Abstract
The advent of precision medicine in non-small cell lung cancer has remarkably altered the direction of research and improved clinical outcomes. The identification of molecular subsets with differential response to targeted therapies began with the identification of epidermal growth factor receptor mutated tumors in subsets of non-small cell lung cancer (NSCLC). Emboldened by unprecedented response rates to kinase inhibitors seen in that subset, the oncologic community searched for other molecular subsets featuring oncogene addiction. An early result of this search was the discovery of NSCLC driven by activating rearrangements of the anaplastic lymphoma kinase (ALK) gene. In an astoundingly brief period following the recognition of ALK-positive NSCLC, details of the biology, clinicopathologic features, development of targeted inhibitors, mechanisms of therapeutic resistance, and new generations of treatment were elucidated. This review summarizes the current understanding of the pathologic features, diagnostic approach, treatment options, resistance mechanisms, and future research areas for ALK-positive NSCLC.
Keywords: adenocarcinoma, crizotinib, oncogene addiction, personalized medicine, precision medicine, targeted therapy
Introduction
Lung cancer accounts for more than a quarter of all cancer-related morality [1]. The identification of molecular subsets of lung cancer with targetable driver mutations has altered the landscape of treatment. Advanced lung cancers that harbor druggable oncogenic alterations are highly responsive to molecularly targeted therapies. Thus, discerning tumor-specific, oncogenic driver mutations from passenger mutations has become a concerted effort in the clinical and research oncology communities, ushering in the era of precision medicine. In 2007, one such molecular alteration, activating fusions of the anaplastic lymphoma kinase (ALK) gene, was discovered in a subset of patients with non-small cell lung cancer (NSCLC) [2]. Only three years later, the first-generation ALK inhibitor crizotinib was found to have a response rate of 57% and 6-month progression free survival of 72% in patients with previously treated advanced NSCLC harboring ALK rearrangement confirmed by fluorescence in situ hybridization (FISH) [3]. In the ensuing years, translational research has yielded further insight into the biology, epidemiology, clinical features, alternative therapies, and mechanisms of resistance in ALK-rearranged NSCLC. Here, we review the current understanding and future directions of biology, epidemiology, clinicopathology, therapy, mechanisms of resistance, and strategies to counter resistance in ALK-rearranged NSCLC.
Biology of ALK-rearranged NSCLC
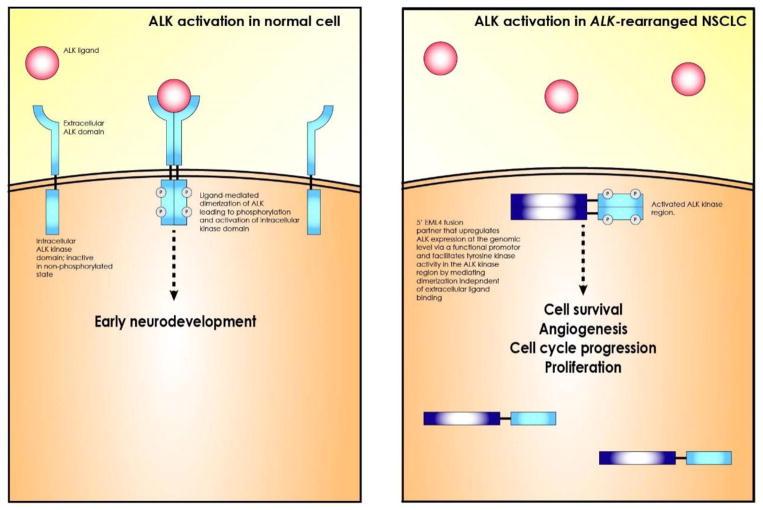
The ALK gene is located on chromosome 2 and encodes a transmembrane tyrosine kinase that is normally restricted at low levels to the small intestine, nervous system, and testes in adult humans [4, 5]. It is developmentally regulated and appears to play a role in neurodevelopment based on studies in mice and Drosophila [6, 7]. Normal activation of ALK is mediated by extracellular, ligand-induced dimerization (Figure 1) [8]. Molecular alterations leading to heightened ALK activation have been implicated in several cancers including non-Hodgkin’s lymphoma, rhabdomyosarcomas, renal cell carcinoma, thyroid cancer, neuroblastoma, and NSCLC [6].
Figure 1.
ALK biology and oncogenesis. Activation of ALK is developmentally regulated and sparse in adult tissue. It appears to be involved in neurodevelopment and is largely restricted to the gut, CNS, and testes in adulthood. Native ALK signaling occurs via extracellular ligand-mediated dimerization of ALK and subsequent autophosphorylation and activation of the intracellular tyrosine kinase domain. The native ligands that bind to and activate ALK have remained unknown, although recent evidence suggests that heparin may be one [74, 75]. In ALK-rearranged NSCLC, ALK activation occurs independently of ligand-mediation. The 5′ fusion partner of ALK provides a functional promotor that escapes normal ALK regulation and expresses a domain in the functional protein that facilitates dimerization. In this way, the kinase domains of separate ALK proteins, which are entirely intracellular in ALK-rearranged cells, are brought into proximity for autophosphorylation. This ultimately results in downstream activation of signaling pathways that enhance cell survival, angiogenesis, cell survival and cell cycle progression [10]. Implicated pathways include STAT3, mTOR, PI3K, Ras, and MEK.
Activating alterations of ALK obviate ligand-dependence, render ALK constitutively active via hyperphosphorylation and/or overexpression, lead to downstream activation of proliferative and anti-apoptotic signals via intracellular pathways (including STAT3, PI3K, mTOR, and MEK), and culminate in oncogenesis [6, 9, 10]. In ALK-activated NSCLC, the predominant molecular event leading to ALK activation is juxtaposition of the N-terminal portion of the protein encoded by the echinoderm microtubule-associated protein like 4 (EML4) gene with the intracellular domain of the ALK tyrosine kinase [2]. Several variants of EML4-ALK rearrangements have been identified. These resulting fusion proteins promote oncogenesis via constitutive activation of downstream pathways (Figure 1). Less commonly, other fusion partners, including KIF5B, and intrinsic activating mutations of ALK have been described [11]. In other malignancies such as diffuse large B cell lymphoma and inflammatory myofiboblastic tumors, other ALK 5′ fusion partners, including Ran-binding protein 2 (RANBP2) and Clathrin, have been described [12, 13]. To date, the EML4-ALK fusion appears unique to NSCLC [6, 14].
Clinicopathologic features
ALK-rearranged NSCLC comprises 2% to 5% of all NSCLC cases [2]. Compared to patients with pan-wild-type NSCLC (i.e. patients who have NSCLC without a known targetable, driver oncogene such as EGFR or ALK), patients who harbor ALK-activated NSCLC tend to be light or never smokers (100% vs 42%), younger (median age 52 years vs 64 years, P=0.005), male (58% vs 32%, P=0.039) [15–17], and at more advanced stage (89% vs 58% with stage IV disease (P=0.051). Histologically, ALK-rearranged NSCLC cases are more likely to be adenocarcinoma histology, in particular signet-ring cell type with abundant intracellular mucin. Molecularly, ALK alterations appear to be mutually exclusive of EGFR and KRAS mutations [2, 15, 16, 18, 19].
Screening
Given the relative infrequency of ALK-rearranged NSCLC, the optimal approach to screening for these cases has received considerable attention [6]. If all advanced NSCLC cases are screened, only 1.6% would be ALK-positive. Employing the screening recommendations of the College of American Pathologists and the Association for Molecular Pathology, if only adenocarcinoma cases are screened (approximately 40% of advanced NSCLC), the rate of ALK-positivity increases to 4%. However, an estimated 13% of ALK-positive cases would be missed. The National Comprehensive Cancer Network recommends screening the slightly broader population of non-squamous histology (which includes large cell, NSCLC not otherwise specified, and other rare histologic subtypes in addition to adenocarcinoma). Restricting screening to adenocarcinoma cases in never smokers (an estimated 6% of advanced NSCLC), the rate of ALK-positivity increases to 14%, but up to 50% of cases are missed. If one were to further enrich advanced NSCLC for ALK-positivity by limiting testing only to those adenocarcinoma cases in never smokers that harbor neither EGFR nor KRAS mutations (representing 2% of advanced NSCLC), an estimated 36% of cases will be ALK-positive, but approximately 55% of ALK-positive cases may be missed.
Methods of testing
Accurate, reproducible, and widely-accessible testing for ALK alterations is essential for clinically meaningful identification and targeting of molecular subtypes. Available assays include fluorescence in situ hybridization (FISH), immunohistochemistry (IHC), reverse transcription polymerase chain reaction (RT-PCR), and DNA sequencing [20–23]. FISH is the most commonly performed and is approved by the United States Food and Drug Administration (FDA). The assay employs uniquely labeled split-signal probes on the 5′ and 3′ termini of ALK. Wild-type ALK appears as a fused signal whereas ALK rearrangements appear as separately colored signals (e.g. fused yellow signal in wild-type and separate red and green signal in EML4-ALK rearrangement) [24]. Sensitivity and specificity with this technique approach 100% using thresholds of >15% of cells and examination of 4+ fields. However, there are challenges to large-scale FISH screening, including cost, equipment requirements, labor training, and time intensiveness.
Immunohistochemistry, due to its low cost, wide availability, and ease of use relative to FISH, has been proposed as an alternative initial screening test and is now FDA-approved [25–27]. IHC was initially fraught with use-limiting insensitive antibodies but newer antibodies (i.e. 5A4 by Novocastra and D5F3 by Cell Signaling Technology with ADVANCE) have demonstrated sensitivities of 96–100% [25, 28]. IHC has been employed as the enrollment biomarker in some clinical trials and has been shown in some case reports to identify ALK-inhibitor responsive tumors that were FISH-negative for ALK rearrangements [22]. Nevertheless, various IHC platforms have differing sensitivity and specificity (Table 1) [29]. It has been proposed IHC and FISH complement one another and that combined testing may enhance the detection of ALK-positive cases [30, 31].
Table 1.
Sensitivity and specificity of FISH by and IHC for the detection of ALK rearrangement in NSCLC cancer by technique and probe
| Screening Test | Sensitivity (%) | Specificity (%) | |
|---|---|---|---|
| FISH | Number of tumor areas examined under high-power microscopy fields | ||
| 2 | 98.6 | 96.6 | |
| 3 | 99.3 | 100 | |
| 4 | 100 | 100 | |
| IHC | Probe and Detection System | ||
| 5A4 by Novocastra with ADVANCE [25] | 100 | 87.5 | |
| 5A4 by Nichirei with Histofine [25] | 100 | 62.5 | |
| D5F3 by Cell Signaling with ADVANCE [25] | 100 | 75 | |
| ALK1 by DAKO with FLEX [25] | 66 | 100 | |
| ALK1 by DAKO with ADVANCE [25] | 66 | 87.5 | |
| 5A4 by Novocastra with i-view [27] | 100 | 95.8 | |
| 5A4 by Novocastra with BenchMark XT [28] | 96 | 100 | |
| D5F3 by Cell Signaling BenchMark XT [28] | 96 | 100 | |
| 5A4 by Novocastra with Bond-MAX [28] | 96 | 100 | |
| 5A4 by Abcam with Bond-MAX and UltraView Universal DAB [31] | 69 | 99.3 | |
| 5A4 by Novocastra with Envision Plus [76] | 93 | 100 | |
| 5A4 by Abcam with Benchmark Ventana [77] | 95 | 100 | |
| D5F3 by Ventana with Optiview [78] | 98 | 100 | |
| 5A4 by Novocastra with Optiview [78] | 98 | 100 |
Abbreviations: FISH, fluorescence in situ hybridization; IHC, immunohistochemistry
T-PCR-based techniques are available for both the detection of ALK rearrangements [29, 32] and for the quantification of the ALK kinase domain, capitalizing on low expression in normal lung tissue relative to ALK-altered tumors. The former relies on specific messenger RNA transcripts and are limited as such. Next generation sequencing has also demonstrated the ability to detect a subset of ALK-rearranged NSCLC not detected by FISH [33, 34] and may have a role in future diagnostics.
Efficacy of first- and second- line crizotinib compared to chemotherapy on ALK-positive NSCLC
Pre-clinical evaluation of ALK inhibitors and subsequent clinical translation developed rapidly after the initial identification of the EML4-ALK rearrangement [2, 35, 36]. This expeditious advancement was aided by previous experience with other tyrosine kinase inhibitors (TKI) and the fact that crizotinib, initially developed as a MET inhibitor but subsequently found to inhibit ALK as well, was already under clinical development [37]. Crizotinib, the first approved targeted therapy for ALK-positive NSCLC, is an oral small-molecule TKI that inhibits ALK, MET, and ROS1 [17, 38, 39]. Crizotinib yielded promising outcomes comparable to EGFR inhibitors in EGFR-mutant NSCLC (Tables 2 and 3). In the first-line setting, crizotinib had an overall response rate (RR) of 74% and median progression-free survival (PFS) of 11 months, which was superior to standard first-line platinum-pemetrexed chemotherapy (RR of 45% and median PFS of 7 months) [40]. In the second-line setting, crizotinib demonstrated similarly impressive superiority over conventional chemotherapy with a RR of 65% versus 20% and median PFS of 8 months versus 3 months [41].
Table 2.
Published clinical trial outcomes in ALK-rearranged NSCLC
| Clinicaltrials.gov Identifier | Trial | Study drug | Trial Phase | Description | RR (%) | Median PFS (months) |
|---|---|---|---|---|---|---|
| NCT00585195 | PROFILE 1001 [79] | crizotinib | 1/2 | Safety of crizotinib in ALK-rearranged NSCLC, mostly in the second line and beyond | 57 | 9.7 |
| NCT00932451 | PROFILE 1005 [80] | crizotinib | 2 | Activity of crizotinib in ALK-rearranged NSCLC | 53 | 8.5 |
| crizotinib | 3 | Outcomes of ALK-rearranged NSCLC treatment in the second line | 65 | 7.7 | ||
| NCT00932893 | PROFILE 1007 [41] | docetaxel | 3 | Outcomes of ALK-rearranged NSCLC treatment in the second line | 6 | 2.6 |
| pemetrexed | 3 | Outcomes of ALK-rearranged NSCLC treatment in the second line | 29 | 4.2 | ||
| NCT01154140 | PROFILE 1014 [40] | crizotinib | 3 | Outcomes of ALK-rearranged NSCLC treatment in the first line | 74 | 10.9 |
| Platinum-based chemotherapy | 3 | Outcomes of ALK-rearranged NSCLC treatment in the first line | 45 | 7.0 | ||
| NCT01283516 | ASCEND-1 [55, 81] | ceritinib | 1 | Efficacy and safety of ceritinib in ALK-rearranged NSCLC in patients who are ALK inhibitor-naïve | 72% in ALK inhibitor-naïve and 56% in ALK inhibitor-pretreated | 18.4 months in ALK inhibitor-naïve and 6.9 months in ALK inhibitor-pretreated |
| JapicCTI*-101264 | AF-001JP [60] | alectinib | 1/2 | Activity and safety of ceritinib in ALK-rearranged NSCLC in ALK inhibitor-naïve patients | 94 | n/a |
| NCT01801111 | NP28673 trial [82] | alectinib | 2 | Activity of alectinib in crizotinib-refractory ALK-rearranged NSCLC | 50 | 8.9 |
| NCT01871805 | Alectinib in ALK - positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicenter, phase 2 trial [83] | alectinib | 2 | Activity of alectinib in crizotinib-refractory ALK-rearranged NSCLC | 48 | 8.1 |
| JapicCTI*-132316 | J-ALEX [84] | alectinib | 3 | Alectinib versus crizotinib in treatment-naive ALK rearranged advanced NSCLC | Not reported | Not reached; PFS HR of alectinib arm to crizotinib arm was 0.35 99.6826% CI: 0.17–0.70, stratified log-rank p<0.0001) |
| crizotinib | 3 | Alectinib versus crizotinib in treatment-naive ALK rearranged advanced NSCLC | Not reported | 10.2 | ||
| NCT01449461 | Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC) [85]. | brigatinib | 1/2 | Safety and efficacy of brigatinib in ALK rearranged NSCLC | 74 (69 in crizotinib-treated subset and 100 in crizotinib-naïve subset) | 14 (11.8 in crizotinib-pre-treated subset) |
| NCT00431015 | Activity of IPI-504, a Novel Heat-Shock Protein 90 Inhibitor, in Patients With Molecularly Defined Non–Small-Cell Lung Cancer [66, 86] | IPI-504 | 2 | Activity of IPI-504 in molecularly defined NSCLC | only 3 patients (partial response in 2 and stable disease in 1) | n/a |
| NCT01031225 | A Multicenter Phase II Study of Ganetespib Monotherapy in Patients with Genotypically Defined Advanced Non–Small Cell Lung Cancer [67] | ganetespib | 2 | Activity and tolerability of ganetespib in the second line in NSCLC | 50 | 8.1 |
Registered with Japan Pharmaceutical Information Center
Abbreviations: ALK, anaplastic lymphoma kinase; NSCLC, non-small cell lung cancer
Table 3.
ALK inhibitor characteristics: dosing, toxicity, IC50, and additional molecular targets
| Drug | Standard Dose | Toxicities | ALK IC50 | Additional Targets |
|---|---|---|---|---|
| Crizotinib [39–41, 87] | 250 mg twice daily (with or without food) | Visual effects (60%), nausea (45%), diarrhea (40%), edema (25%), constipation (25%), decreased appetite (18%), fatigue (15%) | 24 nM | MET, ROS1, VEGFR2, PDGFRβ, IRK, Lck, Tie2, TrkA, TrkB, RON, Axl |
| Ceritinib [55, 57, 81] | 750 mg (five 150-mg capsules) daily (without food) | Nausea (82%; 5% Gr 3–4), diarrhea (75%; 7% Gr 3–4), vomiting (65%; 5% Gr 3–4), fatigue (47%; 5% Gr 3–4), ↑ ALT (35%; 21% Gr 3–4), constipation (32%), abdominal pain (30%), decreased appetite (29%), ↑ AST (25%; 11% Gr 3–4) | 200 pM | IGF1-R, INSR, STK22D |
| Alectinib [56, 59, 88] | 600 mg twice daily | Fatigue (30%), myalgia (17%), edema (15%), ↑ CPK (15%), nausea (15%), ↑ ALT (13%), photosensitivity (13%), constipation (11%) | 3 nM | LTK, GAK |
| Brigatinib (AP26113) [85, 89] | Standard dose not yet established (30–300 mg daily) | nausea (45%), diarrhea (36%), fatigue (36%), cough (26%), headache (26%), Early-onset pulmonary events, observed ≤7 d after starting treatment, included dyspnea, hypoxia, or new pulmonary opacities on chest computed tomography suggestive of pneumonia or pneumonitis (9%) | 0.37 nM | FLT3, ROS1, IGF1E, INSR |
| Entrectinib (RXDX-101) [86, 90, 91] | Standard dose not yet established (800 mg/m2 used in ALK rearranged NSCLC) | paraesthesia (42%) nausea (37%), myalgia (34%), asthenia (27%), dysgeusia (27%), vomiting (21%), arthralgia (19%) and diarrhoea (19%) | 12 nM | ROS1, Pan-TRK |
| Loratinib (PF-06463922) [92–94] | Standard dose not yet established (10–200 mg daily) | hypercholesterolemia (48%), peripheral oedema (23%) and peripheral neuropathy (21%) | 1.3 nM | ROS1, pan-TRK, LTK, FER, FRK, PTK1, PTK2B, FES |
| Belizatinib (TSR-011) [95] | No standard dose (30 to 480 mg total per daily, administered 1,2, or 3 times daily) | fatigue (26.1%), diarrhea (21.7%), QTc prolongation (21.7%), headache (17.4%), decreased appetite (15.2%), urinary tract infection (15.2%), vomiting (15.2%), anemia (13%), asthenia (13%), constipation 13%), dysgeusia (10.9%) | 0.7 nM | Pan-TRK |
Interestingly, in the study that demonstrated crizotinib’s superiority over conventional chemotherapy in ALK-positive NSCLC, pemetrexed demonstrated an advantage over docetaxel in the second line (RR of 29% versus 6% and median PFS of 4.2 months versus 2.6 months). ALK-positivity was previously shown to be a marker for pemetrexed sensitivity when compared to EGFR-mutated or wild-type NSCLC (RR of 46.7 % versus 16.2% versus 4.7%, respectively, P=0.001; PFS 9.2 versus 2.9 versus 1.4 months, P=0.001) [42]. This enhanced sensitivity to pemetrexed is postulated to be a result of decreased thymidylate synthase mRNA levels in ALK-positive cells.
Mechanisms of crizotinib resistance
Unfortunately, resistance to crizotinib invariably occurs. Mechanisms of resistance can be divided into two broad categories: ALK-dominant (i.e. mechanisms dependent on ALK signaling) and ALK-non dominant (i.e. mechanisms that are only partially or independent of ALK signaling) [43, 44]. Among ALK-dominant mechanisms, three means of resistance occur: mutations in the ALK kinase domain [3, 45], copy number gain (CNG) of the ALK fusion gene [44, 46], and central nervous system (CNS) progression. The CNS represents a sanctuary site in which approximately 40% of ALK-positive cases treated with crizotinib develop progression [47], which has been attributed to poor drug penetration of the blood brain barrier [48].
In contrast to EGFR secondary resistance mutations—which are singularly dominated by T790 mutations—the range of mutations within the ALK kinase domain that confer crizotinib resistance appear to be quite wide and broadly distributed [43, 44, 46, 49]. Fundamentally, these mutations impact crizotinib binding and adenosine triphosphate (ATP) affinity. Camidge and Doebele have posited that this difference in range of kinase domain resistance mutations between ALK-positive and EGFR mutant tumors lies in the difference in selective pressure of such mutations [43]. In ALK-positive NSCLC cells with kinase domain mutations, there appears to be no growth disadvantage but, rather, increased proliferation when compared to wild-type [44]. In contrast, EGFR T790M conveys a selective disadvantage to EGFR-positive NSCLC cells compared to wild-type [50, 51]. The lower tolerance of mutations within the EGFR kinase domain relative to the ALK domain suggests higher constraints on the structure of EGFR rendering it less tolerable to amino acid substitutions than is ALK.
ALK non-dominant mechanisms of resistance involve the emergence of a second mutated, overexpressed, or amplified oncogene relative to the pre-treated sample. These include EGFR, KRAS, BRAF, MET, HER2, and KIT [43, 44, 46, 52]. In one case series, lung adenocarcinomas harboring both mutant EGFR and an ALK rearrangement responded to erlotinib [53]. Histologic transformation to small cell lung cancer has also been reported after treatment with the ALK inhibitor alectinib possibly as a mechanism of resistance [54].
Second-generation ALK-inhibitors: certinib, alectinib, and beyond
With the inevitable development of crizotinib resistance came the search for new ALK inhibitors that could overcome the aforementioned mechanisms of resistance. Two second-generation ALK inhibitors, ceritinib [55] and alectinib [56], are currently FDA approved (Tables 2 and 3) Ceritinib is an oral, small-molecule, ATP competitive TKI of ALK [55, 57] that has demonstrated impressive response rates in patients with ALK-rearranged NSCLC in both crizotinib-naïve and crizotinib-resistant patients. Among patients previously treated with crizotinib, the overall RR was 56% (95% CI, 45% to 67%), and median PFS was 6.9 months (95% CI, 5.3 to 8.8). Among patients naïve to crizotinib, the RR was 62% (95% CI, 44% to 78%) [55], and median PFS was 10.4 months (95% CI, 4.6 to could not be estimated). PFS appeared to be similar between patients with and without CNS disease at baseline (6.9 and 7.0 months, respectively; P = 0.37). The impressive response of ceritinib in both crizotinib-resistant and crizotinib-naïve patients may be attributed to several possible mechanisms: increased potency (20 times) against ALK, activity against ALK with secondary mutations in the tyrosine kinase domain, improved CNS activity, and/or inhibition of other tyrosine kinases not targeted by crizotinib, including IGF-1 [55].
Alectinib is a highly-selective ALK inhibitor with activity against both wild-type ALK and ALK harboring secondary mutations conferring crizotinib resistance [56, 58, 59]. In patients naïve to ALK inhibitors and who were resistant to or intolerant of crizotinib, RRs were noted to be 94 % (95 % CI 82% to 98%) and 55%, respectively [56, 60]. Among crizotinib-resistant patients with CNS disease, alectinib demonstrated promising activity with a RR of 52% [56].
What remains to be answered is the optimal sequencing of TKIs in ALK-positive NSCLC to maximize benefit while limiting toxicity, ultimately leading to greatest prolongation of overall survival. As previously mentioned, both ceritinib and alectinib have activity in both crizotinib-naïve and crizotinib-exposed patients. Moreover, PFS seems to be improved when ceritinib is used in the first line as compared to crizotinib. Further head-to-head studies looking at different TKI sequencing will be needed to better clarify this question.
Additionally, the search for and validation of new, more efficacious ALK-inhibitors are underway. These include brigatinib, entrectanib, loratinib, and belizatinib (table 4), for which outcomes data in ongoing clinical trials are pending.
Alternative treatment strategies: HSP90 inhibition and immunotherapy
In addition to the development of newer generation ALK inhibitors, efforts have been made to overcome crizotinib resistance by targeting ALK function via ALK-independent pathways [61]. One such alternative strategy is inhibition of heat shock protein 90 (Hsp90). Hsp90 is a molecular chaperone protein that guides the normal folding and turnover of intracellular growth factors and is implicated in the stabilization of several oncoproteins, including ALK [62–65]. Inhibition of Hsp90 activity results in aggregation and degradation of its client proteins and disrupts associated signaling pathways involved in cellular proliferation. Early preclinical studies with Hsp90 inhibitors in EML4-ALK rearranged NSCLC cells demonstrated encouraging results [61]. Ganetespib, a trazolone inhibitor of Hsp90, when used in used as a single agent in vitro against ALK-positive NSCLC resulted in loss of ALK expression and depletion of several oncogenic signaling proteins. In tumor xenografts, ganetespib resulted in improved survival and antitumor activity comparable to crizotinib. These effects were amplified when used in combination with crizotinib. In cells with induced crizotinib-resistance, whether mediated by secondary mutations in the tyrosine kinase domain of ALK or by ALK amplification, ganetespib overcame these barriers and demonstrated anti-tumor benefit. Early clinical trials have suggested that Hsp90 inhibition may have some activity in patients with ALK rearranged NSCLC (Table 2) [66, 67]. Several ongoing trials are exploring the sequencing of Hsp90 inhibitors alone or in combination with ALK inhibitors.
Another strategy that has gained significant attention in NSCLC therapy is immunotherapy. NSCLC tumor cells have been shown to express programmed cell death ligand 1 (PD-L1), which when bound to programed cell death protein 1 on T cells, initiates apoptosis of T cells. This attenuation of T cell activity provides a mechanism for immune escape in NSCLC [68–70]. Nivolumab is a humanized monoclonal antibody directed against the PD1 and PD-L1 interaction and is currently approved for use in both squamous and non-squamous metastatic NSCLC that has progressed after platinum-based therapy. Checkpoint blockade in ALK-positive NSCLC has yet to be fully elucidated, but in vitro studies have shown that ALK-rearranged NSCLC upregulates PD-L1 expression via activation of PI3K-ALT, MEK-ERK, HIF-1α, and STAT3 signaling [68, 71, 72]. The implications for treatment are currently under investigation in early clinical trials.
Conclusion
ALK-rearranged NSCLC represents an exciting opportunity in the realm of personalized medicine in which identification of a molecular subset of patients with cancer can yield tailored therapy with superior outcomes. There are up to 10,000 cases per year of ALK-rearranged NSCLC in the United States [37, 73] from which further research into improved molecular screening, therapeutic options, and counter-therapies to developed resistance can produce better treatment.
Acknowledgments
Funding: Funded in part by a National Cancer Institute Midcareer Award in Patient Oriented Research (K24CA201543-01; to DEG).
Footnotes
Conflict of interest: The authors report no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Soda M, et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature. 2007;448(7153):561–6. doi: 10.1038/nature05945. [DOI] [PubMed] [Google Scholar]
- 3.Choi YL, et al. EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors. N Engl J Med. 2010;363(18):1734–9. doi: 10.1056/NEJMoa1007478. [DOI] [PubMed] [Google Scholar]
- 4.Iwahara T, et al. Molecular characterization of ALK, a receptor tyrosine kinase expressed specifically in the nervous system. Oncogene. 1997;14(4):439–49. doi: 10.1038/sj.onc.1200849. [DOI] [PubMed] [Google Scholar]
- 5.Morris SW, et al. ALK, the chromosome 2 gene locus altered by the t(2;5) in non-Hodgkin’s lymphoma, encodes a novel neural receptor tyrosine kinase that is highly related to leukocyte tyrosine kinase (LTK) Oncogene. 1997;14(18):2175–88. doi: 10.1038/sj.onc.1201062. [DOI] [PubMed] [Google Scholar]
- 6.Atherly AJ, Camidge DR. The cost-effectiveness of screening lung cancer patients for targeted drug sensitivity markers. Br J Cancer. 2012;106(6):1100–6. doi: 10.1038/bjc.2012.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motegi A, et al. ALK receptor tyrosine kinase promotes cell growth and neurite outgrowth. J Cell Sci. 2004;117(Pt 15):3319–29. doi: 10.1242/jcs.01183. [DOI] [PubMed] [Google Scholar]
- 8.Souttou B, et al. Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem. 2001;276(12):9526–31. doi: 10.1074/jbc.M007333200. [DOI] [PubMed] [Google Scholar]
- 9.Janoueix-Lerosey I, et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature. 2008;455(7215):967–70. doi: 10.1038/nature07398. [DOI] [PubMed] [Google Scholar]
- 10.Grande E, Bolos MV, Arriola E. Targeting oncogenic ALK: a promising strategy for cancer treatment. Mol Cancer Ther. 2011;10(4):569–79. doi: 10.1158/1535-7163.MCT-10-0615. [DOI] [PubMed] [Google Scholar]
- 11.Takeuchi K, et al. KIF5B-ALK, a novel fusion oncokinase identified by an immunohistochemistry-based diagnostic system for ALK-positive lung cancer. Clin Cancer Res. 2009;15(9):3143–9. doi: 10.1158/1078-0432.CCR-08-3248. [DOI] [PubMed] [Google Scholar]
- 12.Ma Z, et al. Fusion of ALK to the Ran-binding protein 2 (RANBP2) gene in inflammatory myofibroblastic tumor. Genes Chromosomes Cancer. 2003;37(1):98–105. doi: 10.1002/gcc.10177. [DOI] [PubMed] [Google Scholar]
- 13.Gascoyne RD, et al. ALK-positive diffuse large B-cell lymphoma is associated with Clathrin-ALK rearrangements: report of 6 cases. Blood. 2003;102(7):2568–73. doi: 10.1182/blood-2003-03-0786. [DOI] [PubMed] [Google Scholar]
- 14.Shinmura K, et al. EML4-ALK fusion transcripts, but no NPM-, TPM3-, CLTC-, ATIC-, or TFG-ALK fusion transcripts, in non-small cell lung carcinomas. Lung Cancer. 2008;61(2):163–9. doi: 10.1016/j.lungcan.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Shaw AT, et al. Clinical features and outcome of patients with non-small-cell lung cancer who harbor EML4-ALK. J Clin Oncol. 2009;27(26):4247–53. doi: 10.1200/JCO.2009.22.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodig SJ, et al. Unique clinicopathologic features characterize ALK-rearranged lung adenocarcinoma in the western population. Clin Cancer Res. 2009;15(16):5216–23. doi: 10.1158/1078-0432.CCR-09-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kwak EL, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong DW, et al. The EML4-ALK fusion gene is involved in various histologic types of lung cancers from nonsmokers with wild-type EGFR and KRAS. Cancer. 2009;115(8):1723–33. doi: 10.1002/cncr.24181. [DOI] [PubMed] [Google Scholar]
- 19.Inamura K, et al. EML4-ALK fusion is linked to histological characteristics in a subset of lung cancers. J Thorac Oncol. 2008;3(1):13–7. doi: 10.1097/JTO.0b013e31815e8b60. [DOI] [PubMed] [Google Scholar]
- 20.Hirsch FR, et al. The tissue is the issue: personalized medicine for non-small cell lung cancer. Clin Cancer Res. 2010;16(20):4909–11. doi: 10.1158/1078-0432.CCR-10-2005. [DOI] [PubMed] [Google Scholar]
- 21.Peled N, et al. Next-generation sequencing identifies and immunohistochemistry confirms a novel crizotinib-sensitive ALK rearrangement in a patient with metastatic non-small-cell lung cancer. J Thorac Oncol. 2012;7(9):e14–6. doi: 10.1097/JTO.0b013e3182614ab5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun JM, et al. A dramatic response to crizotinib in a non-small-cell lung cancer patient with IHC-positive and FISH-negative ALK. J Thorac Oncol. 2012;7(12):e36–8. doi: 10.1097/JTO.0b013e318274694e. [DOI] [PubMed] [Google Scholar]
- 23.Ren S, et al. Atypical negative ALK break-apart FISH harboring a crizotinib-responsive ALK rearrangement in non-small-cell lung cancer. J Thorac Oncol. 2014;9(3):e21–3. doi: 10.1097/JTO.0000000000000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Camidge DR, et al. Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment. Clin Cancer Res. 2010;16(22):5581–90. doi: 10.1158/1078-0432.CCR-10-0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conklin CM, et al. Immunohistochemistry is a reliable screening tool for identification of ALK rearrangement in non-small-cell lung carcinoma and is antibody dependent. J Thorac Oncol. 2013;8(1):45–51. doi: 10.1097/JTO.0b013e318274a83e. [DOI] [PubMed] [Google Scholar]
- 26.Hutarew G, et al. Immunohistochemistry as a screening tool for ALK rearrangement in NSCLC: evaluation of five different ALK antibody clones and ALK FISH. Histopathology. 2014;65(3):398–407. doi: 10.1111/his.12399. [DOI] [PubMed] [Google Scholar]
- 27.Paik JH, et al. Screening of anaplastic lymphoma kinase rearrangement by immunohistochemistry in non-small cell lung cancer: correlation with fluorescence in situ hybridization. J Thorac Oncol. 2011;6(3):466–72. doi: 10.1097/JTO.0b013e31820b82e8. [DOI] [PubMed] [Google Scholar]
- 28.Savic S, et al. Screening for ALK in non-small cell lung carcinomas: 5A4 and D5F3 antibodies perform equally well, but combined use with FISH is recommended. Lung Cancer. 2015;89(2):104–9. doi: 10.1016/j.lungcan.2015.05.012. [DOI] [PubMed] [Google Scholar]
- 29.Weickhardt AJ, et al. Diagnostic assays for identification of anaplastic lymphoma kinase-positive non-small cell lung cancer. Cancer. 2013;119(8):1467–77. doi: 10.1002/cncr.27913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lung J, et al. A sensitive and high throughput TaqMan-based reverse transcription quantitative polymerase chain reaction assay efficiently discriminates ALK rearrangement from overexpression for lung cancer FFPE specimens. Lung Cancer. 2016;94:114–20. doi: 10.1016/j.lungcan.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 31.Cabillic F, et al. Parallel FISH and immunohistochemical studies of ALK status in 3244 non-small-cell lung cancers reveal major discordances. J Thorac Oncol. 2014;9(3):295–306. doi: 10.1097/JTO.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 32.Takeuchi K, et al. Multiplex reverse transcription-PCR screening for EML4-ALK fusion transcripts. Clin Cancer Res. 2008;14(20):6618–24. doi: 10.1158/1078-0432.CCR-08-1018. [DOI] [PubMed] [Google Scholar]
- 33.Ali SM, et al. Comprehensive Genomic Profiling Identifies a Subset of Crizotinib-Responsive ALK-Rearranged Non-Small Cell Lung Cancer Not Detected by Fluorescence In Situ Hybridization. Oncologist. 2016 doi: 10.1634/theoncologist.2015-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ignatius Ou SH, et al. Next-generation sequencing reveals a Novel NSCLC ALK F1174V mutation and confirms ALK G1202R mutation confers high-level resistance to alectinib (CH5424802/RO5424802) in ALK-rearranged NSCLC patients who progressed on crizotinib. J Thorac Oncol. 2014;9(4):549–53. doi: 10.1097/JTO.0000000000000094. [DOI] [PubMed] [Google Scholar]
- 35.Soda M, et al. A mouse model for EML4-ALK-positive lung cancer. Proc Natl Acad Sci U S A. 2008;105(50):19893–7. doi: 10.1073/pnas.0805381105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kwak EL, Camidge DR, Clark J. Clinical activity observed in a phase I dose escalation trial of an oral c-MET and ALK inhibitor, PF-02341066. J Clin Oncol. 2009;27(Suppl):148s. [Google Scholar]
- 37.Gerber DE, Minna JD. ALK inhibition for non-small cell lung cancer: from discovery to therapy in record time. Cancer Cell. 2010;18(6):548–51. doi: 10.1016/j.ccr.2010.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ou SH, et al. Activity of crizotinib (PF02341066), a dual mesenchymal-epithelial transition (MET) and anaplastic lymphoma kinase (ALK) inhibitor, in a non-small cell lung cancer patient with de novo MET amplification. J Thorac Oncol. 2011;6(5):942–6. doi: 10.1097/JTO.0b013e31821528d3. [DOI] [PubMed] [Google Scholar]
- 39.Bergethon K, et al. ROS1 rearrangements define a unique molecular class of lung cancers. J Clin Oncol. 2012;30(8):863–70. doi: 10.1200/JCO.2011.35.6345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Solomon BJ, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167–77. doi: 10.1056/NEJMoa1408440. [DOI] [PubMed] [Google Scholar]
- 41.Shaw AT, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. 2013;368(25):2385–94. doi: 10.1056/NEJMoa1214886. [DOI] [PubMed] [Google Scholar]
- 42.Lee JO, et al. Anaplastic lymphoma kinase translocation: a predictive biomarker of pemetrexed in patients with non-small cell lung cancer. J Thorac Oncol. 2011;6(9):1474–80. doi: 10.1097/JTO.0b013e3182208fc2. [DOI] [PubMed] [Google Scholar]
- 43.Camidge DR, Doebele RC. Treating ALK-positive lung cancer--early successes and future challenges. Nat Rev Clin Oncol. 2012;9(5):268–77. doi: 10.1038/nrclinonc.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doebele RC, et al. Mechanisms of resistance to crizotinib in patients with ALK gene rearranged non-small cell lung cancer. Clin Cancer Res. 2012;18(5):1472–82. doi: 10.1158/1078-0432.CCR-11-2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang S, et al. Crizotinib-resistant mutants of EML4-ALK identified through an accelerated mutagenesis screen. Chem Biol Drug Des. 2011;78(6):999–1005. doi: 10.1111/j.1747-0285.2011.01239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Katayama R, et al. Mechanisms of acquired crizotinib resistance in ALK-rearranged lung Cancers. Sci Transl Med. 2012;4(120):120ra17. doi: 10.1126/scitranslmed.3003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ou SH, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol. 2014;25(2):415–22. doi: 10.1093/annonc/mdt572. [DOI] [PubMed] [Google Scholar]
- 48.Costa DB, et al. CSF concentration of the anaplastic lymphoma kinase inhibitor crizotinib. J Clin Oncol. 2011;29(15):e443–5. doi: 10.1200/JCO.2010.34.1313. [DOI] [PubMed] [Google Scholar]
- 49.Sasaki T, et al. A novel ALK secondary mutation and EGFR signaling cause resistance to ALK kinase inhibitors. Cancer Res. 2011;71(18):6051–60. doi: 10.1158/0008-5472.CAN-11-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chmielecki J, et al. Optimization of dosing for EGFR-mutant non-small cell lung cancer with evolutionary cancer modeling. Sci Transl Med. 2011;3(90):90ra59. doi: 10.1126/scitranslmed.3002356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oxnard GR, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res. 2011;17(6):1616–22. doi: 10.1158/1078-0432.CCR-10-2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koivunen JP, et al. EML4-ALK fusion gene and efficacy of an ALK kinase inhibitor in lung cancer. Clin Cancer Res. 2008;14(13):4275–83. doi: 10.1158/1078-0432.CCR-08-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Popat S, et al. Lung adenocarcinoma with concurrent exon 19 EGFR mutation and ALK rearrangement responding to erlotinib. J Thorac Oncol. 2011;6(11):1962–3. doi: 10.1097/JTO.0b013e31822eec5e. [DOI] [PubMed] [Google Scholar]
- 54.Fujita S, et al. Transformation to SCLC after Treatment with the ALK Inhibitor Alectinib. J Thorac Oncol. 2016;11(6):e67–72. doi: 10.1016/j.jtho.2015.12.105. [DOI] [PubMed] [Google Scholar]
- 55.Shaw AT, et al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med. 2014;370(13):1189–97. doi: 10.1056/NEJMoa1311107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gadgeel SM, et al. Safety and activity of alectinib against systemic disease and brain metastases in patients with crizotinib-resistant ALK-rearranged non-small-cell lung cancer (AF-002JG): results from the dose-finding portion of a phase 1/2 study. Lancet Oncol. 2014;15(10):1119–28. doi: 10.1016/S1470-2045(14)70362-6. [DOI] [PubMed] [Google Scholar]
- 57.Marsilje TH, et al. Synthesis, structure-activity relationships, and in vivo efficacy of the novel potent and selective anaplastic lymphoma kinase (ALK) inhibitor 5-chloro-N2-(2-isopropoxy-5-methyl-4-(piperidin-4-yl)phenyl)-N4-(2-(isopropylsulf onyl)phenyl)pyrimidine-2,4-diamine (LDK378) currently in phase 1 and phase 2 clinical trials. J Med Chem. 2013;56(14):5675–90. doi: 10.1021/jm400402q. [DOI] [PubMed] [Google Scholar]
- 58.Kodama T, et al. Selective ALK inhibitor alectinib with potent antitumor activity in models of crizotinib resistance. Cancer Lett. 2014;351(2):215–21. doi: 10.1016/j.canlet.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 59.Sakamoto H, et al. CH5424802, a selective ALK inhibitor capable of blocking the resistant gatekeeper mutant. Cancer Cell. 2011;19(5):679–90. doi: 10.1016/j.ccr.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 60.Seto T, et al. CH5424802 (RO5424802) for patients with ALK-rearranged advanced non-small-cell lung cancer (AF-001JP study): a single-arm, open-label, phase 1–2 study. Lancet Oncol. 2013;14(7):590–8. doi: 10.1016/S1470-2045(13)70142-6. [DOI] [PubMed] [Google Scholar]
- 61.Sang J, et al. Targeted inhibition of the molecular chaperone Hsp90 overcomes ALK inhibitor resistance in non-small cell lung cancer. Cancer Discov. 2013;3(4):430–43. doi: 10.1158/2159-8290.CD-12-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trepel J, et al. Targeting the dynamic HSP90 complex in cancer. Nat Rev Cancer. 2010;10(8):537–49. doi: 10.1038/nrc2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma J, et al. Emerging treatment for ALK-positive lung cancer. Expert Opin Emerg Drugs. 2016;21(2):147–55. doi: 10.1080/14728214.2016.1183642. [DOI] [PubMed] [Google Scholar]
- 64.Pillai RN, Ramalingam SS. Heat shock protein 90 inhibitors in non-small-cell lung cancer. Curr Opin Oncol. 2014;26(2):159–64. doi: 10.1097/CCO.0000000000000047. [DOI] [PubMed] [Google Scholar]
- 65.Richards MW, et al. Crystal structure of EML1 reveals the basis for Hsp90 dependence of oncogenic EML4-ALK by disruption of an atypical beta-propeller domain. Proc Natl Acad Sci U S A. 2014;111(14):5195–200. doi: 10.1073/pnas.1322892111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sequist LV, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28(33):4953–60. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Socinski MA, et al. A multicenter phase II study of ganetespib monotherapy in patients with genotypically defined advanced non-small cell lung cancer. Clin Cancer Res. 2013;19(11):3068–77. doi: 10.1158/1078-0432.CCR-12-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ota K, et al. Induction of PD-L1 Expression by the EML4-ALK Oncoprotein and Downstream Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21(17):4014–21. doi: 10.1158/1078-0432.CCR-15-0016. [DOI] [PubMed] [Google Scholar]
- 69.Gatalica Z, et al. Programmed cell death 1 (PD-1) and its ligand (PD-L1) in common cancers and their correlation with molecular cancer type. Cancer Epidemiol Biomarkers Prev. 2014;23(12):2965–70. doi: 10.1158/1055-9965.EPI-14-0654. [DOI] [PubMed] [Google Scholar]
- 70.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–64. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hong S, et al. Upregulation of PD-L1 by EML4-ALK fusion protein mediates the immune escape in ALK positive NSCLC: Implication for optional anti-PD-1/PD-L1 immune therapy for ALK-TKIs sensitive and resistant NSCLC patients. Oncoimmunology. 2016;5(3):e1094598. doi: 10.1080/2162402X.2015.1094598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koh J, et al. EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1alpha and STAT3. Oncoimmunology. 2016;5(3):e1108514. doi: 10.1080/2162402X.2015.1108514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jemal A, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 74.Lemke G, Lew ED. A ligand for ALK. Sci Signal. 2015;8(360):fs2. doi: 10.1126/scisignal.aaa5566. [DOI] [PubMed] [Google Scholar]
- 75.Murray PB, et al. Heparin is an activating ligand of the orphan receptor tyrosine kinase ALK. Sci Signal. 2015;8(360):ra6. doi: 10.1126/scisignal.2005916. [DOI] [PubMed] [Google Scholar]
- 76.Sholl LM, et al. Combined use of ALK immunohistochemistry and FISH for optimal detection of ALK-rearranged lung adenocarcinomas. J Thorac Oncol. 2013;8(3):322–8. doi: 10.1097/JTO.0b013e31827db604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McLeer-Florin A, et al. Dual IHC and FISH testing for ALK gene rearrangement in lung adenocarcinomas in a routine practice: a French study. J Thorac Oncol. 2012;7(2):348–54. doi: 10.1097/JTO.0b013e3182381535. [DOI] [PubMed] [Google Scholar]
- 78.Conde E, et al. Accurate identification of ALK positive lung carcinoma patients: novel FDA-cleared automated fluorescence in situ hybridization scanning system and ultrasensitive immunohistochemistry. PLoS One. 2014;9(9):e107200. doi: 10.1371/journal.pone.0107200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Camidge DR, et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 2012;13(10):1011–9. doi: 10.1016/S1470-2045(12)70344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim D-W, Ahn M-J, Shi Y, Martino De Pas T, Yang P-C, Riely GJ, Crinò L, Evans TL, Liu X, Han J-Y, Salgia R, Moro-Sibilot D, Ou SH, Gettinger SN, Wu YL, Lanzalone S, Polli A, Iyer S, Shaw AT. Results of a global phase II study with crizotinib in advanced ALK-positive non-small cell lung cancer (NSCLC) J Clin Oncol. 2012;30 (abstr 7533) [Google Scholar]
- 81.Kim DW, et al. Activity and safety of ceritinib in patients with ALK-rearranged non-small-cell lung cancer (ASCEND-1): updated results from the multicentre, open-label, phase 1 trial. Lancet Oncol. 2016 doi: 10.1016/S1470-2045(15)00614-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ou SH, et al. Alectinib in Crizotinib-Refractory ALK-Rearranged Non-Small-Cell Lung Cancer: A Phase II Global Study. J Clin Oncol. 2016;34(7):661–8. doi: 10.1200/jco.2015.63.9443. [DOI] [PubMed] [Google Scholar]
- 83.Shaw AT, et al. Alectinib in ALK-positive, crizotinib-resistant, non-small-cell lung cancer: a single-group, multicentre, phase 2 trial. Lancet Oncol. 2016;17(2):234–42. doi: 10.1016/S1470-2045(15)00488-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nokihara H, Hida T, Kondo M, Kim YH, Azuma K, Seto T, Takiguchi Y, Nishio M, Yoshioka H, Imamura F, Hotta K, Watanabe S, Goto K, Nakagawa L, Mitsudomi T, Yamamoto N, Kuriki H, Asabe R, Tanaka T, Tamura T. Alectinib (ALC) versus crizotinib (CRZ) in ALK-inhibitor naive ALK-positive non-small cell lung cancer (ALK+ NSCLC): Primary results from the J-ALEX study. J Clin Oncol. 2016;34 (abstr 9008) [Google Scholar]
- 85.Camidge D, Bazhenova RS, Salgia R, Langer CJ, Gold KA, Rosell R, Shaw AT, Weiss GJ, Narasimhan NI, Dorer DJ, Rivera VM, Clackson TP, Conlan MG, Kerstein D, Haluska FG, Gettinger SN. Safety and efficacy of brigatinib (AP26113) in advanced malignancies, including ALK+ non–small cell lung cancer (NSCLC) J Clin Oncol. 2015;33 (abstr 8062) [Google Scholar]
- 86.Patel M, Bauer TM, Liu SV, Drilon AE, Wheler JJ, Shaw AT, Farago AF, Ou S-H, Luo D, Yeh L, Hornby Z, Senderwicz AM, Lim J. STARTRK-1: Phase 1/2a study of entrectinib, an oral Pan-Trk, ROS1, and ALK inhibitor, in patients with advanced solid tumors with relevant molecular alterations. j Clin Oncol. 2015;33 (abstr 2596) [Google Scholar]
- 87.Zou HY, et al. An orally available small-molecule inhibitor of c-Met, PF-2341066, exhibits cytoreductive antitumor efficacy through antiproliferative and antiangiogenic mechanisms. Cancer Res. 2007;67(9):4408–17. doi: 10.1158/0008-5472.CAN-06-4443. [DOI] [PubMed] [Google Scholar]
- 88.Kinoshita K, et al. Design and synthesis of a highly selective, orally active and potent anaplastic lymphoma kinase inhibitor (CH5424802) Bioorg Med Chem. 2012;20(3):1271–80. doi: 10.1016/j.bmc.2011.12.021. [DOI] [PubMed] [Google Scholar]
- 89.Huang WS, et al. Discovery of Brigatinib (AP26113), a Phosphine Oxide-Containing, Potent, Orally Active Inhibitor of Anaplastic Lymphoma Kinase. J Med Chem. 2016;59(10):4948–64. doi: 10.1021/acs.jmedchem.6b00306. [DOI] [PubMed] [Google Scholar]
- 90.Filippo G, De Braud MN, Damian Silvia, Bardazza Benedetta, Martinetti Antonia, Pelosi Giuseppe, Marrapese Giovanna, Palmeri Laura, Cerea Giulio, Valtorta Emanuele, Veronese Silvio, Sartore-Bianchi Andrea, Ardini Elena, Isachi Antonella, Martignoni Marcella, Galvani Arturo, Luo David, Yeh Litain, Senderowicz Adrian Mario, Siena Salvatore. Alka-372-001: First-in-human, phase I study of entrectinib – an oral pan-trk, ROS1, and ALK inhibitor – in patients with advanced solid tumors with relevant molecular alterations. J Clin Oncol. 2015;33 (abstr 2517) [Google Scholar]
- 91.Menichincheri M, et al. Discovery of Entrectinib: A New 3-Aminoindazole As a Potent Anaplastic Lymphoma Kinase (ALK), c-ros Oncogene 1 Kinase (ROS1), and Pan-Tropomyosin Receptor Kinases (Pan-TRKs) inhibitor. J Med Chem. 2016;59(7):3392–408. doi: 10.1021/acs.jmedchem.6b00064. [DOI] [PubMed] [Google Scholar]
- 92.Zou HY, et al. PF-06463922 is a potent and selective next-generation ROS1/ALK inhibitor capable of blocking crizotinib-resistant ROS1 mutations. Proc Natl Acad Sci U S A. 2015;112(11):3493–8. doi: 10.1073/pnas.1420785112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Johnson TW, et al. Discovery of (10R)-7-amino-12-fluoro-2,10,16-trimethyl-15-oxo-10,15,16,17-tetrahydro-2H-8,4-(m etheno)pyrazolo[4,3-h][2,5,11]-benzoxadiazacyclotetradecine-3-carbonitrile (PF-06463922), a macrocyclic inhibitor of anaplastic lymphoma kinase (ALK) and c-ros oncogene 1 (ROS1) with preclinical brain exposure and broad-spectrum potency against ALK-resistant mutations. J Med Chem. 2014;57(11):4720–44. doi: 10.1021/jm500261q. [DOI] [PubMed] [Google Scholar]
- 94.Alice Tsang Shaw TMB, Felip Enriqueta, Besse Benjamin, James Leonard Philip, Clancy Jill S, Mugundu Ganesh, Martini Jean-Francois, Abbattista Antonello, Solomon Benjamin J. Clinical activity and safety of PF-06463922 from a dose escalation study in patients with advanced ALK+ or ROS1+ NSCLC. J Clin Oncol. 2015;33 (abstr 8018) [Google Scholar]
- 95.Hendrik-Tobias Arkenau JCS, Mita Monica M, Dziadziuszko Rafal, Lin Chia-Chi, Yang James CH, Infante Jeffrey R, Anthony Stephen Patrick, Voskoboynik Mark, Su Wu-Chou, De Castro Javier, Natale Ronald B, Zhang Zhi-Yi, Hughes Lorraine, Bobilev Dmitri, Weiss Glen J. Phase (Ph) 1/2a study of TSR-011, a potent inhibitor of ALK and TRK, in advanced solid tumors including crizotinib-resistant ALK positive non-small cell lung cancer. J Clin Oncol. 2015;33 (abstr 8063) [Google Scholar]