMAN DISEASE
Based on the multi-faceted functions of Rbfox proteins in regulating gene expression, and given the phenotypes observed in animal models, one might expect defects in Rbfox networks to be associated with human disease. Indeed, genome-wide studies have repeatedly identified large deletions and translocations in the Rbfox1 gene for small percentages of patients with neurodevelopmental diseases such as autism and epilepsy (summarized in 64). However, evidence implicating Rbfox networks in human disease continues to expand, as defects in Rbfox1 are associated with heart disease and other disorders, and Rbfox2 mutations have now been associated with heart disease 65. In a few cases, reduced Rbfox1 mRNA expression has been confirmed in autistic brains, where Rbfox1 behaves as a “hub” in the ASD gene transcriptome network 66. Reduced Rbfox1 expression has also been reported in failing hearts of patients with dilated cardiomyopathy (DCM) 62.
So far, mechanistic relationships between Rbfox1 gene deletions, aberrant Rbfox1 protein expression, and neurodevelopmental disorders are not well understood. Nor has the incomplete penetrance of neurodevelopmental disorders sometimes observed in families with Rbfox1 deletions been explained 67. A further confounding factor is that Rbfox1 splicing networks can be perturbed not only by direct mutation, but also by defects in Rbfox1-regulatory factors that secondarily disturb splicing 20, 23.
Mutations in the Rbfox1 gene
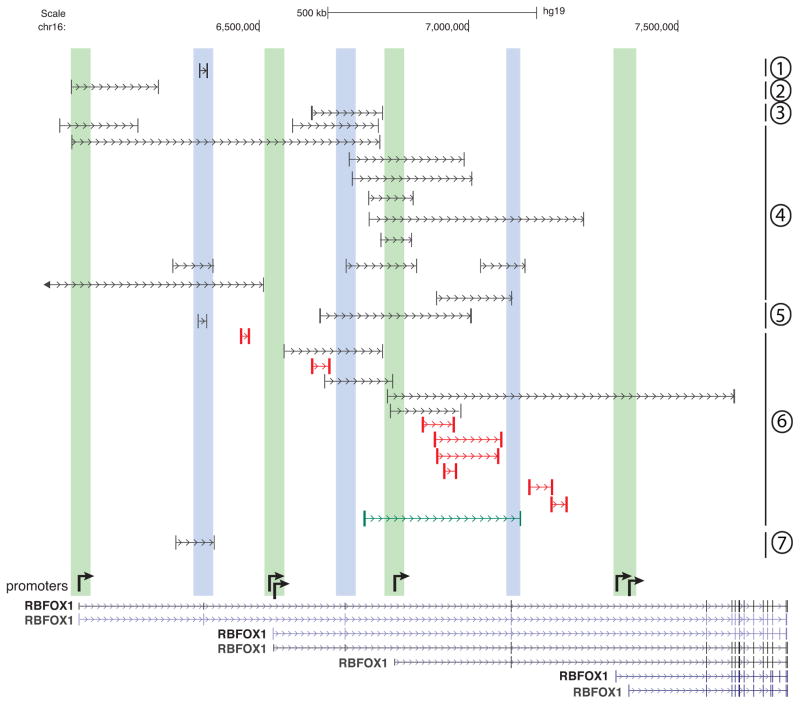
Rbfox1 gene aberrations associated with neurodevelopmental disorders were discovered by virtue of substantial translocations or intragenic deletions mapping to the extraordinarily long 5′ region of the gene. A few of the informative deletions reported in the literature 68–73 are shown in Figure 4. Most deletions remove a first exon (shaded green) or potentially coding internal exons (shaded blue), but a few deletions (depicted in red) apparently encompass only intronic sequence. Not shown here, the farthest upstream promoter region is also disrupted in at least two translocations 74, 75. Mechanistically, these deletions might disrupt Rbfox1 expression by eliminating promoters, disrupting hypothetical transcriptional enhancers, or removing 5′ noncoding exons that impact mRNA stability and translation efficiency. However, given the likelihood that the internal 5′ exons are translated in some brain regions in a promoter-dependent manner (Figure 1), it is possible that functionally important Rbfox1 domains are lost in selected neurons that express extended N-termini.
Figure 4.
Rbfox1 deletions associated with neurodevelopmental disorders in humans. Below are Rbfox1 annotations with arrows depicting presumed promoter locations as described in Figure 1. Green bars, deletions that span promoters; blue bars, deletions that span internal exons likely translated in some contexts as shown in Figure 1. Red lines indicate deletions apparently in introns only. Other Rbfox1 deletions have also been reported 72, 85. Numbers at the right margin indicate the relevant citations for deletions as follows: group 1, 73; 2, 69; 3, 68; 4, 70; 5, 67; 6, 71; 7, 72.
Taken together, other recent observations indicate that the contribution of Rbfox1 (and Rbfox2) mutations to human disease could be far greater than currently appreciated. Intragenic deletions of Rbfox1 have been found in patients with heart disease 65, visual refractive errors 76, 77, and pediatric food allergy 78. Moreover, small mutations within the traditional downstream Rbfox1 coding exons have been reported 67, 79, and exome sequencing studies have suggested that a small proportion of patients with congenital heart disease (CHD) have damaging Rbfox2 mutations 80. In particular, hypoplastic left heart syndrome (HLHS) patients with a truncating nonsense mutation express a shorter Rbfox2 protein that is associated with reduced nuclear localization, reduced expression of transcripts with 3′UTR Rbfox2 binding sites, and reduced activity in splicing 81.
Defects in Rbfox expression secondary to aberrations in other metabolic pathways
Besides direct mutations in the Rbfox1 gene, disturbance in homeostatic mechanisms that maintain normal activity levels of Rbfox1 protein can also cause human disease. Facioscapulohumeral muscular dystrophy (FSHD) is a common neuromuscular disorder associated with myogenic defects and progressive wasting of specific skeletal muscles. Contributing to the pathogenesis in FSHD patients is reduced Rbfox1 mRNA expression that appears to be secondary to over-expression of FRG1, an RBP that binds Rbfox1 mRNA and reduces its half-life 20. Over-expression of FRG1 in mouse muscle and in C2C12 cells reduces Rbfox1 mRNA and induces aberrant splicing of exons that do not interact with FRG1 directly, but rather are Rbfox target exons. Interestingly, mental retardation and autism sometimes occur in severely affected FSHD infants 20.
A second candidate Rbfox1-related disease is Prader-Willi Syndrome (PWS), a neurogenetic disorder typically associated with deletion of a 5–6 Mb region on the paternally derived chromosome 15. This region encodes a class of nuclear sno-lncRNAs (SNORD116) that harbor multiple Rbfox binding motifs and can indirectly modulate splicing of Rbfox2 target exons by functioning as molecular sinks for Rbfox proteins 23. By inference, Rbfox1 and/or Rbfox3 could be partially sequestered by a similar mechanism in the brain, but this has not been demonstrated directly.
Finally, aberrant expression of Rbfox2 or Rbfox1 has been described in diverse disease contexts including diabetic heart and Parkinson’s disease (PD) neurons, respectively 82, 83. In the diabetic heart, up-regulation of Rbfox2 has been proposed to auto-regulate its own splicing, promoting an exon skipping event that generates a dominant negative protein isoform with an aberrant RRM having reduced Rbfox2 activity. In dopaminergic neurons differentiated from iPS cells of Parkinson’s disease (PD) patients, Rbfox1 transcripts are substantially over-expressed in conjunction with aberrant splicing of Rbfox-regulated exons. Whether this effect also involves dominant negative Rbfox isoforms was not reported. Notably, elevated neuronal Rbfox1 has been seen in patients with primary mutations in three different genes (LRRK2, SNCA, and PARKIN) 83. It will be interesting and important to elucidate the metabolic pathways by which Rbfox1 up-regulation in PD neurons and Rbfox2 up-regulation in diabetic hearts is mediated.
CONCLUSIONS
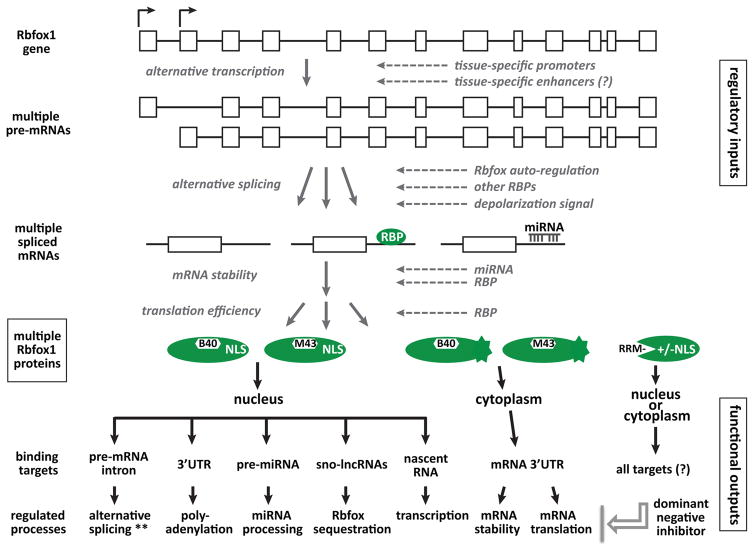
Rbfox protein represent a versatile, multifunctional group of RBPs encoded by a large and complex gene. Differentiating and mature cells of muscle and neuronal lineages each express a particular complement of Rbfox isoforms that can be regulated by numerous inputs at major transcriptional and post-transcriptional steps (shown for Rbfox1 in Figure 5). The resulting pools of nuclear and cytoplasmic Rbfox1 proteins, often co-expressed in the same cells, then bind to specific target sequences at key regulatory sites in the transcriptome to control downstream gene networks.
Figure 5.
Rbfox1 regulatory circuitry. The top half of the figure indicates multiple steps of gene expression at which the expression of Rbfox1 proteins is regulated, while the lower half depicts some of the functional outputs manifested by these proteins. ** alternative splicing regulates coding exons in many target transcripts, but also noncoding exons in others that can induce NMD and reduced transcript levels.
There are many unanswered questions on both sides of the ledger, regarding inputs that regulate Rbfox isoform production, and functional outputs executed by these isoforms. Very little is known about transcriptional controls that activate expression developmentally, at different promoter sites and in different populations of skeletal and cardiac muscle cells, and especially in various neuronal populations in the brain. The functions of N-terminal variants predicted to be encoded by some of the alternative first exons (Figure 1) is unknown. Presumably, tissue- and development-specific transcriptional enhancers play important roles in determining spatiotemporal expression patterns, and these might even be the affected elements in some patients with Rbfox1 gene deletions.
Exciting advances have been made in characterizing developmental processes that are strongly influenced by Rbfox-regulated networks in both cytoplasm and nucleus and, in a few cases, identifying key transcript targets. However, complete Rbfox knockouts in complex tissues remain difficult to interpret. Better understanding of Rbfox regulation might enable more surgical strikes to knock down selected Rbfox isoforms or to limit knockdown to specific neuronal regions relevant to human disorders. Together, hopefully, new studies will provide new insights into normal development, that will also inform therapeutic efforts to treat human disorders that arise from perturbations in Rbfox1 networks.
Acknowledgments
This work was supported by National Institutes of Health [DK094699] and Director, Office of Science and Office of Biological & Environmental Research of the US Department of Energy [DE-AC02-05CH1123].
Footnotes
No conflicts of interest to declare
References
- 1.Barash Y, Calarco JA, Gao W, Pan Q, Wang X, Shai O, Blencowe BJ, Frey BJ. Deciphering the splicing code. Nature. 2010;465:53–59. doi: 10.1038/nature09000. [DOI] [PubMed] [Google Scholar]
- 2.Xiong HY, Alipanahi B, Lee LJ, Bretschneider H, Merico D, Yuen RKC, Hua Y, Gueroussov S, Najafabadi HS, Hughes TR, et al. The human splicing code reveals new insights into the genetic determinants of disease. Science. 2015:347. doi: 10.1126/science.1254806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Damianov A, Ying Y, Lin CH, Lee JA, Tran D, Vashisht AA, Bahrami-Samani E, Xing Y, Martin KC, Wohlschlegel JA, et al. Rbfox Proteins Regulate Splicing as Part of a Large Multiprotein Complex LASR. Cell. 2016;165:606–619. doi: 10.1016/j.cell.2016.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Damianov A, Black DL. Autoregulation of Fox protein expression to produce dominant negative splicing factors. RNA. 2010;16:405–416. doi: 10.1261/rna.1838210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakahata S, Kawamoto S. Tissue-dependent isoforms of mammalian Fox-1 homologs are associated with tissue-specific splicing activities. Nucleic Acids Res. 2005;33:2078–2089. doi: 10.1093/nar/gki338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroyanagi H. Fox-1 family of RNA-binding proteins. Cell Mol Life Sci. 2009;66:3895–3907. doi: 10.1007/s00018-009-0120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carreira-Rosario A, Bhargava V, Hillebrand J, Kollipara RK, Ramaswami M, Buszczak M. Repression of Pumilio Protein Expression by Rbfox1 Promotes Germ Cell Differentiation. Dev Cell. 2016;36:562–571. doi: 10.1016/j.devcel.2016.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin Y, Suzuki H, Maegawa S, Endo H, Sugano S, Hashimoto K, Yasuda K, Inoue K. A vertebrate RNA-binding protein Fox-1 regulates tissue-specific splicing via the pentanucleotide GCAUG. EMBO J. 2003;22:905–912. doi: 10.1093/emboj/cdg089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ponthier JL, Schluepen C, Chen W, Lersch RA, Gee SL, Hou VC, Lo AJ, Short SA, Chasis JA, Winkelmann JC, et al. Fox-2 Splicing Factor Binds to a Conserved Intron Motif to Promote Inclusion of Protein 4. 1R Alternative Exon 16. J Biol Chem. 2006;281:12468–12474. doi: 10.1074/jbc.M511556200. [DOI] [PubMed] [Google Scholar]
- 10.Lambert N, Robertson A, Jangi M, McGeary S, Sharp PA, Burge CB. RNA Bind-n-Seq: quantitative assessment of the sequence and structural binding specificity of RNA binding proteins. Mol Cell. 2014;54:887–900. doi: 10.1016/j.molcel.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray D, Kazan H, Cook KB, Weirauch MT, Najafabadi HS, Li X, Gueroussov S, Albu M, Zheng H, Yang A, et al. A compendium of RNA-binding motifs for decoding gene regulation. Nature. 2013;499:172–177. doi: 10.1038/nature12311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Auweter SD, Fasan R, Reymond L, Underwood JG, Black DL, Pitsch S, Allain FH. Molecular basis of RNA recognition by the human alternative splicing factor Fox-1. EMBO J. 2006;25:163–173. doi: 10.1038/sj.emboj.7600918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuroyanagi H, Ohno G, Mitani S, Hagiwara M. The Fox-1 family and SUP-12 coordinately regulate tissue-specific alternative splicing in vivo. Mol Cell Biol. 2007;27:8612–8621. doi: 10.1128/MCB.01508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sun S, Zhang Z, Fregoso O, Krainer AR. Mechanisms of activation and repression by the alternative splicing factors RBFOX1/2. RNA. 2012 doi: 10.1261/rna.030486.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mauger DM, Lin C, Garcia-Blanco MA. hnRNP H and hnRNP F complex with Fox2 to silence fibroblast growth factor receptor 2 exon IIIc. Mol Cell Biol. 2008;28:5403–5419. doi: 10.1128/MCB.00739-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baraniak AP, Chen JR, Garcia-Blanco MA. Fox-2 mediates epithelial cell-specific fibroblast growth factor receptor 2 exon choice. Mol Cell Biol. 2006;26:1209–1222. doi: 10.1128/MCB.26.4.1209-1222.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weyn-Vanhentenryck Sebastien M, Mele A, Yan Q, Sun S, Farny N, Zhang Z, Xue C, Herre M, Silver Pamela A, Zhang Michael Q, et al. HITS-CLIP and Integrative Modeling Define the Rbfox Splicing-Regulatory Network Linked to Brain Development and Autism. Cell Reports. 2014;6:1139–1152. doi: 10.1016/j.celrep.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dredge BK, Jensen KB. NeuN/Rbfox3 nuclear and cytoplasmic isoforms differentially regulate alternative splicing and nonsense-mediated decay of Rbfox2. PLoS ONE. 2011;6:e21585. doi: 10.1371/journal.pone.0021585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JA, Tang ZZ, Black DL. An inducible change in Fox-1/A2BP1 splicing modulates the alternative splicing of downstream neuronal target exons. Genes Dev. 2009;23:2284–2293. doi: 10.1101/gad.1837009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pistoni M, Shiue L, Cline MS, Bortolanza S, Neguembor MV, Xynos A, Ares M, Jr, Gabellini D. Rbfox1 Downregulation and Altered Calpain 3 Splicing by FRG1 in a Mouse Model of Facioscapulohumeral Muscular Dystrophy (FSHD) PLoS Genet. 2013;9:e1003186. doi: 10.1371/journal.pgen.1003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guven-Ozkan T, Busto Germain U, Schutte Soleil S, Cervantes-Sandoval I, O’Dowd Diane K, Davis Ronald L. MiR-980 Is a Memory Suppressor MicroRNA that Regulates the Autism-Susceptibility Gene A2bp1. Cell Reports. 2016;14:1698–1709. doi: 10.1016/j.celrep.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedrotti S, Giudice J, Dagnino-Acosta A, Knoblauch M, Singh RK, Hanna A, Mo Q, Hicks J, Hamilton S, Cooper TA. The RNA-binding protein Rbfox1 regulates splicing required for skeletal muscle structure and function. Hum Mol Genet. 2015;24:2360–2374. doi: 10.1093/hmg/ddv003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yin QF, Yang L, Zhang Y, Xiang JF, Wu YW, Carmichael GG, Chen LL. Long noncoding RNAs with snoRNA ends. Mol Cell. 2012;48:219–230. doi: 10.1016/j.molcel.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 24.Lovci MT, Ghanem D, Marr H, Arnold J, Gee S, Parra M, Liang TY, Stark TJ, Gehman LT, Hoon S, et al. Rbfox proteins regulate alternative mRNA splicing through evolutionarily conserved RNA bridges. Nat Struct Mol Biol. 2013;20:1434–1442. doi: 10.1038/nsmb.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee JA, Damianov A, Lin CH, Fontes M, Parikshak NN, Anderson ES, Geschwind DH, Black DL, Martin KC. Cytoplasmic Rbfox1 Regulates the Expression of Synaptic and Autism-Related Genes. Neuron. 2016;89:113–128. doi: 10.1016/j.neuron.2015.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim KK, Yang Y, Zhu J, Adelstein RS, Kawamoto S. Rbfox3 controls the biogenesis of a subset of microRNAs. Nat Struct Mol Biol. 2014;21:901–910. doi: 10.1038/nsmb.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y, Zubovic L, Yang F, Godin K, Pavelitz T, Castellanos J, Macchi P, Varani G. Rbfox proteins regulate microRNA biogenesis by sequence-specific binding to their precursors and target downstream Dicer. Nucleic Acids Res. 2016 doi: 10.1093/nar/gkw177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei C, Xiao R, Chen L, Cui H, Zhou Y, Xue Y, Hu J, Zhou B, Tsutsui T, Qiu J, et al. RBFox2 Binds Nascent RNA to Globally Regulate Polycomb Complex 2 Targeting in Mammalian Genomes. Mol Cell. 2016;62:875–889. doi: 10.1016/j.molcel.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Minovitsky S, Gee SL, Schokrpur S, Dubchak I, Conboy JG. The splicing regulatory element, UGCAUG, is phylogenetically and spatially conserved in introns that flank tissue-specific alternative exons. Nucleic Acids Res. 2005;33:714–724. doi: 10.1093/nar/gki210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Das D, Clark TA, Schweitzer A, Yamamoto M, Marr H, Arribere J, Minovitsky S, Poliakov A, Dubchak I, Blume JE, et al. A correlation with exon expression approach to identify cis-regulatory elements for tissue-specific alternative splicing. Nucleic Acids Res. 2007;35:4845–4857. doi: 10.1093/nar/gkm485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Frias MA, Mele A, Ruggiu M, Eom T, Marney CB, Wang H, Licatalosi DD, Fak JJ, Darnell RB. Integrative modeling defines the Nova splicing-regulatory network and its combinatorial controls. Science. 2010;329:439–443. doi: 10.1126/science.1191150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venables JP, Lapasset L, Gadea G, Fort P, Klinck R, Irimia M, Vignal E, Thibault P, Prinos P, Chabot B, et al. MBNL1 and RBFOX2 cooperate to establish a splicing programme involved in pluripotent stem cell differentiation. Nat Commun. 2013;4:2480. doi: 10.1038/ncomms3480. [DOI] [PubMed] [Google Scholar]
- 33.Klinck R, Fourrier A, Thibault P, Toutant J, Durand M, Lapointe E, Caillet-Boudin ML, Sergeant N, Gourdon G, Meola G, et al. RBFOX1 cooperates with MBNL1 to control splicing in muscle, including events altered in myotonic dystrophy type 1. PLoS One. 2014;9:e107324. doi: 10.1371/journal.pone.0107324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim KK, Kim YC, Adelstein RS, Kawamoto S. Fox-3 and PSF interact to activate neural cell-specific alternative splicing. Nucleic Acids Res. 2011;39:3064–3078. doi: 10.1093/nar/gkq1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li YI, Sanchez-Pulido L, Haerty W, Ponting CP. RBFOX and PTBP1 proteins regulate the alternative splicing of micro-exons in human brain transcripts. Genome Research. 2015;25:1–13. doi: 10.1101/gr.181990.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jangi M, Boutz PL, Paul P, Sharp PA. Rbfox2 controls autoregulation in RNA-binding protein networks. Genes Dev. 2014;28:637–651. doi: 10.1101/gad.235770.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baraniak AP, Lasda EL, Wagner EJ, Garcia-Blanco MA. A stem structure in fibroblast growth factor receptor 2 transcripts mediates cell-type-specific splicing by approximating intronic control elements. Mol Cell Biol. 2003;23:9327–9337. doi: 10.1128/MCB.23.24.9327-9337.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo N, Kawamoto S. An intronic downstream enhancer promotes 3′ splice site usage of a neural cell-specific exon. J Biol Chem. 2000;275:33641–33649. doi: 10.1074/jbc.M005597200. [DOI] [PubMed] [Google Scholar]
- 39.Yeo GW, Coufal NG, Liang TY, Peng GE, Fu XD, Gage FH. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nat Struct Mol Biol. 2009;16:130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witten JT, Ule J. Understanding splicing regulation through RNA splicing maps. Trends Genet. 2011;27:89–97. doi: 10.1016/j.tig.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang ET, Sandberg R, Luo S, Khrebtukova I, Zhang L, Mayr C, Kingsmore SF, Schroth GP, Burge CB. Alternative isoform regulation in human tissue transcriptomes. Nature. 2008;456:470–476. doi: 10.1038/nature07509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang C, Zhang Z, Castle J, Sun S, Johnson J, Krainer AR, Zhang MQ. Defining the regulatory network of the tissue-specific splicing factors Fox-1 and Fox-2. Genes Dev. 2008;22:2550–2563. doi: 10.1101/gad.1703108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou HL, Baraniak AP, Lou H. Role for Fox-1/Fox-2 in mediating the neuronal pathway of calcitonin/calcitonin gene-related peptide alternative RNA processing. Mol Cell Biol. 2007;27:830–841. doi: 10.1128/MCB.01015-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohno G, Ono K, Togo M, Watanabe Y, Ono S, Hagiwara M, Kuroyanagi H. Muscle-specific splicing factors ASD-2 and SUP-12 cooperatively switch alternative pre-mRNA processing patterns of the ADF/cofilin gene in Caenorhabditis elegans. PLoS Genet. 2012;8:e1002991. doi: 10.1371/journal.pgen.1002991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohkura N, Takahashi M, Yaguchi H, Nagamura Y, Tsukada T. Coactivator-associated arginine methyltransferase 1, CARM1, affects pre-mRNA splicing in an isoform-specific manner. J Biol Chem. 2005;280:28927–28935. doi: 10.1074/jbc.M502173200. [DOI] [PubMed] [Google Scholar]
- 46.Huang SC, Ou AC, Park J, Yu F, Yu B, Lee A, Yang G, Zhou A, Benz EJ., Jr RBFOX2 promotes protein 4. 1R exon 16 selection via U1 snRNP recruitment. Mol Cell Biol. 2012;32:513–526. doi: 10.1128/MCB.06423-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spitale RC, Flynn RA, Zhang QC, Crisalli P, Lee B, Jung JW, Kuchelmeister HY, Batista PJ, Torre EA, Kool ET, et al. Structural imprints in vivo decode RNA regulatory mechanisms. Nature. 2015;519:486–490. doi: 10.1038/nature14263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Venables JP, Brosseau JP, Gadea G, Klinck R, Prinos P, Beaulieu JF, Lapointe E, Durand M, Thibault P, Tremblay K, et al. RBFOX2 Is an Important Regulator of Mesenchymal Tissue-Specific Splicing in both Normal and Cancer Tissues. Mol Cell Biol. 2013;33:396–405. doi: 10.1128/MCB.01174-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dittmar KA, Jiang P, Park JW, Amirikian K, Wan J, Shen S, Xing Y, Carstens RP. Genome-Wide Determination of a Broad ESRP-Regulated Posttranscriptional Network by High-Throughput Sequencing. Mol Cell Biol. 2012;32:1468–1482. doi: 10.1128/MCB.06536-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mallinjoud P, Villemin JP, Mortada H, Polay Espinoza M, Desmet FO, Samaan S, Chautard E, Tranchevent LC, Auboeuf D. Endothelial, epithelial, and fibroblast cells exhibit specific splicing programs independently of their tissue of origin. Genome Res. 2014;24:511–521. doi: 10.1101/gr.162933.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shapiro IM, Cheng AW, Flytzanis NC, Balsamo M, Condeelis JS, Oktay MH, Burge CB, Gertler FB. An EMT-Driven Alternative Splicing Program Occurs in Human Breast Cancer and Modulates Cellular Phenotype. PLoS Genet. 2011;7:e1002218. doi: 10.1371/journal.pgen.1002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shibata H, Huynh DP, Pulst SM. A novel protein with RNA-binding motifs interacts with ataxin-2. Hum Mol Genet. 2000;9:1303–1313. doi: 10.1093/hmg/9.9.1303. [DOI] [PubMed] [Google Scholar]
- 53.Gehman LT, Stoilov P, Maguire J, Damianov A, Lin CH, Shiue L, Ares M, Jr, Mody I, Black DL. The splicing regulator Rbfox1 (A2BP1) controls neuronal excitation in the mammalian brain. Nat Genet. 2011;43:706–711. doi: 10.1038/ng.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gehman LT, Meera P, Stoilov P, Shiue L, O’Brien JE, Meisler MH, Ares M, Jr, Otis TS, Black DL. The splicing regulator Rbfox2 is required for both cerebellar development and mature motor function. Genes Dev. 2012;26:445–460. doi: 10.1101/gad.182477.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamada N, Ito H, Iwamoto I, Morishita R, Tabata H, Nagata K-i. Role of the cytoplasmic isoform of RBFOX1/A2BP1 in establishing the architecture of the developing cerebral cortex. Molecular Autism. 2015;6:1–13. doi: 10.1186/s13229-015-0049-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hamada N, Ito H, Nishijo T, Iwamoto I, Morishita R, Tabata H, Momiyama T, Nagata K-I. Essential role of the nuclear isoform of RBFOX1, a candidate gene for autism spectrum disorders, in the brain development. Scientific Reports. 2016:6. doi: 10.1038/srep30805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bland CS, Wang ET, Vu A, David MP, Castle JC, Johnson JM, Burge CB, Cooper TA. Global regulation of alternative splicing during myogenic differentiation. Nucleic Acids Res. 2010;38:7651–7664. doi: 10.1093/nar/gkq614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kalsotra A, Xiao X, Ward AJ, Castle JC, Johnson JM, Burge CB, Cooper TA. A postnatal switch of CELF and MBNL proteins reprograms alternative splicing in the developing heart. Proc Natl Acad Sci U S A. 2008;105:20333–20338. doi: 10.1073/pnas.0809045105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuroyanagi H, Kobayashi T, Mitani S, Hagiwara M. Transgenic alternative-splicing reporters reveal tissue-specific expression profiles and regulation mechanisms in vivo. Nat Methods. 2006;3:909–915. doi: 10.1038/nmeth944. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher TL, Arribere JA, Geurts PA, Exner CR, McDonald KL, Dill KK, Marr HL, Adkar SS, Garnett AT, Amacher SL, et al. Rbfox-regulated alternative splicing is critical for zebrafish cardiac and skeletal muscle functions. Dev Biol. 2011;359:251–261. doi: 10.1016/j.ydbio.2011.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh RK, Xia Z, Bland CS, Kalsotra A, Scavuzzo MA, Curk T, Ule J, Li W, Cooper TA. Rbfox2-coordinated alternative splicing of Mef2d and Rock2 controls myoblast fusion during myogenesis. Mol Cell. 2014;55:592–603. doi: 10.1016/j.molcel.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gao C, Ren S, Lee J-H, Qiu J, Chapski DJ, Rau CD, Zhou Y, Abdellatif M, Nakano A, Vondriska TM, et al. RBFox1-mediated RNA splicing regulates cardiac hypertrophy and heart failure. The Journal of Clinical Investigation. 2016;126:195–206. doi: 10.1172/JCI84015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wei C, Qiu J, Zhou Y, Xue Y, Hu J, Ouyang K, Banerjee I, Zhang C, Chen B, Li H, et al. Repression of the Central Splicing Regulator RBFox2 Is Functionally Linked to Pressure Overload-Induced Heart Failure. Cell Rep. 2015;10:1521–1533. doi: 10.1016/j.celrep.2015.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bill BR, Lowe JK, DyBuncio CT, Fogel BL. Orchestration of Neurodevelopmental Programs by RBFOX1: Implications for Autism Spectrum Disorder. International review of neurobiology. 2013;113:251–267. doi: 10.1016/B978-0-12-418700-9.00008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lale S, Yu S, Ahmed A. Complex Congenital Heart Defects in Association with Maternal Diabetes and Partial Deletion of the A2BP1 Gene. Fetal and Pediatric Pathology. 2011;30:161–166. doi: 10.3109/15513815.2010.547555. [DOI] [PubMed] [Google Scholar]
- 66.Voineagu I, Wang X, Johnston P, Lowe JK, Tian Y, Horvath S, Mill J, Cantor RM, Blencowe BJ, Geschwind DH. Transcriptomic analysis of autistic brain reveals convergent molecular pathology. Nature. 2011;474:380–384. doi: 10.1038/nature10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lal D, Reinthaler EM, Altmuller J, Toliat MR, Thiele H, Nurnberg P, Lerche H, Hahn A, Moller RS, Muhle H, et al. RBFOX1 and RBFOX3 Mutations in Rolandic Epilepsy. PLoS ONE. 2013;8:e73323. doi: 10.1371/journal.pone.0073323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gallant NM, Baldwin E, Salamon N, Dipple KM, Quintero-Rivera F. Pontocerebellar hypoplasia in association with de novo 19p13.11p13. 12 microdeletion. American Journal of Medical Genetics Part A. 2011;155:2871–2878. doi: 10.1002/ajmg.a.34286. [DOI] [PubMed] [Google Scholar]
- 69.Sebat J, Lakshmi B, Malhotra D, Troge J, Lese-Martin C, Walsh T, Yamrom B, Yoon S, Krasnitz A, Kendall J, et al. Strong Association of De Novo Copy Number Mutations with Autism. Science. 2007;316:445–449. doi: 10.1126/science.1138659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lal D, Pernhorst K, Klein KM, Reif P, Tozzi R, Toliat MR, Winterer G, Neubauer B, Nürnberg P, Rosenow F, et al. Extending the phenotypic spectrum of RBFOX1 deletions: Sporadic focal epilepsy. Epilepsia. 2015;56:e129–e133. doi: 10.1111/epi.13076. [DOI] [PubMed] [Google Scholar]
- 71.Zhao WW. Intragenic deletion of RBFOX1 associated with neurodevelopmental/neuropsychiatric disorders and possibly other clinical presentations. Mol Cytogenet. 2013;6:26. doi: 10.1186/1755-8166-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fanciulli M, Pasini E, Malacrida S, Striano P, Striano S, Michelucci R, Ottman R, Nobile C. Copy number variations and susceptibility to lateral temporal epilepsy: A study of 21 pedigrees. Epilepsia. 2014;55:1651–1658. doi: 10.1111/epi.12767. [DOI] [PubMed] [Google Scholar]
- 73.Davis LK, Maltman N, Mosconi MW, Macmillan C, Schmitt L, Moore K, Francis SM, Jacob S, Sweeney JA, Cook EH. Rare inherited A2BP1 deletion in a proband with autism and developmental hemiparesis. Am J Med Genet A. 2012;158A:1654–1661. doi: 10.1002/ajmg.a.35396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bhalla K, Phillips HA, Crawford J, McKenzie OL, Mulley JC, Eyre H, Gardner AE, Kremmidiotis G, Callen DF. The de novo chromosome 16 translocations of two patients with abnormal phenotypes (mental retardation and epilepsy) disrupt the A2BP1 gene. J Hum Genet. 2004;49:308–311. doi: 10.1007/s10038-004-0145-4. [DOI] [PubMed] [Google Scholar]
- 75.Martin CL, Duvall JA, Ilkin Y, Simon JS, Arreaza MG, Wilkes K, Alvarez-Retuerto A, Whichello A, Powell CM, Rao K, et al. Cytogenetic and molecular characterization of A2BP1/FOX1 as a candidate gene for autism. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:869–876. doi: 10.1002/ajmg.b.30530. [DOI] [PubMed] [Google Scholar]
- 76.Kiefer AK, Tung JY, Do CB, Hinds DA, Mountain JL, Francke U, Eriksson N. Genome-Wide Analysis Points to Roles for Extracellular Matrix Remodeling, the Visual Cycle, and Neuronal Development in Myopia. PLoS Genet. 2013;9:e1003299. doi: 10.1371/journal.pgen.1003299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Stambolian D, Wojciechowski R, Oexle K, Pirastu M, Li X, Raffel LJ, Cotch MF, Chew EY, Klein B, Klein R, et al. Meta-analysis of genome-wide association studies in five cohorts reveals common variants in RBFOX1, a regulator of tissue-specific splicing, associated with refractive error. Human Molecular Genetics. 2013;22:2754–2764. doi: 10.1093/hmg/ddt116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li J, Fung I, Glessner JT, Pandey R, Wei Z, Bakay M, Mentch FD, Pellegrino R, Wang T, Kim C, et al. Copy Number Variations in CTNNA3 and RBFOX1 Associate with Pediatric Food Allergy. The Journal of Immunology. 2015;195:1599–1607. doi: 10.4049/jimmunol.1402310. [DOI] [PubMed] [Google Scholar]
- 79.Griswold AJ, Dueker ND, Van Booven D, Rantus JA, Jaworski JM, Slifer SH, Schmidt MA, Hulme W, Konidari I, Whitehead PL, et al. Targeted massively parallel sequencing of autism spectrum disorder-associated genes in a case control cohort reveals rare loss-of-function risk variants. Mol Autism. 2015;6:43. doi: 10.1186/s13229-015-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Homsy J, Zaidi S, Shen Y, Ware JS, Samocha KE, Karczewski KJ, DePalma SR, McKean D, Wakimoto H, Gorham J, et al. De novo mutations in congenital heart disease with neurodevelopmental and other congenital anomalies. Science. 2015;350:1262–1266. doi: 10.1126/science.aac9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Verma SK, Deshmukh V, Nutter CA, Jaworski E, Jin W, Wadhwa L, Abata J, Ricci M, Lincoln J, Martin JF, et al. Rbfox2 function in RNA metabolism is impaired in hypoplastic left heart syndrome patient hearts. Scientific Reports. 2016;6:30896. doi: 10.1038/srep30896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nutter CA, Jaworski EA, Verma SK, Deshmukh V, Wang Q, Botvinnik OB, Lozano MJ, Abass IJ, Ijaz T, Brasier AR, et al. Dysregulation of RBFOX2 Is an Early Event in Cardiac Pathogenesis of Diabetes. Cell Reports. 2016;15:2200–2213. doi: 10.1016/j.celrep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin L, Goke J, Cukuroglu E, Dranias MR, VanDongen AM, Stanton LW. Molecular Features Underlying Neurodegeneration Identified through In Vitro Modeling of Genetically Diverse Parkinson’s Disease Patients. Cell Rep. 2016;15:2411–2426. doi: 10.1016/j.celrep.2016.05.022. [DOI] [PubMed] [Google Scholar]
- 84.Kim KK, Adelstein RS, Kawamoto S. Identification of neuronal nuclei (NeuN) as Fox-3, a new member of the Fox-1 gene family of splicing factors. J Biol Chem. 2009;284:31052–31061. doi: 10.1074/jbc.M109.052969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Turner TN, Hormozdiari F, Duyzend MH, McClymont SA, Hook PW, Iossifov I, Raja A, Baker C, Hoekzema K, Stessman HA, et al. Genome Sequencing of Autism-Affected Families Reveals Disruption of Putative Noncoding Regulatory DNA. Am J Hum Genet. 2016;98:58–74. doi: 10.1016/j.ajhg.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]