Abstract
Cucumber is one of the main vegetables in Iranian food basket. A wide range of pesticides are used for crops protection during the cultivation of vegetables such as cucumber due to heavy pest infestation. Analysis of pesticide residues in food and other environmental commodities have become essential requirement for consumers, producers, and food quality control authorities. This study was aimed at determination of pesticides residues in cucumber as a main vegetable in Iranian food basket.
A reliable, rapid and accurate method based on spiked calibration curves and modified QuEChERS sample preparation was developed for determination of 12 pesticide residues in cucumber by gas chromatography-mass spectrometry (GC/MS). The use of spiked calibration standards for constructing the calibration curve substantially reduced adverse matrix-related effects.
The recovery of pesticides at 5 concentration levels (n = 3) was in the range of 80.6-112.3. The method was proved to be repeatable with RSD lower than 20%. The limits of detection and quantification for all pesticides were <10 ng/g and <25 ng/g, respectively. The developed method was used for simultaneous determination of the selected pesticides in 60 greenhouse and garden cucumber samples. Among the 60 analyzed samples, 41.7% of them were contaminated with pesticide residues which 31.7% of samples had pesticide residues lower than maximum residue limit and 10% of samples had residue higher than maximum residue limit.
Key Words: Pesticide, Spiked calibration curve, GC/MS, Cucumber, Multi residue, Iran
Introduction
Cucumber «cucumas sativus» is a frequently consumed vegetable in Iranian daily food basket (1). The increasing public concern about pesticide contamination of food and the environment in recent years has increased the demand for broader and stricter pesticide monitoring. Therefore, it is necessary to develop rapid, reliable and effective analytical methods for the simultaneous determination of the residues of pesticides in order to obtain accurate information about the types and quantity of the pesticides used, to protect the consumers and guarantee the safety of agricultural products (2). The authorities are criticized for monitoring too few samples, which stems from the high cost, complexity, and time-consuming sample preparation procedures involved in current chromatographic methods for pesticides analysis (3).
Over the past decades, approaches to trace level determination of pesticides have moved away from the use of GC with selective detectors including electron capture detection (ECD) (4-6) and nitrogen phosphorus detection (NPD) (7), to mass spectrometer (MS) detectors which are more sensitive and selective (8). The use of mass spectrometry, with its information-rich content and explicit confirmation, is recommended for monitoring pesticide residues in the entire world (9-13). Generally, the complex matrix of agricultural products adversely affects analysis precision, and it is necessary to remove the matrix interference by sample pre-treatment, such as extraction and clean-up steps (14-16).
Conventionally, simultaneous analysis of pesticide residues from different classes in foods is a time-consuming process that often entails several post-extraction clean up steps prior to the analysis. A simple, safe, cheap and high sample throughput method named QuEChERS has been recently proposed for the analysis of pesticide residues (17-21).
This paper presents a rapid multi-residue method of analysis based on QuEChERS extraction procedure using spiked calibration curve to simultaneously determine and confirm 14 pesticide residues in cucumber. The selected pesticides included GC-amenable pesticides, those for which MRL is issued by Institute of Standards of Iran (22).
In order to overcome the adverse matrix-related effects, it was decided to prepare the calibration curves by spiking blank cucumber samples with certain amounts of pesticides and constructing the calibration curve using these spiked samples. The list of selected pesticides along with some of their physico-chemical properties are presented in Table 1.
Table 1.
Physicochemical properties of the selected pesticides
| Compound | Structural group | M.F. | M.W. | M.P. (ºC ) |
|---|---|---|---|---|
| Diazinon | Organophosphorus | C12H21N2O3PS | 304.35 | <120 °C |
| Chlorpyrifos | Organophosphorus | C9H11CL3NO3PS | 350.59 | 41.5-43.5 °C |
| Pirimiphos-methyl | Organophosphorus | C11H20N3O3PS | 305 | 15 °C |
| Beta-Endosufan | Organochlorous | C9H6CL6O3S | 406.93 | 207-209 °C |
| Alpha-Endosulfan | Organochlorous | C9H6CL6O3S | 406.92 | 106 °C |
| Fenvalerate1,2 | Pyrethroid | C25H22CLNO3 | 419.91 | <25 °C |
| Primicarb | Carbamat | C11H18N4O2 | 238.29 | 90.5 °C |
| Carbaryl | Carbamat | C12H11NO2 | 201.22 | 142.2 °C |
| Propargite | Sulfite ester | C19H26O4S | 350.5 | 47 °C |
| Fenpropathrin | Pyrethroid | C22H23NO3 | 349.4 | 47 °C |
| Permethrin1,2 | Pyrethroid | C21H20Cl2O3 | 391.29 | 34 °C |
| Metalaxyl | Acylalanin | C15H21NO4 | 279.34 | 72 °C |
Materials and Methods
Reagent
All pesticides standards were purchased from Dr. Ehrenstorfer Co. (Augsburg, Germany). All organic solvents, intended for extraction, were at least LC grade and purchased from Merck (Darmstadt, Germany). Primary secondary amine (PSA) and graphite carbon black (GCB) were purchased from Supelco (Bellefonte, USA). Bulk quantities of NaCl were obtained from Merck (Darmstadt, Germany). Anhydrous MgSO4 was obtained from SIGMA-Aldrich CO. (Japan). The MgSO4 was baked for 5 h at 500 ºC in a furnace to remove phthalates and residual water.
Apparatus
An Agilent Technologies 6890 Network GC System chromatograph (Wilmington, USA) with a (single quadrupole) SQ detector and equipped with an Agilent 7683B autosampler (Agilent technologies, USA) was used. A HP-5 capillary column (30 m×0.25 mm I.D., 1 μm film thickness)
Real Samples: sixty cucumber samples were collected from Tehran Central Market fruits and vegetables in 2011. Cucumber samples from 4 different cities were prepared that 43 of them were greenhouse products and the others were garden cucumber. In this study, in addition to monitoring 12 pesticides residues in cucumber, the pesticides residues in greenhouse cucumbers were compared with those of garden cucumbers.
Calibration standards
Individual stock standard solutions (1 mg/mL) were prepared in ethyl acetate and stored in the dark at −20 °C. Prior to their use, they were kept for 1 h at ambient temperature. A mixed stock standard solution of pesticides was prepared in ethyl acetate at 10 μg /mL with respect to each pesticide. Spiked calibration curves at 7 levels of 10, 25, 50, 100, 200, 300 and 500 ng/g in triplicates were prepared by addition of 10 μL, 25 μL, 50 μL, 100 μL, 200 μL, 300 μL and 500μL of mixed standard stock solution, respectively, to 10 g portions of blank cucumber samples in each case.
A stock solution of triphenylmethane (TPM) in ethyl acetate at concentration of 1 mg/mL was used as internal standard and an aliquot of 10 μL of TPM solution in ethyl acetate was added to the spiked cucumber sample. The samples so obtained were treated as described in section 2.4.
Sample preparation
An aliquot of 10 μL of internal standard solution (1000 mg/L) was added to 10 g of blended blank cucumber sample in a 50 mL falcon tube and after being left for 1h at ambient temperature in dark, 20 mL (ethanol, toluene, 50%:50%) was added. The mixture was mixed at high speed with vortex mixer for 1min. One gram of NaCl and 2 grams of activated anhydrous MgSO4 was added to the mixture, and mixing was continued for an additional 60 s. The mixture was centrifuged for 5 min at 5000 rpm at -5 ◦C. The supernatant was transferred to a 15 mL falcon tube containing 2 g MgSO4 and 300 mg PSA and 200 mg GCB. After shaking for 1 min and centrifugation for 5 min at 5000 rpm at -5 ◦C, 4 mL of supernatant was transferred to a 5 mL vial and evaporated to dryness under a gentle stream of nitrogen gas. The residue was reconstituted by toluene to obtain 1 mL solution, and after shaking for 3 min, 2 μL of the solution was injected into gas chromatograph.
Recovery studies
For recovery determination, spiked blank cucumber samples at concentration levels of 30, 60, 150, 250, 350 ng/ g were prepared in triplicates and they were kept for 1 h at ambient temperature prior to their use and then treated according to the procedure described in section 2.4. The recoveries were calculated using the calibration curves constructed using spiked samples.
GC/MS analysis
The GC/MS was employed with helium as the carrier gas at a constant flow of 1 mL/min. The oven temperature started at 75 °C and remained at this temperature for 3 min increasing to 120 °C at 25 °C/min ramp rate and then increased to 300 °C at 5 °C/min ramp, holding at 300 °C for 11 min. Injection port was adjusted at 250 °C and splitless injection mode was used.
After acquisition of the total ion chromatogram for the mixed stock standard solutions in scan mode, peaks were identified by their retention time and mass spectra. The most abundant ion that showed no evidence of chromatographic interference and had the highest signal-to-noise ratio was selected for quantification purposes.
Quantitation
The concentrations of pesticides were determined by intrapolation of the relative peak areas for each pesticide to internal standard peak area in the sample on the spiked calibration curve. In order to compensate for losses during sample processing and instrumental analysis, internal standard (TPM) was used.
Results
Gas chromatographic determination
Analysis was performed in the SIM mode based on the use of one target and two or three qualifier ions. Pesticides were identified according to their retention times and target and qualifier ions. The quantitation was based on the peak area ratio of the targets to that of internal standard. Table 2. summarizes pesticides studied with their diagnostic and quantification ions used in SIM mode in this study.
Table 2.
The retention time, diagnostic ions and selected quantification ion for the target pesticides and internal standard
| No. | Compound | Diagnostic ions (m/z) | Quantification ions (m/z) | Retention time (min) |
|---|---|---|---|---|
| 1 | Carbaryl | 144, 115.1, 145.1, 116.1 | 144.1 | 19.869 |
| 2 | Diazinon | 304, 276.1, 179,1 | 304.1 | 26.083 |
| 3 | Pirimicarb | 238.2, 166.1, 138 | 166.1 | 27.216 |
| 4 | Metalaxyl | 206.1, 249,234 | 206.1 | 28.777 |
| 5 | Pirimiphos methyl | 305, 290.1, 276 | 290.1 | 29.282 |
| 6 | Chlorpyrifos | 314, 257.8, 316 | 314.0 | 30.202 |
| 7 | Triphenyl methan | 236.9, 264.9, 338.9 | 244.1 | 30.648 |
| 8 | Alpha-Endosulfan | 236.9, 264.9, 338.9 | 236.9 | 33.365 |
| 9 | Beta-Endosulfan | 339.1, 264.9, 236.9 | 236.9 | 35.573 |
| 10 | Propargite | 350.2, 173.1, 201.1 | 350.2 | 37.390 |
| 11 | Fenpropathrin | 265.1, 208, 349.2 | 265.1 | 38.879 |
| 12 | Permethrin 1 | 183.1,163.1,184.1 | 183.1 | 42.233 |
| 13 | Permethrin 2 | 183.1,163.1,184.1 | 183.1 | 42.500 |
| 14 | Fenvalerate 1 | 419.2, 225.1, 167.1 | 167.1 | 47.256 |
| 15 | Fenvalerate 2 | 419.2, 225.1, 167.1 | 167.1 | 47.911 |
Method validation
Linearity of the calibration curves
The twelve pesticides showed linearity in SIM mode. Linear spiked calibration curves for all the interest pesticides were obtained with correlation factors >0.996. The Calibration data (equation and regression coefficient) of 12 pesticides in spiked cucumber calibration curves is showed in Table 3.
Table 3.
Calibration data (equation and regression coefficient) of 12 pesticides in spiked cucumber calibration curves.
| Compound | Equation | Regression Coefficient |
|---|---|---|
| Carbaryl | y = 0.1899x – 0.016 | 0.999 |
| Diazinon | y = 0.1482x + 0.0003 | 0.999 |
| Pirimicarb | y = 0.6757x - 0.0012 | 0.999 |
| Metalaxyl | y = 0.2803x + 0.0056 | 0.998 |
| Pirimiphos-methyl | y = 0.3587x + 0002 | 0.998 |
| Chlorpyrifos | y = 0.1978x - 0.0001 | 0.999 |
| Alpha-Endosulfan | y = 0.11797x - 0.0023 | 0.999 |
| Beta-Endosulfan | y = 0.0568x + 0.0003 | 0.996 |
| Propargite | y = 0.0965x - 0.0003 | 0.999 |
| Fenpropathrin | y = 0.1351x+ 0.0006 | 0.999 |
| Permethrin 1,2 | y = 0.7747x + 0.0029 | 0.999 |
| Fenvalerate 1,2 | y = 0.1819x + 0.0017 | 0.997 |
LOD and LOQ
Limits of detection (LOD) and limits of quantification (LOQ) of the proposed method were measured in spiked samples and calculated by considering a value 3 and 10 times that of background noise, respectively. The LODs and LOQ for all the pesticides were ≤10 ng/g and ≤25 ng/g respectively.
Accuracy and Precision
Table 4. presents the recovery and repeatability for five concentration levels of pesticides. The recovery of pesticides at 5 concentration levels triplicates was in the range of 80.6-112.3%. In terms of repeatability, the majority of the pesticides gave RSD<20%. The recoveries and repeatabilities are in accordance with the criteria set by SANCO Guideline (23).
Table 4.
Average recoveries (%) and relative standard deviations (%) of pesticides obtained by GC-MS analysis of cucumber samples at 5 spiking levels (n=3).
| Compound | Average recovery (%) (n=3) |
Total Average recovery (%) (n=3) | Range of RDS (%) | ||||
|---|---|---|---|---|---|---|---|
| 30ng/g | 60ng/g | 150ng/g | 250ng/g | 350ng/g | |||
| Carbaryl | 101.3 | 103.2 | 112.4 | 78.1 | 87.3 | 96.5 | 5.4-16.8 |
| Diazinon | 90.7 | 108.5 | 112.6 | 98.1 | 102.4 | 102.5 | 1.8-11.2 |
| Pirimicarb | 112.4 | 117.8 | 114.3 | 102.7 | 108.7 | 111.2 | 10.1-19.9 |
| Metalaxyl | 75.8 | 80.2 | 90.5 | 82.5 | 84.1 | 82.6 | 0.6-24.1 |
| Pirimiphos methyl | 88.4 | 108.2 | 118.2 | 92.2 | 97.6 | 100.9 | 7.1-23.3 |
| Chlorpyrifos | 97.2 | 102.8 | 104.1 | 96.2 | 96.6 | 96.6 | 2.9-5.6 |
| Alpha-Endosulfan | 116.5 | 88.4 | 68.1 | 77.5 | 80.7 | 85.7 | 0.9-9.9 |
| Beta-Endosulfan | 112.4 | 117.8 | 114.3 | 102.7 | 108.7 | 80.6 | 3.3-9.3 |
| Propargite | 75.8 | 80.2 | 90.5 | 82.5 | 84.1 | 112.1 | 1.7-22.8 |
| Fenpropathrin | 88.4 | 108.2 | 118.2 | 92.2 | 97.6 | 91.1 | 2.7-19.1 |
| Permethrin 1, 2 | 80.1 | 95.6 | 101.3 | 92.5 | 96.7 | 96.8 | 3.3-11.0 |
| Fenvalerate1, 2 | 120.6 | 117.8 | 97.7 | 112.5 | 121.3 | 112.3 | 3.7-11.8 |
Discussion
The major source of inaccuracy in pesticide residue analysis by GC-MS, especially in food, is related to the presence of interfering components in the sample, the so-called ‘‘matrix effect’’. In other words, in conventional gas chromatographic analysis, such as the analysis of pesticide residues in foods, co-extracted matrix components may be problematic in obtaining the true data. (13).
Theoretically, elimination of matrix components or active sites in the injection port would surmount the matrix-induced enhancement effect; but, complete and permanent GC system deactivation or comprehensive sample clean up is practically impossible (24).
There are a number of approaches for preventing, reducing, or compensating for the occurrence of matrix effects (13, 21) including the application of alternative calibration methods including the use of (A) matrix-matched calibration method, (25-27), (B) standard addition method, (C) isotopically labeled internal standards (not feasible in multiresidue pesticide analysis due to their unavailability or high price) and (D) usage of analyte protectants (21).
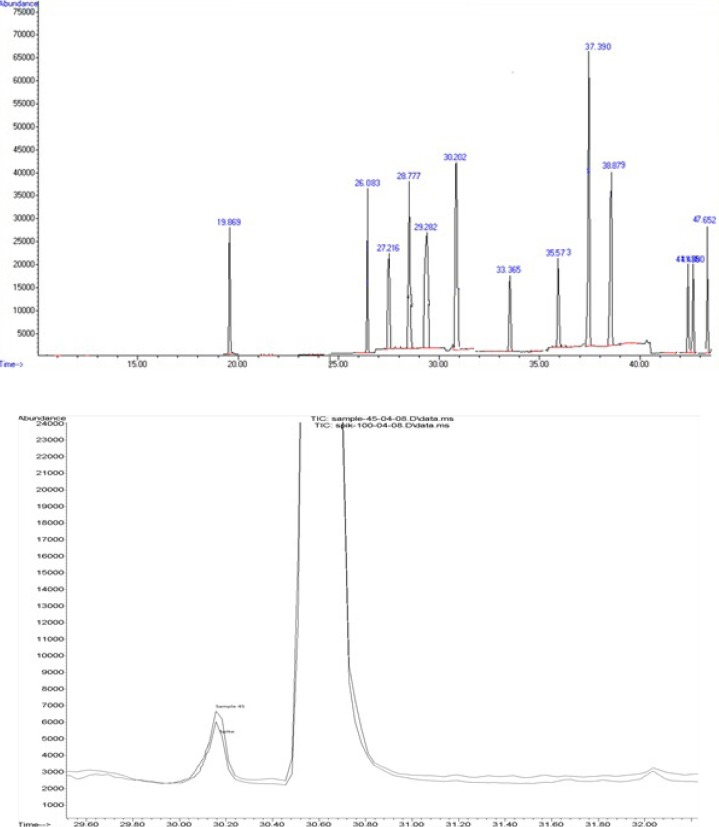
In the present study, we used spiked calibration curves approach to overcome the problems caused by the matrix. In this approach, calibration curves are constructed by the addition of standard solution to blank cucumber samples and these samples are subjected to the same sample preparation procedure which is intended to be used for unknown samples. This way, the standard sample matrices will have the same composition as the unknown samples and therefore the effect of matrix is reflected in both standards and unknown samples. The calibration curve is constructed using these spiked calibration standards and it is easily used to calculate the concentration of analyte(s) in unknown sample without being concerned about the matrix effects. Figure 1. represents an overlaid GC-MS-SIM chromatogram of a cucumber sample spiked at 100 ng/g of diazinone (rt = 26 min), primicarb (rt = 27.2 min) and chlorpyrifos (rt = 30.2 min) and a contaminated cucumber sample with chlorpyrifos.
Figure 1.
An overlaid GC-MS-SIM chromatogram of (a) cucumber sample spiked at 100 ng/g of diazinone (rt=26 min), primicarb (rt=27.2 min) and chlorpyrifos (rt=30.2 min) and (b) contaminated cucumber sample with chlorpyrifos
The recoveries and repeatabilities were in accordance with the criteria set by SANCO Guideline (23).
It appears that the proposed spiked calibration curve method is a proper approach for elimination of matrix effects in pesticide residues analysis. The method could be considered as an alternative method along with matrix-matched standards, standard addition method, isotopically labeled internal standards and usage of analyte protectants, for determination of various classes of pesticide residues. It is rapid, simple, sensitive, selective and rugged.
A comparison of the results for greenhouse cucumbers with the results obtained for garden cucumbers showed that the residues were different in both identity and quantity (Table 5).
Table 5.
Pesticide residues detected on 60 cucumber samples, Iran, 2011.
|
MRL
µg/g |
Range
ng/g |
Mean of Pesticide residue ng/g | Pesticide type (number) | Samples condition (detected vs not-detected) | Samples (number) |
|---|---|---|---|---|---|
| < LOD | 12 pesticide understudy | Pesticide not-detected (26) | Green House (43) | ||
| 40.3 | 40.3 | Fenpropathrin (1) | Pesticide detected (17) | ||
| 1 | 27.7-34.8 | 31.25 | Alpha-Endosulfan(2) | ||
| 1 | 7.6-17.8 | 12.7 | Beta-Endosulfan (2) | ||
| 0.5 | 7.3-194.1 | 57.58 | Metalaxyl (6) | ||
| 0.05 | 66.4-148.2 | 97.13 | Chlorpryfos (6) | ||
| < LOD | 12 pesticide understudy | Pesticide not-detected (8) | Garden (17) | ||
| 0.5 | 36 | 36 | Propargite (1) | Pesticide detected (9) | |
| 0.5 | 40-87.6 | 65.87 | Metalaxyl (8) |
In greenhouse cucumber samples Fenpropathrin, Alpha-Endosulfan, Beta-Endosulfan, Metalaxyl and Chlorpryfos were detected while in garden cucumbers, Propargite and Metalaxyl were detected. The diversity and quantity of pesticides in greenhouse cucumber were higher than garden cucumbers. It seems that the high levels of pesticides residues in greenhouse cucumbers is due to their particular product conditions and higher utilization of pesticides to control pathogens in cultivation of greenhouse cucumbers.
Conclusion
A simple and rapid method was developed to determine 14 pesticide residues in cucumber, a frequently used vegetable in Iranian food basket. The method which consists of a QuEChERS simple sample preparation procedure and GC-SQ-MS-SIM analysis showed a high sensitivity and confirmatory power necessary for the determination of pesticide residues at the levels of maximum residue limits (MRLs) issued in Iran for cucumber. The developed method has the advantage of using spiked calibration curves that minimizes the matrix interferences leading to higher accuracy for pesticides analyses. Among the 60 analyzed cucumber samples, 41.7% of them were contaminated with pesticide residues; 31.7% of the samples had pesticide residues lower than maximum residue limit and 10% of samples had residue higher than maximum residue limit indicating the need for awareness about the greenhouse cucumber production.
Acknowledgment
This study was supported by the Food and Drug Laboratory Research Center (FDLRC), Food and Drug Deputy, Iran’s Ministry of Health and Medical Education. The authors are grateful to the staff members of the Toxicology Laboratory, FDLRC.
References
- 1.National nutritional science and food Technology Research Institute. National report of comprehensive study food consumption pattern and nutrition status in Iran during. 2004;24:2000–2002. [Google Scholar]
- 2.Soon-Kil Cho A. M, Abd El-Aty, Young-Seok Park, Jeong-Heui Choi, Sathya Khay, Cheol-Ah Kang, Byung-Jun Park, Sun-Ju Kimand and Jae-Han Shim A multiresidue method for the analysis of pesticide residues in polished rice (Oryza sativa L) using accelerated solvent extraction and gas chromatography and confirmation by mass. Biomed. Chromatogr. 2007;21:602–9. doi: 10.1002/bmc.792. [DOI] [PubMed] [Google Scholar]
- 3.Kirchner M, Huskova R, Matisov E, Mocak J. Fast gas chromatography for pesticide residues analysis using analyte protectants. J. Chromatogr. A. 2008;1186:271–8. doi: 10.1016/j.chroma.2007.08.089. [DOI] [PubMed] [Google Scholar]
- 4.Dorea HS, Sobrinho LL, Braz J. Analysis of pesticide residue in rice using matrix solid-phase dispersion (MSPD) Chem. Soc. 2004;15:5–12. [Google Scholar]
- 5.Sannino A, Bandini M, Bolzoni L. Application of liquid chromatography with electrospray tandem mass spectrometry to the determination of a new generation of pesticides in processed fruits and vegetables. J. Chromatogr. A. 2004;1036:161–9. doi: 10.1016/j.chroma.2004.02.078. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Shi L, Shan Z, Hu Q. Determination of organochlorine pesticide residues in rice and human and fish fat by simplified two-dimensional gas chromatography. Food Chem. 2007;104:1315–9. [Google Scholar]
- 7.Oishi M, Onishi K, Kano I, Nakazawa IH, Tanabe S. Capillary gas chromatographic determination of thiabendazole in citrus and apple juices. J. AOAC Int. 1994;77:1293–9. [PubMed] [Google Scholar]
- 8.Lehotay SJ. Analysis of pesticide residues in mixed fruit and vegetable extracts by direct sample introduction/ gas chromatography/tandem mass spectrometry. J. AOAC Int. 2000;83:680–97. [PubMed] [Google Scholar]
- 9.Pengyan L, Quingxue L, Yusong M, Jinwei L, Xuan J, Chin J. Analysis of pesticide multiresidues in rice by gas chromatography-mass spectrometry coupled with solid phase extraction. J. Chromatogr. A. 2006;24:228–38. [PubMed] [Google Scholar]
- 10.Libin L, Hashi Y, Yaping Q, Haixia Z, Jinming L. Rapid analysis of multiresidual pesticides in agricultural products by gas chromatography-mass spectrometry. J. Anal. Chem. 2006;34:783–6. [Google Scholar]
- 11.Nguyen TD, Lee B S, Lee BR, Lee DM, Lee GH. multiresidue method for the determination of 109 pesticides in rice using the quick easy cheap effective rugged and safe (QuEChERS) sample preparation method and gas chromatography/mass spectrometry with temperature control and vacuum concentration. Rapid Commun. Mass Spectrom. 2007;21:3115–22. doi: 10.1002/rcm.3199. [DOI] [PubMed] [Google Scholar]
- 12.Liu LB, Hashi Y, Qin YP. Mass spectrometry for measuring multiresidual pesticides in agricultural products. J. Chromatogr. B. 2007;845:61–8. doi: 10.1016/j.jchromb.2006.07.032. [DOI] [PubMed] [Google Scholar]
- 13.Hajslova J, Zrostlikova J. Matrix effects in (ultra) trace analysis of pesticide residues in food and biotic matrices. J. Chromatogr. A. 2003;1000:181–97. doi: 10.1016/s0021-9673(03)00539-9. [DOI] [PubMed] [Google Scholar]
- 14.Ahmed FE. Analyses of pesticides and their metabolites in foods and drinks. Trends Anal. Chem. 2001;20:649–61. [Google Scholar]
- 15.Zambonin CG, Quinto M, Vietro ND, almisano FP. Solid-phase microextraction – gaschromatography mass spectrometry: A fast and simple screening method for the assessment of organophosphorus pesticides residues in wine and fruit juices. Food Chem. 2004;86:269–74. [Google Scholar]
- 16.Albero B, Brunete CS, Tadeo JL. Multiresidue determination of pesticides in juice by solid-phase extraction and gas chromatography–mass spectrometry. Talanta. 2005;66:917–24. doi: 10.1016/j.talanta.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 17.Anastassiades M, Lehotay SJ, Stajnbaher , Schenck FJ. A fast and easy multiresidue method employing acetonitrile extraction/partitioning and «dispersive solid-phase extraction» for the determination of pesticide residues in produce. J. AOAC Int. 2003;86:412–31. [PubMed] [Google Scholar]
- 18.Anastassiades M, Mastovsk K, Lehotay SJ. Evaluation of analyte protectants to improve gas chromatographic analysis of pesticides. J. Chromatogr. A. 2003;1015:163–84. doi: 10.1016/s0021-9673(03)01208-1. [DOI] [PubMed] [Google Scholar]
- 19.Lehotay SJ, Kok A, Hiemstra M, van Bodegraven P. Validation of a fast and easy method for the determination of residues from 229 pesticides in fruits and vegetables using gas and liquid chromatography and mass spectrometric detection. J. AOAC Int. 2005;88:595–613. [PubMed] [Google Scholar]
- 20.Mastovska K, Lehotay SJ. Evaluation of common organic solvents for gas chromatographic analysis and stability of multiclass pesticide residues J. Chromatogr. A. 2004;1040:259–172. doi: 10.1016/j.chroma.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Mastovska K, Lehotay SJ, Anastassiades M. Combination of analyte protectants to overcome matrix effects in routine GC analysis of pesticide residues in food matrixes. Anal. Chem. 2005;77:8129–37. doi: 10.1021/ac0515576. [DOI] [PubMed] [Google Scholar]
- 22.Institute of standards and industrial research of Iran. Maximum pesticide residue limit in cereals. National standard. 2004:6349–6. [Google Scholar]
- 23. [Access date: 10 June 2010]. http://www.inchem.org/documents/jmpr/jmpeval/jmpr 2006.pdf.
- 24.Schenck FJ, Lehotay SJ. Does further clean-up reduce the matrix enhancement effect in gas chromatographic analysis of pesticide residues in food? J. Chromatogr. A. 2000;868:51–61. doi: 10.1016/s0021-9673(99)01137-1. [DOI] [PubMed] [Google Scholar]
- 25.Garrido-Frenich A, Romero-Gonzalez R, Martınez-Vidal JL, Plaza-Bolanos P, Cuadros-Rodrıguez L, Herrera-Abdo MA. Characterization of recovery profiles using gas chromatography- triplequadrupole mass spectrometry for the determination of pesticide residues in meat samples. J. Chromatogr. A. 2006;1133:315–21. doi: 10.1016/j.chroma.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 26.Amirahmadi M, Shoeibi S, Yazdanpanah H, Pirali-Hamedani M, Pakzad SR, Kobarfard F. Effect of cooking process on the residues of three carbamate pesticides in rice. Iran. J. Pharm. Res. 2011;10:119–26. [PMC free article] [PubMed] [Google Scholar]
- 27.Amirahmadi M, Yazdanpanah H, Shoeibi S, Pirali-Hamedani M, Ostad Gholami M, Mohseninia M, Kobarfard F. Simultaneous determination of 17 pesticide residues in rice by GC/MS using a direct sample introduction procedure and spiked calibration curves. Iran. J. Pharm. Res. 2013;12:295–302. [PMC free article] [PubMed] [Google Scholar]