Abstract
Introduction
In 2010, WHO recommended a new first-line treatment for visceral leishmaniasis (VL) in Eastern Africa. The new treatment, a combination of intravenous (IV) or intramuscular (IM) sodium stibogluconate (SSG) and IM paromomycin (PM) was an improvement over SSG monotherapy, the previous first-line VL treatment in the region. To monitor the new treatment’s safety and effectiveness in routine clinical practice a pharmacovigilance (PV) programme was developed.
Methods
A prospective PV cohort was developed. Regulatory approval was obtained in Sudan, Kenya, Uganda and Ethiopia. Twelve sentinel sites sponsored by the Ministries of Health, Médecins Sans Frontières (MSF) and Drugs for Neglected Diseases initiative (DNDi) participated. VL patients treated using the new treatment were consented and included in a common registry that collected demographics, baseline clinical characteristics, adverse events, serious adverse events and treatment outcomes. Six-monthly periodic safety update reports (PSUR) were prepared and reviewed by a PV steering committee.
Results
Overall 3126 patients were enrolled: 1962 (62.7%) from Sudan, 652 (20.9%) from Kenya, 322 (10.3%) from Ethiopia and 190 (6.1%) from Uganda. Patients were mostly male children (68.1%, median age 11 years) with primary VL (97.8%). SSG-PM initial cure rate was 95.1%; no geographical differences were noted. HIV/VL co-infected patients and patients older than 50 years had initial cure rates of 56 and 81.4%, respectively, while 1063 (34%) patients had at least one adverse event (AE) during treatment and 1.92% (n = 60) had a serious adverse event (SAE) with a mortality of 1.0% (n = 32). There were no serious unexpected adverse drug reactions.
Conclusions
This first regional PV programme in VL supports SSG-PM combination as first-line treatment for primary VL in Eastern Africa. SSG-PM was effective and safe except in HIV/VL co-infected or older patients. Active PV surveillance of targeted safety, effectiveness and key VL outcomes such us VL relapse, PKDL and HIV/VL co-infection should continue and PV data integrated to national and WHO PV databases.
Key Points
| The study confirms a sodium stibogluconate-paromomycin (SSG-PM) combination should be used as first-line treatment for primary visceral leishmaniasis (VL) in Eastern Africa according to WHO recommendations. |
| A SSG-PM combination is contraindicated in patients who are HIV/VL co-infected or older than 50 years. |
| Pharmacovigilance (PV) should be strengthened for Neglected Tropical Diseases (NTDs) and PV data integrated to national and WHO PV databases. |
Introduction
Visceral leishmaniasis (VL), also known as kala-azar, is a protozoan infection caused by L. donovani complex and transmitted by phlebotomine sandflies [1]. Globally, approximately 200,000–400,000 new cases of VL are reported each year. Using a case fatality rate (CFR) of 10% there are approximately 20,000–40,000 deaths per year [2]. The Horn of Africa accounts for the second largest number of VL cases globally. The disease is endemic in numerous foci of Sudan, South Sudan, Somalia, Ethiopia, Kenya and Uganda [3]. VL patients present symptoms and signs of persistent systemic infection (including fever, fatigue, weakness, loss of appetite and weight loss) and parasitic invasion of the blood and reticulo-endothelial system such as enlarged lymph nodes, spleen and liver [4]. In the absence of treatment, VL is fatal. Even when VL is treated, mortality is around 5% when using antimonials as monotherapy and the overall CFR may reach 11% among impoverished and displaced populations [5]. Immunosuppressed or malnourished patients are at increased risk of death. Post kala-azar dermal leishmaniasis (PKDL), a dermatosis that is immune inflammatory in nature [6], usually appears 6 months to 1 or more years after apparent cure of VL and may occur earlier or even concurrently with VL in Sudan [7]. PKDL is most common in Eastern Africa, specifically in Sudan where up to 50% of patients presenting with kala-azar develop the condition [8]. PKDL is suspected to play a role in VL transmission, acting as a reservoir for new infections [9]. Hence, an ideal treatment for VL should not only cure the patient, but also reduce the risk for VL relapse and PKDL. A good indicator of definitive cure for VL is the absence of clinical relapse at 6 months [10].
In 2010, WHO recommended SSG-PM combination as the new first-line treatment for VL patients in Eastern Africa [11]. The combination of SSG at 20 mg/kg plus PM given at 15 mg/kg (11 mg base) for 17 days had shown an overall efficacy of 91.4% at 6 months post-treatment. The combination treatment was shown to be non-inferior to SSG monotherapy (91.4 and 93.9%, respectively; difference = 2.5%, 95% confidence interval (CI) −1.3 to 6.3, p = 0.198) and similar in terms of safety outcomes. The main advantages over SSG monotherapy were reduced hospitalization time (from 30 to 17 days), decreased cost and a decreased probability of resistance emergence [12].
A pharmacovigilance (PV) programme to monitor the safety and effectiveness of the new treatment during the early implementation phase was deemed crucial since SSG-PM would be used in settings different from clinical trials where a much larger and more diverse population would be exposed to SSG-PM in a relatively short timeframe. The PV program was developed and implemented based on the Council for International Organizations of Medical Sciences (CIOMs), the International Council on Harmonization (ICH) E2 guidelines and national regulations [13–16]. The information obtained from PV could help refine management guidelines and assist physicians in choosing the best treatment for VL patients in different contexts [17].
Methods
The PV program used a prospective cohort design analogous to the Cohort Event Monitoring (CEM) design [18]. The specific objectives were: (a) to determine the incidence of non-serious adverse events (AEs) during 17 days of treatment with combination SSG-PM and of serious AEs during and after treatment for up to 6 months following the end of treatment (EOT); (b) to identify additional risks that had not been reported in pre-approval clinical studies by reporting and characterizing previously undetected adverse drug reactions (ADRs); (c) to determine whether the occurrence of ADRs was higher in specific groups of patients by correlating unexpected ADRs (i.e. those ADRs which were not consistent with the safety information available for SSG and PM) with age, sex or co-morbidities and drug batch; (d) to monitor the treatment failure rate of SSG-PM; and (e) to investigate between-site variation in terms of SSG-PM effectiveness and safety.
Twelve sentinel health facilities from the Ministry of Health, MSF and DNDi supported sites were selected to participate in the program. Six sites were in Sudan (Bazura, Dooka, Elhawata, Kassab, Tabarak Allah and Um el Kher), three in Ethiopia (Arba Minch, Gondar and Abdurafi), two in Kenya (Kacheliba and Kimalel) and one in Uganda (Amudat).
Paper-based Patient Report Form (PRF) and Serious Adverse Event (SAE) Report Forms were used. The data collected included: patient identity number, age, sex, weight, height, pregnancy status (for women), type of patient (relapse or primary VL), mode of VL diagnosis (clinical, serological and parasitological) and co-morbidities at admission, start date of SSG-PM, duration of treatment, batch numbers (SSG and PM), AEs (serious and non-serious), their onset date, nature of the AE, whether the AE was considered serious, relationship of the event with SSG-PM treatment and whether the AE resulted in treatment discontinuation. Death, initial cure, non-responsiveness, slow-responsiveness or treatment default were recorded to evaluate EOT outcomes.
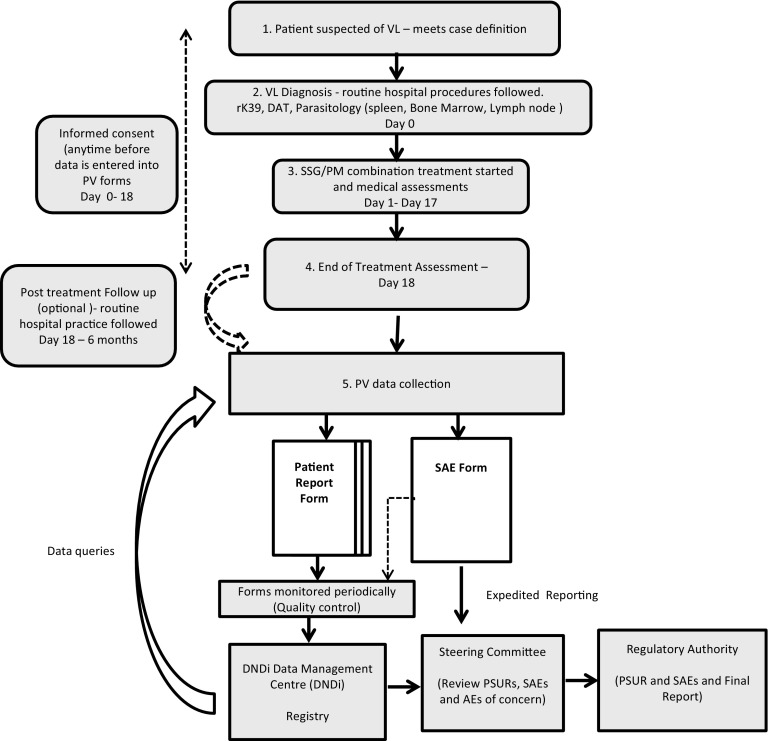
The PRF was adapted from existing national PV forms, and completed upon hospital discharge and during follow-up visits. All data collected were entered in a central drug safety registry with the SAE database kept at the DNDi Africa Data Centre, Nairobi, Kenya. Anonymous, unique patient IDs were used to ensure patient confidentiality. Only DNDi staff had access to the databases. The SAE Forms were used for expedited reporting to the project coordinator who after initial quality check of the SAE Forms forwarded them to the project’s Steering Committee (Fig. 1). At least every 6 months or sooner, SAE data were compiled in Periodic Safety Update Reviews as a line listing of AEs along with a summary of effectiveness data. Depending on the country, PSURs were also sent to the Ministry of Health. In Kenya the reports were sent annually, while in Ethiopia, Sudan and Uganda the Clinical Study was to be provided at the end of the PV.
Fig. 1.
Pharmacovigilance schema and flow. DAT direct agglutination test, DNDi drugs for neglected diseases initiative, PM paromomycin, PRF patient report form, PSUR periodic safety update report form, PV pharmacovigilance, SAE serious adverse event, SSG sodium stibogluconate, VL visceral leishmaniasis
Patients who presented at any of the sentinel sites between April 2011 and November 2013 with symptoms suggesting VL were screened, and upon confirmation of VL diagnosis, SSG-PM treatment was started, in line with routine practices, hospital protocols and clinician decision. Patients were hospitalized for the duration of the treatment (17 days). The procedures for diagnosis, treatment and discharge were consistent with the routine clinical practice at the health centres. Effectiveness and safety assessments for treatment emergent adverse events were conducted during the hospitalization period until the end of treatment. After discharge from day 18, the PV programme planned for passive detection of AEs. The practice of active patient follow-up post-treatment discharge from day 18 necessary to confirm definitive cure was heterogeneous and dependent on the routine site procedures, except for follow-up of safety concerns for which sites received support from the PV study coordinating team. There was also heterogeneous lab capability, for example only Ethiopian sites performed amylase monitoring. Lipase testing was not available at any of the sites. Since patients often travelled from distant remote locations, it was not practical to standardize a follow-up visit after 6 months in the PV program to assess definitive cure. Sites were nonetheless encouraged to report outcomes post discharge, and inform the patients that they should return to the clinic in case of AEs or reappearance of VL symptoms (relapse) or Post Kala Dermal Leishmaniasis (PKDL).
The site clinicians received training on PV, the safety and efficacy of the SSG-PM combination (as determined through clinical trials) and data collection. The PV training was provided by qualified MSF and DNDi staff based on the CIOMS guidelines, the ICH E2 guidelines and available country PV guidelines. Although none of the sites were equipped to perform audiometry, all staff involved in the PV programme received training on possible safety issues including oto-toxicity. The staff were instructed to perform a basic hearing test as part of routine clinical examination. Referral to an ENT specialist was to be organized whenever possible.
Quality control activities were conducted at regular intervals to verify data quality, completeness and consistency with medical records.
The DNDi Data Centre at the Kenya Medical Research Institute (KEMRI), Nairobi, was responsible for data management, statistical analysis and providing the Steering Committee with regular Periodic Safety Update Reports (PSURs). The PV Steering Committee was responsible for reviewing the PSURs, which consisted of data summarized for 6 months, for the period under review, and cumulative data.
Regulatory and Ethical Statement
As the PV program did not involve any additional intervention compared to routine clinical management, minimal risk was involved. Patients’ confidentiality was protected by ensuring that personal data were absent from data collection forms and from the central registry, by providing appropriate training to responsible staff and by restricting access to data to authorized personnel. All patients or their caregivers provided written consent before being enrolled in the PV program. Regulatory approvals were obtained in Sudan, Kenya, Uganda and Ethiopia from the National Medicines and Poisons Board, Pharmacy and Poisons Board, National Drug Authority and Food Medicines Health Care Administration and Control Authority, respectively. In Kenya, the KEMRI Ethical Review Committee also approved the PV program. A waiver was obtained from the MSF Ethics Committee because this concerned collection of data from a routine practice.
Pharmacovigilance (PV) Programme Design
The PV programme was a prospective observational cohort (non-interventional, non-experimental, non-randomized). There was no hypothesis being tested.
PV Programme Participants
Only consenting patients treated with a SSG-PM combination were enrolled. Patients did not receive the combination if they had severe renal, cardiac or other systemic disease based on clinician judgment, or if they were known to be taking an aminoglycoside or were hypersensitive to either SSG or PM. As per MSF clinical protocol, HIV co-infected patients and patients above 50 years of age received AmBisome® rather than SSG-PM in MSF sites. In other sites, those patients who received SSG-PM were thus included in the PV programme.
Treatment
The treatment administered in the PV program was in line with WHO recommendations and national protocols: intravenous (IV) or intramuscular (IM) sodium stibogluconate at 20 mg/kg/day and IM paromomycin at 15 mg/kg/day for 17 days [11]. All patients were hospitalized for the duration of treatment. The SSG-PM combination is co- administered once a day thus compliance was reported according to the number of days SSG-PM was given.
Outcome Measures and Definitions of Outcomes
Safety endpoints were incidence of treatment-emergent adverse events, SAEs and ADRs, as reported by the treating physicians. AEs leading to treatment discontinuation were also described. AEs were analysed by sex and age and/or according to co-morbidity. AE causality assessment with the SSG-PM combination treatment was done by the clinician in charge, according to a simple scale of related/not related/relationship unknown. AEs were defined as ADRs if the AE relationship with a SSG-PM combination could not be excluded (i.e. was reported as related or unknown). AEs were coded by MedDRA (Medical Dictionary for Regulatory Activities)-trained DNDi trial coordination medical team according to MedDRA version 14.0 [19]. AEs were actively reported for the duration of the hospitalization during SSG-PM treatment, and SAEs were passively collected from spontaneous reports after patients were discharged post-treatment.
The primary effectiveness endpoint used in the PV program was initial cure, and was defined as treatment success (by clinical cure and/or parasitology clearance) on day 18 after treatment start (EOT). Clinical cure was defined as fever clearance, improvement of signs and symptoms of the disease, improvement of haematological parameters, reduction of spleen size, in line with the site’s routine practice. Microscopic examination of tissue aspirate (lymph node, spleen or bone marrow) at day 18 was used for parasitology. Any other outcome other than initial cure was considered treatment failure, e.g. non-response, defaulter, slow response, death, treatment discontinuation or PKDL. Patients with missing data were classified as treatment failures. Slow response was defined as partial improvement of clinical symptoms and/or a partial decrease in parasitological load at the EOT. Slow responders were requested to return to the clinic to repeat the test of cure 1 month after discharge. Non-response was defined as no improvement in clinical symptoms and/or positive parasitology test at EOT. Defaulters were patients who failed to complete treatment by absconding further treatments mainly through self-discharge from the hospital. Relapse was defined as a patient who was reported as initially cured by EOT but presented again with clinical symptoms of VL and repeat parasitological diagnosis (microscopy) confirmed VL within 6 months after having been cured. In Eastern Africa VL reinfection is considered more likely rather than VL relapse if a patient presents with confirmed VL more than 6 months after initial cure. Confirmation of reinfection requires molecular diagnosis that was not a practice at any site and is generally unavailable except in research. Other effectiveness endpoints included initial cure by age and sex, by region, country, geography and site; and by co-morbidity. Relapses/PKDL and other EOT outcomes were also described.
Sample Size Calculations
Given the emphasis on safety, the sample size required was based on the probability of detecting AEs with an expected incidence of 1/1000. With a cohort of 2000 patients, there would be an 86% probability of detecting at least one AE with an expected 1/1000 incidence and a 95% probability with a cohort of 3000 patients [20–22]. When the PV program reached a sample size of 3126, the Steering Committee held a final meeting to recommended termination of the PV data collection activities.
Randomization
Randomization was not applicable in the PV programme.
Data Management and Statistical Analysis
Data were double-entered and validated in OpenClinica (http://www.openclinica.com). An in-house Query Management System developed by the Data Centre QMSPlus [23] was used to generate queries. All statistical analyses were performed using STATA version 13.2 (http://www.stata.com).
Continuous data at baseline were summarized using mean [standard deviation (SD)] and median [interquartile range (IQR)]. Binary and categorical data were summarized as raw numbers and percentages. Comparisons between subgroups were done using chi-square tests or ANOVA where applicable.
Analysis Populations
The analysis population was the intention-to-treat (ITT) population, meaning that any patient who received at least one dose of SSG-PM combination treatment was included in the analysis population. When outcome data were missing, the analysis assumed treatment failure. Missing data could arise if the patient did not complete treatment, the investigator failed to make an assessment or the patient left the hospital before assessment was carried out. Treatment effectiveness was determined as the proportion of patients considered cured at end of treatment assessment. The effectiveness was reported together with its 95% CI. Proportions of the other clinical outcomes at EOT were described (death, non-responder, slow responder, defaulted). Sub-group analyses of safety and effectiveness outcomes by initial diagnosis (primary vs. relapse) were also undertaken.
In analysing safety data, the summaries were based on the MedDRA preferred terms and system organ class terms overall and within subgroups. Listings of AEs by body organ system using MedDRA preferred terms were presented along with frequencies for the overall population.
Results
Patient Population: Demography and Co-morbidities
The PV program started in April 2011 in Sudan, Uganda and Kenya and in November 2012 in Ethiopia; enrolment was completed in November 2013. A further 6 months was allowed for follow-up of the last enrolled patients. PV data collection activities thus ended on the 30 May 2014.
Three thousand one hundred and twenty-six patients were enrolled in the PV program. Of these 3126 patients, 1962 (62.7%) were from Sudan; Kenya had 652 (20.9%) patients enrolled, while Ethiopia and Uganda had 322 (10.3%) and 190 (6.1%) patients, respectively. The number of patients enrolled per site were as follows: in Sudan–Bazura 260 (8.32%), Dooka 150 (4.80%), Elhawata 158 (5.05%), Kassab 110 (3.52%), Tabarak Allah 594 (19.00%) and Umelkheir 690 (22.07%); in Kenya–Kacheliba 557 (17.82%) and Kimalel 95 (3.04%); in Uganda–Amudat 190 (6.08%); and in Ethiopia–Gondar 157 (5.02%), Arba Minch 90 (2.88%) and Abdurafi 75 (2.40%).
The median age of the PV population at baseline was 11 years (IQR 6–20). Except in Ethiopia where patients were predominantly males over 15 years of age (95.6%) with a median age of 21 years, patients from Sudan, Kenya and Uganda were predominantly male children aged less than 15 years. Nearly all VL cases (3037 (97.8%)) were reported as primary VL. Seventy (2.2%) patients were classified as relapses at baseline, with rates varying from 0.2% to 7.4% across countries. The highest proportion (14 (7.4%)) was reported in Uganda. Co-morbidities were reported in 279 (8.9%) patients and included severe malnutrition in 33 (3.5%) patients, severe anaemia in 109 (3.5%) patients, tuberculosis (TB) in nine (0.3%) patients and HIV in 18 (0.6%) patients. HIV/VL co-infection was most prevalent in Ethiopia 15 (26.8%); Kenya and Uganda did not report any HIV/VL co-infection (Table 1).
Table 1.
Baseline demographic characteristics
| Overall (n = 3126) | Sudan (n = 1962) | Kenya (n = 652) | Ethiopia (n = 322) | Uganda (n = 190) | |
|---|---|---|---|---|---|
| Age (years) (n = 3111)a | |||||
| Mean (SD) | 14.3 (11.7) | 13.3 (12.5) | 13.6 (9.2) | 22.0 (8.6) | 13.5 (10.5) |
| Median (IQR) | 11 (6–20) | 10 (5–17) | 12 (6–18) | 21 (18–25) | 11 (6–18) |
| Age categories, n (%) (n = 3111) | |||||
| <5 years | 515 (16.6) | 400 (20.5) | 78 (12.0) | 3 (0.9) | 34 (17.9) |
| 5–14 years | 1443 (30.1) | 966 (49.6) | 340 (52.2) | 44 (13.7) | 93 (48.9) |
| 15–34 years | 937 (30.1) | 430 (22.1) | 207 (31.8) | 249 (77.3) | 51 (26.8) |
| 35–50 years | 173 (5.6) | 113 (5.8) | 26 (4.0) | 22 (6.8) | 12 (6.3) |
| >50 years | 43 (1.4) | 38 (2.0) | 1 (0.2) | 4 (1.2) | – |
| Sex, n (%) (n = 3120)b | |||||
| Male | 2126 (68.1) | 1229 (62.6) | 459 (70.8) | 303 (95.6) | 135 (71.1) |
| Female | 994 (31.9) | 733 (37.4) | 192 (29.5) | 14 (4.4) | 55 (29.0) |
| Weight (kg) | |||||
| Mean (SD) | 30.6 (16.9) | 28.1 (17.4) | 31.6 (14.9) | 43.0 (10.7) | 31.3 (16.4) |
| Median (IQR) | 26 (16–45) | 22 (14.5–41) | 28 (19–45.6) | 45 (41–49.4) | 26.8 (18–48) |
| Height (cm) | |||||
| Mean (SD) | 140.5 (28.8) | 133.7 (29.6) | 141.3 (27.1) | 161.1 (16.7) | 139.0 (30.3) |
| Median (IQR) | 145 (118–165.5) | 133.6 (110–160) | 145 (120–165) | 165 (160–170) | 138 (120–168) |
| Diagnosis, n (%) | |||||
| Clinical | 13 (0.4) | 6 (0.3) | 3 (0.5) | 4 (1.3) | 0 |
| Serological | 1344 (43.3) | 832 (42.8) | 501 (76.9) | 11 (3.4) | 0 |
| Parasitological | 852 (27.4) | 593 (30.5) | 100 (15.4) | 159 (49.5) | 0 |
| Combination | 896 (28.9) | 512 (26.4) | 47 (7.2) | 147 (45.8) | 190 (100) |
| Patient type, n (%) | |||||
| Primary | 3037 (97.8) | 1903 (97.8) | 650 (99.8) | 308 (96.2) | 176 (92.6) |
| Relapse | 70 (2.2) | 43 (2.2) | 1 (0.2) | 12 (3.8) | 14 (7.4) |
| Co-morbidities, n (%) | |||||
| HIV | 18 (6.5) | 3 (1.7) | 0 | 15 (26.8) | 0 |
| PTB | 9 (3.2) | 7 (4.1) | 2 (8.3) | 0 | 0 |
| Severe pneumonia | 33 (11.8) | 14 (8.1) | 3 (12.5) | 15 (26.8) | 1 (3.9) |
| Severe malnutrition | 110 (39.4) | 89 (51.5) | 6 (25.0) | 13 (23.2) | 2 (7.8) |
| Severe anaemia (Hb <5 g/dl) | 109 (39.1) | 60 (34.7) | 13 (54.2) | 13 (23.2) | 23 (68.5) |
Hb haemoglobin, HIV human immunodeficiency virus, IQR interquartile range, PTB pulmonary tuberculosis, SD standard deviation
a15 patients with missing data
b6 patients with missing data
Compliance
Two thousand eight hundred and fifty-three (91.3%) patients had 100% compliance (received full course of SSG-PM treatment for 17 days) while 220 (7.0%) of the patients had less than 90% compliance (received treatment for 15 days or less). Of those who had less than 90% compliance, 151 (71.9%) patients had an EOT outcome reported as treatment success. Ethiopia and Sudan reported the highest proportion of patients with less than 90% compliance [11.2% (n = 36) and 7.0% (n = 138), respectively], explained by a high incidence of early deaths. Ethiopia had the highest default rate 4.0% (n = 13) while other countries had less than 1% default rate (Table 2).
Table 2.
Treatment compliance
| Overall | Sudan | Kenya | Ethiopia | Uganda | |
|---|---|---|---|---|---|
| Received full treatment: 100% compliance (17 days' treatment) | 2853 (91.3) | 1791 (91.3) | 605 (92.8) | 281 (87.3) | 176 (92.6) |
| Received 90–99% of expected treatment (15–17 days’ treatment) | 30 (1.0) | 18 (0.9) | 9 (1.4) | 3 (0.9) | 0 |
| Received <90% of expected treatment (<15 days' treatment) | 220 (7.0) | 138 (7.0) | 37 (5.7) | 36 (11.2) | 9 (4.7) |
| Missing | 23 (0.7) | 15 (0.8) | 1 (0.1) | 2 (0.6) | 5 (2.6) |
| Total | 3126 | 1962 | 652 | 322 | 190 |
Safety–Adverse Events and Adverse Reactions
AEs were common, with 34% (n = 1063) of the patients reporting at least one AE. The total number of AEs reported was 1767, the mean number of AE per patient was 1.6, ranging from 1 to 6 (Table 3).
Table 3.
Number of adverse events
| Overall | Sudan | Kenya | Ethiopia | Uganda | Northa | Southb | |
|---|---|---|---|---|---|---|---|
| Number treated | 3126 | 1962 | 652 | 322 | 190 | 2194 | 932 |
| Number of SAEs, n (%) | 60 (1.9) | 39 (2.0) | 3 (0.5) | 16 (5.0) | 2 (1.1) | 56 (2.6) | 6 (0.6) |
| Patients with at least one AE, n (%) | 1063 (34.0) | 607 (30.9) | 215 (33.0) | 221 (68.6) | 20 (10.5) | 627 (28.6) | 436 (46.6) |
| Number of AEs during treatment | 1767 | 1150 | 266 | 331 | 20 | 1475 | 292 |
AE adverse event, SAE serious adverse event
aNorth: Sudan, Ethiopia (Gondar & Abdurafi)
bSouth: Kenya, Uganda, Ethiopia (Arba Minch)
Sixty SAEs were reported, giving an overall SAE rate of 1.9%. Of the 60 SAEs, 16 were considered serious ADRs (SADRs) thus giving an overall SADR rate of 0.5% (Table 4). Any SAE with positive or unknown relationship to SSG-PM treatment was considered a SADR. The most common SAEs were infections and infestations (n = 10), blood and lymphatic system disorders (n = 8), gastrointestinal disorders (n = 8), and ear and labyrinth disorders (n = 7). There were 32 deaths by EOT giving an EOT CFR of 1.0%. The leading causes of death were sepsis related (n = 7), anemia (n = 4) and renal related (n = 3). Of the 16 deaths considered related to SSG-PM treatment, anaemia (n = 4), sudden death (n = 2) and renal-related AEs were the leading causes. Two sudden deaths, one occurring on day 3 in a 70-year-old man who died suddenly at night and the second in a 5-year-old child occurring on day 4, were reported as having unknown relationship. Causes of death could not be confirmed by post-mortem examination. No Suspected Unexpected SADRs (SUSARs) were reported.
Table 4.
List of serious adverse events
| MedDRA system organ class | MedDRA preferred term | Relation to SSG-PM, yes/no | Resulted in death, yes/no |
|---|---|---|---|
| Blood and lymphatic system disorders | Anaemia (n = 8) | Yes (n = 3) No (n = 2) Unknown (n = 3) |
Yes (n = 4) No (n = 4) |
| Cardiac disorders | Cardiac failure (n = 2) | Yes (n = 2) | No (n = 2) |
| Ear and labyrinth disorders | Hypoacusis (n = 6) | Yes (n = 6) | No (n = 6) |
| Tinnitus (n = 1) | Yes | No | |
| Eye disorders | Eye movement disorder (n = 1) | No | Yes |
| Gastrointestinal disorders | Diarrhoea (n = 1) | Unknown | Yes |
| Gastroenteritis (n = 1) | Unknown | No | |
| Haematemesis (n = 1) | No | Yes | |
| Pancreatitis, acute (n = 3) | Yes (n = 2) No (n = 1) |
Yes (n = 1) No (n = 2) |
|
| Rectal haemorrhage (n = 1) | No | Yes | |
| Vomiting (n = 1) | Unknown | Yes | |
| General disorders and administration site conditions | Injection site pain (n = 1) | Yes | No |
| Sudden death (n = 2) | Unknown (n = 2) | Yes (n = 2) | |
| Hepatobiliary disorders | Hepatitis (n = 2) | Yes (n = 2) | No (n = 2) |
| Jaundice (n = 1) | Unknown | Yes | |
| Jaundice cholestatic (n = 1) | Yes | No | |
| Infections and infestations | Malaria (n = 1) | No | Yes |
| Pneumonia (n = 1) | No | Yes | |
| Sepsis (n = 4) | No (n = 4) | Yes (n = 4) | |
| Septic shock (n = 3) | No (n = 3) | Yes (n = 3) | |
| Visceral leishmaniasis (n = 1) | Unknown | Yes | |
| Injury, poisoning and procedural complications | Nerve injury (n = 2) | No (n = 2) | No (n = 2) |
| Investigations | Blood creatinine increased (n = 5) | Yes (n = 4) Unknown (n = 1) |
Yes (n = 2) No (n = 3) |
| Metabolism and nutrition disorders | Dehydration (n = 1) | No | Yes |
| Hypokalaemia (n = 2) | Yes (n = 1) No (n = 1) |
Yes (n = 2) | |
| Malnutrition (n = 2) | No (n = 2) | Yes (n = 2) | |
| Pregnancy, puerperium and perinatal conditions | Abortion, spontaneous (n = 1) | Unknown | No |
| Renal and urinary disorders | Glomerulonephritis and nephrotic syndrome (n = 1) | Yes | No |
| Renal failure (n = 2) | Yes (n = 1) Unknown (n = 1) |
Yes (n = 1) No (n = 1) |
|
| Respiratory, thoracic and mediastinal disorders | Epistaxis (n = 1) | Unknown | Yes |
PM paromocyin, SSG sodium stibogluconate, VL visceral leishmaniasis
The most common treatment-emergent ADRs were injection site pain (n = 735), injection site swelling (n = 94), pyrexia (n = 87), vomiting (n = 51) and abdominal pain (n = 39) (Table 5). During the treatment period, 2.2% (n = 70) of patients discontinued treatment due to various AEs, such as increase in blood creatinine (n = 16), ear pain (n = 10), jaundice (n = 5), anaemia (n = 5) and hearing impairment (n = 4). Increased amylase levels led to treatment discontinuation in two patients. The frequency of PKDL was very low (0.3% (n = 9)) with most of them (9/10) occurring in the sites in the North, with symptoms appearing between 7 and 27 months after treatment, with a mean time of 15 months.
Table 5.
Adverse drug reactions
| MedDRA system organ class term | MedDRA preferred term | N |
|---|---|---|
| Blood and lymphatic system disorders | Anaemia | 8 |
| Ear and labyrinth disorders | Deafness, unilateral | 1 |
| Ear pain | 9 | |
| Hypoacusis | 5 | |
| Tinnitus | 2 | |
| Gastrointestinal disorders | Abdominal discomfort | 5 |
| Abdominal distension | 21 | |
| Abdominal pain | 39 | |
| Abdominal pain upper | 8 | |
| Blood urea increased | 3 | |
| Constipation | 1 | |
| Diarrhoea | 23 | |
| Dysentery | 1 | |
| Dyspepsia | 1 | |
| Haematemesis | 1 | |
| Mouth ulceration | 1 | |
| Nausea | 12 | |
| Pancreatitis, acute | 2 | |
| Stomatitis | 1 | |
| Vomiting | 51 | |
| General disorders and administration site conditions | Chest pain | 1 |
| Chills | 1 | |
| Death | 2 | |
| Fatigue | 1 | |
| Injection site abscess, sterile | 11 | |
| Injection site haemorrhage | 4 | |
| Injection site pain | 735 | |
| Injection site swelling | 94 | |
| Pyrexia | 87 | |
| Hepatobiliary disorders | Jaundice | 6 |
| Infections and infestations | Gastroenteritis | 2 |
| Injection site cellulitis | 1 | |
| Sepsis | 1 | |
| Investigations | Amylase increased | 5 |
| Blood creatinine increased | 20 | |
| Electrocardiogram QT prolonged | 1 | |
| Hepatic enzyme increased | 1 | |
| Metabolism and nutrition disorders | Decreased appetite | 10 |
| Musculoskeletal and connective tissue disorders | Joint swelling | 1 |
| Pain in extremity | 1 | |
| Nervous system disorders | Dizziness | 1 |
| Headache | 15 | |
| Insomnia | 1 | |
| Neuropathy peripheral | 1 | |
| Renal and urinary disorders | Haematuria | 1 |
| Renal failure | 1 | |
| Renal impairment | 1 | |
| Respiratory, thoracic and mediastinal disorders | Cough | 16 |
| Dyspnoea | 2 | |
| Epistaxis | 9 | |
| Sneezing | 1 | |
| Skin and subcutaneous tissue disorders | Rash | 5 |
| Oedema peripheral | 1 | |
| Swelling face | 2 |
MedDRA Medical Dictionary for Regulatory Activities
Effectiveness of SSG-PM Combination
In the 3126 patients, the overall effectiveness at the EOT or initial cure rate of SSG-PM was 95.1% (95% CI 94.4–95.9). There were 1.8% (95% CI 1.3–2.2) slow responders (n = 55), and a non-response rate of 0.3% (95% CI 0.1–0.5; n = 9). Mortality rate at the end of treatment was 0.9% (95% CI 0.5–1.2; n = 27), with 0.9% defaulters (n = 29) (Table 6).
Table 6.
End-of-treatment outcomes
| Overall, N = 3126 | Sudan, N = 1962 | Kenya, N = 652 | Ethiopia, N = 322 | Uganda, N = 190 | North,a n = 2194 | South,b n = 932 | |
|---|---|---|---|---|---|---|---|
| Initial cure, n (%) [95% CI] | 2974 (95.1%) [94.4–95.9] | 1848 (94.2%) [93.2–95.2] | 648 (99.4%) [98.8–100] | 290 (90.1%) [86.8–93.3] | 188 (98.9%) [97.5–100] | 2048 (93.3%) [92.3–94.4] | 926 (99.4%) [98.8–99.9] |
| Deaths, n (%) [95% CI] | 27 (0.9) [0.5–1.2] | 20 (1.0) [0.6–1.5] | 1 (0.2) [0.1–0.5] | 6 (1.9) [0.4–3.3] | 0 | 26 (1.2) [0.7–1.6] | 1 (0.1) [−0.1 to 0.3] |
| Non-response, n (%) [95% CI] | 9 (0.3) [0.1–0.5] | 6 (0.3) [0.1–0.6] | 0 | 3 (0.9) [−0.1 to 2.0] | 0 | 9 (0.4) [0.1–0.7] | 0 |
| Slow response, n (%) [95% CI] | 55 (1.8) [1.3–2.2] | 45 (2.3) [1.6–2.9] | 2 (0.3) [−0.1 to 0.7] | 8 (2.5) [0.8–4.2] | 0 | 53 (2.4) [1.8–3.1] | 2 (0.2) [−0.1 to 0.5] |
| Defaulted, n (%) [95% CI] | 29 (0.9) [0.6–1.3] | 14 (0.7) [0.3–1.1] | 0 | 13 (4.0) [1.9–6.2] | 2 (1.1) [−0.4 to 2.5] | 27 (1.2) [0.8–1.7] | 2 (0.2) [−0.1 to 0.5] |
| Missing, n (%) [95% CI] | 32 (1.0) [0.7–1.4] | 29 (1.5) [1.0–2.1] | 1 (0.2) [0.0–0.9] | 2 (0.6) [0.1–2.2] | 0 | 31 (1.4) [1.0–2.0] | 1 (0.1) [0.0–0.6] |
| Total | 3126 | 1962 | 652 | 322 | 190 | 2194 | 932 |
aNorth: Sudan, Ethiopia (Gondar & Abdurafi)
bSouth: Kenya, Uganda, Ethiopia (Arba Minch)
The overall SSG-PM initial cure rate did not change significantly (95.4%) when HIV/VL co-infected patients were excluded (18/3126). The initial cure rate of SSG-PM combination in Ethiopia was 90.1% (95% CI 86.8–93.3), which was lower than for the PV programme’s overall initial cure rate of 95.1% (95% CI 94.4–95.9) (p < 0.001). When patients with HIV/VL co-infection in Ethiopia were excluded from analysis, the treatment initial cure rate increased to 93%, and it was no longer significantly different from the overall population/other countries. Defining North and South PV sites based on geographical location that could correspond to VL ecologies in Eastern Africa, the overall SSG-PM initial cure rate was higher in the Southern region as compared to the North, 99.4% (95% CI 98.8–99.9) versus 93.3% (95% CI 92.3–94.4) (p < 0.001).
The EOT outcome was also analysed by age, patient type (primary VL or relapse) and co-morbidities at baseline. From Fisher’s exact tests there was a significant difference in initial cure (p < 0.001) for age <35 versus age ≥35 years (96 vs. 85% initial cure), which was more pronounced for patients age ≥50 years (81.8%) (p < 0.001). There was also a significant difference in occurrence of death (p < 0.001) for age <35 versus age ≥35 years (0.5 vs. 5.6% death), which was also more pronounced for age ≥50 years (9.1%). There was a significant difference in initial treatment outcome by patient type (primary vs. relapse). Patients who were primary VL cases had a higher chance of EOT as compared to patients who were VL relapse at baseline (95.7 vs. 87.1%, p < 0.001). The number of relapses was small (n = 70). The treatment initial cure rate in HIV/VL co-infected patients was 56% (95% CI 31–78), significantly lower than that in VL patients without HIV (p < 0.001). Among the other co-morbidities, the EOT effectiveness point estimate was not significantly different from the overall population (89.1–100%).
Sixty-four relapses were passively reported during the PV programme, which represents 2% of the overall VL patients treated.
Missing Data
Missing data accounted for <1% of the total recruited patients. In the ITT analysis, patients with missing data on EOT effectiveness (initial cure) were considered as treatment failures (worst-case analysis). There was no difference in baseline characteristics for missing EOT outcome except for age where there was a significant difference (p = 0.022) between those aged below 15 years, with less than 1% missing data, and those aged above 35 years, with 2.9% missing data.
Discussion
Eastern Africa has a high VL burden with an estimated 29,000–56,000 cases annually [2]. Sudan reports most cases, followed by Ethiopia, Kenya and Uganda. There are two distinct ecological settings in Eastern Africa, the Acacia–Balanites savannah regions in the north, and the savannah and forest areas in the south [5]. Geographically these settings correspond to the North (northern Ethiopia and eastern Sudan) and South (Kenya, Uganda and southern Ethiopia) sites in this PV programme. The significance of these distinct ecologies is the differences in disease transmission and vectors. Genetic variation in the north and south L. donovani species has been reported [24]. Whether this variation accounts for the differences in clinical presentation and outcomes from treatment is unknown.
In this PV programme, collection, monitoring and assessment of the SSG-PM combination was carried out as a regional effort across Eastern Africa to investigate the safety and effectiveness of the combination of SSG-PM during the early post-approval period following the 2010 WHO recommendation of SSG-PM as a new first-line treatment for VL in the region. As a large number of patients were to be exposed in a short period to the new treatment, the PV programme aimed to identify unexpected safety concerns and treatment effectiveness variations in different patient categories and geographies.
There was no new safety signal during the project’s period besides those previously reported for SSG-PM treatment during clinical trials. No SUSAR occurred and the rate of SAEs (1.9%) in this PV programme was comparable to that in the clinical trials [10, 20]. Though not unexpected, of concern were two sudden deaths. Cumulative toxicity has been reported for SSG [25]. Painful injection was the most common ADR, occurring in 23.5% of the patients. The most common cause of treatment discontinuation was increase in blood creatinine and ear pain.
The overall EOT effectiveness was high (95.1% initial cure), consistent with previously reported outcomes in the phase III clinical trial [12], though it was significantly lower in HIV co-infected patients and in patients older than 50 years. This is in line with other publications, which report poorer outcomes in these categories of patients [26, 27]. The overall mortality and non-response rates were 0.92 and 0.3%, respectively. A high EOT effectiveness was reported in a study assessing SSG-PM effectiveness for VL in routine conditions in Sudan that reported initial cure (93%) in primary VL cases. Effectiveness of SSG-PM was higher in primary VL cases as compared to relapse in both the PV programme and Sudan studies. This indicates the need for different management of relapse patients, who fortunately represent only a minority of the VL patients in Eastern Africa (2.2% of patients were VL relapses at baseline). In principle, a case of relapse should be treated with different drugs to those used in the former VL episode. Effectiveness of SSG-PM in the southern region was higher (99.4%) than in the north (93.3%). These regional differences have been previously described for paromomycin monotherapy [28] and liposomal amphotericin B [29]. The difference in effectiveness between northern and southern sites could also be attributed to HIV/VL co-infection and/or older patients, contributing to lower effectiveness (90%, p < 0.001) in Ethiopia. These findings reinforce the need for alternative management of HIV co-infected patients and patients older than 50 years, who ideally should have received liposomal amphotericin B treatment (AmBisome). The 18 cases of HIV/VL co-infected patients enrolled in this PV plan received SSG-PM because no liposomal amphotericin B was available in the treatment centre. This points to the dire need for improved access to medications for VL in Eastern Africa[30].
DNDi, MSF, LEAP (Leishmania East Africa Platform) and partners are currently conducting a clinical trial for new treatment options for HIV/VL co-infection in Ethiopia, to assess safety and efficacy for the currently recommended treatment of liposomal amphotericin B monotherapy (40 mg/kg total dose) and the combination of liposomal amphotericin B with miltefosine. Results should be available in 2016, which will improve understanding of this specific population and how to better manage these cases [31].
PV Limitations
The PV programme was conducted in real-life rural health settings in Eastern African countries where there is heterogeneous practice in terms of VL diagnosis, treatment and patient follow-up post-discharge. VL diagnosis was algorithmic, based on clinical presentation plus serology and/or parasitology according to the national guidelines or health centre practice. The main method of VL diagnosis in the PV programme was by clinical case definition and rapid diagnostic tests (RDTs). Validation of diagnosis by parasitology was available and practiced in six sites. The other six sites either did not have the parasitological diagnosis available or did not include it in the routine diagnosis algorithm. The sensitivity and specificity of RDTs in Eastern Africa varies according to the marketed products, with IT-Leish (BioRad) having the best performance [32], but it is generally believed to be >90% specific. It is therefore unlikely that the heterogeneity in the diagnostic method used by the sites resulted in selection bias, though other biases cannot be discounted [33].
Definitive cure for VL is usually assessed at 6 months; however, the PV programme was not designed to reach conclusions at this end point due to the anticipated variability in post-discharge follow-up practices, and findings should be interpreted with caution. Nevertheless, all patients were instructed to return to the clinic in case of reappearance of VL signs and symptoms. The low relapse rate we observed (2%) suggests a high definitive cure rate, given that the sites included in the PV programme are often the only centres that diagnose and treat VL within the areas of the programme. Patients with reoccurrence of VL symptoms would be able to recognize reappearance of the disease and return to the PV centres for assessment and treatment. If relapses are taken into consideration for an estimation of 6-month effectiveness, the rate of cure is 92.5% (95% CI 91.6–93.4). Even if the relapse rate is underestimated, final cure is likely to be >90% as reported in the Phase III clinical trial [12].
Most sites were scarcely resourced in terms of infrastructure, suffered from staff shortage and high staff turnover, and some were difficult to access. This resulted in variations in the quality and completeness of the data reported, the timeliness of safety reporting and clinical management of individual AEs. Some more difficult to detect AEs may have been missed by site staff such as pancreatitis as amylase testing was only available in Ethiopia. Under-reporting of SAEs after hospital discharge is likely to have occurred in sites with weak patient follow-up procedures. Besides staffing challenges, accurate AE diagnosis and attribution of causes of death that require high technical capability and expertise were not available in remote sites with difficult access to health [34]. Examples were the challenges of establishing the causes of renal-attributed AEs such as nephrotic syndrome and increased creatinine knowing that presence of renal disease affects clinical event rates and requires added vigilance [35]. Misclassification of certain AEs due to different words used in different sites could also be expected [36]. Having foreseen the afore-mentioned challenges, the data collection forms were simplified to the extent possible, a binary assessment of causality used, quality control activities were implemented and frequent retraining conducted throughout the project. Though this effectively minimised missing information, it precluded extensive PV analyses and most of the results presented here are descriptive. This is a common limitation of post-marketing studies based on spontaneous PV reporting [37].
While more structured causality assessment have been developed as a practical tool for assessment of case reports such as WHO-UMC causality [38], these were less practical in the present settings. In line with the CIOMS VI Working Group recommendations, a binary decision system was used by investigators for drug causality, owing to the limited utility of the various gradients of relatedness in data analysis or regulatory reporting. All SAE reports were reviewed by the Steering Committee and relatedness was re-assessed for each individual case. No change in the initial causality assessment made by the investigators occurred.
The PV challenges we encountered are not unique and have been reported in PV programmes for antimalarials [18]. Despite geographical and resource-limited setting, the programme achieved increased awareness and interest in PV, built capacity and yielded clinically practical information that has affected practice. Consideration in the most resource-limited settings during PV planning phase for VL should include support needed to strengthen PV through capacity building and plan for better follow-up of patients post-discharge to identify relapses and PKDL. Beside asymptomatic VL patients, modelling suggests PKDL and relapse patients are important reservoirs of infection [39].
The main advantages of the SSG-PM combination in Eastern Africa are a reduced hospitalization time (from 30 to 17 days), decreased cost and a decreased probability of resistance emergence [40]. Alternative treatment regimens such as single-dose AmBisome®, which provides >95% efficacy in India and Bangladesh [41–43], was tested in Eastern Africa, but with poor results [29]. Other combination regimens of AmBisome® with SSG or with miltefosine have been also assessed in Eastern Africa [44], but none of the regimens tested achieved a minimum 90% cure rate to be taken for further development. New oral chemical entities are currently in development by DNDi, LEAP and partners, but these are not expected to be in the market in the coming 5 years. Therefore, despite the well-known toxicities of its two injectable drugs, SSG-PM stands as the current best option for treatment of primary VL in Eastern Africa.
Conclusion
Given that no new safety signal emerged from the present analysis and the very good treatment outcomes provide further reassurance that the SSG-PM combination is safe and effective even in settings where malnutrition, anaemia and other infectious co-morbidities are prevalent. This PV supports the WHO recommendation for SSG-PM to be used as the first-line VL treatment in Eastern Africa. However, our results show that the combination should not be recommended for HIV/VL co-infected patients and patients more than 50 years old.
This PV programme, the first to be conducted in Eastern Africa on VL, in a large number of patients, demonstrates that through collaborative effort, relevant PV data can be obtained for regional use even from remote rural health settings with limited resources. There is a need to strengthen PV activities in Eastern Africa and dedicated funding should be made available to support sentinel sites in the acquisition and retention of essential PV knowledge and skills. As passive surveillance bears a number of drawbacks in such setting, it would be particularly important to plan for regular pharmaco-epidemiology studies involving active surveillance for a selected period and for selected locations. It is also crucial that PV data is integrated with national PV programmes and databases to contribute to public safety of medicines in public health programmes [15, 45].
Acknowledgements
We dedicate this paper to Prof. Juma Rashid who until his death was an integral part of the team conducting this PV programme. The authors would like to thank all members of the field teams, including nurses and laboratory technicians in all study sites for their contribution to the study. The authors would also like to thank the entire Drugs for Neglected Diseases Initiative (DNDi) Trial Coordination, Data Centre and Finance and Administration teams for their support. We would also like to thank the Ministries of Health of Uganda, Kenya, Ethiopia and Sudan and especially Gedaref state for their support. The report is published with permission from the participating Leishmania East Africa Platform (LEAP) members and from the Director of the Kenya Medical Research Institute (KEMRI).
Compliance with Ethical Standards
Funding
The Drugs for Neglected Diseases initiative is grateful to the following donors for their financial support of this study: Department for International Development (DFID) UK; Spanish Agency for International Development Cooperation (AECID), Spain; Dutch Ministry of Foreign Affairs (DGIS), The Netherlands; Federal Ministry of Education and Research (BMBF) through KfW / Germany and part of the EDCTP2 programme supported by the European Union; Swiss Agency for Development and Cooperation (SDC), Switzerland; Médecins Sans Frontières (Doctors Without Borders), International; Medicor Foundation, Liechtenstein; Fondation Pro Victimis, Switzerland; BBVA Foundation, Spain; and other foundations.
Conflict of interest
Robert Kimutai, Ahmed M. Musa, Simon Njoroge, Raymond Omollo, Fabiana Alves, Asrat Hailu, Eltahir A.G. Khalil, Ermias Diro, Peninah Soipei, Brima Musa, Khalid Salman, Koert Ritmeijer, Francois Chappuis, Juma Rashid, Rezika Mohammed, Asfaw Jameneh, Eyasu Makonnen, Joseph Olobo, Lawrence Okello, Patrick Sagaki, Nathalie Strub, Sally Ellis, Jorge Alvar, Manica Balasegaram, Emilie Alirol and Monique Wasunna declare that they have no conflicts of interest.
Ethical approval
All procedures in this study were in accordance with the 1964 Helsinki Declaration and its amendments. Regulatory approvals were obtained in Sudan, Kenya, Uganda and Ethiopia from the National Medicines and Poisons Board, Pharmacy and Poisons Board, National Drug Authority and Food Medicines Health Care Administration and Control Authority, respectively. In Kenya, the KEMRI Ethical Review Committee also approved the PV program. A waiver was obtained from the MSF Ethics Committee.
Informed consent
All patients or their caregivers provided written consent before being enrolled in the PV programme.
Footnotes
Deceased: Juma Rashid.
References
- 1.Chappuis F, Sundar S, Hailu A, Ghalib H, Rijal S, Peeling RW, et al. Visceral leishmaniasis: what are the needs for diagnosis, treatment and control? Nat Rev Microbiol. 2007;5(11):S7–S16. doi: 10.1038/nrmicro1748. [DOI] [PubMed] [Google Scholar]
- 2.Alvar J, Vélez ID, Bern C, Herrero M, Desjeux P, Cano J, et al. Leishmaniasis worldwide and global estimates of its incidence. PLoS One. 2012;7(5):e35671. doi: 10.1371/journal.pone.0035671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul R. Epidemiology of visceral leishmaniasis. Clin Epidemiol. 2016;(May 2014):147–54. [DOI] [PMC free article] [PubMed]
- 4.Pearson RD, Sousa ADQ. Clinical spectrum of leishmaniasis. Clin Infect Dis. 1995;1996(22):1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Seaman J, Mercer AJ, Sondorp E. The epidemic of visceral leishmaniasis in western Upper Nile, southern Sudan: course and impact from 1984 to 1994. Int J Epidemiol. 1996;25(4):862–871. doi: 10.1093/ije/25.4.862. [DOI] [PubMed] [Google Scholar]
- 6.Awad E, Khalil G, Khidir SA, Musa AM, Musa BY, Elfaki M, et al. A paradigm of paradoxical immune reconstitution syndrome in non-HIV/AIDS patients. J Trop Med. 2013;2013:11–16. doi: 10.1155/2013/275253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khalil EAG, Zijlstra EE, Kager PA, El Hassan AM. Epidemiology and clinical manifestations of Leishmania donovani infection in two villages in an endemic area in eastern Sudan. Trop Med Int Health. 2002;7(1):35–44. [DOI] [PubMed]
- 8.Zijlstra EE, Musa AM, Khalil EAG, El Hassan IM. Review post-kala-azar dermal leishmaniasis. Lancet Infect Dis. 2003;3(February):87–98. doi: 10.1016/S1473-3099(03)00517-6. [DOI] [PubMed] [Google Scholar]
- 9.El Hassan AM, Khalil EAG. Short communication: post-kala-azar dermal leishmaniasis: does it play a role in the transmission of Leishmania donovani in the Sudan? Trop Med Int Health. 2001;6(9):743–744. doi: 10.1046/j.1365-3156.2001.00776.x. [DOI] [PubMed] [Google Scholar]
- 10.Herwaldt BL. Leishmaniasis. Lancet. 1999;354(9185):1191–1199. doi: 10.1016/S0140-6736(98)10178-2. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases. WHO TRS N° 949 March 2010. Available from: http://apps.who.int/iris/bitstream/10665/44412/1/WHO_TRS_949_eng.pdf. Accessed 30 Nov 2016.
- 12.Musa A, Khalil E, Hailu A, Olobo J, Balasegaram M, Omollo R, et al. Sodium stibogluconate (ssg) & paromomycin combination compared to ssg for visceral leishmaniasis in east Africa: a randomised controlled trial. PLoS Negl Trop Dis. 2012;6(6):e1674. doi: 10.1371/journal.pntd.0001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pharmacy and Poisons Board. Guidelines for the National Pharmacovigilance System in Kenya. Ministry of Medical Services and Ministry of Public Health and Sanitation Kenya; 2009.
- 14.CIOMS. Reporting adverse drug reactions: definitions of terms and criteria for their use. Crusius ZBRBI, Venulet JGGKJ, editors. 1999. p. 170.
- 15.Uppsala Monitoring Centre. Safety Monitoring of Medicinal Products, Guidelines for setting up and running a Pharmacovigilance Centre. the Uppsala Monitoring Centre; 2000.
- 16.Administration H, Authority C. Guideline for Adverse Drug Events Monitoring (Pharmacovigilance) Third Edition Food, Medicine and Healthcare Administration and Control Authority of Ethiopia. 2014.
- 17.Arora R, Kapoor R, Gill NS, Aggarwal A, Rana AC. Pharmacovigilance: a way for better tomorrow. Int Res J Pharm. 2011;2(12):43–46. [Google Scholar]
- 18.Suku CK, Hill G, Sabblah G, Darko M, Muthuri G, Abwao E, et al. Experiences and lessons from implementing cohort event monitoring programmes for antimalarials in four african countries: results of a questionnaire-based survey. Drug Saf. 2015;38(11):1115–1126. doi: 10.1007/s40264-015-0331-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MedDRA MSSO. Introductory Guide MedDRA Version 14.0. MSSO-DI-6003-14.0.0 March 2011. Available from: http://www.meddra.org/sites/default/files/guidance/file/intguide_14_0_english.pdf. Accessed 30 Nov 2016.
- 20.WHO. A Practical Handbook on the Pharmacovigilance of Antimalarial Medicines. A Practical Handbook on the Pharmacovigilance of Antimalarial Medicines. 2007. p. 23.
- 21.WHO. A practical handbook on the pharmacovigilance of medicines used in the treatment of tuberculosis, Enhancing the safety of the tb patient. 2012. p. 91.
- 22.WHO. A practical handbook on the pharmacovigilance of antiretroviral medicines. 2013. p. 23.
- 23.Omollo R, Ochieng M, Mutinda B, Omollo T, Owiti R, Okeyo S, et al. Innovative approaches to clinical data management in resource limited settings using open-source technologies. PLoS Negl Trop Dis. 2014;8(9):e3134. doi: 10.1371/journal.pntd.0003134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dawit G. A review on biology, epidemiology and public health significance of leishmaniasis. J Bacteriol Parasitol. 2013;4:2–7. [Google Scholar]
- 25.Cesur S, Bahar K, Erekul S. Death from cumulative sodium stibogluconate toxicity on Kala-Azar. Clin Microbiol Infect. 2002;8:606. doi: 10.1046/j.1469-0691.2002.00456.x. [DOI] [PubMed] [Google Scholar]
- 26.Musa AM, Younis B, Fadlalla A, Royce C, Balasegaram M, Wasunna M, et al. Paromomycin for the treatment of visceral leishmaniasis in Sudan: a randomized, open-label, dose-finding study. PLoS Negl Trop Dis. 2010;4(10):4–10. doi: 10.1371/journal.pntd.0000855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diro E, Lynen L, Mohammed R, Boelaert M, Hailu A, van Griensven J. High parasitological failure rate of visceral leishmaniasis to sodium stibogluconate among HIV co-infected adults in Ethiopia. PLoS Negl Trop Dis. 2014;8(5):e2875. doi: 10.1371/journal.pntd.0002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hailu A, Musa A, Wasunna M, Balasegaram M, Yifru S, Mengistu G, et al. Geographical variation in the response of visceral leishmaniasis to paromomycin in East Africa: a multicentre, open-label, randomized trial. PLoS Negl Trop Dis. 2010;4(10):e709. doi: 10.1371/journal.pntd.0000709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khalil EAG, Weldegebreal T, Younis BM, Omollo R, Musa AM, Hailu W, et al. Safety and efficacy of single dose versus multiple doses of AmBisome for treatment of visceral leishmaniasis in eastern Africa: a randomised trial. PLoS Negl Trop Dis. 2014;8(1):e2613. [DOI] [PMC free article] [PubMed]
- 30.den Boer M, Argaw D, Jannin J, Alvar J. Leishmaniasis impact and treatment access. Clin Microbiol Infect. Eur Soc Clin Microbiol Infect Dis. 2011;17(10):1471–7. [DOI] [PubMed]
- 31.Identifier: C go., NCT02011958. Efficacy Trial of Ambisome Given Alone and Ambisome Given in Combination With Miltefosine for the Treatment of VL HIV Positive Ethiopian Patients. 2016. Available from: https://clinicaltrials.gov/ct2/show/NCT02011958?term=HIV+and+Visceral+Leishmaniasis%26rank=3. Accessed 13 Oct 2016.
- 32.Boelaert M, Verdonck K, Menten J, Sunyoto T, van Griensven J, Chappuis F, et al. Rapid tests for the diagnosis of visceral leishmaniasis in patients with suspected disease. Cochrane database Syst Rev. 2014;6(6):CD009135. [DOI] [PMC free article] [PubMed]
- 33.Psaty BM, Koepsell TD, Lin D, Weiss NS, Siscovick DS, Rosendaal FR, et al. Assessment and control for confounding by indication in observational studies. J Am Geriatr Soc. 1999;47(6):749–754. doi: 10.1111/j.1532-5415.1999.tb01603.x. [DOI] [PubMed] [Google Scholar]
- 34.Sheik-Mohamed A, Velema JP. Where health care has no access: the nomadic populations of sub-Saharan Africa. Trop Med Int Heal. 1999;4(10):695–707. doi: 10.1046/j.1365-3156.1999.00473.x. [DOI] [PubMed] [Google Scholar]
- 35.Jacob D, Marrón B, Rutherford PA, Corporation BH. Pharmacovigilance as a tool for safety and monitoring: a review of general issues and the specific challenges with end-stage renal failure patients. Drug Healthc Patient Saf. 2013;5:105–12. doi: 10.2147/DHPS.S43104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sczech L. Designing a strategy for kidney toxicity monitoring in clinical trials: pitfalls and solutions. Pharmacovigil Rev. 2012;6(4):1–3. [Google Scholar]
- 37.Bate AES. Quantitative signal detection using spontaneous ADR reporting. Pharmacoepidemiol Drug Saf. 18(6):427–36. [DOI] [PubMed]
- 38.World Health Organization The use of the WHO-UMC system for standardized case causality assessment. Uppsala Uppsala Monit Cent. 2005;3:2–7. [Google Scholar]
- 39.Hirve S, Boelaert M, Matlashewski G, Mondal D, Arana B, Kroeger A, et al. Transmission dynamics of visceral leishmaniasis in the indian subcontinent—a systematic literature review. PLoS Negl Trop Dis. 2016;10(8):e0004896. doi: 10.1371/journal.pntd.0004896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO. Costs of medicines in current use for the treatment of leishmaniasis. Technical Report series (TRS). 2010.
- 41.Mondal D, Alvar J, Hasnain MG, Hossain MS, Ghosh D, Huda MM, et al. Efficacy and safety of single-dose liposomal amphotericin B for visceral leishmaniasis in a rural public hospital in Bangladesh: a feasibility study. Lancet Glob Health. 2014;2(1):e51–e57. doi: 10.1016/S2214-109X(13)70118-9. [DOI] [PubMed] [Google Scholar]
- 42.Sundar S, Singh A, Rai M, Chakravarty J. Single-dose indigenous liposomal amphotericin B in the treatment of Indian visceral leishmaniasis: a phase 2 study. Am J Trop Med Hyg. 2015;92(3):513–517. doi: 10.4269/ajtmh.14-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundar S, Chakravarty J, Agarwal D, et al. Single-dose liposomal amphotericin B for visceral leishmaniasis in India. N Engl J Med. 2010;362(2):504–12. [DOI] [PubMed]
- 44.Wasunna M, Njenga S, Balasegaram M, Alexander N, Omollo R, Edwards T, et al. Efficacy and safety of am bisome in combination with sodium stibogluconate or miltefosine and miltefosine monotherapy for african visceral leishmaniasis: phase II randomized trial. PLoS Negl Trop Dis. 2016;10:1–18. doi: 10.1371/journal.pntd.0004880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pal SN, Duncombe C, Falzon D, Olsson S. WHO strategy for collecting safety data in public health programmes : complementing spontaneous reporting systems. Drug Saf. 2013;36:75–81. doi: 10.1007/s40264-012-0014-6. [DOI] [PMC free article] [PubMed] [Google Scholar]