Abstract
Background
De novo transcriptome assembly of short reads is now a common step in expression analysis of organisms lacking a reference genome sequence. Several software packages are available to perform this task. Even if their results are of good quality it is still possible to improve them in several ways including redundancy reduction or error correction. Trinity and Oases are two commonly used de novo transcriptome assemblers. The contig sets they produce are of good quality. Still, their compaction (number of contigs needed to represent the transcriptome) and their quality (chimera and nucleotide error rates) can be improved.
Results
We built a de novo RNA-Seq Assembly Pipeline (DRAP) which wraps these two assemblers (Trinity and Oases) in order to improve their results regarding the above-mentioned criteria. DRAP reduces from 1.3 to 15 fold the number of resulting contigs of the assemblies depending on the read set and the assembler used. This article presents seven assembly comparisons showing in some cases drastic improvements when using DRAP. DRAP does not significantly impair assembly quality metrics such are read realignment rate or protein reconstruction counts.
Conclusion
Transcriptome assembly is a challenging computational task even if good solutions are already available to end-users, these solutions can still be improved while conserving the overall representation and quality of the assembly. The de novo RNA-Seq Assembly Pipeline (DRAP) is an easy to use software package to produce compact and corrected transcript set. DRAP is free, open-source and available under GPL V3 license at http://www.sigenae.org/drap.
Keywords: RNA-Seq, De novo assembly, Compaction, Correction, Quality assessment
Background
Second-generation sequencing platforms have enabled the production of large amounts of transcriptomic data permitting to analyze gene expression for a large variety of species and conditions. For species lacking a reference genome sequence, the now-classical processing pipeline includes a de novo transcriptome assembly step. Assembling an accurate transcriptome reference is difficult because of the raw data variability. This variability comes from different factors: including: 1. The variability of gene expression levels ranging usually between one and millions of copies; 2. The biology of mRNA synthesis which goes through an early stage of pre-mRNA still containing introns and a late state in which mRNA can be decayed; 3. The synthesis from pre-mRNA of numerous alternative transcripts; 4. Potential sample contaminations; 5. Sequencing quality biases; 6. Most of the genome can be expressed in low abundance depending on the biological condition as presented by Djebali et al. (2012) in the results of the ENCODE project.
Today there is no unique best solution to these RNA-Seq assembly problems but several software packages have been proven to generate contig sets comprising most of the expressed transcripts correctly reconstructed. Trinity (Grabherr et al., 2011) and Oases (Schulz et al., 2012) are good examples. The assembled contig sets produced by these packages often contain multiple copies of complete or partial transcripts and also chimeras. Chimeras are structural anomalies of a unique transcript (self-chimeras) or multiple transcripts (multi-transcripts chimeras). They are called “cis” if the transcripts are in the same direction and “trans” if they are in opposite directions. Natural chimeric transcripts exist in some cancer tissues but are rare (Frenkel-Morgenstern et al., 2013). Yang & Smith (2013) have shown the tendency of de novo transcriptome assemblers to produce self-chimeric contigs. The prevalence of the phenomenon depends on the assembly parameters. Multi-transcript chimeras distort contig annotation. The functions of the transcripts merged in the same contig can be very different and therefore the often-unique annotation given to such a chimeric contig does not reflect its content. Assemblies include also contigs corresponding to transcription or sequencing noise a phenomenon often referred as illegitimate transcription (Chelly et al., 1989). These contigs have often low coverage and are not found in the different replicates of the same condition.
Some contigs contain local biological variations or sequencing errors such as substitutions, insertions or deletions. These variations and errors can deeply impact the read alignment rate, create frameshifts which hinder annotation, limit the efficacy of primer design and generate false variations. Assemblies contain also polyA/T tails, which are posttranscriptional marks. They are usually removed before publication. For all these reasons contig sets usually need error correction.
Trinity and Oases have different algorithms, which give them advantages or disadvantages depending on gene expression levels. The main difference comes from their assembly strategy. Trinity chains a greedy algorithm with a de Bruijn graph one and Oases uses multiple de Bruijn graphs with different k-mers. The first step of Trinity is very effective in assembling parts of highly expressed transcripts which will be connected at the second step. As shown by Surget-Groba & Montoya-Burgos (2010), the Oases multi-k assembly approach is able to build contigs corresponding to transcripts with very low to very high expression levels. However, highly expressed genes with multiple transcripts will generate very complex graphs mainly because of the presence of variations or sequencing errors, which will form new paths possibly considered as valid by the assembler and produce numerous erroneous contigs. No assembler is producing the best contig set in all situations. Bio-informaticians and biologists therefore use different strategies to maximize the reference contig set quality (Mbandi et al., 2015; Bens et al., 2016; He et al., 2015; Nakasugi et al., 2014). The simplest approach is to produce a reference set per software package or parameter set, to compare their metrics and choose the best one. It is also possible to merge different results and filter them.
Assemblies can be compared on different criteria. The usual ones are simple contig metrics such as total count, total length, N50, and average length. Assembling equals summarizing (compressing the expression dimension) and therefore a good metric to check the summary quality is the proportion of reads mapped back to the contigs. As a large part of the transcripts correspond to mRNA, it is also possible to use as quality metric the number of correctly reconstructed proteins using a global reference as it is done by CEGMA (Parra, Bradnam & Korf, 2007) or BUSCO (Simão et al., 2015) or using a protein reference set from a phylogenetically closely related organism. Last, some software packages are also rating the contig set or the individual contigs using the above-mentioned criteria (Honaas et al., 2016) or some other for example only related to the way reads map back to the contigs (Smith-Unna et al., 2016; Li et al., 2014; Davidson & Oshlack, 2014).
We have built a de novo RNA-Seq Assembly Pipeline (DRAP) in order to correct the following assembly problems: multiple copies of complete or partial transcripts, chimeras, lowly expressed intergenic transcription, insertion and deletion generated by the assemblers and polyA tails. The pipeline implementation is presented in the next section. The “results and discussion” section compares raw and DRAP assembly metrics for seven different datasets.
Implementation
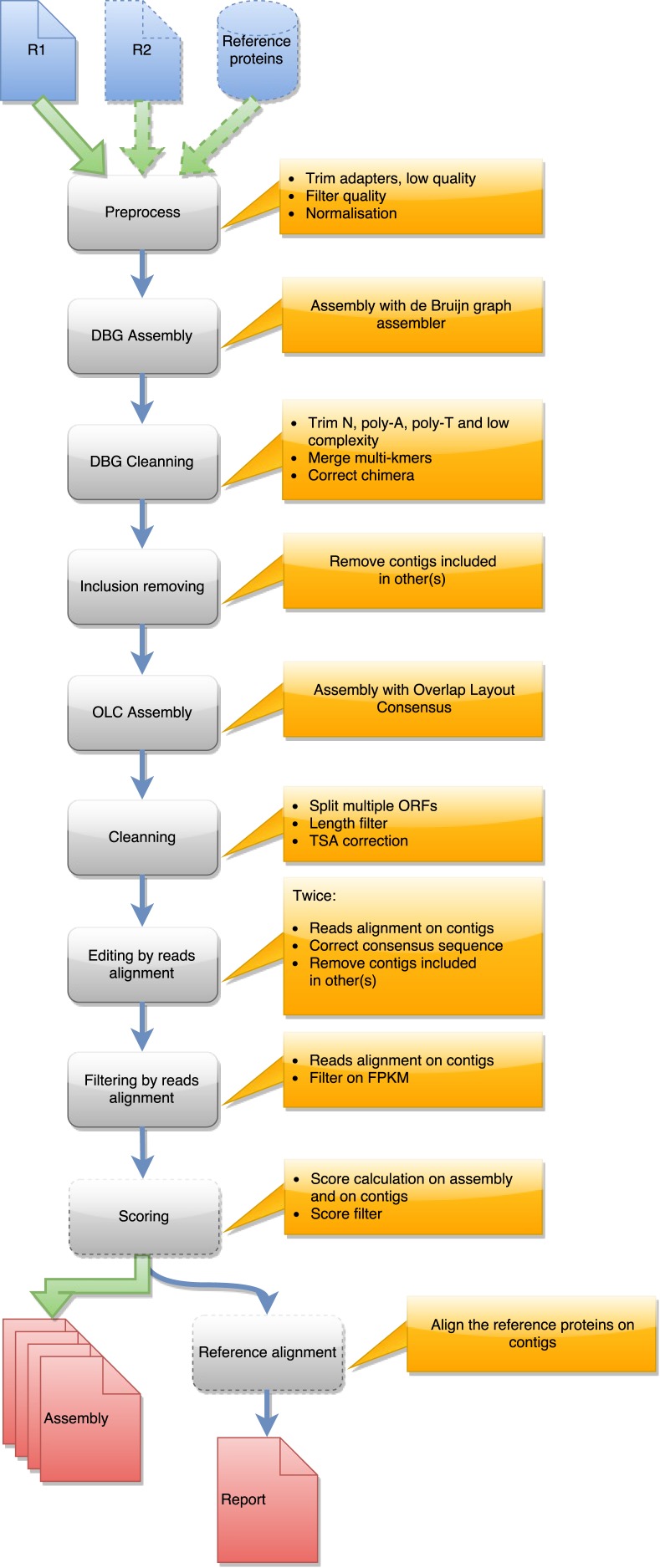
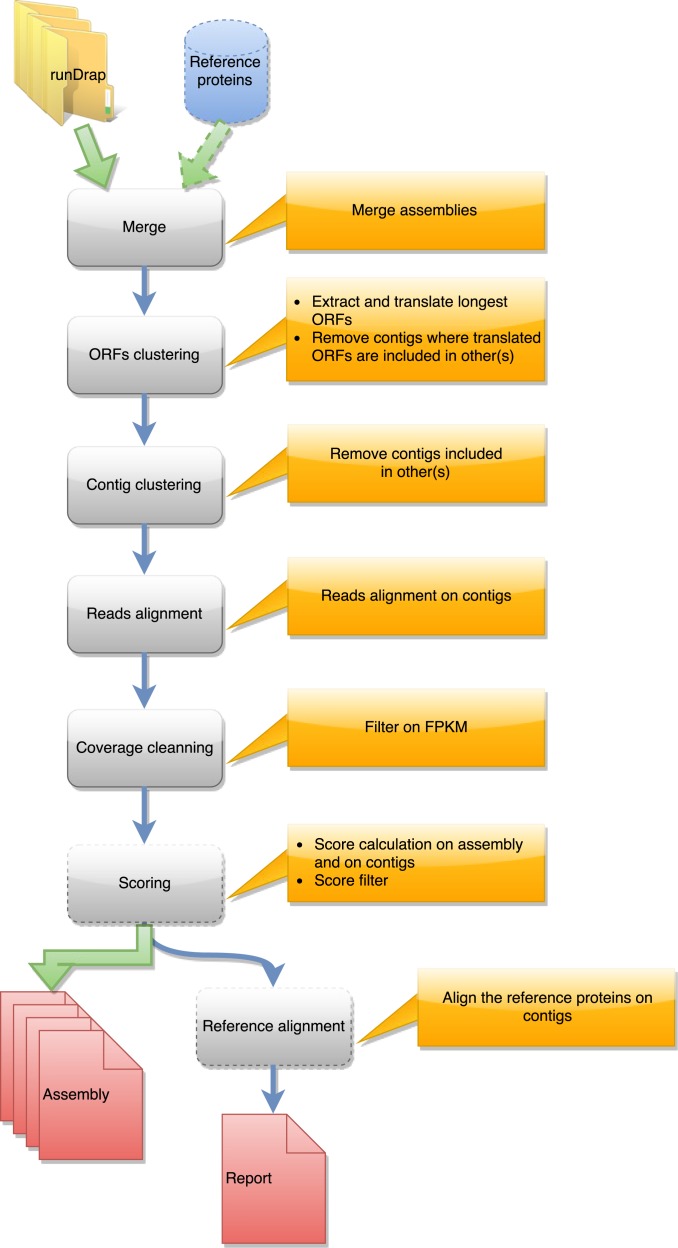
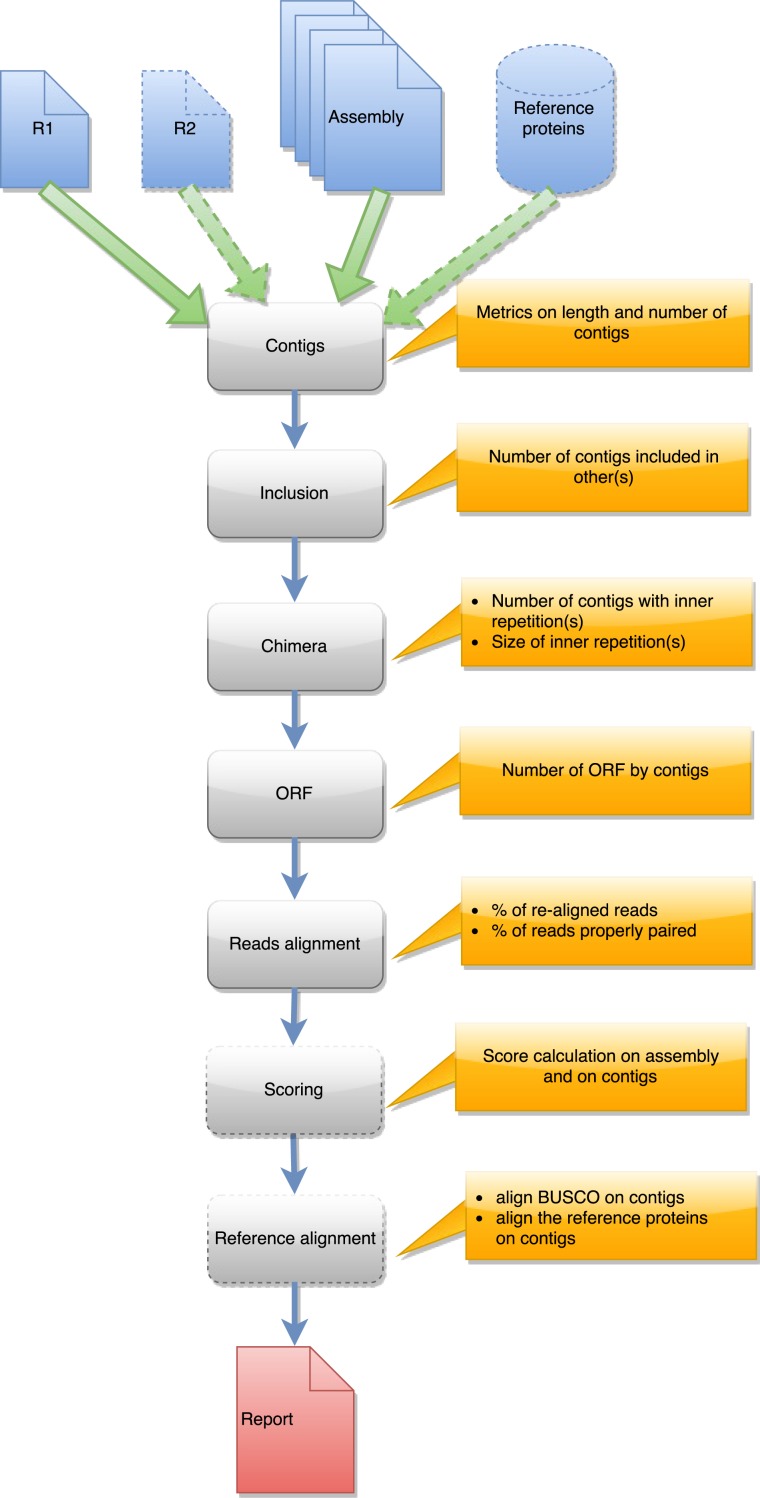
DRAP is written in Perl, Python, and shell. The software is a set of three command-line tools respectively called runDrap, runMeta and runAssessment. runDrap performs the assembly including compaction and correction. It produces a contig set but also a HTML log report presenting different assembly metrics. runAssessment compares different contig sets and gathers the results in a global report. runMeta merges and compacts different contigs sets and should be used for very large datasets for which memory or CPU requirements do not enable a unique global assembly or for highly complex datasets. The modules chained by each tool are presented in a graphical manner in Figs. 1, 2 and 3. Details on the compaction, correction and quality assessment steps of the tools are described hereafter. All software versions, parameters and corresponding default values are presented in Table S1.
Figure 1. Steps in runDRAP workflow.
This workflow is used to produce an assembly from one sample/tissue/development stage. It take as input R1 from single-end sequencing or R1 and R2 from paired-end sequencing and eventually a reference proteins set from closest species with known proteins.
Figure 2. Steps in runMeta workflow.
This workflow is used to produce a merged assembly from several samples/tissues/development stage outputted by runDRAP. Inputs are runDRAP output folders and eventually a reference protein set.
Figure 3. Steps in runAssessment workflow.
This workflow is used to evaluate quality for one assembly or for compare several assemblies produced from the same dataset. Inputs are the assembly/ies, R1 and eventually R2, and a reference protein set.
Contig set compaction
Contig compaction removes redundant and lowly expressed contigs. Four different approaches are used to compact contig sets. The first is only implemented for Oases assemblies and corresponds to the sub-selection of only one contig per locus (NODE) produced by the assembler. Oases resolves the connected component of the de Bruijn graph and for complex sub-graphs generates several longest paths corresponding to different possible forms. These forms have shown (https://sites.google.com/a/brown.edu/bioinformatics-in-biomed/velvet-and-oases-transcriptome) to correspond to subpart of the same transcript, which are usually included one in another. Oases provides the locus (connected component of the assembly graph) of origin of each contig as well as its length and depth. The Oasesv2.0.4BestTransChooser.py script sub-selects the longest and most covered contig of a locus. The second compaction method removes contigs included in longer ones. CD-HIT-EST (Fu et al., 2012) orders the contigs by length and removes all the included ones given identity and coverage thresholds. The third method elongates the contigs through a new assembly step. TGICL (Pertea et al., 2003) performs this assembly in DRAP. The last approach filters contigs using their length or the length of their longest ORF if users are only interested in coding transcripts, and using read coverage according to the idea that lowly covered contigs often correspond to noise. A last optional filter selects contigs using their TransRate quality score when above the calculated threshold (–optimize parameter). By default, runDrap produces eight contigs sets, four include only protein coding transcripts and four others contain all transcripts. Each group comprises a contig set filtered for low coverage with respectively 1, 3, 5 and 10 fragments per kilobase per million (FPKM) thresholds.
Compaction favors assemblies having contigs with multiple ORFs. Because a unique ORF is expected for contig annotation, DRAP splits multi-transcript chimera in mono-ORF contigs.
runMeta also performs a three step compaction of the contigs. The first is based on the contig nucleotide content and uses CD-HIT-EST. The second run CD-HIT on the protein translation of the longest ORF found by EMBOSS getorf. The third, in the same way as runDrap, filters contigs using their length (global or longest ORF), their expression level and optionally their TransRate score producing the eight result files described in the previous paragraph.
Contig set corrections
Contig correction splits chimeras, removes duplicated parts, removes insertions, deletion and polyA/T tails. DRAP corrects contigs in three ways. It first searches self-chimera and removes them by splitting contigs in parts or removing duplicated chimeric elements. An in house script aligns contigs on themselves using bl2seq and keeps only matches having an identity greater or equal to 96%. A contig is defined as a putative chimera if (i) the longest self-match covers at least 60% of the contig length or (ii) the sum of partial non-overlapping self-matches covers at least 80% of its length. In the first case, the putative chimera is split at the start position of the repeated block. In the second case, the contig is only a repetition of a short single block and is therefore discarded. For the second correction step, DRAP searches substitutions, insertions and deletions in the read realignment file. When found it corrects the consensus according to the most represented allele at a given position. Low read coverage alignment areas are usually not very informative therefore only positions having a minimum depth of 10 reads are corrected. The manual assessment made on DRAP assemblies has shown that a second path of this algorithm improves consensus correction. Part of the reads change alignment location after the first correction. runDrap, consequently, runs this step twice.
The last correction script eases the publication of the contig set in TSA (https://www.ncbi.nlm.nih.gov/genbank/tsa): NCBI transcript sequence assembly archive. TSA stores the de novo assembled contig sets of over 1300 projects. In order to improve the data quality, it performs several tests before accepting a new submission. These tests search for different elements such as sequencing adapters or vectors, polyA or polyT and stretches of unknown nucleotides (N). The thresholds used by TSA are presented at https://www.ncbi.nlm.nih.gov/genbank/tsaguide. DRAP performs the same searches on the contig set and corrects the contigs when needed.
Quality assessment
All three workflows create an HTML report. The report is a template including HighCharts (http://www.highcharts.com) graphics and tables using JSON files as database. These files are generated by the different processing steps. The report can therefore also be used to monitor processing progression. Each graphic included in the report can be downloaded in PNG, GIF, PDF or SVG. Some of the graphics can be zoomed in by mouse selecting the area to be enlarged. The report tables can be sorted by clicking on the column headers and exported in CSV format. For runDrap and runMeta, the reports present results of a single contig file.
runAssessment processes one or several contig files and one or several read files. It calculates classical contig metrics, checks for chimeras, searches alignment discrepancies, produces read and fragment alignment rates and assess completeness using an external global reference running BUSCO. If provided, it aligns a set of proteins on the contigs to measure their overlap. Last, it runs TransRate, a contig validation software using four alignment linked quality measures to generate a global quality criterion for each contig and for the complete set. runAssessment does not modify the contig set content but enables users to check and select the best candidate between different assemblies.
Parallel processing and flow control
DRAP runs on Unix machines or clusters. Different steps of the assembly or assessment process are run in parallel mode, if the needed computer infrastructure is available. All modules have been implemented to take advantage of an SGE compliant HPC environment. They can be adapted to other schedulers through configuration file modification.
DRAP first creates a set of directories and shell command files and then launches these files in the predefined order. The ‘–write’ command line parameter forces DRAP to stop after the first step. At this stage, the user can modify the command files for example to set parameters which are not directly accessible from runDRAP, runMeta or runAssessment and then launch the process with the ‘–run’ command line option.
DRAP checks execution outputs at each processing step. If an error has occurred, it adds an error file to the output directory indicating at which step of the processing it happened. After correction, DRAP can be launched again and it will scan the result directory and restart after the last error free step. The pipeline can easily be modified to accept other assemblers by rewriting the corresponding wrapper using the input files and producing correctly named output files.
Results and Discussion
DRAP has been tested on seven different datasets corresponding to five species. These datasets are presented in Table 1 and include five real datasets (Arabidopsis thaliana: At, Bos taurus: Bt, Drosophila melanogaster: Dm, Danio rerio: Dr and Homo sapiens: Hs), one set comprising a large number of diverse samples (Danio rerio multi samples: Dd) and one simulated dataset (Danio rerio simulated: Ds). The simulated reads have been produced using rsem-simulate-reads (version rsem-1.2.18) (Li & Dewey, 2011). The theta0 value was calculated with the rsem-calculate-expression program on read files from the Danio rerio pineal gland sample (SRR1048059). Table 1 also presents for each dataset: the number, length, type (paired or not) and strandedness of the reads, the public accession number, the tissue and experimental condition of origin. The results presented hereafter compare the metrics collected from Trinity, Oases, DRAP Trinity and DRAP Oases assemblies of the six first datasets. The multi sample dataset has been used to compare a strategy in which all reads of the different samples are gathered and processed as one dataset (pooled) to a strategy in which the assemblies are performed by sample and the resulting contigs joined afterwards (meta-assembly). The same assembly pipeline has been used in both strategies, except the contig set merging step, which is specific to the meta-assembly strategy.
Table 1. Datasets.
| Name | Species | Layout | Library | Protocol | ||||
|---|---|---|---|---|---|---|---|---|
| Paired | Stranded | Length (nt) | Nb R1 | SRA ID | Tissue | Condition | ||
| At | Arabidopsis thaliana | Yes | – | 100 | 32,041,730 | SRR1773557 | Root | Full nutrition |
| Yes | – | 100 | 30,990,531 | SRR1773560 | Shoot | Full nutrition | ||
| Yes | – | 100 | 24,898,527 | SRR1773563 | Root | N starvation | ||
| Yes | – | 100 | 54,344,171 | SRR1773569 | Flower | Full nutrition | ||
| Yes | – | 150 | 31,467,967 | SRR1773580 | Shoot | N starvation | ||
| Bt | Bos taurus | Yes | No | 100 | 30,140,101 | SRR2635009 | Milk | Day 70 with low milk production |
| Yes | No | 75 | 15,339,206 | SRR2659964 | Endometrium | – | ||
| Yes | Yes | 50 | 13,542,516 | SRR2891058 | Oviduct | – | ||
| Dd | Danio rerio | Yes | No | 100 | 35,368,936 | SRR1524238 | Brain | 5 months female |
| 54,472,116 | SRR1524239 | Gills | 5 months female | |||||
| 85,672,616 | SRR1524240 | Heart | 5 months male and female | |||||
| 34,032,976 | SRR1524241 | Muscle | 5 months female | |||||
| 59,248,034 | SRR1524242 | Liver | 5 months female | |||||
| 46,371,614 | SRR1524243 | Kidney | 5 months male and female | |||||
| 96,715,965 | SRR1524244 | Bones | 5 months female | |||||
| 43,187,341 | SRR1524245 | Intestine | 5 months female | |||||
| 55,185,501 | SRR1524246 | Embryo | 2 days embryo | |||||
| 24,878,233 | SRR1524247 | Unfertilized eggs | 5 months female | |||||
| 22,026,486 | SRR1524248 | Ovary | 5 months female | |||||
| 59,897,686 | SRR1524249 | Testis | 5 months male | |||||
| Dm | Drosophila melanogaster | Yes | Yes | 75 | 21,849,652 | SRR2496909 | Cell line R4 | Time P17 |
| 21,864,887 | SRR2496910 | Cell line R4 | Time P19 | |||||
| 20,194,362 | SRR2496918 | Cell line R5 | Time P17 | |||||
| 22,596,303 | SRR2496919 | Cell line R5 | Time P19 | |||||
| Dr | Danio rerio | Yes | No | 100 | 5,072,822 | SRR1048059 | Pineal gland | Light |
| 8,451,113 | SRR1048060 | Pineal gland | Light | |||||
| 8,753,789 | SRR1048061 | Pineal gland | Dark | |||||
| 7,420,748 | SRR1048062 | Pineal gland | Dark | |||||
| 9,737,614 | SRR1048063 | Pineal gland | Dark | |||||
| Ds | Danio rerio | Yes | No | 100 | 30,000,000 | Simulated | – | – |
| Hs | Homo sapiens | No | No | 25–50 | 15,885,224 | SRR2569874 | TK6 cells | pretreated with the protein kinase C activating tumor |
| 15,133,619 | SRR2569875 | TK6 cells | pretreated with the protein kinase C activating tumor | |||||
| 19,312,543 | SRR2569877 | TK6 cells | pretreated with the protein kinase C activating tumor | |||||
| 21,956,840 | SRR2569878 | TK6 cells | pretreated with the protein kinase C activating tumor | |||||
Summary Table 2 and Table 3 present the metrics collected for the six first datasets. Table 2 provides metrics related to compaction and correction as Table 3 includes validation metrics and Table 4 collects all three metric types for pooled versus meta-assembly strategies.
Table 2. Compaction and correction in DRAP and standard assembler.
| Dataset | Assembler | Nb contigs | N50 (nt) | L50 (nt) | Sum(nt) | Median length (nt) | Included contigs (%) | Contigs with multi-ORF (%) | Contigs with Multi-prot (%) | Chimeric Contigs (%) | Contigs with Bias* (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| At | Oases | 381,440 | 2,971 | 92,020 | 843,329,264 | 1,816 | 72.75 | 27.89 | 0.26 | 0.80 | 13.88 |
| DRAP Oases | 32,269 | 2,014 | 9,563 | 56,122,047 | 1,547 | 0.00 | 0.24 | 1.40 | 0.04 | 2.78 | |
| Trinity | 95,008 | 2,198 | 19,140 | 130,969,737 | 991 | 4.05 | 15.63 | 1.22 | 0.20 | 11.29 | |
| DRAP Trinity | 54,923 | 1,761 | 15,857 | 80,258,659 | 1,287 | 0.00 | 0.20 | 0.52 | 0.00 | 2.68 | |
| Bt | Oases | 147,163 | 2,739 | 31,441 | 269,085,141 | 1,359 | 71.19 | 7.45 | 0.06 | 0.66 | 6.29 |
| DRAP Oases | 29,685 | 2 441 | 6 029 | 47,727,730 | 1 111 | 0.00 | 0.28 | 0.32 | 0.03 | 1.23 | |
| Trinity | 89,520 | 2,184 | 12,080 | 90,989,611 | 431 | 4.12 | 3.69 | 0.17 | 0.12 | 5.98 | |
| DRAP Trinity | 46,561 | 2,129 | 9,183 | 64,809,448 | 927 | 0.00 | 0.23 | 0.14 | 0.00 | 1.50 | |
| Dm | Oases | 178,696 | 2,220 | 29,086 | 232 776 717 | 756 | 75.48 | 5.14 | 0.18 | 0.35 | 13.11 |
| DRAP Oases | 21,550 | 2,309 | 3,674 | 29,372,261 | 804 | 0.00 | 0.09 | 0.45 | 0.06 | 2.27 | |
| Trinity | 55,214 | 2,266 | 7,126 | 57,209,890 | 438 | 5.19 | 4.58 | 0.95 | 0.22 | 13.33 | |
| DRAP Trinity | 27,236 | 2 146 | 5 240 | 37,249,612 | 914 | 0.00 | 0.07 | 0.31 | 0.00 | 3.59 | |
| Dr | Oases | 702,640 | 2,715 | 114,042 | 1,059,904,844 | 857 | 70.99 | 2.80 | 0.01 | 1.39 | 11.52 |
| DRAP Oases | 46,831 | 2,757 | 9,046 | 82,268,872 | 1,173 | 0.00 | 0.15 | 0.27 | 0.16 | 13.05 | |
| Trinity | 126,210 | 1 279 | 21,003 | 96,279,046 | 418 | 5.56 | 0.81 | 0.08 | 0.56 | 23.63 | |
| DRAP Trinity | 58,114 | 1,644 | 13 022 | 68,900,396 | 866 | 0.00 | 0.07 | 0.12 | 0.00 | 7.41 | |
| Ds | Oases | 131,982 | 2,975 | 28,618 | 280,469,694 | 1 619 | 75.05 | 3.05 | 0.06 | 0.14 | 4.07 |
| DRAP Oases | 21 191 | 3 000 | 4,872 | 46,994,928 | 1,744 | 0.00 | 0.08 | 0.25 | 0.02 | 1.10 | |
| Trinity | 40,335 | 2,398 | 7,159 | 58,571,859 | 910 | 3.12 | 1.82 | 0.37 | 0.09 | 6.47 | |
| DRAP Trinity | 31,113 | 2,381 | 6,492 | 51,580,407 | 1,205 | 0.00 | 0.04 | 0.14 | 0.00 | 1.15 | |
| Hs | Oases | 101,271 | 2,048 | 20,131 | 132,681,065 | 895 | 55.73 | 5.55 | 0.03 | 0.11 | 7.51 |
| DRAP Oases | 30,201 | 1,880 | 5,542 | 34,670,862 | 540 | 0.00 | 0.15 | 0.08 | 0.00 | 0.68 | |
| Trinity | 57,195 | 1,687 | 7,843 | 47,639,190 | 384 | 2.63 | 2.85 | 0.12 | 0.09 | 5.79 | |
| DRAP Trinity | 39,489 | 1,705 | 6,621 | 38,557,758 | 540 | 0.00 | 0.11 | 0.06 | 0.00 | 0.59 |
Notes.
Contigs with consensus variations corrected by DRAP.
Bold values are “best in class” values between raw and DRAP assemblies.
Table 3. Validation DRAP against standard assembler.
| Dataset | Assembler | % contigs by ORF count | Contigs with Complete ORF (%) | % contigs by Proteins count | Nb reference Proteins aligned | Reads mapping (%) | TransRate score * 100 | |||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 0 | 1 | Mapped | Properly paired | |||||
| At | Oases | 18.96 | 53.15 | 65.72 | 94.27 | 5.57 | 23 457 | 97.18 | 90.33 | 2.39 |
| DRAP Oases | 9.90 | 89.86 | 72.38 | 39.38 | 59.22 | 20 895 | 96.53 | 90.21 | 33.16 | |
| Trinity | 38.97 | 45.40 | 40.32 | 81.09 | 17.69 | 20 290 | 93.81 | 85.78 | 10.04 | |
| DRAP Trinity | 13.89 | 85.91 | 55.51 | 69.85 | 29.64 | 17 916 | 92.99 | 85.44 | 24.77 | |
| Bt | Oases | 36.07 | 56.48 | 28.29 | 93.33 | 6.61 | 10 560 | 90.53 | 87.20 | 2.71 |
| DRAP Oases | 32.59 | 67.13 | 25.70 | 67.63 | 32.05 | 10 456 | 91.03 | 88.59 | 23.30 | |
| Trinity | 64.13 | 32.18 | 15.33 | 89.48 | 10.35 | 10 313 | 92.18 | 86.66 | 4.99 | |
| DRAP Trinity | 38.55 | 61.23 | 24.86 | 79.95 | 19.91 | 10 144 | 91.03 | 85.97 | 13.51 | |
| Dm | Oases | 46.19 | 48.67 | 20.27 | 96.43 | 3.39 | 6 873 | 92.86 | 83.24 | 2.21 |
| DRAP Oases | 48.80 | 51.11 | 31.45 | 70.30 | 29.25 | 6 731 | 92.02 | 82.21 | 41.17 | |
| Trinity | 67.53 | 27.89 | 18.49 | 89.63 | 9.42 | 6 494 | 93.24 | 85.07 | 17.56 | |
| DRAP Trinity | 45.94 | 53.99 | 32.23 | 77.76 | 21.93 | 6 358 | 85.77 | 78.09 | 34.23 | |
| Dr | Oases | 56.81 | 40.39 | 23.37 | 97.98 | 2.01 | 15 186 | 85.73 | 75.16 | 0.67 |
| DRAP Oases | 40.20 | 59.65 | 33.43 | 70.89 | 28.84 | 14 901 | 88.26 | 82.84 | 25.19 | |
| Trinity | 66.76 | 32.43 | 9.79 | 92.34 | 7.58 | 10 734 | 84.11 | 75.70 | 5.81 | |
| DRAP Trinity | 39.74 | 60.19 | 20.16 | 82.44 | 17.44 | 11 272 | 81.33 | 75.43 | 18.25 | |
| Ds | Oases | 24.52 | 72.43 | 41.60 | 89.47 | 10.47 | 14 929 | 83.62 | 74.34 | 8.56 |
| DRAP Oases | 12.80 | 87.11 | 53.73 | 35.56 | 64.19 | 14 913 | 90.32 | 88.22 | 59.08 | |
| Trinity | 37.72 | 60.46 | 30.29 | 67.37 | 32.26 | 14 394 | 88.79 | 85.37 | 38.77 | |
| DRAP Trinity | 22.85 | 77.11 | 37.65 | 57.53 | 42.33 | 14 364 | 88.28 | 85.59 | 50.51 | |
| Hs | Oases | 44.51 | 49.94 | 21.18 | 93.04 | 6.93 | 7 554 | 88.30 | NA | NA |
| DRAP Oases | 46.95 | 52.91 | 20.06 | 77.28 | 22.64 | 7 463 | 86.90 | NA | NA | |
| Trinity | 69.02 | 28.13 | 11.70 | 88.53 | 11.35 | 7 199 | 86.76 | NA | NA | |
| DRAP Trinity | 55.48 | 44.41 | 16.07 | 83.46 | 16.48 | 7 124 | 84.08 | NA | NA | |
Notes.
Bold values are “best in class” values between raw and DRAP assemblies.
Table 4. Pooled samples vs meta-assembly strategies on the Danio rerio multi samples dataset (Dd)).
| Assembly strategy | Pooled Oases | Meta Oases | Pooled Trinity | Meta Trinity | |
|---|---|---|---|---|---|
| Compaction | |||||
| Nb seq | 42,726 | 43,049 | 62,327 | 65 271 | |
| N50 (nt) | 3,565 | 3,379 | 2,027 | 2 237 | |
| L50 (nt) | 10,409 | 9,259 | 14,956 | 13,106 | |
| Sum (nt) | 114,371,598 | 99,928,206 | 94,993,910 | 98,421,439 | |
| Median length (nt) | 2,182 | 1,766 | 1,217 | 1,052 | |
| Contigs with multi-ORF (%) | 0.33 | 0.50 | 0.13 | 0.17 | |
| Contigs with multi-prot (%) | 1.39 | 1.73 | 0.64 | 0.95 | |
| Correction | |||||
| Chimeric contigs (%) | 0.11 | 0.21 | 0.00 | 0.00 | |
| Contigs with bias* (%) | 75.19 | 68.00 | 58.79 | 61.88 | |
| Validation | |||||
| % contigs by ORF count | 0 | 24.79 | 38.77 | 37.24 | 50.63 |
| 1 | 74.88 | 60.72 | 62.63 | 49.20 | |
| Contigs with complete ORF (%) | 61.84 | 46.36 | 38.80 | 31.55 | |
| % contigs by proteins count | 0 | 58.52 | 57.15 | 75.23 | 72.02 |
| 1 | 40.09 | 41.13 | 24.13 | 27.03 | |
| Nb reference proteins aligned | 32,367 | 35,432 | 26,041 | 33,385 | |
| Reads mapping (%) | Mapped | 87.38 | 87.57 | 77.82 | 85.19 |
| Properly paired | 78.88 | 80.13 | 70.13 | 77.30 | |
| TransRate score * 100 | 28.66 | 29.49 | 17.97 | 23.36 | |
Notes.
contigs with consensus variations corrected by DRAP.
Bold values are “best in class” values between raw and DRAP assemblies.
Contig set compaction
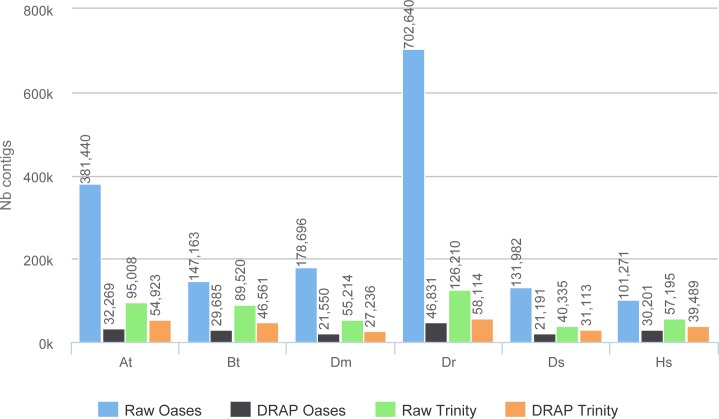
The improvement in compactness is measured by three criteria. The first is the number of assembled contigs presented in Fig. 4. The differences between raw Oases and Trinity assemblies and DRAP assemblies are very significant ranging from 1.3 fold to 15 fold. The impact of DRAP on Oases assemblies (from 3.4 to 15 fold) is much more significant than on Trinity assemblies (from 1.3 to 2,2 fold). Oases multi-k assembly strategy generates a lot of redundant contigs which are not removed at the internal Oases merge step. The second criterion is the percentage of inclusions, i.e., contigs which are part of longer ones. Oases and Trinity inclusion rate range respectively from 55 to 75% and from 2.3 to 5.5% (Table 2). Because of its inclusion removal step this rate is null for DRAP assemblies. The last compaction criteria presented here is the total number of nucleotides in the contigs. The ratios between raw and DRAP assembly sizes for Oases and Trinity range respectively from 3.4 to 14.8 fold and from 1.1 and 2.6 fold (Table 2). All these metrics show that DRAP produces less contigs with less redundancy resulting in an assembly with a much smaller total size.
Figure 4. Number of contigs.
The figure shows for the different assemblers (Oases, DRAP Oases, Trinity, DRAP Trinity) the number of contigs produced for each dataset.
Another metric that can be negatively correlated to compactness, but has to be taken into account, is the number of multi-ORF contigs found in the assemblies. The ratios of multi-ORF contigs found between raw and DRAP assemblies range from 11 and 116 folds (Table 2). DRAP multi-transcript chimera splitting procedure improves significantly this criterion.
In order to check if the compaction step only selects one isoform per gene, we compared the number of genes with several transcripts aligning on different contigs before and after DRAP. A transcript is linked to a contig if its best blat hit has over 90% query identity and 90% query coverage. The test has been performed on the Dr and the Ds datasets assembled with Oases and Trinity. The number of alternative spliced isoforms decreases more, with or without DRAP, in the Oases than in the Trinity assemblies (Table 5). This reduction is of 69% and 23% in the real dataset (Dr) and 83% and 18% in the simulated dataset for Oases and Trinity respectively. However, the spliced forms reduction does not impact the gene representation in the compacted sets (Table 5). Remarkably, the gene representation is increased for the real dataset when processed with DRAP Oases. This results from the different merging strategies used by Oases and DRAP Oases. Using TGICL, DRAP is able, in some cases, to correctly merge gene parts which have been generated by the Oases multi-k assemblies and this more efficiently than the build-in Oases merge procedure.
Table 5. Compaction vs gene representation on Danio rerio simulated dataset (Ds) and Danio rerio dataset (Dr).
| Dataset | Assembly | Nb seq | All genes | Multi-isoform genes | Raw/DRAP assemblies | |
|---|---|---|---|---|---|---|
| All genes (%) | Multi-isoform genes (%) | |||||
| Ds | Raw Oases | 131,982 | 14,396 | 3,593 | −1.74 | −82.99 |
| DRAP Oases | 21,191 | 14,145 | 611 | |||
| Raw Trinity | 40,335 | 12,457 | 1,792 | −2.04 | −17.97 | |
| DRAP Trinity | 31,113 | 12,203 | 1,470 | |||
| Dr | Raw Oases | 702,640 | 11,613 | 2,177 | +10.40 | −69.09 |
| DRAP Oases | 46,831 | 12,821 | 673 | |||
| Raw Trinity | 126,210 | 8,310 | 801 | −2.33 | −22.60 | |
| DRAP Trinity | 58,114 | 8,116 | 620 | |||
Notes.
Bold values are “best in class” values between raw and DRAP assemblies.
Contig set corrections
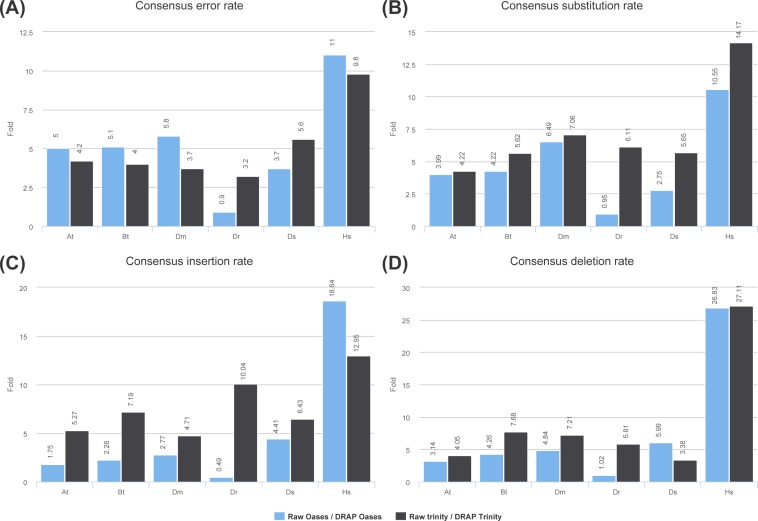
DRAP corrects contigs in two ways: removing self-chimera and rectifying consensus substitutions, insertions and deletions when the consensus does not represent the major allele at the position in the read re-alignment file. Self-chimeras appear in Oases and Trinity contig sets at rate ranging respectively from 0.11 to 1.39 and from 0.09 to 0.56%. In DRAP, the corresponding figures drop to 0.01 to 0.16 and 0.00 to 0.01%. Concerning consensus correction only five datasets can be taken into account i.e., At, Bt, Dm, Ds and Hs. Dr Oases assembly generates such a large number of contigs and total length that it decreases significantly the average coverage and therefore limits the number of positions for which the correction can be made. As shown in Fig. 5 and Table S2, the Dr dataset is an outlier concerning this criteria. Regarding the five other datasets raw versus DRAP correction rates range from 1.7 to 18.6 for insertions, 3.1 to 27.1 for deletions and 2.7 to 14.1 for substitutions. DRAP correction steps lowers significantly the number of positions for which the consensus does not correspond to the major allele found in the alignment. In order to check the positive impact of the correction step, the Danio rerio reference proteome has been aligned to the simulated dataset (Ds) contigs before and after correction. 94.5% of DRAP Oases contigs and 86.2% of DRAP Trinity contigs which have been corrected, have improved alignment scores (Data S1 section “Contig set correction step assessment”).
Figure 5. Consensus error rates.
(A) presents the ratio of the global error rates between raw and DRAP assemblies for each dataset (data from Table 2 colum 12). (B), (C) and (D) present the ratio of the error rates respectively for substitution, insertions and deletions between raw and DRAP assemblies for each dataset (data from Table S2).
Assembly quality assessment
The two previous parts have shown the beneficial impacts of DRAP on the assembly compactness and error rates but this should not impair quality metrics such as read and read pairs alignment rates, number of ORFs, complete ORFs found in the contigs, number of proteins of the known proteome mapped on the contigs or TransRate marks.
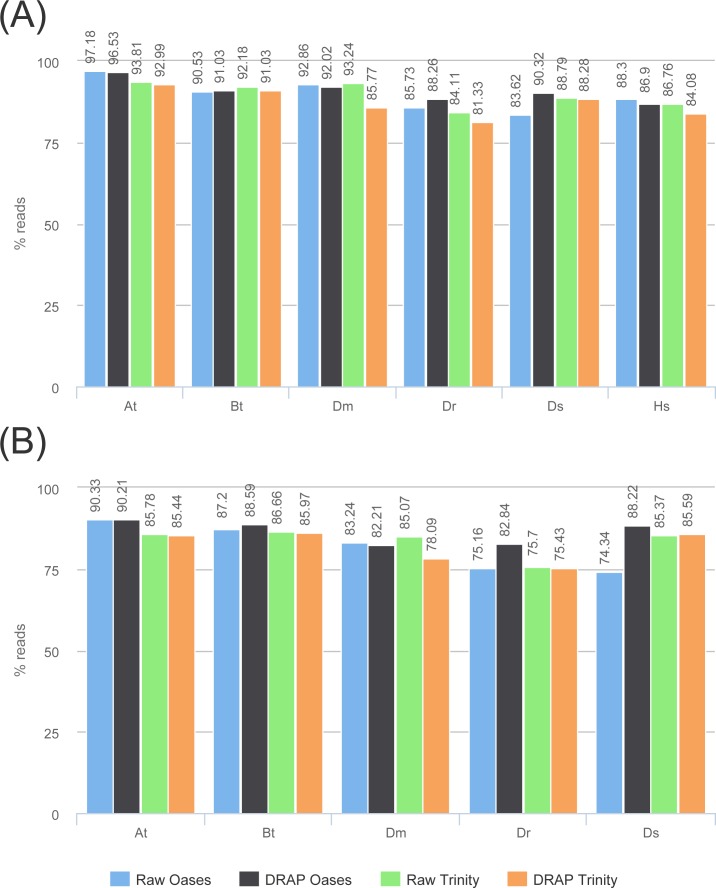
Read and read pair alignment rates differences between raw and DRAP assemblies are usually very low, between 1 and 2% and can sometimes be in favor of DRAP (Fig. 6) . In our test sets, the difference is significant (7.5%) for Dm when comparing Trinity to DRAP Trinity. This comes from the removal by DRAP of a highly expressed transcript (Ensembl: FBtr0100888 mitochondrial large ribosomal RNA) because that does not fulfill the criteria of having at least one 200 base pairs long ORF despite having over 11M reads aligned on the corresponding contig in the Trinity assembly. DRAP Oases assembly was not impacted because it builds a longer contig for this transcript with a long enough ORF to be selected in the additional part.
Figure 6. Reads re-alignment rates.
(A) and (B) show respectively the alignment rates for reads and read pairs for the four assemblies of each dataset.
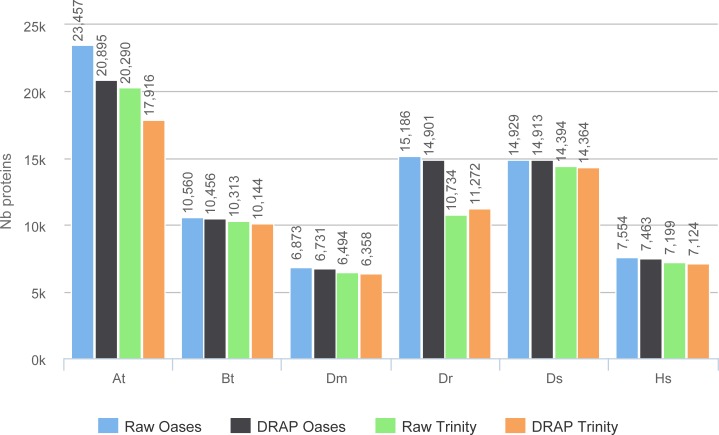
The reference proteome has been aligned on the contigs and matches with over 80% identity and 80% protein coverage have been counted (Fig. 7). These figures give a good overview of the amount of well-reconstructed proteins in the contig sets. For all datasets except one (At) the number of proteins are very close between raw and DRAP results. For this At dataset the difference is of 12.2% for Oases and 13.2% for Trinity. This is due to the FPKM filtering step performed by DRAP and the expression profile of this dataset that mixes different tissues (root, shoot and flower) and conditions (full nutrition and starvation). Contigs corresponding to low expression in one condition do not have sufficient overall expression to pass DRAP expression filter threshold and are therefore eliminated from the final set. Mixed libraries can benefit from the meta-assembly approach presented in the next section.
Figure 7. Proteins realignment rates.
The figure shows the number of proteins which have been aligned on the contig sets with more than 80% identity and 80% coverage for each assembler and dataset.
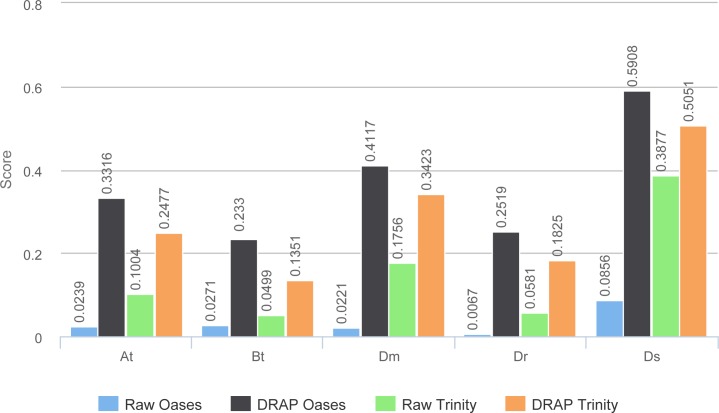
TransRate global scores (Fig. 8) are much higher for DRAP assemblies compared to raw ones. This comes from the compaction performed by DRAP and the limited impact it has on the read alignment rate.
Figure 8. TransRate scores.
The figure presents the TransRate scores of the four assemblers for each dataset.
DRAP has limited negative effect on the assembly quality metrics, and sometimes even improves some of them. Some cases in which multiple libraries are mixed with very distinct conditions can affect the results and it is good practice to systematically compare raw and DRAP assemblies. It is also to be noticed that Oases multi-k strategy outperforms Trinity for all datasets regarding the number of well-reconstructed proteins.
Pooled versus meta-assembly strategies
In the previous sections we compared results from raw and DRAP assemblies. This section compares results from pooled versus meta-assembly strategies both using the DRAP assembly pipeline (Table 4). Because of the read re-alignment filtering thresholds used in DRAP, we expect different metrics between a pooled assembly and merged per sample assembly (meta-assembly). DRAP includes the runMeta workflow, which performs this task.
Differences in compaction and correction are more important between Trinity and Oases than between pooled versus meta-assembly. Pooled assemblies collect significantly worse results for the number of reference proteins and number of read pairs aligned on the contigs. This comes from the filtering strategy which eliminates low-expressed contigs of a given condition when merging all the samples but will keep these contigs in a per sample assembly and meta-assembly strategy. Therefore, we recommend using runMeta when the assembly input samples mix distinct conditions with specific and variable expression patterns.
Assemblies fidelity check using simulated reads
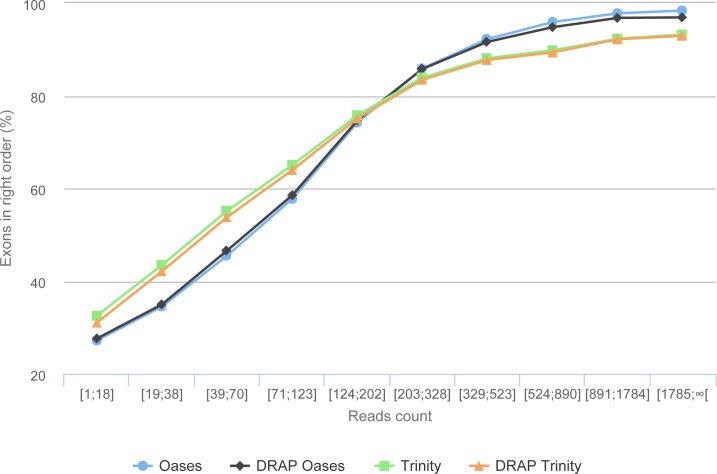
The simulation process links each read with its transcript of origin. With this information it is possible to link contigs and transcripts. Here, the transcript-contig link was calculated using exon content and order in both sets (method explained in Data S1). The results presented in Table 6 first shows that the assembly process loses between 15.76 and 19.97% of the exons compared to the initial transcript set. This loss is close to 22% for all assemblies when the exon order is taken into account. As shown in Fig. 9, this is mainly the case for transcripts with low read coverage. The figures show once more that DRAP has a very limited negative impact on number of retrieved exons in correct order.
Table 6. Structure validation on Danio rerio simulated dataset (Ds).
| Assembly | Retrieved exons | Exons in Right contig | Exons in Right order | Contigs with More than 1 gene | Max number Of genes by contig |
|---|---|---|---|---|---|
| Real assembly | 99.81% | 99.81% | 99.50% | 0.16% (46) | 5 |
| Raw Oases | 80.03% | 77.83% | 77.61% | 2.77% (537) | 221 |
| DRAP Oases | 80.21% | 77.54% | 77.29% | 4.13% (671) | 203 |
| Raw Trinity | 84.24% | 77.30% | 77.10% | 3.65% (717) | 339 |
| DRAP Trinity | 83.30% | 76.65% | 76.47% | 3.17% (602) | 327 |
Notes.
Bold values are “best in class” values between raw and DRAP assemblies.
Figure 9. Gene reconstruction versus expression depth using simulated reads.
The figure presents the proportion of correctly build transcripts (method presented in Data S1 section “Contig validation using exon re-alignment and order checking”) versus the read count per transcript.
Table 6 shows the number of contigs linked to more than one gene. DRAP compaction and ORF splitting feature could have an antagonist impact for this criteria. But depending on the assembler, the figures are in favor or not of DRAP.
Table 6 also presents the maximum number of genes linked to a single contig. These clusters correspond to zink finger gene family members which have been assembled as single contig. Between 92.3 and 93.7% of the clustered transcripts belong to this family. De novo assembly tools are not able to distinguish transcript originating from different gene when the nucleotide content is highly similar.
Conclusion
Different software packages are available to assemble de novo transcriptomes from short reads. Trinity and Oases are commonly used packages which produce good quality references. DRAP assembly pipeline is able to compact and correct contig sets with usually very low quality loss. As no package out performs the others in all cases, producing different assemblies and comparing their metrics is a good general practice.
Supplemental Information
Abbreviations
- RMP
Reads per million
- ORF
Open reading frame
- TSA
Transcript sequences archive
- NCBI
National Center for Bio-Informatics
- DRAP
De novo Rna-seq Assembly Pipeline
- PNG, GIF, PDF or SVG
Image file formats.
Funding Statement
This work was supported by funds from the ANR (http://www.agence-nationale-recherche.fr/) in the frame of the PhyloFish (ANR-10-GENM-017) and the France Génomique (ANR10-INBS-09-08) projects. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Cédric Cabau conceived and designed the experiments, performed the experiments, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Frédéric Escudié performed the experiments, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Anis Djari performed the experiments.
Yann Guiguen and Julien Bobe analyzed the data.
Christophe Klopp conceived and designed the experiments, wrote the paper, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
De novo RNA-seq Assembly Pipeline: http://www.sigenae.org/drap.
References
- Bens et al. (2016).Bens M, Sahm A, Groth M, Jahn N, Morhart M, Holtze S, Hildebrandt TB, Platzer M, Szafranski K. FRAMA: from RNA-seq data to annotated mRNA assemblies. BMC Genomics. 2016;17:54. doi: 10.1186/s12864-015-2349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly et al. (1989).Chelly J, Concordet JP, Kaplan JC, Kahn A. Illegitimate transcription: transcription of any gene in any cell type. Proceedings of the National Academy of Sciences of the United States of America. 1989;86:2617–2621. doi: 10.1073/pnas.86.8.2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson & Oshlack (2014).Davidson NM, Oshlack A. Corset: enabling differential gene expression analysis for de novo assembled transcriptomes. Genome Biology. 2014;15(7):410. doi: 10.1186/s13059-014-0410-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djebali et al. (2012).Djebali S, Davis CA, Merkel A, Dobin A, Lassmann T, Mortazavi A, Tanzer A, Lagarde J, Lin W, Schlesinger F, Xue C, Marinov GK, Khatun J, Williams BA, Zaleski C, Rozowsky J, Rde M, Kokocinski F, Abdelhamid RF, Alioto T, Antoshechkin I, Baer MT, Bar NS, Batut P, Bell K, Bell I, Chakrabortty S, Chen X, Chrast J, Curado J, Derrien T, Drenkow J, Dumais E, Dumais J, Duttagupta R, Falconnet E, Fastuca M, Fejes-Toth K, Ferreira P, Foissac S, Fullwood MJ, Gao H, Gonzalez D, Gordon A, Gunawardena H, Howald C, Jha S, Johnson R, Kapranov P, King B, Kingswood C, Luo OJ, Park E, Persaud K, Preall JB, Ribeca P, Risk B, Robyr D, Sammeth M, Schaffer L, See LH, Shahab A, Skancke J, Suzuki AM, Takahashi H, Tilgner H, Trout D, Walters N, Wang H, Wrobel J, Yu Y, Ruan X, Hayashizaki Y, Harrow J, Gerstein M, Hubbard T, Reymond A, Antonarakis SE, Hannon G, Giddings MC, Ruan Y, Wold B, Carninci P, Guig R, Gingeras TR. Landscape of transcription in human cells. Nature. 2012;489:101–108. doi: 10.1038/nature11233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel-Morgenstern et al. (2013).Frenkel-Morgenstern M, Gorohovski A, Lacroix V, Rogers M, Ibanez K, Boullosa C, Andres Leon E, Ben-Hur A, Valencia A. ChiTaRS: a database of human, mouse and fruit fly chimeric transcripts and RNA-sequencing data. Nucleic Acids Research. 2013;41:D142–D151. doi: 10.1093/nar/gks1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu et al. (2012).Fu L, Niu B, Zhu Z, Wu S, Li W. CD-HIT: accelerated for clustering the next-generation sequencing data. Bioinformatics. 2012;28:3150–3152. doi: 10.1093/bioinformatics/bts565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabherr et al. (2011).Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q, Chen Z, Mauceli E, Hacohen N, Gnirke A, Rhind N, di Palma F, Birren BW, Nusbaum C, Lindblad-Toh K, Friedman N, Regev A. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nature Biotechnology. 2011;29:644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He et al. (2015).He B, Zhao S, Chen Y, Cao Q, Wei C, Cheng X, Zhang Y. Optimal assembly strategies of transcriptome related to ploidies of eukaryotic organisms. BMC Genomics. 2015;16:65. doi: 10.1186/s12864-014-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaas et al. (2016).Honaas LA, Wafula EK, Wickett NJ, Der JP, Zhang Y, Edger PP, Altman NS, Chris Pires J, Leebens-Mack JH, De Pamphilis CW. Selecting superior de novo transcriptome assemblies: lessons learned by leveraging the best plant genome. PLOS ONE. 2016;11:e0146062. doi: 10.1371/journal.pone.0146062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li & Dewey (2011).Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics. 2011;12:323. doi: 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2014).Li B, Fillmore N, Bai Y, Collins M, Thomson JA, Stewart R, Dewey CN. Evaluation of de novo transcriptome assemblies from RNA-Seq data. Genome Biology. 2014;15(12):553. doi: 10.1186/s13059-014-0553-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbandi et al. (2015).Mbandi SK, Hesse U, Van Heusden P, Christoffels A. Inferring bona fide transfrags in RNA-Seq derived-transcriptome assemblies of non-model organisms. BMC Bioinformatics. 2015;16:58. doi: 10.1186/s12859-015-0492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakasugi et al. (2014).Nakasugi K, Crowhurst R, Bally J, Waterhouse P. Combining transcriptome assemblies from multiple de novo assemblers in the allo-tetraploid plant nicotiana benthamiana. PLOS ONE. 2014;9:e91776. doi: 10.1371/journal.pone.0091776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra, Bradnam & Korf (2007).Parra G, Bradnam K, Korf I. CEGMA: a pipeline to accurately annotate core genes in eukaryotic genomes. Bioinformatics. 2007;23:1061–1067. doi: 10.1093/bioinformatics/btm071. [DOI] [PubMed] [Google Scholar]
- Pertea et al. (2003).Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, Lee Y, White J, Cheung F, Parvizi B, Tsai J, Quackenbush J. TIGR Gene Indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics. 2003;19:651–652. doi: 10.1093/bioinformatics/btg034. [DOI] [PubMed] [Google Scholar]
- Schulz et al. (2012).Schulz MH, Zerbino DR, Vingron M, Birney E. Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics. 2012;28:1086–1092. doi: 10.1093/bioinformatics/bts094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simão et al. (2015).Simão FA, Waterhouse RM, Ioannidis P, Kriventseva EV, Zdobnov EM. BUSCO: assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics. 2015;31(19):3210–3212. doi: 10.1093/bioinformatics/btv351. [DOI] [PubMed] [Google Scholar]
- Smith-Unna et al. (2016).Smith-Unna R, Boursnell C, Patro R, Hibberd J, Kelly S. TransRate: reference free quality assessment of de novo transcriptome assemblies. Genome Research. 2016;26(8):1134–1144. doi: 10.1101/gr.196469.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surget-Groba & Montoya-Burgos (2010).Surget-Groba Y, Montoya-Burgos JI. Optimization of de novo transcriptome assembly from next-generation sequencing Data. Genome Research. 2010;20:1432–1440. doi: 10.1101/gr.103846.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang & Smith (2013).Yang Y, Smith SA. Optimizing de novo assembly of short-read RNA-seq data for phylogenomics. BMC Genomics. 2013;14:328. doi: 10.1186/1471-2164-14-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
De novo RNA-seq Assembly Pipeline: http://www.sigenae.org/drap.