Abstract
Background
A novel, insecticide-treated, durable wall lining (ITWL), which mimics indoor residual spraying (IRS), has been developed to provide prolonged vector control when fixed to the inner walls of houses. PermaNet® ITWL is a polypropylene material containing non-pyrethroids (abamectin and fenpyroximate) which migrate gradually to the surface.
Methods
An experimental hut trial was conducted in an area of pyrethroid-resistant Anopheles gambiae s.l. and Anopheles funestus s.s. to compare the efficacy of non-pyrethroid ITWL, long-lasting insecticidal nets (LLIN) (Interceptor®), pyrethroid ITWL (ZeroVector®), and non-pyrethroid ITWL + LLIN.
Results
The non-pyrethroid ITWL produced relatively low levels of mortality, between 40–50% for An. funestus and An. gambiae, across all treatments. Against An. funestus, the non-pyrethroid ITWL when used without LLIN produced 47% mortality but this level of mortality was not significantly different to that of the LLIN alone (29%, P = 0.306) or ITWL + LLIN (35%, P = 0.385). Mortality levels for An. gambiae were similar to An. funestus with non-pyrethroid ITWL, producing 43% mortality compared with 26% for the LLIN. Exiting rates from ITWL huts were similar to the control and highest when the LLIN was present. An attempt to restrict mosquito access by covering the eave gap with ITWL (one eave open vs four open) had no effect on numbers entering. The LLIN provided personal protection when added to the ITWL with only 30% blood-fed compared with 69 and 56% (P = 0.001) for ITWL alone. Cone bioassays on ITWL with 30 min exposure after the trial produced mortality of >90% using field An. gambiae.
Conclusions
Despite high mortality in bioassays, the hut trial produced only limited mortality which was attributed to pyrethroid resistance against the pyrethroid ITWL and low efficacy in the non-pyrethroid ITWL. Hut ceilings were left uncovered and may have served as a potential untreated refuge. By analogy to IRS campaigns, which also do not routinely treat ceilings, high community coverage with ITWL may still reduce malaria transmission. Restriction of eave gaps by 75% proved an inadequate barrier to mosquito entry. The findings represent the first 2 months after installation and do not necessarily predict long-term efficacy.
Keywords: Insecticide-treated wall lining, Long-lasting insecticidal nets, Malaria control, Experimental huts, Pyrethroid resistance
Background
Most malaria-endemic countries have adopted policies to promote universal distribution of long-lasting insecticidal nets (LLINs) free of charge across all age groups, and an estimated 49% of the population in sub-Saharan Africa had access to at least one LLIN in their household in 2013 [1]. However, resistance to pyrethroid insecticides used in all LLINs is now widespread across vector populations and may reduce the level of community protection [2, 3]. Another challenge is maintaining effective year-round protection as during the hot dry seasons when transmission may still occur, some individuals are deterred from sleeping under nets [4]. The development of large holes through wear and tear of net fabrics during normal household use may compromise their protective efficacy despite LLINs retaining insecticidal potency for three years [5]. In areas of hyper-endemic malaria transmission, even when universal coverage (UC) of LLINs is achieved and nets are in good condition, malaria prevalence can remain relatively high unless additional control tools are implemented [6].
Indoor residual spraying (IRS) is a proven vector control method that has been used since the Second World War and was the central feature of the Global Malaria Eradication Campaign between 1955 and 1969, which successfully eliminated malaria from several countries and significantly reduced disease incidence in others [7, 8]. In 2005 the US President’s Malaria Initiative (PMI) revived IRS in sub-Saharan Africa by funding an initial $1.2 billion programme in 15 countries [9]. IRS coverage in sub-Saharan Africa increased substantially from <2% of the at-risk population protected in 2005 to 11%, or 78 million people, by 2010 [1]. A major challenge facing IRS programmes is how to sustain such gains in the face of operational problems, such as vector resistance to insecticides, lack of affordable alternative insecticides and limited resources for recurrent annual campaigns. In addition to the labour costs associated with spraying, high levels of pyrethroid resistance among mosquito populations have necessitated the use of more expensive non-pyrethroid formulations [10]. The commodity cost of PMI-supported IRS campaigns with the organophosphate Actellic CS 300 (pirimiphos methyl CS) has been estimated to be more than four times the expense of using the pyrethroid Icon CS 10 (lambdacyhalothrin CS) to cover the same area [11].
A new product has been developed which mimics the effect of IRS but is designed to control insecticide-resistant mosquitoes for a minimum of three years. Insecticide-treated, durable wall lining (ITWL) is a material that can be fixed to the inner walls and ceilings of houses. The principle is the same as IRS, to kill mosquitoes that land on the ITWL either before or after blood-feeding. If the coverage of ITWL is high enough, the population density and longevity of mosquitoes in the area becomes substantially reduced, together with malaria transmission. There is also the possibility that ITWL could be used to block entry of mosquitoes if the material is extended from floor to ceiling, therefore covering eave spaces. While ITWL, like IRS, could be used to reduce transmission by itself, it is more likely to be an adjunct to LLINs, with the LLIN providing additional personal protection through the barrier and excito-repellent effect. ZeroVector® is a first-generation ITWL containing deltamethrin incorporated into high-density polyethylene shade cloth, which has been evaluated in several countries in sub-Saharan Africa and Asia and consistently received high levels of household acceptability and provided prolonged insecticidal activity of greater than 12 months [12, 13]. With pyrethroid resistance now widespread throughout sub-Saharan Africa, attention has switched to development of a new generation of non-pyrethroid ITWL.
Initial experimental trials of ITWL + LLINs were conducted using plastic sheeting that had been spray treated with a non-pyrethroid (organophosphate) insecticide [14, 15]. These studies produced differing results in Côte d’Ivoire and Burkina Faso, which were attributed to variation in phenotypic resistance to organophosphates and pyrethroids among the respective vector populations [14, 16]. A newer factory-produced product (PermaNet® Lining) has been developed that consists of thin non-woven sheets of cloth made from high-density polypropylene containing a non-pyrethroid insecticide mixture of abamectin (avermectin) and fenpyroximate (pyrazole) which are slowly released together and migrate to the surface of the fibre; neither insecticide has been used in malaria control before. Abamectin is a macrocyclic lactone that acts through chloride channel activation and was discovered in 1981 [17]. Contact bioassays have shown that abamectin is efficacious against house flies [18], cockroaches [19] and fire ants [20] in terms of mortality, and there is evidence for oviposition suppression in blowflies [21]. There are limited data for use against mosquitoes, but ivermectin (also an avermectin) is highly effective in terms of both mortality and oviposition suppression as a cattle parasiticide [22]. Abamectin is widely used in mixtures for control of crop and ornamental pests associated with greenhouse and nursery operators, e.g., abamectin + trifosine (fungicide) is used to control the two-spotted spider mite [23]. Fenpyroximate is a pyrazole in the mitochondrial complex 1 electron transport inhibitors (METI) group of insecticides, which disrupt insect respiration and are in widespread use globally. METI acaricides are extensively used to control Tetranychus spp (spider mites) [24]. To date, there are no published data demonstrating efficacy of these proprietary insecticides against mosquitoes.
Despite the promise of ITWL, the only existing data to support the efficacy of this new product are small-scale, unpublished studies conducted by the manufacturer. In response to the increasing problem of insecticide resistance, PMI has funded a large-scale, cluster-randomized controlled trial (CRT) in Muheza, Tanzania to investigate whether ITWL combined with UC of LLINs provides added protection against malaria compared with LLINs alone [25]. To aid interpretation of the CRT results, an experimental hut trial of the ITWL with or without LLIN was conducted in Muheza against wild free-flying populations of Anopheles funestus sensu stricto (s.s.) and Anopheles gambiae sensu lato (s.l.).
Methods
Insecticide treatments
The following insecticide treatments were tested in the experimental hut trial:
Untreated negative control (all eaves open);
Interceptor® LLIN (alphacypermethrin 200 mg/m2, BASF, Germany) (all eaves open);
Non-pyrethroid ITWL (PermaNet® Lining; abamectin 0.25%, fenpyroximate 1%, Vestergaard Frandsen, Switzerland) (full coverage on walls) (all eaves open);
Non-pyrethroid ITWL + Interceptor® LLIN (all eaves open);
Non-pyrethroid ITWL (partially blocked eaves, only one of the four eaves left open);
Pyrethroid ITWL (ZeroVector®; deltamethrin 4.4 g/kg, Vestergaard Frandsen, Switzerland) (full coverage on walls) (all eaves open).
Insecticide safety
With any new vector control product, it is essential that rigorous mammalian and environmental tests are undertaken to determine whether it is safe to use at the proposed dosages. Both active ingredients have low mammalian toxicity and good safety profiles [25]. The proprietary combination formula in the non-pyrethroid ITWL has passed an initial environmental examination (IEE) conducted by an independent regulatory agency; hazard quotients for continuous habitation in a residence with non-pyrethroid ITWL were far below the acceptable threshold [25].
Experimental hut trial
An experimental hut trial was conducted at the National Institute for Medical Research (NIMR) field station at Zeneti village (5°13′S latitude, 38°39′E longitude and 193 m altitude) where An. gambiae s.l. and An. funestus s.s. are the major malaria vector species. Insecticide susceptibility tests using World Health Organization (WHO) diagnostic dosages showed that An. gambiae s.l. were fully susceptible to pyrethroids (permethrin, deltamethrin) in 2009–2010 [26]. By 2011 there were signs of pyrethroid resistance, with mortality of 75% for permethrin and deltamethrin [27]. In 2014 mortality of An. gambiae s.l. collected from experimental huts was 74% when exposed to deltamethrin, 51% for lambdacyhalothrin and 81% for permethrin [28]. Also in 2014, An. funestus s.s. were found to be resistant to deltamethrin and alphacypermethrin with mortality rates of 75 and 60%, respectively [28]. There is currently no resistance to carbamates or organophosphates.
Experimental huts were constructed to a design described by the WHO [29] and based on the original veranda-hut developed in Tanzania in the 1960s [30, 31] (Fig. 1). In the modern design the eave gap is reduced to 5 cm, the ceiling board is lined with hessian sack cloth, similar to thatch, and the concrete floor surrounded by a water-filled moat [31]. In this trial the veranda traps were not used and the verandas left unscreened. This allowed mosquitoes to freely enter huts through all four eave spaces so the impact of partial eave blocking (only one eave left open) with ITWL could be assessed for some treatments. Inwardly directed eave baffles were installed to prevent egress of mosquitoes that had entered the hut; the only route of exiting was through the window traps [32, 33] (Fig. 1). LLINs were deliberately holed with six 4 × 4-cm holes to simulate wear and tear [29].
Fig. 1.

a East African experimental huts in Zeneti village, Muheza District, northeast Tanzania. b Pyrethroid ITWL (ZeroVector®). c Non-pyrethroid ITWL (PermaNet® Lining) + LLIN (Interceptor®) with eaves partially blocked. d Eave baffles and hessian sack cloth ceiling
Six experimental huts were used in total. An adult volunteer slept in each hut nightly from 20:30–06:00. All were provided with chemoprophylaxis and instructed on use. The six volunteers were rotated between huts on successive nights to reduce any bias due to differences in individual attractiveness to mosquitoes. ITWL treatments were attached to wall boards using Velcro and rotated between huts after every seventh night (six trial nights and one non-trial night for cleaning and aeration with no treatment) for a total duration of 63 nights (49 trial nights). Mosquito collections were conducted using mouth-aspirators between 06:30–08:00 each morning by trained field assistants. White sheets were laid on the concrete floor in the room to ensure dead mosquitoes were more easily visible. Dead and live mosquitoes were collected inside the hut and from the two window traps (not from the verandas, as they were left unscreened). Live mosquitoes were transferred to 150-ml paper cups and provided with 10% glucose solution for scoring delayed mortality after 24, 48, 72 h at the NIMR laboratory. Gonotrophic status was recorded as unfed, blood-fed, semi-gravid, or gravid. All members of the An. gambiae species complex and An. funestus species group identified by morphological characteristics were assumed to be An. gambiae s.l. and An. funestus s.s. based on recent PCR identification [27].
The entomological impact of each treatment was expressed relative to the untreated control in terms of the following:
Induced mortality: percentage of dead mosquitoes in the treated hut at the time of collection and after a 72 h holding period relative to the control hut;
Deterrence: percentage reduction in the number of mosquitoes caught in the treated hut relative to the number caught in the control hut;
Induced exiting (repellency) due to any potential irritant effect of treatment expressed as percentage of mosquitoes collected from the veranda traps of treated huts relative to percentage caught in the veranda trap of the control hut;
Inhibition of blood-feeding: reduction in blood-feeding rate relative to the control. This was calculated using the following model: 100 (Bfu –Bft)/Bfu. where Bfu is the proportion of blood-fed mosquitoes in the untreated control hut and Bft is the proportion of blood-fed mosquitoes in the huts with insecticide treatments.
Supplementary bioassays
WHO cone and cylinder tests
To evaluate the efficacy of non-pyrethroid ITWL, standard WHO cone and cylinder bioassays were performed, based on the WHO protocol for IRS monitoring [29], using insectary-reared, pyrethroid-susceptible An. gambiae Kisumu and pyrethroid-resistant An. gambiae Muleba-Kis strains, and F1 generation of wild An. gambiae s.l. collected from five villages (Kibaoni-Mlingano, Kicheba, Mamboleo-Lusanga, Mkanyageni, Muungano) participating in the CRT. Bioassays were conducted with an exposure of 30 min to new pieces of non-pyrethroid ITWL from multiple batch productions and mortality recorded after 24, 48 and 72 h.
Irritability tests
To characterize any excito-repellent properties of the non-pyrethroid ITWL, 50 non-blood-fed, 2- to 5-day old, insectary-reared, pyrethroid-susceptible An. gambiae Kisumu or F1 generation of wild An. gambiae s.l. collected from Mkanyageni were individually introduced into plastic cones, containing either a piece of untreated netting, new non-pyrethroid ITWL or Interceptor® LLIN, allowed to settle for 60 s, and time elapsed between the first landing and the next take-off of the mosquito was recorded, up to 360 s.
Effective exposure time
To determine the minimum exposure time required to kill 100% of mosquitoes exposed to the non-pyrethroid ITWL, WHO cylinder tests were conducted using progeny of wild An. gambiae s.l. which were exposed to new pieces of non-pyrethroid ITWL for 1.87, 3.75, 7.5, 15, 30 and 60 min and mortality recorded after 24, 48 and 72 h. Five replicates of ten mosquitoes were tested for each exposure time. Parental mosquitoes were collected from two villages participating in the CRT (Kicheba and Mamboleo-Lusanga).
Data analysis
Data were entered into an Excel database and transferred to Stata 11 for processing and analysis (Stata Corp LP, College Station, TX, USA). For the experimental hut trial, the principal aim was to compare the efficacy of the different treatments relative to the untreated control. The outcomes of interest were proportion of mosquitoes blood-fed, dead (i.e., total number of mosquitoes dead by morning plus delayed mortality after holding for a total of 72 h) and exiting on successive nights. Logistic regression for grouped data was used to estimate the outcomes, comparing results for treatments and untreated control, adjusting for clustering by day and for variation between individual sleepers and huts. Negative binomial regression was used to analyse numbers entering the huts (% deterrence). Bioassay data were summarized using proportions and means and binomial confidence intervals, where applicable.
Results
Mortality results (24 and 72 h) are presented in Fig. 2 for An. funestus s.s. and Fig. 3 for An. gambiae s.l. Results showing deterrence, insecticide-induced exiting and reduction in blood-feeding are presented in Table 1 (An. funestus s.s.) and Table 2 (An. gambiae s.l.).
Fig. 2.
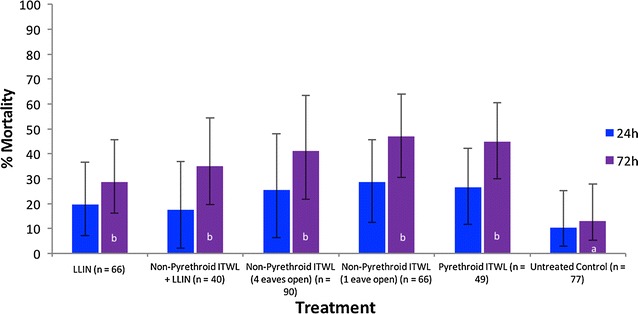
Percentage mortality of pyrethroid-resistant Anopheles funestus s.s. in the experimental hut trial. Percentage mortality was recorded 24 and 72 h after morning collections from huts. If the superscript for a time period (24 or 72 h) is the same, there was no significant difference between treatments (P > 0.05)
Fig. 3.
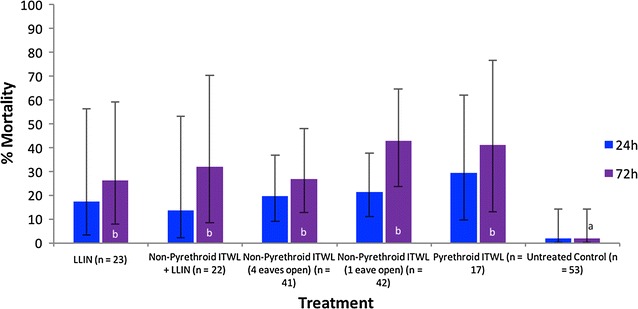
Percentage mortality of pyrethroid-resistant Anopheles gambiae s.l. in the experimental hut trial. Percentage mortality was recorded 24 and 72 h after morning collections from huts. If the superscript for a time period (24 or 72 h) is the same, there was no significant difference between treatments (P > 0.05)
Table 1.
Effect of insecticide-induced deterrence, exiting and blood-feeding of Anopheles funestus s.s. in the experimental hut trial
| Insecticide treatment | N | Deterrence | % Exited by morning | % Insecticide-induced exiting | % Blood-feeding | % Blood-feeding inhibition |
|---|---|---|---|---|---|---|
| LLIN (Interceptor®) | 66ab | 14 | 89b
(75–96) |
33 | 24a
(12–43) |
15 |
| Non-pyrethroid ITWL + LLIN | 40b | 48 | 75bc
(53–89) |
20 | 30ac
(15–50) |
0 |
| Non-pyrethroid ITWL (4 eaves open) | 90ab | 0 | 34d
(19–55) |
0 | 69b
(46–85) |
0 |
| Non-pyrethroid ITWL (1 eave open) | 66ab | 14 | 58ac
(41–73) |
0 | 56bd
(38–73) |
0 |
| Pyrethroid ITWL (ZeroVector®) | 49ab | 36 | 88b
(74–95) |
32 | 45cd
(28–63) |
0 |
| Untreated control | 77a | NA | 60acd
(49–70) |
NA | 29a
(17–44) |
NA |
If the superscript in a column is the same there was no significant difference between treatments (P > 0.05)
Table 2.
Effect of insecticide-induced deterrence, exiting and blood-feeding of Anopheles gambiae s.l. in the experimental hut trial
| Insecticide treatment | N | Deterrence | % Exited by morning | % Insecticide-induced exiting | % Blood-feeding | % Blood-feeding inhibition |
|---|---|---|---|---|---|---|
| LLIN (Interceptor®) | 23b | 57 | 91b
(65–98) |
48 | 44a
(22–68) |
0 |
| Non-pyrethroid ITWL + LLIN | 22b | 59 | 86bc
(64–96) |
45 | 36ab
(21–56) |
0 |
| Non-pyrethroid ITWL (4 eaves open) | 41ab | 23 | 39a
(14–71) |
0 | 73b
(40–92) |
0 |
| Non-pyrethroid ITWL (1 eave open) | 42a | 21 | 60ac
(44–73) |
21 | 55b
(40–69) |
0 |
| Pyrethroid ITWL (ZeroVector®) | 17b | 68 | 65ac
(38–85) |
27 | 29ab
(12–57) |
18 |
| Untreated control | 53a | NA | 47a
(31–64) |
NA | 36a
(21–55) |
NA |
If the superscript in a column is the same there was no significant difference between treatments (P > 0.05)
Anopheles funestus s.s
Mortality 72 h after mosquito collections was relatively low across all treatments (Fig. 2). Interceptor® is a WHOPES-recommended pyrethroid LLIN but produced only 29% mortality. The non-pyrethroid ITWL when used without LLIN produced 47% mortality (eaves partially blocked) but this was not significantly different to that of the LLIN (29%, P = 0.306), non-pyrethroid ITWL + LLIN (35%, P = 0.385), or pyrethroid ITWL (45%, P = 0.306). Mortality of non-pyrethroid ITWL increased between 24 and 72 h after mosquito collections and accounted for 38% of total mortality. However, there was also an unexpected 32% delayed mortality for the pyrethroid LLIN. Levels of mortality to the non-pyrethroid ITWL were consistently low across the 7 week trial, demonstrating no significant decline in bioefficacy (38, 44 and 35% 72 h mortality of An. funesus s.s. after one to two, two to four and >4 weeks, respectively, for non-pyrethroid ITWL with open eaves).
The trial was conducted at the end of the rainy season and numbers of An. funestus s.s. collected were relatively few (n = 77 in the control). There was no significant difference in the numbers that entered huts with non-pyrethroid ITWL when eaves were partially blocked or open (P = 0.9562) (Table 1). The only treatment which produced any measurable deterrence effect was the non-pyrethroid ITWL + LLIN which reduced entry by 48% (P = 0.044). There was also a significant increase in the proportion of An. funestus s.s. that had exited into window traps by morning for the pyrethroid LLIN (P = 0.001) and pyrethroid ITWL (P = 0.001) treatments compared with the untreated hut. The non-pyrethroid ITWL produced no significant increase in exiting (P = 0.082). The LLIN provided added personal protection over the non-pyrethroid ITWL with only 24% blood-fed compared with 69% (P = 0.001) and 56% (P = 0.001) for the two ITWL treatments (eaves open and partially closed). The blood-feeding rate in the untreated hut was surprisingly low, and significantly less than in the huts with ITWL.
Anopheles gambiae sensu lato
The numbers of An. gambiae s.l. collected over the duration of the trial were few (n = 53 in the control), meaning that the sample size was generally too small to assess differences between treatments (except where the effect was particularly large). Mortality levels 72 h after mosquito collections were uniformly low across all treatments, with the non-pyrethroid ITWL producing 43% mortality (eaves partially closed) compared with just 26% for the LLIN (Fig. 3). The mortality levels were similar to those for An. funestus s.s. and there were no significant differences between treatments. There was a degree of delayed mortality between 24 and 72 h, as observed previously with An. funestus s.s. The LLIN (57%, P = 0.007), non-pyrethroid ITWL + LLIN (59%, P = 0.002) and pyrethroid ITWL (68%, P = 0.002) all resulted in significant deterrence of An. gambiae s.l. entering indoors (Table 2). However, the non-pyrethroid ITWL treatment alone did not produce such an effect (P = 0.093 for eaves partially closed, and P = 0.393 for eaves open). As observed with An. funestus s.s., there was no significant difference in the numbers that entered when eaves were partially blocked or open (P = 0.956). The pyrethroid LLIN (48%, P = 0.002) and non-pyrethroid ITWL + LLIN (45%, P = 0.004) also produced a significant increase in the proportion of An. gambiae s.l. that had exited into window traps by morning. The non-pyrethroid ITWL did not increase exiting compared to the untreated hut. The LLIN (44% blood-fed) demonstrated significantly greater blood-feeding inhibition than the non-pyrethroid ITWL (73% for eaves open, and 55% for eaves partially closed).
Bioassay results
WHO cone and cylinder tests
WHO cone and cylinder bioassays with non-pyrethroid ITWL samples from five batch productions, conducted with an exposure time of 30 min, showed no significant difference in mortality between ITWL pieces (95 and 99% mortality after 72 h in cone and cylinder assays, respectively). When replicates were pooled, a direct comparison of cone versus cylinder bioassays demonstrated that overall immediate (after 24 h) and delayed (after 72 h) mortality was significantly lower in cone than cylinder bioassays (23–49 vs 80–100% mortality at 24 h, and 91–97 vs 99–100% mortality at 72 h for cones vs cylinders, respectively; P = 0.001 for all) for all mosquito strains tested (Fig. 4).
Fig. 4.
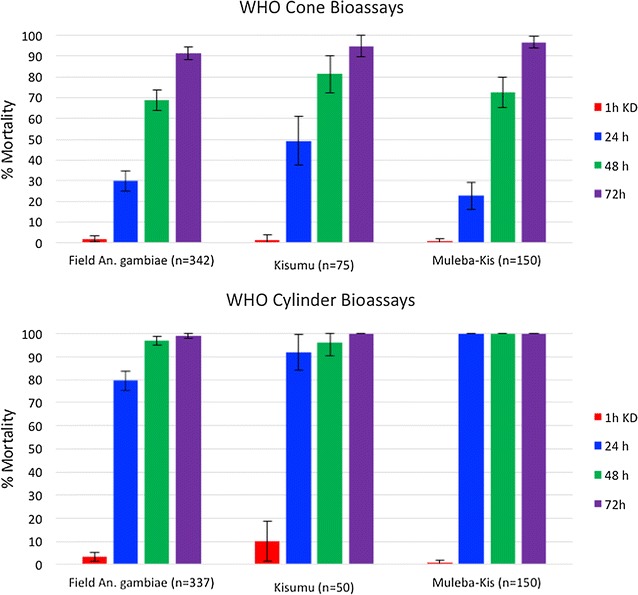
Percentage mortality of pyrethroid-susceptible Anopheles gambiae Kisumu, pyrethroid-resistant Anopheles gambiae Muleba-Kis and F1 offspring of field-collected Anopheles gambiae s.l. in WHO cone and cylinder bioassays on non-pyrethroid ITWL
Irritability tests
Time to first take-off in response to non-pyrethroid ITWL exposure was measured among individual An. gambiae Kisumu and F1 wild An. gambiae s.l. mosquitoes in comparison to untreated netting and a pyrethroid LLIN (Interceptor® LLIN). By 6 min of exposure to non-pyrethroid ITWL, 34 and 44% of An. gambiae Kisumu and wild mosquitoes had taken flight, respectively, compared to 78 and 76% in response to Interceptor® LLIN and 20 and 22% in untreated controls (Fig. 5). No mosquito irritability or excito-repellency was detected in response to non-pyrethroid ITWL exposure, as evidenced by no significant difference in numbers of mosquitoes taking flight, over the 6 min, in response to the non-pyrethroid ITWL compared to the untreated control (P = 0.232 for An. gambiae Kisumu and P = 0.425 for wild An. gambiae s.l.).
Fig. 5.
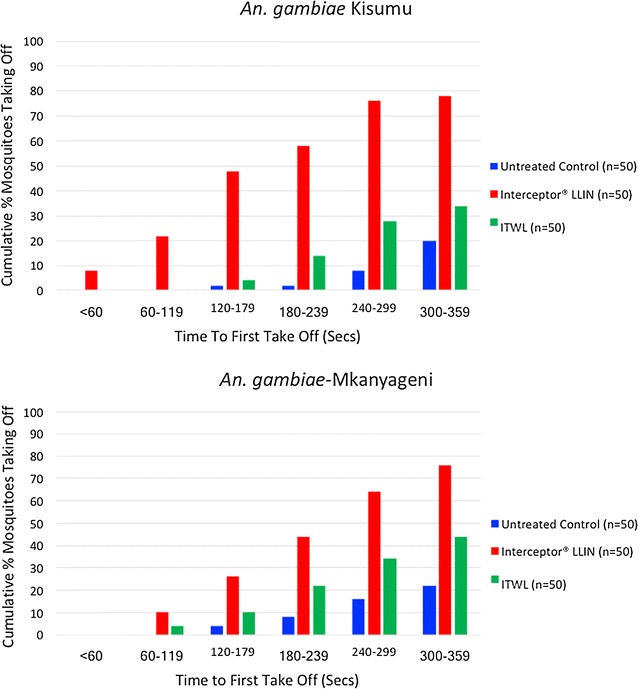
Cumulative percentage of pyrethroid-susceptible Anopheles gambiae Kisumu, and F1 offspring of field-collected Anopheles gambaie s.l. taking off over time, following exposure to untreated netting, Interceptor® LLIN or non-pyrethroid ITWL
Effective exposure time
An exposure time of 7.5 and 15 min to new non-pyrethroid ITWL in WHO cylinder tests killed 88 and 100% of F1 wild An. gambiae s.l. mosquitoes, respectively, after a 72 h holding period (Fig. 6). Exposure time of 15 min was sufficient to kill 100% of mosquitoes within 48 h.
Fig. 6.
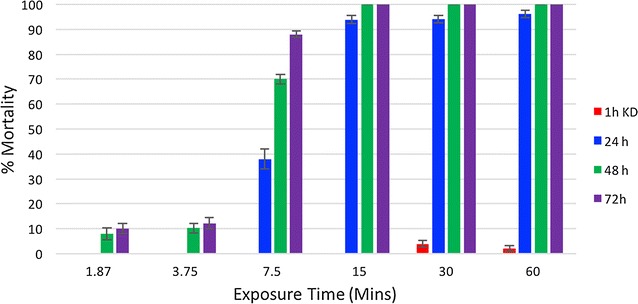
Percentage mortality of F1 offspring of field-collected Anopheles gambiae s.l. in WHO cylinder bioassays, following different exposures times to non-pyrethroid ITWL
Discussion
At present there are no recognized WHO standards for ITWL products. Before progressing to community trials, candidate IRS and LLIN products are evaluated in Phase II experimental huts against an existing gold standard positive control and comparative performance assessed [29, 34]. In this trial the positive control was the WHOPES-recommended pyrethroid LLIN, which produced equivalent levels of mortality to the non-pyrethroid ITWL. However, due to pyrethroid resistance in both of the major vector species, the level of mortality for the LLIN was lower than previous reports from the same site [28]. In 2006 when vector species were fully susceptible to pyrethroids, Interceptor® LLIN (alphacypermethrin) killed 92% of An. gambiae s.l. when unwashed [35], while Olyset® LLIN (permethrin) produced 61% mortality for An. gambiae s.l. and 72% for An. funestus s.s. [36]. The mortality rate with Interceptor® LLIN in this trial was considerably lower at just 26% for An. gambiae s.l. and 29% for An. funestus s.s. and was due to the significant increase in resistance across local vector populations [26]. In this study the non-pyrethroid ITWL also elicited relatively low levels of mortality, between 40 and 50% for An. funestus s.s. and An. gambiae s.l. The level of mortality in free-flying mosquitoes remained consistent over the 7 weeks of the trial.
WHO cone and cylinder bioassays exposing laboratory-reared susceptible and wild resistant An. gambiae s.l. to new pieces of non-pyrethroid ITWL for 30 min produced 90–100% mortality, demonstrating the inherent toxicity of the ITWL insecticides, with no evidence for cross-resistance to pyrethroids. In both assays, mosquito mortality remained low at 24 h, reaching the highest levels after 72 h. While new formulations would ideally produce more immediate mortality, a similar phenomenon has been reported for chlorfenapyr, a pyrrole insecticide which has demonstrated promise as an IRS and net treatment [37, 38]; historically some organochlorines used successfully as IRS (e.g., dieldrin) were also characterized by delayed mortality.
To help interpret the low levels of mortality observed in the main trial, WHO cylinder assays with incremental exposure times were performed to assess the duration of contact time required to kill the field strain (F1 progeny) of An. gambiae s.l. Only 7.5–15 min of contact with the non-pyrethroid ITWL induced 80–100% mortality. In all assays, levels of mosquito mortality were comparable between different rolls of ITWL, excluding differences in manufacturer batch production as a potential confounder in the main trial.
With the demonstration of high mortality in the bioassays, the lower levels in the main trial may be attributable to wild, free-flying mosquitoes spending less time in contact with treated wall surfaces. Shorter resting times are expected when insecticides induce a degree of repellence or irritancy. To further characterize mosquito behaviour in relation to ITWL exposure, time to first flight was measured for An. gambiae Kisumu and F1 wild An. gambiae s.l. in comparison to untreated netting and a pyrethroid LLIN (Interceptor® LLIN). No significant irritability was observed on exposure to the non-pyrethroid ITWL, unlike the pyrethroid LLIN which is known to have excito-repellent properties. These results were supported by the low exiting rates of both An. funestus s.s. and An. gambiae s.l. in non-pyrethroid ITWL huts compared to pyrethroid treatments (Interceptor® LLIN and ZeroVector® ITWL) in the main trial.
In the main trial, only treatments containing pyrethroid interventions significantly reduced vector entry. By comparison, the low levels of deterrence in the non-pyrethroid ITWL huts and the supporting irritability bioassays indicate that the ITWL was not influencing mosquito entry. One possible explanation for the low mortality, exiting and deterrence in the non-pyrethroid ITWL huts, is that vectors were instead resting on the hessian sack cloth ceilings, which remained uncovered throughout the trial, and were not contacting the walls for sufficient time to obtain a lethal insecticide dose. During the trial no data were documented on the location of mosquitoes within the room. While few studies have characterized exactly where African malaria mosquitoes reside within experimental huts, a recent trial of pirimiphos-methyl IRS on wooden panelled walls performed at the same study site also reported unexpectedly low levels of mortality and mosquitoes were noted to be resting on sack cloth-lined ceiling (M. Rowland, unpublished data). Earlier experimental hut trials of insecticide-treated wall lining materials have demonstrated that efficacy is strongly correlated with intervention surface area, with increasing coverage affording higher rates of vector mortality, deterrence and blood-feeding inhibition [39, 40]. If in this trial mosquitoes secured refuge on the ceiling over the non-woven polyethylene wall lining material, this could partly explain the low mortality in the huts. During IRS campaigns in Tanzania, ceilings are not usually sprayed, being too high and inaccessible to spray men, and yet such campaigns can have had a major effect on An. gambiae population density and malaria transmission rates [6].
It has been suggested that ITWL may also impact malaria transmission by functioning as a method of housing improvement, if used to cover eave gaps, preventing mosquito ingress [25]. However, in the present trial, there was no significant differences in vector entry in huts with partially blocked or open eaves. It is possible that host-seeking mosquitoes are able to compensate for a partial restriction of ‘entry points’ if host odour is concentrated from those that remain. ITWL material is not currently designed to act an eave seal or withstand strong winds and house improvements would better look to other means of restricting access.
Conclusions
PermaNet® ITWL is a new malaria vector control strategy, containing two non-pyrethroid insecticides, which is designed to function as a long-lasting IRS, when fixed to the inner walls and ceiling of houses. An experimental hut trial was performed in Muheza, Tanzania, to evaluate performance during 2 months after installation in comparison with a WHOPES-recommended LLIN (Interceptor®) and pyrethroid ITWL (ZeroVector®). The level of mosquito mortality was lower than expected: in the pyrethroid-treated LLIN and pyrethroid ITWL this was explained by insecticide resistance; in the non-pyrethroid ITWL this was attributed to low efficacy; since the ceiling was uncovered some mosquitoes may have secured refuge on this untreated surface. A series of novel, supplementary bioassays characterized the toxicity and mode of action of the non-pyrethroid ITWL product, and its effect on mosquito behaviour. The findings represent the first 2 months after installation and do not necessarily predict longer-term residual efficacy of non-pyrethroid ITWL.
Authors’ contributions
RM, RMO, LAM, FWM, MWR, and WK designed the study and were responsible for data analysis and interpretation. RM, BB, WS, BE, and SW led the entomology field activities and laboratory assays and participated in data collection. GM and JPM were responsible for oversight, management and delivery of the trial. LAM, RMO and MWR drafted the manuscript which was revised by co-authors. All authors read and approved the final manuscript.
Acknowledgements
The authors express their sincere thanks to the residents of Zeneti village, Muheza Distrct for their study participation. We thank Vestergaard Frandsen for their donation of PermaNet® Lining. RMO, LAM, SW and MWR are supported by the Malaria Centre of the London School of Hygiene and Tropical Medicine (http://malaria.lshtm.ac.uk).
This study is made possible by the support of the American people through the US Agency for International Development (USAID). The findings of this study are the sole responsibility of the National Institute for Medical Research, London School of Hygiene and Tropical Medicine, and Kilimanjaro Christian Medical College and do not necessarily reflect the views of USAID or the US Government.
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
The study was approved by the Medical Research Coordinating Committee (MRCC) of the National Institute for Medical Research (NIMR) (NIMR/HQ/R.8a/Vol.IX/1613). Written consent was obtained from all adult volunteer sleepers, the risks of malaria were explained and participants were provided with chemoprophylaxis. Throughout the trial, each volunteer was monitored for fever or possible adverse effects due to the hut treatments.
Funding
This study was funded by the US President’s Malaria Initiative (PMI) through the US Agency for International Development under Translating Research into Action, Cooperative Agreement No. GHS-A-00-09-00015-00, managed by University Research Co., LLC (URC).
Abbreviations
- AI
active ingredient
- CRT
cluster-randomized controlled trial
- CS
capsule suspension
- IEE
initial environmental examination
- IRS
indoor residual spraying
- ITWL
insecticide-treated wall lining
- KCMC
Kilimanjaro Christian Medical College
- LLIN
long-lasting insecticidal net
- LSHTM
London School of Hygiene and Tropical Medicine
- METI
mitochondrial complex 1 electron transport inhibitors
- MRCC
Medical research coordinating committee
- NA
not applicable
- NIMR
National Institute of Medical Research
- PMI
US President’s Malaria Initiative
- UC
Universal coverage
- WHO
World Health Organization
- WHOPES
WHO Pesticide Evaluation Scheme
Contributor Information
Robert Malima, Email: r_malima@hotmail.com.
Basiliana Emidi, Email: ebasi2002@yahoo.co.uk.
Louisa A. Messenger, Email: louisa.messenger@lshtm.ac.uk
Richard M. Oxborough, Email: Richard_Oxborough@abtassoc.com
Bernard Batengana, bmalongo@googlemail.com.
Wema Sudi, Email: sswema@yahoo.com.
Sophie Weston, Email: sophieweston99@gmail.com.
George Mtove, Email: mtoveg2002@gmail.com.
Joseph P. Mugasa, Email: jpmugasa@gmail.com
Franklin W. Mosha, Email: fwmosha@gmail.com
Mark W. Rowland, Email: mark.rowland@lshtm.ac.uk
William Kisinza, Email: wnkisinza@gmail.com.
References
- 1.WHO. Global Malaria Programme. World Malaria Report 2014. Geneva: World Health Organization; 2014. http://www.who.int/malaria/publications/world_malaria_report_2014/en/. Accessed 12 Jan 2017.
- 2.Ranson H, N’Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 2011;27:91–98. doi: 10.1016/j.pt.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Corbel V, Akogbeto M, Damien GB, Djenontin A, Chandre F, Rogier C, et al. Combination of malaria vector control interventions in pyrethroid resistance area in Benin: a cluster randomised controlled trial. Lancet Infect Dis. 2012;12:617–626. doi: 10.1016/S1473-3099(12)70081-6. [DOI] [PubMed] [Google Scholar]
- 4.Singh M, Brown G, Rogerson SJ. Ownership and use of insecticide-treated nets during pregnancy in sub-Saharan Africa: a review. Malar J. 2013;12:268. doi: 10.1186/1475-2875-12-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Irish S, N’Guessan R, Boko P, Metonnou C, Odjo A, Akogbeto M, et al. Loss of protection with insecticide-treated nets against pyrethroid-resistant Culex quinquefasciatus mosquitoes once nets become holed: an experimental hut study. Parasit Vectors. 2008;1:17. doi: 10.1186/1756-3305-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West PA, Protopopoff N, Wright A, Kivaju Z, Tigererwa R, Mosha FW, et al. Indoor residual spraying in combination with insecticide-treated nets compared to insecticide-treated nets alone for protection against malaria: a cluster randomised trial in Tanzania. PLoS Med. 2014;11:e1001630. doi: 10.1371/journal.pmed.1001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Najera JA, Gonzalez-Silva M, Alonso PL. Some lessons for the future from the Global Malaria Eradication Programme (1955–1969) PLoS Med. 2011;8:e1000412. doi: 10.1371/journal.pmed.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pluess B, Tanser FC, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database Syst Rev. 2010;4:CD006657. doi: 10.1002/14651858.CD006657.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.USAID. Lantos-Hyde United States Government Malaria Strategy 2009–2014. https://www.pmi.gov/docs/default-source/default-document-library/pmi-reports/usg_strategy2009-2014.pdf. Accessed 12 Jan 2017.
- 10.Johns B, Cico A. PMI IRS Country Programs: 2014 Comparative cost analysis. Bethesda, MD: Africa Indoor Residual Spraying Project, Abt Associates Inc; 2015. https://www.pmi.gov/docs/default-source/default-document-library/implementing-partner-reports/africa-indoor-residual-spraying-project-pmi-irs-country-programs-2015-comparative-cost-analysis.pdf?sfvrsn=6. Accessed 12 Jan 2017.
- 11.IRS Workshop. Preparing a budget for an IRS campaign http://www.businessfightsaids.org/system/documents/category_77/530/Peter_Mumba_(Abt).pdf?1380748140. Accessed 12 Jan 2017.
- 12.Messenger LA, Matias A, Manana AN, Stiles-Ocran JB, Knowles S, Boakye DA, et al. Multicentre studies of insecticide-treated durable wall lining in Africa and South-East Asia: entomological efficacy and household acceptability during one year of field use. Malar J. 2012;11:358. doi: 10.1186/1475-2875-11-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Messenger LA, Miller NP, Adeogun AO, Awolola TS, Rowland M. The development of insecticide-treated durable wall lining for malaria control: insights from rural and urban populations in Angola and Nigeria. Malar J. 2012;11:332. doi: 10.1186/1475-2875-11-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ngufor C, Chouaibou M, Tchicaya E, Loukou B, Kesse N, N’Guessan R, et al. Combining organophosphate-treated wall linings and long-lasting insecticidal nets fails to provide additional control over long-lasting insecticidal nets alone against multiple insecticide-resistant Anopheles gambiae in Côte d’Ivoire: an experimental hut trial. Malar J. 2014;13:396. doi: 10.1186/1475-2875-13-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ngufor C, Tungu P, Malima R, Kirby M, Kisinza W, Rowland M. Insecticide-treated net wall hangings for malaria vector control: an experimental hut study in north-eastern Tanzania. Malar J. 2014;13:366. doi: 10.1186/1475-2875-13-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ngufor C, Tchicaya E, Koudou B, N’Fale S, Dabire R, Johnson P, et al. Combining organophosphate treated wall linings and long-lasting insecticidal nets for improved control of pyrethroid resistant Anopheles gambiae. PLoS ONE. 2014;9:e83897. doi: 10.1371/journal.pone.0083897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ali A, Nayar JK, Gu WD. Toxicity of a phenyl pyrazole insecticide, fipronil, to mosquito and chironomid midge larvae in the laboratory. J Am Mosq Control Assoc. 1998;14:216–218. [PubMed] [Google Scholar]
- 18.Roush RT, Wright JE. Abamectin: toxicity to house flies (Diptera: Muscidae) resistant to synthetic organic insecticides. J Econ Entomol. 1986;79:562–564. doi: 10.1093/jee/79.3.562. [DOI] [PubMed] [Google Scholar]
- 19.Cochran DG. Efficacy of abamectin fed to German cockroaches (Dictyoptera: Blattellidae) resistant to pyrethroids. J Econ Entomol. 1990;83:1243–1245. doi: 10.1093/jee/83.4.1243. [DOI] [PubMed] [Google Scholar]
- 20.Greenblatt JA, Norton JA, Dybas RA, Harlan DP. Control of the red imported fire ant with abamectin (Affirm), a novel insecticide, in individual mound trials. J Agr Entomol. 1986;3:233–241. [Google Scholar]
- 21.Orton CJ, Watts JE, Rugg D. Comparative effectiveness of avermectins and deltamethrin in suppressing oviposition in Lucilia cuprina (Diptera: Calliphoridae) J Econ Entomol. 1992;85:28–32. doi: 10.1093/jee/85.1.28. [DOI] [PubMed] [Google Scholar]
- 22.Fritz ML, Walker ED, Miller JR. Lethal and sublethal effects of avermectin/milbemycin parasiticides on the African malaria vector, Anopheles arabiensis. J Med Entomol. 2012;49:326–331. doi: 10.1603/ME11098. [DOI] [PubMed] [Google Scholar]
- 23.Cloyd RA. Pesticides-formulations, effects, fate. Pesticide mixtures. In: Stoytcheva PM, editors. Intech. 2011. p. 532–537.
- 24.Van Pottelberge S, Van Leeuwen T, Nauen R, Tirry L. Resistance mechanisms to mitochondrial electron transport inhibitors in a field-collected strain of Tetranychus urticae Koch (Acari: Tetranychidae) Bull Entomol Res. 2009;99:23–31. doi: 10.1017/S0007485308006081. [DOI] [PubMed] [Google Scholar]
- 25.Mtove G, Mugasa JP, Messenger LA, Malima RC, Mangesho P, Magogo F, et al. The effectiveness of non-pyrethroid insecticide-treated durable wall lining to control malaria in rural Tanzania: study protocol for a two-armed cluster randomized trial. BMC Public Health. 2016;16:633. doi: 10.1186/s12889-016-3287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kabula B, Tungu P, Matowo J, Kitau J, Mweya C, Emidi B, et al. Susceptibility status of malaria vectors to insecticides commonly used for malaria control in Tanzania. Trop Med Int Health. 2012;17:742–750. doi: 10.1111/j.1365-3156.2012.02986.x. [DOI] [PubMed] [Google Scholar]
- 27.Kabula B, Tungu P, Malima R, Rowland M, Minja J, Wililo R, et al. Distribution and spread of pyrethroid and DDT resistance among the Anopheles gambiae complex in Tanzania. Med Vet Entomol. 2014;28:244–252. doi: 10.1111/mve.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Report of the eighteenth WHOPES working group meeting. Review of Miranet LN, Pandanet 2.0 LN, Yahe LN, Safenet LN.
- 29.World Health Organization. Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets. 2006:WHO/CDS/NTD/WHOPES/GCDPP/2006.2003.
- 30.Smith A. A verandah-trap hut for studying the house-frequenting habits of mosquitos and for assessing insecticides. I. A description of the verandah-trap hut and of studies on the egress of Anopheles gambiae Giles and Mansonia uniformis (Theo.) from an untreated hut. Bull Entomol Res. 1965;56:161–167. doi: 10.1017/S0007485300057114. [DOI] [PubMed] [Google Scholar]
- 31.Smith A, Webley DJ. A verandah-trap hut for studying the house-frequenting habits of mosquitoes and for assessing insecticides. 3. The effect of DDT on behavior and mortality. Bull Entomol Res. 1969;59:33–46. doi: 10.1017/S000748530000300X. [DOI] [PubMed] [Google Scholar]
- 32.Mosha FW, Lyimo IN, Oxborough RM, Malima R, Tenu F, Matowo J, et al. Experimental hut evaluation of the pyrrole insecticide chlorfenapyr on bed nets for the control of Anopheles arabiensis and Culex quinquefasciatus. Trop Med Int Health. 2008;13:644–652. doi: 10.1111/j.1365-3156.2008.02058.x. [DOI] [PubMed] [Google Scholar]
- 33.Oxborough RM, Kitau J, Mosha FW, Rowland MW. Modified veranda-trap hut for improved evaluation of vector control interventions. Med Vet Entomol. 2015;29:371–379. doi: 10.1111/mve.12123. [DOI] [PubMed] [Google Scholar]
- 34.WHO. Guidelines for laboratory and field-testing of long-lasting insecticidal nets. WHO/HTM/NTD/WHOPES/2013.3. Geneva: World Health Organization; 2013.
- 35.Malima R, Tungu PK, Mwingira V, Maxwell C, Magesa SM, Kaur H, et al. Evaluation of the long-lasting insecticidal net Interceptor LN: laboratory and experimental hut studies against anopheline and culicine mosquitoes in northeastern Tanzania. Parasit Vectors. 2013;6:296. doi: 10.1186/1756-3305-6-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malima RC, Magesa SM, Tungu PK, Mwingira V, Magogo FS, Sudi W, et al. An experimental hut evaluation of Olyset nets against anopheline mosquitoes after seven years use in Tanzanian villages. Malar J. 2008;7:38. doi: 10.1186/1475-2875-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oxborough RM, Kitau J, Matowo J, Mndeme R, Feston E, Boko P, et al. Evaluation of indoor residual spraying with the pyrrole insecticide chlorfenapyr against pyrethroid-susceptible Anopheles arabiensis and pyrethroid-resistant Culex quinquefasciatus mosquitoes. Trans R Soc Trop Med Hyg. 2010;104:639–645. doi: 10.1016/j.trstmh.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 38.N’Guessan R, Ngufor C, Kudom AA, Boko P, Odjo A, Malone D, et al. Mosquito nets treated with a mixture of chlorfenapyr and alphacypermethrin control pyrethroid resistant Anopheles gambiae and Culex quinquefasciatus mosquitoes in West Africa. PLoS ONE. 2014;9:e87710. doi: 10.1371/journal.pone.0087710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diabate A, Chandre F, Rowland M, N’Guessan R, Duchon S, Dabire KR, et al. The indoor use of plastic sheeting pre-impregnated with insecticide for control of malaria vectors. Trop Med Int Health. 2006;11:597–603. doi: 10.1111/j.1365-3156.2006.01605.x. [DOI] [PubMed] [Google Scholar]
- 40.Djènontin A, Chabi J, Baldet T, Irish S, Pennetier C, Hougard JM, et al. Managing insecticide resistance in malaria vectors by combining carbamate-treated plastic wall sheeting and pyrethroid-treated bed nets. Malar J. 2009;8:233. doi: 10.1186/1475-2875-8-233. [DOI] [PMC free article] [PubMed] [Google Scholar]


