Abstract
Background & Aims
Celiac disease is detected using serology and endoscopy analyses. We used multiple statistical analyses of a geographically isolated population in the United States to determine whether a single serum screening can identify individuals with celiac disease.
Methods
We performed a retrospective study of 3555 pediatric patients (18 years old or younger) in the intermountain West region of the United States from January 1, 2008 through September 30, 2013. All patients had undergone serologic analyses for celiac disease, including measurement of antibodies to tissue transglutaminase (TTG) and/or deamidated gliadin peptide (DGP), and had duodenal biopsies collected within the following year. Modified Marsh criteria were used to identify patients with celiac disease. We developed models to identify patients with celiac disease using logistic regression and classification and regression tree (CART) analysis.
Results
Single use of a test for serum level of immunoglobulin A (IgA) against TTG identified patients with celiac disease with 90% sensitivity, 90% specificity, a 61% positive-predictive value (PPV), a 90% negative-predictive value, and an area under the receiver operating characteristic curve value of 0.91; these values were higher than those obtained from assays for IgA against DGP or IgG against TTG plus DGP. Not including the test for DGP antibody caused only 0.18% of celiac disease cases to be missed. Level of TTG IgA 7-fold the upper limit of normal (ULN) identified patients with celiac disease with a 96% PPV and 100% specificity. Using CART analysis, we found a level of TTG IgA 3.2-fold the ULN and above to most accurately identify patients with celiac disease (PPV of 89%). Multivariable CART analysis showed that a level of TTG IgA 2.5-fold the ULN and above was sufficient to identify celiac disease in patients with type 1 diabetes (PPV of 88%). Serum level of IgA against TTG in patients with vs those without trisomy 21 did not affect diagnosis predictability in CART analysis.
Conclusion
In a population-based study, we found that serum level of IgA against TTG can identify patients with celiac disease with PPVs of about 90%. Predictive values increase greatly when levels are markedly above the ULN or when the assay is used in combination with other variables. Measurement of IgG against TTG or DGP does not increase the accuracy of detection of celiac disease based against TTG IgA levels. There is a low risk of false-positive results from serologic analysis in patients with type I diabetes or persistent increases in antibody against TTG on repeat testing.
Keywords: gluten, enteropathy, children, diagnosis
INTRODUCTION
Current North American guidelines to diagnose celiac disease include screening symptomatic and high-risk patients with serum antibodies followed by standard confirmation with duodenal biopsies. European pediatric guidelines offer a less-invasive diagnostic algorithm with increases in antibodies to tissue transglutaminase (TTG) immunoglobulin and endomysial antibodies (EMA), coupled with HLA testing to determine diagnosis (1). In North America this serum-based diagnostic approach has not been uniformly adopted (2–5), although a recent Canadian cohort demonstrated increased utility of a single antibody serology to TTG for celiac disease diagnosis and also analyzed patients who would meet European guidelines for forgoing esophagogastroduodenoscopy (EGD), irrespective of genetic testing (12).
Multiple pediatric studies demonstrate strong correlation between TTG and/or antibody to deamidated gliaden peptide (DGP) levels and duodenal histology (9–14). Given the effects of cost on payers with United States (US) health care reform changes, we sought to determine the most accurate diagnostic tests, which would limit unnecessary testing and costs.
The first aim of this study was to evaluate the utility of elevated TTG IgA antibodies as a single diagnostic test confirmed by duodenal biopsy, with comparison to TTG IgG and DGP IgA and IgG antibody assays. We hypothesized a reliable diagnosis of celiac disease could be made without duodenal biopsy in the proper circumstance. Our second aim was to evaluate if demographic features, including high-risk populations, could predict pediatric celiac disease without biopsy. Thirdly, we evaluated the utility of classification and regression analysis tree (CART) modeling to create a clinical predictive model, a technique not previously utilized.
METHODS
Design, procedure and study population
The state of Utah provides a unique opportunity to study pediatric gastrointestinal disease in a population-based fashion (15). Most pediatric care (primary and subspecialist) takes place within one large healthcare organization, with more than 20 hospitals and clinics covering a large geographically isolated region of the intermountain west. After institutional review board approval, an observational study was performed using an electronic data warehouse for chart review.
Data was obtained from January 1, 2008 through September 30, 2013. We included patients under 19 years of age with a serum IgA, and one or more of the following tests: antibody assays to TTG-IgA, DGP-IgA, TTG-IgG, DGP-IgG. Patients who had duodenal biopsies within 365 days of serology were included. Patients diagnosed with celiac disease from a previous duodenal biopsy were excluded.
Patients who were 18-years-old at serologic testing and who turned 19 before EGD and biopsy were included (n=13). Baseline demographic data obtained from records included age at procedure, sex, body mass index (BMI). Additional diagnoses of Trisomy 21 and Type I Diabetes were also flagged, if documented.
Disease and operational definitions
Modified Marsh criteria were used to define enteropathy (4–6). Marsh scores of 2 or 3 were designated as celiac disease; patients without celiac disease had scores of 0, 1, or 0–1 (6). For pathology reports that did not assign Marsh categories, patients were given Marsh scores of 2 or 3 if the report contained: total villous atrophy, subtotal or partial villous atrophy, with or without crypt hyperplasia. Each pathology report was individually reviewed by 3 pediatric gastroenterologists who confirmed the reports. Individual slides were not reevaluated by a single pathologist given the large number of slides from multiple pathology labs in order to replicate a standard clinical scenario.
Laboratory analyses
The serologic tests employed were manufactured by INOVA Diagnostics Inc. of San Diego, CA. All tests were performed at ARUP Laboratories in Salt Lake City. The quantitative scale for each of the tests followed INOVA Diagnostic’s recommended screening test: positive score was 19 units and higher; less than 19 units is negative; there is no upper limit threshold with this assay (14). ARUP offers a celiac reflexive panel, with a quantitative serum IgA level by indirect fluorescent antibody (IFA) with reflex to IgA or IgG antibodies depending on the individual’s total serum IgA. This testing algorithm will reflex to the TTG IgG and DGP IgG serology for patients with low or deficient IgA for age. We did not include patients with results from outside laboratories with different reference standards.
Thresholds for antibody analysis were chosen at multiples of the upper limit of normal (ULN) IgA and IgG antibody units using the positive ARUP assay value of 19 and above (e.g. a TTG IgA of 95 units would be 5 times the ULN). We then analyzed our pediatric cohort exclusively to measure the optimal cutoff for the most sensitive positive TTG-IgA antibody threshold. We found this to be a TTG of 22, or 1.2 above the laboratory reference (Table 2).
Table 2.
TTG-IgA antibody screening and diagnostic test performance
| TTG-IgA xULN (n) | PPV | NPV | Sens | Spec |
|---|---|---|---|---|
| 1 (763) | 61 | 98 | 90 | 90 |
| 2 (535) | 79 | 97 | 82 | 96 |
| 3 (430) | 89 | 96 | 74 | 99 |
| 4 (380) | 92 | 95 | 68 | 99 |
| 5 (343) | 93 | 94 | 62 | 99 |
| 6 (278) | 95 | 92 | 51 | 100 |
| 7 (221) | 96 | 91 | 41 | 100 |
| 8 (127) | 97 | 89 | 24 | 100 |
| 9 (100) | 98 | 88 | 19 | 100 |
| 10 (80) | 98 | 88 | 21 | 100 |
AUROC, area under receiver operator characteristic curve; IgA, immunoglobulin A; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; TTG, tissue transglutaminase; ULN, upper limit of normal
Variables
The main outcome of interest was celiac disease (Marsh 2 or higher histology). Variables analyzed for prediction of enteropathy included antibody serology, age, sex, body mass index (BMI), IgA level, Type I diabetes mellitus (DM), Trisomy 21. BMI scores were converted to Z scores per year of age, using the 2000 Center for Disease Control (CDC) growth chart standards.
Statistical Analysis
Statistical differences were assessed using t-test, Pearson X2 tests and Wilcoxon-Mann-Whitney test where appropriate. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for serum studies were calculated for all serum samples. Associations among celiac disease patients and candidate variables were assessed using logistic regression. Multivariable analysis was performed with significant variables in sequential univariate logistic regression. These variables were also included in a stepwise CART model to evaluate independent contribution to prediction of celiac disease. Significance was defined as P value < 0.05 throughout. For serum test accuracy in predicting disease, we plotted receiver operating characteristic (ROC) curves and reported area under the ROC (AUROC) to compare serology test performance.
We performed CART analysis as a second multivariable prediction model. With CART analysis, variables are analyzed in a dichotomous tree-pattern at decision node points using the pre-determined predictor variables (results are in sums of squares) to illustrate probabilistic outcomes (See supplemental material).
Missing serology and BMI Z-scores were replaced by using the hotdeck procedure in Stata. The above analyses were performed using Stata-13 statistical software [College Station, TX, StataCorp LP] and CART analysis was performed using JMP Pro 11.2 statistical software [Cary, NC, SAS institute]. This investigation was supported by the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 5UL1TR001067-02 (formerly 8UL1TR000105 and UL1RR025764).
RESULTS
We identified 30413 distinct patients who had serologic testing for celiac disease. There were 3555 distinct patient cases who underwent intestinal biopsies, of which 517 had Marsh 2 or 3 duodenal biopsies. This gave a 1.7% (517/30413) regional prevalence of pediatric celiac disease in screened patients.
Table 1 shows study population characteristics and compares celiac cases to non-cases. Patients with celiac disease tended to be younger, female, with a significantly different median TTG-IgA antibody value than non-celiac patients (p <0.003 between groups).
Table 1.
Cohort characteristics and univariate analysis of celiac disease cases versus non-cases.
| All cases | CD | Controls | P value | |
|---|---|---|---|---|
| N (%) | 3555 | 517 (15) | 3038 (85) | - |
| Girls (%) | 2010 (57) | 330 (64) | 1680 (55) | <0.003 |
| Under 2 years old (%) | 273 (8) | 27 (5) | 246 (8) | 0.023 |
| Mean age (SD) | 10 (5) | 9.4 (4.8) | 10.0 (5.3) | 0.016 |
| Low serum IgAa (%) | 222 (6) | 34 (7) | 188 (6) | 0.736 |
| Trisomy 21 (%) | 58 (2) | 17 (3) | 41 (2) | 0.001 |
| Type I DM (%) | 170 (5) | 88 (17) | 82 (3) | <0.003 |
| BMI Z score (SD) | −0.13 (1.4) | −0.14 (1.4) | −0.12 (1.4) | 0.78 |
BMI, body mass index; DM, diabetes mellitus; IgA, immunoglobulin A;
serum IgA value flaged as “low”
TTG as Single Diagnostic Test
The AUROC curve for disease screening using only TTG IgA antibody values of our cohort was 0.9466. Of these 3513 patients, 763 patients had positive serology (serum assay >22). Table 2 shows the performance of measuring IgA against TTG as a single diagnostic test with regards to each ULN increase. For example, the sensitivity, specificity, PPV, and NPV for patients with a TTG > 1× ULN was 90%, 90%, 61%, and 98% respectively, with AUROC curve of 0.901.
Highly-positive TTG-IgA antibody group
For IgA sufficient patients with antibodies to TTG IgA >7× ULN (n=221), the sensitivity, specificity, PPV and NPV were 41%, 100%, 96%, 91% respectively. Among these patients there were 8 who had normal biopsies (3.6%). The comorbidities identified in this group included: 3 patients with Type I DM, 1 patient with eosinophilic esophagitis, 1 patient with gastritis, 1 with poor growth and 2 patients without comorbidity. Of the patients with DM, 1 patient developed histologic enteropathy on repeat biopsy performed 34 months after initial antibody measurement. Two of these patients had normal antibody to TTG IgA level when the test was repeated. In the patients without DM but high TTG IgA antibody, 2 had negative serology upon repeat testing, and one of those two had a negative repeat duodenal biopsy. No other autoimmune or inflammatory associations were found in the false-positives.
Additional Serology
See Table 3 for results. DGP IgA antibodies as a single serologic test had a sensitivity of 29%, specificity of 93%, PPV 42%, NPV 89% with AUROC 0.6657. In patients with DGP IgA and TTG IgA antibodies (n=1676), the combined performance had an AUROC of 0.9464. There were 3 patients with positive DGP serology but a false-negative TTG, which would diagnose additional celiac disease in 0.18% (3/1676) of our population if DGP IgA antibodies were used in-addition to TTG as a primary screening test.
Table 3.
Single antibody serology test performance
| Serology | AUROC | PPV | NPV | Sens | Spec |
|---|---|---|---|---|---|
| TTG-IgA | 0.9466 | 61 | 98 | 90 | 90 |
| DGP-IgA | 0.6657 | 42 | 89 | 29 | 93 |
| TTG-IgG | 0.5054 | 33 | 94 | 19 | 97 |
| DGP-IgG | 0.5585 | 25 | 86 | 13 | 93 |
AUROC, area under receiver operator characteristic curve; DGP, deamidated gliadin peptide; IgA, immunoglobulin A; IgG, immunoglobulin G; NPV, negative predictive value; PPV, positive predictive value; Sens, sensitivity; Spec, specificity; TTG, tissue transglutaminase; ULN, upper limit of normal
For patients with low or insufficient IgA levels, IgG TTG and DGP levels were used. TTG IgG alone (n=236) had sensitivity, specificity, PPV, NPV of 19%, 97%, 33%, 94%, while DGP-IgG has even worse performance. The AUROC for TTG IgG and DGP IgG combined was 0.5629.
Regression Analysis
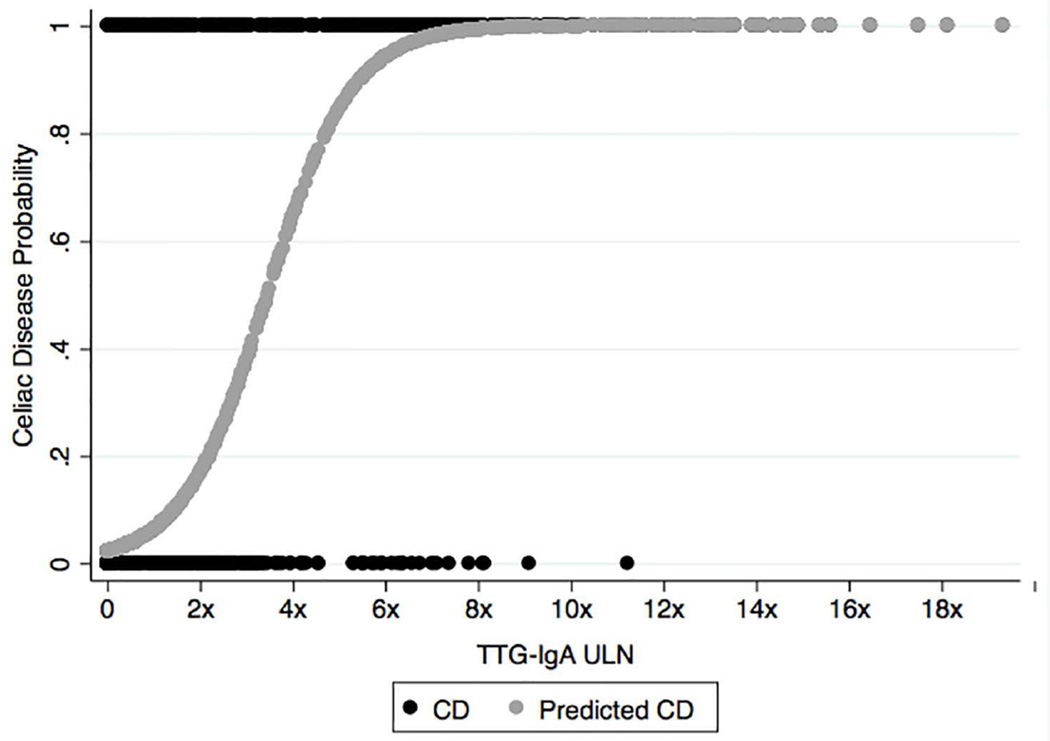
Univariate analysis for the outcome of celiac disease was performed with TTG IgA antibody levels, female sex, Trisomy 21 and Type I DM (Table 1). Neither BMI nor insufficient serum IgA level was significantly associated with celiac disease. Using multivariable logistic regression, Type I Diabetes, Trisomy 21 and TTG-IgA associations remained significant after adjusting for age, BMI and sex (Table 4). Figure 1 illustrates our cohort’s probability of enteropathy using TTG-IgA antibody as a single predictor of disease by applying a logistic curve to cases and non-cases through all antibody values.
Table 4.
Multivariable logistic regression of celiac disease adjusted for age, sex, BMI
| OR (CI) | P value | |
|---|---|---|
| TTG-IgA | 1.05 (1.05, 1.06) | <0.003 |
| Trisomy 21 | 2.6 (1.5, 4.6) | <0.003 |
| Type I DM | 7.5 (5.5, 10.4) | <0.003 |
| Low serum IgAa | 1.05 (0.7, 1.5) | 0.794 |
DM, Diabetes mellitus; IgA, immunoglobulin; OR, odds ratio; TTG, tissue transglutaminase;
serum IgA value flagged as “low”
Figure 1.
Prediction of celiac disease using antibodies to TTG-IgA shown by regression curve. IgA, immunoglobulin A; TTG, tissue transglutaminase; ULN, upper limit normal;
CART Analysis
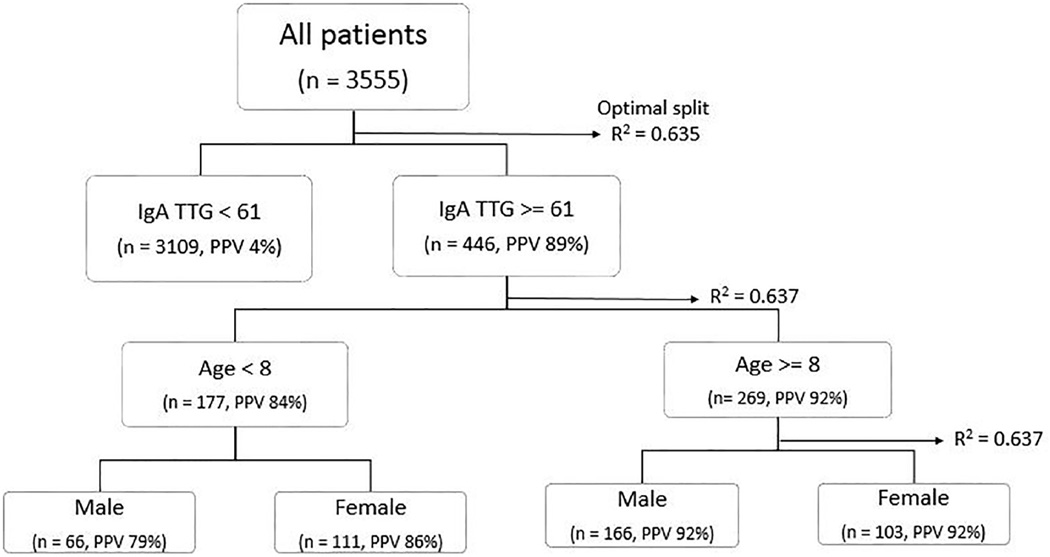
Antibodies to TTG-IgA was the strongest predictor for celiac disease in univariate and multivariable regression. CART analysis revealed the first optimal nominal split point for disease prediction at a TTG-IgA value of 61, or 2.7× ULN (PPV= 89%, R2 = 0.635) (Figure 2). After TTG antibodies, age was the next most significant variable (not including Trisomy 21 or Type I DM given their collinearity). Although age and celiac disease association was not significant with multivariable regression, when broken into categorical age groups there was some association of TTG IgA antibody level and advancing age. CART revealed this with an increasing PPV to 92% for patients equal or older than 8 years old and reduced to 83% for patients younger than 8 (R2 = 0.637).
Figure 2.
CART analysis of celiac disease with TTG-IgA antibody level, age & sex; IgA, immunoglobulin A; PPV, positive predictive value; TTG, tissue transglutaminase;
We then performed two additional CART models, using Type I DM or Trisomy 21 as the second decision node after TTG-IgA (see Supplemental material). For patients with Type I DM, the first optimal predictive cutpoint of antibody to TTG-IgA lowered to 2.5× ULN (raw value 55), with PPV of 87.5% (R2 = 0.092). For Trisomy 21, the optimal antibody value of 3× ULN (raw value 65, 83% PPV, R2 = 0.016) was not any more predictive than testing TTG-IgA antibodies alone.
DISCUSSION
Our sizeable North American pediatric population-based cohort is useful for real-world practice as we demonstrated the diagnostic value of single antibody serology testing using several statistical methods. We showed that a single diagnostic test (antibodies to TTG-IgA), at given thresholds, could determine if duodenal biopsies are absolutely indicated for patients. This single serum test could predict more than 96% of positive biopsies in patients with antibody level of TTG-IgA >7× ULN. Our study is novel in that we suggest practitioners with patients who have this antibody level >7× ULN could offer non-biopsy diagnosis and commence a gluten free diet (GFD). This threshold would be an advantage for patients with early immunological effects from gluten, despite being asymptomatic from a gastrointestinal standpoint.
For patients or practitioners worried about the 4% misclassification at this level, we showed that repeat serum testing could reduce this risk to 2% (5/221 patients). While ESPGHAN offers non-biopsy diagnosis to patients who have antibodies to TTG-IgA >10× ULN with confirmatory genetic testing, we find their guidelines less applicable in practice and more costly given the high price of genetic HLA testing. Additionally, our study evaluates all patients in both primary care and referral populations, rather than limiting analysis to patients with significant GI symptoms or only those evaluated by a gastroenterologist, which also increases this study’s applicability to other patient populations (7–10, 17). We did not use symptomatology as a part of our inclusion criteria or multivariable analysis as required in the ESPGHAN guidelines; however, symptomatology has been shown to be difficult to assign or interpret for pediatric patients who present for celiac disease diagnostics (22).
Neither antibodies to DGP nor IgG TTG/DGP strengthened our pediatric celiac disease prediction, and we would advise against dual antibody tests unless the clinical presentation was concerning for enteropathy and the TTG-IgA value was weakly positive. Type I DM, Trisomy 21, and female sex were all independently associated with an increased risk for celiac disease, but in multivariable analysis, only DM and Trisomy 21 remained significant predictors for disease. Using these dataset models allows clinicians to more accurately estimate the likelihood of celiac disease by matching a patient’s clinical variables paired with an initial TTG-IgA antibody level. While EMA is a very specific test for celiac disease, the cost of this separate serology and its laboratory labor requirements makes it less feasible in routine screening, but can be considered as supportive of disease if patients wish to forego endoscopy.
We used CART analysis as a new tree model that provides optimal multivariable predictive cut points for an increased likelihood of celiac disease. CART analysis is an advantageous clinical tool based on non-parametric decision-making to determine predictive usability of several variables. While logistic regression can only be interpreted as a probability for a given outcome, CART analysis provides a stepwise prediction and provides a best-fit scenario within a clinical situation (17, 18). Using this model, we determined that patients with increased antibodies to TTG (IgA) of 1–3-fold the ULN should undergo EGD for the most definitive diagnosis, whereas practitioner and patient risk–benefit discussions should be offered to patients with levels of TTG-IgA of 3–7-fold the ULN; patients with levels greater than 7-fold the ULN could forego biopsy analysis if results from repeat serum supported findings from the first test (see supplemental material). CART helps show the effects of variables such as age, which may be less-seemingly significant during logistic regression, but has some clinical impact when analyzed step-wise with 1 or 2 other variables (Figure 4).
Reassuringly, our cohort’s false-positive difference is comparable to the prospective European cohort studies. This is interesting, given that their study subjects were all HLA positive, and/or their patients had repeat antibody tests prior to biopsies (reducing their true false-positive rate) (1, 8, 11, 12). Our post-hoc analysis showed that if TTG-IgA serology was repeated and if Type I DM patients were not included (given their increased baseline celiac disease risk), the false-positive rate is even lower than these studies (8, 12).
Our study has several important limitations. While this is a retrospective analysis, we used both disease codes as well as keyword searches to mitigate possibilities for missed data. We did not analyze patients’ genetic makeup given the infrequency of HLA testing in our population. We also did not include DGP-IgG antibody serology, as this single test is not routinely performed in our pediatric cohort. The biopsies are subjective results by different pathologists and we did not review the number or position of duodenal biopsies taken in endoscopy that could lead to a false-negative result; yet, we feel this makes the results more generalizable and provides a standard clinical practice example for patients. Our study did not show a low IgA was statistically associated with a diagnosis of celiac disease, which is explained in that we analyzed IgA insufficient, not true IgA deficient individuals. Because of this, we support the guideline that IgA deficient patients with symptoms or concern for celiac disease undergo biopsies for the most accurate diagnosis (2, 20, 21).
Our findings are consistent with Scandinavian studies that showed positive assays for TTG-IgA antibodies alone was the “best tradeoff between sensitivity, specificity, and cost (12).” With the changing face of U.S. health care reform including rising costs of endoscopy procedures coupled with increased utilization of “high deductible” health care plans (22–24) the decision to proceed with endoscopy may become more difficult for practitioners and families. In a system with capitated health care cost distribution, decreasing a proportion of invasive procedures could result in significant cost savings. Although a formal cost effectiveness analysis regarding our serology testing and diagnosis of pediatric celiac disease has not yet been performed, reducing unnecessary procedures has the potential for reducing costs to any healthcare system and families. Only a minority of patients in this study who underwent EGD had abnormal antibodies to TTG-IgA, suggesting some proportion of endoscopy could be avoided. This was shown with the Stockholm group’s 2012 cost analysis, in which, using the ESPGHAN guidelines, they could have avoided 143 endoscopies and showed a cost reduction of 8% for total diagnostic workup (12). Catching early latent disease in children should result in similar post-diagnosis cost savings. While there is sparse literature on the costs of undiagnosed celiac disease, an adult study in Olmsted County, Minnesota demonstrated a 39% reduction of direct medical costs the year after enteropathy confirmation compared to the prior year (22). Our results highlight the need for formal decision or cost-effectiveness analysis to guide medical decision-making. Future studies can address these knowledge gaps.
For parents and practitioners who fear the impact or lifestyle costs of false-positive diagnosis without biopsy, repeating TTG-IgA antibody serology (while still on a gluten-containing diet) after initial measurement can reduce risk of misclassification and unnecessary implementation of a GFD (patients with >5× ULN TTG-IgA had false-positive reduction of 5 to 2%, and from 4 to 2% with TTG-IgA >7× ULN upon retesting). Additionally, some parents may be concerned about the social impacts of a GFD, but quality of life is similar in children with and without celiac disease (25). Furthermore, compliance with GFD one year after a non-biopsy diagnosis was reported as “always” or “often” in more than 98% of European adolescents coupled with appropriate antibody reduction (26). Ideally, future studies will show reduced lifetime complications in patients with early detection by serology, prior to development of enteropathy.
Celiac disease remains a challenging diagnosis for patients who have weakly positive TTG or DGP antibody titers or those with elevated titers but who remain asymptomatic (7, 22). As a single screening test, antibodies to TTG-IgA has the best predictive performance compared to DGP or IgG antibody serology in multiple clinical scenarios and this knowledge should inform practitioners to avoid unnecessary ordering of additional lab tests. CART analysis is an objective decision tree model that incorporates individual characteristics and TTG-IgA antibody values to show likelihood of celiac disease diagnosis, while logistic regression suggests many patients could safely forego biopsies at values >7× ULN. As healthcare reform continues, these findings will help guide clinical decision-making and allow the best use of limited health care resources.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Dubé C, Rostom A, Sy R, Cranney A, et al. The prevalence of celiac disease in average-risk and at-risk Western European populations: a systematic review. Gastro. 2005 Apr;128(4 Suppl 1):S57–S67. doi: 10.1053/j.gastro.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 2.Fasano A, et al. Prevalence of celiac disease in at-risk and not-at-risk groups in the United States: a large multicenter study. Arch Intern Med. 2003 Feb 10;163(3):286–292. doi: 10.1001/archinte.163.3.286. [DOI] [PubMed] [Google Scholar]
- 3.Rostom A, Dubé C, Cranney A, et al. Celiac disease. Evid Rep Technol Assess (Summ) 2004 Jun;(104):1–6. [PMC free article] [PubMed] [Google Scholar]
- 4.Hill ID, et al. Guideline for the Diagnosis and Treatment of Celiac Disease in Children: Recommendations of the North American Society for Pediatric Gastroenterology, Hepatology and Nutrition. J Pediatr Gastroenterol Nutr. 2005 Jan;40(1):1–1. doi: 10.1097/00005176-200501000-00001. [DOI] [PubMed] [Google Scholar]
- 5.Rostom A, Murray JA, Kagnoff MF. American Gastroenterological Association (AGA) Institute technical review on the diagnosis and management of celiac disease. Gastro. 2005;128:38–46. doi: 10.1053/j.gastro.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 6.Oberhuber G. Histopathology of celiac disease. Biomed Pharmacother. 2000;54:368–372. doi: 10.1016/S0753-3322(01)80003-2. [DOI] [PubMed] [Google Scholar]
- 7.Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: Diagnosis and management of celiac disease. Am J Gastroenterol. 2013;108:656. doi: 10.1038/ajg.2013.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Husby S, et al. ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. 2012 Jan;54(1):136–160. doi: 10.1097/MPG.0b013e31821a23d0. [DOI] [PubMed] [Google Scholar]
- 9.Gidrewicz D, Potter K, Trevenen CL, Lyon M, Butzner JD. Evaluation of the ESPGHAN Celiac Guidelines in a North American Pediatric Population. Am J Gastroenterol. 2015 May;110(5):760–767. doi: 10.1038/ajg.2015.87. [DOI] [PubMed] [Google Scholar]
- 10.Donaldson MR, Book LS, Lieferman KM, Zone JJ, Neuhausen SL. Strongly positive tissue transglutaminase antibodies are associated with Marsh 3 histopathology in adult and pediatric celiac disease. J Clin Gastroenterol. 2008;42:256–260. doi: 10.1097/MCG.0b013e31802e70b1. [DOI] [PubMed] [Google Scholar]
- 11.Webb C, et al. Celiac disease can be predicted by high levels of anti-tissue transglutaminase antibodies in population-based screening. J Pediatr Gastroenterol Nutr. 2015 Jun;60(6):787–791. doi: 10.1097/MPG.0000000000000688. [DOI] [PubMed] [Google Scholar]
- 12.Olen O, et al. Antibodies against deamidated gliadin peptides and tissue transglutaminase for diagnosis of pediatric celiac disease. J Pediatr Gastroenterol Nutr. 2012;55(6):695–700. doi: 10.1097/MPG.0b013e3182645c54. [DOI] [PubMed] [Google Scholar]
- 13.Barker CC, Mitton C, Jevon G, Mock T. Can Tissue Transglutaminase antibody titers replace small-bowel biopsy to diagnose celiac disease in select pediatric populations? Pediatrics. 2005;115(5):1341–1346. doi: 10.1542/peds.2004-1392. [DOI] [PubMed] [Google Scholar]
- 14.Vivas S, et al. Duodenal biopsy may be avoided when high transglutaminase antibody titers are present. World J Gastro. 2009 Oct 14;14(38):4775–4780. doi: 10.3748/wjg.15.4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deneau M, Jensen MK, Holmen J, Williams MS, Book LS, Guthery SL. Primary sclerosing cholangitis, autoimmune hepatitis, and overlap in Utah children: epidemiology and natural history. Hepatology. 2013 Oct;58(4):1392–400. doi: 10.1002/hep.26454. [DOI] [PubMed] [Google Scholar]
- 16.ARUP. Laboratory Test Directory: Tissue Transglutaminase (tTG) Antibody, IgA with Reflex to Endomysial Antibody, IgA by IFA. [Accessed February 1, 2015]; http://ltd.aruplab.com/Tests/Pub/0050734. [Google Scholar]
- 17.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. Belmont, CA: Wadsworth, Inc; 1984. [Google Scholar]
- 18.Lewis RJ. An Introduction to Classification and Regression Tree (CART) Analysis. Presented at the 2000 Annual Meeting of the Society for Academic Emergency Medicine; San Francisco, California. [Google Scholar]
- 19.Rosen A, et al. Usefulness of symptoms to screen for celiac disease. Pediatrics. 2014 Feb;133(2):211–218. doi: 10.1542/peds.2012-3765. [DOI] [PubMed] [Google Scholar]
- 20.Cataldo F, Marino V, Ventura A, Bottaro G, Corazza GR. Prevalence and clinical features of selective immunoglobulin A deficiency in coeliac disease: an Italian multicentre study. Italian Society of Paediatric Gastroenterology and Hepatology (SIGEP) and “Club del Tenue” Working Groups on Coeliac Disease. Gut. 1998;42(3):362–365. doi: 10.1136/gut.42.3.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chow MA, Lebwohl B, Reilly NR, Green PHR. Immunoglobulin A Deficiency in Celiac Disease. J Clin Gastro. 2012 Nov-Dec;46(10):850–854. doi: 10.1097/MCG.0b013e31824b2277. [DOI] [PubMed] [Google Scholar]
- 22.Collins SR, Rasmussen PW, Doty MM, Beutel S. Too high a price: out-of-pocket health care costs in the United States. Findings from the Commonwealth Fund Health Care Affordability Tracking Survey. September–October 2014. Issue Brief (Commonwealth Fund) 2014 Nov;29:1–11. [PubMed] [Google Scholar]
- 23.Shenkin B, et al. AAP Policy Statement High-Deductible Health Plans (Committee on Child Health Financing) Pediatrics. 2014;133(5):e1461–e1470. doi: 10.1542/peds.2014-0555. [DOI] [PubMed] [Google Scholar]
- 24.Long KH, Rubio-Tapia A, Wagie AE, et al. The economics of coeliac disease: a population-based study. Aliment Pharmacol Ther. 2010;32:261–269. doi: 10.1111/j.1365-2036.2010.04327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myleus, et al. Health-related quality of life is not impaired in children with undetected as well as diagnosed celiac disease: a large population based cross-sectional study. BMC Public Health. 2014 May 5;14:425. doi: 10.1186/1471-2458-14-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb, et al. High adherence to a Gluten-Free Diet in Adolescents with Screening-Detected Celiac Disease. J Pediatr Gastroenterol Nutr. 2015;60(1):54–59. doi: 10.1097/MPG.0000000000000571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.