Abstract
Individuals with body dysmorphic disorder (BDD) and obsessive-compulsive disorder (OCD) are categorized within the same major diagnostic group and both show regional brain hyperactivity in the orbitofrontal cortex (OFC) and the basal ganglia during symptom provocation. While recent studies revealed that degree connectivity of these areas is abnormally high in OCD and positively correlates with symptom severity, no study has investigated degree connectivity in BDD. We used functional magnetic resonance imaging (fMRI) to compare the local and distant degree of functional connectivity in all brain areas between 28 unmedicated BDD participants and 28 demographically matched healthy controls during a face-processing task. Correlational analyses tested for associations between degree connectivity and symptom severity assessed by the BDD version of the Yale-Brown obsessive-compulsive scale (BDD-Y-BOCS). Reduced local amygdalar connectivity was found in participants with BDD. No differences in distant connectivity were found. BDD-Y-BOCS scores significantly correlated with the local connectivity of the posterior-lateral OFC, and distant connectivity of the posterior-lateral and post-central OFC, respectively. These findings represent preliminary evidence that individuals with BDD exhibit brain-behavioral associations related to obsessive thoughts and compulsive behaviors that are highly similar to correlations previously found in OCD, further underscoring their related pathophysiology. This relationship could be further elucidated through investigation of resting-state functional connectivity in BDD, ideally in direct comparison with OCD and other obsessive-compulsive and related disorders.
Keywords: body dysmorphic disorder, obsessive-compulsive disorder, amygdala, orbitofrontal cortex, connectivity
1. Introduction
The most recent version of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (American Psychiatric Association, 2013) categorizes body dysmorphic disorder (BDD), a severely impairing (Phillips et al., 2005; Phillips and Diaz, 1997) psychiatric condition characterized by preoccupation with perceived defects in a person’s own appearance, in the chapter of obsessive-compulsive and related disorders (OCRD) (American Psychiatric Association, 2013), after it had been viewed as a somatoform disorder in previous editions. This diagnostic change is based on several overlapping features, including clinical observations that OCRD including BDD are all characterized by obsessive thoughts and/or repetitive, compulsive behaviors. Moreover, the severity of these symptoms can be reliably assessed using analogous psychometric expert ratings in BDD and OCD (Goodman et al., 1989; Phillips et al., 1997).
In addition to these similarities in clinical phenotype, the recent change in classification intensifies efforts studying all OCRD to understand the brain circuitry of compulsivity (van den Heuvel et al., 2015), and motivates the search for neurobiological abnormalities common to intensively (such as OCD) and less intensively studied OCRD (such as BDD). For example, a recent study predicted and confirmed lower striatal dopamine D2/3 receptor availability in BDD based on previous examinations of patients with OCD (Denys et al., 2004; Figee et al., 2014). A further common phenomenon observed in both BDD and OCD is regional hyperactivation patterns in frontostriatal brain areas during symptom provocation. More specifically, relative hyperactivity in the orbitofrontal cortex (OFC) and the striatum has been demonstrated in BDD participants when being presented with their own face compared to familiar faces (Feusner et al., 2010), and abnormally high responses in these areas is a well-known and replicated finding in participants with OCD presented with stimuli triggering their OCD symptom profile (Breiter et al., 1996; Rauch et al., 1994; Simon et al., 2010).
Considering the fundamental intrinsic network structure of the brain, the field of connectomics has received increasing interest in psychiatry recently (Menon, 2011; Taylor, 2014). This discipline uses graph theoretical approaches to compute metrics indexing brain network properties on the basis of structural and functional magnetic resonance imaging (MRI) data (Buckner et al., 2009; Bullmore and Sporns, 2009; Sepulcre et al., 2010). For example, degree connectivity (also termed node degree or centrality) quantifies the number of connections of all regions, and thus identifies the most central areas in the network. Noteworthy, degree connectivity can be quantified both for local and distant connections, and on the basis of resting-state as well as task functional MRI data (Sepulcre et al., 2010). Connectomics is thought to have the potential to provide novel insights into brain correlates of psychopathology (Taylor, 2014), while at the same time allowing testing of predictions inferred from existing brain circuit models of neuropsychiatric conditions. In OCD, abnormally high degree connectivity of the OFC and the basal ganglia (Beucke et al., 2013; Hou et al., 2014), and positive correlations between OCD symptom severity and degree connectivity of the OFC and striatum were found in unmedicated patients. Importantly, contemporary neuroanatomical models of OCD (Milad and Rauch, 2012) and compulsivity (van den Heuvel et al., 2015) suspect abnormalities not only in frontostriatal, but also in limbic circuits, referencing observations of heightened responses of the amygdala in some symptom provocation studies (Breiter et al., 1996; Simon et al., 2014; Simon et al., 2010), and during processing of OCD-related words (van den Heuvel et al., 2005), respectively, in patients with OCD. Still there have been very few neuroimaging studies in BDD, and no study has investigated degree connectivity in participants with BDD to date.
The present study sought to investigate degree connectivity abnormalities in unmedicated participants with BDD using fMRI data acquired during a face-processing task. Even though differences in study design and data acquisition between the present study in BDD and the previous study in OCD (Beucke et al., 2013) did not permit a direct comparison of the two patient groups, we sought to use an established approach (Sepulcre et al., 2010) previously applied in OCD (Beucke et al., 2013) to test whether connectivity abnormalities previously reported in OCD can also be found in BDD participants. Based on the similarities in diagnostic classification, symptomatology and abnormal regional activation patterns, we hypothesized that individuals with BDD would exhibit similar degree connectivity abnormalities previously described in OCD, i.e. higher degree connectivity of the OFC and the basal ganglia in those with BDD as compared to healthy controls, and positive correlations between the degree connectivity of these regions with BDD symptom severity. Further, given observations of amygdala hyperactivity in OCD and the emphasis on limbic circuit alterations in recent neuroanatomical models of OCD and compulsivity, we also tested for altered amygdala connectivity in patients with BDD.
2. METHODS
2.1. Participants
Twenty-eight individuals meeting the DSM-IV diagnosis of BDD who were recruited from the community, as well as 28 mentally healthy controls that were matched for demographic variables (age, gender, and education) participated in the study. All were right-handed. The included participants were all unmedicated; they had not taken psychiatric medications for at least 8 weeks prior to the imaging study. None of the participants were being treated with cognitive-behavioral therapy. All participants had normal/corrected visual acuity, as tested with a Snellen eye chart.
Participants were interviewed by a licensed psychiatrist (J.D.F.) and diagnoses were made using the BDD Diagnostic Module (Phillips, 1995), a 6-item clinician-administered structured interview based on DSM-IV criteria for BDD. The Mini International Neuropsychiatric Interview (MINI) (Sheehan et al., 1998) was used to determine comorbid diagnoses and to screen healthy controls. BDD symptom severity was assessed in BDD participants using the BDD version of the Yale-Brown Obsessive-Compulsive Scale (BDD-YBOCS) (Phillips et al., 1997). Comorbid diagnoses included major depression (n = 12), generalized anxiety disorder (n = 4), dysthymia (n = 3), social phobia (n = 2), and agoraphobia (n = 1). Eleven participants had BDD as their single diagnosis. Table 1 displays demographic data of BDD and control groups. Potential differences regarding demographic variables were explored using t-tests for independent samples. T-values, means and standard deviations of questionnaire data and demographic comparisons are presented in Table 1.
Table 1.
Demographic data of participants with BDD and healthy controls; comparisons are based on two-sample t-tests.
| N = 28 vs. 28 | BDD (M±SD) | Control(M±SD) | T (p value) |
|---|---|---|---|
| Sex [female (male)] | 23 (5) | 24 (4) | |
| Age | 23.0 (4.6) | 22.8 (4.9) | 0.03 (0.98) |
| Education (years) | 14.7 (2.7) | 14.4 (3.4) | 0.15 (0.88) |
| BABS | 14.2 (3.6) | ||
| BDD-Y-BOCS | 30.0 (5.7) | ||
| MADRS | 17.4 (7.7) | 1.8 (1.7) | −11.1 (< 0.001) |
| HAM-A | 10.2 (6.2) | 0.9 (1.3) | −7.0 (< 0.001) |
| Mean interscan movement | 0.05 (0.02) | 0.05 (0.02) | 0.10 (0.92) |
Abbreviations: BDD, body dysmorphic disorder; M, mean; SD, standard deviation; BABS, Brown Assessment of Beliefs Scale; Y-BOCS, Yale-Brown Obsessive Compulsive Scale; MADRS, Montgomery Asberg Depression Scale; HAM-A, Hamilton Anxiety Rating Scale.
BDD participants were eligible if they had a score of ≥20 on the BDD-YBOCS. Exclusion criteria for participants included cardiac pacemakers or other metallic implants or artifacts, pregnancy, neurological illness, prior head trauma, thyroid disorders, or diabetes mellitus. Individuals with comorbid Axis I disorders besides major depression, dysthymia, panic disorder, agoraphobia, social phobia, or generalized anxiety disorder were excluded. These diagnoses were allowed given their frequency in BDD, and our wish to recruit a representative clinical sample, however we required the BDD was the primary diagnosis in all included cases. HC participants could not meet any criteria for Axis I disorders, including substance use disorders. All participants gave written informed consent according to the institutional guidelines before enrollment. The local ethics committee approved the study.
2.2. Clinical Scales and questionnaires
The severity of BDD symptoms was evaluated using the BDD version of the Yale-Brown Obsessive Compulsive Scale (BDD-Y-BOCS) (Phillips et al., 1997). In addition, the Montgomery-Asberg Depression Rating Scale (MADRS) (Montgomery and Asberg, 1979), the Hamilton Anxiety Rating Scale (HAMA) (Hamilton, 1960), and the Brown Assessment of Beliefs Scale (BABS) (Eisen et al., 1998) were administered at the initial assessment. The Edinburgh Handedness Inventory (Oldfield, 1971) was used to classify handedness.
2.3. Task Procedure
Participants engaged in a matching task of pictures of others’ faces frequency low spatial frequency (LSF), normal spatial frequency (NSF), and high spatial frequency (HSF) during the imaging session, as previously described (Feusner et al., 2007). Further, a baseline control condition (CON) was included, consisting of gray ovals which had approximately the same size and luminosity as the faces stimuli.
2.4. MRI Procedures
A total of 133 volumes (T2*-weighted single-shot gradient echo planar imaging sequence) were acquired on a 3 T Siemens Trio system using the following parameters: TR=2500 ms, TE=25 ms, 32 interleaved slices, isotropic 3 mm voxel size, 0.75 mm gap, flip angle= 80 deg, FOV=192 mm, 64 × 64 matrix, aligned parallel to the anterior-posterior commissure line, and 176 anatomical slices were acquired using the MPRAGE sequence (spatial resolution 1×1×1 mm, TR=1900 ms, TE=2.26 ms, flip angle= 9 deg, 256×256 matrix). In order to reduce head motion, participants’ heads were immobilized by foam cushions. Earplugs were provided to attenuate scanner noise.
2.5. Image Processing and Group Analyses
The present study applied the identical degree connectivity data analysis pipeline that has been used previously (Beucke et al., 2013; Buckner et al., 2009; Sepulcre et al., 2010). Image preprocessing included slice time correction, spatial normalization to the atlas space of the Montreal Neurological Institute (MNI), and temporal filtering to retain frequencies below 0.08 Hz. Regression of nuisance variables (six-parameter rigid body head motion, the signal averaged over the whole brain, the lateral ventricles, and over a region centered in the deep cerebral white matter) was used to remove spurious or regionally nonspecific variance. Further, fMRI data was spatially smoothed using a 4 mm FWHM kernel. Using these preprocessed functional runs, the local and distant degree of connectivity in the entire brain was determined through correlation of each voxel’s blood oxygen-level dependent (BOLD) time-series to the BOLD time-series of all other voxels, followed by using the resulting Pearson’s correlation coefficient matrix to count the number of voxels where the correlation with the BOLD time-series exceeds the predefined, optimized statistical threshold of r > .25 that has been used in previous degree connectivity studies (Buckner et al., 2009; Sepulcre et al., 2010). Two different kinds of degree connectivity maps were computed from the correlation matrix: (a) local maps, derived by counting for each voxel’s BOLD time-series the number of voxels where the correlation with the BOLD time-series exceeded a predefined optimized threshold (Buckner et al., 2009) (r > .25) inside a 12 mm radius sphere around each voxel; (b) distant maps, derived by the same procedure, but exclusively considering voxels outside the 12 mm radius sphere. In previous implementations of this graph theoretical metric, the spherical radius of 12 mm has been shown to be optimal in separating local and distant functional connections in the human brain (Sepulcre et al., 2010). Histograms of degree connectivity maps were derived on the individual level in order to assure normal distribution. Statistical parametric mapping (SPM8; Wellcome Department of Cognitive Neurology, London, UK) was used for smoothing connectivity maps with a 8mm kernel and for analyses at the group level. Group comparisons (two-sample t-tests) were performed, testing for differences in local and distant hub connectivity between BDD participants and controls. As it is known that group differences in interscan movement can lead to reductions in correlational strength between voxel time-series and erroneous interpretations of altered connectivity (Van Dijk et al., 2012), each participants’ mean interscan movement was calculated using a previously described procedure (Van Dijk et al., 2012), and the averaged mean interscan movement was compared between groups and used as a covariate in group comparisons. Correction for multiple comparisons was performed at the cluster level using Monte Carlo simulations for fMRI data as implemented in the AlphaSim program (http://afni.nimh.nih.gov/pub/dist/doc/program_help/AlphaSim.html) and the statistical threshold of P < .05. In addition, regions of interest analyses that were based on previous studies in OCD and BDD were performed in all analyses using one combined mask of bilateral orbitofrontal, caudate, putamen, pallidum, amygdala and midbrain masks generated from the Automatic Anatomic Labeling (AAL) tool of the Wake Forest University WFU PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas)) and a corrected threshold of PFWE < 0.05.
Further, we tested for correlations between local and distant degree connectivity and BDD symptom severity by including BDD-Y-BOCS total scores as a covariate in two random effects models containing local and distant connectivity maps of BDD participants, respectively. In this voxelwise whole-brain correlation analysis, only clusters exceeding the above-mentioned corrected threshold were used for further post-hoc assessment (i.e. Pearson correlation coefficient (r) between averaged voxel estimates from significant clusters and BDD-Y-BOCS scores in SPSS, Version 19). All variables used for post-hoc assessment were tested for normal distribution using Kolmogorov-Smirnov tests.
3. RESULTS
3.1. Degree Connectivity: Group comparisons
Significant local connectivity within-group (i.e. main) (P < .05, FWE whole-brain corrected) effects were evident in both BDD patients and the corresponding healthy control group (Figure 1A and Table 2), revealing most pronounced effects in lateral occipital, parietal and medial prefrontal regions. Two-sample t-tests showed that BDD patients had significantly (P < .05, cluster-corrected) lower local connectivity in the right amygdala (Table 2 and Figure 1B) as compared to healthy controls (HC > BDD). No regions were observed indicating a significantly higher local degree of connectivity in BDD participants than in controls.
FIG. 1.
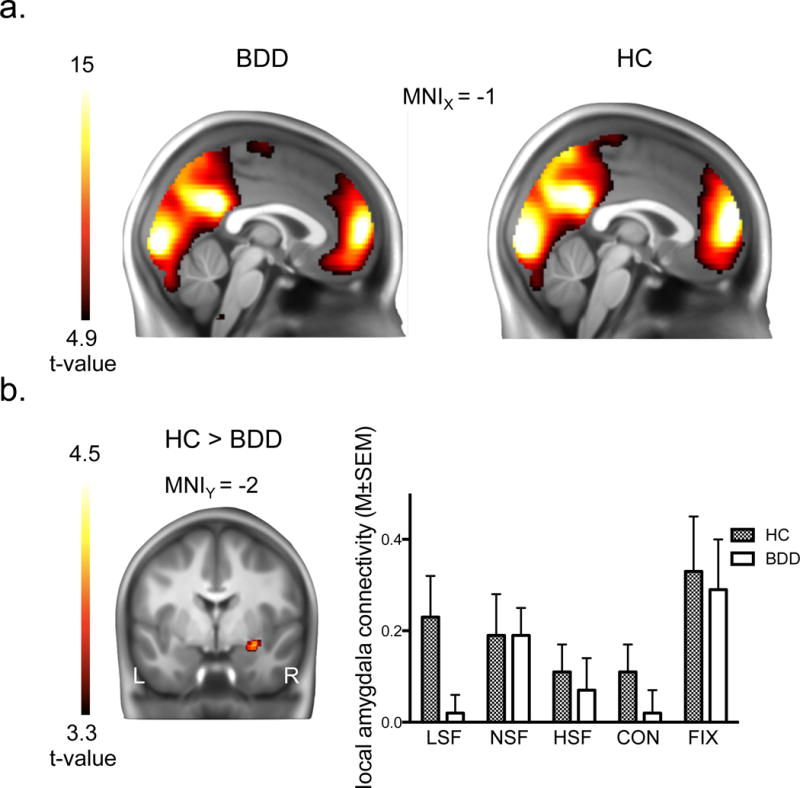
Local degree connectivity effects. (a.) main local degree connectivity effects in medial occipital, parietal and prefrontal brain areas, displayed separately for participants with body dysmorphic disorder (BDD) and healthy controls (HC); (b.) significantly lower local right amygdala connectivity in BDD participants as compared to HC, and post-hoc bar graphs indicating local amygdala connectivity across the different task conditions. The minimum t-value threshold of 3.3 used for display purposes of between-group effects is equivalent to a threshold of p < 0.001, uncorrected, and the minimum t-value threshold of 4.9 used for display of within-group effects corresponds to a whole-brain corrected family-wise error corrected threshold of p < 0.05. Abbreviations: LSF, low spatial frequency; NSF, normal spatial frequency; HSF, high spatial frequency; CON, control task; FIX, fixation.
Table 2. Local Degree connectivity maps.
Within-group (main effects) and between-group effects in participants with BDD and controls. The peak of significant clusters is indicated in bold, in contrast to sub-clusters.
| Controls | BDD | Differencec | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anatomya | Statisticb | Anatomy | Statistic | Anatomy | Statistic | ||||||||
| Local map effect region | x, y, z | z Score | CS | BA | x, y, z | z Score | CS | BA | Direction | x, y, z | z Score | CS | BA |
| Inferior occipital gyrus | 30, −92, −6 | > 8 | 25903 | 18 | 30,−92,−6 | > 8 | 25377 | 18 | |||||
| 18, −94, −4 | > 8 | 17 | 18,−94,−4 | > 8 | 17 | ||||||||
| Lingual gyrus | −10, −92, −4 | > 8 | 18 | −10,−92,−4 | > 8 | 18 | |||||||
| Inferior Parietal Lobule | 62,−28,22 | 5.60 | 132 | 40 | |||||||||
| Medial frontal gyrus | −2, 56, 10 | > 8 | 6102 | 9 | −2,56,8 | > 8 | 5583 | 9 | |||||
| 0, 50, −16 | 7.04 | 10 | 0,−14,70 | 5.47 | 188 | 6 | |||||||
| 2, 22, 44 | 4.59 | 6 | |||||||||||
| Superior frontal gyrus | 2, 22, 62 | 4.71 | 13 | 6 | 32,60,4 | 6.65 | 10 | ||||||
| −10, 48, 46 | 6.79 | 8 | −28,54,26 | 6.46 | 9 | ||||||||
| Inferior frontal gyrus | −48, 22, −10 | 5.29 | 34 | 47 | −50,22,−6 | 5.87 | 220 | 47 | |||||
| Transverse temporal gyrus | 60,−14,10 | 4.54 | 41 | ||||||||||
| Superior temporal gyrus | −60,−30,18 | 4.87 | 29 | 42 | |||||||||
| Amygdala | HC > BDD | 30,−2,−14 | 3.80 | 72 | |||||||||
Abbreviations: HC, healthy controls; BDD, body dysmorphic disorder; CS, cluster size; BA, Brodmann area.
Peaks of local map effect coordinates (x, y, z) are given in Montreal Neurological Institute (MNI) space.
Magnitude statistics for within-group (i.e., main) effects correspond to a minimum whole-brain corrected threshold of PFWE < 0.05 and 10 contiguous voxels.
Between-group effects are thresholded at P < 0.025 (cluster-corrected for multiple comparisons).
As a post hoc analysis, we examined local amygdala connectivity for subdivisions of the task by computing local degree connectivity maps for fixation, CON, LSF, NSF, and HSF periods separately, revealing most pronounced amygdala degree connectivity reductions in the LSF condition in BDD participants (Figure 1B), which however did not survive correction for multiple comparisons when tested voxel-wise using a post hoc region of interest analysis (right amygdala: MNIXYZ = 32, 2, −20, z = 2.66; left amygdala: MNIXYZ = −26, 6, −18, z = 2.75; both P < .005, uncorrected). Post hoc analyses for the different task conditions were also used to test for significant within-group effects, as no significant within-group effects of amygdala connectivity occurred across the entire experiment. Significant (whole-brain FWE corrected) within-group effects in the amygdala were observed in the LSF condition in both groups (Supplemental Figure 2), revealing that local amygdala connectivity was significantly high during LSF, but not across the entire experiment.
Significant distant connectivity within-group (i.e. main) (P < .05, FWE whole-brain corrected) effects were evident in medial and superior prefrontal areas, in the precuneus and the cerebellum in both groups (Supplemental Table 1). No regions were observed indicating group differences between BDD participants and controls.
3.2. Brain-Behavioral Associations: Correlations between degree connectivity and BDD symptom severity
Voxelwise correlational analyses between degree connectivity and overall BDD symptom severity (BDD-Y-BOCS total scores) revealed significant positive correlations (P < .05, cluster-corrected) between overall BDD symptom severity (BDD-Y-BOCS total scores) and the degree of local connectivity in the right OFC and a cluster including the left midbrain and thalamus (Figure 2A and Table 3). The degree of distant connectivity in the right OFC also showed significant positive associations with BDD-Y-BOCS total scores (Figure 2B and Table 3). Post-hoc assessment of the degree connectivity estimates of the regions showing significant correlations in voxelwise analyses showed significant (Pearson correlation coefficients, 2-tailed in SPSS) correlations between BDD overall symptom severity and the degree of local connectivity in the right OFC (r = .78, p < 0.001) and the left midbrain/thalamus (r = .64, p < 0.001), and the degree of distant connectivity in the right OFC (r = .65, p < 0.001).
FIG. 2.
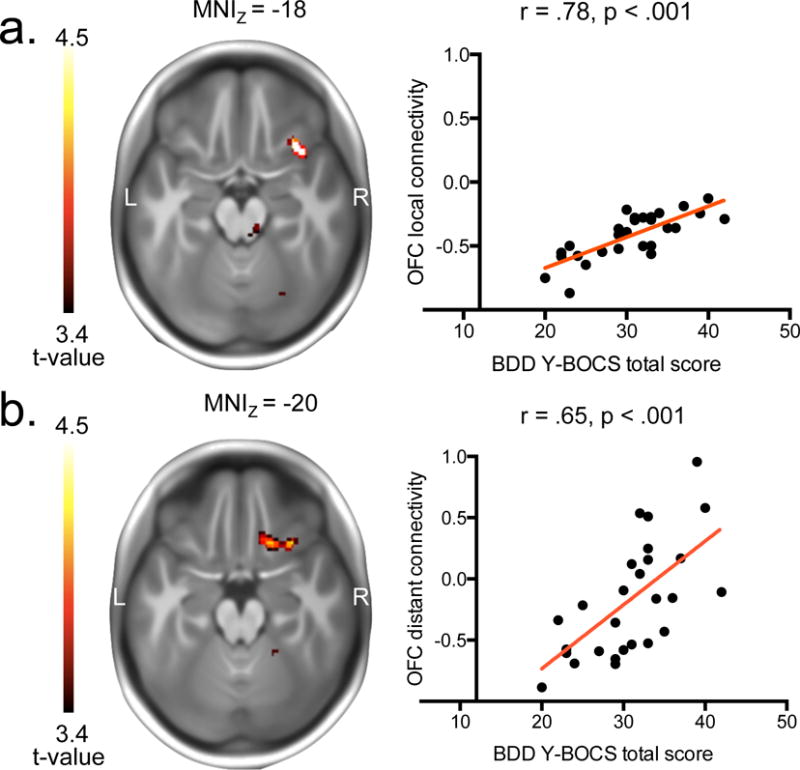
Brain-behavioral relationships in participants with BDD. (a.) Positive correlation between overall BDD symptom severity (BDD-Y-BOCS total scores) and local degree connectivity (voxel estimates averaged over the entire significant cluster from correlational analyses between BDD-Y-BOCS scores and local maps) in the posterior-lateral orbitofrontal cortex. (b.) Positive correlation between overall BDD symptom severity (BDD-Y-BOCS total scores) and distant degree connectivity (voxel estimates averaged over the entire significant cluster from correlational analyses between BDD-Y-BOCS scores and distant maps) in the posterior-lateral and posterior-central orbitofrontal cortex. The minimum t-value threshold of 3.4 used for display purposes of correlational effects corresponds to a threshold of p < 0.001, uncorrected.
Table 3. Correlational analysis results.
Peaks of significant correlations between BDD-Y-BOCS total scores and local and distant degree connectivity in the right orbitofrontal cortex and the left midbrain/thalamus revealed by a voxel-wise whole-brain analysis. The peak of significant clusters is indicated in bold, in contrast to sub-clusters, and effects are thresholded at P<.025.
| degree connectivity | correlated region | Direction | MNIxyza | z Score | CS | BA |
|---|---|---|---|---|---|---|
| local | right orbitofrontal cortex | positive | 34, 20, −20 | 4.29 | 91 | 47 |
| left midbrain/thalamus | positive | −4, −18, −4 | 3.72 | 202 | ||
| 10, −30, −20 | 3.22 | |||||
| distant | right orbitofrontal cortex | positive | 32, 22, −20 | 3.67 | 119 | 47 |
| 18, 22, −18 | 3.67 | 47 |
Abbreviations: CS, cluster size; BA, Brodmann area.
Coordinates (x, y, z) are given in Montreal Neurological Institute (MNI) space.
Significant correlations in a priori regions of interest (one combined mask of bilateral orbitofrontal, caudate, putamen, pallidum and midbrain masks generated from the Automatic Anatomic Labeling (AAL) tool of the Wake Forest University WFU PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas)) using a corrected threshold of PFWE < 0.025.
Additional correlational analyses between degree connectivity and HAM-A and MADRS were conducted to test whether OFC degree connectivity would be correlated not only with BDD symptom severity, but also with anxiety and depressive symptoms, mostly revealing significant correlations with the connectivity of posterior brain areas, but no correlations with OFC connectivity (Supplemental Table 2). No correlations were observed between OFC and amygdalar degree connectivity and task performance (reaction times and percentage of correct matches in the faces task).
4. DISCUSSION
Based on the observation that BDD and OCD share similarities in symptom expression and regional brain hyperactivation during symptom provocation, the present study investigated degree connectivity in individuals with BDD to test for abnormal global network alterations and associations with symptom severity mirroring abnormalities previously identified in patients with OCD. Strikingly, global BDD symptom severity correlated with local and distant degree connectivity of the same region in the right posterior-lateral OFC that has been previously associated with symptom severity in OCD. At the same time, a reduction in local connectivity of the amygdala was observed, although no group differences in either the basal ganglia or the OFC were observed. Thus, the present findings provide initial evidence of highly similar brain-behavioral associations across OCRD, but also suggest that there might be deviating connectivity abnormality profiles within patient groups categorized in OCRD. Both of these conclusions have to be considered preliminary and need to be confirmed by studies that directly compare the disorders.
Consistent with our hypotheses, BDD symptom severity positively correlated with the local and distant degree connectivity of the right OFC. This finding is remarkable for several reasons. First, the anatomical peak locations identified by our voxelwise correlational analyses are almost identical to the coordinates of voxelwise correlations reported in OCD previously (Beucke et al., 2013) (local: MNIXYZ = 34, 20, −20 (BDD) and MNIXYZ = 38, 22, −14 (OCD); distant: MNIXYZ = 32, 22, −20 (BDD) and MNIXYZ = 34, 20, −16 (OCD)). According to a recent connectivity-based parcellation providing subdivisions of the OFC (Kahnt et al., 2012), these coordinates all belong to a posterior-lateral OFC subregion known to be connected with lateral and dorsomedial prefrontal as well as inferior parietal and lateral temporal cortices in healthy subjects. Functionally, the lateral orbitofrontal cortex appears to be involved in avoidance of punishment and escape from danger (Kringelbach and Rolls, 2004), as well as in goal-directed behavioral choice, and may be involved in ritualized behavioral responses (Hollerman et al., 2000; Milad and Rauch, 2012). Within the OFC, the only noticeable deviation from this pattern was represented by the distant connectivity correlational analysis results in BDD participants, where the posterior-lateral OFC cluster extended to the posterior-central OFC subregion (Figure 2B), which is connected to the hypothalamic basal forebrain and the ventral striatum in healthy individuals (Kahnt et al., 2012). Second, analogous assessments of symptom severity underlie the previous and present correlational analyses. More precisely, the fact that the BDD-Y-BOCS (Phillips et al., 1997) represents an adaptation of the original Y-BOCS (Goodman et al., 1989), i.e. the instrument designed to measure OCD symptom severity, secures that the assessment of the underlying behavior of interest (i.e., primarily measuring obsessive thoughts and compulsive behaviors) was done in a comparably valid manner. Third, correlational findings were observed despite the fact that the present study used visual task data, whereas the previous OCD study used resting-state data (a limitation which is discussed in detail below). Finally, individuals with BDD in the present study were unmedicated. This is worth noting because in the previous studies in OCD, correlations between OFC degree connectivity and symptom severity were found in unmedicated (Beucke et al., 2013), but not in medicated (Beucke et al., 2013; Hou et al., 2014) individuals, suggesting that antidepressant medication reduces the association between symptom severity and OFC degree connectivity. Taken together, these correlational findings indicate parallel brain-behavioral associations in two OCRD. Whether this extends to other OCRD needs to be confirmed by similarly testing for correlations between OC symptom severity and degree connectivity in OCRD conditions such as hoarding and skin picking disorder.
Despite these correlational findings, the OFC and the basal ganglia did not show significantly higher degree connectivity in individuals with BDD as compared with healthy controls, results contrary to our prediction based on the abnormalities in patients with OCD. Of note, the degree connectivity group differences observed in unmedicated patients with OCD previously (Beucke et al., 2013) were found in medial, central and anterior-lateral OFC, thus also not in posterior-lateral OFC where the correlational findings were observed in the present BDD and in the previous OCD study (Beucke et al., 2013).
The only group difference that emerged in the present study was the finding of reduced local connectivity of the right amygdala. Post-hoc analyses revealed that local connectivity of the right amygdala was lower in BDD participants compared to controls across all task conditions, and that the difference was most pronounced during viewing of low spatial frequency face images (Figure 1B). An extensive literature documents the important role of the amygdala in the affective processing of faces and facial expressions (e.g. see (Whalen and Phelps, 2009) for review); thus this abnormality is potentially interesting considering the characteristic preoccupation of patients with own and others’ appearances (most often, faces), as well as others’ facial expressions in reaction to them (Buhlmann et al., 2006). In a previous fMRI study using others’ faces of varying spatial frequencies, the BDD group demonstrated greater amygdala activity than healthy controls for low and high spatial frequencies but not for unaltered images (Feusner et al., 2007). In the current study, separate post hoc analyses of local connectivity patterns by specific task periods suggests that the connectivity is lowest for low spatial frequency faces (however it should be noted that these post hoc sub-analyses are less stable in their correlation estimates as they are based on fewer time points). In addition, there is evidence of abnormal amygdalar sensitization with repeated viewing of fearful faces (Hutcheson et al., 2014), as well as associations between BDD symptom severity and regional amygdala volume (Feusner et al., 2009). In the current study, given that local connectivity corresponds to a 12mm sphere around the significant amygdala cluster voxels, these could reflect reduced connectivity between amygdala and other limbic and/or striatal structures including the hippocampus, putamen, and/or ventral anterior insula. Further studies are necessary to directly test connectivity between amygdala and these local structures to elucidate the specificity of the dysconnectivity pattern found in this study.
At the same time, several factors have to be taken into consideration regarding this finding. First, a previous study using different spatial frequencies of own facial images did not identify activation abnormalities in the amygdala (Feusner et al., 2010) (although this could be considered abnormal lack of expected reactivity, given that the BDD participants had higher subjective aversiveness ratings across stimuli than healthy controls). In addition, in the current study there was no correlation between amygdala connectivity and symptom severity. Moreover, regional activity patterns may or may not be linked with connectivity patterns; thus integrating across different studies and analyses is challenging. Nevertheless, the current study suggests amygdala dysconnectivity, which adds to the growing evidence of aberrant amygdala function in BDD.
Similarly, there are limitations that impede comparisons of the current study with the previous studies in OCD. An obvious limitation of the present study is the absence of a direct comparison between patients with OCD and individuals with BDD. Further, although brain areas of extensive degree connectivity generally maintain their numerous connections across different task states (Sepulcre et al., 2010), significant differences in areas specifically required by the task have been noted when directly comparing resting-state and task states (Sepulcre et al., 2010). Therefore, it is possible that the observed group difference partially arose as a consequence of the experimental manipulation, and resting-state studies in individuals with BDD are required to resolve this issue. In this context, it should be noted that the goal of this study was to identify global (i.e. task-independent) network differences in individuals with BDD, and that the numbers of data points within each task condition was insufficient for separate analyses. Further, in order to investigate whether not only healthy controls and BDD, but also OCD and BDD might differ in amygdala degree connectivity, future studies should compare these two patient groups directly in addition to comparisons to healthy controls. In this context, it should be noted that within OCD, there is some inconsistency regarding degree connectivity of the amygdala, with a recent study showing reductions in amygdala connectivity (Gottlich et al., 2014), but the majority of studies showing no connectivity differences in this region (Anticevic et al., 2014; Beucke et al., 2013; Hou et al., 2014).
In conclusion, the present study is the first to graph-theoretical methods on neuroimaging data of individuals with BDD, and identifies correlations between degree connectivity and symptom severity, strikingly mirroring brain-behavioral associations previously observed in patients with OCD. Reductions in local amygdala connectivity were also found in individuals with BDD, which adds to the growing, although still incomplete, evidence of aberrant amygdala function in BDD. Finally, these results encourage future study designs that include multiple OCRD patient groups to test for commonalities in brain-behavioral relationships between degree connectivity of the orbitofrontal cortex and obsessive-compulsive symptoms, and to also investigate potential distinct connectivity profiles within OCRD.
Supplementary Material
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th 2013. [Google Scholar]
- Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, Li CS, Pittenger C. Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive-compulsive disorder. Biological Psychiatry. 2014;75:595–605. doi: 10.1016/j.biopsych.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucke JC, Sepulcre J, Talukdar T, Linnman C, Zschenderlein K, Endrass T, Kaufmann C, Kathmann N. Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry. 2013;70:619–629. doi: 10.1001/jamapsychiatry.2013.173. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, Kendrick AD, Davis TL, Jiang A, Cohen MS, Stern CE, Belliveau JW, Baer L, O’Sullivan RL, Savage CR, Jenike MA, Rosen BR. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Archives of General Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. Journal of Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhlmann U, Etcoff NL, Wilhelm S. Emotion recognition bias for contempt and anger in body dysmorphic disorder. Journal of Psychiatric Research. 2006;40:105–111. doi: 10.1016/j.jpsychires.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nature Reviews Neuroscience. 2009;10:186–198. doi: 10.1038/nrn2575. [DOI] [PubMed] [Google Scholar]
- Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biological Psychiatry. 2004;55:1041–1045. doi: 10.1016/j.biopsych.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown Assessment of Beliefs Scale: reliability and validity. American Journal of Psychiatry. 1998;155:102–108. doi: 10.1176/ajp.155.1.102. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Moody T, Hembacher E, Townsend J, McKinley M, Moller H, Bookheimer S. Abnormalities of visual processing and frontostriatal systems in body dysmorphic disorder. Archives of General Psychiatry. 2010;67:197–205. doi: 10.1001/archgenpsychiatry.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, Bookheimer S. Visual information processing of faces in body dysmorphic disorder. Archives of General Psychiatry. 2007;64:1417–1425. doi: 10.1001/archpsyc.64.12.1417. [DOI] [PubMed] [Google Scholar]
- Feusner JD, Townsend J, Bystritsky A, McKinley M, Moller H, Bookheimer S. Regional brain volumes and symptom severity in body dysmorphic disorder. Psychiatry Research. 2009;172:161–167. doi: 10.1016/j.pscychresns.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figee M, de Koning P, Klaassen S, Vulink N, Mantione M, van den Munckhof P, Schuurman R, van Wingen G, van Amelsvoort T, Booij J, Denys D. Deep brain stimulation induces striatal dopamine release in obsessive-compulsive disorder. Biological Psychiatry. 2014;75:647–652. doi: 10.1016/j.biopsych.2013.06.021. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Archives of General Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gottlich M, Kramer UM, Kordon A, Hohagen F, Zurowski B. Decreased limbic and increased fronto-parietal connectivity in unmedicated patients with obsessive-compulsive disorder. Human Brain Mapping. 2014;35:5617–5632. doi: 10.1002/hbm.22574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, Schultz W. Involvement of basal ganglia and orbitofrontal cortex in goal-directed behavior. Progress in Brain Research. 2000;126:193–215. doi: 10.1016/S0079-6123(00)26015-9. [DOI] [PubMed] [Google Scholar]
- Hou JM, Zhao M, Zhang W, Song LH, Wu WJ, Wang J, Zhou DQ, Xie B, He M, Guo JW, Qu W, Li HT. Resting-state functional connectivity abnormalities in patients with obsessive-compulsive disorder and their healthy first-degree relatives. Journal of Psychiatry & Neuroscience. 2014;39:304–311. doi: 10.1503/jpn.130220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson NL, Lawrence KE, Moody T, Khalsa SS, Strober M, Feusner JD. Reactivity and habituation to fearful face stimuli in body dysmorphic disorder and anorexia nervosa. American College of Neuropsychopharmacology; Phoenix, AZ: 2014. [Google Scholar]
- Kahnt T, Chang LJ, Park SQ, Heinzle J, Haynes JD. Connectivity-based parcellation of the human orbitofrontal cortex. Journal of Neuroscience. 2012;32:6240–6250. doi: 10.1523/JNEUROSCI.0257-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Progress in neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends in Cognitive Sciences. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends in Cognitive Sciences. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British journal of Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Coles ME, Menard W, Yen S, Fay C, Weisberg RB. Suicidal ideation and suicide attempts in body dysmorphic disorder. The Journal of clinical psychiatry. 2005;66:717–725. doi: 10.4088/jcp.v66n0607. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Diaz SF. Gender differences in body dysmorphic disorder. The Journal of Nervous and Mental disease. 1997;185:570–577. doi: 10.1097/00005053-199709000-00006. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the Yale-Brown Obsessive Compulsive Scale. Psychopharmacology Bulletin. 1997;33:17–22. [PubMed] [Google Scholar]
- Phillips KA, A K, Pope H. Diagnostic Instruments for body dysmorphic disorder; Paper presented at: 148th Annual Meeting of the American Psychiatric Association; May 20–25; Miami, FL. 1995. [Google Scholar]
- Rauch SL, Jenike MA, Alpert NM, Baer L, Breiter HC, Savage CR, Fischman AJ. Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Archives of General Psychiatry. 1994;51:62–70. doi: 10.1001/archpsyc.1994.03950010062008. [DOI] [PubMed] [Google Scholar]
- Sepulcre J, Liu H, Talukdar T, Martincorena I, Yeo BT, Buckner RL. The organization of local and distant functional connectivity in the human brain. PLoS Computational Biology. 2010;6:e1000808. doi: 10.1371/journal.pcbi.1000808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- Simon D, Adler N, Kaufmann C, Kathmann N. Amygdala hyperactivation during symptom provocation in obsessive-compulsive disorder and its modulation by distraction. NeuroImage Clinical. 2014;4:549–557. doi: 10.1016/j.nicl.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D, Kaufmann C, Müsch K, Kischkel E, Kathmann N. Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 2010;47:728–738. doi: 10.1111/j.1469-8986.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- Taylor SF. Using Graph Theory to Connect the Dots in Obsessive-Compulsive Disorder. Biological Psychiatry. 2014;75:593–594. doi: 10.1016/j.biopsych.2014.01.012. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, Denys D, Goudriaan AE, Veltman DJ. Brain circuitry of compulsivity. European Neuropsychopharmacology. 2015 doi: 10.1016/j.euroneuro.2015.12.005. [DOI] [PubMed] [Google Scholar]
- van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, van Balkom AJ, van Oppen P, van Dyck R. Disorder-specific neuroanatomical correlates of attentional bias in obsessive-compulsive disorder, panic disorder, and hypochondriasis. Archives of General Psychiatry. 2005;62:922–933. doi: 10.1001/archpsyc.62.8.922. [DOI] [PubMed] [Google Scholar]
- Van Dijk KR, Sabuncu MR, Buckner RL. The influence of head motion on intrinsic functional connectivity MRI. NeuroImage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whalen PH, Phelps EA. The human amygdala. The Guilford Press; New York, London: 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


