Abstract
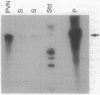
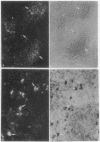
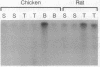
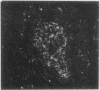
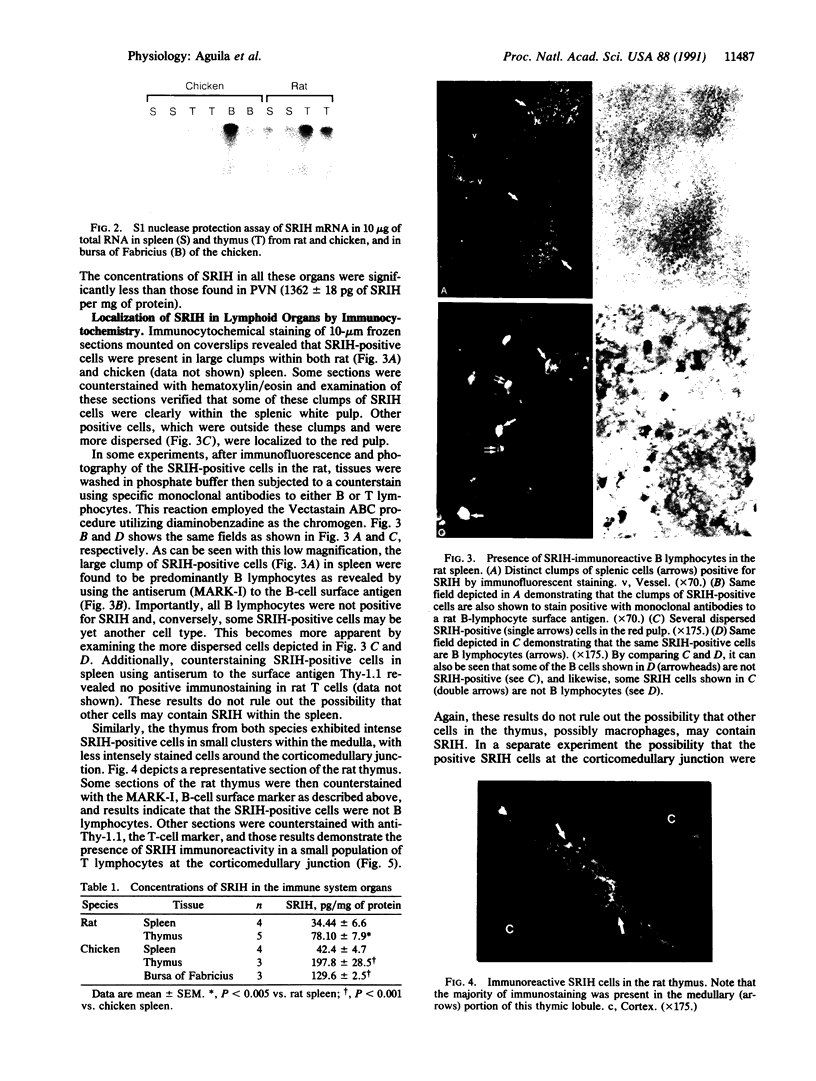
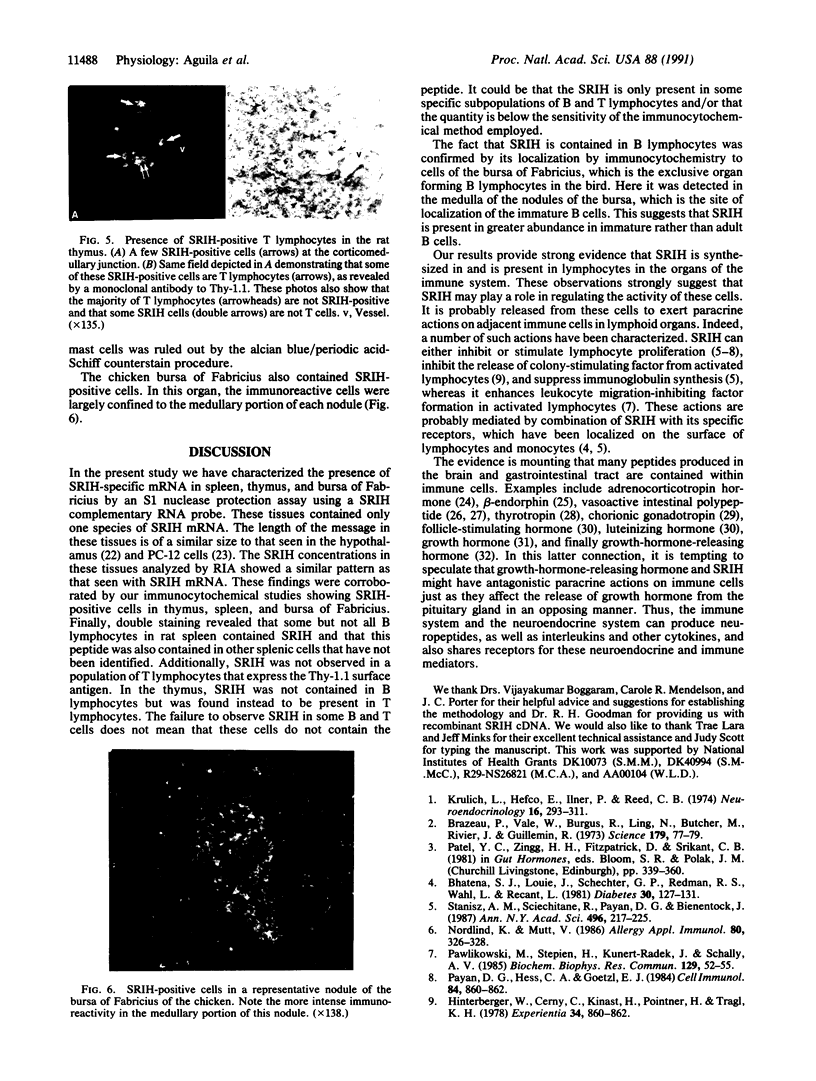
Because several peptides originally found in the pituitary as within the central nervous system have been localized in lymphoid tissues and because somatostatin (somatotropin-release-inhibiting hormone, SRIH) can act on cells of the immune system, we searched for this peptide in lymphoid organs. We demonstrated that SRIH mRNA exists in lymphoid tissue, albeit in smaller levels than in the periventricular region of the hypothalamus, the brain region that contains the highest level of this mRNA. SRIH mRNA was found in the spleen and thymus of male rats and in the spleen, thymus, and bursa of Fabricius of the chicken. Its localization in the bursa indicates that the peptide must be present in B lymphocytes since this is the site of origin of B lymphocytes in birds. The SRIH concentration in these lymphoid organs as determined by radioimmunoassay was greater in the thymus than in the spleen of the rat. These concentrations were 50 times less than those found in the periventricular region of the hypothalamus, the site of the perikarya of SRIH-containing neurons. In the chicken, as in the rat, the concentration of SRIH was greater in the thymus than in the spleen; it was present in the bursa of Fabricius, also in higher concentration than in the spleen. Fluorescence immunocytochemistry revealed the presence of SRIH-positive cells in clusters inside the white pulp and more dispersed within the red pulp of the spleen of both the rat and the chicken. The thymus from these species also contained SRIH-positive cells within the medulla and around the corticomedullary junction. In the chicken, there were large clusters of SRIH-positive cells in the medullary portion of each nodule of the bursa of Fabricius. Preabsorption of the primary antiserum or replacing this antiserum with normal rabbit serum verified the specificity of staining. Sequential immunostaining of the same sections from rat spleen using first SRIH antibody and subsequently a monoclonal antibody against a rat B-cell surface antigen revealed the presence of SRIH immunoreactivity in some, but not all, B cells. Other cell types in spleen not yet identified also stained positively with the SRIH antibody but were not reactive to monoclonal antibodies to rat Thy-1.1, a marker for all the thymic T lymphocytes. The possibility that SRIH is present in other populations of cells in the spleen cannot be ruled out. Sequential immunostaining of the same sections of rat thymus revealed the presence of SRIH immunoreactivity in a small population of T lymphocytes in the medulla, as revealed by the Thy-1.1 marker. The SRIH-positive cells were nonimmunoreactive when exposed to the B-cell marker; however, the possibility that SRIH is present in other cells was not investigated. Thus, our results indicate that SRIH is synthesized and stored in cells of the immune system. SRIH may be secreted from these cells to exert paracrine actions that alter the function of immune cells in spleen and thymus.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed C. E., Dees W. L., Ojeda S. R. The immature rat ovary is innervated by vasoactive intestinal peptide (VIP)-containing fibers and responds to VIP with steroid secretion. Endocrinology. 1986 Apr;118(4):1682–1689. doi: 10.1210/endo-118-4-1682. [DOI] [PubMed] [Google Scholar]
- Arimura A., Sato H., Coy D. H., Schally A. V. Radioimmunoassay for GH-release inhibiting hormone. Proc Soc Exp Biol Med. 1975 Mar;148(3):784–789. doi: 10.3181/00379727-148-38631. [DOI] [PubMed] [Google Scholar]
- Bhathena S. J., Louie J., Schechter G. P., Redman R. S., Wahl L., Recant L. Identification of human mononuclear leukocytes bearing receptors for somatostatin and glucagon. Diabetes. 1981 Feb;30(2):127–131. doi: 10.2337/diab.30.2.127. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Dees W. L., Ahmed C. E., Ojeda S. R. Substance P- and vasoactive intestinal peptide-containing fibers reach the ovary by independent routes. Endocrinology. 1986 Aug;119(2):638–641. doi: 10.1210/endo-119-2-638. [DOI] [PubMed] [Google Scholar]
- Feramisco J. R., Smart J. E., Burridge K., Helfman D. M., Thomas G. P. Co-existence of vinculin and a vinculin-like protein of higher molecular weight in smooth muscle. J Biol Chem. 1982 Sep 25;257(18):11024–11031. [PubMed] [Google Scholar]
- Harbour-McMenamin D., Smith E. M., Blalock J. E. Production of immunoreactive chorionic gonadotropin during mixed lymphocyte reactions: a possible selective mechanism for genetic diversity. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6834–6838. doi: 10.1073/pnas.83.18.6834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinterberger W., Cerny C., Kinast H., Pointner H., Tragl K. H. Somatostatin reduces the release of colony-stimulating activity (CSA) from PHA-activated mouse spleen lymphocytes. Experientia. 1978 Jul 15;34(7):860–862. doi: 10.1007/BF01939667. [DOI] [PubMed] [Google Scholar]
- Kedzierski W., Porter J. C. Quantitative study of tyrosine hydroxylase mRNA in catecholaminergic neurons and adrenals during development and aging. Brain Res Mol Brain Res. 1990 Jan;7(1):45–51. doi: 10.1016/0169-328x(90)90072-l. [DOI] [PubMed] [Google Scholar]
- Khorram O., DePalatis L. R., McCann S. M. Development of hypothalamic control of growth hormone secretion in the rat. Endocrinology. 1983 Aug;113(2):720–728. doi: 10.1210/endo-113-2-720. [DOI] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 1987;155:397–415. doi: 10.1016/0076-6879(87)55027-3. [DOI] [PubMed] [Google Scholar]
- Krulich L., Hefco E., Illner P., Read C. B. The effects of acute stress on the secretion of LH, FSH, prolactin and GH in the normal male rat, with comments on their statistical evaluation. Neuroendocrinology. 1974;16(5-6):293–311. doi: 10.1159/000122576. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lygren I., Revhaug A., Burhol P. G., Giercksky K. E., Jenssen T. G. Vasoactive intestinal polypeptide and somatostatin in leucocytes. Scand J Clin Lab Invest. 1984 Jun;44(4):347–351. doi: 10.3109/00365518409083818. [DOI] [PubMed] [Google Scholar]
- Montminy M. R., Goodman R. H., Horovitch S. J., Habener J. F. Primary structure of the gene encoding rat preprosomatostatin. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3337–3340. doi: 10.1073/pnas.81.11.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Low M. J., Tapia-Arancibia L., Reichlin S., Mandel G., Goodman R. H. Cyclic AMP regulates somatostatin mRNA accumulation in primary diencephalic cultures and in transfected fibroblast cells. J Neurosci. 1986 Apr;6(4):1171–1176. doi: 10.1523/JNEUROSCI.06-04-01171.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montminy M. R., Sevarino K. A., Wagner J. A., Mandel G., Goodman R. H. Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6682–6686. doi: 10.1073/pnas.83.18.6682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordlind K., Mutt V. Influence of beta-endorphin, somatostatin, substance P and vasoactive intestinal peptide on the proliferative response of human peripheral blood T lymphocytes to mercuric chloride. Int Arch Allergy Appl Immunol. 1986;80(3):326–328. doi: 10.1159/000234073. [DOI] [PubMed] [Google Scholar]
- Pawlikowski M., Stepien H., Kunert-Radek J., Schally A. V. Effect of somatostatin on the proliferation of mouse spleen lymphocytes in vitro. Biochem Biophys Res Commun. 1985 May 31;129(1):52–55. doi: 10.1016/0006-291x(85)91401-9. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Blalock J. E. Human lymphocyte production of corticotropin and endorphin-like substances: association with leukocyte interferon. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7530–7534. doi: 10.1073/pnas.78.12.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith E. M., Morrill A. C., Meyer W. J., 3rd, Blalock J. E. Corticotropin releasing factor induction of leukocyte-derived immunoreactive ACTH and endorphins. 1986 Jun 26-Jul 2Nature. 321(6073):881–882. doi: 10.1038/321881a0. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Phan M., Kruger T. E., Coppenhaver D. H., Blalock J. E. Human lymphocyte production of immunoreactive thyrotropin. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6010–6013. doi: 10.1073/pnas.80.19.6010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanisz A. M., Scicchitano R., Payan D. G., Bienenstock J. In vitro studies of immunoregulation by substance P and somatostatin. Ann N Y Acad Sci. 1987;496:217–225. doi: 10.1111/j.1749-6632.1987.tb35769.x. [DOI] [PubMed] [Google Scholar]
- Weigent D. A., Blalock J. E. Immunoreactive growth hormone-releasing hormone in rat leukocytes. J Neuroimmunol. 1990 Sep-Oct;29(1-3):1–13. doi: 10.1016/0165-5728(90)90142-a. [DOI] [PubMed] [Google Scholar]
- Werner H., Koch Y., Baldino F., Jr, Gozes I. Steroid regulation of somatostatin mRNA in the rat hypothalamus. J Biol Chem. 1988 Jun 5;263(16):7666–7671. [PubMed] [Google Scholar]