Abstract
More than 75% of hospital-acquired or nosocomial urinary tract infections are initiated by urinary catheters, which are used during the treatment of 15–25% of hospitalized patients. Among other purposes, urinary catheters are primarily used for draining urine after surgeries and for urinary incontinence. During catheter-associated urinary tract infections, bacteria travel up to the bladder and cause infection. A major cause of catheter-associated urinary tract infection is attributed to the use of non-ideal materials in the fabrication of urinary catheters. Such materials allow for the colonization of microorganisms, leading to bacteriuria and infection, depending on the severity of symptoms. The ideal urinary catheter is made out of materials that are biocompatible, antimicrobial, and antifouling. Although an abundance of research has been conducted over the last forty-five years on the subject, the ideal biomaterial, especially for long-term catheterization of more than a month, has yet to be developed. The aim of this review is to highlight the recent advances (over the past 10 years) in developing antimicrobial materials for urinary catheters and to outline future requirements and prospects that guide catheter materials selection and design.
Keywords: catheter-associated urinary tract infections, antimicrobial coatings, antifouling coatings, urinary catheters, urinary catheter coatings, biocides
Graphical Abstract
1. Introduction
1.1. Urinary Catheter
Urinary catheters have been used since the third century B.C., by the Greeks, Egyptians and Chinese, but the first malleable urinary catheter on record was only made in 1779 by a goldsmith, Bernard [1]. Some of the first materials used to make urinary catheters were copper, tin, bronze, gold, lead, papyrus, onion stems, dried reeds and palm leaves. In recent times, materials such as gum-elastic, plastic (poly(vinylchloride), PVC), polyurethanes, silicone and latex rubbers have been used for their superior malleability [2, 3]. These materials have been developed over the years to include most of the characteristics desirable in a catheter: high tensile strength, soft and pliable, inherently chemical resistant, biocompatible and able to meet flow requirements while maintaining a minimally invasive circumference or French profile. Some of the strengths and weaknesses of different urinary catheter materials have been listed in Table 1, and these characteristics have led the emergence of silicone as the material of choice for urinary catheters despite a few of its disadvantages [1, 4, 5]. While latex was originally used alone, or modified with either hydrogel or Teflon coatings, its unsuitable properties like poor UV and chemical resistance, poor adherence, and possible allergic reactions leave much to be desired [1]. It has also been observed using scanning electron microscopy (SEM) that the rough surface of latex can also promote biofilm formation [6]. Therefore, silicone is now more commonly used as a base catheter material since it circumvents many of the problems faced by latex catheters.
Table 1.
Comparison of strength and weaknesses of commonly used urinary catheter materials.
| Material | Advantages | Disadvantages |
|---|---|---|
| Latex rubber |
|
|
| Silicone |
|
|
| PTFE coating |
|
|
| Polyvinyl chloride |
|
|
| Polyurethane |
|
|
Besides the evolution of materials, catheter design has also undergone several changes over the years including the balloon used to hold the catheter onto the urinary bladder and development of the modern indwelling catheter, also called the ‘Foley’ catheter, designed by Dr. Frederick B. Foley in mid 1930s [1]. This evolution of materials and design has been steered by the efficiency and comfort the catheter is able to provide to the patient along with availability of materials and technology. Currently, medical device companies like C. R. Bard, Inc. (Murray Hill, NJ), Coloplast (Minneapolis, MN) and Teleflex (Morrisville, NC) manufacture urinary catheters that contain advanced formulations with silver alloy (for example, gold and palladium) coatings and hydrogels that claim to dramatically reduce infection rates down by 3.7 times the standard urinary catheters.
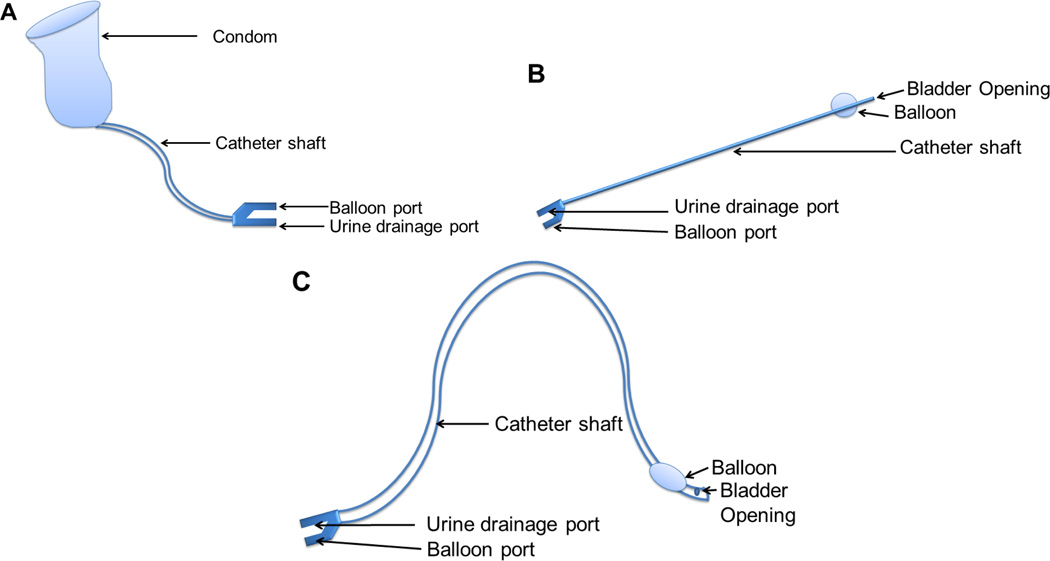
Urinary catheters are used to manage urinary incontinence, urinary retention, and/or after prostrate or genital surgical procedures. In simple terms, urinary catheters are used to remove urine from the body. If the body is unable to remove urine for some reason, pressure builds on the urinary bladder and as a result, kidney failure can occur. Currently, there are several types of catheter designs that serve different purposes as shown in Figure 1. The condom or single-use catheter is used for males who may have mental disabilities and have trouble urinating. These types of catheters are changed daily. The intermittent or short term use catheter is used for a few weeks. This is commonly used for postoperative care when the patient is unable to urinate by themselves and need assistance. The long-term use or Foley catheters are typically used for several months at a time by patients with urine retention problems including those with spinal cord injury/disease, multiple sclerosis, enlarged prostate, or cerebrovascular accident. A management system/protocol for the insertion and removal of urinary catheters is maintained by different hospital systems. This includes the use of gloves, handwashing, sterile barrier, no-touch insertion techniques and training [7]. However, despite the care taken to avoid contamination and subsequent infections, catheters are still susceptible to accumulation of microbes. In urinary catheters, these microbes can accumulate to form single species biofilms, which can cover even short term non-Foley catheters in a period as short as 24 hours, which can ultimately develop multispecies biofilms causing infections if not detected at an early stage. Infection occurs in 10–50% of patients undergoing non-Foley or short-term urinary catheterization (7 days) but virtually all patients undergoing Foley or long-term catheterization (>28 days) become infected [8]. Foley catheters are most susceptible to infection as bacteria can collect and grow rapidly over time if not identified. This infection is called catheter-associated urinary tract infection (CAUTI), an infection that has stimulated antimicrobial materials research for urinary catheters.
Figure 1.
Types of Urinary Catheters: (A) Condom or single use catheter: used only in males for ~1-week period; (B) Intermittent or short term use catheter: used for a few weeks to months; (C) Foley or long term use catheter: used for a few months up to a year.
1.2. The Problem with Urinary Catheters: Long-term Catheterization and Catheter-associated Urinary Tract Infections
Catheter-associated urinary tract infections account for over 1 million cases in the US alone [9] and almost 80% of the nosocomial infections worldwide [10]. Annual treatment costs exceed $350 million every year, which illustrate the urgency of the situation [10]. Some of the symptoms and signs of normal urinary tract infections (UTIs) and CAUTIs overlap, [11] as summarized in Table 2 of this review but the term CAUTI is assigned to patients who have been catheterized for over 24 hours and show signs and symptoms of CAUTI within 48 hours of catheterization. Urinary tract infection is defined as an invasion of any part of the urinary system by a bacterial or fungal pathogen [12, 13]. Nosocomial CAUTI is defined as the new appearance of bacteriuria or funguria in the urine at a concentration greater than 105 CFU mL−1 according to the Centers for Disease Control and Prevention. CAUTIs are caused by the invasion and colonization of pathogens through the route of the urinary catheter [14]. Such infections can cause mild to severe symptoms and pose a major cause for concern, as 15–25% of hospitalized patients use urinary catheters [14]. Catheter-associated urinary tract infection, when left untreated, may cause infections in the kidneys (pyelonephritis) and bloodstream (septicemia) [15], leading to sepsis or, in extreme cases, even death. The urinary catheter, a partially implanted device, can cause a patient to be highly prone to infections mostly because of cross contamination from the drainage bag and the rich microbial flora in the skin. This susceptibility increases with the duration of catheterization, which allows bacteria to flourish.
Table 2.
Urinary tract infection signs and symptoms
| Symptoms | Signs |
|---|---|
|
|
Long-term catheterization is needed when a patient suffers from urinary incontinence which entails inserting the catheter into the bladder for several months or years [1]. A major hindrance regarding the use of these long-term catheters is their ineffectiveness to prevent infection. Infections occur due to free-floating (planktonic) bacteria or encrustation of bacteria developing biofilms on catheter surfaces [16]. According to Dr. J. L. Brusch, an infectious disease specialist in Cambridge, Massachusetts, 90–100% patients who undergo long-term catheterization develop bacteriuria and 80% nosocomial UTIs are caused by catheters while only 5–10% are related to genitourinary operation [17]. For these reasons, the emergence of research in CAUTI and urinary catheters has been influenced by the enormous amount of health risks and healthcare-associated economic pressures caused by CAUTIs.
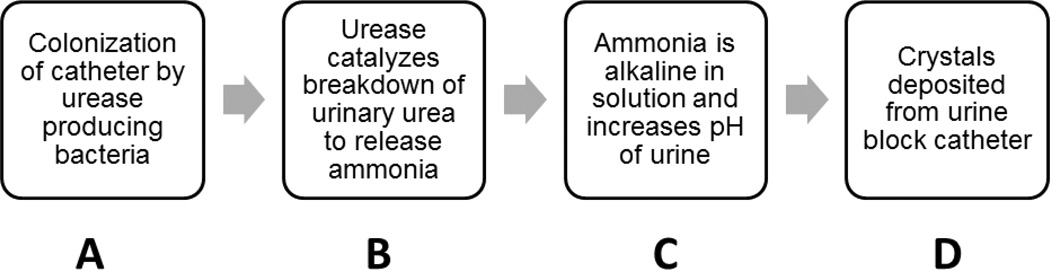
Two main issues that afflict urinary catheters and make it harder to treat CAUTIs are encrustation and biofilm formation [8]. While they are two different mechanisms caused by different factors, they can overlap and make conditions worse in an infection. Encrustation (Figure 2) begins with the colonization of the catheter by urease-positive pathogens. Some urease-positive pathogens are P. mirabilis, M. morganii, P. aeruginosa, K. pneumoniae and P. vulgaris [18]. Urease is an enzyme that catalyzes the hydrolysis of urea into ammonia and carbamate. The presence of urine in urinary catheters creates a suitable environment for urease-positive pathogens. Ammonia is alkaline, and increases the pH of urine, leading to deposition of calcium and magnesium phosphate crystals on the catheter, which eventually leads to complete occlusion of the catheter through encrustation or crystalline biofilms [19]. One of the most common bacteria that causes encrustation is the urease-positive bacteria Proteus mirabilis [20]. P. mirabilis is a gram-negative, rod-shaped bacterium and causes 90% of all Proteus infections in humans and 20–45% of catheterization related infections [21]. In 1993, Stickler et al. presented a case study in which the patient’s catheter was completely blocked within 4–5 days of use [22]. The biofilms in the catheter contained elevated levels of mineral deposits. The ability of P. mirabilis to colonize all available types of indwelling catheters allows it to form secure biofilms in the catheterized tract and cause persistent catheter blockage. The infection can be diagnosed by an increase in the urine’s pH, fishy odor (produced by the bacteria) and P. mirabilis can be detected by its inability to metabolize lactose (on a MacConkey agar plate). A common treatment for Proteus infections is the use of antibiotics in urinary catheters, which can break down the biofilms formed by these persistent pathogens.
Figure 2.
Flowchart showing the process of encrustation caused by urease producing bacteria: (A) Urease producing bacteria colonize the catheter with the help of biofilms (B) The urease produced by the bacteria breaks down urinary urea to release ammonia (C) The presence of ammonia in urine raises its pH. (D) The alkalinity of urine causes precipitation of salt crystals that are deposited on the catheter and cause blockage.
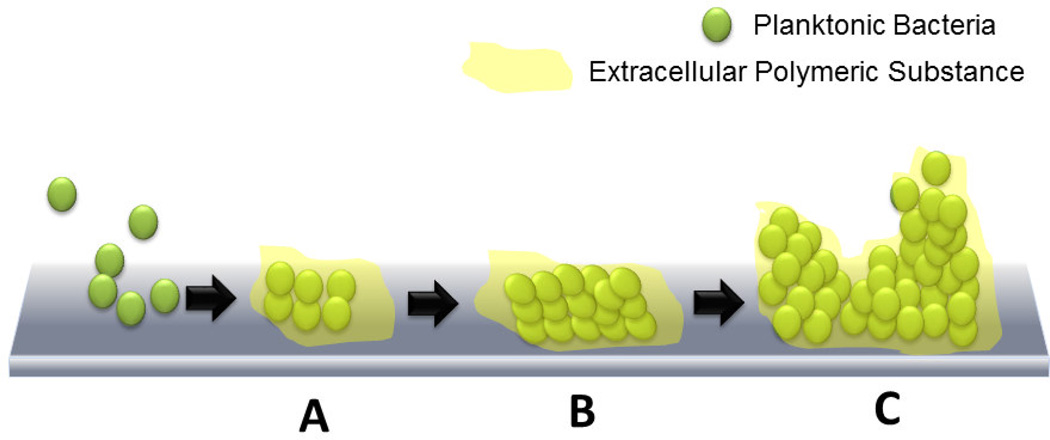
Biofilms are another major problem faced by urinary catheter patients because of the inherent property of urine to deposit minerals once infection by any microbe has occurred [18]. Free-floating, or planktonic, bacteria come across a surface submerged in the fluid and within minutes become attached. These attached bacteria produce slimy, extracellular polymeric substances (EPS) that colonize the surface (Figure 3) and form the conditioning film. Extracellular polymeric substance production allows the emerging biofilm community to develop a complex, three-dimensional structure that is influenced by a variety of environmental factors. Biofilm communities develop within hours. Scanning electron microscopy and transmission electron microscopy have been used to document biofilms in urinary catheters removed from patients [23]. Biofilms have been reported to be approximately 200 µm in thickness and occasionally reach a thickness of ~500 µm [24]. The rate of bacterial cell attachment depends on the number and types of bacteria in the urine or environment to which the catheter is exposed, the flow rate of liquid through the catheter, and the physicochemical characteristics of the surface of the catheter. It has been found that catheter surfaces that display both hydrophobic and hydrophilic properties attract the widest variety of CAUTI pathogens [25]. The bacteria can also propagate other biofilm communities by detaching in parts and attaching themselves elsewhere on the surface. A major hindrance in attacking and eliminating these biofilms is the extracellular polymeric substance that protects the cells, which allows the biofilm to exude high tolerance to stress from antibiotics and other biocidal treatments [26]. In fact, a biofilm’s tolerance to antibiotics has been attributed to three possible characteristics of the biofilm [27]: 1) slow penetration of antibiotics due to the matrix formed by the exopolysaccharides [28]; 2) formation of a resistant phenotype called persister cells that remain in a transient dormant state and can cause recurrent infections [29]; and 3) an altered environment within the biofilm that is composed of different anaerobic niches, concentration gradients and local accumulation of acids and inhibitive waste products. Hence, a major research development that has propagated the advancement in antimicrobial urinary catheter materials is the discovery of bacteria that cause CAUTIs, build single species biofilms and ultimately cause co-infection by forming multi-species biofilms (Table 3) [30, 31]. This has allowed researchers to develop mechanisms and bacteria specific or broad spectrum biocidal techniques to prevent CAUTIs. Some of the most common bacteria associated with CAUTIs are S. aureus, E. coli, P. aeruginosa. mirabilis, S. epidermidis, E. faecalis, and K. pneumoniae [8]. Several studies reveal that it is important to focus on the prevention of the biofilm rather than focus on planktonic bacteria as slow growth of the biofilms can confer resistance [32]. For example, in a rabbit catheter model study, only the highest dosage (400 mg/kg) of the antibiotic, amdinocillin could eradicate an E. coli biofilm formed on the catheter [33]. Another study showed how vancomycin concentration in an S. aureus biofilm was inversely related to the biofilm growth but it was also unable to completely eradicate the biofilm [34]. This meant that a biofilm’s resistance was related to the diminished effect of the antibiotic in the biofilm rather than poor penetration of the antibiotic. Another major hurdle in eradication of CAUTIs has been the increased incidence of infection due to polymicrobial infections, also called coinfection. As we know, single species biofilms can develop to form multi-species biofilms, studies to understand this effect on CAUTIs are important. One study found out that coinfection by P. mirabilis and P. stuartii caused an increase in the incidence of bacteremia and urolithiasis in a mouse model [35]. In fact, a detailed review on infection and coinfection by P. mirabilis has been published by Armbruster and Mobley [20]. This development in the body of knowledge of known pathogens of CAUTI has helped in understanding the mechanism of biofilm formation in specific bacteria, which aids in designing target specific biocides. While bacteria form the majority in the pool of pathogens, fungi are not far behind in CAUTIs. Ramage et al. have reported that C. albicans are frequently found in CAUTI biofilms and is the cause of 10–15% of cases [36]. Antifungal therapy and catheter removal have been described as the best therapies for treatment. A major drawback of studying and eliminating all pathogens associated with CAUTI is that a percentage of the pathogens in biofilms cannot be cultured by traditional microbial methods [37]. So even though they can be observed using microscopy, they cannot be cultured traditionally. Frank et al. devised a way to work around this problem using rRNA-based molecular phylogenetic methods to identify pathogens that form CAUTI biofilms [38]. This method did not require any culturing and relied on searching for the genomic sequences with BLASTN search and molecular-phylogenetic analysis. This study showed how further molecular studies could be conducted to find clinically-relevant microbes involved in CAUTIs across different regions of the world.
Figure 3.
Biofilm formation process: (A) Free-floating, or planktonic, bacteria come across a surface submerged in the fluid and within minutes become attached. These attached bacteria produce slimy extracellular polymeric substances (EPS) and colonize the surface. (B) EPS production allows the emerging biofilm community to develop a complex, three-dimensional structure that is influenced by a variety of environmental factors. (C) Biofilm communities develop within hours.
Table 3.
Microorganisms causing CAUTI
| Microorganism name | Type | References |
|---|---|---|
| Bacillus subtilis | Gram positive bacteria | [1, 2] |
| Enterococcus faecalis | Gram positive bacteria | [3–6] |
| Enterococcus faecium | Gram positive bacteria | [7–9] |
| Staphylococcus aureus | Gram positive bacteria | [3, 6, 10–20] |
| Staphylococcus epidermidis | Gram positive bacteria | [3, 4, 12, 18, 21] |
| Escherichia coli | Gram negative bacteria | [3, 4, 6, 7, 10–12, 14, 16, 19, 22– 26] |
| Klebsiella pneumoniae | Gram negative bacteria | [3, 4, 6, 7, 12] |
| Morganella morganii | Gram negative bacteria | [4, 12] |
| Proteus mirabilis | Gram negative bacteria | [1, 3, 4, 6, 7, 12, 27–29] |
| Providencia spp. | Gram negative bacteria | [4, 6, 7, 12] |
| Pseudomonas aeruginosa | Gram negative bacteria | [4, 7, 12, 13, 25, 30–33] |
| Candida albicans | Fungi | [3, 7–9] |
| Candida glabarata | Fungi | [7] |
| Candida tropicalis | Fungi | [7] |
I. Goncalves, A.S. Abreu, T. Matama, A. Ribeiro, A.C. Gomes, C. Silva, A. Cavaco-Paulo, Enzymatic synthesis of poly(catechin)-antibiotic conjugates: an antimicrobial approach for indwelling catheters, Appl Microbiol Biotechnol 99(2) (2015) 637–51.
C.G. Kumar, P. Sujitha, Green synthesis of Kocuran-functionalized silver glyconanoparticles for use as antibiofilm coatings on silicone urethral catheters, Nanotechnology 25(32) (2014) 325101.
R.M. Donlan, Biofilms and device-associated infections, Emerging Infectious Diseases 7(2) (2001) 277–281.
R.M. Donlan, J.W. Costerton, Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms, Clinical Microbiology Reviews 15(2) (2002) 167–193.
F.R. Dametto, C.C.R. Ferraz, B.P.F. de Almeida Gomes, A.A. Zaia, F.B. Teixeira, F.J. de Souza- Filho, In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis, Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology 99(6) (2005) 768–772.
G.L. Jones, C.T. Muller, M. O'Reilly, D.J. Stickler, Effect of triclosan on the development of bacterial biofilms by urinary tract pathogens on urinary catheters, Journal of Antimicrobial Chemotherapy 57(2) (2006) 266–272.
M.E. Rupp, T. Fitzgerald, N. Marion, V. Helget, S. Puumala, J.R. Anderson, P.D. Fey, Effect of silver-coated urinary catheters: Efficacy, cost-effectiveness, and antimicrobial resistance, American Journal of Infection Control 32(8) (2004) 445–450.
J.R. Johnson, B. Johnston, M.A. Kuskowski, In vitro comparison of nitrofurazone- and silver alloy-coated foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms, Antimicrob Agents Chemother 56(9) (2012) 4969–72.
X. Sun, Z. Cao, N. Porteous, Y. Sun, An N-halamine-based rechargeable antimicrobial and biofilm controlling polyurethane, Acta Biomater 8(4) (2012) 1498–506.
J.S. Kim, E. Kuk, K.N. Yu, J.-H. Kim, S.J. Park, H.J. Lee, S.H. Kim, Y.K. Park, Y.H. Park, C.-Y. Hwang, Y.-K. Kim, Y.-S. Lee, D.H. Jeong, M.-H. Cho, Antimicrobial effects of silver nanoparticles, Nanomedicine: Nanotechnology, Biology and Medicine 3(1) 95–101.
F.C. Fang, Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity, Journal of Clinical Investigation 99(12) (1997) 2818–2825.
J.W. Warren, Catheter-associated Urinary Tract Infections, Infectious Disease Clinics of North America 11(3) (1997).
J.M. Schierholz, L.J. Lucas, A. Rump, G. Pulverer, Efficacy of silver-coated medical devices, Journal of Hospital Infection 40(4) (1998) 257–262.
Q.L. Feng, J. Wu, G.Q. Chen, F.Z. Cui, T.N. Kim, J.O. Kim, A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus, Journal of Biomedical Materials Research 52(4) (2000) 662–668.
C. Slater-Radosti, G. Van Aller, R. Greenwood, R. Nicholas, P.M. Keller, W.E. DeWolf, F. Fan, D.J. Payne, D.D. Jaworski, Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus, Journal of Antimicrobial Chemotherapy 48(1) (2001) 1–6.
A.D. Fuchs, J.C. Tiller, Contact-Active Antimicrobial Coatings Derived from Aqueous Suspensions, Angewandte Chemie (2006).
R.O. Darouiche, M.D. Mansouri, P.V. Gawande, S. Madhyastha, Antimicrobial and antibiofilm efficacy of triclosan and DispersinB® combination, Journal of Antimicrobial Chemotherapy 64(1) (2009) 88–93.
J.B. Kaplan, K. LoVetri, S.T. Cardona, S. Madhyastha, I. Sadovskaya, S. Jabbouri, E.A. Izano, Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci, J Antibiot 65(2) (2012) 73–77.
D. Kowalczuk, G. Ginalska, T. Piersiak, M. Miazga-Karska, Prevention of biofilm formation on urinary catheters: comparison of the sparfloxacin-treated long-term antimicrobial catheters with silver-coated ones, J Biomed Mater Res B Appl Biomater 100(7) (2012) 1874–82.
M. Honda, Y. Kawanobe, K. Ishii, T. Konishi, M. Mizumoto, N. Kanzawa, M. Matsumoto, M. Aizawa, In vitro and in vivo antimicrobial properties of silver-containing hydroxyapatite prepared via ultrasonic spray pyrolysis route, Materials Science and Engineering C(33) (2013) 5000–5018.
J.J. Curtin, R.M. Donlan, Using Bacteriophages To Reduce Formation of Catheter-Associated Biofilms by Staphylococcus epidermidis, Antimicrobial Agents and Chemotherapy 50(4) (2006) 1268–1275.
I. Sondi, B. Salopek-Sondi, Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria, Journal of Colloid and Interface Science 275(1) (2004) 177–182.
W.-R. Li, X.-B. Xie, Q.-S. Shi, H.-Y. Zeng, Y.-S. Ou-Yang, Y.-B. Chen, Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli, Applied Microbiology and Biotechnology 85(4) (2010) 1115–1122.
G. Regev-Shoshani, M. Ko, A. Crowe, Y. Av-Gay, Comparative efficacy of commercially available and emerging antimicrobial urinary catheters against bacteriuria caused by E. coli in vitro, Urology 78(2) (2011) 334–9.
P.J. Nowatzki, R.R. Koepsel, P. Stoodley, K. Min, A. Harper, H. Murata, J. Donfack, E.R. Hortelano, G.D. Ehrlich, A.J. Russell, Salicylic acid-releasing polyurethane acrylate polymers as anti-biofilm urological catheter coatings, Acta Biomater 8(5) (2012) 1869–80.
A.V. Fuchs, S. Ritz, S. Pütz, V. Mailänder, K. Landfester, U. Ziener, Bioinspired phosphorylcholine containing polymer films with silver nanoparticles combining antifouling and antibacterial properties, Biomaterials Science 1(5) (2013) 470.
L. Carson, S.P. Gorman, B.F. Gilmore, The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli, FEMS Immunology & Medical Microbiology 59(3) (2010) 447–455.
S.M. Jacobsen, M.E. Shirtliff, Proteus mirabilis biofilms and catheter-associated urinary tract infections, Virulence 2(5) (2011) 460–5.
C.E. Armbruster, H.L.T. Mobley, Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis, Nat Rev Micro 10(11) (2012) 743–754.
B.J. Nablo, T.-Y. Chen, M.H. Schoenfisch, Sol−Gel Derived Nitric-Oxide Releasing Materials that Reduce Bacterial Adhesion, Journal of the American Chemical Society 123(39) (2001) 9712–9713.
W. Fu, T. Forster, O. Mayer, J.J. Curtin, S.M. Lehman, R.M. Donlan, Bacteriophage Cocktail for the Prevention of Biofilm Formation by Pseudomonas aeruginosa on Catheters in an In Vitro Model System, Antimicrobial Agents and Chemotherapy 54(1) (2010) 397–404.
K.S. Liao, S.M. Lehman, D.J. Tweardy, R.M. Donlan, B.W. Trautner, Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters, Journal of applied microbiology 113(6) (2012) 1530–1539.
C.Y. Loo, P.M. Young, W.H. Lee, R. Cavaliere, C.B. Whitchurch, R. Rohanizadeh, Superhydrophobic, nanotextured polyvinyl chloride films for delaying Pseudomonas aeruginosa attachment to intubation tubes and medical plastics, Acta Biomater 8(5) (2012) 1881–90.
Despite all of the research effort that has gone into finding techniques and methods to solve the problem of infections caused by urinary catheters, most approaches have failed because of the rising problems associated with microbial resistance. It is also safe to assume that microbial studies alone will not eradicate CAUTIs, researchers need to understand the interaction of the microbes with the materials and their evolution as infection progresses. Next, we will discuss antimicrobial resistance developed by planktonic and biofilm bacteria.
1.3. The case of Antimicrobial Resistance in CAUTIs
Alexander Fleming’s serendipitous discovery of penicillin marked the beginning of the modern medical era of antibiotics and has likely saved more lives than most other medical advances in history. But as early as 1946, he also noted that, “There is probably no chemotherapeutic drug to which in suitable circumstances the bacteria cannot react by in some way acquiring ‘fastness’ (resistance)”. According to the WHO, antimicrobial resistance happens when microbes change upon exposure to antimicrobial drugs. This causes them to develop resistance and as a result, infections do not subside. Antibiotic resistance has led to the development of “superbugs” that are resistant to many antimicrobial therapies that in turn has compounded the problem of nosocomial infections.
The WHO has reported several instances of rising resistance among commonly found nosocomial pathogens [39]. Resistance to several antibiotics have been reported so far: carbapenem resistant K. pneumoniae, fluoroquinolone resistant E. coli, multidrug resistant S. aureus (MRSA) and colistin resistant Enterobacteriaceae. It is important to note that these bacteria are also commonly found in CAUTIs, and hence their infection raises issues of resistance to the antimicrobial agents used in urinary catheter materials.
A long term study was conducted by Wazait et al. between 1996 to 2001 in the UK to collect information on catheter urine samples to identify change in bacterial profile and antibiotic resistance in CAUTI [40]. The samples were collected in 1996, 1998 and 2001. E. coli and Enterococcus were the most common pathogens. Frequencies were 35.6%, 32.5% and 26.6% for E. coli and 11.8%, 15.3% and 22.0% for Enterococcus for 1996, 1998 and 2001. The results also indicated a change in pattern of antibiotic resistance along with this change in frequency of the bacteria profile. In 1996, bacteria were least resistant to ciprofloxacin (8.0%), co-amoxiclav (18.5%) and cephalexin (25.4%) but in 2001 the resistance changes to co-amoxiclav (22.5%), ciprofloxacin (27.2%) and nitrofurantoin (28.8%). As of now eight pathogen groups that make up 80% of antimicrobial resistant bacteria found in nosocomial infections are MRSA (8.5%); vancomycin resistant Enterococcus (3%); extended spectrum cephalosporin-resistant K. pneumoniae and K. oxytoca (2%), E. coli (2%) and Enterobacter spp. (2%); carbapenem resistant P. aeruginosa, K. pneumoniae and K. oxytoca (<1%), E. coli (<1%) and Enterobacter spp. (<1%) [41]. Studies like the two highlighted above should be performed in other regions of the world to identify and appropriately detect any change in the resistance profile for CAUTI pathogens. This would aid and supplement microbial studies along with the development of antimicrobial coatings that can attack even multidrug resistant CAUTI pathogens.
1.4. Improving Interaction between the Urinary System and the Urinary Catheter
Various approaches can help to prevent CAUTI, such as better handling of catheters, fabricating urinary catheter coatings, improving catheter design, and emphasizing short-term use. However, this review focuses on urinary catheter coating materials designed to prevent CAUTI either by their antifouling or biocidal properties, or both. While the other approaches can help in preventing CAUTI, sometimes patient comfort in case of design and need for long-term use can hinder the utilization of these approaches. In contrast, fabricating biocidal and antifouling materials is a simpler task as long as the materials do not pose side effects like development of antimicrobial resistance and patient allergy. As healthcare-associated costs have risen over the past two decades and population around the world has increased, the need for better catheter materials has substantially increased. Hence, research on antifouling and biocidal materials for catheters has centered on designing the most competent, yet simple, material in terms of use and fabrication. Although the sophisticated antimicrobial coatings for urinary catheters may cost more than the standard urinary catheters, they make up for this cost in the long run by preventing nosocomial infections, the treatment of which is generally not covered by most insurance policies.
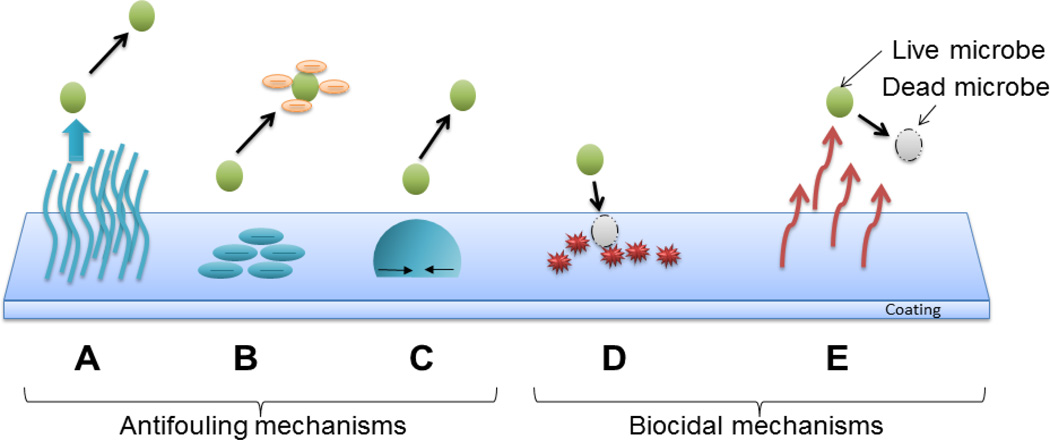
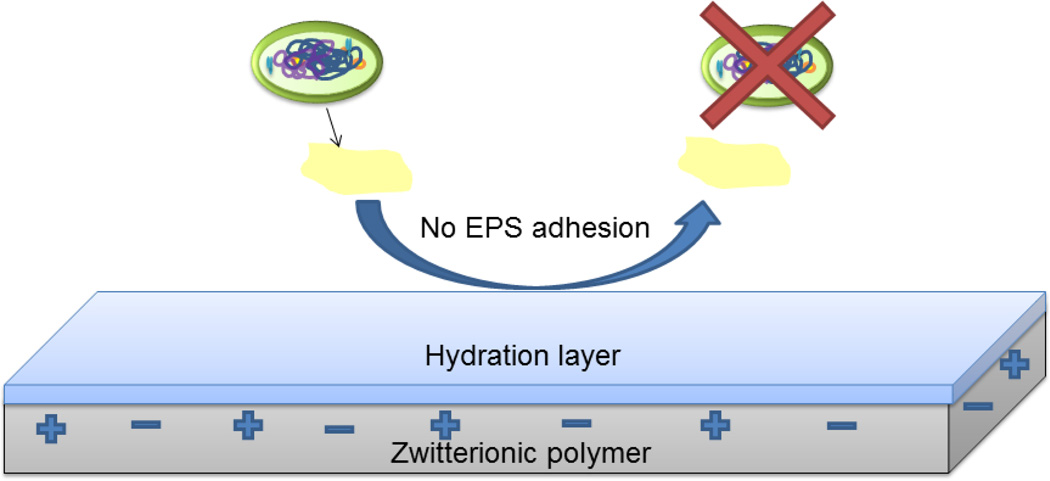
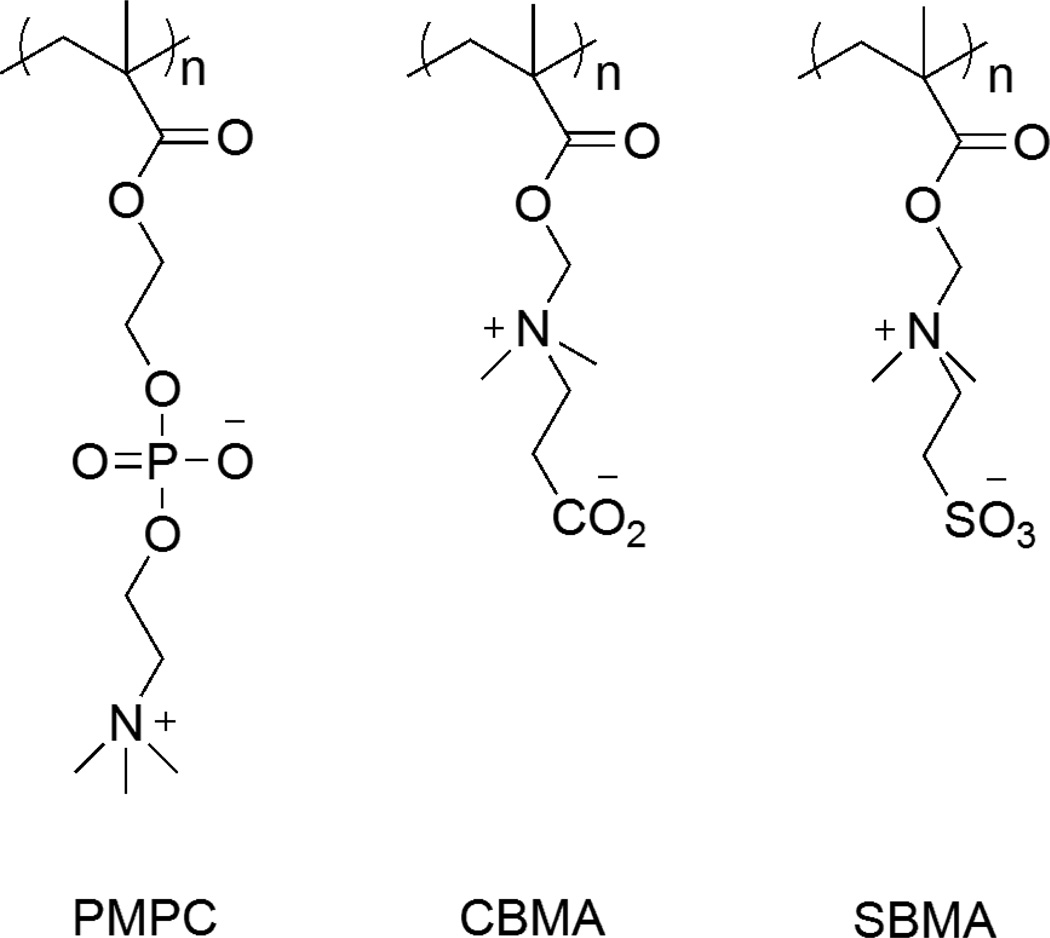
Antifouling coatings do not kill the microbes directly but instead prevent the attachment of bacteria on the surfaces that allow the formation of biofilms [42, 43]. Mechanisms of antifouling materials include steric repulsion, electrostatic repulsion and low surface energy (Figure 4 A, B and C) to keep foulants from attaching to the surface of the catheter. This prevents the formation of conditioning films for planktonic bacteria that ultimately form biofilms, the stage at which is hardest to treat with antimicrobial agents. Generally, materials that are antifouling by the mechanism of steric repulsion are bioinert in nature [44, 45]. This means that they tend to avoid any interaction with their surrounding environment. The two main types of antifouling materials currently in research are made of hydrophilic materials (e.g. SAM-OEG, PEG, POEGMA) [46–48] and polyzwitterions (e.g. polyMPC, polyCBMA, polySBMA) [49–51]. They repel foulants by forming a barrier of hydration layer on the surface [52, 53]. This hydration layer is formed through hydrogen bonding and/or ionic solvation. When the proteins approach the surface, water molecules are released from the surface and the polymer is compressed. This leads to an increase in enthalpy due to polymer dehydration and decrease in entropy due to chain compression. According to thermodynamics, both of these events are unfavorable and hence these surfaces tend to repel proteins or other foulants by the mechanism of steric repulsion [44, 46].
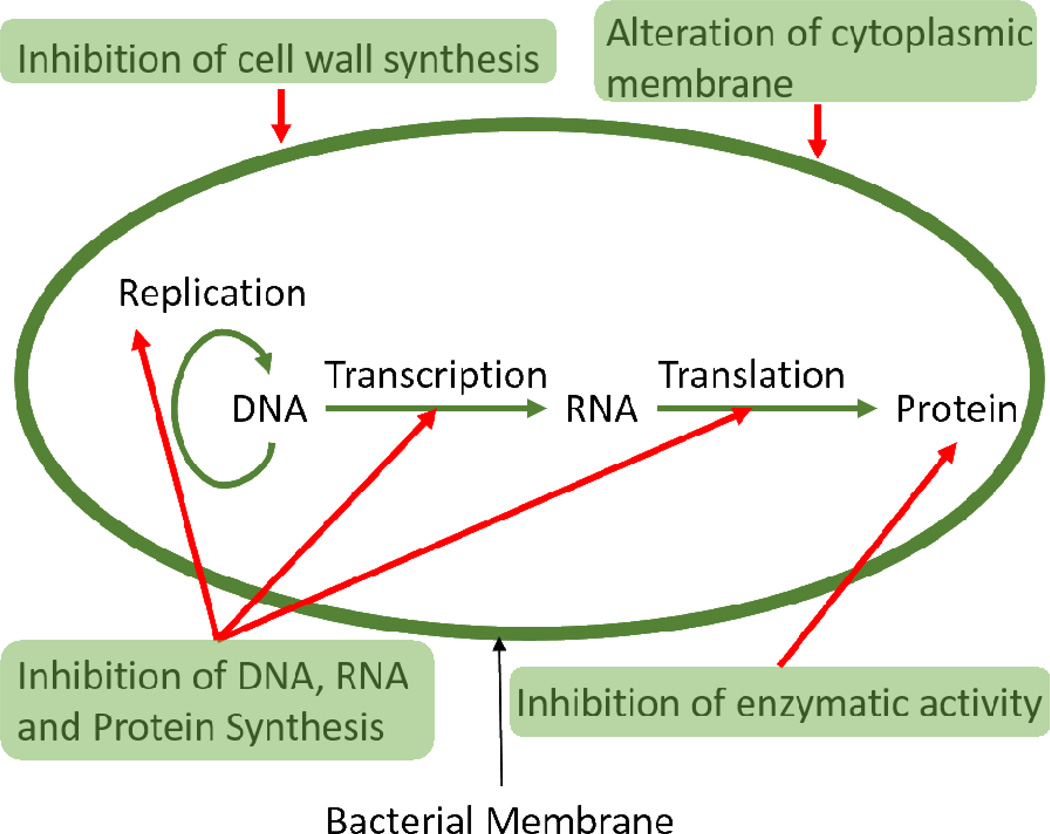
Figure 4.
Antimicrobial Mechanisms: (A) Exclusion Steric repulsion: Polymers attached to coating surfaces provide physical barriers to proteins, cells and microbes. (B) Electrostatic repulsion: Charges on coatings prevent the attachment of microbes. (C) Low surface energy: Reduction of external microbial adhesion by the use of low energy surfaces. (D) Biocide releasing: These coatings release biocides, such as silver ion and nitric oxide, and kill microbes. (E) Contact-active: These polymer coatings don’t release biocides but kill multi-resistant microbes upon contact.
On the other hand, biocidal urinary catheter materials are designed to kill the microbes instead of minimizing their deposition. These catheters are essential because they protect the patients from infection and encrustation development. Several biocidal materials have been developed to combat the problem of CAUTIs and decrease associated hospital care costs. Clinically tested biocidal coatings have silver or antibiotics as the active ingredient. While these agents are predominant in the clinical field, many other agents (triclosan, chlorhexidine, nitric oxide, enzymes, peptides) or agent carriers (liposomes, polymers) are currently in the research stage, and will be discussed in the next section below. The most common mechanisms for biocidal actions fall into 5 basic categories according to their mechanism of action: 1) Inhibition of cell wall synthesis (e.g. chlorhexidine, penicillin and vancomycin) 2) Inhibition of protein synthesis (e.g. silver ions, nitric oxide and tetracyclines like minocycline) 3) Inhibition of nucleic acid synthesis (e.g. sparfloxacin, quinolones, nitric oxide and rifampin) 4) Effects on cell membrane sterols (e.g. silver ions, triclosan, antimicrobial peptides and antifungal agents like amphotericin B) 5) Inhibition of unique metabolic steps (e.g. nitrofuran, triclosan, bacteriophages and sulfonamide). Cell walls are not present in humans and mammals but are an important component in bacteria. Biocides can inhibit the formation of peptidoglycan and dephosphorylation of phospholipid carrier in peptidoglycans, which ultimately leads to death of the microbe. For inhibition of protein synthesis, biocides attack by attaching themselves to the ribosomal subunits (50s and 30s) in bacteria. Proteins are necessary for multiplication and survival in bacteria and thus their disruption causes cell death. Nucleic acids are the key for replication. When biocides inhibit mRNA synthesis, DNA gyrase, topoisomerases and nucleic acid synthesis, they can kill bacteria. Cell membrane sterols are altered by some biocides but since cell membranes are present in both mammalian and bacterial cells, these biocides can also cause cytotoxicity. For this reason, these materials a typically used as the final line of defense against bacteria. Alteration of cell metabolism by inhibiting the synthesis of cofactors for nucleic acid synthesis and mycolic acid synthesis can also cause bacteria death by biocides. These biocidal agents can either be embedded in the polymer and be released to kill bacteria (Figure 4 D) or they can be covalently bonded or crosslinked to the surface of the materials to kill microbes on contact (Figure 4 E). Examples of agents which are mostly used in biocide release antimicrobial coatings are silver ions, triclosan, chlorhexidine, chlorine, tributyltin, nitric oxide, and antibiotics [54]. A commonly studied example of contact active biocidal agent is quaternary ammonium compounds [55, 56]. It is important to note here that some of the biocidal mechanisms of different agents can overlap (e.g. silver ions and antibiotics have two mechanisms in common) since these agents are categorized according to their structure and not mechanism of killing and some agents employ more than one mechanism of killing (e.g. silver, nitric oxide). In case of biocidal coatings that release biocides, the materials leach out their antimicrobial agent and hence do not let the microbe come in contact with the catheter. This can aid in preventing encrustation and biofilm formation. However, leaching of the biocide can also prove to be harmful, as we discuss later in the case of triclosan.
Antimicrobial and antifouling materials are the focal point of research in catheter materials, as they offer the potential for complete protection against CAUTIs [57]. These materials are proven to be efficient in preventing CAUTIs as they have a higher microbial cytotoxic ability [58]. However, it is also important to note that because of the high resistance of biofilms to antimicrobial agents, catheters that elute biocides to prevent contact of contact with urinary catheter would increase the efficiency of the coatings compared to contact active methods [59, 60]. This elution should be from both inner and outer surface of the catheters.
Although the problem of CAUTIs is complex and still a major challenge, materials that combine known and novel antimicrobial agents with an increase in effectiveness have shown tremendous progress. Examples include the use of silver alloy, a commonly used antimicrobial material, modified with plasma and silver nitrate and the use of antibiotics like rifampin and sparfloxacin in combination with antiseptics such as triclosan to increase the agent’s antimicrobial efficacy. While the list of antimicrobial agents along with new formulations continues to expand to combat the problem of CAUTIs, some of the more commonly studied urinary catheter coating materials (both in clinical trial and/or research) are highlighted in this article, along with a discussion of future research directions. In this review, the antimicrobial agents/materials for urinary catheters have been categorized broadly into two categories: Clinically tested antimicrobial catheter agents/materials and researched (but not clinically tested) antimicrobial catheter agents/materials. Since it would be complicated to present them in smaller, sub-categories organized by their antifouling and biocidal mechanisms, we have categorized them into antimicrobial agents/materials and carriers of antimicrobial agents. The materials described herein are not an exhaustive list of the antimicrobial coatings that have been developed to date, but rather highlights of the major agent/material categories.
2. Antimicrobial Urinary Catheter Coating Agents/Materials in Clinical Trials and/or Research
2.1. Clinically-Tested Antimicrobial Agents/Materials
2.1.1. Silver
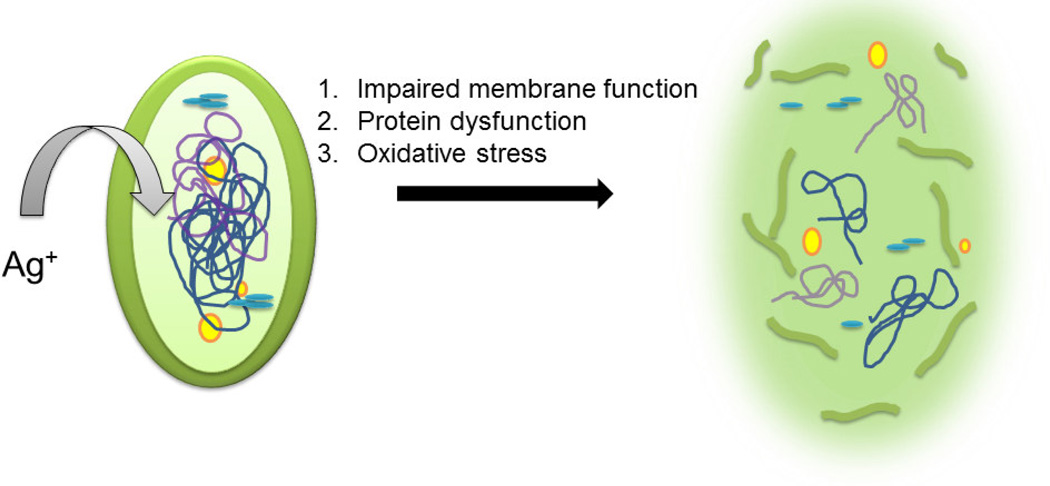
Silver is one of the few antimicrobial agents for urinary catheter coatings (along with other medical devices) that is approved by the FDA. Even low concentrations of Ag ions are enough to kill microbes. Its mechanisms for killing bacteria include 1) impaired membrane function by loss of membrane potential, 2) protein dysfunction by destruction of Fe-S cluster, and 3) oxidative stress by antioxidant depletion [61–65]. A visual description of what happens to the bacterial cell is shown in Figure 5. The multifunctional mechanism of antimicrobial activity demonstrated by Ag makes it a potent biocide and one of the most popular antimicrobial agents used in medical device coating formulations. For medical device coatings, Ag ion releasing coatings can be designed in the form of Ag coatings, Ag alloy (with gold, palladium), Ag-containing polymers and Ag nanoparticles (NPs) [66–74]. These coatings have evolved from pure silver coatings, which do not have enough antimicrobial efficacy and deteriorated rather quickly. Silver is susceptible to oxidation in aqueous environments and releases Ag ions which are highly biocidal in nature. However, this fast release of ions also means that the release needs to be controlled and sustained. Most studies in clinical trials are focused on Ag-alloy coatings, while research has also focused on Ag-NPs. This is because of the poor solubility of silver salts, which results in lower antimicrobial efficacy. On the other hand, the large surface-to-volume ratio of NPs gives them an edge in antimicrobial efficacy. However, an important factor that can affect the efficacy of Ag-NPs is its tight incorporation with the catheter material to prevent fast and excessive release of ions which could prove cytotoxic to patients [71].
Figure 5.
Silver ions act as biocides with the help of one or more of the above three mechanisms. In the diagram, we see that the cell membrane has been damaged by silver ions. Also, respiration and DNA replication are inhibited because the silver ions damage the integrity of the cellular structure.
One of the earliest studies using Ag as a biocide for urinary catheter coating dates back to 1949 [1]. This research was controversial, with several supporting studies [3, 75–79], and those that contradicted the findings, claiming that silver coated catheters are not as effective against E. coli and S. aureus as previously considered [72, 80, 81]. Most of the studies compare silver-alloy coatings with antibiotics or nitrofural (trade name Furacin) catheters. Nitrofural is a bactericidal compound that is frequently used in ointments as an antibiotic. It has been currently discontinued in the US. In a major study conducted over a 40-month period (2007–10) on 7102 patients in the UK [82], silver-alloy coated latex catheters (Bardex IC, Bard Medical, Crawley, UK) were found to be less effective in reducing infection or cost of catheterization when compared to antibiotic coatings. However, the same study also found that for short-term use, silver-alloy coatings were useful, as they did not cause discomfort like the nitrofural catheter [82, 83]. It is also important to keep in mind that there is a difference between the predicted and observed infection caused by silver-alloy coatings. The latency period before exposure of bacteria until the development of symptoms and duration of exposure to the bacteria should be taken into account [84]. In 2012, Johnson et al. examined the adherence of 11 CAUTI causing microorganisms including E. coli, P. mirabilis, S. aureus (MRSA) and C. albicans in order to compare nitrofural-coated catheters (manufactured by Rochester Medical Group) and silver-alloy coated catheters (manufactured by C. R. Bard, Inc.) [66]. It was found that after an overnight incubation, nitrofural-coated catheters turned out to be more effective against bacterial adherence and biofilm formation. Counts from both inoculum broth and catheter sonication were taken. Besides including 11 species of pathogens, this study was further supported by a biofilm assay, different methods of analysis and a direction for clinical studies. While these studies generally found nitrofural catheters more effective than silver-alloy catheters, silver-alloy still exists in the market because of its FDA approval and cost efficiency. Besides this, comfort of patients has also played a big role in supporting the silver-alloy coatings market.
While conventional silver-alloy coating was previously compared to traditional antimicrobial agents, in the past few years silver-alloy has been combined with other materials to improve its antimicrobial efficacy. An epidemiological screening study conducted in 2004 by Rupp et al. found that silver-alloy, hydrogel-coated catheters (C. R. Bard, Inc.) reduced the occurrence of CAUTIs from 6.13/1000 catheters in 1999–2000 to 2.62/1000 catheters in 2001–2002 (P= 0.002) [70]. The susceptibility tests also found that no microbes were resistant to silver. In 2014, another study was conducted with silver-alloy hydrogel catheters (C. R. Bard, Inc.) and patients from 7 acute care hospitals [68]. According to the criteria of National Healthcare Safety Network (NHSN), a 58% relative reduction occurred in the number of CAUTIs when silver-alloy hydrogel catheters were compared to standard catheters. The study was successful in proving that silver-alloy hydrogel catheters decreased CAUTI occurrences according to both clinical and NHSN criteria when compared to standard catheters. In 2015, a novel coating was prepared using plasma and silver nitrate wet treatments by Aflori et al. [73]. Silicon-coated latex catheters were plasma treated followed by a wet treatment of the catheters in sodium hydroxide and silver nitrate solution for a week. XPS analysis was done to confirm the presence of Ag2+ ions on the catheter surface. Culture media and inoculation was performed in order to test the sensitivity of C. albicans. The catheters treated with plasma and silver nitrate gave the best results in the antibiograms for biofilm formation and bacterial adhesion. The shadow diameter for oxygen plasma treated catheters followed by wet silver treatment was 10 mm while the control catheter with no treatment showed no resistance to the fungus. Recently in Japan, Honda et al. examined an Ag ion-releasing hydroxyapatite material that was prepared by using an ultrasonic spray pyrolysis technique (USSP). [67]. USSP is a simple method to produce nanomaterials. In this case, the precursors for the Ag-containing hydroxyapatite powders were Ca(NO3)2·4H2O, (NH4)2HPO4, HNO3, and AgNO3. These solutions were pyrolyzed using the ultrasonic vibrator and resulted in sample preparation. The in vitro tests were carried out using S. aureus and the in vivo tests for imaging were done using a bioluminescent strain of S. aureus, XEN-29. The material was effective against bacterial colonization and biofilm formation. These types of materials that release Ag ions at low concentration levels (5%) will be helpful in developing robust materials with efficient antimicrobial capacity for urinary catheters. While the above-listed combinations of silver-alloy with novel materials have shown promising antimicrobial efficacy, a standard protocol for antimicrobial efficacy requirements has not been established for Ag. Furthermore, these novel combinations of antimicrobial agents need to be tested for their cytotoxicity before market introduction.
Besides being studied in bulk form, Ag has also been extensively examined in the form of nanoparticles [62, 71, 85–89]. Some of the applications for nanoparticles include biomedical (drug delivery, gene transfer) optical and electronic fields. Although antimicrobial nanoparticles have been extensively reported, their use in urinary catheter coatings have not been explored widely [6, 90]. These antimicrobial particles can be made of several materials (metals, semiconductors, oxides, ceramics, organics) but the most commonly studied are Ag nanoparticles [71]. Phosphorylcholines with silver nanoparticles have been proven to have both antifouling and biocidal properties. The films on the substrates were prepared by inverse mini-emulsion polymerization followed by reduction of Ag and colloid deposition. These hybrid films killed more than 99% of the E. coli colonies at concentrations up to 1 × 105 CFU mL−1 within one hour of exposure [91]. Kocuran functionalized silver glyconanoparticles have been studied and proven to have antifouling and biocidal properties against S. aureus and E. coli. Kocuran is the exopolysaccharide from Kocuria rosea strain BS-1. 100 mM of aqueous AgNO3 was mixed with kocuran suspension and stirred at 200 rpm for 15 mins at 50°C. The Kocuran-capped NPs were then rinsed with distilled water and centrifuged for further use. Unlike commercialized silver coatings, this coating was shown to be biocompatible and non-toxic in nature [88]. Silver nanoparticles continue to be a field of aggressive research and it is expected to be studied more in the future with an emphasis on completely eradicating infection of urinary catheters by being 100% effective.
The exhaustive list of studies performed on silver coatings in the form of silver-alloys and silver alloy combinations proves that research on finding the ideal antimicrobial coating for urinary catheters has suggested using conventional antimicrobial compounds. However, despite the enormity of reports suggesting silver to be a safe and effective antimicrobial agent, it effectiveness in urinary catheters has proven limited. Another drawback of silver coatings is that due to its high costs and absence of completely reliable group of reports, research on silver as an antimicrobial agent will likely continue through the advent of new combinations that will substantially increase its antimicrobial efficacy and decrease cytotoxicity.
2.1.2. Antibiotics
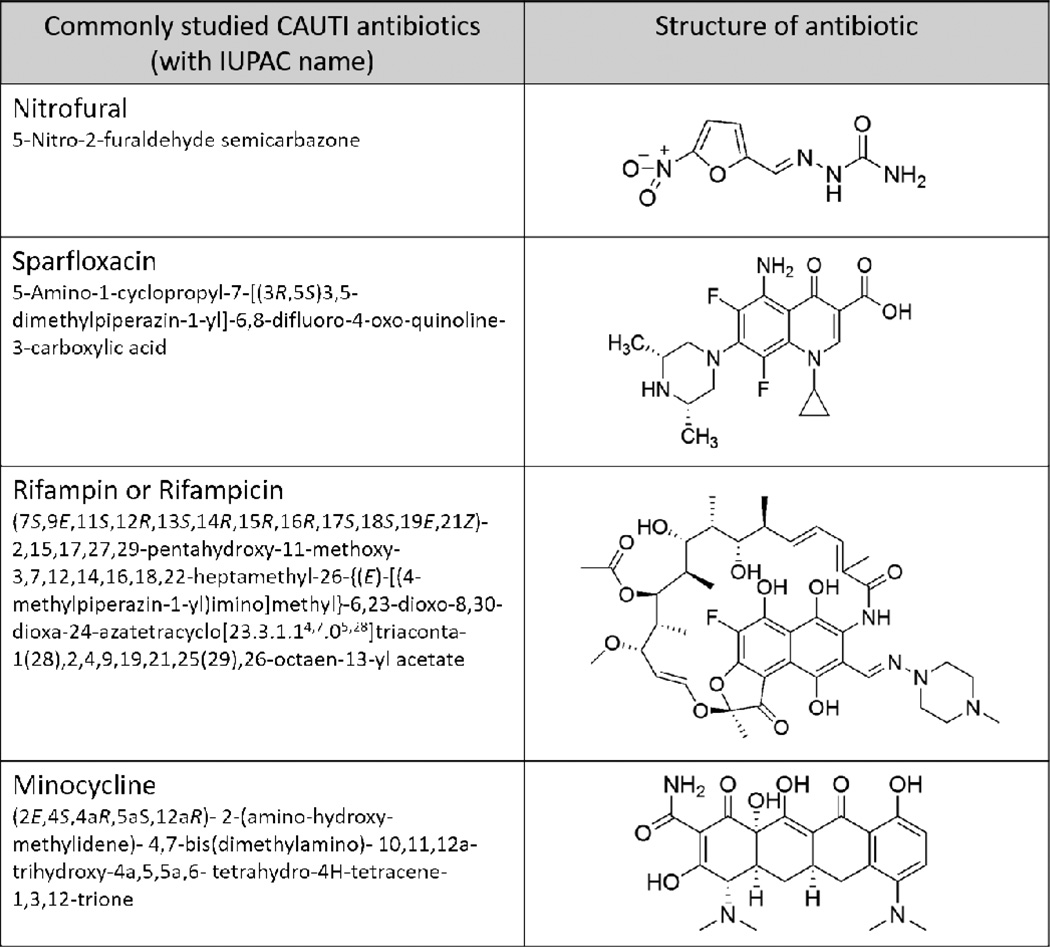
Antibiotics are low molecular weight compounds isolated from one living organism that kill or inhibit the growth of other organisms. Antibiotics may have e.g., antibacterial, antifungal, antiviral, antiparasitic, or even anticancer activity. They have been studied extensively over the years [10, 43, 66, 72, 82–84, 92–99] and studies suggest that antibiotic resistant bacteria can develop against a wide range of antibiotics including ones that do not enter the cell like vancomycin. However, many antibiotics have proven to be more efficient than silver-alloy coatings in studies for infections caused by most gram-negative and gram-positive bacteria [66, 92]. In this review, we will focus on commonly studied antibiotics which have been relevant in shaping the research for fabrication of urinary catheter antimicrobial coatings (Figure 6).
Figure 6.
A list of the commonly studied antibiotics that have been used as antimicrobial agent in urinary catheter coating studies.
Nitrofural impregnated catheters are the most commonly studied catheters as they are commercially available [66]. Nitrofural has been found to be a direct inhibitor of DNA replication at concentrations of 200 µM for 20 minutes [100]. However, this is only a temporary solution and replication can resume once the nitrofural is removed since this happens independently of DNA damage. Studies comparing nitrofural Foley catheters and silver-alloy coated catheters have found that nitrofural was more effective in preventing planktonic growth and biofilm formation in addition to being cost effective [66, 92, 93, 95]. It has also been noted that in certain cases, nitrofural catheters caused discomfort in short term catheterization [83]. Although nitrofural catheters have been successfully used, it is yet to be determined if these should be modified for short-term purposes because of the discomfort issue. Also, nitrofural has been known to cause mammary and ovarian tumors in animal subjects [101] and hence has been listed under prohibited drugs for food animals under Group I by FDA. This drawback of nitrofural has led to the slowdown of research in this field and it has not been under investigation in recent years.
Some other antibiotics studied in the prevention of CAUTIs are sparfloxacin, minocycline and rifampin. Sparfloxacin (5-Amino-1-cyclopropyl-7-[(3R,5S)3,5-dimethylpiperazin-1-yl]-6,8-difluoro-4-oxo-quinoline-3-carboxylic acid) (SPA) is a aminodifluoroquinolone-based antibiotic and displays its antibacterial activity by inhibiting DNA gyrase, which assists in DNA replication of bacteria. Its trade name in the United States is Zargam. In 2012, researchers observed that sparfloxacin treated long-term catheters demonstrate better antimicrobial and antibiofilm activity in both agar and broth diffusion tests when compared to silver-alloy coated catheters and untreated catheter controls [72]. Two types of SPA coatings were prepared: 1) heparin coated catheters were oxidized with sodium periodate and then exposed to SPA in organic medium that formed Schiff’s base with a final SPA concentration of 1 mg/mL and; 2) heparin coated catheters were activated with glycidol, oxidized with sodium periodate and subsequently exposed to SPA in organic medium that formed Schiff’s base with a final SPA concentration of 1 mg/mL. These catheters were able to prevent the invasion and colonization of E. coli and S. aureus while maintaining broad-spectrum activity for 6 months.
Minocycline-rifampin (MR) impregnated silicone catheters have showed inhibition zones (<10mm) in the cell culture studies but not enough to prove antimicrobial efficacy against E. coli and P. aeruginosa [97]. Rifampin is known to be a DNA transcription inhibitor which inhibits bacterial RNA polymerase, thereby preventing transcription [102]. The coatings were prepared by covering the silicone catheters with tridodecyl methyl ammonium chloride (TDMAC) cationic surfactant and 1000 mg of MR (anionic structure). Another study used careful combinations of rifampin, sparfloxacin and triclosan which were impregnated into catheters for long-term broad spectrum antibiofilm activity against P. mirabilis, S. aureus, and E. coli [96]. The antimicrobial catheters were found to be more efficient in preventing bacterial growth than commercially available clinically relevant catheters. 7 to 12 weeks of the test catheter use in vitro (flow conditions) had reduced infections and complications. Zone of inhibition test (Serial Plate Transfer Test, SPTT) was done to produce zones of up to 100 days or until no zones of inhibition were produced. While the silver-processed and nitrofural catheters showed no zone of inhibition within two days of incubation, the antimicrobial impregnated catheters showed zones of inhibition for more than 100 days (with a reduction in zone diameter between 17%–36%).
While antibiotics may seem to be a good alternative to the cytotoxicity problems caused by silver-alloy catheter coatings, the largest issue associated with antibiotics is the inherent problem of bacterial resistance [27, 103], which can render these antibiotics useless after second or third application. Antibiotic resistance has become more challenging since biofilms require higher doses of antibiotics and, in turn, the common infections caused by the bacteria lead to an increase in the resistance rate. Therefore, a better understanding of antibiotic resistance is required in order to further develop coatings with potent doses of antibiotics without the development of resistant bacterial strains.
2.2. Antimicrobial Agents/Materials in Research (not yet clinically tested)
In this section, antimicrobial agents (both biocidal and antifouling) are introduced (Section 2.2.1–2.2.7) followed by the carriers of biocidal agents (Section 2.2.8–2.2.9).
2.2.1. Chlorhexidine
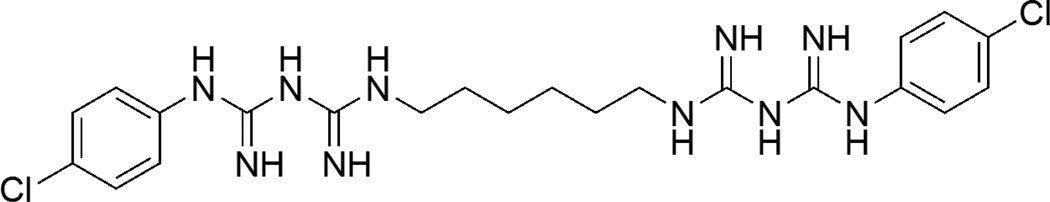
Chlorhexidine (N,N‴′1,6-Hexanediylbis[N′-(4-chlorophenyl)(imidodicarbonimidic diamide)]) is a cationic bisbiguanide and has a low mammalian toxicity [104]. It is bacteriostatic (i.e. a reversible reaction) at low levels and bactericidal (i.e. an irreversible reaction) at high levels where the level activity depends on the species of the bacteria [105]. It is a common antimicrobial agent used in oral consumer products [106]. The mode of action includes destruction of the cytoplasmic membrane because of its affinity to negatively charged bacterial cell surfaces, since chlorhexidine is positively charged. Figure 7 shows the chemical structure of chlorhexidine.
Figure 7.
Chemical structure of chlorhexidine
While chlorhexidine has been used in the drainage bags and other forms for urinary catheters for a long time, it has only been recently tested as a coating on urinary catheters. For antimicrobial activity in urinary catheters, chlorhexidine has been mainly used in the form of nanoparticles [107, 108], but it can also be embedded with controlled leaching from polymer matrices [109]. In one of the studies, ethylene vinyl acetate (EVA) was functionalized with chlorhexidine hematophosphate nanoparticles (CHX NNPs) which inhibited the growth of methicillin-resistant S. aureus (MRSA) and P. aeruginosa [108]. CHX NNPs had previously been adsorbed to glass and titanium, but this study was the first to successfully embed CHX NNPs in a biomaterial. Polymers were dipped in rapidly stirring colloidal suspensions (99% of CHX bound with NNPs) for 30 secs. They were then rinsed with deionized water and air-dried. To verify immobilization, AFM and SEM were performed. Although this study was not performed with urinary catheters specifically in mind, EVA is commonly used to form urinary catheters and moreover the pathogens studied are commonly found in CAUTIs.
Urinary tract infections have been found to affect 50% of catheterized canines and a 2013 study [109] conducted by Segev et al. investigated the chlorohexidine release from urinary catheters in dogs. A varnish coat containing 1% chlorhexidine (with 5 g ethyl cellulose, 4 g PEG, 1 % CHX in 100 mL ethanol) was brushed on the catheter to make a uniform coating. Urine samples were collected right after catheterization and right before the removal of catheters for dipstick analysis and sediment evaluation. The urinary catheter once removed was cut into three portions: upper (near the urinary bladder), middle and lower (proximal end of urinary catheter). The CFU count from these portions was performed after a 24 h incubation in 37°C. The median CFU of all the portions from the study group was found to be lower than the control group (around 10 CFU/15 mm catheter and 105 CFU/15 mm catheter respectively). Confocal laser scanning microscopy and SEM was used to study biofilm formation. The local release of chlorhexidine helped in decreasing systemic effect and hence reduced any potential adverse effect. However, a follow up with a well-designed toxicity and safety study need to confirm these coatings safe to patients.
In 2015, chlorhexidine-loaded polycaprolactone nanospheres (synthesized by high-pressure emulsification-solvent evaporation method) were spray-coated on silicone catheters and it was observed that these materials were effective over a period of 15 days, which is three times the span of effectiveness for chlorhexidine mixed with polymers [107]. The results from this study show that nanospheres could prove superior in the sustained release of chlorhexidine to prevent bacterial growth for a longer period of time than bulk polymers.
From the listed studies, chlorhexidine nanoparticles look very promising with a better understanding of release behavior. Chlorhexidine’s use also is advantageous when compared to antibiotics since is effective for a longer time without the development of bacterial resistance. Reports of reactions to chlorhexidine have been rare. Thus, it seems that once enough data from results of chlorhexidine coated urinary catheters are generated, this technology would be a very patient-friendly and effective antimicrobial coating.
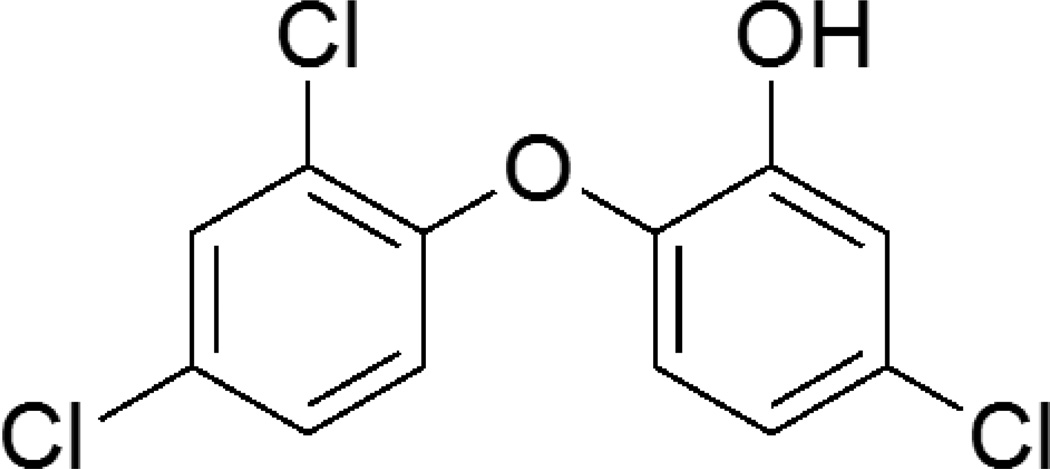
2.2.2. Triclosan
Triclosan (2, 4, 4’ –trichloro- 2’-hydroxydiphenyl ether) is a broad spectrum antimicrobial agent found in consumer products such as soaps and detergents [110]. At low concentrations, triclosan is bacteriostatic by inhibiting fatty acid synthesis, but at high concentrations it becomes biocidal by targeting bacterial membranes and cytoplasm [111]. Fatty acids are necessary for building membranes, and the enoyl-acyl carrier protein reductase (ENR) enzyme that triclosan targets is found only in bacteria and hence does not affect human cells. Slater-Radosti et al. studied the mechanism of triclosan on S. aureus and confirmed ENR inhibition [112]. It has been shown that triclosan targets the RNA and not the DNA [110]. As seen in Figure 8, it is a chlorinated aromatic compound.
Figure 8.
Chemical structure of triclosan (2, 4, 4’ –trichloro- 2’-hydroxydiphenyl ether)
Even though triclosan has been mainly used in the form of a fluid filled into the retention balloon of the catheter [113, 114], it has also been impregnated into the coating material of catheters [115–117]. In 2003, Gaonkar et al. studied the effect of triclosan impregnated (proprietary method) urinary catheters on an in vitro model of the urinary tract [117]. A zone of inhibition assay was performed with a mixed culture of microbes. The bladder portion of the model was cultured daily to check for bacterial growth. The silicone catheter with chlorhexidine, silver sulfadiazine, and triclosan (CXST) was more effective in preventing the growth of S. aureus and S. epidermidis for a longer time (23–24 days) as compared to the nitrofural-treated catheters (9–11 days). This study confirmed that the low levels of CXST used could provide longer term antimicrobial efficacy with low risk of antimicrobial resistance.
Triclosan has also been studied in combination with plant-derived antimicrobials [118]. In 2015, Jordan et al. studied the effect of silicone catheters impregnated with triclosan and plant-derived antimicrobials (eugenol and terpinen-4-ol) using agar diffusion tests which targeted the effectiveness against encrustation, a major problem in urinary catheters that leads to blockage. Impregnation was done by blending two different solutions containing PDMS, silica filler, hydrophilic Cabosil filler, platinum catalyst, crosslinker and the test antimicrobial or the control solvent followed by curing the solutions for 10 mins at 90°C. Solvent used for triclosan was 2-propanol and acetone was used for eugenol and terpenin-4-ol. The study showed that the dip-coating of silicone with 0.2% of triclosan improved the efficiency of the catheter in preventing encrustation caused by P. mirabilis. Increasing the concentration to 1% of triclosan (dissolved in 2-propanol) showed antimicrobial activity (15 out of 18 isolates in agar diffusion test) while a lower concentration of 0.2% of triclosan showed antibiofilm activity for up to 11 weeks. This study was also able to demonstrate that a simple dip coating technique was sufficient to coat the catheter effectively and retain its antimicrobial effects for over 7 days in a running in vitro bladder model.
While the results of very low concentrations of triclosan used in these formulations definitely show promise, the use of triclosan remains controversial due to its cytotoxicity to mammalian cells and development of bacterial resistance. It is currently under review by FDA because it has been noticed in animal studies that triclosan alters hormonal regulation and can increase antimicrobial resistance against antibiotics.
2.2.3. Antimicrobial Peptides (AMPs)
Antimicrobial peptides are short strands of amino acids that have antimicrobial activity against bacteria, fungi, enveloped viruses and even transformed or cancerous cells. Antimicrobial peptides are part of the innate immune response and are also called host defense peptides (HDP). AMPs can function as immunomodulators. An immunomodulator functions as an agent that can modify the immune response or the functioning of the immune system by stimulating antibody formation or by inhibiting white blood cell activity. Antimicrobial peptides vary greatly in their structural motifs and can induce antimicrobial effects either by disrupting the membranes or by passing through the membranes and targeting the intracellular components (Figure 9) [119]. Epand and Vogel have classified antimicrobial peptides into different groups: 1) Amphipathic and hydrophobic helices (linear peptides) 2) β sheet peptides and small proteins 3) Peptides with thioether rings 4) Peptides with unique amino acid compositions and 5) Lipopeptides terminating in an amino alcohol and macro-cyclic knotted peptides.
Figure 9.
The different mechanisms for antimicrobial activity by antimicrobial peptides
Previous reviews have mentioned the use of peptides [10, 43] to create antimicrobial coatings for urinary catheters. These peptides are cationic because of the presence of lysine and arginine as the main amino acid component. They are regarded as excellent antimicrobial agents because of the broad spectrum of cellular mechanisms responsible for antimicrobial activity and also the development of resistance against peptides is negligible. AMPs and mammalian cell membranes are both positively charged, which repels AMPs [120]. In 2002, Shai described that AMPs can target either in a nonreceptor-mediated or receptor mediated mechanism [121]. AMPs can kill either via damaging membranes or by acting on more than one anionic target. However, some of the issues with AMPs are their suboptimal coating properties, potential toxicity, pH sensitivity and high cost of synthesis [10]. Some of these issues have been addressed recently, where Mishra et al. immobilized peptides on silicone catheters [122]. A sulfhydryl coupling was used to immobilize the antimicrobial peptide, Lasio-III on the silicone urinary catheter. Antimicrobial activity was checked in both physiologically relevant conditions and in artificial urine medium. This activity lasted for at least 4 days against both gram negative and gram positive bacteria. According to the study, it was the first “proof-of-concept” study that has reported the efficacy of immobilized AMPs on a silicone catheter by sulfhydryl coupling. In another study conducted in 2015, an AMP, CWR11, was tethered to the surface of a polydimethylsiloxane (PDMS) film with the help of polydopamine (DOPA) [123]. The DOPA coating undergoes oxidative crosslinking, along with chemical bond formation with any surface silanol groups to provide robust coatings that are highly crosslinked. This study was promising, as the coatings were able to retain antibiofilm and biocidal activity for 21 days. It has been claimed that different architecture types of AMPs could increase antimicrobial activity [58]. Studies have also been conducted regarding the design of the AMPs [124] and their conjugation with allyl glycidyl ether (AGE) polymer brushes [125]. These polymer brushes with AMPs showed no biofilm formation and had a faster rate of killing than immobilized peptides.
The versatility in the antimicrobial mechanisms of AMPs make them viable candidates for use in urinary catheter coatings. It is also seen that these antimicrobials can be easily tethered to the surfaces of routinely used medical device polymers such as PDMS and other types of silicone for catheters. However, few studies have been performed and the complex mechanism of AMP antimicrobial actions need further investigation before use in antimicrobial coatings becomes widespread to prevent any accidental repercussions.
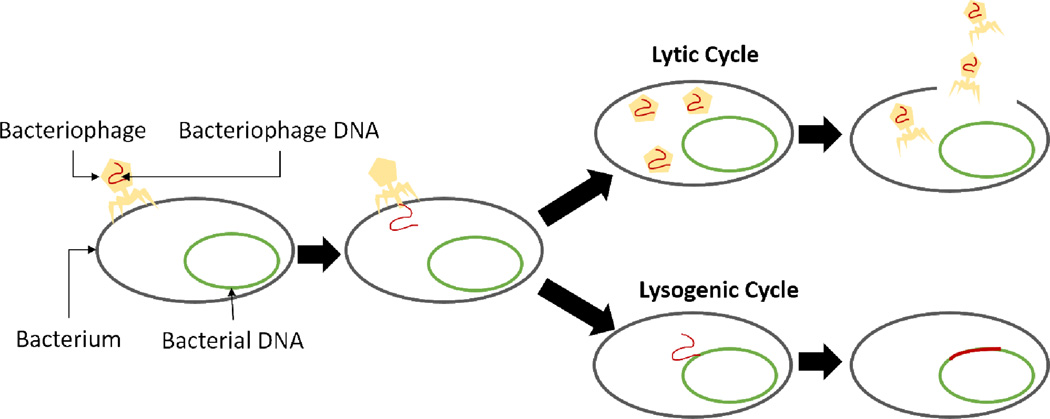
2.2.4. Bacteriophages
In the simplest terms, bacteriophages are the natural predators of bacteria. They are selective and can disrupt various metabolic pathways in bacteria. Bacteriophages are viruses that can enter bacteria and duplicate. A bacteriophage can reproduce in two ways (Figure 10): lytic (this destroys the host cell and its membrane) and lysogenic (bacterium continues to live and reproduce normally). Lytic phages are commonly used as antimicrobial agents as they are considered very effective and they are also abundantly available at the site of infection. Although bacteria can develop resistance to phages, studies have claimed that phage cocktails containing different viruses prevent this from taking place. Because of the antimicrobial selectivity and low cytotoxicity to mammalian cells, bacteriophages have recently been investigated in great detail for medical device coatings.
Figure 10.
The reproductive cycles of bacteriophage indicating the lytic cycle that destroys the bacterial membrane resulting in the host cell’s death.
One of the earliest studies for CAUTI pathogens with bacteriophages was performed in 2006 [126]. The study used hydrogel-coated catheters that were pretreated with a coagulase-negative bacteriophage (lytic S. epidermidis bacteriophage 456) which prevent the formation of S. epidermidis biofilms. For pretreatment of catheters with phages, catheter lumens are exposed to phage culture. In this study, each catheter segment of the modified drip flow bioreactor (mDFR) was filled with phage culture and incubated at 37°C for 1 hour before their removal. In contrast, control catheters were made by exposing their lumens to heat inactivated phages (80°C incubation for 3 hours). After the preparation of the test and control catheter segments, they were aseptically transferred to the mDFR for exposure to the S. epidermidis culture. Significant reduction of biofilm was found on the treated catheters compared to standard untreated catheters (silicone Foley catheters) after a 24-hour exposure period. The use of drip flow bioreactors in this study was one of the first studies done for bacteriophages and hence it was important in determining the effects of lytic phages on this common CAUTI pathogen. After this study, others have continued to study bacteriophages in the form of cocktails to prevent the development of antimicrobial resistance [127–129].
In 2015, silicone hydrogel coated catheters pretreated with phage cocktails were observed to have antimicrobial activity [130]. The pretreatment of the silicone hydrogel coated catheters consisted of dipping them in P. aeruginosa and P. mirabilis bacteriophages and incubating for 1 hour. The coating reduced biofilm formation for 72–96 h by both the CAUTI pathogens. A continuous flow of artificial urine containing 1 × 103 CFU mL−1 of the pathogens was used as the medium. The P. aeruginosa biofilm counts were reduced by 4 log CFU cm−2 (p<0.01) and P. mirabilis counts were reduced by >2 log CFU cm−2 (p<0.01) over 48 h. It was significant in showing that mixed-species biofilm could be targeted by the tuning the material composition.
Despite the considerable research in antimicrobial coatings fabricated with bacteriophages, this field needs more studies to prove reliable and usable for clinical settings. Bacteriophages have high antimicrobial effectivity and what makes them even more exciting as a potential antimicrobial agent for CAUTI pathogens is their advantage over antibiotics for not promoting the development of antimicrobial resistance. More research toward the development of multipathogen antimicrobial coatings by fabricating phage cocktail containing coatings are anticipated in the near future.
2.2.5. Enzymes
Enzymes as active component of antimicrobial coatings have been utilized recently in the field of urinary catheters. Antimicrobial enzymes are major components of immune systems of living organisms that fight pathogenic microorganisms. These enzymes can act through various mechanisms: degrading structural components of microorganisms (hydrolytic enzymes), inducing production of antimicrobial substances in the living organism (oxidative enzymes), preventing bacterial quorum sensing (quorum quenching enzymes) which ultimately prevents cell aggregation and production of virulent compounds. Hydrolytic enzymes can be further categorized into proteolytic (e.g. subtilisin, and lysostaphin) [131–133], polysaccharide hydrolyzing (e.g. alpha amylase, dispersin B, chitinases, beta-glucanases, lysozyme and alginate lyase) [134, 135], DNases [136] and bacteriophage lysins [137]. These enzymes attack major constituents of the cells or degrade compounds that help adhere cells to each other in biofilms and have demonstrated successful antimicrobial activity against commonly found pathogens in CAUTIs like Pseudomonas, Streptococcus, and Bacillus, along with the multidrug resistant strain of S. aureus (MRSA). Oxidative enzymes produce hydrogen peroxide (H2O2) which is used by peroxidases to attack bacterial cells. H2O2 is also used by peroxidases to oxidize halides to more potent antimicrobial agents. Some of the commonly studied oxidative enzymes are glucose oxidase [138], cellobiose dehydrogenase [139, 140], superoxide dismutase, myeloperoxidase, lactoperoxidase [141] and horseradish peroxidase. Quorum quenching enzymes attack acylhomoserine lactone (AHL), the signaling molecule in bacteria without which the bacteria are unable to communicate with each other and hence cannot produce virulence compounds [142]. This prevents the formation of biofilms. Some of the quorum quenching enzymes include AHL lactonase, AHL acylase and paraoxonases.
In case of urinary catheters and other medical devices, enzymes can be immobilized onto the surfaces either reversibly or irreversibly. Reversible immobilization methods include methods through which the enzymes can be easily removed. These include chelation or metal binding [143], formation of disulfide bonds [143] and adsorption of the enzymes through physical and ionic bonds [144]. However, irreversible methods are generally preferred because of the improved stability and lower amounts of leaching. Common irreversible methods of immobilization include crosslinking using linker molecules, entrapment, microencapsulation and covalent bonding.
As mentioned earlier, antimicrobial enzymes have only been recently studied in urinary catheters. A group in Austria has been able to both incorporate and immobilize cellobiose dehydrogenase on PDMS through different methods [139, 145, 146]. CDH is produced by a wood degrading fungus and uses cello-oligosaccharides as electron donors to produce H2O2. In 2014, Thallinger et al. demonstrated the ability of a CDH/cellobiose system to inhibit several CAUTI pathogens including MRSA by generating H2O2 in the presence of either cellobiose or extracellular polysaccharaides (EPS) [139]. This antimicrobial system was incorporated into the catheter’s lubricant which is an easy, effective, and cost reducing strategy. The study was important in demonstrating the ability of CDH to kill microbes on demand when biofilms were formed. Also, CDH combined with glycoside hydrolase showed an increase in antimicrobial activity in the presence of EPS. In 2015, for the first time, CDH nanoparticles were grafted to the surface of PDMS by Lipovsky et al. This was done using an ultrasonic system in which the PDMS sheets were dipped into to the enzyme solution and irradiated with high intensity ultrasonic waves [146]. The immobilization was characterized using E-SEM, AFM and water contact angle measurements. LIVE/DEAD assay results for S. aureus showed that the antimicrobial activity of the coated PDMS sheets depended on the enzyme concentration and the sonication time. This was an approach that allowed rapid production of CDH coatings. Another method of CDH immobilization was developed by Thallinger et al. [145], who treated the surface of amino-functionalized PDMS with glutaraldehyde and subsequently grafted CDH to the remaining aldehyde groups at the interface. The coating was able to maintain its antimicrobial activity like its previous study [139] even in artificial urine medium.
Enzymes as active components of antimicrobial coatings have many advantages over antibiotics and other currently used antimicrobial agents. Firstly, they are specific for particular pathogens. This means that they can kill specific pathogens without disturbing the other necessary bacteria required by the living organism. Secondly, bacterial resistance to enzymes is very rare. However, this has to regulated by not providing more than the lethal dose for the pathogens, so as to not develop resistance over time. Antimicrobial enzymes are also safer when compared to other antimicrobial agents as they are natural, non-reactive and non-toxic to living organisms. However, besides these advantages, enzymes also pose some disadvantages in the current market. Compared to cheaper alternatives like silver and antibiotics, the production and purification of antimicrobial enzymes to be used for coatings is expensive. Another big disadvantage is that they are proteins so they can get denatured in extreme conditions (e.g. sterilization of device, storage and transport).
2.2.6. Nitric Oxide
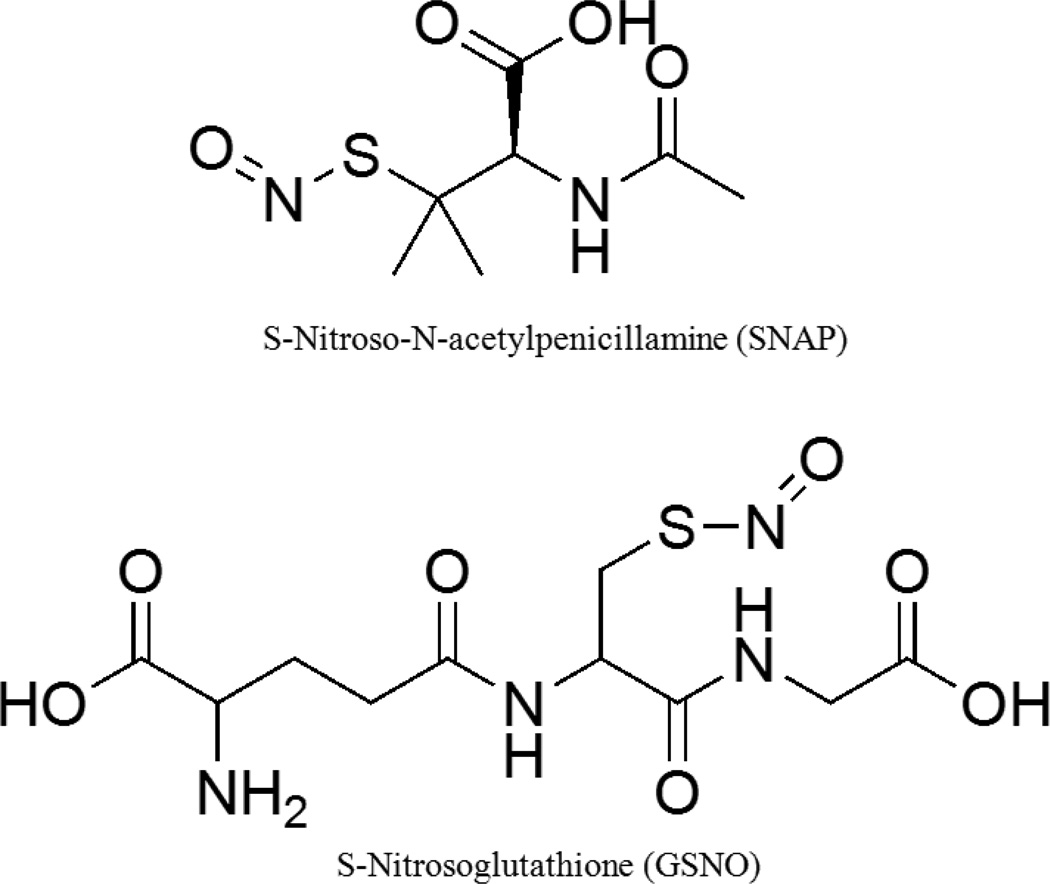
Nitric oxide (NO) has been a well-known antimicrobial agent since the 1980s [147]. It also has several other biological functions like inhibition of platelet aggregation, production of oxygen free radicals, promotes angiogenesis, causes vasodilation and acts as a neurotransmitter [148]. In endothelial cells, NO is produced via the enzymatic oxidation of L-arginine by nitric oxide synthases and this production is increased during infections in case of inducible nitric oxide synthase [149]. Nitric oxide’s antimicrobial mechanisms include nitrosation of amines and thiols, lipid peroxidation, tyrosine nitration and DNA cleavage (Figure 11) [149, 150]. Some commonly used NO donors in the medical research laboratories are S-nitrosothiols like S-Nitroso-N-acetyl-DL-penicillamine (SNAP) and S-nitrosoglutathione (GSNO) (Figure 12).
Figure 11.
Antimicrobial mechanisms of nitric oxide include nitrosation of amines and thiols in the extracellular matrix, lipid peroxidation and tyrosine nitration in the cell wall, and DNA cleavage in the cellular matrix.
Figure 12.
Structures of two commonly studied NO donors: S-nitroso-N-acetyl-DL-penicillamine and S-nitrosoglutathione
Polymers have been impregnated (solvent evaporation and solvent swelling) with nitric oxide donors to localize NO release as it has a short half-life of merely few seconds [151]. In 2013, Brisbois et al. found that E2As polymer films doped (solvent evaporation method) with NO donors could generate a stable flux of NO for a period of 20 days. Due to the low storage capacity of NO [152], research with NO releasing polymers as an antimicrobial coating has been limited, [153] but this study showed that the NO donor, SNAP, is surprisingly stable in the polymer used and retains 82% of the initial SNAP amount even after 2 months of storage in 37°C. While NO has been studied widely as a general antimicrobial agent in medical devices [151, 153–159], very few studies have been conducted with NO or NO donor impregnated urinary catheters [92, 160].
In 2011, a research group in University of British Columbia found that nitrofural coated catheters and nitric oxide impregnated Foley catheters both had the same amount of microbial efficacy against a 103 CFU mL−1 bacterial load [92]. In this study the performance of a silicone Foley catheter was compared with the same silicone Foley catheter impregnated with NO (in an exposure chamber), a silver-coated antimicrobial catheter and a nitrofural-coated Foley catheter. These catheters were exposed to the E. coli culture (103 CFU mL−1) for 24 hours at 37°C. It was observed that the control and Ag-coated catheters had >1 × 108 CFU mL−1 of E. coli while the nitrofural-coated and NO impregnated catheters had a very high antimicrobial efficacy (< 2 × 102 CFU mL−1). Both planktonic and biofilm growth was inhibited in the nitrofural-coated and NO impregnated catheters. While nitrofural is currently not FDA approved in the USA, NO proves to be a viable candidate for further studies in developing antimicrobial coatings for urinary catheters.
In 2015, a study found that a synthetic NO donor, SNAP, could be impregnated into catheters via a solvent swelling method and hence reduce biofilm formation rates for up to 14 days [160]. The catheter tubings were soaked in SNAP/THF solution for 24 hours in dark and then dried for 72 hours in the fume hood. Under physiological conditions, the catheters were found to have a NO surface-flux of between 0.8 and 1.4 × 10−10 mol min−1 cm−2 up to a month which is an effective flux rate to avoid infection. The NO flux was monitored with the help of a chemiluminescence analyzer at 37°C in real time. A UV-vis spectrometer was used to measure SNAP diffusion from the catheter surfaces while being immersed in PBS solution. Studies revealed low or no leaching confirming the longevity of the antimicrobial effects. The catheters were found to be effective against S. epidermidis and P. mirabilis. Toxicity assessment on a L-929 mouse fibroblast cell-line toxicity model also confirmed a fully biocompatible coating. The tubing scored a 0 on 3-point grading scale, which is the safest grading. While this study was successful in showing the effectivity of long term NO release from urinary catheters, it also left concerns regarding the depletion of the NO donor from the polymer as there was only a limited reservoir of SNAP present in the catheter coating, which would ultimately be exhausted.
While concerns of storage stability and controlled release plague the antimicrobial utilization of nitric oxide, it is also important to note that since nitric oxide is physiologically available, it does not pose threats of foreign body reaction. The studies performed to date confirm that the controlled release of NO should be further researched with a focus on NO donors that can be cross-linked to avoid the problem of NO donor diffusion into the physiological environment and thereby preventing the exhaustion of the NO donor reservoir.
2.2.7. Polyzwitterions
A zwitterion is a neutral molecule containing both a positive and a negative charge. As long as the summation of the charges remains neutral, there can be more than one positive and negative charge on the molecule. Zwitterionic polymers fall under the category of antifouling materials with electrostatic and steric repulsion characteristics. Similar to hydrophilic coatings, zwitterions also form hydration layers but through tight electrostatic interactions unlike the comparatively loose Van der Waals’ force of hydrophilic coatings. The zwitterionic hydration layers are formed by hydrogen bonding between the groups on the zwitterion and water molecules at the coating interface (Figure 13). The charge neutral characteristic of a zwitterionic polymer allows it to form a hydration shell/layer around the polymer via electrostatic interactions [161]. This acts as a barrier against foulants because the hydration layer does not allow proteins to settle down on the surface of the device which otherwise would promote bacterial adhesion. This barrier is known to be denser and thicker than the hydration shell formed by PEG. Compared with the directional arrangement of water molecules in the hydration shell formed via hydrogen bonds in case of PEG, the dipole arrangement of water molecules in the hydration shell formed via electrostatic interactions by zwitterionic molecules are closer to free water. This makes the zwitterionic materials superior to PEGbased materials in repelling biological foulants and more biocompatible [162]. The second mechanism of antifouling by zwitterionic polymers is steric hindrance. When the foulants come in contact with zwitterionic polymer chains, compressing the excluded volume of and lowering their motility, the system Gibbs free energy increases. So the polymer chains tend to recover to the swelling state and stop the foulants from getting in touch with the surface [44]. Three of the most commonly studied are phosphorylcholines, sulfobetaines and carboxybetaines (Figure 14) [163]. They are typically presented as pendant groups bound to polymethacrylate or polyacrylamide backbones. The structural versatility granted to zwitterionic coatings due to the ability to attach different functional groups to these polymers gives them an advantage over other polymers used for biological applications [164].
Figure 13.
Zwitterionic mechanism of antifouling: The hydration layer formed by the electrostatic hydrogen bonds between the water molecules and zwitterions prevent the attachment of the extrapolymeric substance (EPS) produced by the microbial cells. The EPS helps the microbes in attaching to the coatings. However, in the case of zwitterionic coatings, the hydration layer prevents this attachment and inhibits antimicrobial attachment to the device.
Figure 14.
Structures of some commonly used zwitterionic polymers for antifouling surfaces
The antifouling nature of zwitterions has been known for a long time and they have been studied for a variety of medical device coatings. One of the first antifouling zwitterionic materials to be investigated were phosphorylcholine containing polymers. Zwaal et al. found that erythrocytes have an asymmetric lipid bilayer membrane which makes their inner surface of the membrane thrombogenic but gives anti-thrombogenic properties to the outer surface [165]. The outer side of the membrane is composed majorly of phosphatidylcholine, a zwitterionic molecule. Chapman et al. found that negatively charged phospholipids were thrombogenic and phosphorylcholine containing surfaces were not. This study attracted interest in the antifouling and biocompatible properties of zwitterionic materials and was termed biomembrane mimicry or biomimicry, in which the surfaces of material behaved like membranes and repelled attachment of biomacromolecules. Two groups have been instrumental in spearheading the research in 2-methacryloyloxyethyphosphorylcholines (MPC), Nakabayashi and Ishihara [166, 167] in Japan and Chapman [168, 169] in UK. The group in Japan specializes in copolymers of MPC with butyl methacrylate (C4, MPC-co-BMA) while the group in UK focuses on copolymers of MPC with n-dodecyl methacrylate (C12, MPC-co-DMA). Both of these polymers have reliable antifouling properties [170–172]. Due to their poor mechanical properties these polymers are not suitable as the base material for medical devices, but they have been exploited as surface coatings. Phosphorylcholine coated (PC) polymers have been studied in some detail for urinary catheter coatings [173, 174]. Russell et al. demonstrated that PC polymers were more resistant to fibrinogen and E. coli adhesion compared to uncoated catheters [174]. The catheter materials were latex, silicone, polyurethanes and polyvinyl chloride. It was also found that the PC coated catheters were able to reduce encrustation in the presence of an artificial urine medium with P. mirabilis. This result was further supported by a clinical trial of three months with ureteral stents. At the end of the clinical trial, PC coated catheters had 30% coverage of biofilms compared to 70% coverage with uncoated stents. These results have been helpful in setting up a company called Urotech (Germany) which manufactures PC coated catheters and several other medical devices for urology. Another crucial study was the in vivo testing of PC-coated urinary catheters and clinical trial of PC-coated ureteral stents [173]. It was found that though encrustation was reduced in the stents, they were not reduced in catheters. Hence, from this study it is seen that the perspective of encrustation mechanism is important. Encrustation can take place in different ways for different urological devices and hence need to be studied in more detail.
Sulfobetaine methacrylate (SBMA) is another common zwitterionic polymer that has been studied for medical device applications and recently in 2014 for a urinary catheter coating application. In 2014, Blanco et al. found that functionalization of silicone catheters with zwitterionic moieties provided antibiofilm properties to the catheter against P. aeruginosa and S. aureus [175]. The coating was primarily composed of PDMS which was plasma-activated and preaminated. The natural phenolic compound gallic acid (GA) was then grafted to this preaminated surface with laccase as the catalyst. The tethered GA residues were activated by laccases to produce phenoxy radicals. Radical polymerization of zwitterionic sulfobetaine methacrylate monomers took place on this surface in a grafting from approach. Attenuated total reflectance-Fourier transform infrared spectroscopy (ATR-FTIR), x-ray photoelectron spectroscopy (XPS), atomic force microscopy (AFM) and contact angle measurements were performed to characterize the surface coatings. FITC labeled bovine serum albumin (BSA) protein was used to perform protein adhesion testing on the treated and untreated catheters. Hydrophilic, hydrophobic and zwitterion coated surfaces were compared and it showed that hydrophilic nature of coatings is not enough to prevent protein adhesion while the zwitterion coatings showed no protein adhesion. This proved that the hydration layer formed on zwitterion coatings due to electrostatic interactions prevents the hydrophobic interactions between the protein and the untreated device coatings. Static biofilm tests for P. aeruginosa and S. aureus were performed by incubation of the bacteria for 18 h. Bacteria viability test and direct fluorescence microscopy showed that biofilm formation was reduced by 80% compared to uncoated catheters. This study was important in designing an environmentally friendly material that would not require hazardous starting materials like organic peroxides and organic solvents whilst at the same time being as effective as it’s counterparts in the medical coating industry.
These studies of zwitterionic antifouling effect on catheters were important to gain more information about biocompatible coatings with antimicrobial efficacy against CAUTIs. However, overtime the hydration layer may become breached and the proteins can adhere on the device coatings. Therefore, it would be important to now direct studies towards finding superhydrophilic zwitterionic coatings that can keep the hydration layer stable for more than a month, the duration for which the urinary catheters are usually used. Therefore, superhydrophilic zwitterionic polymers crosslinked to biocide releasing polymers with long term stability have the potential to be strong antimicrobial candidates against CAUTIs.
2.2.8. Polymeric Coating Modifications
Polymers have been used to immobilize many types of antimicrobials including biocide releasing chemicals (like silver ions) or antibiofilm agent releasing substances (like nitric oxide). The methods employed to immobilize antimicrobial agents include: 1) “graft to” techniques in which agents are covalently coupled to the surface of the polymer; 2) physical adsorption of agents in which non covalent but strong multidentate interactions are employed to attach agents to the polymers, interactions could be hydrogen bonding, ionic bonding or steric attraction; 3) surface initiated immobilization involves synthesis of the antimicrobial on the surface of the polymer by the use of a covalently bonded initiator and; 4) “as formed” immobilization involves the synthesis of the agent within the substrate when it is formed [176, 177]. These immobilization strategies are based on the compatibility of the agent with the polymer itself. For example, if an agent is required in high concentration for antimicrobial efficacy, it would be best to use a polymer that allows for high release of the agent. Each of the strategies mentioned have their own advantages and disadvantages. While “graft to” involves covalent bonding, sometimes physisorption of agents could result in better immobilization due to the multidentate interactions present. Hence intrinsic and surface properties of polymers are also taken into consideration while immobilizing agents. Many studies conducted over the past decade have shown tremendous growth in the quality and efficacy of these antimicrobial and antifouling polymers [82, 91, 94, 178–183]. However, for the specific needs of urinary catheters, modified polymer coatings have been limited as these materials have to go through several clinical trials to be available in the market.
Polytetrafluoroethylene (PTFE) has been studied as a control catheter to compare its antimicrobial effects against silver-alloy coated and nitrofural impregnated catheters [82]. The PTFE coated latex catheters were considered as control, and 7102 patients were randomly chosen and underwent consented catheterization. At the end of 6 weeks, health status and incidences of CAUTI proved to be the same for each of the three groups. This study was an example of how silver-alloy and nitrofural catheters, despite claiming to be more effective, can work only as well as PTFE. Grafted polyethylene glycol (PEG) based polymers have also been studied for urinary catheter coatings and exhibit good antifouling properties [178]. In 2014, a study showed that a Ag-based coating composed of a mixture of AgNO3 and surface exposed PEG conjugated to the base material with 3,4-dihydroxyphenylalanine (mPEG-DOPA3) was able to reduce bacteriuria in a rabbit catheter model [182]. The DOPA functionality was used to form a crosslinked polymer network on the catheter surface, while the surface exposed PEG provided antifouling properties. The significant clearance of E. coli from the urine proved that Ag-based mPEG-DOPA3 catheters could be used to prevent CAUTIs. 90% of the rabbits with control (uncoated polyurethane) catheters showed E. coli growth while 40–50% of the coated catheters were infected at the end of the seven-day study.
Modifications of polyurethane (PU) catheters have also demonstrated positive effects [179, 180]. Polyurethanes have the advantage of being very versatile as compared to other polymers and are known to be tough, biocompatible and hemocompatible. Another crucial advantage is that they can be processed in several ways including extrusion, injection molding, film blowing and solution dipping. A salicyclic acid releasing polyurethane acrylate polymer coated on the inner lumen of the catheter was able to show antibiofilm effects against P. aeruginosa and E. coli [179]. This was achieved by the hydrolysis of salicyclic acid from the polymer backbone, leaving the polymer coating intact on the inside of the catheter. In another study, 5,5-dimethylhydantoin (DMH), an N-halamine precursor was covalently linked to the surface of PU [180] with 1,6-hexamethylene diisocyanate (HDI) as the coupling agent. The grafted DMH was then transformed into N-halamines by immersing the coating in 10% bleach solution. Organic N-halamines are compounds containing one or more nitrogen-halogen covalent bonds that are normally formed by the halogenation of imide, amide, or amine groups [184]. Their antimicrobial activity involves a halogen exchange reaction with microorganisms which causes expiration of the cells. This N-halamine generating coating demonstrated antibiofilm and biocidal properties with a broad-spectrum activity that lasted for 6 months. The innovation was that the functionality of this coating could be recharged by the regeneration of the N-halamines through chlorination (immersing in 10% bleach solution) [180]. Successful antimicrobial activity after 10 cycles of “quenching-recharging” chlorine contents of the polymer confirmed that the functional groups could be recharged and hence the coating holds promise for efficient long-term use. In another study conducted in 2014, poly(catechins)-antibiotic conjugates were used as coatings on silicone and polyurethane catheters [181]. Two coatings, polycatechin with trimethoprim (TMP) and polycatechin with TMP and sulfamethoxazole (SMZ), showed high antimicrobial activity. They were also biocompatible with mammalian cells in cytotoxicity testing. These exceptional results show that with a few modifications, PU coatings could be further explored in clinical trials and may be ready for the market in the near future.
Polyvinyl chlorine (PVC) coatings have been found to have antimicrobial efficacy in intubation tubes and medical plastics but haven’t been specifically studied as urinary catheter coatings [94]. They have good mechanical strength and their lightweight characteristic adds to the advantage of using a PVC coated medical device. In this particular study, unmodified PVC coatings were compared to PVC coatings that were dissolved in tetrahydrofuran (THF, a good solvent for PVC) and non-solvent (ethanol, methanol) combinations. The study found that while unmodified PVC which is only processed using THF had a smooth and hydrophobic surface (contact angle of 80°), the hydrophobicity of this surface could be increased (contact angle increased from 73° to 150°) using 15% to 35% v/v of ethanol in the polymer solvent mixture. This addition of the non-solvent to the polymer mixture attributed to its porous structure with micron-sized PVC particles and hence gave it a near superhydrophobic surface. This superhydrophobic surface was able to delay the attachment of P. aeruginosa bacteria from 6 hours to 24 hours when compared to the unmodified polymer. The authors also noted that while the bacterial study showed appreciable results, the introduction of the pores could change the mechanical strength of the polymer and future work should focus on including antimicrobial agents in these polymers to increase its antimicrobial efficacy.
The listed modifications of polymer coatings show that much work remains to be done to understand the mechanism of antimicrobial action and improve the ultimate outcome. While polymer coatings for other medical devices have been studied more extensively, it is important that more polymers be studied in the case of urinary catheter coatings. In the future, easily employable modifications of polymers to render them highly effective as antimicrobial coatings would be appreciated as physical modifications that are permanent (like superhydrophobicity with nanostructures) could prevent the evolution of antimicrobial-agent resistant bacteria in case of CAUTIs.
2.2.9. Liposomes
Liposomes are artificially prepared spherical lipid vesicles that consist of one or more phospholipid bilayers (Figure 15) [185]. To form liposomes, lipids are first dissolved in an organic solvent. The organic solvent is then removed and the clear lipid film acquired is dispersed in aqueous media. The lipid films are swollen in this media and the swollen hydrated lipid stacks then form vesicles on agitation [186]. When phospholipids are scattered in water, the hydrophobic tails tend to turn inward and the hydrophilic heads face the surrounding aqueous media, forming concentric layers of phospholipids. Liposomes are categorized according to the number of lamellae they are composed of: unilamellar vesicles and multilamellar vesicles. The unilamellar vesicles are further classified according to their sizes: small unilamellar vesicles (SUV, 15–50 nm in dia.), large unilamellar vesicles (LUV, 100–400 nm in diameter) and giant unilamellar vesicles (GUV, 1µM and larger). Multilammelar vesicles are also classified into large multilamellar (LMV, 200nm–3µM) and multivesicular vesicles (MVV, 200nm–3µM). The different sized vesicles can be prepared by various mechanical dispersion techniques (e.g. sonication of LMV can yield SUV while extrusion of LMV can yield LUV). Some of the advantages of having catheters coated with liposomes are that they can carry hydrophilic, hydrophobic and amphipathic drugs and these drugs can be protected from external disturbances ensuring their sustained release [187]. This allows the catheter to be coated with liposomes having broad spectrum antimicrobial activity. These materials can be highly customized according to the needs; for example they can be anionic or cationic in order to accommodate a desired molecule payload [6]. One of the advantages of using liposomes is that the encapsulated drugs are protected from faster degradation or reaction with non-specific entities that increase the rate of drug delivery. Liposomes are also considered to be highly biocompatible as compared to other coatings [6].
Figure 15.
A liposome has at least one phospholipid bilayered membrane. It consists of a hydrophobic tail and a hydrophilic tail. Drugs can be inserted within the liposome and delivered into the subject.
In 1998, a group at the University of Toronto was able to synthesize a poly (ethylene glycol)–gelatin–liposome mixture that was applied to a pretreated (phenylazido-modified gelatin) silicone catheter [188]. Gelatin hydrogels were used because of their biocompatibility and biodegradability along with the ability to withstand temperatures up to 80°C. These catheters contained ciprofloxacin, a commonly used broad spectrum antibiotic, with an initial concentration of 185±16 µg cm−2. The release rate of ciprofloxacin was enough to produce a growth-inhibition zone of 39±1 mm (P. aeruginosa) while bacterial adhesion was not observed at all during the seven-day test period. The PEG-gelatin formulation was proclaimed a superior biocompatible material for catheters which otherwise can cause inflammation. In 1999, the same group studied the coating in a rabbit model for E. coli [189]. Compared to positive urine cultures obtained from control hydrogel coatings in 3.5 days, positive urine cultures from the liposome-ciprofloxacin embedded hydrogel was obtained after 5.3 days. Even though the study of liposomes has not led to a major breakthrough as of yet, studies such as this reveal that liposomes can be effectively used to deliver a variety of antimicrobial drugs through urinary catheter coatings.
3. Future Perspectives and Challenges
Currently, research on antimicrobial agents/materials for urinary catheters is aggressive and a direct result of the need to find viable solutions to CAUTIs. The interest in agents such as NO and antimicrobial enzymes also suggests that research will not slowdown in this area in the near future. For this reason, there are a couple of challenges that researchers need to focus on whether they are improving formulations with clinically tested materials or diving into ‘new research’ with urinary catheter antimicrobial agents. These challenges can be healthcare and socio-economy based.
The presence of antibiotic resistance along with the already present villains, biofilms and encrustation of urinary catheters will remain the major focus on developing antimicrobial coatings for urinary catheters. The emergence of superbugs has led to panic in the public, which can affect the use of antibiotic containing catheters in patients. Antimicrobial coatings that attack pathogens through multi-mechanism approaches will ultimately win this war. Multi-mechanism approaches reduce the ability of pathogens to become resistant and hence are an effective strategy against resistance. While multi-mechanism methods can be employed by using just one antimicrobial agent, synergistic approaches are also another effective strategy to design antimicrobial coatings. In case of synergistic approaches, urinary catheters could be composed of either one or two biocidal agents along with an antifouling topcoat [190, 191]. This would greatly increase antimicrobial efficacy by having one agent that prevent all biomacromolecules from attaching to the surface while at the same time killing any pathogens. This method could also be employed to use the combined strategies at different point of time during the catheter use. One effective multi-mechanism strategy has been the use of nano/micro structures as coatings [192]. Most have been developed by mimicking the characteristics of a shark’s skin. A shark’s skin has certain specific roughness that prevents marine organisms from attaching to its skin. In addition to micropatterning, researchers have also looked into light activated antimicrobial surfaces [193]. Light activated antimicrobial surfaces include species that produce reactive oxygen species on exposure to light and destroy bacterial cell walls.
Another challenge that antimicrobial coatings face is cytotoxicity [194]. This could either be through contact with the antimicrobial agent or through leaching of the agent into the patient’s system. Researchers will need to conduct thorough in vitro and in vivo leaching and cytotoxicity studies to rule out any problematic side-effects.
A marketing challenge that researchers will also need to address before introducing new materials is the comfort factor. This especially applies to long-term use catheters. While nitrofural-based catheters have been able to provide antimicrobial efficacy, they have been known to be uncomfortable to patients. Healthcare officials also need to care for a patient’s overall welfare. This means that catheter coatings which can be lubricated well and are flexible with small enough outer diameter (patient comfort) and wide inner lumen (for preventing blockage) are preferred.
Conclusion
CAUTI is one of the leading nosocomial infections in the world and has led to the study of various novel ways to prevent the infection. This has made “antimicrobial agents/materials for urinary catheters” a widely studied subject. Studies such as synthesizing novel gendine for antimicrobial gloves [195] could aid in preventing infections in the hospital settings. However, even after meticulous care and insertion of the catheters in sterilized conditions, infections still occur. This shows that the simple impregnation of antimicrobial agents in catheters with sterile conditions is not sufficient for the prevention of CAUTI. For this reason, catheters with holistic antimicrobial effects need to be developed [196] and marketed after efficient and well-studied clinical trials. Currently the market is dominated by silver-alloy and antibiotic coated catheters. As mentioned in the review, silver-alloy coatings have been popular because of their widely studied characterizations but they have been found to have less microbial efficacy than antibiotics. However, the increased usage of antibiotics causes antibiotic resistance and hence new methods have been developed to combat these resistant microbes. This shows a greater need for understanding the biofilm structure and its mechanisms so that antimicrobial coatings can be designed more efficiently.
Foley catheters have not gone through a design change in a long time and hence can be uncomfortable for patients. Some fundamental changes need to be introduced into the catheters: better drainage without and residual urine in the urethra so that bacteria does not grow, wider luminal internal diameter to provide better flow and lesser chances of encrustation initiated blockage and smoother surface to prevent foulant adhesion. As of now, the market has replaced a lot of Foley catheters with Nelatone catheters, which have been found to be more efficient as tested in vivo [197].
Regarding the research of materials to manufacture urinary catheter coatings, standards for antimicrobial properties have not been established as yet and clinical studies have not always proved to agree with each other. This puts material scientists and engineers in a tight spot on deciding the crucial factors to look for while researching the ideal antimicrobial coating. This is the reason why only a few studies have been done with a focus on urinary catheters exposed to dynamic conditions and subsequently have materials approved for clinical trials. There is a need for better standards and ways to prevent CAUTIs. These materials have to be tested very vigorously using characterization processes that are robust and can be used to achieve 100% efficiency for antimicrobial effects of the catheter material. Thus, looking at the various materials and the results, development of antimicrobial urinary catheter materials will remain a current and broad interest for the upcoming decade.
Statement of Significance.
This review article intends to provide an expansive insight into the various antimicrobial agents currently being researched for urinary catheter coatings. According to CDC, approximately 75% of urinary tract infections are caused by urinary catheters and 15–25% of hospitalized patients undergo catheterization. In addition to these alarming statistics, the increasing cost and health related complications associated with Catheter Associated UTIs make the research for antimicrobial urinary catheter coatings even more pertinent. This review provides a comprehensive summary of the history, the latest progress in development of the coatings and a brief conjecture on what the future entails for each of the antimicrobial agents discussed.
Acknowledgments
The authors acknowledge the support from the National Institutes of Health, Grant K25HL111213.
Abbreviations used in text
- CAUTI(s)
Catheter-associated urinary tract infection(s)
- UTI
Urinary tract infection
- CFU
Colony forming unit
- HICPAC
Healthcare Infection Control Practices Advisory Committee
- EPS
Extracellular polymeric substances
- SEM
Scanning electron microscopy
- SPTT
Serial plate transfer test
- PTFE
Polytetrafluoroethylene
- PEG
Polyethylene glycol
- PU
Polyurethane
- DMH
5,5-dimethylhydantoin
- TMP
Trimethoprim
- SMZ
Sulfamethoxazole
- PVC
Polyvinyl chlorine
- EVA
Ethylene vinyl acetate
- 4AP12
4-amide-piperidine-C12
- ENR
Enoyl-acyl carrier protein reductase
- FDA
Food and Drug administration
- AMP(s)
Antimicrobial peptides
- PDMS
Polydimethylsiloxane
- AGE
Allyl glycidyl ether
- ATR-FTIR
Attenuated total reflectance-Fourier transform infrared spectroscopy
- XPS
X-ray photoelectron spectroscopy
- AFM
Atomic force microscopy
- FITC
Fluorescein isothiocyanate
- BSA
Bovine serum albumin
- NO
Nitric oxide
- SNAP
S-Nitroso-N-acetyl-DL-penicillamine
- GSNO
S-nitrosoglutathione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lawrence EL, Turner IG. Materials for urinary catheters: a review of their history and development in the UK. Medical Engineering & Physics. 2005 doi: 10.1016/j.medengphy.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 2.Nacey J, Delahijnt B. The evolution and development of the urinary catheter. Australian and New Zealand Journal of Surgery. 1993;63(10):815–819. doi: 10.1111/j.1445-2197.1993.tb00347.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JR, Kuskowski MA, Wilt TJ. Systematic Review: Antimicrobial Urinary Catheters To Prevent Catheter-Associated Urinary Tract Infection in Hospitalized Patients. 2006 doi: 10.7326/0003-4819-144-2-200601170-00009. [DOI] [PubMed] [Google Scholar]
- 4.Curtis J, Klykken P. A Comparative Assessment of Three Common Catheter Materials. Dow Corning. 2008 [Google Scholar]
- 5.s.t.p.i.t. association. Advantages of Flexible PVC (vs. other Polymers) 2012 < https://plasticsindustry.org/FVPC/VinylFAQ/content.cfm?ItemNumber=3845&navItemNumber=3846>. [Google Scholar]
- 6.Siddiq DM, Darouiche RO. New strategies to prevent catheter-associated urinary tract infections. Nat Rev Urol. 2012;9(6):305–314. doi: 10.1038/nrurol.2012.68. [DOI] [PubMed] [Google Scholar]
- 7.Fink R, Gilmartin H, Richard A, Capezuti E, Boltz M, Wald H. Indwelling urinary catheter management and catheter-associated urinary tract infection prevention practices in Nurses Improving Care for Healthsystem Elders hospitals. Am J Infect Control. 2012;40(8):715–720. doi: 10.1016/j.ajic.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 8.Donlan RM. Biofilms and device-associated infections. Emerging Infectious Diseases. 2001;7(2):277–281. doi: 10.3201/eid0702.010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tambyah PA, Maki DG. Catheter-associated urinary tract infection is rarely symptomatic: a prospective study of 1,497 catheterized patients. Arch Intern Med. 2000 doi: 10.1001/archinte.160.5.678. [DOI] [PubMed] [Google Scholar]
- 10.Lo J, Lange D, Chew B. Ureteral Stents and Foley Catheters-Associated Urinary Tract Infections: The Role of Coatings and Materials in Infection Prevention. Antibiotics. 2014;3(1):87–97. doi: 10.3390/antibiotics3010087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller S. Catheter-related UTI. 2014 < https://www.nlm.nih.gov/medlineplus/ency/article/000483.htm>.
- 12.Ashley Bowen WJGH. Urinary Tract Infections: A Primer for Clinicians. 2007 < http://www.medscape.org/viewarticle/556040>. [Google Scholar]
- 13.Wagenlehner FME, Naber KG. Treatment of Bacterial Urinary Tract Infections: Presence and Future. European Urology. 2006;49(2):235–244. doi: 10.1016/j.eururo.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 14.Warren JW. Catheter-associated Urinary Tract Infections. Infectious Disease Clinics of North America. 1997;11(3) doi: 10.1016/s0891-5520(05)70376-7. [DOI] [PubMed] [Google Scholar]
- 15.Bursle EC, Dyer J, Looke DF, McDougall DA, Paterson DL, Playford EG. Risk factors for urinary catheter associated bloodstream infection. J Infect. 2015;70(6):585–591. doi: 10.1016/j.jinf.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: something needs to be done. J Intern Med. 2014;276(2):120–129. doi: 10.1111/joim.12220. [DOI] [PubMed] [Google Scholar]
- 17.Brusch JL. Catheter-Related Urinary Tract Infection. 2013 2013. [Google Scholar]
- 18.Donlan RM, Costerton JW. Biofilms: Survival Mechanisms of Clinically Relevant Microorganisms. Clinical Microbiology Reviews. 2002;15(2):167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Broomfield RJ, Morgan SD, Khan A, Stickler DJ. Crystalline bacterial biofilm formation on urinary catheters by urease-producing urinary tract pathogens: a simple method of control. J Med Microbiol. 2009;58(Pt 10):1367–1375. doi: 10.1099/jmm.0.012419-0. [DOI] [PubMed] [Google Scholar]
- 20.Armbruster CE, Mobley HLT. Merging mythology and morphology: the multifaceted lifestyle of Proteus mirabilis. Nat Rev Micro. 2012;10(11):743–754. doi: 10.1038/nrmicro2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez G. Proteus infections. 2015 < http://emedicine.medscape.com/article/226434-overview#showall>. [Google Scholar]
- 22.Stickler D, King J, Nettleton J, Winters C. The structure of urinary catheter encrusting bacterial biofilms. Cells and Materials. 1993;3(3):315–320. [Google Scholar]
- 23.Trautner BW, Darouiche RO. Role of biofilm in catheter-associated urinary tract infection. American journal of infection control. 2004;32(3):177–183. doi: 10.1016/j.ajic.2003.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ganderton L, Chawla J, Winters C, Wimpenny J, Stickler D. Scanning electron microscopy of bacterial biofilms on indwelling bladder catheters. European Journal of Clinical Microbiology and Infectious Diseases. 1992;11(9):789–796. doi: 10.1007/BF01960877. [DOI] [PubMed] [Google Scholar]
- 25.Brisset L, Vernet-Garnier V, Carquin J, Burde A, Flament J, Choisy C. In vivo and in vitro analysis of the ability of urinary catheter to microbial colonization. Pathologie-biologie. 1996;44(5):397–404. [PubMed] [Google Scholar]
- 26.Rendueles O, Kaplan JB, Ghigo JM. Antibiofilm polysaccharides. Environmental microbiology. 2013;15(2):334–346. doi: 10.1111/j.1462-2920.2012.02810.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. The lancet. 2001;358(9276):135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- 28.Lynch AS, Robertson GT. Bacterial and fungal biofilm infections. Annu. Rev. Med. 2008;59:415–428. doi: 10.1146/annurev.med.59.110106.132000. [DOI] [PubMed] [Google Scholar]
- 29.Lewis K. Persister cells, dormancy and infectious disease. Nature Reviews Microbiology. 2007;5(1):48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 30.Jacobsen SM, Shirtliff ME. Proteus mirabilis biofilms and catheter-associated urinary tract infections. Virulence. 2011;2(5):460–465. doi: 10.4161/viru.2.5.17783. [DOI] [PubMed] [Google Scholar]
- 31.H.I.C.P.A. Committee. Guideline for Prevention of Catheter-associated Urinary Tract Infections, 2009. 2009 < http://www.cdc.gov/hicpac/cauti/005_background.html>.
- 32.Brown MR, Allison DG, Gilbert P. Resistance of bacterial biofilms to antibiotics: a growth-rate related effect? The Journal of antimicrobial chemotherapy. 1988;22(6):777–780. doi: 10.1093/jac/22.6.777. [DOI] [PubMed] [Google Scholar]
- 33.Olson ME, Nickel JC, Khoury AE, Morck DW, Cleeland R, Costerton JW. Amdinocillin treatment of catheter-associated bacteriuria in rabbits. The Journal of infectious diseases. 1989;159(6):1065–1072. doi: 10.1093/infdis/159.6.1065. [DOI] [PubMed] [Google Scholar]
- 34.Darouiche RO, Dhir A, Miller AJ, Landon GC, Raad, Musher DM. Vancomycin penetration into biofilm covering infected prostheses and effect on bacteria. The Journal of infectious diseases. 1994;170(3):720–723. doi: 10.1093/infdis/170.3.720. [DOI] [PubMed] [Google Scholar]
- 35.Armbruster CE, Smith SN, Yep A, Mobley HL. Increased incidence of urolithiasis and bacteremia during Proteus mirabilis and Providencia stuartii coinfection due to synergistic induction of urease activity. The Journal of infectious diseases. 2014;209(10):1524–1532. doi: 10.1093/infdis/jit663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ramage G, Martínez JP, López-Ribot JL. Candida biofilms on implanted biomaterials: a clinically significant problem. FEMS Yeast Research. 2006;6(7):979–986. doi: 10.1111/j.1567-1364.2006.00117.x. [DOI] [PubMed] [Google Scholar]
- 37.Nickel JC, Downey JA, Costerton JW. Ultrastructural study of microbiologic colonization of urinary catheters. Urology. 1989;34(5):284–291. doi: 10.1016/0090-4295(89)90327-0. [DOI] [PubMed] [Google Scholar]
- 38.Frank DN, Wilson SS, Amand ALS, Pace NR. Culture-independent microbiological analysis of foley urinary catheter biofilms. PloS one. 2009;4(11):e7811. doi: 10.1371/journal.pone.0007811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.W. H. Organization. Antimicrobial Resistance. 2016
- 40.Wazait HD, Patel HRH, Veer V, Kelsey M, Van Der Meulen JHP, Miller RA, Emberton M. Catheter-associated urinary tract infections: prevalence of uropathogens and pattern of antimicrobial resistance in a UK hospital (1996–2001) BJU International. 2003;91(9):806–809. doi: 10.1046/j.1464-410x.2003.04239.x. [DOI] [PubMed] [Google Scholar]
- 41.Sievert DM, Ricks P, Edwards JR, Schneider A, Patel J, Srinivasan A, Kallen A, Limbago B, Fridkin S. Antimicrobial-Resistant Pathogens Associated with Healthcare-Associated Infections Summary of Data Reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009–2010. Infection Control & Hospital Epidemiology. 2013;34(1):1–14. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 42.Tenke P, Koves B, Nagy K, Hultgren SJ, Mendling W, Wullt B, Grabe M, Wagenlehner FM, Cek M, Pickard R, Botto H, Naber KG, Bjerklund Johansen TE. Update on biofilm infections in the urinary tract. World J Urol. 2012;30(1):51–57. doi: 10.1007/s00345-011-0689-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Campoccia D, Montanaro L, Arciola CR. A review of the biomaterials technologies for infection-resistant surfaces. Biomaterials. 2013;34(34):8533–8554. doi: 10.1016/j.biomaterials.2013.07.089. [DOI] [PubMed] [Google Scholar]
- 44.Jeon S, Lee J, Andrade J, De Gennes P. Protein—surface interactions in the presence of polyethylene oxide: I. Simplified theory. Journal of Colloid and Interface Science. 1991;142(1):149–158. [Google Scholar]
- 45.Jeon S, Andrade J. Protein—surface interactions in the presence of polyethylene oxide: II. Effect of protein size. Journal of Colloid and Interface Science. 1991;142(1):159–166. [Google Scholar]
- 46.Li L, Chen S, Zheng J, Ratner BD, Jiang S. Protein adsorption on oligo (ethylene glycol)-terminated alkanethiolate self-assembled monolayers: the molecular basis for nonfouling behavior. The Journal of Physical Chemistry B. 2005;109(7):2934–2941. doi: 10.1021/jp0473321. [DOI] [PubMed] [Google Scholar]
- 47.Hamilton-Brown P, Gengenbach T, Griesser HJ, Meagher L. End terminal, poly (ethylene oxide) graft layers: surface forces and protein adsorption. Langmuir. 2009;25(16):9149–9156. doi: 10.1021/la900703e. [DOI] [PubMed] [Google Scholar]
- 48.Barbey R, Lavanant L, Paripovic D, Schüwer N, Sugnaux C, Tugulu S, Klok H-A. Polymer brushes via surface-initiated controlled radical polymerization: synthesis, characterization, properties, and applications. Chemical reviews. 2009;109(11):5437–5527. doi: 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- 49.Feng W, Zhu S, Ishihara K, Brash JL. Protein resistant surfaces: Comparison of acrylate graft polymers bearing oligo-ethylene oxide and phosphorylcholine side chains. Biointerphases. 2006;1(1):50–60. doi: 10.1116/1.2187495. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z, Zhang M, Chen S, Horbett TA, Ratner BD, Jiang S. Blood compatibility of surfaces with superlow protein adsorption. Biomaterials. 2008;29(32):4285–4291. doi: 10.1016/j.biomaterials.2008.07.039. [DOI] [PubMed] [Google Scholar]
- 51.Kuang J, Messersmith PB. Universal surface-initiated polymerization of antifouling zwitterionic brushes using a mussel-mimetic peptide initiator. Langmuir. 2012;28(18):7258–7266. doi: 10.1021/la300738e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen S, Li L, Zhao C, Zheng J. Surface hydration: principles and applications toward low-fouling/nonfouling biomaterials. Polymer. 2010;51(23):5283–5293. [Google Scholar]
- 53.Wang R, Kreuzer H, Grunze M. Molecular conformation and solvation of oligo (ethylene glycol)-terminated self-assembled monolayers and their resistance to protein adsorption. The Journal of Physical Chemistry B. 1997;101(47):9767–9773. [Google Scholar]
- 54.Fuchs AD, Tiller JC. Contact-Active Antimicrobial Coatings Derived from Aqueous Suspensions. Angewandte Chemie. 2006 doi: 10.1002/anie.200602738. [DOI] [PubMed] [Google Scholar]
- 55.He W, Zhang Y, Li J, Gao Y, Luo F, Tan H, Wang K, Fu Q. A Novel Surface Structure Consisting of Contact-active Antibacterial Upper-layer and Antifouling Sub-layer Derived from Gemini Quaternary Ammonium Salt Polyurethanes. Scientific Reports. 2016;6:32140. doi: 10.1038/srep32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Poverenov E, Shemesh M, Gulino A, Cristaldi DA, Zakin V, Yefremov T, Granit R. Durable contact active antimicrobial materials formed by a one-step covalent modification of polyvinyl alcohol, cellulose and glass surfaces, Colloids and surfaces. B. Biointerfaces. 2013;112:356–361. doi: 10.1016/j.colsurfb.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 57.Hasan J, Crawford RJ, Ivanova EP. Antibacterial surfaces: the quest for a new generation of biomaterials. Trends Biotechnol. 2013;31(5):295–304. doi: 10.1016/j.tibtech.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 58.Salwiczek M, Qu Y, Gardiner J, Strugnell RA, Lithgow T, McLean KM, Thissen H. Emerging rules for effective antimicrobial coatings. Trends Biotechnol. 2014;32(2):82–90. doi: 10.1016/j.tibtech.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 59.Danese PN. Antibiofilm approaches: prevention of catheter colonization. Chemistry & biology. 2002;9(8):873–880. doi: 10.1016/s1074-5521(02)00192-8. [DOI] [PubMed] [Google Scholar]
- 60.Knudsen BE, Chew BH, Denstedt JD. Drug-eluting biomaterials in urology: the time is ripe. BJU international. 2005;95(6):726–727. doi: 10.1111/j.1464-410X.2005.05388.x. [DOI] [PubMed] [Google Scholar]
- 61.Kumar R, Münstedt H. Silver ion release from antimicrobial polyamide/silver composites. Biomaterials. 2005;26(14):2081–2088. doi: 10.1016/j.biomaterials.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 62.Kim JS, Kuk E, Yu KN, Kim J-H, Park SJ, Lee HJ, Kim SH, Park YK, Park YH, Hwang C-Y, Kim Y-K, Lee Y-S, Jeong DH, Cho M-H. Antimicrobial effects of silver nanoparticles, Nanomedicine: Nanotechnology. Biology and Medicine. 3(1):95–101. doi: 10.1016/j.nano.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 63.Lansdown AB. Silver. I: Its antibacterial properties and mechanism of action. J Wound Care. 2002;11(4):125–130. doi: 10.12968/jowc.2002.11.4.26389. [DOI] [PubMed] [Google Scholar]
- 64.Feng QL, Wu J, Chen GQ, Cui FZ, Kim TN, Kim JO. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. Journal of Biomedical Materials Research. 2000;52(4):662–668. doi: 10.1002/1097-4636(20001215)52:4<662::aid-jbm10>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 65.Lemire JA, Harrison JJ, Turner RJ. Antimicrobial activity of metals: mechanisms, molecular targets and applications. Nat Rev Micro. 2013;11(6):371–384. doi: 10.1038/nrmicro3028. [DOI] [PubMed] [Google Scholar]
- 66.Johnson JR, Johnston B, Kuskowski MA. In vitro comparison of nitrofurazone- and silver alloy-coated foley catheters for contact-dependent and diffusible inhibition of urinary tract infection-associated microorganisms. Antimicrob Agents Chemother. 2012;56(9):4969–4972. doi: 10.1128/AAC.00733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honda M, Kawanobe Y, Ishii K, Konishi T, Mizumoto M, Kanzawa N, Matsumoto M, Aizawa M. In vitro and in vivo antimicrobial properties of silver-containing hydroxyapatite prepared via ultrasonic spray pyrolysis route. Materials Science and Engineering. 2013;C(33):5000–5018. doi: 10.1016/j.msec.2013.08.026. [DOI] [PubMed] [Google Scholar]
- 68.Lederer JW, Jarvis WR, Thomas L, Ritter J. Multicenter cohort study to assess the impact of a silver-alloy and hydrogel-coated urinary catheter on symptomatic catheter-associated urinary tract infections. J Wound Ostomy Continence Nurs. 2014;41(5):473–480. doi: 10.1097/WON.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McKee C, Williams D. Effect of Silver-Alloy Urinary Catheters in Reducing the Rate of CAUTI’s in Patients Requiring Short-term Catheterization: A Review of the Literature. Digital Commons @ West Chester University, West Chester University of Pennsylvania; 2014. [Google Scholar]
- 70.Rupp ME, Fitzgerald T, Marion N, Helget V, Puumala S, Anderson JR, Fey PD. Effect of silver-coated urinary catheters: Efficacy, cost-effectiveness, and antimicrobial resistance. American Journal of Infection Control. 2004;32(8):445–450. doi: 10.1016/j.ajic.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 71.Knetsch MLW, Koole LH. New Strategies in the Development of Antimicrobial Coatings: The Example of Increasing Usage of Silver and Silver Nanoparticles. Polymers. 2011;3(4):340–366. [Google Scholar]
- 72.Kowalczuk D, Ginalska G, Piersiak T, Miazga-Karska M. Prevention of biofilm formation on urinary catheters: comparison of the sparfloxacin-treated long-term antimicrobial catheters with silver-coated ones. J Biomed Mater Res B Appl Biomater. 2012;100(7):1874–1882. doi: 10.1002/jbm.b.32755. [DOI] [PubMed] [Google Scholar]
- 73.Aflori M, Miron C, Dobromir M, Drobota M. Bactericidal effect on Foley catheters obtained by plasma and silver nitrate treatments. High Performance Polymers. 2015;27(5):655–660. [Google Scholar]
- 74.Leuck AM, Johnson JR, Hunt MA, Dhody K, Kazempour K, Ferrieri P, Kline S. Safety and efficacy of a novel silver-impregnated urinary catheter system for preventing catheter-associated bacteriuria: a pilot randomized clinical trial. Am J Infect Control. 2015;43(3):260–265. doi: 10.1016/j.ajic.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Saint S, Elmore JG, Sullivan SD, Emerson SS, Koepsell TD. The efficacy of silver alloy-coated urinary catheters in preventing urinary tract infection: a meta-analysis. The American journal of medicine. 1998;105(3):236–241. doi: 10.1016/s0002-9343(98)00240-x. [DOI] [PubMed] [Google Scholar]
- 76.Liedberg H, Lundeberg T. Silver Alloy Coated Catheters Reduce Catheter-associated Bacteriuria. British journal of urology. 1990;65(4):379–381. doi: 10.1111/j.1464-410x.1990.tb14760.x. [DOI] [PubMed] [Google Scholar]
- 77.Akiyama H, Okamoto S. Prophylaxis of indwelling urethral catheter infection: clinical experience with a modified Foley catheter and drainage system. The Journal of urology. 1979;121(1):40–42. doi: 10.1016/s0022-5347(17)56652-5. [DOI] [PubMed] [Google Scholar]
- 78.Johnson JR, Roberts PL, Olsen RJ, Moyer KA, Stamm WE. Prevention of Catheter-Associated Urinary-Tract Infection with a Silver-Oxide coated Urinary Catheter - Clinical And Microbiologic Correlates. Journal of Infectious Diseases. 1990;162(5):1145–1150. doi: 10.1093/infdis/162.5.1145. [DOI] [PubMed] [Google Scholar]
- 79.Stamm WE. Catheter-associated Urinary tract infections: Epidemiology, Pathogenesis, and Prevention. The American Journal of Medicine. 1991;91 doi: 10.1016/0002-9343(91)90345-x. [DOI] [PubMed] [Google Scholar]
- 80.Riley DK, Classen DC, Stevens LE, Burke JP. A large randomized clinical trial of a silver-impregnated urinary catheter: lack of efficacy and staphylococcal superinfection. The American journal of medicine. 1995;98(4):349–356. doi: 10.1016/S0002-9343(99)80313-1. [DOI] [PubMed] [Google Scholar]
- 81.Schierholz JM, Lucas LJ, Rump A, Pulverer G. Efficacy of silver-coated medical devices. Journal of Hospital Infection. 1998;40(4):257–262. doi: 10.1016/s0195-6701(98)90301-2. [DOI] [PubMed] [Google Scholar]
- 82.Pickard R, Lam T, Maclennan G, Starr K, Kilonzo M, McPherson G, Gillies K, McDonald A, Walton K, Buckley B, Glazener C, Boachie C, Burr J, Norrie J, Vale L, Grant A, N'Dow J. Types of urethral catheter for reducing symptomatic urinary tract infections in hospitalised adults requiring short-term catheterisation: multicentre randomised controlled trial and economic evaluation of antimicrobial- and antiseptic-impregnated urethral catheters (the CATHETER trial) Health Technol Assess. 2012;16(47):1–197. doi: 10.3310/hta16470. [DOI] [PubMed] [Google Scholar]
- 83.Pickard R, Lam T, MacLennan G, Starr K, Kilonzo M, McPherson G, Gillies K, McDonald A, Walton K, Buckley B, Glazener C, Boachie C, Burr J, Norrie J, Vale L, Grant A, N'Dow J. Antimicrobial catheters for reduction of symptomatic urinary tract infection in adults requiring short-term catheterisation in hospital: a multicentre randomised controlled trial. The Lancet. 2012;380(9857):1927–1935. doi: 10.1016/S0140-6736(12)61380-4. [DOI] [PubMed] [Google Scholar]
- 84.Akre O, Thulin H, Bottai M. Assessing catheter-associated urinary tract infection. The Lancet. 2013;381(9877):1535. doi: 10.1016/S0140-6736(13)60972-1. [DOI] [PubMed] [Google Scholar]
- 85.Li W-R, Xie X-B, Shi Q-S, Zeng H-Y, Ou-Yang Y-S, Chen Y-B. Antibacterial activity and mechanism of silver nanoparticles on Escherichia coli. Applied Microbiology and Biotechnology. 2010;85(4):1115–1122. doi: 10.1007/s00253-009-2159-5. [DOI] [PubMed] [Google Scholar]
- 86.Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances. 2009;27(1):76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 87.Lok C-N, Ho C-M, Chen R, He Q-Y, Yu W-Y, Sun H, Tam PK-H, Chiu J-F, Che C-M. Proteomic Analysis of the Mode of Antibacterial Action of Silver Nanoparticles. Journal of Proteome Research. 2006;5(4):916–924. doi: 10.1021/pr0504079. [DOI] [PubMed] [Google Scholar]
- 88.Kumar CG, Sujitha P. Green synthesis of Kocuran-functionalized silver glyconanoparticles for use as antibiofilm coatings on silicone urethral catheters. Nanotechnology. 2014;25(32):325101. doi: 10.1088/0957-4484/25/32/325101. [DOI] [PubMed] [Google Scholar]
- 89.Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. Journal of Colloid and Interface Science. 2004;275(1):177–182. doi: 10.1016/j.jcis.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 90.Wang R, Neoh KG, Kang ET, Tambyah PA, Chiong E. Antifouling coating with controllable and sustained silver release for long-term inhibition of infection and encrustation in urinary catheters. J Biomed Mater Res B Appl Biomater. 2015;103(3):519–28. doi: 10.1002/jbm.b.33230. [DOI] [PubMed] [Google Scholar]
- 91.Fuchs AV, Ritz S, Pütz S, Mailänder V, Landfester K, Ziener U. Bioinspired phosphorylcholine containing polymer films with silver nanoparticles combining antifouling and antibacterial properties. Biomaterials Science. 2013;1(5):470. doi: 10.1039/c2bm00155a. [DOI] [PubMed] [Google Scholar]
- 92.Regev-Shoshani G, Ko M, Crowe A, Av-Gay Y. Comparative efficacy of commercially available and emerging antimicrobial urinary catheters against bacteriuria caused by E. coli in vitro. Urology. 2011;78(2):334–339. doi: 10.1016/j.urology.2011.02.063. [DOI] [PubMed] [Google Scholar]
- 93.Leone M. Prevention of CAUTI: simple is beautiful. The Lancet. 2012;380(9857):1891–1892. doi: 10.1016/S0140-6736(12)61515-3. [DOI] [PubMed] [Google Scholar]
- 94.Loo CY, Young PM, Lee WH, Cavaliere R, Whitchurch CB, Rohanizadeh R. Superhydrophobic, nanotextured polyvinyl chloride films for delaying Pseudomonas aeruginosa attachment to intubation tubes and medical plastics. Acta Biomater. 2012;8(5):1881–1890. doi: 10.1016/j.actbio.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 95.Kilonzo M, Vale L, Pickard R, Lam T, N'Dow J, Catheter Trial G. Cost effectiveness of antimicrobial catheters for adults requiring short-term catheterisation in hospital. Eur Urol. 2014;66(4):615–618. doi: 10.1016/j.eururo.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 96.Fisher LE, Hook AL, Ashraf W, Yousef A, Barrett DA, Scurr DJ, Chen X, Smith EF, Fay M, Parmenter CD, Parkinson R, Bayston R. Biomaterial modification of urinary catheters with antimicrobials to give long-term broadspectrum antibiofilm activity. J Control Release. 2015;202:57–64. doi: 10.1016/j.jconrel.2015.01.037. [DOI] [PubMed] [Google Scholar]
- 97.Salvarci A, Koroglu M, Gurpinar T. Evaluation of antimicrobial activities of minocycline and rifampin-impregnated silicone surfaces in an in vitro urinary system model. J Pak Med Assoc. 2015 [PubMed] [Google Scholar]
- 98.Nicolle LE. Catheter-related urinary tract infection: practical management in the elderly. Drugs Aging. 2014;31(1):1–10. doi: 10.1007/s40266-013-0089-5. [DOI] [PubMed] [Google Scholar]
- 99.Wollin TA, Tieszer C, Riddell JV, Denstedt JD, Reid G. Bacterial biofilm formation, encrustation, and antibiotic adsorption to ureteral stents indwelling in humans. Journal of endourology / Endourological Society. 1998;12(2):101–111. doi: 10.1089/end.1998.12.101. [DOI] [PubMed] [Google Scholar]
- 100.Ona KR, Courcelle CT, Courcelle J. Nucleotide Excision Repair Is a Predominant Mechanism for Processing Nitrofurazone-Induced DNA Damage in Escherichia coli. Journal of Bacteriology. 2009;191(15):4959–4965. doi: 10.1128/JB.00495-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hiraku Y, Sekine A, Nabeshi H, Midorikawa K, Murata M, Kumagai Y, Kawanishi S. Mechanism of carcinogenesis induced by a veterinary antimicrobial drug, nitrofurazone, via oxidative DNA damage and cell proliferation. Cancer Letters. 2004;215(2):141–150. doi: 10.1016/j.canlet.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 102.Wehrli W. Rifampin: Mechanisms of Action and Resistance. Review of Infectious Diseases. 1983;5(Supplement 3):S407–S411. doi: 10.1093/clinids/5.supplement_3.s407. [DOI] [PubMed] [Google Scholar]
- 103.Jayaraman R. Antibiotic resistance: an overview of mechanisms and a paradigm shift. Current Science. 2009;96(11) [Google Scholar]
- 104.Denton GW. Chlorhexidine, Disinfection, sterilization and preservation. 4th. Philadelphia: Lea and Febiger; 1991. pp. 274–289. [Google Scholar]
- 105.Jones CG. Chlorhexidine: is it still the gold standard? Periodontology 2000. 1997;15(1):55–62. doi: 10.1111/j.1600-0757.1997.tb00105.x. [DOI] [PubMed] [Google Scholar]
- 106.Dametto FR, Ferraz CCR, de Almeida Gomes BPF, Zaia AA, Teixeira FB, de Souza-Filho FJ. In vitro assessment of the immediate and prolonged antimicrobial action of chlorhexidine gel as an endodontic irrigant against Enterococcus faecalis, Oral Surgery. Oral Medicine, Oral Pathology, Oral Radiology, and Endodontology. 2005;99(6):768–772. doi: 10.1016/j.tripleo.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 107.Phuengkham H, Nasongkla N. Development of antibacterial coating on silicone surface via chlorhexidine-loaded nanospheres. J Mater Sci Mater Med. 2015;26(2):78. doi: 10.1007/s10856-015-5418-2. [DOI] [PubMed] [Google Scholar]
- 108.Wood NJ, Maddocks SE, Grady HJ, Collins AM, Barbour ME. Functionalization of ethylene vinyl acetate with antimicrobial chlorhexidine hexametaphosphate nanoparticles. Int J Nanomedicine. 2014;9:4145–4152. doi: 10.2147/IJN.S65343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Segev G, Bankirer T, Steinberg D, Duvdevani M, Shapur NK, Friedman M, Lavy E. Evaluation of Urinary Catheters Coated with Sustained-Release Varnish of Chlorhexidine in Mitigating Biofilm Formation on Urinary Catheters in Dogs. J Vet Intern Med. 2013;27:39–46. doi: 10.1111/j.1939-1676.2012.01027.x. [DOI] [PubMed] [Google Scholar]
- 110.Bhargava HN, Leonard PA. Triclosan: Applications and safety. American Journal of Infection Control. 1996;24(3):209–218. doi: 10.1016/s0196-6553(96)90017-6. [DOI] [PubMed] [Google Scholar]
- 111.Regos J, Hitz HR. Investigations on the mode of action of Triclosan, a broad spectrum antimicrobial agent. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Erste Abteilung Originale. Reihe A: Medizinische Mikrobiologie und Parasitologie. 1974;226(3):390–401. [PubMed] [Google Scholar]
- 112.Slater-Radosti C, Van Aller G, Greenwood R, Nicholas R, Keller PM, DeWolf WE, Fan F, Payne DJ, Jaworski DD. Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus. Journal of Antimicrobial Chemotherapy. 2001;48(1):1–6. doi: 10.1093/jac/48.1.1. [DOI] [PubMed] [Google Scholar]
- 113.Williams GJ, Stickler DJ. Effect of triclosan on the formation of crystalline biofilms by mixed communities of urinary tract pathogens on urinary catheters. Journal of Medical Microbiology. 2008;57(9):1135–1140. doi: 10.1099/jmm.0.2008/002295-0. [DOI] [PubMed] [Google Scholar]
- 114.Jones GL, Muller CT, O'Reilly M, Stickler DJ. Effect of triclosan on the development of bacterial biofilms by urinary tract pathogens on urinary catheters. Journal of Antimicrobial Chemotherapy. 2006;57(2):266–272. doi: 10.1093/jac/dki447. [DOI] [PubMed] [Google Scholar]
- 115.Gaonkar TA, Caraos L, Modak S. Efficacy of a Silicone Urinary Catheter Impregnated with Chlorhexidine and Triclosan Against Colonization With Proteus mirabilis and Other Uropathogens. Infection Control & Hospital Epidemiology. 2007;28(05):596–598. doi: 10.1086/513449. [DOI] [PubMed] [Google Scholar]
- 116.Darouiche RO, Mansouri MD, Gawande PV, Madhyastha S. Antimicrobial and antibiofilm efficacy of triclosan and DispersinB® combination. Journal of Antimicrobial Chemotherapy. 2009;64(1):88–93. doi: 10.1093/jac/dkp158. [DOI] [PubMed] [Google Scholar]
- 117.Gaonkar TA, Sampath LA, Modak SM. Evaluation of the Antimicrobial Efficacy of Urinary Catheters Impregnated With Antiseptics in an In Vitro Urinary Tract Model. Infection Control & Hospital Epidemiology. 2003;24(07):506–513. doi: 10.1086/502241. [DOI] [PubMed] [Google Scholar]
- 118.Jordan RPC, Malic S, Waters MG, Stickler DJ, Williams DW. Development of an antimicrobial urinary catheter to inhibit urinary catheter encrustation. Microbiology Discovery. 2015;3(1):1. [Google Scholar]
- 119.Epand RM, Vogel HJ. Diversity of antimicrobial peptides and their mechanisms of action. Biochimica et Biophysica Acta (BBA) - Biomembranes. 1999;1462(1–2):11–28. doi: 10.1016/s0005-2736(99)00198-4. [DOI] [PubMed] [Google Scholar]
- 120.Oren Z, Shai Y. Mode of action of linear amphipathic α-helical antimicrobial peptides. Peptide Science. 1998;47(6):451–463. doi: 10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 121.Shai Y. Mode of action of membrane active antimicrobial peptides. Peptide Science. 2002;66(4):236–248. doi: 10.1002/bip.10260. [DOI] [PubMed] [Google Scholar]
- 122.Mishra B, Basu A, Chua RRY, Saravanan R, Tambyah PA, Ho B, Chang MW, Leong SSJ. Site specific immobilization of a potent antimicrobial peptide onto silicone catheters: evaluation against urinary tract infection pathogens. Journal of Materials Chemistry B. 2014;2(12):1706. doi: 10.1039/c3tb21300e. [DOI] [PubMed] [Google Scholar]
- 123.Lim K, Chua RR, Bow H, Tambyah PA, Hadinoto K, Leong SS. Development of a catheter functionalized by a polydopamine peptide coating with antimicrobial and antibiofilm properties. Acta Biomater. 2015;15:127–138. doi: 10.1016/j.actbio.2014.12.015. [DOI] [PubMed] [Google Scholar]
- 124.Xiang L. School of Chemical and Biomedical Engineering. Singapore: Nanyang Technologocal University; 2013. The Production and Engineering of Antimicrobial Peptides And their Application in the coating of Urinary Catheters. [Google Scholar]
- 125.Li X, Li P, Saravanan R, Basu A, Mishra B, Lim SH, Su X, Tambyah PA, Leong SS. Antimicrobial functionalization of silicone surfaces with engineered short peptides having broad spectrum antimicrobial and salt-resistant properties. Acta Biomater. 2014;10(1):258–266. doi: 10.1016/j.actbio.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 126.Curtin JJ, Donlan RM. Using Bacteriophages To Reduce Formation of Catheter-Associated Biofilms by Staphylococcus epidermidis. Antimicrobial Agents and Chemotherapy. 2006;50(4):1268–1275. doi: 10.1128/AAC.50.4.1268-1275.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carson L, Gorman SP, Gilmore BF. The use of lytic bacteriophages in the prevention and eradication of biofilms of Proteus mirabilis and Escherichia coli. FEMS Immunology & Medical Microbiology. 2010;59(3):447–455. doi: 10.1111/j.1574-695X.2010.00696.x. [DOI] [PubMed] [Google Scholar]
- 128.Fu W, Forster T, Mayer O, Curtin JJ, Lehman SM, Donlan RM. Bacteriophage Cocktail for the Prevention of Biofilm Formation by Pseudomonas aeruginosa on Catheters in an In Vitro Model System. Antimicrobial Agents and Chemotherapy. 2010;54(1):397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liao KS, Lehman SM, Tweardy DJ, Donlan RM, Trautner BW. Bacteriophages are synergistic with bacterial interference for the prevention of Pseudomonas aeruginosa biofilm formation on urinary catheters. Journal of applied microbiology. 2012;113(6):1530–1539. doi: 10.1111/j.1365-2672.2012.05432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lehman SM, Donlan RM. Bacteriophage-mediated control of a two-species biofilm formed by microorganisms causing catheter-associated urinary tract infections in an in vitro urinary catheter model. Antimicrob Agents Chemother. 2015;59(2):1127–1137. doi: 10.1128/AAC.03786-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hedstrom L. Serine Protease Mechanism and Specificity. Chemical Reviews. 2002;102(12):4501–4524. doi: 10.1021/cr000033x. [DOI] [PubMed] [Google Scholar]
- 132.Longhi C, Scoarughi GL, Poggiali F, Cellini A, Carpentieri A, Seganti L, Pucci P, Amoresano A, Cocconcelli PS, Artini M, Costerton JW, Selan L. Protease treatment affects both invasion ability and biofilm formation in Listeria monocytogenes. Microbial Pathogenesis. 2008;45(1):45–52. doi: 10.1016/j.micpath.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 133.Kumar JK. Lysostaphin: an antistaphylococcal agent. Applied Microbiology and Biotechnology. 2008;80(4):555–561. doi: 10.1007/s00253-008-1579-y. [DOI] [PubMed] [Google Scholar]
- 134.Proctor VA, Cunningham FE, Fung DYC. The chemistry of lysozyme and its use as a food preservative and a pharmaceutical. C R C Critical Reviews in Food Science and Nutrition. 1988;26(4):359–395. doi: 10.1080/10408398809527473. [DOI] [PubMed] [Google Scholar]
- 135.Oulahal-Lagsir N, Martial-Gros A, Bonneau M, Blum LJ. "Escherichia coli-milk" biofilm removal from stainless steel surfaces: synergism between ultrasonic waves and enzymes. Biofouling. 2003;19(3):159–168. doi: 10.1080/08927014.2003.10382978. [DOI] [PubMed] [Google Scholar]
- 136.Kaplan JB, LoVetri K, Cardona ST, Madhyastha S, Sadovskaya I, Jabbouri S, Izano EA. Recombinant human DNase I decreases biofilm and increases antimicrobial susceptibility in staphylococci. J Antibiot. 2012;65(2):73–77. doi: 10.1038/ja.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Borysowski J, Weber-Dabrowska B, Gorski A. Bacteriophage endolysins as a novel class of antibacterial agents. Experimental biology and medicine (Maywood, N.J.) 2006;231(4):366–377. doi: 10.1177/153537020623100402. [DOI] [PubMed] [Google Scholar]
- 138.Finnegan M, Linley E, Denyer SP, McDonnell G, Simons C, Maillard J-Y. Mode of action of hydrogen peroxide and other oxidizing agents: differences between liquid and gas forms. Journal of Antimicrobial Chemotherapy. 2010;65(10):2108–2115. doi: 10.1093/jac/dkq308. [DOI] [PubMed] [Google Scholar]
- 139.Thallinger B, Argirova M, Lesseva M, Ludwig R, Sygmund C, Schlick A, Nyanhongo GS, Guebitz GM. Preventing microbial colonisation of catheters: antimicrobial and antibiofilm activities of cellobiose dehydrogenase. International journal of antimicrobial agents. 2014;44(5):402–408. doi: 10.1016/j.ijantimicag.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 140.Nyanhongo GS, Sygmund C, Ludwig R, Prasetyo EN, Guebitz GM. An antioxidant regenerating system for continuous quenching of free radicals in chronic wounds. European Journal of Pharmaceutics and Biopharmaceutics. 2013;83(3):396–404. doi: 10.1016/j.ejpb.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 141.Tenovuo J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: efficacy and safety. Oral Diseases. 2002;8(1):23–29. doi: 10.1034/j.1601-0825.2002.1o781.x. [DOI] [PubMed] [Google Scholar]
- 142.Pearson JP, Van Delden C, Iglewski BH. Active efflux and diffusion are involved in transport of Pseudomonas aeruginosa cell-to-cell signals. J Bacteriol. 1999;181(4):1203–1210. doi: 10.1128/jb.181.4.1203-1210.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cabral J, Novais J, Kennedy J. Immobilization studies of whole microbial cells on transition metal activated inorganic supports. Applied microbiology and biotechnology. 1986;23(3–4):157–162. [Google Scholar]
- 144.Roig M. Immobilised cells and enzymes—A practical approach: Edited by J Woodward. pp 177. IRL Press, Oxford. 1985.£ 13. ISBN-947946-21-7. Biochemical Education. 1986;14(2):91–91. [Google Scholar]
- 145.Thallinger B, Brandauer M, Burger P, Sygmund C, Ludwig R, Ivanova K, Kun J, Scaini D, Burnet M, Tzanov T, Nyanhongo GS, Guebitz GM. Cellobiose dehydrogenase functionalized urinary catheter as novel antibiofilm system. J Biomed Mater Res B Appl Biomater. 2015 doi: 10.1002/jbm.b.33491. [DOI] [PubMed] [Google Scholar]
- 146.Lipovsky A, Thallinger B, Perelshtein I, Ludwig R, Sygmund C, Nyanhongo GS, Guebitz GM, Gedanken A. Ultrasound coating of polydimethylsiloxanes with antimicrobial enzymes. Journal of Materials Chemistry B. 2015;3(35):7014–7019. doi: 10.1039/c5tb00671f. [DOI] [PubMed] [Google Scholar]
- 147.Nathan CF, Hibbs JB. Role of nitric oxide synthesis in macrophage antimicrobial activity. Current Opinion in Immunology. 1991;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- 148.Yamamoto T, Bing RJ. Nitric Oxide Donors. Proceedings of the Society for Experimental Biology and Medicine. 2000;225(3):200–206. doi: 10.1046/j.1525-1373.2000.22525.x. [DOI] [PubMed] [Google Scholar]
- 149.Fang FC. Perspectives series: host/pathogen interactions. Mechanisms of nitric oxide-related antimicrobial activity. Journal of Clinical Investigation. 1997;99(12):2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fang FC. Antimicrobial actions of nitric oxide. Nitric oxide : biology and chemistry/official journal of the Nitric Oxide Society. 2012;27(Supplement):S10. [Google Scholar]
- 151.Brisbois EJ, Handa H, Major TC, Bartlett RH, Meyerhoff ME. Long-term nitric oxide release and elevated temperature stability with S-nitroso-N-acetylpenicillamine (SNAP)-doped Elast-eon E2As polymer. Biomaterials. 2013;34(28):6957–6966. doi: 10.1016/j.biomaterials.2013.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Nathan C. Nitric oxide as a secretory product of mammalian cells. The FASEB Journal. 1992;6(12):3051–3064. [PubMed] [Google Scholar]
- 153.Nablo BJ, Chen T-Y, Schoenfisch MH. Sol−Gel Derived Nitric-Oxide Releasing Materials that Reduce Bacterial Adhesion. Journal of the American Chemical Society. 2001;123(39):9712–9713. doi: 10.1021/ja0165077. [DOI] [PubMed] [Google Scholar]
- 154.Halpenny GM, Mascharak PK. Emerging Antimicrobial Applications of Nitric Oxide (NO) and NO-Releasing Materials. Anti-Infective Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry - Anti-Infective Agents) 2010;9(4):187–197. [Google Scholar]
- 155.Brisbois EJ, Bayliss J, Wu J, Major TC, Xi C, Wang SC, Bartlett RH, Handa H, Meyerhoff ME. Optimized polymeric film-based nitric oxide delivery inhibits bacterial growth in a mouse burn wound model. Acta Biomaterialia. 2014;10(10):4136–4142. doi: 10.1016/j.actbio.2014.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Brisbois EJ, Davis RP, Jones AM, Major TC, Bartlett RH, Meyerhoff ME, Handa H. Reduction in Thrombosis and Bacterial Adhesion with 7 Day Implantation of -Nitroso--acetylpenicillamine (SNAP)-Doped Elast-eon E2As Catheters in Sheep. J Mater Chem B Mater Biol Med. 2015;3(8):1639–1645. doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Brisbois EJ, Handa H, Meyerhoff ME. Recent Advances in Hemocompatible Polymers for Biomedical Applications. In: Puoci F, editor. Advanced Polymers in Medicine. Switzerland: Springer International Publishing Switzerland; 2015. [Google Scholar]
- 158.Handa H, Brisbois EJ, Major TC, Refahiyat L, Amoako KA, Annich GM, Bartlett RH, Meyerhoff ME. In vitro and in vivo study of sustained nitric oxide release coating using diazeniumdiolate-doped poly(vinyl chloride) matrix with poly(lactide-co-glycolide) additive. Journal of Materials Chemistry B. 2013;1(29):3578–3587. doi: 10.1039/C3TB20277A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Handa H, Major TC, Brisbois EJ, Amoako KA, Meyerhoff ME, Bartlett RH. Hemocompatibility comparison of biomedical grade polymers using rabbit thrombogenicity model for preparing nonthrombogenic nitric oxide releasing surfaces. Journal of Materials Chemistry B. 2014;2(8):1059–1067. doi: 10.1039/C3TB21771J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Colletta A, Wu J, Wo Y, Kappler M, Chen H, Xi C, Meyerhoff ME. S-Nitroso-N-acetylpenicillamine (SNAP) Impregnated Silicone Foley Catheters: A Potential Biomaterial/Device To Prevent Catheter-Associated Urinary Tract Infections. ACS Biomaterials Science & Engineering. 2015;1(6):416–424. doi: 10.1021/acsbiomaterials.5b00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Chen S, Zheng J, Li L, Jiang S. Strong resistance of phosphorylcholine self-assembled monolayers to protein adsorption: insights into nonfouling properties of zwitterionic materials. Journal of the American Chemical Society. 2005;127(41):14473–14478. doi: 10.1021/ja054169u. [DOI] [PubMed] [Google Scholar]
- 162.Bernstein R, Belfer S, Freger V. Bacterial attachment to RO membranes surface-modified by concentration-polarization-enhanced graft polymerization. Environmental science & technology. 2011;45(14):5973–5980. doi: 10.1021/es1043694. [DOI] [PubMed] [Google Scholar]
- 163.Harding JL, Reynolds MM. Combating medical device fouling. Trends Biotechnol. 2014;32(3):140–146. doi: 10.1016/j.tibtech.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 164.Mi L, Jiang S. Integrated Antimicrobial and Nonfouling Zwitterionic Polymers. Angewandte Chemie International Edition. 2014;53(7):1746–1754. doi: 10.1002/anie.201304060. [DOI] [PubMed] [Google Scholar]
- 165.Zwaal R, Comfurius P, Van Deenen L. Membrane asymmetry and blood coagulation. 1977 doi: 10.1038/268358a0. [DOI] [PubMed] [Google Scholar]
- 166.Kojima M, Ishihara K, Watanabe A, Nakabayashi N. Interaction between phospholipids and biocompatible polymers containing a phosphorylcholine moiety. Biomaterials. 1991;12(2):121–124. doi: 10.1016/0142-9612(91)90189-h. [DOI] [PubMed] [Google Scholar]
- 167.Ueda T, Oshida H, Kurita K, Ishihara K, Nakabayashi N. Preparation of 2-methacryloyloxyethyl phosphorylcholine copolymers with alkyl methacrylates and their blood compatibility. POLYMER JOURNAL-TOKYO. 1992;24:1259–1259. [Google Scholar]
- 168.Hayward JA, Chapman D. Biomembrane surfaces as models for polymer design: the potential for haemocompatibility. Biomaterials. 1984;5(3):135–142. doi: 10.1016/0142-9612(84)90047-4. [DOI] [PubMed] [Google Scholar]
- 169.Bird RlR, Hall B, Chapman D, Hobbs K. Material thrombelastography: An assessment of phosphorylcholine compounds as models for biomaterials. Thrombosis research. 1988;51(5):471–483. doi: 10.1016/0049-3848(88)90113-2. [DOI] [PubMed] [Google Scholar]
- 170.Iwasaki Y, Kurita K, Ishihara K, Nakabayashi N. Effect of methylene chain length in phospholipid moiety on blood compatibility of phospholipid polymers. Journal of Biomaterials Science, Polymer Edition. 1995;6(5):447–461. doi: 10.1163/156856294x00437. [DOI] [PubMed] [Google Scholar]
- 171.Ishihara K, Nomura H, Mihara T, Kurita K, Iwasaki Y, Nakabayashi N. Why do phospholipid polymers reduce protein adsorption? 1997 doi: 10.1002/(sici)1097-4636(199802)39:2<323::aid-jbm21>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 172.Cheng G, Zhang Z, Chen S, Bryers JD, Jiang S. Inhibition of bacterial adhesion and biofilm formation on zwitterionic surfaces. Biomaterials. 2007;28(29):4192–4199. doi: 10.1016/j.biomaterials.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Stickler DJ, Evans A, Morris N, Hughes G. Strategies for the control of catheter encrustation. International Journal of Antimicrobial Agents. 2002;19(6):499–506. doi: 10.1016/s0924-8579(02)00091-2. [DOI] [PubMed] [Google Scholar]
- 174.Russell JC. Bacteria, Biofilms, and Devices: The Possible Protective Role of Phosphorylcholine Materials. Journal of Endourology. 2000;14(1):39–42. doi: 10.1089/end.2000.14.39. [DOI] [PubMed] [Google Scholar]
- 175.Diaz Blanco C, Ortner A, Dimitrov R, Navarro A, Mendoza E, Tzanov T. Building an antifouling zwitterionic coating on urinary catheters using an enzymatically triggered bottom-up approach. ACS Appl Mater Interfaces. 2014;6(14):11385–11393. doi: 10.1021/am501961b. [DOI] [PubMed] [Google Scholar]
- 176.Green J-BD, Fulghum T, Nordhaus MA. Immobilized antimicrobial agents: A critical perspective. Chem. Rev. 2009;109(11):5437–5527. [Google Scholar]
- 177.Green J-BD, Fulghum T, Nordhaus MA. A review of immobilized antimicrobial agents and methods for testing. Biointerphases. 2011;6(4):MR13–MR28. doi: 10.1116/1.3645195. [DOI] [PubMed] [Google Scholar]
- 178.Hamming LM, Messersmith PB. Fouling Resistant Biomimetic Poly(Ethylene Glycol)Based Grafted Polymer Coatings. Material Matters. 2008;3.3(52) [Google Scholar]
- 179.Nowatzki PJ, Koepsel RR, Stoodley P, Min K, Harper A, Murata H, Donfack J, Hortelano ER, Ehrlich GD, Russell AJ. Salicylic acid-releasing polyurethane acrylate polymers as antibiofilm urological catheter coatings. Acta Biomater. 2012;8(5):1869–1880. doi: 10.1016/j.actbio.2012.01.032. [DOI] [PubMed] [Google Scholar]
- 180.Sun X, Cao Z, Porteous N, Sun Y. An N-halamine-based rechargeable antimicrobial and biofilm controlling polyurethane. Acta Biomater. 2012;8(4):1498–1506. doi: 10.1016/j.actbio.2011.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Goncalves I, Abreu AS, Matama T, Ribeiro A, Gomes AC, Silva C, Cavaco-Paulo A. Enzymatic synthesis of poly(catechin)-antibiotic conjugates: an antimicrobial approach for indwelling catheters. Appl Microbiol Biotechnol. 2015;99(2):637–651. doi: 10.1007/s00253-014-6128-2. [DOI] [PubMed] [Google Scholar]
- 182.MacPhee R, Koepsel J, Welch I, Dalsin J, Tailly T, Cadieux P, Burton J, Razvi H. Mp8-18 Use of Novel Antimicrobial Coatings on Urinary Catheters for Prevention of E. Coli Infection in a Rabbit Model. The Journal of Urology. 2014;191(4):e81. [Google Scholar]
- 183.Russo JL. Biomedical Engineering. UConn; 2014. Inhibition of Urinary Tract Infections and Biofilm Formation on Long-term Indwelling Urethral Catheters through Antibacterial Coatings. [Google Scholar]
- 184.Worley SD, Williams DE, Crawford RA. Halamine water disinfectants. Critical Reviews in Environmental Control. 1988;18(2):133–175. [Google Scholar]
- 185.Rooijen NV, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. Journal of Immunological Methods. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 186.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, Samiei M, Kouhi M, Nejati-Koshki K. Liposome: classification, preparation, and applications. Nanoscale Research Letters. 2013;8(1):102–102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Lasic DD, Papahadjopoulos D. Medical applications of liposomes. Elsevier; 1998. [Google Scholar]
- 188.DiTizio V, Ferguson GW, Mittelman MW, Khoury AE, Bruce AW, DiCosmo F. A liposomal hydrogel for the prevention of bacterial adhesion to catheters. Biomaterials. 1998;19(20):1877–1884. doi: 10.1016/s0142-9612(98)00096-9. [DOI] [PubMed] [Google Scholar]
- 189.Pugach JL, DiTizio V, Mittelman MW, Bruce AW, DiCosmo F, Khoury AE. Antibiotic hydrogel coated Foley catheters for prevention of urinary tract infection in a rabbit model. The Journal of Urology. 1999;162(3, Part 1):883–887. doi: 10.1097/00005392-199909010-00084. [DOI] [PubMed] [Google Scholar]
- 190.Hu R, Li G, Jiang Y, Zhang Y, Zou J-J, Wang L, Zhang X. Silver–Zwitterion Organic–Inorganic Nanocomposite with Antimicrobial and Antiadhesive Capabilities. Langmuir. 2013;29(11):3773–3779. doi: 10.1021/la304708b. [DOI] [PubMed] [Google Scholar]
- 191.Díaz C, Miñán A, Schilardi P, de Mele MFL. Synergistic antimicrobial effect against early biofilm formation: micropatterned surface plus antibiotic treatment. International journal of antimicrobial agents. 2012;40(3):221–226. doi: 10.1016/j.ijantimicag.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 192.Reddy ST, Chung KK, McDaniel CJ, Darouiche RO, Landman J, Brennan AB. Micropatterned surfaces for reducing the risk of catheter-associated urinary tract infection: an in vitro study on the effect of sharklet micropatterned surfaces to inhibit bacterial colonization and migration of uropathogenic Escherichia coli. Journal of endourology. 2011;25(9):1547–1552. doi: 10.1089/end.2010.0611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Zolfaghari PS, Packer S, Singer M, Nair SP, Bennett J, Street C, Wilson M. In vivo killing of Staphylococcus aureus using a light-activated antimicrobial agent. BMC microbiology. 2009;9(1):27. doi: 10.1186/1471-2180-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Gliga AR, Skoglund S, Odnevall Wallinder I, Fadeel B, Karlsson HL. Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Particle and Fibre Toxicology. 2014;11(1):11. doi: 10.1186/1743-8977-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Reitzel R, Rosenblatt J, Jiang Y, Hachem R, Raad I. Disposable gendine antimicrobial gloves for preventing transmission of pathogens in health care settings. Am J Infect Control. 2014;42(1):55–59. doi: 10.1016/j.ajic.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 196.Guggenbichler J-P, Böswald M, Lugauer S, Krall D-IT. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection. 1999;27(1):S16–S23. doi: 10.1007/BF02561612. [DOI] [PubMed] [Google Scholar]
- 197.Nouri S, Sharif MR, Hosseinpour M, Farokhi S, Sharif MH. A Comparison Between Foley and Nelatone Urinary Catheters in Causing Urinary Tract Infection in Animal Models. Nurs Midwifery Stud. 2015;4(1) [PMC free article] [PubMed] [Google Scholar]