Abstract
Background
An obesity-related single-nucleotide polymorphism (SNP) of the Tribbles pseudokinase 2 gene (TRIB2) was shown to have underwent adaptive evolution in the last glacial period, suggesting a selective advantage of this SNP in human populations in cold environments. In order to verify this hypothesis, the effect of the TRIB2 SNP on the expression of genes involved in adaptive thermogenesis was tested using messenger RNAs prepared from adipose tissues of Japanese adults.
Methods
Complementary DNA was prepared from subcutaneous adipose tissues (SAT) and visceral adipose tissues (VAT) obtained from 48 Japanese adults. Transcript levels of 15 selected genes, including five genes that are upregulated in development of thermogenic adipocytes, were measured by using real-time polymerase chain reaction. Differences in transcript levels between the TRIB2 SNP genotype groups (AA genotype versus AT + TT genotype) were assessed using t test.
Results
Of the five thermogenic genes, DIO2, CIDEA, PPARGC1A, and PRDM16 showed significantly higher transcript levels in SAT of individuals with the AA genotype relative to those with the AT + TT genotype (P < 0.05). However, only 2 out of the 10 non-thermogenic genes exhibited differences in transcript levels according to genotype. Additionally, in silico prediction indicated that this SNP likely affects the expression of nearby genes including TRIB2.
Conclusion
The higher expression levels of thermogenic genes in individuals homozygous for the adaptive variant of TRIB2 SNP suggest a greater propensity for induction of thermogenesis in adipose tissues in cold environments.
Keywords: TRIB2, SNP, Thermogenesis, Obesity
Background
The primary cause of obesity is an imbalance between caloric intake and energy expenditure. Susceptibility to obesity in present human populations is thought to be shaped by past genetic adaptation to famine [1, 2]. Additionally, lower ambient temperatures may exert selective pressure on genetic variations that influence susceptibility to obesity, as cold-induced thermogenesis substantially increases energy expenditure [3]. It may be hypothesized that genotypes linked to higher thermogenic capacity and leaner phenotypes were adaptive in archaic human populations during glacial periods [4]. A single-nucleotide polymorphism (SNP) in the uncoupling protein 1 gene, an essential gene for thermogenesis in brown adipocytes, has been shown to support the role of adaptation to cold climates in shaping the susceptibility to obesity [5, 6].
A previous study reported that a functional SNP in the 3′ untranslated region of the tribbles pseudokinase 2 gene (TRIB2) strongly influences visceral fat accumulation in Japanese adults. The obesity-resistance-associated allele of TRIB2 underwent positive natural selection in East Asians during the last glacial maximum, suggesting that this TRIB2 variant links past adaptation to present resistance to obesity [7]. TRIB2 suppresses the differentiation of adipocytes by promoting the degradation of the CCAAT/enhancer binding protein beta (CEBPB), a transcription factor that acts during early stages of brown adipocyte development [8, 9]. Human brown adipocytes are thought to be present only during early life in humans; however, recent studies have indicated the existence of active brown adipocytes in adult humans [10]. Moreover, it has been shown that energy-storing white adipocytes (or/and their progenitor cells) may be transformed into thermogenic beige/brite adipocytes via a series of stimuli; these thermogenic adipocytes resemble brown adipocytes but possess distinctive developmental lineages [11]. The adaptive TRIB2 allele, which is associated with the reduction of visceral fat, may contribute to resistance to cold environments by increasing the activity of these thermogenic adipocytes. In the present study, we examined the effects of the previously reported TRIB2 SNP on the expression of genes involved in thermogenesis in the adipose tissues of Japanese adults.
Methods
Subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) samples were obtained from 48 Japanese adults. These participants were enrolled from among patients admitted to the Jichi Medical University Hospital for gastrointestinal surgery or gynecological surgery. All participants provided written informed consent. SAT was collected from the abdominal skin incision and VAT from the extirpated organ, omentum, or mesenterium during surgical procedures. Complementary DNA (cDNA) and genomic DNA were prepared from adipose tissue specimens. Details of participants and samples were described in the previous paper [12]. The TRIB2 SNP (dbSNP: rs1057001) was genotyped using TaqMan Genotyping Assays and ABI PRISM 7900HT [7]. Transcription levels of selected genes were measured using TaqMan Gene Expression Assays or RT-PCR with SYBR Green on an ABI PRISM 7900HT. The list of target genes is shown in Table 1. Transcript levels of the target genes were normalized to that of the actin beta gene (measured by the TaqMan method) using the delta Ct method. Statistical tests were performed using SPSS version 23. Epigenetic modification patterns near rs1057001 and tightly linked variants (r 2 > 0.8 in the 1000 Genome Project East Asians) were retrieved using Haploreg4.1 [13]. The design of the present study was approved by the ethical committee of Jichi Medical University.
Table 1.
Tested genes
| Symbol | Gene name | Function | Assay type and nucleotide sequences of primersa |
|---|---|---|---|
| CIDEA | Cell death-inducing DFFA-like effector A | Development of brown/beige adipocytes | 5′-CAGCTCGCCCTTTCCGGGTC 5′-CGAGGGCATCCAGAGTCTTGCT |
| DIO2 | Iodothyronine deiodinase 2 | Thermogenesis | 5′-AGAGGGACTGCGCTGCGTCT 5′-TGCACCACACTGGAATTGGGGG |
| PDRM16 | PR/SET domain 16 | Development of brown/beige adipocytes | 5′-GAACCAGGCATATGCAATGATGCTG 5′-CCAGCCCGTCAGAGGTGGTTG |
| PPARGC1A | PPARG coactivator 1 alpha | Development of brown/beige adipocytes | 5′-AGACACCGCACGCACCGAAAT 5′-AGCTGTCATACCTGGGCCGACG |
| UCP1 | Uncoupling protein 1 | Thermogenesis | 5′-CGGCCTCTACGACACGGTCCA 5′-ACGACCTCTGTGGGTTGCCCAA |
| ACC | Acetyl-CoA carboxylase | Fatty acid synthesis | TaqMan |
| DGAT1 | Diacylglycerol O-acyltransferase 1 | Triglyceride synthesis | 5′-GCCCCCAACAAGGACGGAGAC 5′-CCACACACCAGTTCAGGATGCCA |
| FASN | Fatty acid synthase | Fatty acid synthesis | TaqMan |
| SCD1 | Stearoyl-CoA desaturase 1 | Fatty acid synthesis | TaqMan |
| SREBP1 | Sterol regulatory element-binding protein1 | Master regulator of fatty acid synthesis | TaqMan |
| ADIPOQ | Adiponectin | Adipocytokine | TaqMan |
| FABP4 | Fatty acid-binding protein 4 | Incorporation of fatty acids | 5′-TGGGGGTGTCCTGGTACATGTGC 5′-ACGCCTTTCATGACGCATTCCACC |
| ADRB3 | Adrenoceptor beta 3 | Lipolysis | TaqMan |
| GLUT4 | Facilitated glucose transporter, member 4 | Incorporation of glucose | 5′-TTGGCCCTGGCCCCATTCCT 5′-GCCCCATAGCCTCCGCAACA |
| LEP | Leptin | Adipocytokine | 5′-TAGGAATCGCAGCGCCAGCGG 5′-ATCCGCACAGGGTTCCCCAATG |
aPrimer sequences are shown for genes quantified by RT-PCR with SYBR Green
Results and discussion
The genotypes of rs1057001 in participants were AA = 26, AT = 20, and TT = 2. The proportion of the three genotypes was in Hardy-Weinberg equilibrium status (P = 0.421, chi square test, degrees of freedom = 1). Individuals with TT genotype were infrequent and thus combined with individuals with AT genotype for further statistical analyses. Genotype groups did not show differences in terms of the proportion of males and females (P = 0.066, Fisher’s exact test), age (P = 0.253, t test), and body mass index (P = 0.960, t test).
The transcript levels of tested genes are shown in Table 2. We tested CIDEA, DIO2, PPARGC1A, PRDM16, and UCP1, which were previously linked to brown adipose tissue activity as well as induction of beige/brite adipocytes in mice and humans [14–18]. The UCP1 transcript was undetectable in the majority of the adipose tissue samples. Transcripts of the other four genes were present at higher levels in individuals with the AA genotype than in those with the AT + TT genotype (P < 0.05, t test). The level of PPARGC1A expression in SAT was significant, at 5% after the Bonferroni correction (numbers of successfully measured gene = 14). The genotypic differences in the transcript levels of thermogenic genes were more notable in SAT, in which induction of beige/brite adipocytes was observed in mice and humans [11]. Furthermore, CIDEA and PRDM16 showed higher expression levels in SAT than in VAT (P < 0.05, after the Bonferroni correction was applied), supporting the greater prepotency of SAT for thermogenesis. We additionally tested the effect of TRIB2 genotypes on the expression of genes for de novo lipogenesis in white adipocytes (ACC, DGAT1, FASN, SCD1, SREBP1) and genes commonly expressed in white and brown adipocytes (ADIPOQ, ADRB3, FABP4, GLUT4, and LEP). Of the 10 genes, only ADIPOQ and GLUT4 in SAT showed significant differences in expression between the genotype groups (P < 0.05, t test).
Table 2.
Transcription levels of the tested genes in the TRIB2 genotype groups
| Gene symbol | Mean (SD) transcription level in subcutaneous adipose tissues | Ratio | P | Mean (SD) transcription level in visceral adipose tissues | Ratio | P | ||
|---|---|---|---|---|---|---|---|---|
| AA | AT + TT | AA | AT + TT | |||||
| CIDEA | 0.4474 (0.1986) | 0.2999 (0.2094) | 1.45 | 0.016 | 0.2962 (0.2511) | 0.1897 (0.1572) | 1.55 | 0.095 |
| DIO2 | 0.0012 (0.0017) | 0.0005 (0.0003) | 2.51 | 0.036 | 0.0012 (0.0009) | 0.0006 (0.0007) | 2.00 | 0.015 |
| PPARGC1A | 0.0023 (0.0015) | 0.0012 (0.0005) | 1.80 | 0.002 | 0.0017 (0.0009) | 0.0012 (0.0009) | 1.51 | 0.034 |
| PRDM16 | 0.0074 (0.0058) | 0.0041 (0.0033) | 1.73 | 0.021 | 0.0028 (0.0022) | 0.0018 (0.0014) | 1.58 | 0.084 |
| ACC | 0.0054 (0.0038) | 0.0082 (0.0061) | 0.65 | 0.065 | 0.0045 (0.003) | 0.006 (0.0034) | 0.75 | 0.112 |
| DGAT1 | 0.0405 (0.024) | 0.044 (0.0227) | 0.96 | 0.61 | 0.0935 (0.0371) | 0.1052 (0.0652) | 0.88 | 0.440 |
| FASN | 0.2635 (0.1727) | 0.4259 (0.3704) | 0.60 | 0.069 | 0.2006 (0.1452) | 0.2739 (0.2178) | 0.72 | 0.172 |
| SCD1 | 0.8676 (0.8784) | 1.3172 (1.7992) | 0.64 | 0.266 | 0.7727 (0.7134) | 0.8298 (1.0251) | 0.72 | 0.822 |
| SREBP1 | 0.0238 (0.0126) | 0.0366 (0.0268) | 0.64 | 0.050 | 0.0255 (0.0345) | 0.0213 (0.0138) | 1.19 | 0.577 |
| ADIPOQ | 0.5757 (0.2606) | 0.8252 (0.4946) | 0.69 | 0.041 | 0.4176 (0.3337) | 0.4953 (0.2959) | 0.85 | 0.402 |
| ADRB3 | 0.002 (0.0021) | 0.0017 (0.0023) | 1.13 | 0.667 | 0.0017 (0.0018) | 0.0021 (0.0027) | 0.80 | 0.590 |
| FABP4 | 1.683 (0.84) | 1.2587 (0.6681) | 1.31 | 0.068 | 0.7458 (0.4979) | 0.718 (0.4251) | 1.09 | 0.839 |
| GLUT4 | 0.0463 (0.0462) | 0.0241 (0.0192) | 1.86 | 0.035 | 0.0246 (0.016) | 0.0175 (0.0141) | 1.45 | 0.114 |
| LEP | 0.0803 (0.0847) | 0.0653 (0.0539) | 1.24 | 0.477 | 0.0189 (0.0151) | 0.0222 (0.0321) | 1.02 | 0.643 |
AA and AT + TT indicate TRIB2 genotypes. “Ratio” indicates mean expression levels of AA where the mean expression levels of AT + TT were set to be 1. Data for UCP1 are not shown as UCP1 transcripts were not detectable in the adipose tissue samples. SD indicates standard deviation
The previous study showed that the A allele of rs1057001 is linked to lower transcription levels of TRIB2 in adipose tissues [7]. We additionally investigated epigenetic modifications of rs1057001 and short nucleotide sequence variants with tight linkage disequilibrium status with rs1057001 (Fig. 1). Several of these variants were found in genome sequences with signatures of gene regulatory elements, including histone enhancer marks, DNase hypersensitive sites, and alteration of protein-binding motifs. These variants are considered to coordinately alter expression levels of TRIB2 in various tissues. The transcription level of a microRNA-encoding gene (MIR3125) as well as of TRIB2 showed significant correlations with genotypes of rs1057001 or the tightly linked variants. Although the physiological function of MIR3125 is still unknown, this gene is thought to contribute to the genotype-related effects of the TRIB2 variant of the thermogenic genes.
Fig. 1.
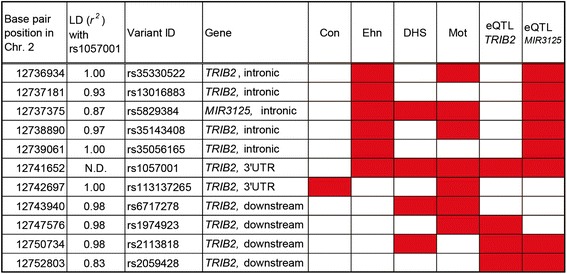
Functional annotation of the short nucleotide sequence variants in strong linkage disequilibrium (LD) with rs1057001. Red color indicates SNPs located in an evolutionarily conserved site (Con), in enhancer histone marks in adipocytes (Ehn), in DNase hypersensitive site (DHS), and in protein-binding motif sequences (Mot). Nucleotide sequence variants associated with expression levels of nearby genes (TRIB2 and/or MIR3125) in previous studies are also indicated in red. The functional annotation of the nucleotide sequence variants was performed using Haploreg4.1
Higher expression levels of genes involved in the differentiation and function of brown and beige/brite adipocytes may indicate a greater propensity for thermogenesis in individuals with the AA genotype in response to cold stimuli. This observation may support the hypothesis that the A allele contributed to cold adaptation in ancestors of East Asians in the last glacial period [7]. The suppression of Trib2 has been shown to reduce the degradation of CEBPB, a key transcription factor involved in brown adipocyte differentiation in murine models [8, 9]. This suggests that the AA genotype, which is linked with suppressed expression of TRIB2, is associated with more potent differentiation of thermogenic adipocytes. Transcription levels of UCP1, an important component of the thermogenic machinery of brown and beige/brite adipocytes, were very low in the present adipose tissue samples. UCP1 expression is known to occur at very low levels in non-stimulated white adipose tissues in mice and humans [18, 19]. The present adipose tissue samples, which were obtained from patients who were not subject to cold simulation, did not exhibit high levels of UCP1 expression.
The present study has several potential limitations, which should be addressed in future work; (1) the observed gene expression pattern may be confounded by non-adipocyte cell populations, mainly stromal vascular cells, in the adipose tissues. (2) It is unclear whether the abdominal SAT mirrors the white adipose tissues in the supraclavicular region, which represents the main site of expression of thermogenic genes in human beige/brite adipocytes [19]. (3) The molecular mechanisms underlying the modulation of thermogenic gene expression by TRIB2 remain unknown.
In conclusion, the present study provides evidence for the role of TRIB2 in energy expenditure in adipose tissues. These findings are believed to explain the previously proposed positive natural selection for the TRIB2 cold-adaptive variant during the last glacial period.
Acknowledgments
We are grateful to the participants of the present study.
Funding
This work was partly supported by the MEXT KAKENHI Grant Numbers 26291096 and 26251050.
Availability of data and materials
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
KN conceived this study. SI provided the DNA and cDNA samples. KN performed the experiments. KN wrote the manuscript. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable
Ethics approval and consent to participate
The design of this study was approved by the Ethical Committee of the Jichi Medical University. All participants provided written informed consent.
Abbreviations
- ACC
Acetyl-CoA carboxylase
- ADIPOQ
Adiponectin
- ADRB3
Adrenoceptor beta 3
- cDNA
Complementary DNA
- CEBPB CCAAT
Enhancer binding proteinbeta
- CIDEA
Cell death-inducing DFFA-like effector A
- DGAT1
Diacylglycerol O-acyltransferase 1
- DIO2
Iodothyronine deiodinase 2
- DNA
Deoxyribonucleic acid
- FABP4
Fatty acid-binding protein 4
- FASN
Fatty acid synthase
- GLUT4
Facilitated glucose transporter, member 4
- LD
Linkage disequilibrium
- LEP
Leptin
- PCR
Polymerase chain reaction
- PDRM16
PR/SET domain 16
- PPARGC1A
PPARG coactivator 1 alpha
- RNA
Ribonucleic acid
- RT-PCR
Real-time PCR
- SAT
Subcutaneous adipose tissues
- SCD1
Stearoyl-CoA desaturase 1
- SNP
Single-nucleotide polymorphism
- SREBP1
Sterol regulatory element binding protein1
- TRIB2
Tribbles pseudokinase 2
- UCP1
Uncoupling protein 1
- VAT
Visceral adipose tissues
Contributor Information
Kazuhiro Nakayama, Phone: +81-285-58-7341, Phone: +81-285-44-4902, Email: nakayama@jichi.ac.jp.
Sadahiko Iwamoto, Email: siwamoto@jichi.ac.jp.
References
- 1.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. 1962;14:353–362. [PMC free article] [PubMed] [Google Scholar]
- 2.Prentice AM, Hennig BJ, Fulford AJ. Evolutionary origins of the obesity epidemic: natural selection of thrifty genes or genetic drift following predation release? Int J Obesity. 2008;32:1607–1610. doi: 10.1038/ijo.2008.147. [DOI] [PubMed] [Google Scholar]
- 3.Nishimura T, Motoi M, Egashira Y, Choi D, Aoyagi K, Watanuki K. Seasonal variation of non-shivering thermogenesis (NST) during mild cold exposure. J Physiol Anthropol. 2015;34:11. doi: 10.1186/s40101-015-0051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sellayah D, Cagampang FR, Cox RD. On the evolutionary origins of obesity: a new hypothesis. Endocrinology. 2014;155:1573–1588. doi: 10.1210/en.2013-2103. [DOI] [PubMed] [Google Scholar]
- 5.Hancock AM, Clark VJ, Qian Y, Di Rienzo A. Population genetic analysis of the uncoupling proteins supports a role for UCP3 in human cold resistance. Mol Biol Evol. 2011;28:601–614. doi: 10.1093/molbev/msq228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoneshiro T, Ogawa T, Okamoto N, Matsushita M, Aita S, Kameya T, et al. Impact of UCP1 and beta3AR gene polymorphisms on age-related changes in brown adipose tissue and adiposity in humans. Int J Obesity. 2013;37:993–998. doi: 10.1038/ijo.2012.161. [DOI] [PubMed] [Google Scholar]
- 7.Nakayama K, Ogawa A, Miyashita H, Tabara Y, Igase M, Kohara K, et al. Positive natural selection of TRIB2, a novel gene that influences visceral fat accumulation, in East Asia. Hum Genet. 2013;132:201–217. doi: 10.1007/s00439-012-1240-9. [DOI] [PubMed] [Google Scholar]
- 8.Naiki T, Saijou E, Miyaoka Y, Sekine K, Miyajima A. TRB2, a mouse Tribbles ortholog, suppresses adipocyte differentiation by inhibiting AKT and C/EBPbeta. J Biol Chem. 2007;282:24075–24082. doi: 10.1074/jbc.M701409200. [DOI] [PubMed] [Google Scholar]
- 9.Kajimura S, Seale P, Kubota K, Lunsford E, Frangioni JV, Gygi SP, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoneshiro T, Saito M. Activation and recruitment of brown adipose tissue as anti-obesity regimens in humans. Ann Med. 2015;47:133–141. doi: 10.3109/07853890.2014.911595. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Seale P. Control of brown and beige fat development. Nat Rev Mol Cell Biol. 2016;17:691–702. doi: 10.1038/nrm.2016.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Munkhtulga L, Nagashima S, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, et al. Regulatory SNP in the RBP4 gene modified the expression in adipocytes and associated with BMI. Obesity. 2010;18:1006–1014. doi: 10.1038/oby.2009.358. [DOI] [PubMed] [Google Scholar]
- 13.Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically linked variants. Nucleic Acids Res. 2012;40:D930–D934. doi: 10.1093/nar/gkr917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, et al. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petrovic N, Walden TB, Shabalina IG, Timmons JA, Cannon B, Nedergaard J. Chronic peroxisome proliferator-activated receptor gamma (PPARgamma) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J Biol Chem. 2010;285(10):7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seale P, Kajimura S, Yang W, Chin S, Rohas LM, Uldry M, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homoe P, Loft A, et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17:798–805. doi: 10.1016/j.cmet.2013.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets during and/or analyzed during the current study are available from the corresponding author on reasonable request.


