Abstract
Objective
Although storage alters red blood cells, several recent, randomized trials found no differences in clinical outcomes between patients transfused with red blood cells stored for shorter vs. longer periods of time. The objective of this study was to see if storage impairs the in vivo ability of erythrocytes to traverse the microcirculation and deliver oxygen at the tissue level.
Methods
A subset of subjects from a clinical trial of cardiac surgery patients randomized to receive transfusions of red blood cells stored ≤10d or ≥21d were assessed for thenar eminence and cerebral tissue hemoglobin oxygen saturation (StO2) using near-infrared spectroscopy, and sub-lingual microvascular blood flow using side-stream darkfield videomicroscopy.
Results
Among 55 subjects, there was little change in the primary endpoint (thenar eminence StO2 from before to after transfusion of one unit) and the change was similar in the two groups: +1.7%(95%CI:−0.3,3.8) for shorter-storage and +0.8%(95%CI:−1.1,2.9) for longer-storage (p=0.61). Similarly, no significant differences were observed for cerebral StO2 or sublingual microvascular blood flow. These parameters were also not different from preoperatively to one day postoperatively, reflecting the absence of a cumulative effect of all red blood cell units transfused during this period.
Conclusions
There were no differences in thenar eminence or cerebral StO2, or sublingual microcirculatory blood flow, in cardiac surgery patients transfused with red blood cells stored ≤10d or ≥21d. These results are consistent with the clinical outcomes in the parent study, which also did not differ, indicating that storage may not impair oxygen delivery by red blood cells in this setting.
INTRODUCTION
The changes occurring in RBCs under conventional storage conditions have been well described(1) and have led to the hypothesis that they might impair RBC function in the transfusion recipient.(2) A number of observational, usually retrospective, studies comparing clinical outcomes in patients receiving RBCs stored for different periods of time have yielded conflicting results,(3,4) including studies which were conducted in cardiac surgery patients.(5,6) The Red Cell Storage Duration Study (RECESS), the parent trial for the study reported here, compared clinical outcomes among patients undergoing complex cardiac surgery who were randomized to receive RBC stored ≤10 days or ≥21 days.(7) Other randomized clinical trials addressed the clinical impact of RBC storage duration in neonates(8) and critically ill adults (9) but none of these three trials demonstrated any differences in clinical outcome measures. A large, randomized study in children with severe anemia measured lactate clearance as well as clinical endpoints and also found no differences between the patients receiving RBCs stored 1–10 days vs. 25–35 days.(10)
Relatively few studies in humans have addressed the effect of RBC storage at the tissue level using physiologic endpoints such as tissue oxygen saturation of hemoglobin(11–14) or microcirculatory blood flow,(15,16) and none of them were designed to correlate such physiologic measurements with clinical outcomes. The primary objective of the NIH funded RECESS Ancillary Physiologic Study (RECAP) was to determine if the storage duration of RBCs affects tissue oxygen saturation of hemoglobin, as measured by near infra-red spectroscopy (NIRS), and microcirculatory blood flow, as measured using side-stream darkfield (SDF) videomicroscopy, in patients who had undergone complex cardiac surgery, and whether these measurements would be consistent with the clinical outcomes observed in the parent trial. The intention of this study was to examine events at the tissue level which might provide insight into the apparent paradox between the well-documented changes that occur to RBCs during storage and the lack of measurable clinical impact.
MATERIALS and METHODS
Oversight
RECAP (NCT01274390) was a multicenter, prospective, clinical trial which enrolled patients at four sites belonging to the Transfusion Medicine and Hemostasis Clinical Trials Network (TMHCTN). RECAP was conducted as an ancillary trial to a parent study carried out by the network, RECESS (NCT00991341), and was independently funded by NHLBI/NIH with no commercial support. The four enrolling sites for RECAP were Duke University, the Johns Hopkins University, Massachusetts General Hospital and the University of Pittsburgh. The Data Statistical Coordinating Center was New England Research Institutes (NERI). The study was designed by the authors and approved by the institutional review boards at each participating hospital. Study subjects provided written informed consent. The same data and safety monitoring board oversaw the parent study and RECAP.
Study Patients
The patients participating in this study were a subset of subjects who were enrolled in the RECESS study at four of the participating sites. Patients were eligible for the parent study(11) if they were scheduled for complex cardiac surgery via median sternotomy and were likely to require RBC transfusion as determined by a Transfusion Risk Understanding Score (TRUST) of 3 or higher.(17) Additional eligibility criteria for RECAP were age at least 18 years and scheduled coronary artery bypass grafting, valve repair or replacement, or a combination. Patients were approached and consented for RECAP at the same time they were consented for the parent study. Randomization could not be done any earlier than the day before surgery. Subjects who could not be randomized were no longer eligible for RECAP.
Study Design and Treatment Protocols
For the parent study, subjects were randomly assigned, in a 1:1 ratio, to receive units of RBCs stored for ≤10 days or ≥21 days for all transfusions from the time of randomization through the earliest of day 28, death or hospital discharge. Randomization was balanced by site (18) with the use of a centralized computer system. All units were pre-storage leukoreduced RBCs collected in standard, licensed additive solution systems and were not irradiated or washed. The expiration dates of the units were not obscured, but the central laboratory (Shapiro Laboratory, Center for Vascular Biology Research, Beth Israel Deaconess Medical Center, Boston, MA), which analyzed the primary raw electronic data files for the oxygenation and microcirculatory flow measurements, was blinded to study arm assignment.
Assessments, Monitoring and Outcome Measures
Tissue Hemoglobin Oxygen Saturation
The oxygen saturation of tissue hemoglobin (StO2) was measured using NIRS. Sensors were applied to the patient’s thenar eminence (InSpectra 650; Hutchinson Technology, Hutchinson, MN) and forehead (Foresight; CAS Medical Systems, Branford CT) prior to the induction of general anesthesia, and were left in place at least 24 h after the patient left the operating room. Data were continuously recorded every 2 seconds, stored in the devices, and then were transferred to the central laboratory for analysis using a proprietary file transfer software (Studymaker Microscan File Manager, Studymaker, Newton, MA). Analysis of the raw data is described in the Supplement.
Microcirculatory Blood Flow
The sub-lingual microcirculation was visualized using SDF videomicroscopy (Microscan; Microvision Medical, Inc., Amsterdam, The Netherlands) as previously described.(19) Multiple videoclips of up to a minute each were captured at the 4 time points defined below. The videoclips were stored on a laptop computer and uploaded to the central laboratory using the same proprietary file transfer software and cloud storage. Details of the processing and analysis of the videoscans are described in the Supplement. The microcirculatory parameters reported in this study were: proportion of perfused small vessels (PPV) and the change in PPV (ΔPPV); microcirculatory flow index for small vessels (MFI and ΔMFI); perfused small vessel density (PVD and ΔPVD); and total small vessel density (TVD and ΔTVD). For the perfusion parameters (PPV, ΔPPV, PVD, ΔPVD), any vessel segment with a flow score greater than or equal to 2 (sluggish or continuous flow) was considered perfused. Following the guidelines of a consensus report, MFI was estimated as the average over 4 quadrants of the field of view of the most prominent flow in the vessels that occupied each quadrant.(20)
Timing of Measurements
Measurements were made at four time points: within 6 h prior to surgery; within 2 h prior to a post-operative RBC transfusion that took place in the intensive care unit (ICU) at any point within the 24 h following surgery; within 2 h after that post-operative RBC transfusion; and 24±4 h after the end of surgery. If measurements could not be obtained before and after the first post-operative transfusion, or if the first transfusion included more than 1 unit, the measurements could be made before and after a subsequent RBC transfusion, as long as that single unit of RBC was transfused in the ICU within 24 h after the conclusion of surgery. For each patient, the first RBC transfusion for which measurements could be obtained was considered the “index” transfusion. The measurements within 2 h before and after the index transfusion were intended to capture transient changes in tissue oxygenation, whereas the pre-operative and 24 h post-operative measurements would reflect the cumulative impact of the transfusion of multiple units of RBCs.
Statistical Analysis
All analyses were conducted by a statistician (S.G.) at the Data and Statistical Coordinating Center (NERI). Data on the changes in physiologic parameters were compared between treatment groups using analysis of covariance (ANCOVA), with adjustment for the pre-transfusion value. Binary and categorical variables were compared between treatment groups using Fisher’s exact test, and effect sizes were calculated using Cramer’s phi statistic. Continuous variables were compared using Kruskal-Wallis tests, and effect sizes were calculated as the absolute difference in means divided by the pooled standard deviation.
The primary endpoint was the change in the peripheral StO2 from within 2 h before transfusion to within 2 h after a post-operative RBC transfusion in the ICU (ΔStO2). The protocol-defined secondary endpoints were: the change in the cerebral oxygen saturation of hemoglobin, and the changes in four parameters of microcirculatory blood flow (ΔMFI, ΔPPV, ΔPVD and ΔTVD) over this same time period. Other secondary outcomes were the changes in all six of these measurements from the period 6 hours prior to surgery to 24±4 h after surgery. No imputation was done for missing data points.
Subjects were considered to be analyzable for pre- to post-transfusion changes in a RECAP outcome if they had been successfully randomized in RECESS, underwent a cardiac surgical procedure, had an index transfusion in the ICU, and had valid data for the outcome being analyzed. Similarly, subjects were considered evaluable for changes from pre-surgery to one day post-surgery if they were randomized in RECESS, had surgery, received at least one RBC unit during this time period, and had valid data for the outcome. As specified in the protocol, an intention-to-treat analysis was conducted with data from all participants who met the criteria for evaluation listed above, regardless of what storage-duration RBCs were actually received. Secondary per-protocol analyses were carried out in a similar way, including only subjects who received RBCs in accordance with their assigned treatment group from randomization through one day after surgery.
RESULTS
Patients and Treatment Assignments
Between January 2011 and January 2014, 130 subjects in the parent trial also consented to participate in RECAP (Figure 1). Of these, 7 were ineligible for RECAP: 2 had a planned surgical procedure that did not meet the inclusion criteria for RECAP, and 5 could not be randomized in RECESS because the blood bank could not meet the crossmatch request with units of RBC for both storage durations. There were 6 eligible RECAP subjects (3 in each treatment arm) who did not undergo surgery. Of the remaining subjects, 59 were randomized to the arm receiving RBC stored ≤10 d, and 58 to the arm receiving RBC stored ≥21 d. Of these, 23 in the ≤10 d arm and 32 in the ≥21 d arm received at least 1 unit of RBC within 24±4h of leaving the operating room (OR) for which measurements could be obtained (index transfusions).
Figure 1.
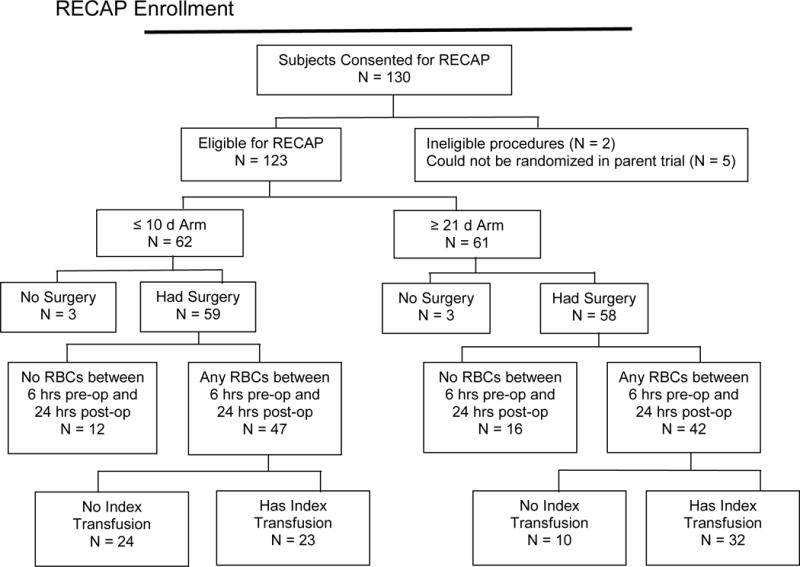
CONSORT Diagram for the RECESS Ancillary Physiologic Study. An index transfusion is the first post-operative single unit of RBCs for which the study measurements could be made.
The baseline characteristics of patients in the two arms of the study were similar (Table 1), including the frequency of significant co-morbidities and the distribution of ABO blood groups. The baseline Multiple Organ Dysfunction Score (MODS)(21) was lower in the ≥21 d arm (p≤0.01) but the difference was less than 1.0 point and both values were very low. Serum creatinine was also lower in this group, but the levels were mostly within the reference range and the differences between the groups were small (≤ 0.5 mg/dL). The characteristics of the surgical procedures were also comparable between the two groups (Table 2). The baseline characteristics of the RECAP subjects were very similar to those for the parent study, RECESS, as a whole.(7) (See Table S1 in Supplementary Material.)
Table 1.
Preoperative Characteristics of RECAP Study Subjects
| Characteristica | RECAP Subjects who underwent cardiac surgery and received ≥1 RBC transfusion 6 hours pre-op to 24 hours post-op |
RECAP Subjects who had an index transfusion | ||||||
|---|---|---|---|---|---|---|---|---|
| RBC ≤ 10 d Arm (n=47) |
RBC ≥ 21 d Arm (n=42) |
P valueb | Effect Sizec | RBC ≤ 10 d Arm (n=23) |
RBC ≥ 21 d Arm (n=32) |
P valueb | Effect Sizec | |
| Age, years | 70 (66, 79) | 72 (63, 80) | 0.94 | 0.06 | 69 (65, 79) | 71 (61, 79) | 0.84 | 0.16 |
| Male Gender | 22 (47%) | 19 (45%) | 1.00 | 0.02 | 12 (52%) | 14 (44%) | 0.59 | 0.08 |
| White | 40 (85%) | 29 (74%)d | 0.28 | 0.13 | 20 (87%) | 22 (71%)e | 0.20 | 0.19 |
| Weight, kg | 81 (69, 92) | 75 (68, 86) | 0.41 | 0.22 | 80 (70, 92) | 81 (70, 86) | 0.90 | 0.06 |
| Height, cm | 165 (158, 178) | 165 (160, 171) | 0.68 | 0.18 | 165 (160, 180) | 165 (161, 174) | 0.60 | 0.25 |
| BMI, kg/m2 | 28 (25, 31) | 28 (25, 32) | 0.84 | 0.14 | 28 (25, 31) | 28 (25, 32) | 0.60 | 0.04 |
| Hemoglobin, g/dL | 11.2 (10.4, 12.1) | 11.8 (10.7, 12.8) | 0.26 | 0.23 | 10.9 (10.2, 12.1) | 11.6 (10.4, 12.5) | 0.20 | 0.37 |
| Platelet count, × 109/L | 198 (166, 243) | 225 (173, 256) | 0.33 | 0.19 | 196 (148, 235) | 225 (170, 249) | 0.16 | 0.40 |
| Creatinine, mg/dL | 1.2 (1.0, 1.5) | 1.0 (0.8, 1.3) | <0.01 | 0.63 | 1.4 (1.0, 1.8) | 0.9 (0.8, 1.2) | <0.01 | 1.06 |
| TRUSTf – mean (±standard deviation) | 4.00 (0.72) | 3.71 (0.86) | 0.04 | 0.36 | 3.96 (0.71) | 3.63 (0.91) | 0.05 | 0.41 |
| Baseline MODSg – mean (±standard deviation) | 0.89 (0.76) | 0.52 (0.71) | 0.01 | 0.50 | 1.13 (0.82) | 0.44 (0.62) | <0.01 | 0.98 |
| Hypertension | 43 (91%) | 38 (90%) | 1.00 | 0.02 | 21 (91%) | 28 (88%) | 1.00 | 0.06 |
| Diabetes | 21 (45%) | 22 (52%) | 0.53 | 0.08 | 13 (57%) | 17 (53%) | 1.00 | 0.03 |
| Smoking History | ||||||||
| Past smoker | 20 (43%) | 12 (29%) | 0.37 | 0.15 | 9 (39%) | 10 (31%) | 0.82 | 0.09 |
| Current smoker | 7 (15%) | 8 (19%) | 4 (17%) | 7 (22%) | ||||
| No smoking history | 20 (43%) | 22 (52%) | 10 (43%) | 15 (47%) | ||||
| History of COPDh | 14 (30%) | 10 (24%) | 0.63 | 0.07 | 8 (35%) | 8 (25%) | 0.55 | 0.11 |
| History of myocardial infaction | 12 (26%) | 14 (33%) | 0.49 | 0.09 | 5 (22%) | 8 (25%) | 1.00 | 0.04 |
| History of CHFi | 12 (26%) | 13 (31%) | 0.64 | 0.06 | 9 (39%) | 9 (28%) | 0.56 | 0.12 |
| History of stroke or TIAj | 9 (19%) | 6 (14%) | 0.58 | 0.06 | 5 (22%) | 4 (13%) | 0.47 | 0.12 |
| History of vascular surgery | 13 (28%) | 8 (19%) | 0.45 | 0.08 | 7 (30%) | 6 (19%) | 0.35 | 0.13 |
| Left Ventricular Ejection Fraction, % | 55 (35, 55) | 48 (34, 55) | 0.45 | 0.11 | 44 (30, 55) | 50 (35, 55) | 0.34 | 0.35 |
| Group O | 18 (38%) | 17 (40%) | 0.69 | 0.16 | 8 (35%) | 14 (44%) | 0.18 | 0.31 |
| Group A | 22 (47%) | 22 (52%) | 10 (43%) | 17 (53%) | ||||
| Group B | 5 (11%) | 3 (7%) | 3 (13%) | 1 (3%) | ||||
| Group AB | 2 (4%) | 0 (0%) | 2 (9%) | 0 (%) | ||||
Unless otherwise noted, the median and first and third quartiles are reported for continuous characteristics and number and percent are reported for categorical characteristics.
P-values for continuous variables are from a Kruskall-Wallis non-parametric test; p-values for categorical variables are from Fisher’s exact test.
Effect sizes for binary and categorical variables were calculated using Cramer’s phi statistic and were calculated as the absolute difference in means divided by the pooled standard deviation for continuous variables.
Data unknown for 3 subjects in the ≥21 d arm
Data unknown for 1 subject in the ≥21 d arm
Transfusion Requirements Understanding Scoring Tool (20)
Multiple Organ Dysfunction Score (25)
Chronic Obstructive Pulmonary Disease
Congestive Heart Failure
Transient Ischemic Attack
Table 2.
Characteristics of Surgical Procedures of RECAP Subjects
| Surgery Characteristicsa | RECAP Subjects who underwent cardiac surgery and received ≥1 RBC transfusion 6 hours pre-op to 24 hours post-op |
RECAP Subjects who had an index transfusion | ||||||
|---|---|---|---|---|---|---|---|---|
| RBC ≤ 10 d Arm (n=47) |
RBC ≥ 21 d Arm (n=42) |
P valueb | Effect Sizec | RBC ≤ 10 d Arm (n=23) |
RBC ≥ 21 d Arm (n=32) |
P valueb | Effect Sizec | |
| Procedure Types | ||||||||
| CABGd only | 8 (17 %) | 8 (19 %) | 0.62 | 0.20 | 4 (17%) | 6 (19%) | 0.64 | 0.27 |
| Valve repair/replacement only | 7 (15%) | 3 (7%) | 2 (9%) | 3 (9%) | ||||
| CABG + valve replacement/repair | 3 (6%) | 4 (10%) | 0 (0%) | 2 (6%) | ||||
| CABG + valve replacement/repair + other | 15 (32%) | 9 (21%) | 9 (39%) | 6 (19%) | ||||
| CABG +Other | 8 (17 %) | 9 (21 %) | 4 (17%) | 7 (22%) | ||||
| Valve replacement/repair + other | 6 (13%) | 9 (21%) | 4 (17%) | 8 (25%) | ||||
| Surgical Access | ||||||||
| Primary Median Sternotomy | 34 (72 %) | 33 (79 %) | 0.71 | 0.11 | 15 (65%) | 24 (75%) | 0.53 | 0.18 |
| Repeat Median Sternotomy | 12 (26 %) | 9 (21 %) | 7 (30%) | 8 (25%) | ||||
| Thoracotomy | 1 (2 %) | 0 (0 %) | 1 (4%) | 0 (0%) | ||||
| Operative Details | ||||||||
| Cardiopulmonary bypass used | 47 (100%) | 41 (98%) | 0.47 | 0.11 | 23 (100%) | 31 (97%) | 1.00 | 0.12 |
| Cardiopulmonary bypass duratione, min | 150.5 (102.0, 207.0)f | 157.0 (105.0, 212.0) | 0.76 | 0.12 | 160.5 (100.0, 238.0)f | 157.0 (102.0, 212.0) | 0.67 | 0.22 |
| Aortic cross-clamp duration, min | 99.5 (68.0, 126.0)f | 97.0 (68.0, 115.0)f | 0.66 | 0.13 | 100.0 (70.0, 121.0)f | 92.0 (68.0, 115.0)f | 0.48 | 0.20 |
| Time in OR, h | 6.8 (5.9, 8.3) | 6.7 (5.7, 8.0) | 0.51 | 0.18 | 7.0 (6.2, 8.8) | 6.5 (5.7, 7.8) | 0.21 | 0.42 |
The median and first and third quartiles are reported for continuous characteristics and number and percent are reported for categorical characteristics.
P-values for continuous variables are from a Kruskal-Wallis non-parametric test; p-values for categorical variables are from Fisher’s exact test.
Effect sizes for binary and categorical variables were calculated using Cramer’s phi statistic and were calculated as the absolute difference in means divided by the pooled standard deviation for continuous variables.
Coronary artery bypass grafting
Calculated only for patients undergoing cardiopulmonary bypass
Data missing for 1 subject
Transfusions
The storage duration of the RBC units transfused to the RECAP patients intra-operatively and within 24 h of leaving the OR is shown in Figure 2 and Table 3. The mean (±SD) storage duration for RBCs transfused to subjects assigned to receive units stored ≤10 d was 8.2±4.7 d; for units transfused to subjects in the ≥21 d arm, the mean (±SD) storage duration was 27.9±7.4 d. The median storage times (quartile 1, quartile 3) were 7 d (6,9) and 28 d (23,34) respectively. Adherence to the study arm assignment was high, with 92% of units transfused to subjects in the ≤10 d arm and 93% of units transfused to subjects in the ≥21 d arm meeting study requirements. Of the subjects in the shorter storage arm of the study, 87% received only units stored ≤10 d, while 93% of subjects in the longer storage-arm received only units stored ≥21 d.
Figure 2.
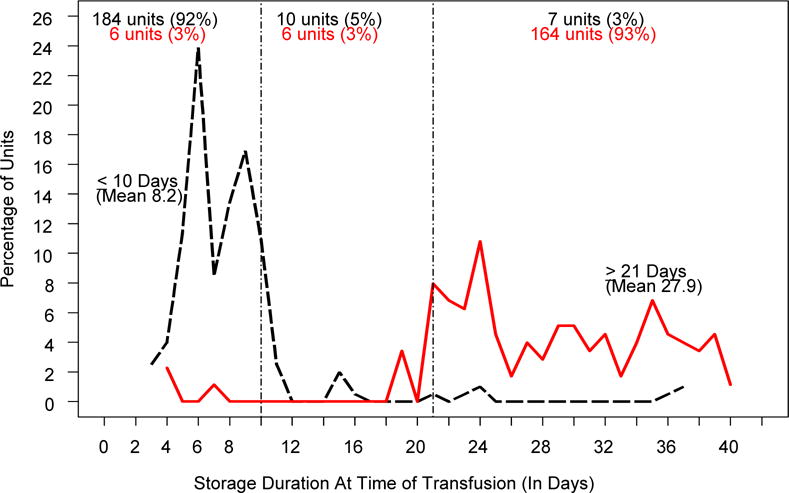
Storage Duration of RBC Units at Time of Transfusion. The black line and text refer to the ≤10 day arm, and the red line and text refer to the ≥21 day arm.
Table 3.
All Transfusions From the Time of Entering the Operating Room until 24 h after Leaving the OR
| Parameter | RBC ≤ 10 d Arm (n=201 units in 47 subjects) | RBC ≥ 21 d Arm (n=176 units in 42 subjects) | P valuea | Effect Sizeb |
|---|---|---|---|---|
| Storage Time of RBC | ||||
| Mean – d (± SD) | 8.2 (4.7) | 27.9 (7.4) | <0.01 | 3.23 |
| Median – d (Q1, Q3) | 7.0 (6.0, 9.0) | 28.0 (23.0, 34.0) | ||
| Mean longest storage – d (± SD) | 9.0 (5.6) | 31.90 (7.1) | <0.01 | 3.61 |
| Mean shortest storage – d (± SD) | 6.5 (2.0) | 25.2 (6.6) | <0.01 | 3.91 |
| Units per protocol – n (%) | 184 (92%) | 164 (93%) | 0.55 | 0.03 |
| Transfused subjects receiving all units per protocol – n (%) | 41 (87%) | 39 (93%) | 0.49 | 0.09 |
| Units RBC Transfused | ||||
| Total units (U) | 201 | 176 | ||
| Total subjects | 47 | 42 | ||
| Mean U/subject – n (± SD) | 4.3 (3.9) | 4.2 (2.9) | 0.42 | 0.03 |
| Median U/subject – n (Q1, Q3) | 3.0 (2.0, 6.0) | 3.5 (2.0, 5.0) | ||
| Subjects > 6 Units – n (%) | 9/47 (19%) | 7/42 (17%) | 0.79 | 0.03 |
| Units out-of-ABO group – n (%) | 9 (4%) | 7 (4%) | 1.00 | 0.01 |
| Subjects out-of-ABO group – n (%) | 4 (9%) | 4 (10%) | 1.00 | 0.02 |
| Unitsc Platelets Transfused | ||||
| Total units | 68 | 65 | ||
| Total subjects – n (%) | 30 (64%) | 25 (60%) | 0.83 | 0.04 |
| Mean U/subject – n (± SD) | 2.3 (2.0) | 2.6 (2.5) | 0.56 | 0.15 |
| Median U/subject – n (Q1, Q3) | 2.0 (1.0, 3.0) | 2.0 (1.0, 3.0) | ||
| Units FFP transfused | ||||
| Total units | 108 | 60 | ||
| Total subjects – n (%) | 28 (60%) | 20 (48%) | 0.29 | 0.12 |
| Mean U/subject – n (± SD) | 3.9 (3.9) | 3.0 (2.2) | 0.52 | 0.26 |
| Median U/subject – n (Q1, Q3) | 2.0 (2.0, 5.0) | 2.0 (1.0, 5.0) | ||
| Units cryoprecipitate transfused | ||||
| Total units | 13 | 16 | ||
| Total subjects – n (%) | 8 (17%) | 12 (29%) | 0.21 | 0.14 |
| Mean U/subject – n (± SD) | 1.6 (1.1) | 1.3 (0.5) | 0.71 | 0.38 |
| Median U/subject – n (Q1, Q3) | 1.0 (1.0, 2.0) | 1.0 (1.0, 2.0) | ||
Abbreviations: SD, standard deviation; Q1, first quartile, Q3 third quartile
P-values for continuous variables are from a Kruskal-Wallis non-parametric test; p-values for categorical variables are from Fisher’s exact test.
Effect sizes for binary and categorical variables were calculated using Cramer’s phi statistic and were calculated as the absolute difference in means divided by the pooled standard deviation for continuous variables.
1 U ≡ 1 U apheresis platelets or 6 U whole blood platelets
The mean number of RBCs transfused up to and including the index transfusion, for subjects with an index transfusion, was 3.6± 2.8 units (median 3.0 units) in the ≤ 10 day arm and 2.9±2.3 units (median 2.5 units) in the ≥21 day arm (p=0.30). The mean number of RBCs transfused through 24 hours post-op was greater than 4 units per subject for both groups, and a similar proportion of subjects received more than 6 units of RBCs. (Table 3) The volumes of other blood components transfused to the patients in both arms were also similar.
Physiologic Measurements
As shown in Table S2, the subjects in the two arms of the study were comparable with respect to several key clinical characteristics within 2 h preceding the transfusion of a single unit of RBC in the ICU post-operatively: systolic and diastolic blood pressure, heart rate, blood O2 saturation, and level of respiratory and inotropic support. Figure 3 and Table 4 show the results of the StO2 measurements at the thenar eminence as determined by NIRS within 2 h before and after transfusion of this post-operative RBC transfusion, time points which might be expected to detect real, albeit transient, changes in tissue oxygenation. Prior to the post-operative RBC transfusion, the two groups of patients also showed similar levels of thenar eminence StO2 with means of approximately 78%. Following transfusion of one unit of RBC, the StO2 levels were again comparable between the two groups, with very little change compared to the pre-transfusion levels. The change in StO2 from pre- to post-transfusion was only an increase of 1.7% (95%CI:−0.3,3.8) in the ≤10 d group and 0.8% (95%CI:−1.1,2.7) in the ≥21 d group; the difference was not statistically significant. Based on the number of subjects and the observed standard deviation for change in thenar oxygen saturation, this comparison had 80% power to detect a 0.8% difference between treatment groups, a difference not likely to be clinically significant. As shown in Figure 3C, the changes in StO2 tended to be slightly higher for patients with lower pre-transfusion values than patients with higher pre-transfusion values. However, the slopes were very similar between treatment groups (interaction p-value 0.92).
Figure 3.
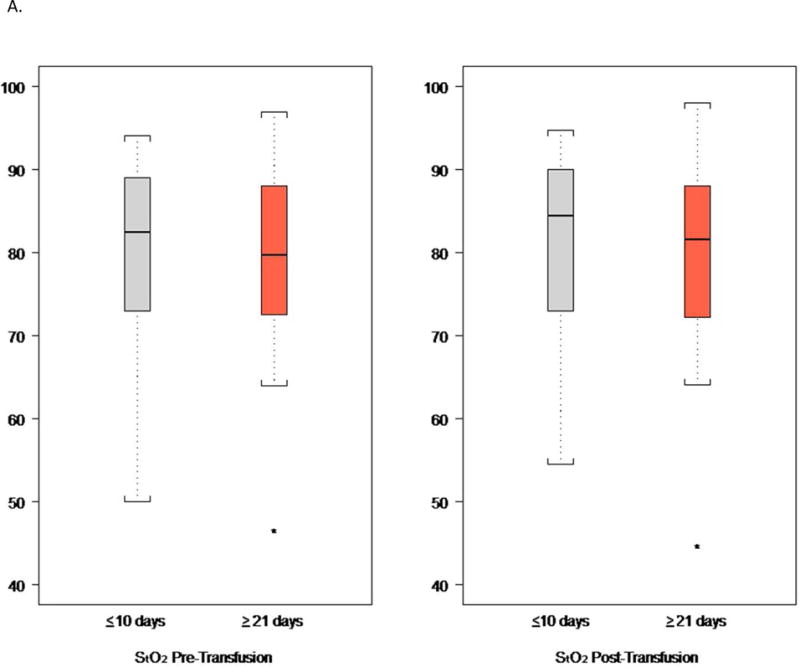
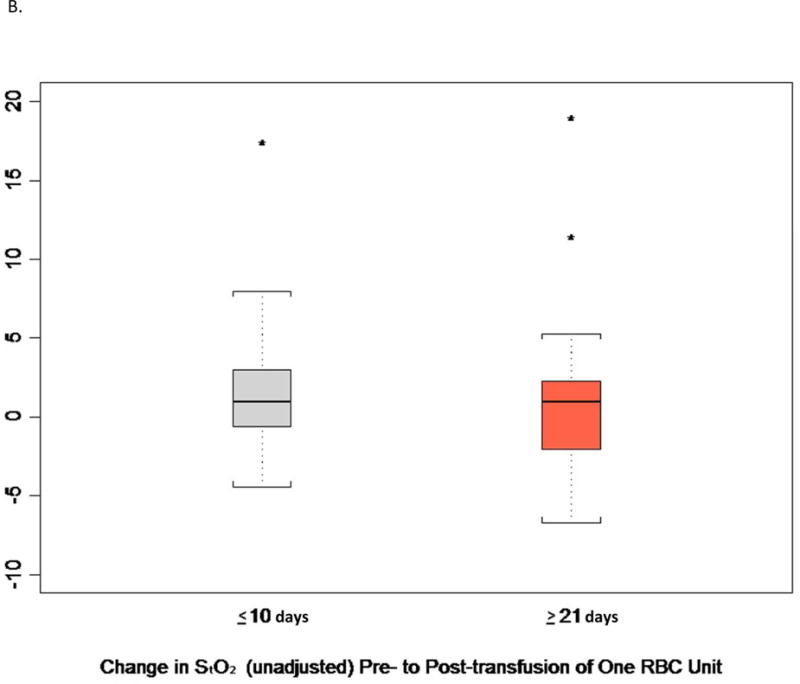
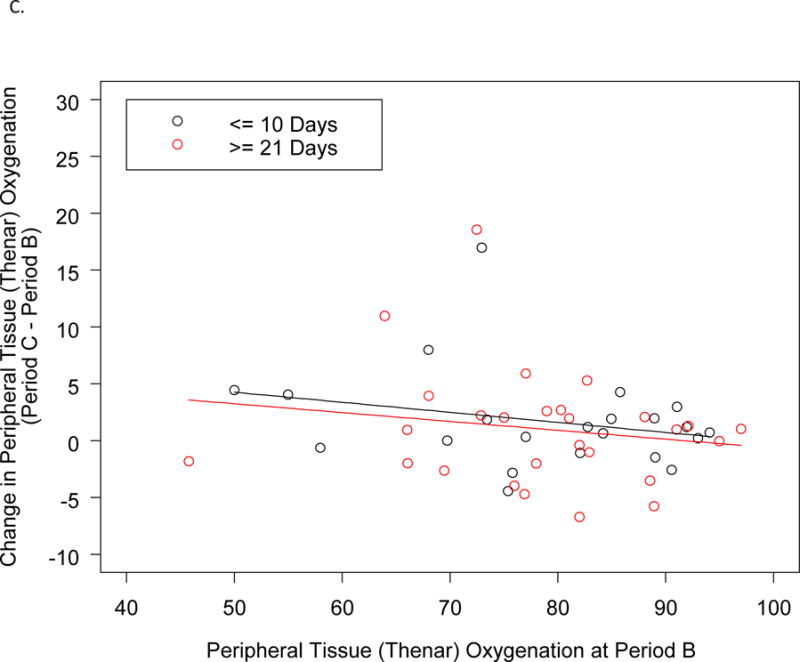
Thenar Eminence StO2.
A. StO2 Before and After a Single Unit RBC (Index) Transfusion.
B. Change in StO2 Before and After a Single Unit RBC (Index) Transfusion.
C. Change in Thenar Oxygenation from Before to After a Single Unit RBC (Index) Transfusion as a Function of the Pre-transfusion Oxygenation.
Videoclip: Blood flow in sub-lingual microcirculation as visualized by side-stream darkfield imaging and videomicroscopy.
Table 4.
Primary and Secondary Outcome Measures for RECAP
| Outcome Measures All are mean values (95% CI) adjusted for baseline | RBC ≤ 10 d Arm | RBC ≥ 21 d Arm | P value | Effect Sizeb |
|---|---|---|---|---|
| Change pre to post index RBC transfusion | ||||
| Peripheral Δ StO2 (%)a | 1.7 (−0.3, 3.8) n=22 |
0.8 (−1.1, 2.7) n=26 |
0.52 | 0.18 |
| Cerebral Δ StO2 (%) | 2.6 (1.3, 3.9) n=19 |
1.6 (0.5, 2.7) n=28 |
0.23 | 0.27 |
| Δ Microvascular flow index,c | −0.05 (−0.4, 0.3) n=5 |
−0.1 (−0.4, 0.3) n=6 |
0.89 | 0.55 |
| Δ Proportion perfused vessels (%)c | 0.4 (−12.9, 13.7) n=5 |
−2.8 (−14.8, 9.1) n=6 |
0.71 | 0.80 |
| Δ Perfused vessel density (mm/mm2)c | −2.7 (−6.8, 1.4) n=5 |
−3.5 (−7.2, 0.2) n=6 |
0.74 | 0.11 |
| Δ Total vessel density (mm/mm2)c | −3.2 (−8.2, 1.8) n=5 |
−3.4 (−8.0, 1.2) n=6 |
0.94 | 0.21 |
| Change pre – op to 24 h post surgery | ||||
| Peripheral Δ StO2 (%) | 3.3 (−0.9, 7.5) n=14 |
3.6 (−0.2, 7.5) n=17 |
0.91 | 0.01 |
| Cerebral Δ StO2 (%) | 0.3 (−2.0, 2.7) n=19 |
−0.5 (−2.8, 1.9) n=19 |
0.64 | 0.27 |
| Δ Microvascular flow indexc | 0.1 (−0.2, 0.3) n=6 |
−0.01 (−0.2, 0.2) n=11 |
0.54 | 0.52 |
| Δ Proportion perfused vessels (%)c | −3.5 (−10.7, 3.7) n=6 |
−1.6 (−6.9, 3.7) n=11 |
0.65 | 0.03 |
| Δ Perfused vessel density (mm/mm2)c | −0.7 (−5.5, 4.1) n=6 |
−1.6 (−5.1, 1.9) n=11 |
0.74 | 0.35 |
| Δ Total vessel density (mm/mm2)c | 0.3 (−4.9, 5.4) n=6 |
−1.3 (−5.1, 2.4) n=11 |
0.61 | 0.21 |
Primary outcome measure
Effect sizes for binary and categorical variables were calculated using Cramer’s phi statistic and were calculated as the absolute difference in means divided by the pooled standard deviation for continuous variables.
As measured by SDF imaging
StO2 = tissue O2 saturation as measured by NIRS
Comparable results were observed for measurements of cerebral StO2 levels by NIRS (Table 4). The pre-transfusion cerebral StO2 levels were comparable between the two groups, although lower than the readings at the thenar eminence (around 62%) and showed a statistically significant but small increase after transfusion in both groups. The increases in the mean cerebral StO2 from pre- to post-transfusion were only 2.6% (95% CI: 1.3,3.9) in the ≤10 d group and 1.6% (95% CI:0.5,2.7) in the ≥21 d group, and did not differ from one another.
The videomicroscopy images of the sub-lingual microcirculation were analyzed for four parameters characterizing blood flow. There were no clinically important changes in response to RBC transfusion, nor any apparent difference in that response between the two study groups, but the number of observations was too small to be statistically meaningful.
Similar comparisons were made for the changes in these parameters from within 6 h before the start of surgery to 24±4 h after surgery to assess the possible cumulative effect of multiple units of RBCs. The subjects in both arms received on average approximately 4 units of RBCs in this time period (Table 3) which represents approximately one third of their RBC mass. There was a slightly greater change in the thenar eminence ΔStO2 (3–4%) than was observed before and after the transfusion of a single unit, but there was not a significant difference between the two arms. Similarly, the modest changes in the other parameters of tissue oxygenation and microcirculatory blood flow were not different between the subjects receiving shorter- or longer-storage RBCs.
The treatment group comparisons of physiologic changes were also not statistically significant in the per-protocol analyses (all p-values > 0.35).
DISCUSSION
This study used FDA-approved, minimally invasive techniques for monitoring tissue hemoglobin oxygen saturation (NIRS) and blood flow through the microcirculation (SDF videomicroscopy). NIRS captures the level of hemoglobin oxygen saturation in small vessels and capillaries. It has been used in multiple clinical situations to gauge effectiveness of resuscitation efforts, vascular flow sufficiency, and transfusion efficacy in neonates,(22) septic patients,(23) critical care patients,(24) patients undergoing surgery,(25) and outpatients.(26) SDF videomicroscopy has been used to interrogate the microcirculation of critically ill patients, especially those with sepsis,(27) as well as to assess the effect of RBC transfusion in the setting of sepsis,(23,28) cardiac surgery(29) and trauma.(30) Although RBC transfusion was associated with improved tissue StO2 levels in many of these studies, this was not the case in all of them. For example, when RBCs stored > 21 d were transfused to trauma patients, tissue oxygen saturation decreased.(11)
The primary finding of this study is that there were no differences in tissue oxygen saturation or microvascular blood flow between patients who received RBC stored ≤10 d vs. ≥21 d. This finding is consistent with the results of the parent trial, RECESS, which also found no differences in clinical outcomes including the primary outcome measure, change in the MODS through 7 days, or any of the other secondary outcomes.(12) Although the RECAP subset was not large enough to compare these clinical outcomes independently with any statistical power, the similarity between the baseline patient characteristics and surgical characteristics of the RECAP study population and the entire RECESS cohort, as well as their comparable clinical outcomes (See Table S1 in Supplement) suggests that storage of RBCs for 21 days or more does not affect their function in cardiac surgery patients as measured by these clinical and physiologic endpoints. The results of three other large, randomized clinical trials in vulnerable populations, adult(9) and neonatal ICU patients,(8) and children with severe anemia(10) likewise found no differences in clinical outcomes among subjects receiving RBC stored for shorter vs. longer periods of time. Thus, the absence of measurable physiologic differences between patients receiving shorter- vs. longer-storage RBC is reflected in the absence of observable clinical effects as well. The apparently equivalent clinical and physiologic effects of RBCs stored for greater or lesser amounts of time have been consistently observed in randomized trials despite the well described changes which occur in banked erythrocytes. This consistency makes it less likely that the clinical endpoints used to date have been too insensitive to detect subtle defects in RBC function at the tissue level.
The second finding is that independent of storage duration, RBC transfusion had very little effect on tissue oxygen saturation or microcirculatory flow in the subjects in this study. One possible reason is that banked RBCs, no matter how briefly they have been stored, fail to deliver oxygen to tissues. Another possibility is that even the techniques deployed in this study to assess these parameters were not sensitive to the effects of RBC transfusion. However, among patients with very low hemoglobin levels or existing hypoxemia, RBC transfusion has been shown to improve tissue oxygenation as measured by NIRS(11–14) and microcirculatory blood flow as visualized by SDF imaging.(15,16) A third possible explanation for the absence of significant changes after RBC transfusion in this study is that most of these patients were neither oxygen-deprived nor supply limited. This observation raises the question of the utility of these post-operative RBC transfusions. In a medically resource-rich setting, RBCs are often transfused to prevent tissue ischemia, despite the fact that the minimum hemoglobin concentration required to achieve this goal has not been defined in most clinical situations, and may not be the best marker to identify which patients are likely to benefit from transfusion. Indeed, it can be argued that patients who are in bed, and not exercising require relatively modest arterial oxygen content. Consistent with this supposition, studies comparing restrictive and liberal RBC transfusion strategies have generally found that there is no clinical detriment to maintaining patients at the lower of the two hemoglobin levels tested(31) including in the setting of cardiac surgery.(32,33)
One key strength of the study is its link to a large parent study which was powered for clinical endpoints and complemented the physiologic measurements reported here. In addition, RECAP was performed at four different facilities, enhancing the generalizability of the findings, and randomization was balanced by site thereby minimizing the effects of differences in patient populations and clinical practices among participating hospitals. Adherence to the study intervention was excellent with greater than 90% of RBC units transfused to the study subjects having been stored for the assigned period of time, and achieved a wide separation in the median RBC storage times (21 d) between the two arms.
However, there are several limitations to the study. Enrollment was lower than anticipated and additional sites could not be added due to the limitation in the number of devices available for the study. In addition, fewer patients received post-operative RBC transfusions than originally projected based on historical data, which may be attributable to the implementation of patient blood management programs(34) and the gradual adoption of more conservative transfusion practices based on clinical trials data.(32,33) Missing data points also highlight the logistical challenges of obtaining some of these timed measurements in unstable patients in the ICU setting, especially for the videomicroscopy which requires active intervention.
CONCLUSIONS
The use of non-invasive technology to assess tissue oxygen saturation and microcirculatory blood flow in the RECAP cohort of patients has enabled the measurement of physiologic correlates to the clinical outcomes observed in the parent study, RECESS. The physiologic measurements are consistent with the clinical endpoints; both show that outcomes are not different among cardiac surgery patients in the ICU who receive RBC stored for ≤10 d vs. ≥21 d.
Supplementary Material
Perspective Statement.
The impact of red cell storage on clinical outcomes has been controversial, but recent randomized trials have demonstrated no differences between patients receiving red cells stored for shorter vs. longer periods. In a subset of cardiac surgery patients in one of these trials, we found no differences in tissue oxygenation and microcirculatory blood flow, consistent with the clinical outcomes.
Acknowledgments
The authors would like to acknowledge the leadership of the TMHCTN and RECESS for its input into the early design phases and their on-going support. We would especially like to recognize the early guidance of Dr. Gregory Bielman (U MN). The authors wish to thank the nurses and physicians in the operating rooms and intensive care units of the four enrolling institutions for their cooperation and support. We also recognize the contributions of individuals whose efforts were material to the successful realization of this study: Cristina Brueggeman, RN (MGH); Marcy Lindley, the research coordinators from the Multidisciplinary Acute Care Research Organization, Arthur Boujoukos, MD, Penny Sappington, MD, Vinay Badhwar, MD and Lawrence Wei, MD (U Pittsburgh); Rika Ohkuma, MD (Johns Hopkins); Kerri Hayes, BS and Brian Harty, MS (NERI).
Financial Support:
National Heart, Lung and Blood Institute; 1 R01 HL101382-01; Evelyn and Robert Luick Memorial Fund (CPS); K12 HL109068-02 (HG)
None of the sponsors participated in the design of the study, collecting, analyzing and interpreting the data, writing the report or deciding to submit the report for publication.
Abbreviations Used
- ANCOVA
Analysis of covariance
- ICU
Intensive care unit
- Δ MFI
Microcirculatory flow index for small vessels (MFI) and the change in MFI
- MODS
Multiple Organ Dysfunction Score
- NIRS
Near infra-red spectroscopy
- NERI
New England Research Institutes
- OR
Operating Room
- ΔPVD
Perfused small vessel density (PVD) and the change in PVD
- ΔPPV
Proportion of perfused small vessels (PPV) and the change in PPV
- RBC
Red blood cells
- RECESS
Red Cell Storage Duration Study
- RECAP
RECESS Ancillary Physiologic Study
- SDF
Side-stream darkfield
- SD
Standard deviation
- StO2
Tissue hemoglobin oxygen saturation
- ΔTVD
Total small vessel density (TVD) and the change in TVD
- TMHCTN
Transfusion Medicine and Hemostasis Clinical Trials Network
- TRUST
Transfusion Risk Understanding Score
IRB Approvals
| Duke University Medical Center | 12/07/2009 | 21198 |
| Johns Hopkins Hospital | 10/25/2012 | NA_00075888 |
| Massachusetts General Hospital | 04/07/2010 | 2009 P002612/1 |
| University of Pittsburgh | 09/12/2012 | PRO12060386 |
| New England Research Institutes | 03/18/2010 | #788.10 |
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement:
None of the authors reports any conflicts of interest.
Central Picture (Same as Fig. 3 B)
Change in Thenar Eminence StO2 Pre- to Post-Transfusion of RBC Stored ≤ 10d or ≥ 21d
Central Message
Red cell storage did not impair tissue oxygenation or microcirculation in cardiac surgery patients, consistent with lack of difference in clinical outcomes.
Contributor Information
Christopher P. Stowell, Blood Transfusion Service, Department of Pathology, Massachusetts General Hospital, Harvard Medical School.
Glenn Whitman, Department of Surgery, Division of Cardiac Surgery, Johns Hopkins Hospital.
Suzanne Granger, New England Research Institutes.
Hernando Gomez, Department of Critical Care Medicine, Center for Critical Care Nephrology, University of Pittsburgh.
Susan F. Assmann, New England Research Institutes.
Michael J. Massey, Center for Vascular Biology Research, Beth Israel Deaconess Medical Center.
Nathan I. Shapiro, Center for Vascular Biology Research, Beth Israel Deaconess Medical Center, Harvard Medical School.
Marie E. Steiner, Department of Pediatrics, University of Minnesota.
Elliott Bennett-Guerrero, Department of Anesthesiology, Duke University Medical Center.
References
- 1.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. Evolution of adverse changes in stored RBCs. Proc Nat Acad Sci USA. 2007;104:17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimrin AB, Hess JR. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 2009;96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x. [DOI] [PubMed] [Google Scholar]
- 3.Triulzi D, Yazer MH. Clinical studies of the effect of blood storage on patient outcomes. Transfus Aph Sci. 2010;43:95–106. doi: 10.1016/j.transci.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Sun J, Soloman SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46:1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 6.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 7.Steiner M, Ness PM, Assmann SF, Triulzi DJ, Sloan SR, Delaney M, et al. Duration of red-cell storage and cardiac surgery outcomes. New Engl J Med. 2015;372:1419–29. doi: 10.1056/NEJMoa1414219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fergusson DA, Hébert P, Hogan DL, LeBel L, Rouvinez-Bouali N, Smyth JA, et al. Effect of fresh red blood cell transfusions on clinical outcomes in premature, very low-birth-weight infants: the ARIPI randomized trial. J Amer Med Assoc. 2012;308:1443–1451. doi: 10.1001/2012.jama.11953. [DOI] [PubMed] [Google Scholar]
- 9.Lacroix J, Hébert P, Fergusson D, Tinmouth A, Cook DJ, Marshall JC, et al. Age of transfused blood in critically ill adults. New Engl J Med. 2015;372:1410–18. doi: 10.1056/NEJMoa1500704. [DOI] [PubMed] [Google Scholar]
- 10.Dhabangi A, Ainomugisha B, Cserti-Gzdewich C, Ddungu H, Kyeyune D, Musisi E, et al. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the TOTAL randomized clinical trial. J Amer Med Assoc. 2015;314:2514–23. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 11.Kiraly LN, Underwood S, Differding JA, Schreiber MA. Transfusion of aged packed red blood cells results in decreased tissue oxygenation on critically injured trauma patients. J Trauma. 2009;67:29–32. doi: 10.1097/TA.0b013e3181af6a8c. [DOI] [PubMed] [Google Scholar]
- 12.Roberson RS, Lockhart E, Shapiro NI, Bandarenko N, McMahon TJ, Massey MJ, et al. Impact of transfusion of autologous 7- versus 42-day old AS-3 red blood cells on tissue oxygenation and the microcirculation in healthy volunteers. Transfusion. 2013;52:2459–64. doi: 10.1111/j.1537-2995.2012.03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weinburg JA, MacLennan PA, Vandromme-Cusick MJ, Magnotti LJ, Kerby JD, Rue LW, et al. The deleterious effect of red blood cell storage on microvascular response to transfusion. J Trauma Acute Care Surg. 2013;75:807–12. doi: 10.1097/TA.0b013e3182a74a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhabangi A, Ainomugisha B, Cserti-Gazdewich C, Ddungu H, Kyeyune D, Musisi A, Opoka R, Stowell CP, Dzik WH. Cerebral oximetry in Ugandan children with severe anemia: clinical categories and response to transfusion. J Amer Med Assoc Pediatrics. 2016 doi: 10.1001/jamapediatrics.2016.1254. Published online August 08, 2016. [DOI] [PubMed] [Google Scholar]
- 15.Yuruk K, Milstein DMJ, Bezemer R, Bartels SA, Biemond BJ, Ince C. Transfusion of banked red blood cells and the effects on hemorrheology and microvascular dynamics in anemic hematology outpatients. Transfusion. 2013;53:1346–52. doi: 10.1111/j.1537-2995.2012.03905.x. [DOI] [PubMed] [Google Scholar]
- 16.Ayhan B, Yuruk K, Koene S, Sahin A, Ince C, Aypar U. The effect of non-leukoreduced red blood cell transfusions on microcirculation in mixed surgical patients. Transfus Aph Sci. 2013;49:212–22. doi: 10.1016/j.transci.2013.01.016. [DOI] [PubMed] [Google Scholar]
- 17.Alghamdi AA, Davis A, Brister S, Corey P, Logan A. Development and validation of Transfusion Risk Understanding Scoring Tool (TRUST) to stratify cardiac surgery patients according to their blood transfusion needs. Transfusion. 2006;46:1120–1129. doi: 10.1111/j.1537-2995.2006.00860.x. [DOI] [PubMed] [Google Scholar]
- 18.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27:365–75. doi: 10.1016/0021-9681(74)90015-0. [DOI] [PubMed] [Google Scholar]
- 19.Goedhart PT, Khalilzada M, Bezemer R, Merza J, Ince CM. Sidestream Dark Field (SDF) Imaging: a novel stroboscopic LED ring-based imaging modality for clinical assessment of the microcirculation. Optics Express. 2007;15:15101–14. doi: 10.1364/oe.15.015101. [DOI] [PubMed] [Google Scholar]
- 20.De Backer D, Hollenberg S, Boerma C, Goedhart P, Büchele G, Ospina-Tasco G, et al. How to evaluate the microcirculation: report of a round table conference. Crit Care. 2007;11:R101–10. doi: 10.1186/cc6118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marshall JC, Cook DJ, Christou NV, Bernard JR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of complex clinical outcome. Crit Care Med. 1995;23:1638–52. doi: 10.1097/00003246-199510000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Sandal G, Oguz SS, Erdeve O, Akar M, Uras N, Dilmen U. Assessment of red blood cell transfusion and transfusion duration on cerebral and mesenteric oxygenation using near-infrared spectroscopy in preterm infants with symptomatic anemia. Transfusion. 2014;54:1100–5. doi: 10.1111/trf.12359. [DOI] [PubMed] [Google Scholar]
- 23.Donati A, Damiani E, Luchetti MM, Domizi R, Scorcella C, Carsetti A, et al. Microcirculatory effects of the transfusion of leukodepleted or non-leukodepleted red blood cells in patients with sepsis: a pilot study. Crit Care. 2014;18:R33–43. doi: 10.1186/cc13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Creteur J, Neves AP, Vincent JL. Near infrared spectroscopy technique to evaluate the effects of red blood cell transfusion on tissue oxygenation. Crit Care. 2009;13(Suppl 5):S11–18. doi: 10.1186/cc8009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Torella F, Haynes SL, McCollum CN. Cerebral and peripheral oxygen saturation during red cell transfusion. J Surg Res. 2003;110:217–21. doi: 10.1016/s0022-4804(03)00037-4. [DOI] [PubMed] [Google Scholar]
- 26.Yuruk K, Bartels SA, Milstein DMJ, Bezemer R, Biemond BJ, Ince C. Red blood cell transfusions and tissue oxygenation in anemic hematology outpatients. Transfusion. 2012;52:641–6. doi: 10.1111/j.1537-2995.2011.03312.x. [DOI] [PubMed] [Google Scholar]
- 27.Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–31. doi: 10.1097/01.ccm.0000138558.16257.3f. [DOI] [PubMed] [Google Scholar]
- 28.Sakr Y, Chierego M, Piagnerelli M, Verdant C, Dubois MJ, Koch M, et al. Microvascular response to red blood cell transfusion in patients with severe sepsis. Crit Care Med. 2007;35:1639–44. doi: 10.1097/01.CCM.0000269936.73788.32. [DOI] [PubMed] [Google Scholar]
- 29.Yuruk K, Almac E, Bezemer R, Goedhart P, de Mol B, Ince C. Blood transfusions recruit the microcirculation during cardiac surgery. Transfusion. 2011;41:961–7. doi: 10.1111/j.1537-2995.2010.02971.x. [DOI] [PubMed] [Google Scholar]
- 30.Weinburg JA, MacLennan PA, Vandromme-Cusick MJ, Angnotti JM, Magnotti LJ, Kerby JD, et al. Microvascular response to red blood cell transfusion in trauma patients. Shock. 2012;37:276–81. doi: 10.1097/SHK.0b013e318241b739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson JL, Carless PA, Hébert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, et al. Transfusion requirements after cardiac surgery: The TRACS randomized controlled trial. J Amer Med Assoc. 2010;304:1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 33.Murphy GJ, Pike K, Rogers CA, Wordsworth S, Stokes WA, Angelini GD, et al. Liberal or restrictive transfusion after cardiac surgery. New Engl J Med. 2015;372:1997–108. doi: 10.1056/NEJMoa1403612. [DOI] [PubMed] [Google Scholar]
- 34.Ness PM, Frank SM. Enhancing patient blood management: a long-term FOCUS. Lancet. 2015;355:157–9. doi: 10.1016/S0140-6736(14)62344-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


