Abstract
Background
The purpose of this study is to determine whether larger abdominopelvic abscess drains reduce the time required for abscess resolution, or the probability of tube occlusion.
Methods
144 consecutive patients who underwent abscess drainage at a single institution were reviewed retrospectively. Results: Larger initial drain size did not reduce drainage time, drain occlusion, or drain exchanges (p>0.05). Subgroup analysis did not find any type of collection that benefitted from larger drains. A multivariate model predicting drainage time showed that large collections (>200 ml) required 16 days longer drainage time than small collections (<50 ml). Collections with a fistula to bowel required 17 days longer drainage time than collections without a fistula. Initial drain size and the viscosity of the fluid in the collection had no significant effect on drainage time in the multivariate model.
Conclusions
8 F drains are adequate for initial drainage of most serous and serosanguineous collections. 10 F drains are adequate for initial drainage of most purulent or bloody collections.
Keywords: abscess, drainage, fistula
Introduction
Since its initial description in 1978 (1), image-guided percutaneous abscess drainage has become the treatment of choice for the drainage of intra-abdominal fluid collections (2). It is successful in over 90% of patients (3, 4), and has a lower complication rate than surgery (5). Percutaneous abscess drainage is typically performed using ultrasound or CT guidance. Various factors can affect the success of intra-abdominal abscess drainage, such as the location and size of the abscess, number of loculations, presence of phlegmon (6, 7), presence of fistulas (8), and viscosity of its contents (8).
Abscess drainage is one of the most common procedures in interventional radiology, but the size of tube to place in each abscess is largely driven by intuition and personal preference. Many different types of drains are commercially available, ranging from 5 – 20 F for locking loop drains, and 6 – 36 F for straight drains. The most commonly used drains are 8 – 14 F, and academic centers are more likely to place ≥ 14 F drains compared to private practice centers (9).
A few older studies with small patient numbers have examined the effects of abscess drain size on success rates and drainage times. A meta-analysis by Park et al (8) in 1993 found similar drainage times for small and large diameter catheters, but they did not account for the characteristics of the collection drained. A randomized trial by Gobien et al (10) in 1985 found no differences in success rates or drainage times between 8 F locking loop drains (25 patients) and 12 – 18 F straight drains (18 patients). A retrospective review by Rothlin et al (11) in 1998 showed no differences in drainage times or success rates between 7 F locking loop drains (40 patients) and 14 F sump drains (24 patients), but they did not account for the characteristics of the collection drained.
In general, retrospective studies would be expected to underestimate any benefits of larger abscess drains. If larger drains work better, this effect will be counteracted by the fact that interventional radiologists tend to select larger drains for more viscous collections. A proper evaluation of the effects of drain size would account for the characteristics of the collections drained, or would randomize patients to different drain sizes.
The last randomized trial of abscess drain sizes was published in 1985. Since then, there has been a decrease in the rate of surgical management of abscesses, increase in CT imaging, improved CT and ultrasound image quality, and newer catheter designs. The purpose of this study is to re-examine this common question of what size abscess drain to place, in a modern setting.
Materials and Methods
The Institutional Review Board approved this retrospective study based on a chart and imaging review of 144 consecutive patients at a single cancer center who underwent image-guided abscess drainage by interventional radiology between August 2013 and August 2014. A variety of different drains were used, most commonly Multipurpose Drainage and Dawson-Mueller catheters (Cook, Bloomington, IN). Dawson-Mueller catheters were typically used for smaller collections.
We examined total drainage time, whether the catheter occluded, and whether the catheter was exchanged for any reason, as a function of the initial drain size, the attending who performed the initial drainage, and the characteristics of the collection. The size of the collection was based on the amount aspirated at the time of placement, which was classified into 3 groups: small (<50 ml), medium (50 – 200 ml), or large (>200 ml). The viscosity of the fluid was classified as thin (serous, serosanguineous, or bilious) or thick (purulent, feculent, or bloody), based on the description of the fluid aspirated at the time of drain placement. Drain occlusion was determined by review of abscess drain exchange reports and presence of occlusion on corresponding fluoroscopic images.
Interventional radiology attendings were classified into two groups based on the average size drain placed. Attendings who on average placed > 10 F drains were classified as “big tube” attendings (7 of 15), and attendings who on average placed ≤ 10 F drains were classified as “small tube” attendings. “Big tube” attendings typically (>50% of the time) placed 10 F drains in thin collections, and 10 or 12 F drains in thick collections. “Small tube” attendings typically placed 8 F drains in thin collections, and 10 F drains in thick collections. This created a natural experiment, where patients were effectively randomized to different drain sizes based on operator preference.
After drain placement, the collection was completely drained in the procedure room, and the output recorded. Drains were typically flushed with normal saline two to three times per day to maintain drain patency. Drains were evaluated daily by an interventional radiology fellow or nurse practitioner, and discussed with an attending. Tissue plasminogen activator (tPA) was often injected into the drain when there was residual undrained fluid in a thick or loculated collection (12). Abscess drains were typically removed when there was less than 20 ml / day output with a functioning tube, minimal residual collection, and resolution of symptoms (fever, leukocytosis, pain, etc).
p values for differences between average drainage times was calculated using ANOVA (3 groups) or a two-tail t test with unequal variances (2 groups). p values for differences between the fraction of tubes that occluded or required exchange or tPA were evaluated using a two-tail z-test, comparing “big tube” versus “small tube” attendings, and ≤ 8 F versus ≥ 12 F tubes. Total drainage time was predicted using a linear regression model, using collection characteristics (viscosity and size), presence of a fistula to bowel, and drain size. Probability of occlusion was predicted using a logistic regression model, using collection characteristics (viscosity and size), presence of a fistula to bowel, and drain size. The threshold probability for the logistic regression prediction was selected to maximize the sum of the sensitivity and specificity for predicting occlusion (13).
Results
Out of the 144 collections drained, 39 collections contained thin fluid (serous or serosanguineous), 100 contained thick fluid (purulent or bloody), and 5 collections had no data recorded on fluid characteristics. The average initial drain size was 9.9 F (range: 6 F – 18 F), and almost all (97%) were 8, 10, or 12 F. The average total drainage time was 28 days.
“Small tube” and “big tube” attendings drained collections with similar characteristics: there was no significant difference in the collection viscosity, collection size, or presence of fistula to bowel. Despite similar collection characteristics, the “big tube” attendings placed significantly larger drains (p=1.4×10−8).
When comparing abscesses drained by “small tube” attendings versus “big tube” attendings, there was no difference in average drainage time (p=0.61, Table 1), occlusion (p=0.64, Table 2), fraction of tubes that were injected with tPA (p=0.81), or fraction of tubes that required drain exchange (p=0.36).
Table 1.
Average drainage time in days as a function of type of collection, drain size, and attending type. Larger tubes do not reduce drainage time.
| Collection type | Num. | Average drainage time (days) | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial tube size | Attending type | |||||||
| ≤ 8 F | 10 F | ≥ 12 F | p | Small tube | Big tube | p | ||
| all | 144 | 21 | 29 | 32 | 0.26 | 26 | 29 | 0.61 |
| thin | 39 | 21 | 32 | 32 | 0.36 | 25 | 30 | 0.49 |
| thick | 100 | 22 | 29 | 32 | 0.63 | 28 | 29 | 0.95 |
| small | 51 | 15 | 24 | 13 | 0.50 | 21 | 18 | 0.72 |
| medium | 48 | 26 | 30 | 26 | 0.91 | 28 | 29 | 0.97 |
| large | 45 | 31 | 33 | 44 | 0.52 | 34 | 36 | 0.79 |
| large and thick | 30 | n/a | 34 | 45 | 0.35 | 28 | 41 | 0.32 |
| fistula to bowel | 14 | 31 | 35 | 63 | 0.34 | 25 | 52 | 0.07 |
Table 2.
Fraction of drains that occluded, as a function of type of collection, drain size, and attending type. Larger tubes do not reduce tube occlusion.
| Collection type | Num. | Fraction of drains that occluded | ||||||
|---|---|---|---|---|---|---|---|---|
| Initial tube size | Attending type | |||||||
| ≤ 8 F | 10 F | ≥ 12 F | p | Small tube | Big tube | p | ||
| all | 144 | 23% | 23% | 29% | 0.63 | 26% | 23% | 0.64 |
| thin | 39 | 27% | 26% | 20% | 0.77 | 26% | 25% | 0.93 |
| thick | 100 | 23% | 24% | 32% | 0.60 | 29% | 23% | 0.48 |
| small | 51 | 29% | 23% | 25% | 0.86 | 29% | 22% | 0.58 |
| medium | 48 | 14% | 25% | 22% | 0.69 | 21% | 24% | 0.80 |
| large | 45 | 17% | 21% | 36% | 0.39 | 29% | 23% | 0.67 |
| large and thick | 30 | n/a | 25% | 40% | 0.40 | 43% | 26% | 0.40 |
| fistula to bowel | 14 | 0% | 14% | 25% | n/a | 0% | 22% | n/a |
When comparing ≤ 8 F, 10 F, and ≥ 12 F tubes, there was no difference in average drainage time (Table 1) or occlusion (Table 2). Subgroup analysis did not find any type of collection (for example, large and thick collections) that benefitted from larger drains.
A multivariate model was developed to predict total drainage time based on drain size, collection viscosity, collection size, and presence of fistula to bowel (Table 3). This model could only predict 10% of the variance in drainage times (Figure 1). The model parameters show that large collections required 16 days longer drainage time than small collections. The collection size had a bigger effect on the drainage time than the collection viscosity. Collections with a fistula to bowel required 17 days longer drainage time than collections without a fistula. This multivariate model also shows no benefit to larger tube sizes, after accounting for characteristics of the collection.
Table 3.
Predicting total drainage time as a function of collection characteristics (viscosity and size) and drain size. The predicted drainage time (days) is obtained by adding together the applicable numbers from the table. Additional drainage time for each feature was determined using multiple linear regression.
| Feature | Additional drainage time (days) | 95% CI† | p | |
|---|---|---|---|---|
| baseline | 13.1 | 1.0 – 25.2 | 0.034 * | |
|
| ||||
| collection viscosity | thick | 2.0 | −8.5 – 12.5 | 0.71 |
| thin | 0.0 | |||
|
| ||||
| collection size | small | 0.0 | ||
| medium | 8.2 | −2.9 – 19.2 | 0.15 | |
| large | 16.4 | 5.0 – 27.7 | 0.0050 * | |
|
| ||||
| drain size | ≤ 8 F | 0.0 | ||
| 10 F | 4.9 | −7.0 – 16.8 | 0.42 | |
| ≥ 12 F | 5.9 | −9.7 – 21.5 | 0.46 | |
|
| ||||
| fistula to bowel | 17.2 | 2.2 – 32.2 | 0.025 * | |
CI = confidence interval
Figure 1.
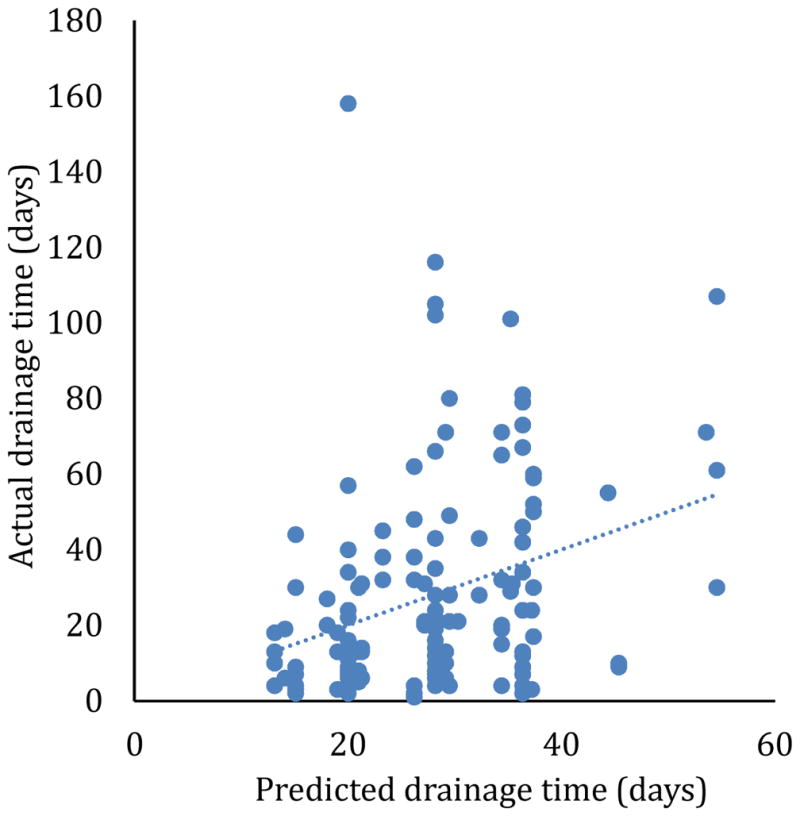
Predicted versus actual drainage time, using the model in Table 3. The correlation between the predicted and actual drainage times has r2 = 0.10, and the regression line has a non-zero slope (p=8.9×10−5).
A multivariate model could not accurately predict which drains are likely to occlude (Table 4).
Table 4.
Predicting the probability of the drain getting occluded, as a function of collection characteristics (viscosity and size) and drain size. Model parameters were calculated using logistic regression. This model has a sensitivity of 49% and specificity of 61%, which is barely better than random guessing.
| Feature | Odds ratio | |
|---|---|---|
| baseline | 0.37 | |
|
| ||
| collection viscosity | thick | 0.98 |
| thin | 1 | |
|
| ||
| collection size | small | 1 |
| medium | 0.80 | |
| large | 0.87 | |
|
| ||
| drain size | ≤ 8 F | 1 |
| 10 F | 0.98 | |
| ≥ 12 F | 1.42 | |
|
| ||
| fistula to bowel | 0.48 | |
Discussion
The simplest way to examine the effect of abscess drain size on the total drainage time and probability of drain occlusion would be to retrospectively examine abscess drains of different sizes. However, such an analysis would likely be flawed due to selection bias, because larger drains are presumably placed in response to collection characteristics, such as viscosity. Therefore, we examined subgroups of collections with similar sizes and viscosities. This subgroup analysis showed no effect of drain size on drainage times or probability of occlusion, for various specific types of collections. Even large and viscous collections did not show any benefit to placing larger abscess drains. Multivariate analyses also showed no reduction in drainage time or probability of occlusion for larger tubes, after correcting for the characteristics of the collection drained.
The most convincing evidence would be a randomized controlled trial. However, the 15 interventional radiologists in our group have a range of different opinions on the optimal drain size to place for different types of collections. The “big tube” attendings placed significantly larger drains, even though there was no significant difference in the characteristics of the collections drained. This effectively randomized patients to different drain sizes, depending on which attending was assigned to place their drain. There was no difference in outcomes (total drainage time, probability of drain occlusion, or fraction of tubes that required drain exchange) between the attendings who preferred bigger tubes and attendings who preferred smaller tubes.
Based on these results, we conclude that larger initial drain size does not reduce the total drainage time or probability of occlusion. However, this conclusion only applies for the range of initial drain sizes used in this study, which was typically 8 – 12 F. In this study, no 8 F drains were placed in large thick collections, so we do not have any data to evaluate 8F drains in that setting. We do know that the “small tube” attendings typically (>50% of the time) placed 8 F drains in thin (serous or serosanguineous) collections, and 10 F drains in thick (purulent or bloody) collections, and that their results were similar to their colleagues who placed larger drains.
This study only addresses the initial tube size for drainage. 85% of tubes were exchanged at least once before removal, and 50% were upsized. However, smaller drains were no more likely to require exchange than larger drains. Drains were typically upsized if they were occluded, if there was a large residual collection, or if there was leakage around the tube. Upsizing poorly functioning drains is standard practice, and there is no evidence in this paper to argue against this practice. On average, the initial drain was in place for 50% of the total drainage time, so we do expect the choice of initial drain size to affect the total drainage time.
Our practice is to routinely use intracavitary tPA to improve drainage from thick and loculated collections, and we frequently saw a dramatic increase in drain output after tPA. In this study, tPA was used in 33% of abscesses. It is possible that the use of tPA enables the use of smaller tubes than would otherwise be possible.
Flow rate increases proportional to the fourth power of the inner diameter of a drain, and inversely proportional to the viscosity of the fluid (Poiseulle’s law). Experimentally, 12 F (outer diameter) drains have 2 – 3 times higher flow rates than 8 F drains, and this ratio is independent of viscosity (14). Although increased flow rate helps rapidly drain the collection while the patient is on the procedure table, it did not translate into shorter time before drain removal in this study. An 8 F drain can output 133 L of water or 27 L of unclotted blood per day (14, 15), which is more than adequate for any medical application. In terms of drain occlusion, an 8 F drain has an average inner diameter of 1.8 mm, and a 12 F drain has an average inner diameter of 2.7 (14). If an abscess contains solid particulate matter between 1.8 and 2.7 mm in size, then we would expect an advantage to the 12 F drain. In practice, we did not see a significant reduction in occlusion with 12 F drains, suggesting that particulate matter in this specific size range is not common in clinical practice.
Larger drains are less susceptible to kinking (14), which could potentially be beneficial for obese patients. In addition, certain applications require large bore drains, such as maintaining access for pancreatic necrosectomy.
Limitations of the current study include its retrospective design, and limited range of initial drain sizes (8 – 12 F).
In conclusion, 8 F drains are adequate for initial drainage of most serous and serosanguineous collections, and 10 F drains are adequate for most purulent or bloody collections. Larger tubes may be required for poorly draining collections, collections with particulate matter, or to reduce tube kinking. In contrast, most academic centers routinely place ≥ 12 F drains for abscesses (9). We anticipate that the smaller drains recommended here should result in less invasive procedures and improved patient comfort.
Summary.
8 F drains are adequate for initial drainage of most serous and serosanguineous collections. 10 F drains are adequate for initial drainage of most purulent or bloody collections. Larger initial drain size did not reduce drainage time, drain occlusion, or drain exchanges.
Acknowledgments
This work was supported in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Conflicts of interest: None
Prior publication or presentations: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gerzof SG, Robbins AH, Birkett DH. Computed tomography in the diagnosis and management of abdominal abscesses. Gastrointestinal Radiology. 1978;3(1):287–94. doi: 10.1007/BF01887081. [DOI] [PubMed] [Google Scholar]
- 2.vanSonnenberg E, Wittich GR, Goodacre BW, et al. Percutaneous abscess drainage: update. World J Surg. 2001 Mar;25(3):362–9. doi: 10.1007/s002680020386. discussion 70-2. Epub 2001/05/09. eng. [DOI] [PubMed] [Google Scholar]
- 3.Robert B, Yzet T, Regimbeau JM. Radiologic drainage of post-operative collections and abscesses. J Visc Surg. 2013 Jun;150(3 Suppl):S11–8. doi: 10.1016/j.jviscsurg.2013.05.005. Epub 2013/06/25. eng. [DOI] [PubMed] [Google Scholar]
- 4.Gee MS, Kim JY, Gervais DA, et al. Management of abdominal and pelvic abscesses that persist despite satisfactory percutaneous drainage catheter placement. AJR Am J Roentgenol. 2010 Mar;194(3):815–20. doi: 10.2214/AJR.09.3282. Epub 2010/02/23. eng. [DOI] [PubMed] [Google Scholar]
- 5.Johnson WC, Gerzof SG, Robbins AH, Nabseth DC. Treatment of abdominal abscesses: comparative evaluation of operative drainage versus percutaneous catheter drainage guided by computed tomography or ultrasound. Ann Surg. 1981 Oct;194(4):510–20. doi: 10.1097/00000658-198110000-00014. Epub 1981/10/01. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller PR, vanSonnenberg E, Ferrucci JT., Jr Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part II: Current procedural concepts. Radiology. 1984 May;151(2):343–7. doi: 10.1148/radiology.151.2.6709903. Epub 1984/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 7.vanSonnenberg E, Mueller PR, Ferrucci JT., Jr Percutaneous drainage of 250 abdominal abscesses and fluid collections. Part I: Results, failures, and complications. Radiology. 1984 May;151(2):337–41. doi: 10.1148/radiology.151.2.6709901. Epub 1984/05/01. eng. [DOI] [PubMed] [Google Scholar]
- 8.Park JK, Kraus FC, Haaga JR. Fluid flow during percutaneous drainage procedures: an in vitro study of the effects of fluid viscosity, catheter size, and adjunctive urokinase. AJR Am J Roentgenol. 1993 Jan;160(1):165–9. doi: 10.2214/ajr.160.1.8416618. Epub 1993/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 9.Jaffe TA, Nelson RC, Delong DM, Paulson EK. Practice patterns in percutaneous image-guided intraabdominal abscess drainage: survey of academic and private practice centers. Radiology. 2004 Dec;233(3):750–6. doi: 10.1148/radiol.2333032063. [DOI] [PubMed] [Google Scholar]
- 10.Gobien RP, Stanley JH, Schabel SI, et al. The effect of drainage tube size on adequacy of percutaneous abscess drainage. Cardiovasc Intervent Radiol. 1985;8(2):100–2. doi: 10.1007/BF02552867. Epub 1985/01/01. eng. [DOI] [PubMed] [Google Scholar]
- 11.Rothlin MA, Schob O, Klotz H, et al. Percutaneous drainage of abdominal abscesses: are large-bore catheters necessary? Eur J Surg. 1998 Jun;164(6):419–24. doi: 10.1080/110241598750004229. [DOI] [PubMed] [Google Scholar]
- 12.Beland MD, Gervais DA, Levis DA, et al. Complex abdominal and pelvic abscesses: efficacy of adjunctive tissue-type plasminogen activator for drainage. Radiology. 2008 May;247(2):567–73. doi: 10.1148/radiol.2472070761. [DOI] [PubMed] [Google Scholar]
- 13.Kaivanto K. Maximization of the sum of sensitivity and specificity as a diagnostic cutpoint criterion. J Clin Epidemiol. 2008 May;61(5):517–8. doi: 10.1016/j.jclinepi.2007.10.011. Epub 2008/04/09. eng. [DOI] [PubMed] [Google Scholar]
- 14.Macha DB, Thomas J, Nelson RC. Pigtail catheters used for percutaneous fluid drainage: comparison of performance characteristics. Radiology. 2006 Mar;238(3):1057–63. doi: 10.1148/radiol.2383050578. [DOI] [PubMed] [Google Scholar]
- 15.Elert G. The physics hypertextbook. [cited 2015 2015-02-28]; Available from: http://physics.info/viscosity/


