Abstract
Background
Avascular necrosis of the femoral head (AVNFH) typically constitutes 5 to 15% of all complications of low-energy femoral neck fractures, and due to an increasingly ageing population and a rising prevalence of femoral neck fractures, the number of patients who develop AVNFH is increasing. However, there is no consensus regarding the relationship between blood lipid abnormalities and postoperative AVNFH. The purpose of this retrospective study was to investigate the relationship between blood lipid abnormalities and AVNFH following the femoral neck fracture operation among an elderly population.
Methods
A retrospective, comparative study was performed at our institution. Between June 2005 and November 2009, 653 elderly patients (653 hips) with low-energy femoral neck fractures underwent closed reduction and internal fixation with cancellous screws (Smith and Nephew, Memphis, Tennessee). Follow-up occurred at 1, 6, 12, 18, 24, 30, and 36 months after surgery. Logistic multi-factor regression analysis was used to assess the risk factors of AVNFH and to determine the effect of blood lipid levels on AVNFH development. Inclusion and exclusion criteria were predetermined to focus on isolated freshly closed femoral neck fractures in the elderly population. The primary outcome was the blood lipid levels. The secondary outcome was the logistic multi-factor regression analysis.
Results
A total of 325 elderly patients with low-energy femoral neck fractures (AVNFH, n = 160; control, n = 165) were assessed. In the AVNFH group, the average TC, TG, LDL, and Apo-B values were 7.11 ± 3.16 mmol/L, 2.15 ± 0.89 mmol/L, 4.49 ± 1.38 mmol/L, and 79.69 ± 17.29 mg/dL, respectively; all of which were significantly higher than the values in the control group. Logistic multi-factor regression analysis showed that both TC and LDL were the independent factors influencing the postoperative AVNFH within femoral neck fractures.
Conclusions
This evidence indicates that AVNFH was significantly associated with blood lipid abnormalities in elderly patients with low-energy femoral neck fractures. The findings of this pilot trial justify a larger study to determine whether the result is more generally applicable to a broader population.
Keywords: Avascular necrosis of the femoral head, Total cholesterol, Low-density lipoprotein, Femoral neck fracture, Harris Hip Score
Background
Avascular necrosis of the femoral head (AVNFH) is usually considered to be related to reduced blood flow as a consequence of a surgical approach and mostly occurs in patients over the age of 50 who are admitted with low-energy femoral neck fractures [1–3]. The precise pathogenesis of AVNFH remains unclear. Hyperlipidaemia involvement in the pathogenesis of AVNFH has been proposed based on human and animal studies. It should be primarily considered to be related to an interruption of the vascular supply to the bone leading to bone ischaemia and cellular necrosis [4–6]. Previous studies have confirmed that the excessive use of cortical hormones induces AVNFH and lipid metabolism disorders [5, 7, 8]. However, to our knowledge, no detailed description of AVNFH has been reported in the literature [4, 9].
Whether dysregulated lipid metabolism was related to the occurrence of AVNFH after surgery has rarely been reported [10, 11]. In addition, the reported incidence of postoperative AVNFH in different series has varied greatly [12, 13].
The purpose of this retrospective study was to investigate the relationship between blood lipid abnormalities and AVNFH following a femoral neck fracture operation among the elderly population. Our hypothesis was that AVNFH was significantly associated with blood lipid abnormalities in elderly patients with low-energy femoral neck fractures.
Methods
General data
This study was reviewed and approved by the review board of the First Affiliated Hospital at Sun Yat-sen University, Guangzhou, China, and an exemption for informed consent was obtained from our investigational ethical review board. The study was conducted in compliance with the provisions of the Declaration of Helsinki and EN 540.
Between June 2005 and November 2009, 653 patients with an isolated fresh femoral neck fracture (653 hips) undergoing closed reduction and internal fixation with cancellous screws (Smith and Nephew, Memphis, Tennessee) were identified from our orthopaedic trauma database. WY performed the clinical investigation of the patients. All surgeries were finished at our institution by senior orthopaedists (WY, XCZ, XZ, and KZ). The surgical procedures were based on standard protocols for cancellous screws, as recommended by device manufacturers and as described previously by Nagi et al. [1]. Fractures were assessed for Garden classification. Follow-up occurred at 1, 6, 12, 18, 24, 30, and 36 months after surgery. The study was also to evaluate the level and determinants of change in physical activity during the follow-up period. Obvious dysfunction would be excluded. Harris Hip Score (HHS) was used for functional evaluation during examination. A retrospective evaluation of the clinical data and radiographic information was performed at each visit.
Inclusion and exclusion criteria
Inclusion criteria included an isolated freshly closed femoral neck fracture, age ranging from 50–94 years, the ability to walk independently either without assistance or with auxiliary equipment before the fracture, low-energy femoral neck fractures, and no chronic illness (chronic heart failure, chronic kidney disease, chronic obstructive pulmonary disease, cancer) or major surgery contraindications. Exclusion criteria included age outside the inclusion range, <37 months of follow-up, alcohol abuse, long-term use of hormone drugs, pre-existing femoral head necrosis, multiple traumatic injuries, malabsorption syndrome, metabolic abnormalities, hypertension, developmental dysplasia of the hip (DDH), severe arthrosis/arthritis, severe comorbidities, any diseases affecting the blood supply to the femoral head, bed-ridden status, an American Society of Anesthesiologists (ASA) score of V, language barrier, mental retardation, hemiplegia, or incomplete preoperative data.
Diagnostic criteria
The diagnostic criteria were as follows: if the X-ray is still clearly showing a visible fracture line 6 months or longer after surgery in conjunction with clinical symptoms, the fracture was defined as non-union. If the X-ray, computed tomography (CT), or magnetic resonance imaging (MRI) scan displayed changes of radionuclide imaging or femoral density, including cystic degeneration, hardening, or uneven density, the patient was diagnosed with AVNFH. Mechanical failure was defined as either a loss of alignment of at least 10° or shortening of at least 2 cm. Deep surgical site infections and reoperations were additionally quantified.
Detection index
Approximately 2 mL of preoperative fasting venous blood was centrifuged to obtain serum. The Hitachi 7000 automatic biochemical analyzer tested the blood lipid levels, including triglyceride (TG), total cholesterol (TC), low-density lipoprotein (LDL), high-density lipoprotein (HDL), apolipoprotein-B (Apo-B), and apolipoprotein-A1 (Apo-A1).
Statistical analysis
All continuous data were expressed as the mean ± standard deviation (SD), and the ratio data were expressed as N and %; all these data were analysed using the Wilcoxon rank sum test (Mann–Whitney U test). Quantitative variables were analysed using the two-tailed Student’s t test, and categorical variables were analysed by the χ 2 test or Fisher’s exact test where appropriate. Multi-factor logistic regression analysis was used to analyse the different influencing factors. Two-tailed P values <0.05 were deemed statistically significant. In principle, the χ 2 test was employed. However, the Fisher’s exact test was used for the analysis of the cases in which the expected frequency was not attained. The software SPSS (version 22.0.0, SPSS Inc., Chicago, Illinois, IBM, New York, NY) was used to analyse the data.
Results
General data comparison
There were 328 of 653 patients (50.2%) excluded based on inclusion and exclusion criteria, leaving 325 patients with a mean age of 74 years (range 50–94 years) who met the inclusion criteria and were available for analysis (two groups: AVNFH [n = 160] and control [n = 165], Fig. 1). The average body mass index (BMI) was 25.4 (range 14.0–38.0). There were 143 right hips and 182 left hips. The gender distribution was 43.7% male and 56.3% female. The patient demographics are shown in Table 1. All patients had successful operations, including 160 cases of postoperative AVNFH patients, accounting for 49.2%. There were no meaningful differences in gender, age, ASA scale, fracture lateralization, BMI, femoral neck bone mineral density (FNBMD), Garden classification, or preoperative blood lipid levels between the groups (P > 0.05) (Tables 1 and 2).
Fig. 1.
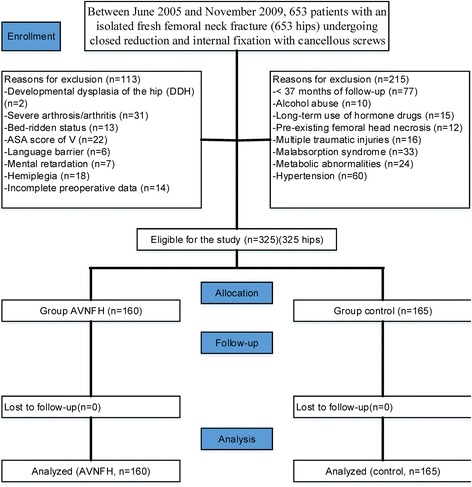
Flow diagram demonstrating methods for identification of studies to investigate the relationship between blood lipid abnormalities and AVNFH following the femoral neck fracture operation among an elderly population
Table 1.
Patient demographics in the two groups
| Variable | AVNFH (n = 160) | Control (n = 165) | P value |
|---|---|---|---|
| Age (years) | 75.2 ± 13.37 | 73.0 ± 11.97 | 0.12*a |
| Sex (M:F) | 68:92 | 74:91 | 0.67*b |
| ASA scale, no. | 0.22*c | ||
| I | 23 | 18 | |
| II | 41 | 37 | |
| III | 67 | 76 | |
| IV | 29 | 34 | |
| V | 0 | 0 | |
| Laterality (L/R) | 93/67 | 89/76 | 0.45*b |
| BMI (kg/m2) | 26.2 ± 5.62 | 24.6 ± 6.50 | 0.19*a |
| FNBMD | 2.3 ± 0.91 | 2.4 ± 1.06 | 0.09*a |
| Garden classification (no.) | 0.15*c | ||
| I | 13 | 23 | |
| II | 35 | 34 | |
| III | 43 | 48 | |
| IV | 69 | 60 |
AVNFH avascular necrosis of the femoral head, ASA American Society of Anesthesiologists, BMI body mass index, FNBMD femoral neck bone mineral density
*No statistically significant values
aAnalysed using an independent samples t test
bAnalysed using the chi-square
cAnalysed using the Mann–Whitney test
Table 2.
Preoperative comparison of average blood lipid levels between the two groups
| Variable | AVNFH (n = 160) | Control (n = 165) | P value |
|---|---|---|---|
| TC (mmol/L) | 4.53 ± 0.37 | 4.48 ± 0.33 | 0.15*a |
| TG (mmol/L) | 1.64 ± 0.42 | 1.59 ± 0.53 | 0.32*a |
| HDL (mmol/L) | 2.43 ± 0.63 | 2.50 ± 0.24 | 0.17*a |
| LDL (mmol/L) | 3.02 ± 0.36 | 2.97 ± 0.14 | 0.94*a |
| Apo-A1 (mg/dL) | 102.46 ± 16.93 | 105.41 ± 21.39 | 0.17*a |
| Apo-B (mg/dL) | 57.32 ± 7.40 | 55.50 ± 11.77 | 0.10*a |
AVNFH avascular necrosis of the femoral head, TC total cholesterol, TG triglyceride, HDL high-density lipoprotein, LDL low-density lipoprotein, Apo-A1 apolipoprotein A1, Apo-B apolipoprotein-B
*No statistically significant values
aAnalysed using an independent samples t test
Comparison of fracture treatment
There were no between-group significant differences in the combined Garden index and HHS (P > 0.05). At an average follow-up of 42 months (range 37–46 months), 57 mechanical failures (17.5%) occurred. The mean time to diagnosis of AVNFH was 32 months (range 10–38), with 12 mechanical failures occurring within 8 weeks after surgery. No significant differences were observed in terms of reduction mode, Garden index, operation interval, and weight-bearing activity time (Table 3).
Table 3.
Comparison of the treatment of patients with femoral neck fractures between the two groups
| Variable | AVNFH (n = 160) | Control (n = 165) | P value |
|---|---|---|---|
| HHS | 72.3 ± 11.25 | 75.2 ± 9.4 | 0.01*a |
| Garden index | 0.57*b | ||
| I | 42 | 46 | |
| II | 56 | 52 | |
| III | 44 | 34 | |
| IV | 18 | 33 | |
| Injury operation interval | 0.48*b | ||
| <24 h | 26 | 32 | |
| 24–48 h | 45 | 58 | |
| 48–72 h | 53 | 35 | |
| >72 h | 31 | 40 | |
| Weight-bearing activity time (<8 months/≥8 months) | 37/123 | 49/116 | 0.18*c |
| Mechanical failure | 14.4% (23/160) | 20.6% (34/165) | 0.14*c |
AVNFH avascular necrosis of the femoral head, HHS Harris Hip Score
*No statistically significant values
aAnalysed using an independent samples t test
bAnalysed using the Mann–Whitney test
cAnalysed using the chi-square test
Between-group comparisons of the average blood lipid levels
Follow-up occurred at 1, 6, 12, 18, 24, 30, and 36 months after surgery. In the AVNFH group, the TC, TG, LDL, and Apo-B levels were 7.11 ± 3.16 mmol/L, 2.15 ± 0.89 mmol/L, 4.49 ± 1.38 mmol/L, and 79.69 ± 17.29 mg/dL, respectively, which were significantly higher than those of the control group, while HDL and Apo-A1 levels were 1.41 ± 0.43 mmol/L and 114.96 ± 19.85 mg/dL, respectively, which were significantly lower than those of the control group. All of these differences were statistically significant (P = 0.00) (Table 4).
Table 4.
Postoperative comparison of average blood lipid levels between the two groups
| Variable | AVNFH (n = 160) | Control (n = 165) | P value |
|---|---|---|---|
| TC (mmol/L) | 7.11 ± 3.16 | 5.69 ± 1.45 | 0.00*a |
| TG (mmol/L) | 2.15 ± 0.89 | 1.79 ± 0.65 | 0.00*a |
| HDL (mmol/L) | 1.41 ± 0.43 | 2.12 ± 0.73 | 0.00*a |
| LDL (mmol/L) | 4.49 ± 1.38 | 3.07 ± 0.69 | 0.00*a |
| Apo-A1 (mg/dL) | 114.96 ± 19.85 | 136.13 ± 28.64 | 0.00*a |
| Apo-B (mg/dL) | 79.69 ± 17.29 | 72.81 ± 13.59 | 0.00*a |
AVNFH avascular necrosis of the femoral head, TC total cholesterol, TG triglyceride, HDL high-density lipoprotein, LDL low-density lipoprotein, Apo-A1 apolipoprotein A1, Apo-B apolipoprotein-B
*Statistically significant values
aAnalysed using an independent samples t test
Logistic multi-factor regression analysis of AVNFH
Logistic multi-factor regression analysis exhibited that in addition to the traditional common indicators, TC and LDL were the independent factors that influenced postoperative AVNFH in patients with a femoral neck fracture (Table 5).
Table 5.
Logistic regression analysis of factors was applied to identify variables independently associated with AVNFH following femoral neck fractures
| Influence factors | β | SE | OR | 95% CI | χ 2 | P value |
|---|---|---|---|---|---|---|
| TC | 0.762 | 0.392 | 1.23 | 1.10~2.43 | 3.29 | 0.002* |
| TG | 2.112 | 0.751 | 6.49 | 1.08~4.17 | 21.36 | 0.102 |
| HDL | 1.855 | 0.554 | 4.78 | 2.16~5.40 | 5.04 | 0.290 |
| LDL | 1.484 | 0.603 | 4.41 | 1.33~6.69 | 9.37 | 0.001* |
| Apo-A1 | 0.448 | 0.835 | 9.35 | 1.26~8.85 | 4.99 | 0.080 |
| Apo-B | 0.566 | 0.676 | 5.41 | 1.33~6.04 | 21.02 | 0.107 |
TC total cholesterol, TG triglyceride, HDL high-density lipoprotein, LDL low-density lipoprotein, Apo-A1 apolipoprotein A1, Apo-B apolipoprotein-B, SE standard error, OR odds ratio, CI confidence interval
*Statistically significant values
Discussion
AVNFH is a pathological process of bone cells, haematopoietic bone marrow cells, and fat cell necrosis caused by partial or complete ischaemia of the femoral head due to different reasons [14, 15]. AVNFH is divided into two categories (traumatic and non-traumatic). In recent years, because of an ageing population and an increase in traffic accidents and other traumatic events, the incidence of femoral neck fracture has been increasing year after year [16]. Although the healing rate of femoral neck fracture was significantly increased with the constant updating of internal fixation techniques and materials, the incidence of AVNFH failed to decrease accordingly and remained between 10 and 30% [2, 17]. The incidence of AVNFH in this study was 24.5%, which confirmed those reports. Therefore, how to identify high-risk patients early to minimize the incidence of AVNFH following femoral neck fracture has become an important issue in the field of orthopaedics.
Numerous studies have indicated that dysregulation of lipid metabolism may be one of the most important contributors in AVNFH [7, 18, 19]. Mielants noticed that non-traumatic AVNFH was associated with increased serum lipoprotein and TG levels in patients [20]. Iio reported 103 AVNFH patients, of whom 69 had increased cholesterol and TG [21]. In an experimental study of sex hormones in a femoral head necrosis model, it was found that the first change observed was increased blood fat levels, and the second change occurred in the bone. Some researchers found that serum TG and femoral neck bone density were positively correlated in postmenopausal women [2, 3, 6]. The current study also found that the incidence of AVNFH was significantly higher in the patients with hyperlipidaemia compared to a normal group after the reduction of femoral neck fracture. This difference was statistically significant (P < 0.05). Logistic multi-factor regression analysis showed that both TC and LDL were independent risk factors of AVNFH. The results of a study by Okpala et al. were consistent with the conclusions of our study showing that the TC and LDL levels in the AVNFH group were significantly higher than those in the control group, and they also believed that TC and LDL could be considered as independent factors and diagnostic criteria for AVNFH [7].
Regarding the pathogenic mechanism of AVNFH induced by the dysregulation of lipid metabolism, the present study suggested that on the one hand, hyperlipidaemia damages vascular endothelial cells to create pro-thrombotic conditions: the ability of vascular endothelial cells to synthesize nitric oxide (NO) decreases resulting in the dysfunction of vascular contraction and relaxation, which affects bone microcirculation [13, 18, 22]. On the other hand, hyperlipidaemia leads to the formation of fat emboli in the peripheral blood, causing bone microvascular obstructions, increasing the intraosseous pressure, and further aggravating the dysfunction of bone microcirculation [10, 23]. In addition, adipocyte hypertrophy, fat accumulation, and fatty marrow in the femoral head increases bone marrow microcirculation pressure, which contributes to the ischaemia, hypoxia, metabolic disorders, and edema observed in bone marrow tissue, resulting in secondary intracranial pressure increases and further aggravating ischaemia and hypoxia. This creates a vicious cycle, eventually resulting in consequences such as bone dystrophy or bone necrosis [10, 13, 24]. Increased blood viscosity, decreased erythrocyte deformability, and microcirculation congestion also damages the blood supply to the femoral neck fracture site after surgery, which contributes to the relative ease of the development of ischaemic necrosis of the femoral head [25, 26].
There are several limitations to our study. First, a small sample size may have introduced bias. However, the focus of our study is to assess an area that has not been studied extensively in the literature. Second, because this is a retrospective study with problems that are inherent to this methodology, patient- and surgeon-related confounders may have existed. Third, we may not have addressed all potential confounding variables in our analyses. Despite these limitations, this analysis presents long-term follow-up results and is the first to evaluate covariates. A prospective randomised study is needed to assess the relationship between blood lipid abnormalities and AVNFH following the femoral neck fracture operation among an elderly population.
Conclusions
TC and LDL can be considered as diagnostic criteria for AVNFH after surgical repair of femoral neck fracture. Early intervention in patients with dyslipidaemia may be implemented to prevent or delay the occurrence and development of AVNFH in the early stages.
Acknowledgements
The authors would like to acknowledge Dr Xiaohui Li for his assistance with the technical help.
Funding
Funding for this research was received from the Shanghai Municipal Health and Family Planning Commission.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Authors’ contributions
WY performed the data collection and analysis and participated in manuscript writing. LZ, DZ, and RC performed the database setup and statistical analysis. WY, XCZ, XZ, and KZ performed the operations. XCZ and MZ participated in the study design and coordination and helped to draft the manuscript. All of the authors have read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
This is not applicable as no identifying personal information is being published in this manuscript.
Ethics approval and consent to participate
The study was approved by the institutional ethics review board of the First Affiliated Hospital, Sun Yat-sen University, and an exemption for informed consent was obtained from our investigational ethical review board.
Abbreviations
- Apo-A1
Apolipoprotein-A1
- Apo-B
Apolipoprotein-B
- ASA
American Society of Anaesthesiologists
- AVNFH
Avascular necrosis of femoral head
- BMI
Body mass index
- CCD
Collodiaphyseal angle
- CD
Computed tomography
- DDH
Developmental dysplasia of the hip
- FNBMD
Femoral neck bone mineral density
- HDL
High-density lipoprotein
- HHS
Harris Hip Score
- LDL
Low-density lipoprotein
- MRI
Magnetic resonance imaging
- NO
Nitric oxide
- SD
Standard deviation
- TC
Total cholesterol
- TG
Triglyceride
Contributor Information
Xianshang Zeng, Email: xianshangzh@126.com.
Ke Zhan, Email: 1987zhan@163.com.
Lili Zhang, Email: allys19841126@163.com.
Dan Zeng, Email: 2447218947@qq.com.
Weiguang Yu, Email: 10211270007@fudan.edu.cn.
Xinchao Zhang, Email: zhangxc666@aliyun.com.
Mingdong Zhao, Email: zhaomingdong@medmail.com.cn.
Zhicheng Lai, Email: 2017328989@qq.com.
Runzhen Chen, Email: 330197665@qq.com.
References
- 1.Nagi ON, Gautam VK, Marya SK. Treatment of femoral neck fractures with a cancellous screw and fibular graft. J Bone Joint Surg Br. 1986;68(3):387–91. doi: 10.1302/0301-620X.68B3.3733802. [DOI] [PubMed] [Google Scholar]
- 2.Popelka O, Skala-Rosenbaum J, Bartoska R, Waldauf P, Krbec M, Dzupa V. Fracture type and injury-to-surgery interval as risk factors for avascular necrosis of the femoral head after internal fixation of intracapsular femoral neck fracture. Acta Chir Orthop Traumatol Cech. 2015;82(4):282–7. [PubMed] [Google Scholar]
- 3.Davies-Tuck ML, Hanna F, Davis SR, Bell RJ, Davison SL, Wluka AE, et al. Total cholesterol and triglycerides are associated with the development of new bone marrow lesions in asymptomatic middle-aged women - a prospective cohort study. Arthritis Res Ther. 2009;11(6). doi:10.1186/ar2873 [DOI] [PMC free article] [PubMed]
- 4.Emet M, Atac K, Aydin A, Altay N, Saritemur M. Spotted lipid sign floating on the blood to differentiate obscured open fractures from simple wound lacerations. Am J Emerg Med. 2015;33(2). doi:10.1016/j.ajem.2014.08.009. [DOI] [PubMed]
- 5.Raffiani G. Exercise/diet induced reversal and cure of bilateral multifocal femoral stage ii secondary osteonecrosis (avascular necrosis) Bone. 2010;47:S313-S. [Google Scholar]
- 6.Kuribayashi M, Fujioka M, Takahashi KA, Arai Y, Ishida M, Goto T, et al. Vitamin E prevents steroid-induced osteonecrosis in rabbits. Acta Orthop. 2010;81(1):154–60. doi: 10.3109/17453671003628772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okpala L, Ren HM, Ghebrerneskel K, Ibegbulam O, Ugochukwu CC, Crawford M. Blood lipid abnormalities in sickle cell disease are pancellular and clinically important. Blood. 2005;106(11):658A-A. [Google Scholar]
- 8.Kim T-H, Baek J-I, Hong JM, Choi S-J, Lee H-J, Cho H-J, et al. Significant association of SREBP-2 genetic polymorphisms with avascular necrosis in the Korean population. BMC Med Genet. 2008;9. doi:10.1186/1471-2350-9-94. [DOI] [PMC free article] [PubMed]
- 9.Sheen C, Vincent T, Barrett D, Horwitz EM, Hulitt J, Strong E, et al. Statins are active in acute lymphoblastic leukaemia (ALL): a therapy that may treat ALL and prevent avascular necrosis. Br J Haematol. 2011;155(3):403–7. doi: 10.1111/j.1365-2141.2011.08696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kuroda T, Tanabe N, Wakamatsu A, Takai C, Sato H, Nakatsue T, et al. High triglyceride is a risk factor for silent osteonecrosis of the femoral head in systemic lupus erythematosus. Clin Rheumatol. 2015;34(12):2071–7. doi: 10.1007/s10067-015-3075-y. [DOI] [PubMed] [Google Scholar]
- 11.Ayina CNA, Sobngwi E, Essouma M, Noubiap JJN, Boudou P, Ngoa LSE, et al. Osteoprotegerin in relation to insulin resistance and blood lipids in sub-Saharan African women with and without abdominal obesity. Diabetol Metab Syndr. 2015;7. doi:10.1186/s13098-015-0042-3 [DOI] [PMC free article] [PubMed]
- 12.Rabinovitz H, Friedensohn A, Leibovitz A, Gabay G, Rocas C, Habot B. Effect of chromium supplementation on blood glucose and lipid levels in type 2 diabetes mellitus elderly patients. Int J Vitam Nutr Res. 2004;74(3):178–82. doi: 10.1024/0300-9831.74.3.178. [DOI] [PubMed] [Google Scholar]
- 13.Xu ZH, Dai XY, Yao Y, Shi DQ, Chen DY, Dai J, et al. Higher levels of serum triglycerides were associated with postoperative deep vein thrombosis after total hip arthroplasty in patients with nontraumatic osteonecrosis of the femoral head. Int J Low Extrem Wounds. 2016;15(1):41–4. doi: 10.1177/1534734615580017. [DOI] [PubMed] [Google Scholar]
- 14.Shuai B, Shen L, Yang YP, Xie J, Shou ZX, Wei B. Low plasma adiponectin as a potential biomarker for osteonecrosis of the femoral head. J Rheumatol. 2010;37(10):2151–5. doi: 10.3899/jrheum.100342. [DOI] [PubMed] [Google Scholar]
- 15.Kabata T, Kubo T, Matsumoto T, Hirata T, Fujioka M, Takahashi KA, et al. Onset of steroid-induced osteonecrosis in rabbits and its relationship to hyperlipaemia and increased free fatty acids. Rheumatology. 2005;44(10):1233–7. doi: 10.1093/rheumatology/keh721. [DOI] [PubMed] [Google Scholar]
- 16.Miyanishi K, Yamamoto T, Irisa T, Yamashita A, Jingushi S, Noguchi Y, et al. A high low-density lipoprotein cholesterol to high-density lipoprotein cholesterol ratio as a potential risk factor for corticosteroid-induced osteonecrosis in rabbits. Rheumatology. 2001;40(2):196–201. doi: 10.1093/rheumatology/40.2.196. [DOI] [PubMed] [Google Scholar]
- 17.Lu BB, Li KH. Lipoic acid prevents steroid-induced osteonecrosis in rabbits. Rheumatol Int. 2012;32(6):1679–83. doi: 10.1007/s00296-011-1846-6. [DOI] [PubMed] [Google Scholar]
- 18.Arlet J. Nontraumatic avascular necrosis of the femoral head. Past, present, and future. Clin Orthop Relat Res. 1992;277:12–21. [PubMed] [Google Scholar]
- 19.Wang GJ, Sweet DE, Reger SI, Thompson RC. Fat-cell changes as a mechanism of avascular necrosis of the femoral head in cortisone-treated rabbits. J Bone Joint Surg Am. 1977;59(6):729–35. doi: 10.2106/00004623-197759060-00003. [DOI] [PubMed] [Google Scholar]
- 20.Mielants H, Veys EM, DeBussere A, van der Jeught J. Avascular necrosis and its relation to lipid and purine metabolism. J Rheumatol. 1975;2(4):430–6. [PubMed] [Google Scholar]
- 21.Iio H, Ake Y, Saegusa Y, Mizuno K. The effect of lipid peroxide on osteoblasts and vascular endothelial cells: the possible role of ischemia-reperfusion in the progression of avascular necrosis of the femoral head. Kobe J Med Sci. 1996;42(6):361–73. [PubMed] [Google Scholar]
- 22.Zhao DW, Yu M, Hu K, Wang W, Yang L, Wang BJ, et al. Prevalence of nontraumatic osteonecrosis of the femoral head and its associated risk factors in the Chinese population: results from a nationally representative survey. Chinese Med J. 2015;128(21):2843–50. doi: 10.4103/0366-6999.168017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bian Y, Qian W, Li H, Zhao RC, Shan WX, Weng X. Pathogenesis of glucocorticoid-induced avascular necrosis: a microarray analysis of gene expression in vitro. Int J Mol Med. 2015;36(3):678–84. doi: 10.3892/ijmm.2015.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Orth P, Anagnostakos K. Coagulation abnormalities in osteonecrosis and bone marrow edema syndrome. Orthopedics. 2013;36(4):290–300. doi: 10.3928/01477447-20130327-08. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi R, Yamamoto T, Motomura G, Ikemura S, Iwasaki K, Zhao G, et al. Effects of an anti-platelet drug on the prevention of steroid-induced osteonecrosis in rabbits. Rheumatology. 2012;51(5):789–93. doi: 10.1093/rheumatology/ker197. [DOI] [PubMed] [Google Scholar]
- 26.Okazaki S, Nishitani Y, Nagoya S, Kaya M, Yamashita T, Matsumoto H. Femoral head osteonecrosis can be caused by disruption of the systemic immune response via the toll-like receptor 4 signalling pathway. Rheumatology. 2009;48(3):227–32. doi: 10.1093/rheumatology/ken462. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


