Abstract
While the function of vitamin D in regulating calcium homeostasis is well established, there has been growing interest in its role in the prevention of numerous chronic diseases, including cardiovascular disease (CVD). There is mounting epidemiological evidence suggesting that vitamin D deficiency is linked to increased CVD risk. However, the results of previous vitamin D supplementation trials have yielded mixed results in regards to cardiovascular health, and the results of on-going large-scale randomized controlled trials are not yet available. Further complicating the issue, calcium supplementation, which is often prescribed concurrently with vitamin D, has been associated with increased CVD risk in some (but not all) studies. Thus, it is currently unclear whether vitamin D supplements, particularly for those that are deficient, can help prevent the development of CVD. In addition, there has not been uniform consensus regarding the threshold of 25-hydroxyvitamin D levels that constitutes “sufficiency” across organizational guidelines. This review will provide an update on the most recent evidence regarding the effects of vitamin D and calcium supplements on CVD clinical outcomes, summarize ongoing vitamin D trials, and discuss the current but remarkably disparate recommendations regarding vitamin D deficiency screening and supplementation.
Keywords: Vitamin D, Calcium, Supplementation, Cardiovascular Disease, Prevention
Introduction
Cardiovascular disease (CVD) is a leading cause of mortality worldwide, estimated to account for 30% of all deaths [1]. Thus, identifying modifiable risk factors and treatments remains a high priority for CVD prevention. Vitamin D deficiency, primarily known for its role in bone and mineral regulation, has garnered interest for its potential role in many other disease processes, including CVD. There is strong evidence from observational studies that have linked low serum levels of 25-hydroxyvitamin D [25(OH)D] with poor cardiovascular outcomes [2]. Vitamin D is an attractive interventional target, as serum 25(OH)D levels are modifiable and treatment is relatively inexpensive. Furthermore, as it is estimated that approximately one billion people in the world have either deficient or insufficient levels of 25(OH)D, vitamin D intervention can potentially impact a large population [3].
Despite encouraging results from observational epidemiological studies, randomized controlled trials (RCTs) of vitamin D supplements have yielded mixed results regarding cardiovascular outcomes [4-8]. However, to date, no large-scale trial of vitamin D supplements with CVD as a primary outcome has been completed [9]. While several large-scale RCTs are currently underway, the lack of persuasive evidence has resulted in differing guidelines and practices regarding the definition of vitamin D deficiency, populations to be screened, and the ideal level of supplementation.
Because vitamin D promotes the intestinal absorption of calcium, vitamin D is often prescribed in conjunction with calcium supplementation, particularly in populations at risk for bone loss, such as post-menopausal women and older adults [10]. However, there is now some evidence that calcium supplementation may increase risk for CVD [11]. This underscores the necessity in evaluating calcium and vitamin D supplementation effects on not only bone, but on cardiovascular outcomes as well.
Vitamin D Metabolism and Measurement
Vitamin D is a fat-soluble pro-hormone that is a precursor to the active steroid hormone 1,25-dihydroxyvitamin D [1,25(OH)2D]. There are 3 possible sources of vitamin D – food, pill supplements, and sunlight. Specifically, vitamin D is obtained either exogenously as vitamin D3 (cholecalciferol) or D2 (ergocalciferol) from diet or supplements, or endogenously through D3 production in the skin. Traditionally, endogenous cutaneous synthesis was the main process by which individuals obtain vitamin D, accounting for 90% of an individual's vitamin D requirement [12]. However recently, there has been an increased use of vitamin D supplements in the U.S. population [13]. There are few natural food sources of vitamin D, primarily oily fish, liver, and egg yolks; thus many individuals rely on fortified food or dietary supplements for vitamin D intake [14, 15].
In the skin, ultraviolet B (UVB) radiation from sunlight converts 7-dehydrocholesterol into previtamin D3, which then isomerizes to vitamin D3. However, several factors affect the skin's ability to produce vitamin D3, including age, race, use of sunscreen, season, and geographic location. Older people have less 7-dehydrocholesterol in their epidermis, decreasing their capacity to produce vitamin D3 [16]. Certain races, including African Americans, tend to have lower 25(OH)D levels because the increased pigmentation in darker skin inhibits vitamin D production [17]. Likewise, sunscreen use, seasons with less sunlight, and latitudes further from the equator reduce the amount of UVB radiation that enters the skin, thereby reducing vitamin D production [12].
Following either consumption or endogenous synthesis, vitamin D is then hydroxylated by 25-hydroxylase in the liver, to become 25-hydroxyvitamin D [25(OH)D]. This is the main circulating form of vitamin D, and is used to define an individual's vitamin D status. 25(OH)D will then undergo a second hydroxylation by 1α-hydroxylase to form the active metabolite, 1,25(OH)2D (calcitriol). This 1-hydroxylation primarily occurs in the kidneys and is responsible for the majority of calcitriol production; however, there are extra-renal forms of 1α-hydroxylase, which is also found in cells implicated in the pathogenesis of CVD, such as vascular smooth muscle cells (VSMCs), endothelial cells, and macrophages [18-20]. This allows for local production of calcitriol, which is thought to play an autocrine/paracrine function. Although there are 1,25(OH)2D assays, it is not considered an accurate indicator of overall vitamin D status, due to 1,25(OH)2D's shorter half-life and lower circulating concentration compared to 25(OH)D, as well as parathyroid hormone (PTH)'s tight regulation of its level in the blood [21]. In fact, 1,25(OH)2D levels are often normal in individuals with vitamin D deficiency, and therefore do not accurately represent vitamin D stores.
While 25(OH)D is currently the best measure of vitamin D status, it is not necessarily representative of an individual's bioavailable vitamin D. Importantly, 85-90% of all circulating 25(OH)D is bound to vitamin D binding protein (VDBP), 10-15% is bound to albumin, and less than 1% is free [22, 23]. Because clinical assays do not distinguish between bound versus unbound 25(OH)D, an individual with low total 25(OH)D may have adequate bioavailable 25(OH)D.
Racial differences of Vitamin D and CVD
Blacks have lower total 25(OH)D than whites, but paradoxically low 25(OH)D appears to be a stronger risk factor for CVD in whites compared to blacks [24-27]. One prior study had concluded that blacks may have similar levels of bioavailable vitamin D as whites due to less overall VDBP [28], a finding that might potentially explain this racial paradox. However, studies evaluating 25(OH)D with VDBP genotypes have yielded mixed results [24, 25, 29], and more recent evidence has disputed the existence of racial differences in VDBP concentration [30, 31]. Thus, differences in VDBP do not appear to be the explanation for why 25(OH)D is a stronger CVD risk factor in whites than blacks. Further research is needed in this area.
Vitamin D Mechanisms on CVD
While the mechanisms by which vitamin D deficiency relates to CVD are uncertain, low vitamin D levels are thought to increase CVD risk by contributing to established cardiovascular risk factors, including hypertension, diabetes, and inflammation (Figure 1).
Fig. 1.
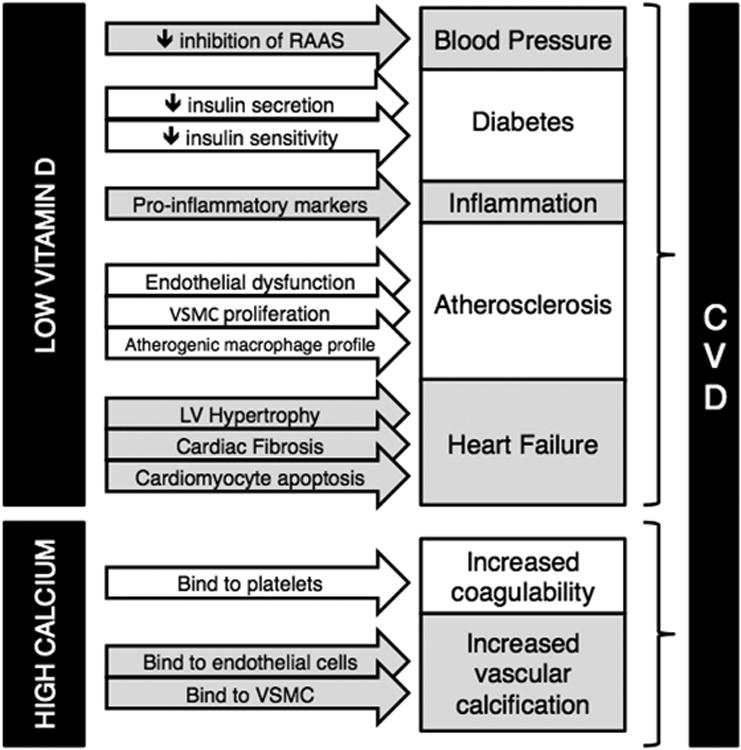
Proposed mechanisms by which vitamin D deficiency and high calcium levels (typically from calcium supplementation and not dietary calcium sources) may increase cardiovascular disease risk. RAAS: renin-angiotensin-aldosterone system; VSMC: vascular smooth muscle cell; LV: left ventricular
Hypertension
Most clinical studies, although not all, have found an inverse association between 25(OH)D serum levels and blood pressure [32-34]. A recent meta-analysis of eight studies with 283,537 participants found that individuals in the top third of serum 25(OH)D levels had a 30% lower risk of developing hypertension compared to those in the bottom third [33]. Furthermore, Vimaleswaran et al. provided evidence for a potential causal relationship between low vitamin D levels and increased blood pressure in a Mendelian randomization study [35]. Activated vitamin D is thought to affect blood pressure through inhibition of the renin-angiotensin-aldosterone system (RAAS) [36-38].
Nonetheless, results of clinical trials that tested the effects of vitamin D supplements on blood pressure have predominantly had null results. While some small clinical trials have shown positive results [39], the highest quality trials and most meta-analyses of vitamin D supplementation trials have documented a null relationship [40, 41]. In the recent DAYLIGHT trial with 534 pre-hypertensive/hypertensive participants, there was no difference in blood pressure between those treated with 4000 IU/day of vitamin D compared to those only treated with 400 IU/day [42]. However, a recently published meta-analysis reported that while vitamin D did not reduce blood pressure in the overall population, daily vitamin D dosing of >800 IU/day for <6 months in participants older than 50 years old significantly reduced both systolic and diastolic blood pressure [5]. Further evidence is needed.
Diabetes
Low vitamin D levels are thought to promote diabetes through increased insulin resistance and pancreatic beta-cell dysfunction resulting in decreased insulin secretion [43, 44]. Most epidemiologic studies show an association between low 25(OH)D serum levels and increased incidence of type 2 diabetes mellitus [45, 46]. Grubler et al. recently published a RCT (n=185) which found that vitamin D supplementation of 2,800 IU/day for 8 weeks in obese hypertensive patients with vitamin D deficiency reduced HbA1c levels by 3.52 mmol per mol of vitamin D [47]. However, Jorde et al. performed a RCT with a larger sample size (n=511) and longer intervention time (5 years) and found no evidence that vitamin D supplementation of 20,000 IU/week over a 5-year period prevented the progression from pre-diabetes to diabetes [4]. Their study population on average was not vitamin D deficient (mean 25(OH)D = 24 ng/ml); however the authors reported that subgroup analysis in subjects with low baseline 25(OH)D yielded results similar to the main trial results. Furthermore, several systematic reviews and meta-analyses also concluded that vitamin D supplementation did not reduce HbA1c levels or improve beta cell function and insulin resistance [48-51].
Inflammation
Suboptimal levels of vitamin D are also thought to contribute to an increased inflammatory profile. Pro-inflammatory cytokines, such as IL-1, Il-2, Il-6, and tumor necrosis factor-α (TNF-α) are down-regulated by calcitriol [52]. Furthermore, vitamin D may protect against endothelial dysfunction, promote an anti-atherogenic macrophage phenotype, and protect against VSMC changes that would result in increased inflammatory molecules and overall increased risk of atherosclerosis [53]. Yet, results of vitamin D supplementation on inflammation are mixed. One study of 332 participants found that one year of vitamin D supplementation decreased serum IL-6 levels, although other inflammatory markers including C-reactive protein (CRP) levels were increased [54]. However, a meta-analysis of vitamin D supplementation in obese and overweight participants suggested that supplemental vitamin D did not have a statistically significant impact on inflammatory markers such as CRP, TNF-α, and IL-6 [55]. Additional research needs to be done.
Cardiac Structure/Function
Vitamin D may also influence CVD risk by directly affecting the heart. Animal studies have shown evidence that low vitamin D levels contribute to left ventricular hypertrophy, cardiac fibrosis, reduced ejection fraction, and cardiomyocyte apoptosis [56-58]. However, few studies examine the association between 25(OH)D and myocardial structure and function in humans. Further research needs to be done to clarify this relationship [59].
Calcium Mechanisms on CVD
As the physiology of vitamin D and calcium are intertwined, it is perhaps unsurprising that there is growing evidence that calcium itself plays a role in CVD. In general, calcium is thought to protect against CVD development through several potential mechanisms, including lowering blood pressure, decreasing lipid levels and improving glycemic control [60].
Despite these potential benefits, there has been recent concern that calcium supplements might increase CVD risk. Although this topic remains highly controversial, there are several proposed mechanisms by which transiently elevated calcium levels (conferred by calcium supplement intake) could contribute to cardiovascular risk (Figure 1). High serum calcium levels may increase blood coagulability, as platelets have calcium-sensing receptors and may be activated with elevated serum calcium. A randomized trial of 100 post-menopausal women found that calcium supplementation reduced the time to clot initiation compared to placebo [61]. It is also thought that the rapid bolus of calcium from supplementation, unlike dietary calcium, may acutely elevate serum calcium and promote vascular calcification [62]. However, observational studies have yielded inconsistent evidence for the association between supplemental calcium intake and arterial calcification [63].
Calcium, often taken alongside vitamin D, has long been prescribed to reduce the risk of osteoporosis and other bone diseases. While calcium can be obtained in dietary forms, such as dairy products, certain vegetables, and fortified foods, many people do not achieve the recommended intake levels through diet alone. It is estimated that approximately 50% of the U.S. adult population takes calcium supplements [64]. The Institute of Medicine (IOM) recommends a daily intake level of 1,000 mg/day for men 19–70 years old and women 19–50 years old and 1,200 mg/day for individuals who are older [65]. Gallagher et al. found that hypercalcemia occurred in 8.8% of participants and hypercalciuria occurred in 30.6% of participants at a calcium intake of 1,200 mg/day [66]. Thus, it is possible that even at recommended levels of calcium supplementation, individuals may be at risk of the purported adverse cardiovascular effects of high calcium levels.
Observational Studies and Mendelian Randomization Studies on Vitamin D and CVD
Vitamin D deficiency has been associated with increased all-cause mortality [67-69] and increased risk of CVD outcomes, including cardiovascular mortality [2, 68], myocardial infarction (MI) [70, 71], coronary heart disease (CHD) [25, 26], stroke [27, 29, 72], and heart failure (HF) [24].
In a 2015 analysis of the Copenhagen vitamin D (CopD) study (n=247,574), both high and low levels of serum 25(OH)D were associated with increased incidence of cardiovascular outcomes, including CVD, stroke, and acute myocardial mortality [73]. Serum 25(OH)D was non-linearly associated with CVD mortality in a “reverse J-shaped” pattern, consistent with another study which found increased mortality risk at high vitamin D levels >50 ng/ml [69]. Interestingly, in the CopD study, the lowest CVD mortality risk occurred at 70 nmol/L (28 ng/ml), a level lower than the Endocrine Society's guidelines for vitamin D sufficiency of 30 ng/ml [74], but higher than the level deemed adequate for health (20 ng/ml) by the IOM [65].
While these observational studies yield promising results, there are substantial limitations. With any observational study, there is concern about confounding, that is, some key variables are either not measured or are crudely assessed. Individuals with adequate vitamin D levels might also be engaging in other health-promoting behaviors such as physical activity and healthy diet. Furthermore, these observational studies cannot prove causation in the relationship between vitamin D and cardiovascular outcomes. It is possible that the results are due to reverse causation, in which low 25(OH)D status may be reflective of poor health in general. For example, if individuals are less healthy, they may be less mobile and are less likely to spend time outdoors, and therefore have less exposure to sunlight and synthesize less vitamin D. Additionally, individuals in poor health may be obese, allowing for vitamin D sequestering in adipose tissue and consequently a lower 25(OH)D status [75].
Although RCTs are the gold standard for proving causation, Mendelian randomization studies can provide insight into causation, as genetic studies are largely free of the confounding factors that plague observational studies. Manousaki et al. recently performed such an analysis using data from the SUNLIGHT consortium (n=33,996) and found that genetically low 25(OH)D levels were not associated with increased risk of coronary artery disease [76]. This is consistent with another Mendelian randomization study, which found an association between genetically low 25(OH)D concentrations and all-cause mortality, but no association with cardiovascular mortality [77]. Although not conclusive, these two studies suggest that the positive results from observational studies may be due to confounding factors rather than a true causal relationship between vitamin D and cardiovascular outcomes.
Observational Studies of Calcium and CVD Outcomes
Observational studies have yielded mixed results regarding whether calcium intake is associated with increased risk of CVD. An analysis of 72,245 women in the prospective cohort Nurses' Health Study with 24 years follow-up found that women taking >1,000 mg/day calcium supplements had a 29% lower risk of incident CHD compared to those not taking supplements, but did not have a statistically significant different risk of incident stroke [78]. Similarly, an analysis of 6,236 participants in the Multi-Ethnic Study of Atherosclerosis (MESA) did not find a strong association between dietary or supplementary calcium with incident CVD events over 10 years of follow-up [79].
In contrast, another recent analysis of 5,448 adults, also from MESA, found that while higher overall dietary calcium intake was associated with a decreased risk of incident coronary atherosclerosis as measured by the coronary artery calcium (CAC) score, the use of calcium supplements was associated with 22% increased risk for incident CAC [80]
Finally, a meta-analysis of prospective cohort studies, involving 757,304 participants, found a U-shaped association between dietary calcium intake and cardiovascular mortality. Total daily dietary calcium intake (i.e. from food sources) that were either higher or lower than 800 mg were associated with increasing risk of cardiovascular mortality [81]. However, meta-analysis of 6 studies did not find calcium supplements to be associated with cardiovascular mortality [81].
. These mixed results suggest that further clinical trials must be done to evaluate the impact of calcium supplementation on cardiovascular outcomes.
Randomized Controlled Trials (RCT) of Vitamin D and CVD Outcomes
Unfortunately, RCT results thus far have not been promising. However, most published trials have either had a relatively small sample size, did not include CVD outcomes as a specified outcome prior to study start, or did not collect CVD outcomes in a rigorous fashion. There are multiple large-scale vitamin D supplementation trials currently underway. The largest of which are the VITamin D and OmegA-3 TriaL (VITAL), a placebo-controlled, double-blind 2×2 factorial trial of over 25,875 multi-ethnic participants randomized to 2,000 IU/day of vitamin D3 and omega-3 fatty acid supplements for 5 years [82], and the D-Health Trial, a placebo-controlled trial with 21,315 participants randomized to 60,000 IU/month of vitamin D3 for 5 years [83].
Table 1 provides a list of additional ongoing clinical trials of vitamin D supplementation for outcomes related to CVD or its risk factors [82-89]. These studies have their limitations. Most ongoing trials are studying older populations, and the results may not be generalizable to younger individuals. Furthermore, only one trial required a low vitamin D status [25(OH)D < 30 ng/ml] as an enrollment criterion. If vitamin D intervention is effective, the effect would likely be most pronounced in vitamin D deficient individuals; thus benefits may not be seen in a population that started with adequate vitamin D levels.
Table 1.
Summary of ongoing large, randomized controlled trials with vitamin D supplementation as an intervention. Note that outcomes listed are only those related to cardiovascular disease risk factors or outcomes. Participant demographics of sex and race were included for trials with published baseline demographics. Only trials with a sample size of 400 participants or more were included
| Study | Sample Size |
Participants | Participant Demographics |
Intervention (vs. placebo) |
Intervention Period |
Primary Endpoint |
Other endpoints |
Location | Primary End Date |
|---|---|---|---|---|---|---|---|---|---|
| VIDA84 | 5,110 | 50-84 years | 58% M, 42% F 83.2% White 16.8% Other | 100,000 IU/month | 3.3 years (median) | CVD | Blood pressure, HR variability, Arterial waveform | New Zealand | 2016 |
| EVITA85 | 400 | CHF patients, 18-79 years, 25(OH)D <30ng/ml | 4,000 IU/day | 3 years | All-cause mortality | Event-free survival | Germany | 2016 | |
| VITAL82 | 25,875 | ≥50 years M ≥55 years F | 49.4% M, 50.6% F 75.3% White 20.2% Black 4.5% Other | 2,000 IU/day | 5 years | Cancer, CVD | United States | 2017 | |
| VIDAL (Feasibility Study)86 | 1,600 | 65-84 years | 100,000 IU/month | 2 years | Cause - specific mortality | United Kingdom | 2017 | ||
| D2D87 | 2,362 | Prediabetes ≥30 years | 4,000 IU/day | 3 years | Diabetes | United States | 2017 | ||
| DO-Heafth88 | 2,152 | ≥70 years | 2,000 IU/day | 3 years | Blood Pressure | Europe | 2017 | ||
| FIND89 | 2,495 | ≥60 years | 1600 IU/day, 3200 IU/day | 5 years | CVD, Cancer | Finland | 2018 | ||
| D-Health83 | 21,315 | 60-84 years | 54.1% M, 45.9% F 94.5% White 5.5% Other | 60,000 lU/month | 5 years | All-cause mortality | Total CV events, Diabetes, High blood pressure | Australia | 2019 |
As we await the results of these ongoing trials, meta-analyses of completed RCTs may shed light on the effect of vitamin D supplementation on CVD outcomes. In a 2015 meta-analysis of 13 RCTs of oral vitamin D supplementation in adults with chronic kidney disease, there was no significant effect on all-cause mortality, cardiovascular mortality, or serious cardiovascular adverse events compared [90]. Of note, the trials in this meta-analysis were not initially designed to collect data on mortality or cardiovascular events, and therefore may not accurately reflect true outcomes. Another meta-analysis of 21 vitamin D trials found no statistically significant difference in those supplemented with vitamin D compared to placebo for the outcomes of cardiac failure, MI, and stroke [7].
The Vitamin D Treating Patients with Chronic Heart Failure (VINDICATE) study was a randomized, placebo-controlled, double blind trial of vitamin D supplementation in patients with HF and vitamin D deficiency [91]. The study found that while one year of 4,000 IU/day of vitamin D3 did not improve 6-minute walk distance, their primary outcome, those receiving vitamin D supplementation showed a 6.1% improvement in ejection fraction and a decrease in left ventricular end diastolic diameter. While this sample was relatively small (n=229) and not particularly generalizable (participants were all male HF patients), it does provide some new encouraging evidence on the potential effects of vitamin D supplementation. Conversely, a recent meta-analysis of 7 RCTs investigating whether vitamin D supplementation has protective effects in patients with chronic HF, found that while vitamin D may decrease PTH levels and some inflammatory markers, there was no statistically significant improvement in left ventricular function [92]. However, the meta-analysis was limited by the small sample sizes and relatively short follow-up duration of the included trials; the VINDICATE trial was both larger in size and had a longer follow-up compared to all trials included in the meta-analysis.
RCTs of Calcium Supplementation and CVD
Since the Auckland Calcium Study, which first introduced the notion that calcium supplements may increase cardiovascular risk, there has been increased interest in investigating the effects on calcium supplementation on CVD outcomes [93].
The results of calcium supplementation trials have been mixed. A meta-analysis of 15 RCTs (8,151 participants) of calcium supplementation >500 mg/day found that calcium supplements were associated with a 27% increased risk of MI compared to placebo [94]. However, more recently, in 2014 Lewis et al. published results of a 3-year intervention of 1200 mg calcium supplementation vs. placebo in older women (n=1103) on common carotid artery intimal medial thickness (CCA-IMT) and carotid atherosclerosis [95]. They found no difference in CCA-IMT or carotid atherosclerosis between the two groups. Furthermore, they found that participants with the highest tertile of total calcium had decreased carotid atherosclerosis compared to those in the lowest tertile (OR=0.67, 95% CI 0.5-0.9). Thus, this trial did not yield evidence that supports the claim that calcium supplementation increases carotid atherosclerosis, although this was a surrogate end-point.
While these trials evaluated the effect of calcium supplementation alone, in clinical practice, calcium is often co-administered with vitamin D. Therefore, it is paramount to study the effect of the co-administered supplement on cardiovascular health, as the effects could differ from the monotherapies alone. If vitamin D does have a protective effect against CVD, it could attenuate the possible negative effects of calcium on cardiovascular health.
With the exception of the landmark Women's Health Initiative (WHI) study, in which 36,282 postmenopausal women were randomized to either 1000 mg calcium with 400 IU vitamin D daily or placebo, few trials have examined the effect of co-administered calcium and vitamin D (CaD) supplementation [96]. However, the results of the WHI study have presented uncertainty. Initially, Hsia et al. found that CaD supplementation was not associated with increased or decreased coronary or cerebrovascular risk [8]. Yet, in a meta-analysis that incorporated a secondary analysis of WHI data that removed participants with personal use of calcium supplements (for concern regarding potential interaction) and combined those results with the results from 7 other studies, Bolland et al. found that calcium supplements with or without vitamin D had a 24% increased risk of MI compared to placebo [11]. There are several limitations to this study, including controversy about removing users of personal calcium supplements from the Bolland analysis, lack of cardiovascular endpoints as primary outcomes, and the lack of adherence in taking study pills during the WHI study itself [96]. Further RCTs with CaD supplementation and cardiovascular outcomes need to be done to clarify whether CaD has a beneficial or harmful effect on cardiovascular health.
Current Vitamin D Guidelines
Given the absence of strong evidence, there is a lack of consensus regarding vitamin D deficiency screening and management. Several organizations have issued recommendations concerning either populations who should be screened for vitamin D deficiency, laboratory cutoff values to be considered deficient, or recommended intake of vitamin D (Table 2) [65, 74, 97-102]. However, these recommendations are all based on evidence for optimal bone health. To our knowledge, no organization has issued guidelines for vitamin D intake specifically to prevent CVD, as the current evidence is insufficient to support such a recommendation.
Table 2.
Summary of guidelines regarding vitamin D deficiency screening and supplementation. Note that all threshold and treatment values are to promote optimal serum 25-hydroxyvitamin D levels for bone health, and not the prevention of cardiovascular disease.
| Agency (Year of Guideline) | Country | Screening | Deficiency Threshold | Recommended Dietary Intake | Upper Level Intake |
|---|---|---|---|---|---|
| Institute of Medicine (2010) | United States, Canadaa | Deficient: <20 ng/ml | 600 lU/day (19-70years) 800 IU/day (≥70 years) | 4,000 IU/day | |
| Endocrine Society (2011) | International | Individuals at risk for deficiencyc | Deficient: <20 ng/ml Insufficient: 21 -29 ng/ml Sufficient: ≥30 ng/ml | Minimum 600 IU/day (may need 1,500-2,000 IU/day to ensure 30 ng/ml or higher) | 4,000 IU/dayb |
| United States Preventative Services Task Force (2014) | United States | Symptomatic adults | |||
| American Geriatric Society (2013) | United States | Not necessary if supplementation within recommended limits | Deficient: <30 ng/ml | 1,000 IU/day (>65 years) | 10,000 IU/day |
| National Osteoporosis Foundation (2014) | United States | 800-1,000 IU/day (>50 years) | |||
| Scientific Advisory Committee on Nutrition (2016) | United Kingdom | Deficient: <25 ng/mL | 400 IU/day | 4,000 IU/day | |
| National Institute for Health and Care Excellence (2014) | United Kingdom | Individuals symptomatic or at high risk for deficiencyc | 400 IU/day for at-risk groups |
IOM is a U.S. organization, but the IOM Vitamin D report was funded by the U.S. and Canadian governments.
The Endocrine Society recognizes that supplementation up to 10,000 IU/day in people aged 19 years and older may be needed to correct for deficiency.
Risk factors for deficiency include: African-American and Hispanic ethnicity, pregnant or lactating women, older adults with history of falls or fractures, obesity (BMI >30 kg/m2), granuloma-forming disorders, malabsorption syndromes, chronic kidney disease, liver failure, conditions that cause weakening of bone, certain medications including anticonvulsants
Two of the most widely cited recommendations are the IOM's 2010 guidelines [65] and the Endocrine Society's 2011 guidelines [74]. Notably, despite their widespread acceptance, there are several major discrepancies between the two documents [103]. While the IOM does not consider a serum 25(OH)D level above 20 ng/ml to confer additional benefit for bone health, the Endocrine Society considers a serum 25(OH)D level of 30 ng/ml to be optimal. Both organizations differ in their recommended daily intake to achieve these vitamin D levels. Additionally, while both advise against routine vitamin D screening in the general population, the Endocrine Society considers additional groups to be at risk for deficiency compared to the IOM, including African-American and Hispanic populations, older adults with history of fracture, and obese individuals.
In the face of varying guidelines, physicians understandably may express confusion about whom to screen and treat in their clinical practice. A study of the National Ambulatory Medical Care Survey found that the rate of diagnoses for vitamin D deficiency increased from 2007 to 2010 [104]. Most diagnoses were for non-specified disease, thus vitamin D screening was likely used for preventative care, rather than for diagnostic purposes in symptomatic patients. However, these surveys were administered prior to the update in the IOM and Endocrine Society guidelines, so it is possible that vitamin D screening has declined following the release of the guidelines. Additionally, a small study by Tarn et al. suggested that the uncertainty inherent in the varying vitamin D guidelines does not get conveyed to patients, resulting in patients getting unnecessarily screened and treated for vitamin D deficiency [105]. While not representative of all clinical practices, it underlines the importance of providing consistent vitamin D guidelines to minimize variations in patient care.
Conclusion
In summary, despite intriguing evidence from animal studies and observational studies that low vitamin D is associated with increased risk for CVD, there is insufficient evidence at this time to prove a causal relationship and to prove that vitamin D supplementation is effective in reducing cardiovascular risk. Although calcium supplementation is widely used for maintaining bone health, there is some evidence that it may increase CVD risk. Due to the seemingly conflicting effects of low vitamin D and high calcium levels on CVD, further research is needed to examine the effect of combined CaD supplementation on specific cardiovascular outcomes. Until the results of ongoing large-scale RCTs are available, it is important for clinicians to be aware of the differing vitamin D guidelines to best care for their patients and weigh the potential risks and benefits before prescribing supplementation.
Acknowledgments
Funding: Erin Michos is supported by a grant R01NS072243 from NIH/NINDS and the Blumenthal Scholars Award in Preventive Cardiology. Kathleen Chin was funded by the Hopkins Scholars Program.
Footnotes
Conflict of Interest Disclosures: The authors have nothing to disclose related to this article. Dr. Michos reports receiving an honorarium from Siemens Diagnostics (modest) for work unrelated to this topic.
References
- 1.Wong ND. Epidemiological studies of CHD and the evolution of preventive cardiology. Nat Rev Cardiol. 2014;11(5):276–289. doi: 10.1038/nrcardio.2014.26. [DOI] [PubMed] [Google Scholar]
- 2.Wang L, Song Y, Manson JE, et al. Circulating 25-hydroxy-vitamin D risk of cardiovascular disease: a meta-analysis of prospective studies. Circ Cardiovasc Qual Outcomes. 2012;5(6):819–829. doi: 10.1161/CIRCOUTCOMES.112.967604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87(4):1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 4.Jorde R, Sollid ST, Svartberg J, et al. Vitamin D 20,000 IU per Week for Five Years Does Not Prevent Progression From Prediabetes to Diabetes. J Clin Endocrinol Metab. 2016;101(4):1647–1655. doi: 10.1210/jc.2015-4013. [DOI] [PubMed] [Google Scholar]
- 5.Golzarand M, Shab-Bidar S, Koochakpoor G, Speakman JR, Djafarian K. Effect of vitamin D3 supplementation on blood pressure in adults: An updated meta-analysis. Nutr Metab Cardiovasc Dis. 2016;26(8):663–673. doi: 10.1016/j.numecd.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Bolland MJ, Grey A, Gamble GD, Reid IR. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes: a trial sequential meta-analysis. The Lancet Diabetes & Endocrinology. 2014;2(4):307–320. doi: 10.1016/S2213-8587(13)70212-2. [DOI] [PubMed] [Google Scholar]
- 7.Ford JA, MacLennan GS, Avenell A, et al. Cardiovascular disease and vitamin D supplementation: trial analysis, systematic review, and meta-analysis. Am J Clin Nutr. 2014;100(3):746–755. doi: 10.3945/ajcn.113.082602. [DOI] [PubMed] [Google Scholar]
- 8.Hsia J, Heiss G, Ren H, et al. Calcium/vitamin D supplementation and cardiovascular events. Circulation. 2007;115(7):846–854. doi: 10.1161/CIRCULATIONAHA.106.673491. [DOI] [PubMed] [Google Scholar]
- 9.Prentice RL, Pettinger MB, Jackson RD, et al. Health risks and benefits from calcium and vitamin D supplementation: Women's Health Initiative clinical trial and cohort study. Osteoporos Int. 2013;24(2):567–580. doi: 10.1007/s00198-012-2224-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657–666. doi: 10.1016/S0140-6736(07)61342-7. [DOI] [PubMed] [Google Scholar]
- 11.Bolland MJ, Grey A, Avenell A, Gamble GD, Reid IR. Calcium supplements with or without vitamin D and risk of cardiovascular events: reanalysis of the Women's Health Initiative limited access dataset and meta-analysis. BMJ. 2011;342:d2040. doi: 10.1136/bmj.d2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr. 2004;80(6 Suppl):1678S–1688S. doi: 10.1093/ajcn/80.6.1678S. [DOI] [PubMed] [Google Scholar]
- 13.Schleicher RL, Sternberg MR, Lacher DA, et al. The vitamin D status of the US population from 1988 to 2010 using standardized serum concentrations of 25-hydroxyvitamin D shows recent modest increases. Am J Clin Nutr. 2016;104(2):454–461. doi: 10.3945/ajcn.115.127985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Calvo MS, Whiting SJ. Survey of current vitamin D food fortification practices in the United States and Canada. J Steroid Biochem Mol Biol. 2013;136:211–213. doi: 10.1016/j.jsbmb.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Gahche J, Bailey R, Burt V, et al. NCHS Data Brief. 61. 2011. Dietary supplement use among U.S adults has increased since NHANES III (1988-1994) pp. 1–8. [PubMed] [Google Scholar]
- 16.MacLaughlin J, Holick MF. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76(4):1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris SS. Vitamin D and African Americans. J Nutr. 2006;136(4):1126–1129. doi: 10.1093/jn/136.4.1126. [DOI] [PubMed] [Google Scholar]
- 18.Somjen D, Weisman Y, Kohen F, et al. 25-hydroxyvitamin D3-1alpha-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation. 2005;111(13):1666–1671. doi: 10.1161/01.CIR.0000160353.27927.70. [DOI] [PubMed] [Google Scholar]
- 19.Zehnder D, Bland R, Chana RS, et al. Synthesis of 1, 25-dihydroxyvitamin D(3) by human endothelial cells is regulated by inflammatory cytokines: a novel autocrine determinant of vascular cell adhesion. J Am Soc Nephrol. 2002;13(3):621–629. doi: 10.1681/ASN.V133621. [DOI] [PubMed] [Google Scholar]
- 20.Monkawa T, Yoshida T, Hayashi M, Saruta T. Identification of 25-hydroxyvitamin D3 1alpha-hydroxylase gene expression in macrophages. Kidney Int. 2000;58(2):559–568. doi: 10.1046/j.1523-1755.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 21.Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Ann Epidemiol. 2009;19(2):73–78. doi: 10.1016/j.annepidem.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bikle DD, Gee E, Halloran B, Kowalski MA, Ryzen E, Haddad JG. Assessment of the free fraction of 25-hydroxyvitamin D in serum and its regulation by albumin and the vitamin D-binding protein. J Clin Endocrinol Metab. 1986;63(4):954–959. doi: 10.1210/jcem-63-4-954. [DOI] [PubMed] [Google Scholar]
- 23.Yousefzadeh P, Shapses SA, Wang X. Vitamin D Binding Protein Impact on 25-Hydroxyvitamin D Levels under Different Physiologic and Pathologic Conditions. Int J Endocrinol. 2014;2014:981581. doi: 10.1155/2014/981581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lutsey PL, Michos ED, Misialek JR, et al. Race and Vitamin D Binding Protein Gene Polymorphisms Modify the Association of 25-Hydroxyvitamin D and Incident Heart Failure: The ARIC (Atherosclerosis Risk in Communities) Study. JACC Heart Fail. 2015;3(5):347–356. doi: 10.1016/j.jchf.2014.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michos ED, Misialek JR, Selvin E, et al. 25-hydroxyvitamin D levels, vitamin D binding protein gene polymorphisms and incident coronary heart disease among whites and blacks: The ARIC study. Atherosclerosis. 2015;241(1):12–17. doi: 10.1016/j.atherosclerosis.2015.04.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson-Cohen C, Hoofnagle AN, Ix JH, et al. Racial differences in the association of serum 25-hydroxyvitamin D concentration with coronary heart disease events. JAMA. 2013;310(2):179–188. doi: 10.1001/jama.2013.7228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Michos ED, Reis JP, Post WS, et al. 25-Hydroxyvitamin D deficiency is associated with fatal stroke among whites but not blacks: The NHANES-III linked mortality files. Nutrition. 2012;28(4):367–371. doi: 10.1016/j.nut.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider AL, Lutsey PL, Selvin E, et al. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Neurol. 2015;22(8):1220–1227. doi: 10.1111/ene.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henderson CM, Lutsey PL, Misialek JR, et al. Measurement by a Novel LC-MS/MS Methodology Reveals Similar Serum Concentrations of Vitamin D-Binding Protein in Blacks and Whites. Clin Chem. 2016;62(1):179–187. doi: 10.1373/clinchem.2015.244541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denburg MR, Hoofnagle AN, Sayed S, et al. Comparison of Two ELISA Methods and Mass Spectrometry for Measurement of Vitamin D-Binding Protein: Implications for the Assessment of Bioavailable Vitamin D Concentrations Across Genotypes. J Bone Miner Res. 2016;31(6):1128–1136. doi: 10.1002/jbmr.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgaz A, Orsini N, Larsson SC, Wolk A. Blood 25-hydroxyvitamin D concentration and hypertension: a meta-analysis. J Hypertens. 2011;29(4):636–645. doi: 10.1097/HJH.0b013e32834320f9. [DOI] [PubMed] [Google Scholar]
- 33.Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28(3):205–221. doi: 10.1007/s10654-013-9790-2. [DOI] [PubMed] [Google Scholar]
- 34.Jorde R, Figenschau Y, Emaus N, Hutchinson M, Grimnes G. Serum 25-hydroxyvitamin D levels are strongly related to systolic blood pressure but do not predict future hypertension. Hypertension. 2010;55(3):792–798. doi: 10.1161/HYPERTENSIONAHA.109.143990. [DOI] [PubMed] [Google Scholar]
- 35.Vimaleswaran KS, Cavadino A, Berry DJ, et al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–729. doi: 10.1016/S2213-8587(14)70113-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Kong J, Deb DK, Chang A, Li YC. Vitamin D receptor attenuates renal fibrosis by suppressing the renin-angiotensin system. J Am Soc Nephrol. 2010;21(6):966–973. doi: 10.1681/ASN.2009080872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 38.Grubler MR, Gaksch M, Kienreich K, et al. Effects of Vitamin D Supplementation on Plasma Aldosterone and Renin-A Randomized Placebo-Controlled Trial. J Clin Hypertens (Greenwich) 2016;18(7):608–613. doi: 10.1111/jch.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forman JP, Scott JB, Ng K, et al. Effect of vitamin D supplementation on blood pressure in blacks. Hypertension. 2013;61(4):779–785. doi: 10.1161/HYPERTENSIONAHA.111.00659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beveridge LA, Struthers AD, Khan F, et al. Effect of Vitamin D Supplementation on Blood Pressure: A Systematic Review and Meta-analysis Incorporating Individual Patient Data. JAMA Intern Med. 2015;175(5):745–754. doi: 10.1001/jamainternmed.2015.0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon? Eur J Epidemiol. 2014;29(1):1–14. doi: 10.1007/s10654-013-9874-z. [DOI] [PubMed] [Google Scholar]
- 42.Arora P, Song Y, Dusek J, et al. Vitamin D therapy in individuals with prehypertension or hypertension: the DAYLIGHT trial. Circulation. 2015;131(3):254–262. doi: 10.1161/CIRCULATIONAHA.114.011732. [DOI] [PubMed] [Google Scholar]
- 43.Chiu KC, Chu A, Go VL, Saad MF. Hypovitaminosis D is associated with insulin resistance and beta cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. doi: 10.1093/ajcn/79.5.820. [DOI] [PubMed] [Google Scholar]
- 44.Kayaniyil S, Vieth R, Retnakaran R, et al. Association of vitamin D with insulin resistance and beta-cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care. 2010;33(6):1379–1381. doi: 10.2337/dc09-2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Afzal S, Bojesen SE, Nordestgaard BG. Low 25-hydroxyvitamin D and risk of type 2 diabetes: a prospective cohort study and metaanalysis. Clin Chem. 2013;59(2):381–391. doi: 10.1373/clinchem.2012.193003. [DOI] [PubMed] [Google Scholar]
- 46.Reis JP, Michos ED, Selvin E, Pankow JS, Lutsey PL. Race, vitamin D-binding protein gene polymorphisms, 25-hydroxyvitamin D, and incident diabetes: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2015;101(6):1232–1240. doi: 10.3945/ajcn.115.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grubler MR, Gaksch M, Kienreich K, et al. Effects of vitamin D supplementation on glycated haemoglobin and fasting glucose levels in hypertensive patients: a randomized controlled trial. Diabetes Obes Metab. 2016 doi: 10.1111/dom.12709. [DOI] [PubMed] [Google Scholar]
- 48.George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta-analysis. Diabet Med. 2012;29(8):e142–150. doi: 10.1111/j.1464-5491.2012.03672.x. [DOI] [PubMed] [Google Scholar]
- 49.Seida JC, Mitri J, Colmers IN, et al. Clinical review: Effect of vitamin D3 supplementation on improving glucose homeostasis and preventing diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2014;99(10):3551–3560. doi: 10.1210/jc.2014-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nigil Haroon N, Anton A, John J, Mittal M. Effect of vitamin D supplementation on glycemic control in patients with type 2 diabetes: a systematic review of interventional studies. J Diabetes Metab Disord. 2015;14:3. doi: 10.1186/s40200-015-0130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jamka M, Wozniewicz M, Jeszka J, Mardas M, Bogdanski P, Stelmach-Mardas M. The effect of vitamin D supplementation on insulin and glucose metabolism in overweight and obese individuals: systematic review with meta-analysis. Sci Rep. 2015;5:16142. doi: 10.1038/srep16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114(2):379–393. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 53.Yin K, Agrawal DK. Vitamin D and inflammatory diseases. J Inflamm Res. 2014;7:69–87. doi: 10.2147/JIR.S63898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Beilfuss J, Berg V, Sneve M, Jorde R, Kamycheva E. Effects of a 1-year supplementation with cholecalciferol on interleukin-6, tumor necrosis factor-alpha and insulin resistance in overweight and obese subjects. Cytokine. 2012;60(3):870–874. doi: 10.1016/j.cyto.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 55.Jamka M, Wozniewicz M, Walkowiak J, Bogdanski P, Jeszka J, Stelmach-Mardas M. The effect of vitamin D supplementation on selected inflammatory biomarkers in obese and overweight subjects: a systematic review with meta-analysis. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1089-5. [DOI] [PubMed] [Google Scholar]
- 56.Gupta GK, Agrawal T, DelCore MG, Mohiuddin SM, Agrawal DK. Vitamin D deficiency induces cardiac hypertrophy and inflammation in epicardial adipose tissue in hypercholesterolemic swine. Exp Mol Pathol. 2012;93(1):82–90. doi: 10.1016/j.yexmp.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gardner DG, Chen S, Glenn DJ. Vitamin D and the heart. Am J Physiol Regul Integr Comp Physiol. 2013;305(9):R969–977. doi: 10.1152/ajpregu.00322.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bae S, Singh SS, Yu H, Lee JY, Cho BR, Kang PM. Vitamin D signaling pathway plays an important role in the development of heart failure after myocardial infarction. J Appl Physiol (1985) 2013;114(8):979–987. doi: 10.1152/japplphysiol.01506.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Ballegooijen AJ, Snijder MB, Visser M, et al. Vitamin D in relation to myocardial structure and function after eight years of follow-up: the Hoorn study. Ann Nutr Metab. 2012;60(1):69–77. doi: 10.1159/000336173. [DOI] [PubMed] [Google Scholar]
- 60.Rautiainen S, Wang L, Manson JE, Sesso HD. The role of calcium in the prevention of cardiovascular disease--a review of observational studies and randomized clinical trials. Curr Atheroscler Rep. 2013;15(11):362. doi: 10.1007/s11883-013-0362-4. [DOI] [PubMed] [Google Scholar]
- 61.Bristow SM, Gamble GD, Stewart A, Horne AM, Reid IR. Acute effects of calcium supplements on blood pressure and blood coagulation: secondary analysis of a randomised controlled trial in post-menopausal women. Br J Nutr. 2015;114(11):1868–1874. doi: 10.1017/S0007114515003694. [DOI] [PubMed] [Google Scholar]
- 62.Spence LA, Weaver CM. Calcium intake, vascular calcification, and vascular disease. Nutr Rev. 2013;71(1):15–22. doi: 10.1111/nure.12002. [DOI] [PubMed] [Google Scholar]
- 63.Anderson JJ, Klemmer PJ. Risk of high dietary calcium for arterial calcification in older adults. Nutrients. 2013;5(10):3964–3974. doi: 10.3390/nu5103964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mangano KM, Walsh SJ, Insogna KL, Kenny AM, Kerstetter JE. Calcium intake in the United States from dietary and supplemental sources across adult age groups: new estimates from the National Health and Nutrition Examination Survey 2003-2006. J Am Diet Assoc. 2011;111(5):687–695. doi: 10.1016/j.jada.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ross AC, Manson JE, Abrams SA, et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine what clinicians need to know. J Clin Endocrinol Metab. 2011;96(1):53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gallagher JC, Smith LM, Yalamanchili V. Incidence of hypercalciuria and hypercalcemia during vitamin D and calcium supplementation in older women. Menopause. 2014;21(11):1173–1180. doi: 10.1097/GME.0000000000000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Garland CF, Kim JJ, Mohr SB, et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. 2014;104(8):e43–50. doi: 10.2105/AJPH.2014.302034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schottker B, Jorde R, Peasey A, et al. Vitamin D mortality: meta-analysis of individual participant data from a large consortium of cohort studies from Europe and the United States. BMJ. 2014;348:g3656. doi: 10.1136/bmj.g3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168(15):1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brondum-Jacobsen P, Benn M, Jensen GB, Nordestgaard BG. 25-hydroxyvitamin d levels and risk of ischemic heart disease, myocardial infarction, and early death: population-based study and meta-analyses of 18 and 17 studies. Arterioscler Thromb Vasc Biol. 2012;32(11):2794–2802. doi: 10.1161/ATVBAHA.112.248039. [DOI] [PubMed] [Google Scholar]
- 71.Aleksova A, Belfiore R, Carriere C, et al. Vitamin D Deficiency in Patients with Acute Myocardial Infarction: An Italian Single-Center Study. Int J Vitam Nutr Res. 2015;85(1-2):23–30. doi: 10.1024/0300-9831/a000220. [DOI] [PubMed] [Google Scholar]
- 72.Chowdhury R, Stevens S, Ward H, Chowdhury S, Sajjad A, Franco OH. Circulating vitamin D, calcium and risk of cerebrovascular disease: a systematic review and meta-analysis. Eur J Epidemiol. 2012;27(8):581–591. doi: 10.1007/s10654-012-9729-z. [DOI] [PubMed] [Google Scholar]
- 73.Durup D, Jorgensen HL, Christensen J, et al. A Reverse J-Shaped Association Between Serum 25-Hydroxyvitamin D and Cardiovascular Disease Mortality: The CopD Study. J Clin Endocrinol Metab. 2015;100(6):2339–2346. doi: 10.1210/jc.2014-4551. [DOI] [PubMed] [Google Scholar]
- 74.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 75.Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr. 2000;72(3):690–693. doi: 10.1093/ajcn/72.3.690. [DOI] [PubMed] [Google Scholar]
- 76.Manousaki D, Mokry LE, Ross S, Goltzman D, Richards JB. Mendelian Randomization Studies do not Support a Role for Vitamin D in Coronary Artery Disease. Circ Cardiovasc Genet. 2016 doi: 10.1161/CIRCGENETICS.116.001396. [DOI] [PubMed] [Google Scholar]
- 77.Afzal S, Brondum-Jacobsen P, Bojesen SE, Nordestgaard BG. Genetically low vitamin D concentrations and increased mortality: Mendelian randomisation analysis in three large cohorts. BMJ. 2014;349:g6330. doi: 10.1136/bmj.g6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Paik JM, Curhan GC, Sun Q, et al. Calcium supplement intake and risk of cardiovascular disease in women. Osteoporos Int. 2014;25(8):2047–2056. doi: 10.1007/s00198-014-2732-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Raffield LM, Agarwal S, Hsu FC, et al. Nutr Metab Cardiovasc Dis. 2016. The association of calcium supplementation and incident cardiovascular events in the Multi-ethnic Study of Atherosclerosis (MESA) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Anderson JJB, Kruszka B, Delaney JAC, et al. Calcium Intake and the Risk of Coronary Arterial Calcification and its Progression among Older Adults: 10-year follow-up of the Multi-Ethnic Study of Atherosclerosis (MESA) J Am Heart Assoc. 2016 doi: 10.1161/JAHA.116.003815. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang X, Chen H, Ouyang Y, et al. Dietary calcium intake and mortality risk from cardiovascular disease and all causes: a meta-analysis of prospective cohort studies. BMC Med. 2014;12:158. doi: 10.1186/s12916-014-0158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pradhan AD, Manson JE. Update on the Vitamin D and OmegA-3 trial (VITAL) J Steroid Biochem Mol Biol. 2016;155(Pt B):252–256. doi: 10.1016/j.jsbmb.2015.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neale RE, Armstrong BK, Baxter C, et al. The D-Health Trial: A randomized trial of vitamin D for prevention of mortality and cancer. Contemp Clin Trials. 2016;48:83–90. doi: 10.1016/j.cct.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 84.Scragg R, Waayer D, Stewart AW, et al. The Vitamin D Assessment (ViDA) Study: design of a randomized controlled trial of vitamin D supplementation for the prevention of cardiovascular disease, acute respiratory infection, falls and non-vertebral fractures. J Steroid Biochem Mol Biol. 2015 doi: 10.1016/j.jsbmb.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 85.Vitamin D and Mortality in Heart Failure (EVITA) https://clinicaltrials.gov/ct2/show/NCT01326650.
- 86.Vitamin D and Longevity (VIDAL) Trial: Randomised Feasibility Study. http://www.isrctn.com/ISRCTN46328341.
- 87.Pittas AG, Dawson-Hughes B, Sheehan PR, et al. Rationale and design of the Vitamin D Type 2 Diabetes (D2d) study: a diabetes prevention trial. Diabetes Care. 2014;37(12):3227–3234. doi: 10.2337/dc14-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.DO-HEALTH / Vitamin D3-Omega3-Home Excercise-Healthy Ageing and Longevity Trial (DO-HEALTH) https://clinicaltrials.gov/ct2/show/study/NCT01745263.
- 89.Finnish Vitamin D Trial (FIND) https://clinicaltrials.gov/ct2/show/NCT01463813.
- 90.Mann MC, Hobbs AJ, Hemmelgarn BR, Roberts DJ, Ahmed SB, Rabi DM. Effect of oral vitamin D analogs on mortality and cardiovascular outcomes among adults with chronic kidney disease: a meta-analysis. Clin Kidney J. 2015;8(1):41–48. doi: 10.1093/ckj/sfu122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Witte KK, Byrom R, Gierula J, et al. Effects of Vitamin D on Cardiac Function in Patients With Chronic HF: The VINDICATE Study. J Am Coll Cardiol. 2016;67(22):2593–2603. doi: 10.1016/j.jacc.2016.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jiang WL, Gu HB, Zhang YF, Xia QQ, Qi J, Chen JC. Vitamin D Supplementation in the Treatment of Chronic Heart Failure: A Meta-analysis of Randomized Controlled Trials. Clin Cardiol. 2016;39(1):56–61. doi: 10.1002/clc.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Radford LT, Bolland MJ, Mason B, et al. The Auckland calcium study: 5-year post-trial follow-up. Osteoporos Int. 2014;25(1):297–304. doi: 10.1007/s00198-013-2526-z. [DOI] [PubMed] [Google Scholar]
- 94.Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ. 2010;341:c3691. doi: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lewis JR, Zhu K, Thompson PL, Prince RL. The effects of 3 years of calcium supplementation on common carotid artery intimal medial thickness and carotid atherosclerosis in older women: an ancillary study of the CAIFOS randomized controlled trial. J Bone Miner Res. 2014;29(3):534–541. doi: 10.1002/jbmr.2117. [DOI] [PubMed] [Google Scholar]
- 96.Challoumas D, Stavrou A, Pericleous A, Dimitrakakis G. Effects of combined vitamin D--calcium supplements on the cardiovascular system: should we be cautious? Atherosclerosis. 2015;238(2):388–398. doi: 10.1016/j.atherosclerosis.2014.12.050. [DOI] [PubMed] [Google Scholar]
- 97.American Geriatrics Society Workgroup on Vitamin DSfOA: Recommendations abstracted from the American Geriatrics Society Consensus Statement on vitamin D for Prevention of Falls and Their Consequences. J Am Geriatr Soc. 2014;62(1):147–152. doi: 10.1111/jgs.12631. [DOI] [PubMed] [Google Scholar]
- 98.LeFevre ML, Force USPST. Screening for vitamin D deficiency in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2015;162(2):133–140. doi: 10.7326/M14-2450. [DOI] [PubMed] [Google Scholar]
- 99.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25(10):2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.SACN Vitamin D and Health Report. Scientific Advisory Committee on Nutrition. 2016 [Google Scholar]
- 101.Vitamin D increasing supplement use among at-risk groups. National Institute for Health and Care Excellence. 2014 [Google Scholar]
- 102.Pilz S, Verheyen N, Grubler MR, Tomaschitz A, Marz W. Vitamin D and cardiovascular disease prevention. Nat Rev Cardiol. 2016;13(7):404–417. doi: 10.1038/nrcardio.2016.73. [DOI] [PubMed] [Google Scholar]
- 103.Rosen CJ, Abrams SA, Aloia JF, et al. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab. 2012;97(4):1146–1152. doi: 10.1210/jc.2011-2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Huang KE, Milliron BJ, Davis SA, Feldman SR. Surge in US outpatient vitamin D deficiency diagnoses: National Ambulatory Medical Care Survey analysis. South Med J. 2014;107(4):214–217. doi: 10.1097/SMJ.0000000000000085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tarn DM, Paterniti DA, Wenger NS. Provider Recommendations in the Face of Scientific Uncertainty: An Analysis of Audio-Recorded Discussions about Vitamin D. J Gen Intern Med. 2016;31(8):909–917. doi: 10.1007/s11606-016-3667-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


