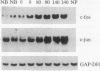
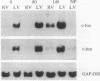
Abstract
The effect of changes in left ventricular (LV) systolic force generation on cardiac c-fos and c-jun protooncogene expression was studied by using isolated beating hearts from male Wistar rats. An isovolumic buffer-perfused heart preparation was utilized in which coronary flow and heart rate were held constant and increments in LV balloon volume were used to generate defined levels of LV systolic wall stress. Using Northern and slot-blot analyses, we found that LV tissue from control hearts that generated high levels of LV systolic wall stress expressed 3- to 4.4-fold higher c-fos and c-jun mRNA levels in comparison with tissue from the respective flaccid right ventricles, and in comparison with LV tissue from hearts that generated minimal LV systolic wall stress. To distinguish the role of passive LV diastolic wall stretch from active LV force generation, we found that distension of the LV balloon per se did not have a significant effect on protooncogene induction in hearts perfused with 2,3-butanedione monoxime, which prevents systolic cross-bridge cycling and force generation. In additional hearts studied at a constant LV balloon volume to generate an LV end-diastolic pressure of 10 mm Hg, c-fos mRNA levels were proportional to the magnitude of peak LV systolic wall stress (r = 0.823, P less than 0.05). In these protocols, Fos protein was localized by immunohistochemistry in myocyte nuclei with minimal staining in fibroblasts and vascular smooth muscle. When c-fos and c-jun mRNA expression was compared in hearts with chronic LV hypertrophy due to ascending aortic banding and age-matched control hearts that generated similar incremental levels of LV systolic wall stress, significantly lower levels of c-fos and c-jun mRNA were measured in the hypertrophied hearts. However, there was no difference in protooncogene mRNA expression in response to stimulation by the Ca2+ ionophore A23187. These data suggest that, in this isolated isovolumic beating heart preparation, the active generation of an acute increment in LV systolic force independent of passive diastolic myocardial stretch causes a rapid induction of both c-fos and c-jun, which is down-regulated in the presence of established LV hypertrophy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abate C., Luk D., Gentz R., Rauscher F. J., 3rd, Curran T. Expression and purification of the leucine zipper and DNA-binding domains of Fos and Jun: both Fos and Jun contact DNA directly. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1032–1036. doi: 10.1073/pnas.87.3.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P., Ricci R., Olivetti G. Quantitative structural analysis of the myocardium during physiologic growth and induced cardiac hypertrophy: a review. J Am Coll Cardiol. 1986 May;7(5):1140–1149. doi: 10.1016/s0735-1097(86)80236-4. [DOI] [PubMed] [Google Scholar]
- Bauters C., Moalic J. M., Bercovici J., Mouas C., Emanoil-Ravier R., Schiaffino S., Swynghedauw B. Coronary flow as a determinant of c-myc and c-fos proto-oncogene expression in an isolated adult rat heart. J Mol Cell Cardiol. 1988 Feb;20(2):97–101. doi: 10.1016/s0022-2828(88)80023-3. [DOI] [PubMed] [Google Scholar]
- Blanchard E. M., Smith G. L., Allen D. G., Alpert N. R. The effects of 2,3-butanedione monoxime on initial heat, tension, and aequorin light output of ferret papillary muscles. Pflugers Arch. 1990 Apr;416(1-2):219–221. doi: 10.1007/BF00370248. [DOI] [PubMed] [Google Scholar]
- Fort P., Marty L., Piechaczyk M., el Sabrouty S., Dani C., Jeanteur P., Blanchard J. M. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985 Mar 11;13(5):1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman W. Cardiac hypertrophy: useful adaptation or pathologic process? Am J Med. 1980 Oct;69(4):576–584. doi: 10.1016/0002-9343(80)90471-4. [DOI] [PubMed] [Google Scholar]
- Grossman W., Jones D., McLaurin L. P. Wall stress and patterns of hypertrophy in the human left ventricle. J Clin Invest. 1975 Jul;56(1):56–64. doi: 10.1172/JCI108079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. T., Gopal T. V., Moulton A. D., Nienhuis A. W. Inducible production of c-fos antisense RNA inhibits 3T3 cell proliferation. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4794–4798. doi: 10.1073/pnas.83.13.4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama S., Grossman W., Wei J. Y. Effect of age on myocardial adaptation to volume overload in the rat. J Clin Invest. 1988 Jun;81(6):1850–1857. doi: 10.1172/JCI113530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoyama S., Wei J. Y., Izumo S., Fort P., Schoen F. J., Grossman W. Effect of age on the development of cardiac hypertrophy produced by aortic constriction in the rat. Circ Res. 1987 Sep;61(3):337–345. doi: 10.1161/01.res.61.3.337. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson T., Allard M. F., Sreenan C. M., Doss L. K., Bishop S. P., Swain J. L. The c-myc proto-oncogene regulates cardiac development in transgenic mice. Mol Cell Biol. 1990 Jul;10(7):3709–3716. doi: 10.1128/mcb.10.7.3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent R. L., Hoober J. K., Cooper G., 4th Load responsiveness of protein synthesis in adult mammalian myocardium: role of cardiac deformation linked to sodium influx. Circ Res. 1989 Jan;64(1):74–85. doi: 10.1161/01.res.64.1.74. [DOI] [PubMed] [Google Scholar]
- Kira Y., Kochel P. J., Gordon E. E., Morgan H. E. Aortic perfusion pressure as a determinant of cardiac protein synthesis. Am J Physiol. 1984 Mar;246(3 Pt 1):C247–C258. doi: 10.1152/ajpcell.1984.246.3.C247. [DOI] [PubMed] [Google Scholar]
- Komuro I., Katoh Y., Kaida T., Shibazaki Y., Kurabayashi M., Hoh E., Takaku F., Yazaki Y. Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J Biol Chem. 1991 Jan 15;266(2):1265–1268. [PubMed] [Google Scholar]
- Kruijer W., Cooper J. A., Hunter T., Verma I. M. Platelet-derived growth factor induces rapid but transient expression of the c-fos gene and protein. Nature. 1984 Dec 20;312(5996):711–716. doi: 10.1038/312711a0. [DOI] [PubMed] [Google Scholar]
- Lompre A. M., Schwartz K., d'Albis A., Lacombe G., Van Thiem N., Swynghedauw B. Myosin isoenzyme redistribution in chronic heart overload. Nature. 1979 Nov 1;282(5734):105–107. doi: 10.1038/282105a0. [DOI] [PubMed] [Google Scholar]
- Lorell B. H., Wexler L. F., Momomura S., Weinberg E., Apstein C. S. The influence of pressure overload left ventricular hypertrophy on diastolic properties during hypoxia in isovolumically contracting rat hearts. Circ Res. 1986 May;58(5):653–663. doi: 10.1161/01.res.58.5.653. [DOI] [PubMed] [Google Scholar]
- Mitchell R. L., Henning-Chubb C., Huberman E., Verma I. M. c-fos expression is neither sufficient nor obligatory for differentiation of monomyelocytes to macrophages. Cell. 1986 May 23;45(4):497–504. doi: 10.1016/0092-8674(86)90281-3. [DOI] [PubMed] [Google Scholar]
- Morgan H. E., Baker K. M. Cardiac hypertrophy. Mechanical, neural, and endocrine dependence. Circulation. 1991 Jan;83(1):13–25. doi: 10.1161/01.cir.83.1.13. [DOI] [PubMed] [Google Scholar]
- Mulvagh S. L., Michael L. H., Perryman M. B., Roberts R., Schneider M. D. A hemodynamic load in vivo induces cardiac expression of the cellular oncogene, c-myc. Biochem Biophys Res Commun. 1987 Sep 15;147(2):627–636. doi: 10.1016/0006-291x(87)90977-6. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Peterson M. B., Lesch M. Protein synthesis and amino acid transport in the isolated rabbit right ventricular papillary muscle. Effect of isometric tension development. Circ Res. 1972 Sep;31(3):317–327. doi: 10.1161/01.res.31.3.317. [DOI] [PubMed] [Google Scholar]
- Ray A., Aumont M. C., Aussedat J., Bercovici J., Rossi A., Swynghedauw B. Protein and 28S ribosomal RNA fractional turnover rates in the rat heart after abdominal aortic stenosis. Cardiovasc Res. 1987 Aug;21(8):587–592. doi: 10.1093/cvr/21.8.587. [DOI] [PubMed] [Google Scholar]
- Sassone-Corsi P., Sisson J. C., Verma I. M. Transcriptional autoregulation of the proto-oncogene fos. Nature. 1988 Jul 28;334(6180):314–319. doi: 10.1038/334314a0. [DOI] [PubMed] [Google Scholar]
- Schuermann M., Neuberg M., Hunter J. B., Jenuwein T., Ryseck R. P., Bravo R., Müller R. The leucine repeat motif in Fos protein mediates complex formation with Jun/AP-1 and is required for transformation. Cell. 1989 Feb 10;56(3):507–516. doi: 10.1016/0092-8674(89)90253-5. [DOI] [PubMed] [Google Scholar]
- Schunkert H., Dzau V. J., Tang S. S., Hirsch A. T., Apstein C. S., Lorell B. H. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J Clin Invest. 1990 Dec;86(6):1913–1920. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz K., de la Bastie D., Bouveret P., Oliviéro P., Alonso S., Buckingham M. Alpha-skeletal muscle actin mRNA's accumulate in hypertrophied adult rat hearts. Circ Res. 1986 Nov;59(5):551–555. doi: 10.1161/01.res.59.5.551. [DOI] [PubMed] [Google Scholar]
- Simpson P. C. Role of proto-oncogenes in myocardial hypertrophy. Am J Cardiol. 1988 Oct 5;62(11):13G–19G. doi: 10.1016/0002-9149(88)90026-4. [DOI] [PubMed] [Google Scholar]
- Smith D. M., Sugden P. H. Stimulation of left-atrial protein-synthesis rates by increased left-atrial filling pressures in the perfused working rat heart in vitro. Biochem J. 1983 Dec 15;216(3):537–542. doi: 10.1042/bj2160537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Xenophontos X. P., Gordon E. E., Morgan H. E. Effect of intraventricular pressure on protein synthesis in arrested rat hearts. Am J Physiol. 1986 Jul;251(1 Pt 1):C95–C98. doi: 10.1152/ajpcell.1986.251.1.C95. [DOI] [PubMed] [Google Scholar]