Abstract
Introduction/Background
Traditional imaging assessment criteria may not correlate well with clinical benefit from vascular endothelial growth factor pathway-directed therapy in metastatic renal cancer. Preclinical data suggest tumor growth is preceded by a rise in Ktrans, a parameter derived from dynamic contrast enhanced (DCE)-MRI that reflects vascular permeability. We thus hypothesized that Ktrans may be a predictive biomarker for pazopanib.
Patients and Methods
Patients with metastatic renal cancer were treated with pazopanib at 800 mg oral daily until disease progression. MRI of the abdomen and pelvis with a DCE-MRI sequence was obtained at baseline and every 8 weeks.
Results
Seventy-three DCE-MRIs were completed and 66 were technically assessable. Of the 17 patients with at least one post-baseline DCE-MRI, 16 (94%) had a decline in Ktrans. Changes in Ktrans compared to baseline after 1, 8, 16, and 24 weeks were −49%, −65%, −63%, and −53%, respectively (P = 0.0052, RM ANOVA). The median Ktrans nadir occurred at 8 weeks. The median progression free survival (PFS) was 32.1 weeks. PFS was longer in patients with a higher baseline Ktrans values (P = 0.036, log-rank). Baseline Ktrans did not reach significance in a Cox-proportional hazard model including clinical prognostic index and prior treatments (P = 0.083).
Conclusion
We demonstrate that Ktrans is a pharmacodynamic biomarker for pazopanib therapy in metastatic renal cancer. Due to the small sample size, the predictive capacity of Ktrans recovery could not be assessed, but baseline Ktrans correlated with progression free survival.
Keywords: Renal cancer, DCE-MRI, VEGF receptor tyrosine kinase, Ktrans, progression
INTRODUCTION
Clear cell renal cancer is characterized by inactivation of the von Hippel-Lindau (VHL) pathway, which leads to upregulation of the HIF2α transcription factor and vascular endothelial growth factor (VEGF).1 This process results in angiogenesis that is necessary for growth, invasion, and metastases. Modern antineoplastic therapies have exploited this dependency by targeting the VEGF pathway. Pazopanib is one such FDA approved agent.2, 3 While generally efficacious, the duration of benefit with VEGF pathway inhibitors, such as pazopanib, is variable. Furthermore, traditional imaging assessment criteria may not correlate well with clinical outcome since these drugs often result in growth inhibition, as opposed to direct cytotoxicity.4 Thus, it remains difficult to determine whether a patient continues to derive benefit from treatment at clinical re-assessment with each cycle. Given that these agents can have significant toxicities impacting quality of life, identification and validation of novel biomarkers in this space is critical.
Quantitative and semi-quantitative parameters derived from dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) have been identified as biomarkers for VEGF pathway-directed agents.4–8 Some studies have also demonstrated predictive and prognostic value for DCE-MRI-based biomarkers.6, 7 Ktrans is one parameter that reflects perfusion rate and capillary permeability. Studies in renal cancer have demonstrated that it declines an average of 30% upon treatment with VEGF targeted therapy, making it a potentially important pharmacodynamic biomarker.6, 7, 9 The initial change in Ktrans is dose dependent, but has not been shown to correlate with time to progression in prior studies. There remains limited clinical data on longitudinal assessment of Ktrans during VEGF pathway directed therapy. Pre-clinical animal models strongly suggest that changes in blood flow and perfusion, as assessed by Ktrans, precede tumor growth.10, 11 We thus hypothesized that recovery of Ktrans is predictive of progression in metastatic renal cancer patients treated with pazopanib. In order to address this question, we conducted a clinical trial with serial assessments of Ktrans during treatment with pazopanib for patients with metastatic renal cancer.
PATIENTS AND METHODS
Patients
Patients with histologically confirmed metastatic clear cell renal cancer without prior pazopanib therapy were eligible. Other requirements included measurable disease with at least one lesion in the abdomen or pelvis ≥ 20 mm on MRI or CT scan, WHO performance status 0 to 2, and normal organ function defined as GFR > 30 mL/min, AST/ALT ≤ 2.5 × upper limit of normal, and total bilirubin ≤ 1.5 upper limit of normal. Exclusion criteria included patients with central nervous system metastases, uncontrolled hypertension (defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥ 90mmHg), pregnancy, and anti-cancer therapy within 14 days of study enrollment.
Study Design
Patients were treated with pazopanib at 800 mg oral daily without food (GlaxoSmithKline) on 28-day cycles until disease progression as measured using Response Evaluation Criteria in Solid Tumors. Standard MRI of the abdomen and pelvis was obtained at baseline and every 8 weeks ± 1 week for tumor burden assessment. An investigational dynamic contrast enhanced (DCE) sequence was added to the standard image acquisition sequences to calculate Ktrans and associated physiologic parameters. An additional investigational DCE-MRI imaging procedure was conducted 1 week ± 1 day after initiation of drug therapy. The protocol included a total of 5 DCE-MRI procedures per patient. Toxicity monitoring using National Cancer Institute Common Toxicity Criteria version 3.0 was performed and dose modifications made for grade 3 or 4 toxicities. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the University of Chicago’s institutional review board.
MRI Acquisition
MR images were acquired on a 1.5T Philips Achieva scanner (Philips Healthcare, Andover, MA), using an 8-channel torso local coil. Gadobenate dimeglumine (Multihance, Bracco, Cranbury Township, NJ) was used as the contrast agent, at standard dose of 0.1 mmol/kg (0.2 mL/kg). For patients with compromised kidney function (GFR < 60 mL/min/1.73m2), one half of the standard dose was administered.
The standard clinical part of the MRI scan covered the whole abdomen and included axial and coronal non-fat suppressed single-shot FSE sequences (TR/TE = 1800/80 ms, flip angle = 90°), an axial fat suppressed T2-weighted sequence (TR/TE = 1800/80 ms, flip angle = 90°), an axial diffusion-weighted MRI (fat suppressed spin-echo EPI, TR/TE = 7155/67 ms, flip angle = 90°, b = 800 s/mm2), and an axial breath-hold T1-weighted fat suppressed sequence (TR/TE = 4.07/1.98 ms, flip angle =10°), acquired before and 3 minutes post-contrast administration (delayed phase).
Research sequences were acquired in a 36 mm thick axial slab centered over the largest cross-section of a selected metastatic lesion in an area with minimal MRI, especially motion related, artifacts. Limited coverage was necessary to insure high temporal resolution with adequate signal to noise ratios. The location of the slab was determined by the clinical radiologist (A.O.) based on previous CT or MRI imaging, and was selected at the level of largest lesion cross-section. The location of the slab was kept constant in subsequent scans, relative to the spine. An axial non-fat suppressed T1-weighted dual-echo DCE-MRI 3D sequence (TR/TE1/TE2 = 8.5/3.0/6.0 ms, flip angle = 10°, in-plane resolution = 2 mm, field-of-view = 380 × 356 mm, slice thickness = 3 mm both acquired and reconstructed, number of slices = 12, partial Fourier factors = 0.7 and 0.85 in the Y and Z directions, SENSE acceleration factor = 1.2 in the anterior/posterior direction, time resolution = 9.8 s) was acquired for 16 minutes, with 10 dynamic phases acquired prior to contrast agent injection, and the rest acquired post-contrast. The clinical delayed phase whole abdomen T1-weighted sequence was nested into the research DCE-MRI sequence at 3 minutes post-contrast administration, which did not affect the gain or other pre-scan parameters.
Contrast Agent Concentration Calculation
Contrast agent concentration was calculated as an average over regions of interest (ROIs) outlined over the lesion, muscle, and adipose tissue and over the aorta cross-section in slices in which they were visible. The edge slices (first and last 3 slices) had reduced flip angles due to the non-ideal slab profile, and were not used for calculation. Concentration versus time curves for lesion, muscle, and arterial ROIs were calculated using the reference tissue method published earlier.12 In short, the approximate inverse proportionality of signal to T1 in the short TR T1-weighted DCE-MRI sequence was utilized. Based on the tabulated value of native T1 in adipose tissue (T1ref = 288 ms),13 and its average pre-contrast signal intensity Sref(0), the pre-contrast and post-contrast T1, and hence the contrast agent concentration as a function of time C(t) in each ROI could be approximated using Eq. 1:
| [1] |
where R = 5.6 L/mmol/s (as obtained from the manufacturer data sheet) is the relaxivity of the contrast agent at 1.5T, and S(0) and S(t) are average signal in the ROI pre-contrast and as a function of time, respectively.
Ktrans Calculation
Prior to further analysis, the C(t) curves for tumor and muscle were smoothed via a fit to the 5-parameters empirical mathematical model (EMM), shown previously to provide excellent fits to the concentration uptake curves in tissues.14–16 Two compartment modeling17 was used to iteratively fit smoothed C(t) curves to Eq 2:
| [2] |
where Ktrans is the volume transfer constant between the intravascular extracellular space and extravascular extracellular space (EES), ve is the volume of EES per unit volume of tissue, and Cp(t) is the arterial input function (AIF). The AIF was obtained as the contrast concentration curve from the arterial ROI for each patient, and was corrected for hematocrit by dividing the contrast agent concentration in the arterial ROI by (1- hematocrit), with hematocrit = 0.45, i.e., Cp(t) = Carterial(t)/(1–0.45).
Statistical Analysis
The primary study endpoint was to determine whether a rise in Ktrans from nadir is predictive of subsequent tumor growth. Secondary endpoints included the association between changes in Ktrans and pazopanib therapy. The pre-defined principal metric assessed, R, was the log ratio of Ktrans at time ti to the lowest Ktrans among the first 2 (baseline or 1 week post therapy), a time-dependent covariate. The main analysis was to assess whether the time-dependent variable R had a significant impact on the hazard rate for disease progression in a Cox proportional hazards model also incorporating a baseline clinical prognostic index (good/intermediate/poor)18, 19 and prior VEGF pathway inhibitor therapy (yes/no) as covariates. We assumed that the median time to progression in this patient population is 8 months and that Ktrans variability and change with therapy are similar to that seen in prior studies.9 Exploratory objectives included correlating baseline Ktrans with progression free survival (PFS). Kaplan-Meier estimates and log-rank tests were computed to evaluate the association between changes in Ktrans and PFS.
Trial size was based on prior data showing that the standard deviation in R from baseline to 4 weeks was approximately 0.40.9 A clinically meaningful effect was defined as a 50% increase in the hazard rate per one standard deviation increase in R. In order to have 80% power to detect an effect of this magnitude, a total of 48 events (disease progressions) were required. Assuming a median time to progression of 8 months, enrollment of 65 patients was planned.
RESULTS
Patient Characteristics and Feasibility of a Complex Biomarker Assessment
Due to poor accrual the study was closed after 20 patients were enrolled over the course of two years. Baseline characteristics of patients are summarized in Table 1. The median age was 63 (range, 50 – 82) and median number of prior therapies was two (range, 0 – 5). Fourteen (70%) patients were male. The median number of completed 28-day treatment cycles on study was four (range, 1 – 21). Eighteen (90%) patients underwent a baseline MRI with a calculable Ktrans and were included in the analysis, while 17 patients (85%) also underwent at least one post-baseline MRI with a calculable Ktrans. A median of 4 MRIs were obtained per patient.
Table 1.
Baseline Characteristics for Patients Enrolled on Trial
| Characteristic | No. of Patients (N = 20) |
|---|---|
| Sex | |
| Male | 6 |
| Female | 14 |
| Age | |
| Median | 63 |
| Range | 50–82 |
| Race/ethnicity | |
| White | 13 |
| African American | 2 |
| Hispanic | 2 |
| Not reported | 3 |
| Number of Prior Therapies | |
| 0 | 5 |
| 1 | 5 |
| 2 | 4 |
| 3 | 3 |
| 4 | 2 |
| 5 | 1 |
| Clinical Prognostic Index | |
| Good | 4 |
| Intermediate | 8 |
| Poor | 5 |
| Not available | 3 |
Change in Ktrans With Pazopanib Therapy
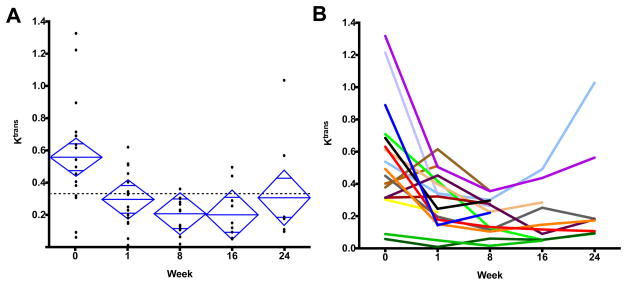
Of the 18 patients assessed, 73 DCE-MRIs were completed and 66 were technically assessable. The median baseline Ktrans value was 0.472 min−1, and the mean was 0.538 min−1 with a standard deviation of 0.339 min−1. One patient did not have any assessable DCE-MRI studies after baseline due to poor uptake of contrast. Of the 17 patients with at least one post-baseline DCE-MRI, 16 (94%) had a decline in Ktrans (Figure 1). Mean Ktrans log ratios (±SD) compared to baseline after 1, 8, 16, and 24 weeks were −0.680 (±0.729), −1.049 (±0.634), −0.998 (±0.757), −0.755 (±0.952), respectively, corresponding to changes of −49%, −65%, −63%, and −53% (P = 0.0052, RM ANOVA). The recovery of mean Ktrans at week 24 compared to the prior time point was not statistically significant (P = 0.40, Wilcoxon signed rank). Patients showed variability in the timing of Ktrans decline and recovery as depicted in Figure 1B. At week 24 the Ktrans values showed a broad range between 0.092 and 1.028 min−1.
Figure 1.
(A) Individual dynamic contrast magnetic resonance imaging (DCE-MRI) derived Ktrans values (min−1) plotted at each time point. The top and bottom of each diamond represent the 95% confidence interval for each group. (B) Ktrans values (min−1) plotted across each time point by individual patient. Each color represents an individual patient, with trend line shown over the course of the study.
Ktrans and Clinical Outcome
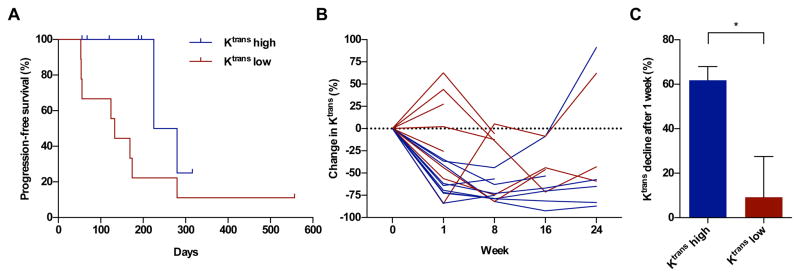
The median progression free survival (PFS) was 32.1 weeks. Kaplan-Meier analysis showed a significant difference in PFS in patients grouped dichotomously around the median baseline Ktrans (P = 0.036, log-rank, Figure 2A). Patients with higher baseline values showed deeper reductions in Ktrans after therapy (Figure 2B and 2C). The mean percent reduction in Ktrans after one week of therapy was 61.8% in the group with higher baseline values versus 9.2% in the group with lower baseline values (P = 0.017, two-tailed t-test). Baseline Ktrans did not reach significance in a Cox-proportional hazard model including clinical prognostic index and prior treatments (P = 0.083). With our accrued sample size, the primary study endpoint, whether Ktrans recovery as a time-dependent covariate impacted hazard rate for disease progression, could not be assessed.
Figure 2.
(A). Kaplan-Meier estimates for progression-free survival (PFS) of patients with low and high baseline Ktrans values (min−1). The cut off value was defined as the median Ktrans value, which was 0.472 min−1. The PFS difference between groups was statistically significant (P = 0.036, log-rank). (B) Percent change in Ktrans from baseline in individual patients colored by the groups of patients with high and low baseline Ktrans values. (C) Bar graph comparing the depth of Ktrans decline at one week for patients with high versus low baseline Ktrans (P = 0.017, two-tailed t-test).
DISCUSSION
This prospective, non-randomized, imaging biomarker study demonstrates that Ktrans, a quantitative parameter derived from DCE-MRI, is a pharmacodynamic biomarker for pazopanib therapy in metastatic renal cancer. To date, this is the first study to report the effect of pazopanib on a quantitative DCE-MRI-derived biomarker. The median Ktrans nadir occurred eight weeks after initiating therapy, and a decline in Ktrans was nearly universal, occurring in 94% of patients. Only a single patient did not show a decline, but data from only one follow up MRI for that patient was available at week one. It is likely that a decline was simply not captured due to the absence of assessment beyond one week. The declines in Ktrans noted in our study were consistent with prior reports on other anti-angiogenic therapies in metastatic renal cancer.9 Interestingly, we observed variability among individual patients with some reaching their nadir at week one, while others continued to decline at the final 24-week assessment.
This study also assessed clinical outcomes in relation to Ktrans values. It was hypothesized that recovery of Ktrans would predict progression on pazopanib therapy. Indeed, there was a trend towards Ktrans recovery by 24 weeks, which preceded the median progression free survival by 8 weeks. Unfortunately, due to the small sample size, the primary endpoint of Ktrans recovery as a time-dependent covariate could not be assessed. Thus, the predictive capacity of Ktrans recovery remains uncertain. It is notable that at week 24, the majority of patients still had Ktrans values below their baseline. Thus, increasing the duration of serial DCE-MRI assessments beyond 24 weeks in future studies might capture more definitive Ktrans recovery data in a greater fraction of patients. Baseline Ktrans values did exhibit a significant association with progression free survival, an observation that is consistent with data reported in prior studies.9 We also found that higher baseline Ktrans was associated with deeper declines at week 1. While there may be difficulty in utilizing absolute baseline Ktrans levels between different populations, the percent reduction from baseline could be assessed at week one for an individual patient and might be consistent and reliable predictive measure to evaluate in future studies.
Despite not reaching its primary endpoint, this study was unique as the first to report serial DCE-MRI evaluations longitudinally over 24 weeks in renal cancer patients treated with VEGF therapy. It also demonstrated the feasibility of performing a complex biomarker study using a multidisciplinary team of investigators. There remain challenges in the recruitment of patients for a study evaluating a novel biomarker for an FDA-approved therapy. Nonetheless, this study validated DCE-MRI-derived Ktrans as a non-invasive, pharmacodynamic biomarker. Its baseline value correlates with progression free survival for pazopanib therapy in metastatic renal cancer, thus demonstrating its consistency across different anti-angiogenic drug therapies.
Clinical Practice Points.
Dynamic contrast enhanced magnetic resonance imaging (DCE-MRI) is an emerging modality with potentially more sensitivity in assessing tumor growth compared to traditional imaging. Ktrans is an important DCE-MRI-derived parameter that changes with alteration in tumor vascular permeability. Clinical studies have shown that in some cancers, such as renal carcinoma, Ktrans declines shortly after initiation of vascular endothelial growth factor-targeted therapies. This study is the first to describe longitudinal serial assessments of Ktrans in metastatic renal cancer patients treated with pazopanib. We report that Ktrans consistently declined upon initiation of therapy, and that higher baseline Ktrans correlated with longer progression-free survival. The data reported have implications for future studies using DCE-MRI as a pharmacodynamic biomarker. This technique may ultimately lead to more accurate tools to determine disease progression at an earlier time point than traditional imaging assessment, which could inform therapeutic changes at an earlier time point when treatment is no longer effective and possibly still causing toxicities impacting quality of life.
Acknowledgments
Funding: Supported by NIH grants T32 CA009566, T32 GM007019 (R.F.S.)
Abbreviations
- DCE-MRI
Dynamic contrast enhanced magnetic resonance imaging
- VEGF
Vascular endothelial growth factor
- PFS
Progression-free survival
Footnotes
This study was registered with ClinicalTrials.gov as NCT01599832
Presented at 51st Annual Meeting of the American Society of Clinical Oncology.
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shen C, Kaelin WG. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013;23:18–25. doi: 10.1016/j.semcancer.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harris PA, Boloor A, Cheung M, et al. Discovery of 5-[[4-[(2,3-dimethyl-2H-indazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor. J Med Chem. 2008;51:4632–4640. doi: 10.1021/jm800566m. [DOI] [PubMed] [Google Scholar]
- 3.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–1068. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 4.Ammari S, Thiam R, Cuenod CA, et al. Radiological evaluation of response to treatment: application to metastatic renal cancers receiving anti-angiogenic treatment. Diagn Interv Imaging. 2014;95:527–539. doi: 10.1016/j.diii.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002;16:407–422. doi: 10.1002/jmri.10176. [DOI] [PubMed] [Google Scholar]
- 6.Liu G, Rugo HS, Wilding G, et al. Dynamic contrast-enhanced magnetic resonance imaging as a pharmacodynamic measure of response after acute dosing of AG-013736, an oral angiogenesis inhibitor, in patients with advanced solid tumors: results from a phase I study. J Clin Oncol. 2005;23:5464–5473. doi: 10.1200/JCO.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 7.Morgan B, Thomas AL, Drevs J, et al. Dynamic contrast-enhanced magnetic resonance imaging as a biomarker for the pharmacological response of PTK787/ZK 222584, an inhibitor of the vascular endothelial growth factor receptor tyrosine kinases, in patients with advanced colorectal cancer and liver metastases: results from two phase I studies. J Clin Oncol. 2003;21:3955–3964. doi: 10.1200/JCO.2003.08.092. [DOI] [PubMed] [Google Scholar]
- 8.Jackson A, O’Connor JP, Parker GJ, Jayson GC. Imaging tumor vascular heterogeneity and angiogenesis using dynamic contrast-enhanced magnetic resonance imaging. Clin Cancer Res. 2007;13:3449–3459. doi: 10.1158/1078-0432.CCR-07-0238. [DOI] [PubMed] [Google Scholar]
- 9.Hahn OM, Yang C, Medved M, et al. Dynamic contrast-enhanced magnetic resonance imaging pharmacodynamic biomarker study of sorafenib in metastatic renal carcinoma. J Clin Oncol. 2008;26:4572–4578. doi: 10.1200/JCO.2007.15.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhatt RS, Wang X, Zhang L, et al. Renal cancer resistance to antiangiogenic therapy is delayed by restoration of angiostatic signaling. Mol Cancer Ther. 2010;9:2793–2802. doi: 10.1158/1535-7163.MCT-10-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang D, Ding Y, Zhou M, et al. Interleukin-8 mediates resistance to antiangiogenic agent sunitinib in renal cell carcinoma. Cancer Res. 2010;70:1063–1071. doi: 10.1158/0008-5472.CAN-09-3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Medved M, Karczmar G, Yang C, et al. Semiquantitative analysis of dynamic contrast enhanced MRI in cancer patients: Variability and changes in tumor tissue over time. J Magn Reson Imaging. 2004;20:122–128. doi: 10.1002/jmri.20061. [DOI] [PubMed] [Google Scholar]
- 13.Han E, Gold G, Stainsby J, Wright G, Beaulieu C, Brittain J. In-Vivo T1 and T2 Measurements of Muskuloskeletal Tissue at 3T and 1.5T. 2003;11:450–450. [Google Scholar]
- 14.Fan X, Medved M, River JN, et al. New model for analysis of dynamic contrast-enhanced MRI data distinguishes metastatic from nonmetastatic transplanted rodent prostate tumors. Magn Reson Med. 2004;51:487–494. doi: 10.1002/mrm.10737. [DOI] [PubMed] [Google Scholar]
- 15.Fan X, Medved M, Foxley S, et al. Multi-slice DCE-MRI data using P760 distinguishes between metastatic and non-metastatic rodent prostate tumors. MAGMA. 2006;19:15–21. doi: 10.1007/s10334-005-0022-y. [DOI] [PubMed] [Google Scholar]
- 16.Fan X, Medved M, Karczmar GS, et al. Diagnosis of suspicious breast lesions using an empirical mathematical model for dynamic contrast-enhanced MRI. Magn Reson Imaging. 2007;25:593–603. doi: 10.1016/j.mri.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tofts PS, Brix G, Buckley DL, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10:223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: memorial sloan-kettering cancer center experience. Clin Cancer Res. 2004;10:6302S–6303S. doi: 10.1158/1078-0432.CCR-040031. [DOI] [PubMed] [Google Scholar]
- 19.Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23:832–841. doi: 10.1200/JCO.2005.05.179. [DOI] [PubMed] [Google Scholar]