Abstract
The goal of this research is to study the effect of polyanhydride chemistry on the immune response induced by a prophylactic cancer vaccine based on biodegradable polyanhydride particles. To achieve this goal, different compositions of polyanhydride copolymers based on 1,8-bis-(p-carboxyphenoxy)- 3,6-dioxaoctane (CPTEG), 1,6-bis-(p-carboxyphenoxy)-hexane (CPH), and sebacic anhydride (SA) were synthesized by melt polycondensation, and polyanhydride copolymer particles encapsulating a model antigen, ovalbumin (OVA), were then synthesized using a double emulsion solvent evaporation technique. The ability of three different compositions of polyanhydride copolymers (50:50 CPTEG:CPH, 20:80 CPTEG:CPH, and 20:80 CPH:SA) encapsulating OVA to elicit immune responses was investigated. In addition, the impact of unmethylated oligodeoxynucleotides containing deoxycytidyl-deoxyguanosine dinucleotides (CpG ODN), an immunological adjuvant, on the immune response was also studied. The immune response to cancer vaccines was measured after treatment of C57BL/6J mice with two subcutaneous injections, seven days apart, of 50 μg OVA encapsulated in particles composed of different polyanhydride copolymers with or without 25 μg CpG ODN. In vivo studies showed that 20:80 CPTEG:CPH particles encapsulating OVA significantly stimulated the highest level of CD8+ T lymphocytes, generated the highest serum titers of OVA-specific IgG antibodies, and provided longer protection against tumor challenge with an OVA-expressing thymoma cell line in comparison to formulations made from other polyanhydride copolymers. The results also revealed that vaccination with CpG ODN along with polyanhydride particles encapsulating OVA did not enhance the immunogenicity of OVA. These results accentuate the crucial role of the copolymer composition of polyanhydrides in stimulating the immune response and provide important insights on rationally designing efficacious cancer vaccines.
Keywords: polyanhydrides, antitumor immune response, biodegradable polymer, antigen, adjuvant, cancer vaccine
Graphical Abstract
1. Introduction
Cancer is one of the leading causes of death worldwide [1]. In the United States, cancer is the second leading cause of death, exceeded only by cardiovascular disease, and accounts for one out of every four deaths [2], whilst approximately 14.1 million cancer cases were diagnosed globally and 8.2 million cancer patients died in 2012 [3]. Several conventional cancer treatments such as chemotherapy, surgery, and radiotherapy are available, however, current treatment approaches may not offer sufficient clinical benefits, and require improvement to enhance survival in cancer patients [4, 5]. Vaccines for cancer treatment have received an increased interest in recent years [6]. In fact, the promising results of cancer vaccines have culminated in the FDA approval of the first therapeutic cancer vaccine, Sipuleucel-T (Provenge™), for treatment of prostate cancer [7, 8].
Tumor antigen-based cancer vaccines can stimulate tumor-specific immune responses. These immune responses have the potential to not only eradicate the cancer, but also generate long-lasting memory responses to guard against tumor recurrence [6]. Compared to soluble cancer vaccine formulations, tumor antigens encapsulated in biodegradable polymeric particles have been shown to sustain antigen release and provide long-term protection against tumor challenge by improving the immune response towards the antigen [9, 10]. The reasons for the improved immune responses are many-fold. One reason is because particulate delivery systems protect encapsulated antigen(s) from degradation by host enzymes [10–12]. Also, particle-based vaccines can be designed to present similar signals to immune cells as pathogens. Therefore, particulate delivery systems are internalized more readily and efficiently by antigen presenting cells than their soluble counterparts. This enhanced cellular uptake of particulate delivery systems leads to an induction of stronger immune responses compared to soluble antigen [13, 14]. Furthermore, several studies have shown that particle-based vaccine formulations induced cell-mediated immunity, which is a key component of the immune response against tumors [15–21].
Polyanhydrides are a class of degradable polymers that possess favorable properties in terms of their suitability as cancer vaccine delivery systems. Polyanhydrides are FDA-approved polymers that degrade through surface erosion [22], which results in nearly zero-order release of encapsulated drug and protein molecules [23–25]. This can facilitate long-lasting immunity [26]. In addition, changing the type of monomers or varying the molar ratios in the copolymer composition of polyanhydrides can significantly alter the degradation rate from a week to several years, thereby regulating the release kinetics of the payload [25, 27]. Amphiphilic polyanhydride copolymers have been reported to successfully maintain protein stability and provide sustained release of proteins [28–35]; consequently, they are excellent delivery systems for acid-sensitive payloads. Furthermore, since polyanhydrides are inert and their degradation products are safe, they have high biocompatibility and are suitable for in vivo drug/antigen delivery [24]. Additionally, polyanhydrides have been reported to have inherent adjuvant properties and polyanhydride particles can act as Toll-like receptor (TLR) agonists on various TLRs including TLRs 2, 4, and 5 [19, 20, 36–47].
In this study, the impact of polyanhydride chemistry on the stimulation of immune responses and protection against tumor challenge was investigated. Based on 1,8-bis-(p-carboxyphenoxy)-3,6-dioxaoctane (CPTEG), 1,6-bis-(p-carboxyphenoxy) hexane (CPH), and sebacic anhydride (SA) (Figure 1), three different compositions of polyanhydride copolymers (i.e., 50:50 CPTEG:CPH, 20:80 CPTEG:CPH, and 20:80 CPH:SA) were used to synthesize particles encapsulating ovalbumin (OVA) and their in vivo efficacy was tested in a mouse model. OVA, a major protein in chicken egg white, is a well-studied model antigen comprised of 385 amino acid residues with a molecular weight of 44 kDa [48]. In this research, the effect of unmethylated oligodeoxynucleotides containing deoxycytidyl-deoxyguanosine dinucleotides (CpG ODN) as an immunological adjuvant was also studied. CpG ODN is a TLR 9 agonist that has been advanced to clinical trials such as a phase I clinical trial in patients with previously treated chronic lymphocytic leukemia (B cell lymphoma), a phase I clinical trial in patients with recurrent glioblastoma, and a phase II clinical trial in patients with non-small cell lung cancer [49]. CpG ODN has been reported to promote T helper cell type-1 (Th1) immune response [14, 50]. However, Joshi et al. found that the co-delivery of CpG ODN and OVA in 50:50 CPTEG:CPH particles did not promote Th1 immune responses [51], which was attributed to observations that 50:50 CPTEG:CPH copolymer activates dendritic cells in a similar fashion to lipopolysaccharide [45], which has been previously reported to abrogate the function of CpG ODN [52]. This finding was further investigated in this research to deduce whether this apparent abrogation of CpG ODN function is specific to the 50:50 CPTEG:CPH copolymer or instead is a possible universal characteristic of polyanhydride copolymers.
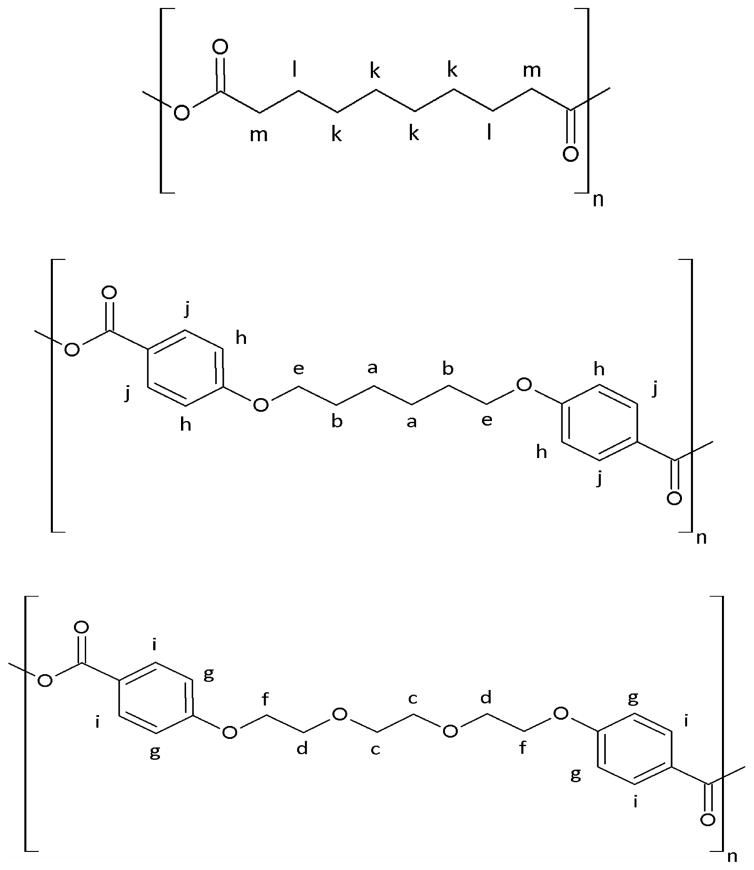
Figure 1.
Chemical structures of poly (SA), poly (CPH), and poly (CPTEG); from top to the bottom.
2. Materials and methods
2.1 Synthesis of polyanhydride copolymers
Polyanhydride copolymers with molar ratio compositions 50:50 CPTEG:CPH, 20:80 CPTEG:CPH and 20:80 CPH:SA were synthesized by melt polycondensation of acetylated monomers, as described previously [28, 53]. Briefly, monomers were mixed together at the specified molar ratios. Acetic anhydride was then added to the monomer mixture and reacted for 30 minutes at 125 °C. The acetic anhydride was removed using a rotary evaporator and the resulting viscous liquid was polymerized in an oil bath at 140 °C for the CPTEG:CPH copolymers (or at 180 °C for 20:80 CPH:SA copolymer) under high vacuum (<0.3 torr) for 90 minutes. To purify the copolymers, the crude copolymers were dissolved in methylene chloride and isolated by precipitation in dry petroleum ether. The purified copolymers were dried under vacuum overnight.
2.2 Characterization of polyanhydride copolymers
The purity of the synthesized polyanhydride copolymers was examined using 1H nuclear magnetic resonance (1H NMR) spectroscopy. The number-average molecular weight of polyanhydride copolymers was estimated by end group analysis from 1H NMR spectra obtained on a Varian VXR-300 MHz NMR spectrometer (Varian Inc., Palo Alto, CA) using deuterated chloroform as a solvent. In addition, gel permeation chromatography (GPC) with a Waters HPLC 277 system (Waters, Milford, MA) and GPC columns (Varian Inc.) was used to determine the molecular weight of the polyanhydride copolymers. The static contact angle between the films of the polyanhydride copolymers and water droplets was measured using a model 100 contact angle goniometer (Ramé-Hart, Mountain Lakes, NJ) equipped with a high resolution CMOS camera with a 6–60x magnification lens (Thor Labs, Newton, NJ). Briefly, polyanhydride copolymers were dissolved at 10% w/v in methylene chloride (Fisher Scientific, Fair lawn, NJ). Solutions were cast onto glass coverslips (Leica, Buffalo Grove, IL) and dried at room temperature. Droplets with a volume of 10 μL of deionized water purified with a Milli-Q UV Plus System (Millipore, Bedford, MA) were dispensed onto the surface of the films. Multiple images of water droplets were captured and the measurements were taken in triplicate. Using ImageJ software, the shape of the droplet was analyzed to determine the static contact angle from the right and left edges of the droplets.
2.3 Synthesis of polyanhydride particles encapsulating OVA
Particles were prepared using a water-in-oil-in-water (w/o/w) double emulsion, solvent evaporation method derived from Intra et al. [54]. In brief, 3 mg of purified endotoxin-free chicken egg white OVA (Sigma, St. Louis, MO) was dissolved in 100 μL of 1% w/v poly(vinyl alcohol) (PVA) (Sigma) solution. Using a Model FB-120 Sonic Dismembrator equipped with an ultrasonic converter probe CL-18 (Fisher Scientific, Pittsburgh, PA) set at an amplitude of 40%, this aqueous solution was sonicated for 30 seconds in 1.5 mL of methylene chloride containing 200 mg of one of the polyanhydride copolymers (50:50 CPTEG:CPH, 20:80 CPTEG:CPH, or 20:80 CPH:SA). This primary emulsion was then sonicated in 8 mL of 1% w/v PVA solution under the same previously used sonication conditions. The obtained secondary emulsion was then immediately added to 22 mL of 1% w/v PVA stirring solution in a fume hood. The double emulsion was stirred for two hours to allow evaporation of methylene chloride. After stirring, the particles were collected by centrifugation for 5 minutes using a Sorvall Legend XTR Centrifuge (Thermo Scientific, Waltham, MA) set at 2880 × g. The obtained particles were washed twice with nanopure water and then resuspended in 5 mL of nanopure water. Particle suspensions were frozen at −85 °C for one hour and then lyophilized for 24 hours using a FreeZone 4.5 freeze dry system (Labconco Corporation, Kansas City, MO) set at 0.08 mBar and − 53 °C. Lastly, the particles were stored at −20 °C until use.
2.4 Characterization of polyanhydride particles encapsulating OVA
Suspensions of particles were prepared in nanopure water in order to characterize the synthesized particles. Size distribution and surface charge were measured using a dynamic light scattering (DLS) Zetasizer Nano ZS instrument (Malvern, Southborough, MA). Approximately 1 mL of particles in suspension was added to a polystyrene cuvette (Sarstedt Inc., Newton, NC) and folded capillary cell (Malvern) to measure particle size and zeta potential, respectively. Particles were also characterized for their shape and surface morphology using a Hitachi S-4800 scanning electron microscope (SEM) (Hitachi High-Technologies, Ontario, Canada). A small drop of particles in suspension was plated onto a silicon wafer chip (Ted Pella Inc., Redding, CA) mounted on a SEM pin stub specimen mount. This drop was left to completely dry at room temperature. Then, the silicon wafer chip was coated with gold-palladium using an argon beam K550 sputter coater (Emitech Ltd., Kent, U.K.) for 3 minutes. Finally, SEM images were captured using a Hitachi S-4800 SEM at 2 kV accelerating voltage.
2.5 Quantification of OVA encapsulated in polyanhydride particles
Particles were initially degraded in order to release OVA, which was then quantified using a micro bicinchoninic acid (BCA) assay. Briefly, 5–7 mg of particles from each batch were accurately weighted and dispersed in 1 mL of 0.2 N NaOH. Suspensions of particles were incubated overnight in an incubator shaker (New Brunswick Scientific Co. Inc., Edison, NJ) set at 37 °C and 300 rpm. Solutions were centrifuged for 5 minutes using an Eppendorf Centrifuge 5415-D (Eppendorf AG, Hauppauge, NY) set at 5,000 × g. A Micro BCA™ Protein Assay Kit (Thermo Scientific, Rockford, IL) was used to determine the protein concentration in supernatants after being neutralized by 0.3 N HCl to approximately pH 7.0. The supernatants were incubated with Micro BCA™ reagents for two hours at 37 °C, following which the absorbance of the solutions at 562 nm was measured using a SpectraMax® Plus 384 microplate reader (Molecular Devices LLC, Sunnyvale, CA). The results were expressed as the amount of OVA per milligram of particles as described in equation (1). The percent encapsulation efficiency was also calculated as described in equation (2).
| (1) |
| (2) |
2.6 Prophylactic vaccinations using a murine tumor model
Six to eight week-old female wild-type C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were treated with subcutaneous (rear dorsal flank) injections of the following treatment groups (n=5 mice per group): (I) naïve (i.e., unvaccinated), (II) 50:50 CPTEG:CPH/OVA, (III) 50:50 CPTEG:CPH/OVA plus soluble CpG ODN, (IV) 20:80 CPTEG:CPH/OVA, (V) 20:80 CPTEG:CPH/OVA plus soluble CpG ODN, (VI) 20:80 CPH:SA/OVA, and (VII) 20:80 CPH:SA/OVA plus soluble CpG ODN. For mice treated with soluble CpG ODN 1826 (molecular weight 6,171 g.mol−1) (Integrated DNA Technologies, Coralville, IA), the suspensions of polyanhydride particles encapsulating OVA in 1X phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY) were mixed with CpG ODN solution immediately prior to vaccination. Doses of 50 μg of OVA and 25 μg of CpG ODN per mouse were consistently used. Each mouse was primed on day 0 and boosted on day 7 with the same indicated treatment that was used for priming. On day 14, OVA-specific CD8+ T lymphocyte levels were measured in the peripheral blood harvested via submandibular bleeds. On day 28, OVA-specific IgG1 and IgG2C antibody titers were measured in the serum harvested via submandibular bleeds. On day 35, mice were subcutaneously challenged with tumor cells (see below) and the tumor growth was monitored over time. In this study, blank particles made of polyanhydride copolymer alone (i.e., no antigen) were not used as a control group since they have already been tested in a previous study and did not stimulate any detectable immune responses (since OVA is not an integral part of those particles), nor did they provide any prophylactic protection to the mice challenged with tumor cells [51].
2.7 Estimation of OVA-specific CD8+ T lymphocyte levels in the peripheral blood
The frequency of OVA-specific CD3+ CD8+ T lymphocytes was measured on day 14 (post-prime vaccination) by tetramer staining and direct immunofluorescence as described previously by Karan et al. [55]. H-2Kb SIINFEKL class I iTAg™ MHC Tetramer (Kb-OVA257) labeled with phycoerythrin (PE) (MBLI, Woburn, MA) was used in this assay. Surface CD8 and CD3 were stained with fluorescein isothiocyanate (FITC)-labeled rat anti-mouse CD8 (eBioscience, San Diego, CA) and PE-Cy5-labeled hamster anti-mouse CD3 (eBioscience) antibodies, respectively. Samples were acquired using a BD FACScan flow cytometer (Becton, Dickinson, Franklin Lakes, NJ) and analyzed with FlowJo software (Tree Star, Ashland, OR). Results are expressed as percentage of total CD3+ CD8+ T lymphocytes in peripheral blood that were positive to tetramer staining.
2.8 Estimation of anti-OVA antibody levels in the peripheral blood
The levels of OVA-specific IgG antibody subtypes, IgG1 and IgG2C, were measured in sera using an enzyme-linked immune-sorbent assay (ELISA). In brief, blood samples were collected through submandibular bleeds and incubated for one hour at room temperature. After incubation, blood clots were removed and the samples were centrifuged for 10 minutes using an Eppendorf Centrifuge 5804-R (Eppendorf, Westbury, NY) set at 3,000 × g and 4 °C. Supernatants (serum samples) were collected, serially diluted, and incubated overnight at room temperature in an Immulon® 2HB Flat Bottom Microtiter 96-well plates (Thermo Scientific, Rochester, NY) which had been previously coated with 100 μL of 5 μg.mL−1 of OVA solution in PBS. Plates were washed with PBS-Tween 20 solution, and followed by incubation for three hours at room temperature with either goat anti-mouse IgG1 (or goat anti-mouse IgG2C) antibody conjugated with alkaline phosphatase (Southern Biotech, Birmingham, AL). After three hours of incubation, plates were washed with PBS-Tween 20 solution to remove excess antibody and followed by incubation with p-nitrophenylphosphate (pNPP) in TRIS buffer (Sigma) in the dark. After 30 minutes, the absorbance was measured at 405 nm using a SpectraMax® Plus 384 microplate reader.
2.9 Tumor challenge
On day 35 post-prime vaccination, mice were subcutaneously challenged with 2 × 106 OVA-expressing E.G7 cells (American Type Culture Collection, Manassas, VA) suspended in 100 μL of sterile 1X PBS (contralateral to vaccination site). Tumor growth was monitored regularly for the subsequent two months and tumor volumes were calculated as described in equation (3). Mice were euthanized when the tumor size exceeded 20 mm at the largest diameter or 10 mm in the height. All animal experiments were performed in accordance with the University of Iowa guidelines for the care and handling of the laboratory animals.
| (3) |
2.10 Statistical analysis
In this study, CD8+ T lymphocyte levels, OVA-specific IgG antibody serum titers, and tumor volumes of all groups were compared by one-way analysis of variance (ANOVA) followed by a Tukey post-test to compare all pairs of groups. Statistical analysis was performed using Prism 6 (GraphPad Prism, La Jolla, CA). Survival analysis was performed by the Mantel-Cox log-rank test using SAS 9.3 software (SAS Institute Inc., Cary, NC). Values with p<0.05 were considered to be statistically significant.
3. Results
3.1 Characterization of polyanhydride copolymers
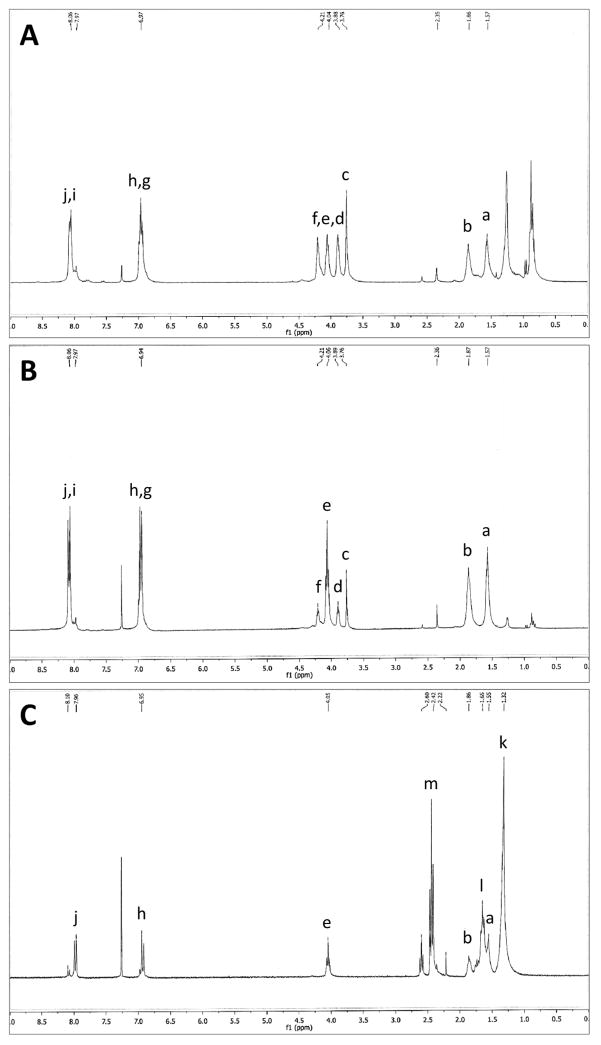
The purity of polyanhydride copolymers was verified using 1H NMR spectroscopy. The NMR spectra indicated that the actual composition of the polyanhydride copolymers was consistent with the starting molar ratios of the monomers. The designation of peaks of polyanhydride copolymers in 1H NMR spectra (Figure 2) demonstrates the purity of the synthesized copolymers. Deuterated chloroform used in NMR analysis has a chemical shift at approximately δ = 7.26 ppm. In the 50:50 CPTEG:CPH and 20:80 CPTEG:CPH copolymers, the protected protons, A and B, in the inner chain of CPH monomer have chemical shifts at δ = 1.57 ppm and δ = 1.86 ppm, respectively. The chemical shifts of the acetylated end groups in the 50:50 CPTEG:CPH and 20:80 CPTEG:CPH copolymers appeared at δ = 2.35 ppm and δ = 2.36 ppm, respectively. The inner chain protons, C to F, close to the electronegative oxygen atoms, have chemical shifts between δ = 3.76 ppm and δ = 4.21 ppm. Peaks of protons, G to J, on the aromatic groups, appeared at chemical shifts between δ = 6.94 ppm and δ = 8.06 ppm. In the 20:80 CPH:SA copolymer, the peak at chemical shifts δ = 1.32 ppm and δ = 1.65 ppm represents the protons K and L respectively, of the inner chain of SA. The chemical shift of the acetylated end groups was at δ = 2.22 ppm. The peak at chemical shift δ = 2.42 ppm represents the protons, M, of the methylenes next to the anhydride bond. CPH had peaks at the same chemical shifts. GPC analysis revealed that the synthesized 50:50 CPTEG:CPH, 20:80 CPTEG:CPH, and 20:80 CPH:SA copolymers had number-average molecular weights of 8,222, 10,942, and 20,154 g.mol−1, respectively. These results are consistent with previous work [36, 51, 56, 57]. As expected, the water contact angle was directly correlated with the CPH content in the polynahydride copolymers (Table 1). Increasing the CPH content in the polyanhydride copolymer had increased the hydrophobicity and decreased the interaction with the water droplet which leads to decreased wetting and raised contact angle (Figure 3).
Figure 2.
1H NMR spectra of (A) 50:50 CPTEG:CPH, (B) 20:80 CPTEG:CPH, and (C) 20:80 CPH:SA copolymers.
Table 1.
Contact angle measurements of polyanhydride copolymers.
| 50:50 CPTEG:CPH | 20:80 CPTEG:CPH | 20:80 CPH:SA | |
|---|---|---|---|
| Water contact angle (°) | 81.2 ± 1.2 | 97.6 ± 3.4 | 75.5 ± 2.5 |
Figure 3.
Images of water droplets dispensed onto the films of (A) 50:50 CPTEG:CPH, (B) 20:80 CPTEG:CPH, and (C) 20:80 CPH:SA copolymers.
3.2 Characterization of polyanhydride particles encapsulating OVA
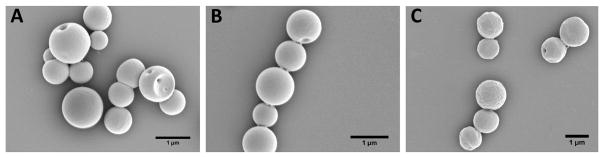
As described in the materials and methods section, polyanhydride particles encapsulating OVA were prepared using a w/o/w double emulsion, solvent evaporation method. The average size of particles was in the range 1020–1100 nm while the average zeta potential values were between −33 and −37 mV. Particles exhibited a narrow size distribution with average polydispersity index values less than 0.2 (Table 2). The average loading capacity of OVA in the 50:50 CPTEG:CPH, 20:80 CPTEG:CPH, 20:80 CPH:SA particles was 5.39, 4.51, and 10.09 μg of OVA per milligram of polyanhydride particles, respectively. In addition, the average encapsulation efficiency of OVA in 50:50 CPTEG:CPH, 20:80 CPTEG:CPH, and 20:80 CPH:SA particles was 25.1, 21.1, and 47.1%, respectively (Table 3). The percentage encapsulation efficiency was calculated based on 70% polymer recovery during particle synthesis. Electron photomicrographs showed that polyanhydride particles encapsulating OVA possessed a spherical shape with smooth surface morphology (Figure 4).
Table 2.
Particle size and zeta potential of polyanhydride particles encapsulating OVA.
| Particle size (nm) | Polydispersity index (PDI) | Zeta potential (mV) | |
|---|---|---|---|
| 50:50 CPTEG:CPH particles encapsulating OVA | 1020 ± 10 | 0.154 ± 0.087 | −35.60 ± 0.36 |
| 20:80 CPTEG:CPH particles encapsulating OVA | 1097 ± 55 | 0.124 ± 0.078 | −36.23 ± 0.32 |
| 20:80 CPH:SA particles encapsulating OVA | 1024 ± 56 | 0.037 ± 0.033 | −33.35 ± 0.07 |
Table 3.
Loading capacity and encapsulation efficiency of OVA encapsulated in polyanhydride particles.
| Loading capacity of OVA (μg per mg of particles) | Encapsulation efficiency (%) | |
|---|---|---|
| 50:50 CPTEG:CPH particles encapsulating OVA | 5.39 ± 0.18 | 25.1 ± 0.8 |
| 20:80 CPTEG:CPH particles encapsulating OVA | 4.51 ± 0.16 | 21.1 ± 0.7 |
| 20:80 CPH:SA particles encapsulating OVA | 10.09 ± 0.07 | 47.1 ± 0.3 |
Figure 4.
Electron photomicrographs of prepared polyanhydride particles encapsulating OVA. (A) 50:50 CPTEG:CPH; (B) 20:80 CPTEG:CPH; and (C) 20:80 CPH:SA. Scale bar = 1 μm.
3.3 Immunogenicity of different formulations of polyanhydride particles encapsulating OVA
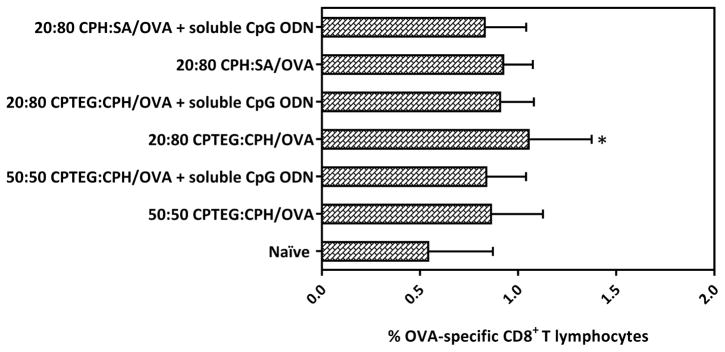
On day 14 post-prime vaccination with polyanhydride particles encapsulating OVA +/− soluble CpG ODN, the levels of CD8+ T lymphocytes were measured in the peripheral blood. All polyanhydride particle formulations encapsulating OVA +/− soluble CpG ODN caused a marginal increase in the levels of CD8+ T lymphocytes in peripheral blood from vaccinated mice compared to that from naïve (unvaccinated) mice. However, only the peripheral blood of mice in group (IV), which were vaccinated with 20:80 CPTEG:CPH/OVA, displayed increases in CD8+ T lymphocyte levels that were significantly higher (p<0.05) compared to the naïve mice (Figure 5). The expression of CD8+ T lymphocytes was not further enhanced upon co-delivery of soluble CpG ODN with polyanhydride particles encapsulating OVA.
Figure 5.
Percent OVA-specific CD8+ T lymphocytes in the peripheral blood of mice vaccinated with different polyanhydride particles encapsulating OVA ± soluble CpG ODN. On day 14 post-prime vaccination with indicated treatments, OVA-specific CD8+ T lymphocyte levels (expressed as a percentage of CD3+ CD8+ cells derived from peripheral blood) were measured. Asterisk symbol (*) refers to the statistically significant difference/s between the naïve and other vaccinated groups. Data are plotted as mean ± SD, n = 5. *p<0.05.
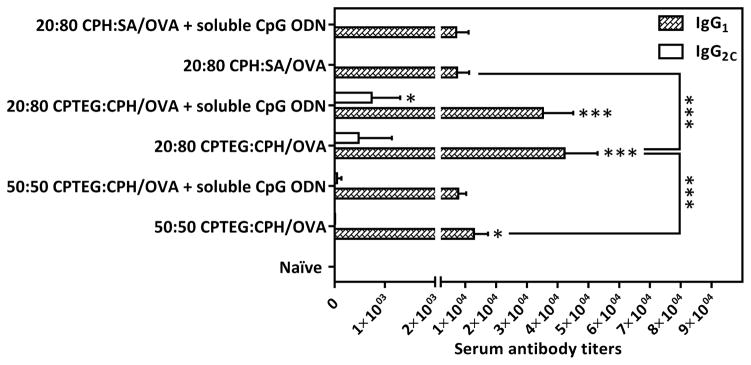
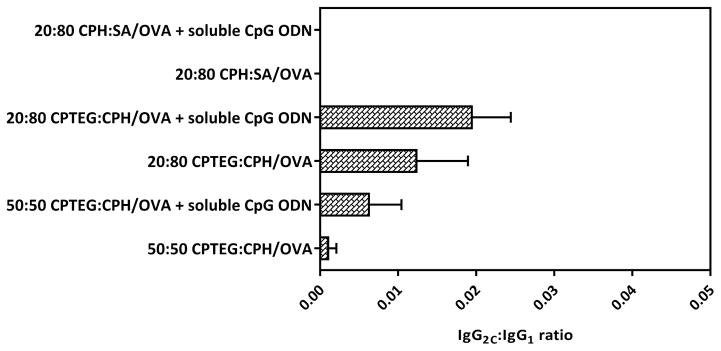
Using ELISA, the serum titers of OVA-specific IgG1 and IgG2C antibodies were measured on day 28 post-prime vaccination (Figure 6). As expected, all prophylactic polyanhydride vaccine formulations resulted in higher in vivo OVA-specific IgG1 antibody serum titers compared to the titers in naïve mice. In addition, the sera of mice vaccinated with 20:80 CPTEG:CPH/OVA +/− soluble CpG ODN showed the highest serum titers of OVA-specific IgG1 and IgG2C antibodies compared to sera from other mice. Serum titers of OVA-specific IgG2C generally remained low except in mice vaccinated with 20:80 CPTEG:CPH/OVA with soluble CpG ODN and to a lesser extent in mice vaccinated with 20:80 CPTEG:CPH/OVA alone. Also, only sera from mice in the group (V) vaccinated with 20:80 CPTEG:CPH/OVA with soluble CpG ODN displayed increases in the IgG2C serum titers that were statistically significant compared to the naïve group. In this study, sera from all the vaccinated mice showed higher titers of OVA-specific IgG1 antibody compared to IgG2C levels and co-delivery of soluble CpG ODN along with polyanhydride particles encapsulating OVA did not result in a significant increase in the IgG2C to IgG1 ratios (Figure 7).
Figure 6.
Titers of OVA-specific IgG1 and IgG2C antibodies in sera of mice vaccinated with different polyanhydride particles encapsulating OVA ± soluble CpG ODN. On day 28 post-prime vaccination with indicated treatments, OVA-specific IgG1 and IgG2C serum titers were measured using ELISA. Asterisk symbol (*) refers to the statistically significant difference/s between the naïve and other vaccinated groups unless otherwise specified. Data are plotted as mean ± SD, n = 5. *p<0.05, **p<0.01, ***p<0.001.
Figure 7.
Ratios of IgG2C to IgG1 on day 28. Data are plotted as mean ± SEM, n = 5.
3.4 Assessment of tumor protection
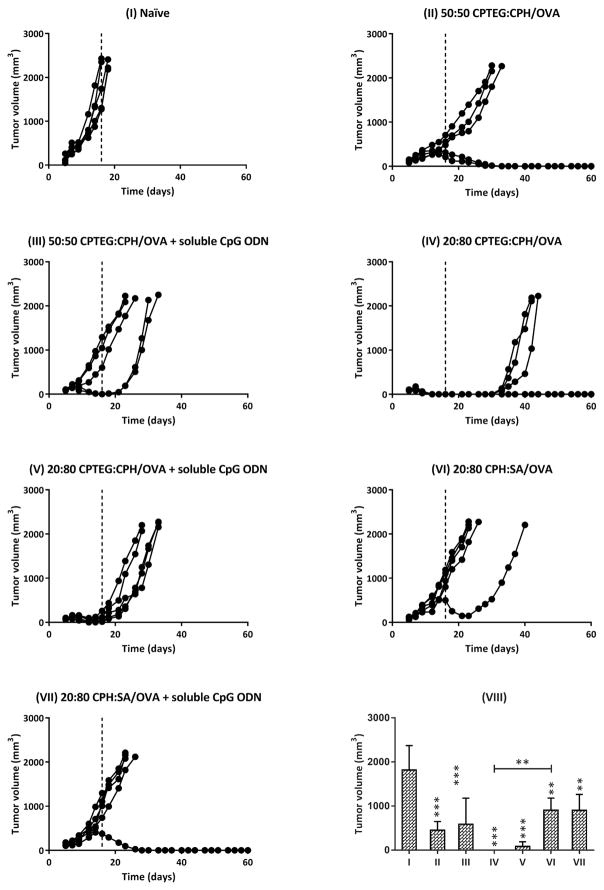
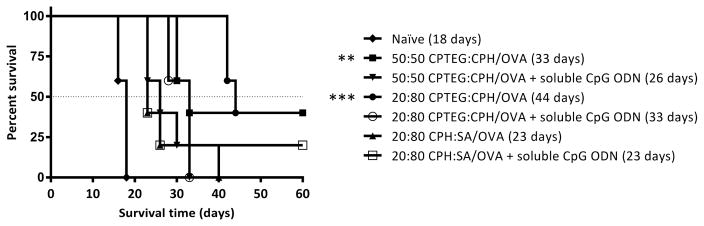
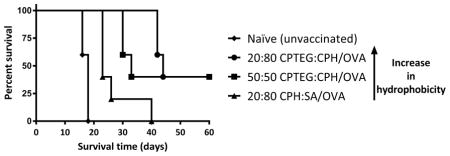
All mice were subcutaneously challenged with the OVA-expressing thymoma cell line on day 35 post-prime vaccination and it was observed that the naïve mice had rapid tumor growth whereas mice that received the prophylactic cancer vaccine formulations showed reduced tumor growth and progression to varying degrees (Figure 8). Interestingly, mice vaccinated with 20:80 CPTEG:CPH/OVA displayed a substantial delay in the onset of tumor growth, whilst other formulations not involving 20:80 CPTEG:CPH did not delay the onset of tumor appearance compared to the naïve controls. On day 18 post-tumor challenge, all the naïve mice were euthanized due to large tumor volumes. Tumor volumes of all mice on day 16 (post-tumor challenge) were statistically compared and it was found that vaccinated mice (all groups) had significantly smaller tumor volumes than the naïve mice (Figure 8). In particular, mice vaccinated with 20:80 CPTEG:CPH/OVA had no detectable tumor volumes on day 16 and this was also significantly lower than tumor volumes of mice vaccinated with 20:80 CPH:SA/OVA. There were no statistically significant differences between the tumor volumes of mice vaccinated with polyanhydride/OVA formulations alone versus tumor volumes of mice vaccinated with polyanhydride/OVA formulations plus soluble CpG ODN. Survival analysis revealed that mice vaccinated with 50:50 CPTEG:CPH/OVA and 20:80 CPTEG:CPH/OVA had significantly extended survival in comparison to the naïve mice. No other statistically significant differences were observed. In addition, mice vaccinated with 20:80 CPTEG:CPH/OVA had the longest survival with a median survival time of 44 days (Figure 9).
Figure 8.
Prophylactic antitumor effect of vaccinating mice with different polyanhydride/OVA formulations ± soluble CpG ODN. Mice were primed (day 0) and boosted (day 7) with indicated treatments (I to VII) before being challenged with tumor cells (day 35). Tumor growth was monitored regularly post challenge for up to 2 months. Each curve represents the tumor growth for each individual mouse. Tumor volumes from each group were statistically compared at day 16 (VIII). Asterisk symbol (*) refers to the statistically significant difference/s between the naïve and other vaccinated groups unless otherwise specified. Data are plotted as mean ± SD, n = 5. *p<0.05, **p<0.01, ***p<0.001.
Figure 9.
Survival curve of mice vaccinated with different polyanhydride/OVA formulations ± soluble CpG ODN. Mice were primed (day 0) and boosted (day 7) with the indicated treatment before being challenged with tumor cells (day 35). Survival analysis was performed using the Mantel-Cox log-rank test. The numerals in the brackets refer to the median survival time while asterisk symbol (*) refers to the statistically significant difference/s between the naïve and other vaccinated groups (n = 5). **p<0.01, ***p<0.001.
4. Discussion
In this work, immune responses to cancer vaccine formulations based on three different OVA-loaded particle chemistries synthesized from different polyanhydride copolymers were evaluated. Previously, we reported on the immunogenicity and antitumor potential of 50:50 CPTEG:CPH particles [51]. The current study sought to build upon the observed antitumor activity by comparing two additional polyanhydride chemistry-based formulations (20:80 CPTEG:CPH and 20:80 CPH:SA). Particles prepared by a double emulsion solvent evaporation technique possessed a comparable size regardless of the type of polyanhydride copolymer used. The presence of deprotonated carboxylic groups may account for the negative charges on the surface of polyanhydride particles encapsulating OVA. The encapsulation efficiency of OVA appeared to be inversely correlated to the increased hydrophobicity of polyanhydride copolymers, which is in the following order: 20:80 CPH:SA < 50:50 CPTEG:CPH < 20:80 CPTEG:CPH as demonstrated by static contact angle measurements. The more hydrophobic the polyanhydride copolymer, the lower the loading efficiency of the water soluble OVA. Polyanhydrides degrade by hydrolysis of hydrolytically sensitive anhydride bonds and predominately undergo surface erosion [24]. Polyanhydride copolymers used in this study have been previously shown to be biodegradable and their degradation profiles have been reported [56, 58]. The least hydrophobic polymers in this category are aliphatic polyanhydrides such as poly (SA), which have a rapid degradation rate [24, 25]. In contrast, hydrophobic aromatic polyanhydrides such as poly (CPH) have much slower erosion rates [24, 27]. Thus, the degradation rate significantly decreases as the content of poly (CPH) in the polyanhydride copolymer increases [24]. Consequently, particles based on the CPH-rich 20:80 CPTEG:CPH and to a lesser extent 50:50 CPTEG:CPH are more hydrophobic than the SA-rich 20:80 CPH:SA particles and degrade more slowly, providing longer availability of OVA as indicated by the in vitro release profile of OVA from polyanhydride copolymers [28, 31, 59].
The surface properties of potentially phagocytosable particles influence their interactions with antigen presenting cells, which in turn can affect their binding affinity and subsequent cellular uptake and ultimately the magnitude of the immune response [60–62]. The effect of particle hydrophobicity is a key factor affecting particle opsonization (i.e., promoting phagocytosis), wherein hydrophobic particles are more readily opsonized than hydrophilic particles [63–65]. The surface properties of particles directly affect the adsorption of opsonins and, in turn, the extent of phagocytosis [63], and it has also been reported that hydrophobic particles induce more robust immune responses than hydrophilic particles, which may be attributed to “danger signals” that are enhanced with increased hydrophobicity [63, 66, 67]. In addition to facilitating cellular uptake by immune cells, hydrophobic particles provide a slow and continuous release of the antigen and are considered to have higher in vivo biocompatibility than hydrophilic particles [62, 67].
In this research, in vivo studies revealed that cancer vaccines based on different compositions of polyanhydride copolymer particles stimulated immune responses of varying magnitudes. In addition, the results showed that vaccination with the most hydrophobic formulation (i.e., 20:80 CPTEG:CPH/OVA) provided the greatest protection against subsequent tumor challenge, with a median survival time of 44 days, in comparison to other formulations. Vaccination with the moderate hydrophobicity formulation (i.e., 50:50 CPTEG:CPH/OVA) also stimulated antigen-specific immune responses and provided protection against tumor challenge, but to a lesser extent than that provided by the more hydrophobic 20:80 CPTEG:CPH/OVA formulation. Although mice vaccinated with 50:50 CPTEG:CPH/OVA had enhanced median survival time (33 days) than mice vaccinated with the least hydrophobic 20:80 CPH:SA/OVA formulation, there were no statistical differences between the two cancer vaccine formulations in terms of both cellular and humoral immune responses. One possible explanation for this finding is that the hydrophobicity of both formulations may be below a threshold necessary to have a significant effect on uptake by antigen presenting cells, as discussed by previous studies [68].
Regardless of polyanhydride copolymer composition, co-delivery of CpG ODN with polyanhydride particles encapsulating OVA did not promote Th1 immune response as the IgG2C to IgG1 ratios remained very small. Mice vaccinated with polyanhydride/OVA formulations plus soluble CpG ODN had either similar or even shorter median survival times than mice vaccinated with the corresponding polyanhydride/OVA formulations alone. These results are consistent with previous observations where vaccination with CpG ODN along with 50:50 CPTEG:CPH/OVA reduced rather than improved the immunogenicity of OVA [51]. Such results are in contrast to those obtained with poly(lactic-co-glycolic acid) and poly(diaminosulfide) particles co-encapsulating OVA and CpG ODN where CpG ODN was reported to enhance the immunogenicity of these formulations [18, 21, 69]. These studies add to the body of literature attesting to the inherent adjuvanticity exhibited by polyanhydride particles [19, 20, 36–47].
5. Conclusions
Treatment of mice with cancer vaccines based on different polyanhydride copolymers encapsulating OVA resulted in a stimulation of the tumor-specific immune response with different magnitudes. This clearly indicates that polyanhydride chemistry plays a substantial role in stimulating the immune response. Vaccination with 20:80 CPTEG:CPH/OVA, the most hydrophobic formulation, stimulated the strongest cellular and humoral immune responses and provided the longest survival outcome without adding any other adjuvant. However, the prophylactic protection was incomplete and therefore further improvements and optimizations are needed in order to further enhance cancer vaccine efficacy. The most important finding in this study is that the copolymer composition of polyanhydride particle-based vaccines can have a direct effect on the magnitude of the antitumor immune response and should be selected carefully in order to achieve optimal cancer vaccine efficacy.
Statement of Significance.
Compared to soluble cancer vaccine formulations, tumor antigens encapsulated in biodegradable polymeric particles have been shown to sustain antigen release and provide long-term protection against tumor challenge by improving the immune response towards the antigen. Treatment of mice with cancer vaccines based on different polyanhydride copolymers encapsulating OVA resulted in stimulation of tumor-specific immune response with different magnitudes. This clearly indicates that polyanhydride chemistry plays a substantial role in stimulating the immune response. Vaccination with 20:80 CPTEG:CPH/OVA, the most hydrophobic formulation, stimulated the strongest cellular and humoral immune responses and provided the longest survival outcome without adding any other adjuvant. The most important finding in this study is that the copolymer composition of polyanhydride particle-based vaccines can have a direct effect on the magnitude of the antitumor immune response and should be selected carefully in order to achieve optimal cancer vaccine efficacy.
Acknowledgments
A.K.S. and B.N. acknowledge support from the Iowa State University Nanovaccine Initiative. B.N. gratefully acknowledges the Vlasta Klima Balloun Faculty Chair. A.K.S. gratefully acknowledges support from the National Cancer Institute at the National Institutes of Health (P50 CA97274/UI Mayo Clinic Lymphoma SPORE grant and P30 CA086862 Cancer Center support grant) and the Lyle and Sharon Bighley Professorship. The authors declare no conflict of interests.
Footnotes
Disclosure
The authors declare that there is no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Butterfield LH. Cancer vaccines. British Medical Journal. 2015;350:h988. doi: 10.1136/bmj.h988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.United States Cancer Statistics: 1999–2012 Incidence and Mortality Web-based Report. Atlanta, GA: National Cancer Institute; 2015. [Google Scholar]
- 3.Cancer Facts and Figures 2016. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 4.Bonavida B. Molecular Mechanisms of Tumor Cell Resistance to Chemotherapy: Targeted Therapies to Reverse Resistance. Springer; New York: 2013. [Google Scholar]
- 5.Geinitz H, Roach M, van As N. Radiotherapy in Prostate Cancer: Innovative Techniques and Current Controversies. Springer; Berlin Heidelberg: 2014. [Google Scholar]
- 6.Finn OJ. Vaccines for cancer prevention: a practical and feasible approach to the cancer epidemic. Cancer Immunology Research. 2014;2(8):708–13. doi: 10.1158/2326-6066.CIR-14-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo C, Manjili MH, Subjeck JR, Sarkar D, Fisher PB, Wang XY. Therapeutic cancer vaccines: past, present, and future. Advances in Cancer Research. 2013;119:421–75. doi: 10.1016/B978-0-12-407190-2.00007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. Pharmacy and Therapeutics. 2011;36(4):197–202. [PMC free article] [PubMed] [Google Scholar]
- 9.Lin CY, Lin SJ, Yang YC, Wang DY, Cheng HF, Yeh MK. Biodegradable polymeric microsphere-based vaccines and their applications in infectious diseases. Human Vaccines & Immunotherapeutics. 2015;11(3):650–56. doi: 10.1080/21645515.2015.1009345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmed KK, Geary SM, Salem AK. Applying biodegradable particles to enhance cancer vaccine efficacy. Immunologic Research. 2014;59(1–3):220–28. doi: 10.1007/s12026-014-8537-9. [DOI] [PubMed] [Google Scholar]
- 11.O’Hagan DT, Rappuoli R. Novel approaches to pediatric vaccine delivery. Advanced Drug Delivery Reviews. 2006;58(1):29–51. doi: 10.1016/j.addr.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine antigen delivery systems for stimulating cellular immune responses. Human Vaccines & Immunotherapeutics. 2013;9(12):2584–90. doi: 10.4161/hv.26136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bachmann MF, Jennings GT. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nature Reviews Immunology. 2010;10(11):787–96. doi: 10.1038/nri2868. [DOI] [PubMed] [Google Scholar]
- 14.Barros CM, Wafa EI, Chitphet K, Ahmed K, Geary SM, Salem AK. Production of Adjuvant-Loaded Biodegradable Particles for Use in Cancer Vaccines. In: Fox CB, editor. Vaccine Adjuvants: Methods and Protocols. Humana Press; 2016. pp. 201–13. [DOI] [PubMed] [Google Scholar]
- 15.Slobbe L, Medlicott N, Lockhart E, Davies N, Tucker I, Razzak M, Buchan G. A prolonged immune response to antigen delivered in poly (epsilon-caprolactone) microparticles. Immunology & Cell Biology. 2003;81(3):185–91. doi: 10.1046/j.1440-1711.2003.01155.x. [DOI] [PubMed] [Google Scholar]
- 16.Hamdy S, Molavi O, Ma Z, Haddadi A, Alshamsan A, Gobti Z, Elhasi S, Samuel J, Lavasanifar A. Co-delivery of cancer-associated antigen and Toll-like receptor 4 ligand in PLGA nanoparticles induces potent CD8+ T cell-mediated anti-tumor immunity. Vaccine. 2008;26(39):5046–57. doi: 10.1016/j.vaccine.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Evans JT, Ward JR, Kern J, Johnson ME. A single vaccination with protein-microspheres elicits a strong CD8 T-cell-mediated immune response against Mycobacterium tuberculosis antigen Mtb8.4. Vaccine. 2004;22(15–16):1964–72. doi: 10.1016/j.vaccine.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Joshi VB, Geary SM, Salem AK. Biodegradable particles as vaccine delivery systems: size matters. AAPS Journal. 2013;15(1):85–94. doi: 10.1208/s12248-012-9418-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross KA, Loyd H, Wu W, Huntimer L, Ahmed S, Sambol A, Broderick S, Flickinger Z, Rajan K, Bronich T, Mallapragada S, Wannemuehler MJ, Carpenter S, Narasimhan B. Hemagglutinin-based polyanhydride nanovaccines against H5N1 influenza elicit protective virus neutralizing titers and cell-mediated immunity. International Journal of Nanomedicine. 2015;10:229–43. doi: 10.2147/IJN.S72264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huntimer LM, Ross KA, Darling RJ, Winterwood NE, Boggiatto P, Narasimhan B, Ramer-Tait AE, Wannemuehler MJ. Polyanhydride nanovaccine platform enhances antigen-specific cytotoxic T cell responses. Technology. 2014;02(02):171–75. [Google Scholar]
- 21.Geary SM, Hu Q, Joshi VB, Bowden NB, Salem AK. Diaminosulfide based polymer microparticles as cancer vaccine delivery systems. Journal of Controlled Release. 2015;220(Pt B):682–90. doi: 10.1016/j.jconrel.2015.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matijević E. Fine Particles in Medicine and Pharmacy. Springer; US: 2011. [Google Scholar]
- 23.Gopferich A, Tessmar J. Polyanhydride degradation and erosion. Advanced Drug Delivery Reviews. 2002;54(7):911–31. doi: 10.1016/s0169-409x(02)00051-0. [DOI] [PubMed] [Google Scholar]
- 24.Katti DS, Lakshmi S, Langer R, Laurencin CT. Toxicity, biodegradation and elimination of polyanhydrides. Advanced Drug Delivery Reviews. 2002;54(7):933–61. doi: 10.1016/s0169-409x(02)00052-2. [DOI] [PubMed] [Google Scholar]
- 25.Jain JP, Modi S, Domb AJ, Kumar N. Role of polyanhydrides as localized drug carriers. Journal of Controlled Release. 2005;103(3):541–63. doi: 10.1016/j.jconrel.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 26.Lee A, Ferrari M, Lee J. BioMEMS and Biomedical Nanotechnology: Volume I: Biological and Biomedical Nanotechnology. Springer; US: 2007. [Google Scholar]
- 27.Kumar N, Langer RS, Domb AJ. Polyanhydrides: an overview. Advanced Drug Delivery Reviews. 2002;54(7):889–910. doi: 10.1016/s0169-409x(02)00050-9. [DOI] [PubMed] [Google Scholar]
- 28.Torres MP, Determan AS, Anderson GL, Mallapragada SK, Narasimhan B. Amphiphilic polyanhydrides for protein stabilization and release. Biomaterials. 2007;28(1):108–16. doi: 10.1016/j.biomaterials.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petersen LK, Sackett CK, Narasimhan B. High-throughput analysis of protein stability in polyanhydride nanoparticles. Acta Biomaterialia. 2010;6(10):3873–81. doi: 10.1016/j.actbio.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Carrillo-Conde B, Schiltz E, Yu J, Chris Minion F, Phillips GJ, Wannemuehler MJ, Narasimhan B. Encapsulation into amphiphilic polyanhydride microparticles stabilizes Yersinia pestis antigens. Acta Biomaterialia. 2010;6(8):3110–19. doi: 10.1016/j.actbio.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 31.Lopac SK, Torres MP, Wilson-Welder JH, Wannemuehler MJ, Narasimhan B. Effect of polymer chemistry and fabrication method on protein release and stability from polyanhydride microspheres. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2009;91(2):938–47. doi: 10.1002/jbm.b.31478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Determan AS, Wilson JH, Kipper MJ, Wannemuehler MJ, Narasimhan B. Protein stability in the presence of polymer degradation products: consequences for controlled release formulations. Biomaterials. 2006;27(17):3312–20. doi: 10.1016/j.biomaterials.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 33.Vela Ramirez JE, Roychoudhury R, Habte HH, Cho MW, Pohl NL, Narasimhan B. Carbohydrate-functionalized nanovaccines preserve HIV-1 antigen stability and activate antigen presenting cells. Journal of Biomaterials Science: Polymer Edition. 2014;25(13):1387–406. doi: 10.1080/09205063.2014.940243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ross KA, Loyd H, Wu W, Huntimer L, Wannemuehler MJ, Carpenter S, Narasimhan B. Structural and antigenic stability of H5N1 hemagglutinin trimer upon release from polyanhydride nanoparticles. Journal of Biomedical Materials Research Part A. 2014;102(11):4161–68. doi: 10.1002/jbm.a.35086. [DOI] [PubMed] [Google Scholar]
- 35.Haughney SL, Petersen LK, Schoofs AD, Ramer-Tait AE, King JD, Briles DE, Wannemuehler MJ, Narasimhan B. Retention of structure, antigenicity, and biological function of pneumococcal surface protein A (PspA) released from polyanhydride nanoparticles. Acta Biomaterialia. 2013;9(9):8262–71. doi: 10.1016/j.actbio.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kipper MJ, Wilson JH, Wannemuehler MJ, Narasimhan B. Single dose vaccine based on biodegradable polyanhydride microspheres can modulate immune response mechanism. Journal of Biomedical Materials Research Part A. 2006;76(4):798–810. doi: 10.1002/jbm.a.30545. [DOI] [PubMed] [Google Scholar]
- 37.Torres MP, Wilson-Welder JH, Lopac SK, Phanse Y, Carrillo-Conde B, Ramer-Tait AE, Bellaire BH, Wannemuehler MJ, Narasimhan B. Polyanhydride microparticles enhance dendritic cell antigen presentation and activation. Acta Biomaterialia. 2011;7(7):2857–64. doi: 10.1016/j.actbio.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tamayo I, Irache JM, Mansilla C, Ochoa-Reparaz J, Lasarte JJ, Gamazo C. Poly(anhydride) nanoparticles act as active Th1 adjuvants through Toll-like receptor exploitation. Clinical and Vaccine Immunology. 2010;17(9):1356–62. doi: 10.1128/CVI.00164-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vela Ramirez JE, Tygrett LT, Hao J, Habte HH, Cho MW, Greenspan NS, Waldschmidt TJ, Narasimhan B. Polyanhydride Nanovaccines Induce Germinal Center B Cell Formation and Sustained Serum Antibody Responses. Journal of Biomedical Nanotechnology. 2016;12(6):1303–11. doi: 10.1166/jbn.2016.2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ross KA, Haughney SL, Petersen LK, Boggiatto P, Wannemuehler MJ, Narasimhan B. Lung deposition and cellular uptake behavior of pathogen-mimicking nanovaccines in the first 48 hours. advanced healthcare materials. 2014;3(7):1071–77. doi: 10.1002/adhm.201300525. [DOI] [PubMed] [Google Scholar]
- 41.Phanse Y, Carrillo-Conde BR, Ramer-Tait AE, Broderick S, Kong CS, Rajan K, Flick R, Mandell RB, Narasimhan B, Wannemuehler MJ. A systems approach to designing next generation vaccines: combining alpha-galactose modified antigens with nanoparticle platforms. Scientific Reports. 2014;4:3775. doi: 10.1038/srep03775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Phanse Y, Carrillo-Conde BR, Ramer-Tait AE, Roychoudhury R, Pohl NL, Narasimhan B, Wannemuehler MJ, Bellaire BH. Functionalization of polyanhydride microparticles with di-mannose influences uptake by and intracellular fate within dendritic cells. Acta Biomaterialia. 2013;9(11):8902–09. doi: 10.1016/j.actbio.2013.06.024. [DOI] [PubMed] [Google Scholar]
- 43.Huntimer L, Wilson Welder JH, Ross K, Carrillo-Conde B, Pruisner L, Wang C, Narasimhan B, Wannemuehler MJ, Ramer-Tait AE. Single immunization with a suboptimal antigen dose encapsulated into polyanhydride microparticles promotes high titer and avid antibody responses. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2013;101(1):91–98. doi: 10.1002/jbm.b.32820. [DOI] [PubMed] [Google Scholar]
- 44.Ulery BD, Petersen LK, Phanse Y, Kong CS, Broderick SR, Kumar D, Ramer-Tait AE, Carrillo-Conde B, Rajan K, Wannemuehler MJ, Bellaire BH, Metzger DW, Narasimhan B. Rational design of pathogen-mimicking amphiphilic materials as nanoadjuvants. Scientific Reports. 2011;1:198. doi: 10.1038/srep00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen LK, Ramer-Tait AE, Broderick SR, Kong CS, Ulery BD, Rajan K, Wannemuehler MJ, Narasimhan B. Activation of innate immune responses in a pathogen-mimicking manner by amphiphilic polyanhydride nanoparticle adjuvants. Biomaterials. 2011;32(28):6815–22. doi: 10.1016/j.biomaterials.2011.05.063. [DOI] [PubMed] [Google Scholar]
- 46.Ulery BD, Kumar D, Ramer-Tait AE, Metzger DW, Wannemuehler MJ, Narasimhan B. Design of a protective single-dose intranasal nanoparticle-based vaccine platform for respiratory infectious diseases. PLOS One. 2011;6(3):e17642. doi: 10.1371/journal.pone.0017642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petersen LK, Xue L, Wannemuehler MJ, Rajan K, Narasimhan B. The simultaneous effect of polymer chemistry and device geometry on the in vitro activation of murine dendritic cells. Biomaterials. 2009;30(28):5131–42. doi: 10.1016/j.biomaterials.2009.05.069. [DOI] [PubMed] [Google Scholar]
- 48.Galazka VB, Dickinson E, Ledward DA. Emulsifying properties of ovalbumin in mixtures with sulphated polysaccharides: effects of pH, ionic strength, heat and high-pressure treatment. Journal of the Science of Food and Agriculture. 2000;80:1219–29. [Google Scholar]
- 49.Jahrsdorfer B, Weiner GJ. CpG oligodeoxynucleotides as immunotherapy in cancer. Update on Cancer Therapeutics. 2008;3(1):27–32. doi: 10.1016/j.uct.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Levenson EA, Kiick KL. DNA-polymer conjugates for immune stimulation through Toll-like receptor 9 mediated pathways. Acta Biomaterialia. 2014;10(3):1134–45. doi: 10.1016/j.actbio.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joshi VB, Geary SM, Carrillo-Conde BR, Narasimhan B, Salem AK. Characterizing the antitumor response in mice treated with antigen-loaded polyanhydride microparticles. Acta Biomaterialia. 2013;9(3):5583–89. doi: 10.1016/j.actbio.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gould MP, Greene JA, Bhoj V, DeVecchio JL, Heinzel FP. Distinct modulatory effects of LPS and CpG on IL-18-dependent IFN-gamma synthesis. Journal of Immunology. 2004;172(3):1754–62. doi: 10.4049/jimmunol.172.3.1754. [DOI] [PubMed] [Google Scholar]
- 53.Kipper MJ, Shen E, Determan A, Narasimhan B. Design of an injectable system based on bioerodible polyanhydride microspheres for sustained drug delivery. Biomaterials. 2002;23(22):4405–12. doi: 10.1016/s0142-9612(02)00181-3. [DOI] [PubMed] [Google Scholar]
- 54.Intra J, Salem AK. Fabrication, characterization and in vitro evaluation of poly(D,L-lactide-co-glycolide) microparticles loaded with polyamidoamine-plasmid DNA dendriplexes for applications in nonviral gene delivery. Journal of Pharmaceutical Sciences. 2010;99(1):368–84. doi: 10.1002/jps.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karan D, Krieg AM, Lubaroff DM. Paradoxical enhancement of CD8 T cell-dependent anti-tumor protection despite reduced CD8 T cell responses with addition of a TLR9 agonist to a tumor vaccine. International Journal of Cancer. 2007;121(7):1520–28. doi: 10.1002/ijc.22873. [DOI] [PubMed] [Google Scholar]
- 56.Torres MP, Vogel BM, Narasimhan B, Mallapragada SK. Synthesis and characterization of novel polyanhydrides with tailored erosion mechanisms. Journal of Biomedical Materials Research Part A. 2006;76(1):102–10. doi: 10.1002/jbm.a.30510. [DOI] [PubMed] [Google Scholar]
- 57.Lee WC, Chu IM. Preparation and degradation behavior of polyanhydrides nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2008;84(1):138–46. doi: 10.1002/jbm.b.30854. [DOI] [PubMed] [Google Scholar]
- 58.Shen E, Kipper MJ, Dziadul B, Lim MK, Narasimhan B. Mechanistic relationships between polymer microstructure and drug release kinetics in bioerodible polyanhydrides. Journal of Controlled Release. 2002;82(1):115–25. doi: 10.1016/s0168-3659(02)00125-6. [DOI] [PubMed] [Google Scholar]
- 59.Carrillo-Conde B, Garza A, Anderegg J, Narasimhan B. Protein adsorption on biodegradable polyanhydride microparticles. Journal of Biomedical Materials Research Part A. 2010;95(1):40–48. doi: 10.1002/jbm.a.32815. [DOI] [PubMed] [Google Scholar]
- 60.Thomann-Harwood LJ, Kaeuper P, Rossi N, Milona P, Herrmann B, McCullough KC. Nanogel vaccines targeting dendritic cells: contributions of the surface decoration and vaccine cargo on cell targeting and activation. Journal of Controlled Release. 2013;166(2):95–105. doi: 10.1016/j.jconrel.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 61.Musyanovych A, Dausend J, Dass M, Walther P, Mailander V, Landfester K. Criteria impacting the cellular uptake of nanoparticles: a study emphasizing polymer type and surfactant effects. Acta Biomaterialia. 2011;7(12):4160–68. doi: 10.1016/j.actbio.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 62.Nel AE, Madler L, Velegol D, Xia T, Hoek EM, Somasundaran P, Klaessig F, Castranova V, Thompson M. Understanding biophysicochemical interactions at the nano-bio interface. Nature Materials. 2009;8(7):543–57. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 63.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cellular and Molecular Life Sciences. 2009;66(17):2873–96. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Advanced Drug Delivery Reviews. 2009;61(6):428–37. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Owens DEr, Peppas NA. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. International Journal of Pharmaceutics. 2006;307(1):93–102. doi: 10.1016/j.ijpharm.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 66.Moyano DF, Goldsmith M, Solfiell DJ, Landesman-Milo D, Miranda OR, Peer D, Rotello VM. Nanoparticle hydrophobicity dictates immune response. Journal of the American Chemical Society. 2012;134(9):3965–67. doi: 10.1021/ja2108905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raghuvanshi RS, Katare YK, Lalwani K, Ali MM, Singh O, Panda AK. Improved immune response from biodegradable polymer particles entrapping tetanus toxoid by use of different immunization protocol and adjuvants. International Journal of Pharmaceutics. 2002;245(1–2):109–21. doi: 10.1016/s0378-5173(02)00342-3. [DOI] [PubMed] [Google Scholar]
- 68.Haughney SL, Ross KA, Boggiatto PM, Wannemuehler MJ, Narasimhan B. Effect of nanovaccine chemistry on humoral immune response kinetics and maturation. Nanoscale. 2014;6(22):13770–78. doi: 10.1039/c4nr03724c. [DOI] [PubMed] [Google Scholar]
- 69.Zhang XQ, Dahle CE, Baman NK, Rich N, Weiner GJ, Salem AK. Potent antigen-specific immune responses stimulated by codelivery of CpG ODN and antigens in degradable microparticles. Journal of Immunotherapy. 2007;30(5):469–78. doi: 10.1097/CJI.0b013e31802fd8c6. [DOI] [PubMed] [Google Scholar]