Abstract
The adolescent period in mammals is a critical period of brain maturation and thus represents a time of susceptibility to environmental insult, e.g. psychosocial stress and/or drugs of abuse, which may cause lasting impairments in brain function and behavior and even precipitate symptoms in at risk individuals. One likely effect of these environmental insults is to increase oxidative stress in the developing adolescent brain. Indeed, there is increasing evidence that redox dysregulation plays an important role in the development of schizophrenia and other neuropsychiatric disorders and that GABA interneurons are particularly susceptible to alterations in oxidative stress. The current study sought to model this adolescent neurochemical “stress” by exposing mice to the dopamine transporter inhibitor GBR12909 (5 mg/kg; IP) during adolescence (postnatal day 35–44) and measuring the resultant effect on locomotor behavior and probabilistic reversal learning as well as GABAergic interneurons and oxidative stress in adulthood. C57BL6/J mice exposed to GBR12909 showed increased activity in a novel environment and increased impulsivity as measured by premature responding in the probabilistic reversal learning task. Adolescent GBR12909-exposed mice also showed decreased parvalbumin (PV) immunoreactivity in the prefrontal cortex, which was accompanied by increased oxidative stress in PV+ neurons. These findings indicate that adolescent exposure to a dopamine transporter inhibitor results in loss of PV in GABAergic interneurons, elevations in markers of oxidative stress, and alterations in behavior in adulthood.
Keywords: Adolescence, mice, GBR 12909, oxidative stress, paravalbumin interneurons, impulsivity
1. Introduction
Adolescence is a critical window of time during which psychological and neuroendocrinological changes take place (Spear 2000; Marco et al. 2011). Emergence of psychopathology can result if this transition period is perturbed through genetically programmed abnormalities (e.g. problems with synaptic pruning) or environmental influences (Andersen & Teicher 2008; Adriani & Laviola 2004). Indeed, several neuropsychiatric disorders emerge during adolescence, e.g. psychosis, anxiety and mood disorders, substance abuse, eating disorders, and personality disorders, and are all thought to involve disturbances in typical adolescent brain maturation (Paus et al. 2008 for review). Adolescence is also a time of increased vulnerability to stress and increased risky and thrill-seeking behavior, as well as a time in which many individuals are exposed for the first time to psychostimulants, both recreationally and therapeutically (Laviola et al. 1999). Changes in behavior during the adolescent period of development parallel progressive changes in cortical areas, particularly in the prefrontal cortex (PFC) (Casey et al 2000).
GABAergic inhibitory neurons, particularly parvalbumin (PV)+ interneurons, are essential to maintaining the appropriate excitatory/inhibitory (E/I) balance that is critical to experience-dependent refinement of synaptic connections and cognitive function (Huang et al. 2009). PV+ interneurons help maintain oscillatory activity of cortical networks in the gamma frequency range (30–80 Hz) (Bartos et al. 2007), which help coordinate neuronal communication between circuits and brain regions (Uhlhaas et al. 2010). Disruptions in gamma oscillations are associated with cognitive impairments in several neuropsychiatric disorders (Marin 2012; Uhlhaas et al. 2010). Frontal cortical-inhibitory circuits, including PV+ interneurons are relatively immature at birth and undergo a prolonged maturation, reaching maturity at the end of the adolescence period (Chattopadhyaya et al. 2004; Micheva and Beaulieu 1996; Reynolds and Beasley 2001). In rodents, PV+ neurons start to express PV after the first postnatal week and acquire their mature fast spiking properties after 2–3 weeks of postnatal life (de Lecea et al. 1995; del Rio et al. 1994; Gonchar et al. 2008; Pangratz-Fuehrer & Hestrin 2011).
PV fast-spiking interneurons utilize high levels of energy to maintain their fast-spiking properties and generate gamma oscillations (Kann et al. 2014). Thus, PV interneurons are particularly susceptible to oxidative stress effects (Behrens et al. 2007; Behrens & Sejnowski 2009; Powell et al. 2012). Several neurodevelopmental disorders such as schizophrenia (Do et al. 2012; Akyol et al 2002; Dadheech et al 2006; Dadheech et al. 2008), autism (Chauhan et al. 2012; Frustaci et al. 2012; Rossignol & Frye 2011; Villagonzalo et al. 2010), and ADHD (Bulut et al. 2013; Bulut et al. 2007; Ceylan et al. 2012; Guney et al. 2015; but see also Oztop et al. 2012) have been associated with redox dysregulation. Our work and that of others have shown that early postnatal redox imbalance alters the maturation of PV-interneurons. For example, decreased antioxidant capacity during early postnatal periods in rodents produces a loss in PV-interneurons and induces cognitive deficits relevant to schizophrenia (Cabungcal et al. 2007). Mice with a genetic deficiency in GSH synthesis have a reduced number of PV-interneurons, decreased kainate-induced gamma oscillations, and display schizophrenia-like behaviors in adulthood (Do et al., 2009). Additionally, early postnatal exposure to the NMDA antagonist ketamine produces loss of PV immunoreactivity in the absence of PV cell death (Powell et al. 2012), an effect shown to be mediated by an IL-6-Nox2-dependent pathway (Behrens et al. 2007, 2008; Dugan et al. 2009).
Psychological stress and drugs of abuse during adolescence are risk factors for psychiatric illness, including schizophrenia. Several groups have hypothesized that dopamine hyperstimulation during adolescence may represent a common biological consequence of psychological adversity and vulnerability for later development of neuropsychiatric disorders (Selten et al. 2013; Selemon & Zecevic 2015; Lieberman et al. 1997). Dopaminergic innervation, predominantly of forebrain areas, continues to mature during adolescence (Kalsbeek et al. 1988; Wahlstrom et al. 2010). Modulation of PFC circuits by dopamine (DA) peaks during adolescence in non-human primates and rodents (Tarazi & Baldessarini 2000; Brenhouse & Andersen 2011), and the rewarding effects of psychostimulants change during transitions between adolescence and adulthood (Andersen et al 2001; Adriani & Laviola 2003). Adolescent rats are more sensitive to the hyperactivity and DA release induced by DA uptake inhibitors (e.g. GBR12909) compared to adult rats (Walker et al. 2010). Adolescent exposure to methylphendidate results in long-lasting upregulation of Grik2 (kainite 2 subunit of ionotropic glutamate receptor) and Htr7 (5-HT7 receptor) in striatum (Adriani et al 2006). GABAergic neurons, specifically fast-spiking PV+ inhibitory neurons, show changes in DA-induced excitation patterns and in expression of N-methyl-D-aspartate (NMDA) receptors during the transition from adolescence to adulthood (Tseng & O'Donnell 2005). Activation of D1 DA receptors enhances excitability while activation of D2 DA receptors exerts mild inhibitory effects on PV+ inhibitory neurons in preadolescent PFC (Gorelova et al 2002). However, this pattern of DA modulation of PV+ interneurons changes from adolescence to adulthood (Tseng & O'Donnell 2007a; Tseng & O'Donnell 2007b), and only PV+ interneurons show sensitivity to D1 agonists during adolescence (Tseng and O’Donnell 2007a,b).
Because adolescence is a developmental period of increased maturation of both the DA system and GABAergic inhibitory circuits and a period of vulnerability to neuropsychiatric disorders that may involve increased mesolimbic dopamine signalling, we investigated whether hyperdopaminergia during early adolescence can lead to long-term alterations in PV+ neurons and oxidative stress markers and produce behavioral alterations in mice in adulthood. GBR12909 is a high affinity, long-acting DA transporter (DAT) inhibitor (Heikkila et al. 1984), which induces a hyperdopaminergic state when administered systemically and is known to produce oxidative stress by producing reactive oxygen species (ROS) via the catabolism of DA (Meiser et al 2013; Grima et al. 2003). Acute administration of GBR12909 decreased levels of GSH and increased lipid peroxidation in the hippocampus and PFC of mice up to 24h after injection (Queiroz et al 2015). Hence, we exposed adolescent mice to GBR12909. Mice were tested in the behavioral pattern monitor (BPM) to measure locomotor activity and investigatory behavior and in a probabilistic reversal learning task to measure learning and cognitive flexibility in adulthood. Oxidative stress and levels of PV immunohistochemistry were also evaluated in the PFC.
2. Experimental Procedures
2.1 Animals and drug administration
C57BL/6 male and female mice (The Jackson Laboratory; Bar Harbor, ME) were bred in our animal facility. Mice were housed in a temperature-controlled (21 + 1 C) vivarium on a 12-h reversed light/dark cycle (1900–0700h light). Food and water were provided ad libitum except during behavioral testing. During probabilistic learning training and testing, mice were food restricted to maintain weight at 85% of their free-feeding weight. All mice were tested during the dark phase of the cycle, i.e. their active, awake phase. Mice were maintained in Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC)-approved facilities and all procedures were approved by the UCSD Institutional Animal Care and Use Committee. Pups were weaned on postnatal day (PND) 24, housed in groups of three to four per cage, and assigned to either the saline exposed group (n=20 male; n=14 female) or the GBR12909-exposed group (n=20 male; n=14 female). Starting on PND 35 mice received either GBR12909 (Sigma-Aldrich, St Louis, MO, USA, 5 mg/kg s.c.) or saline (5 ml/kg) for 10 days until PND 44, encompassing the period of middle adolescence (Laviola et al. 2003; Spear 2000). GBR12909 dose and treatment schedule were based on previous studies showing that adolescent exposure to this dose increased oxidative stress (Cabungcal et al. 2013). Behavioral testing began at 12 weeks of age (~PND 84), which is considered adulthood in mice. Laboratory rodents are regarded as adults from PND 60 onwards (Spear 2000).
2.2. Behavioral Testing
2.2.1. Mouse Behavioral Pattern Monitor
In order to assess locomotor activity and investigatory behavior (rearing, holepokes), 12 week old male (n=20 Saline-exposed; n=20 GBR-exposed) and female (n=14 Saline-exposed; n=14 GBR-exposed) mice were tested in 10 Plexiglas mouse behavior pattern monitor (mBPM) chambers (San Diego Instruments, San Diego, CA) as described previously (Halberstadt et al 2011). The chambers were located within frames containing 12 x 24 infrared beams 1cm above the floor (2.5 cm apart along the length and width of the chamber) to detect the location of the mouse. Infrared photo beams located 2.5 cm above the floors were used to detect rearing behavior. Eleven holes (three in each long wall, two holes in the front and back walls, and three along the center of the floor) containing photo beams detected holepoking as a measure of investigatory behavior. After mice were acclimated to the testing room for 60 minutes, mice were then gently placed into the BPM chambers and tested for 60 minutes. Distance travelled (horizontal locomotor activity), rearings (vertical activity), and hole pokes were measured.
2.2.2. Probabilistic reversal learning task
Training and testing was conducted in 15, five-hole operant chambers enclosed in ventilated sound-attenuating boxes (Med Associates Inc., St. Albans, VT). Each chamber consisted of an array of 5 square apertures arranged horizontally on a curved wall 2.5 cm above the floor. A food-delivery magazine was located on the opposite wall at floor level and a house light mounted at the ceiling. Infrared beams located 3 mm from the aperture’s opening detected the nose poke response. The food delivery magazine delivered reward in the form of strawberry milkshake (Nestle®) by a peristaltic pump (Lafayette Instruments, Lafayette, IN, USA). An infrared beam, 5 mm above the floor, detected head entries into the magazine. The control of the stimuli and recording responses were managed by a SmartCtrl Package 8-In/16-Out with additional interfacing by MED-PC for Windows (Med Associates Inc., St. Albans, VT, USA) using custom programming.
A subset of the male mice (n=15 Saline-exposed; n=15 GBR-exposed) were tested for probabilistic reversal learning starting at 6 months of age. Mice were trained to retrieve the strawberry milkshake from the magazine for 3 consecutive days in a 10-min session, during which reward was delivered every 15s into the lighted magazine well. Head entries into the magazine extinguished the magazine light until delivery of the next reward. On day 4 mice were trained in a 30-min session to nose poke in one of the four holes opposite to the reward magazine. All trials were initiated by head entries into the reward magazine. This session was repeated Monday thru Friday until >70 responses were recorded within the session for 2 consecutive days. Once mice reached stable levels of responding, they were trained daily in 30-minute sessions on the probabilistic learning task. All trials were initiated by the head entry into the food magazine. Upon removal of the head from the magazine a 2s intertrial interval began, after which two of the apertures were illuminated. A nose poke into the target aperture resulted in delivery of “reward” (in food magazine) 80% of the time and in punishment (extinction of aperture light and no reward delivery with an illumination of house light for 4s timeout) 20% of the time. Nosepoke into the nontarget aperture resulted in reward 20% of the time and punishment 80% of the time. Target and nontarget apertures were counterbalanced as target aperture was located on the right for half of the mice and on the left for the other half. If a response was made at any aperture before illumination of target or nontarget aperture, a premature response was recorded. A premature response led to a time-out period in which all holes were unresponsive and no reward was earned. The next trial began when the house light was extinguished and the mouse initiated the trial by nose-poking into the food magazine. Daily sessions continued until mice reached criterion performance (>90% of the responses into the target location, >50 trials per session), which was considered indicative of successful learning of target location. Once animals reached criterion, the contingencies were reversed the next day such that the target location became the nontarget and the previously nontarget aperture became the target aperture. Once mice reached criterion performance in the reversal learning phase, its reward contingencies were reversed again on the next day. When animals reached the criterion in re-reversed phase, reward contingencies were switched again the next day and so on. The probabilistic reversal learning task was conducted for 39 experimental days. During this period, reward contingencies were changed back and forth for each mouse as long as it managed to reach performance criteria. The following measures were analyzed:
Days to criterion: Number of days required to reach criterion performance in initial learning phase
Days to first reversal: Number of days required to reach criterion performance after reversed task contingencies for the first time
Total reversals: Total number of reversals completed during the 39 days of the experiment.
Percentage premature responses: Number of premature responses per session divided by the number of total trials per session.
Percentage Perseverative responses: Number of additional responses in the same aperture after the initial nose-poke divided by the number of trails per session.
Reward latency: Time taken to retrieve reward after correct response.
Target response latency: Time taken to nosepoke in the target aperture
Non-target response latency: Time taken to nosepoke in the non-target aperture
2.3 Immunohistochemistry
To examine the effects of adolescent GBR12909–induced changes in PV+ interneurons, PV immunoreactivity was quantified in PFC and hippocampus in mice at approximately 9 months of age. Mice that had been behaviorally tested (n=4/group) were perfused with 4% paraformaldehyde and brains were sliced for immunohistochemistry as described (Behrens et al. 2007, 2008). Coronal brain slices (50 μm) obtained between Bregma 2.8 to 1.8 mm for the prelimbic region, and Bregma −1.3 to −2.3 mm for dorsal hippocampus, were immunostained with Vectastain for detection of parvalbumin using a polyclonal antibody (Swant, Switzerland). For total counts in the prelimbic region, all PV+ cells within a viewing grid (0.5 x 0.75 mm) placed over the prelimbic region were counted across 6 slices (Figure 3B). For the PV+ cells in the different regions of the dorsal hippocampus, the images for 5 slices were analyzed as described (Dugan et al 2009). The sum of cells across all slices in each region was calculated using the Abercrombie correction as described (Abercrombie 1946). In this correction, the possibility of double counting of cells is avoided according to the following equation P = A (M/(L + M)), where P is the average number of cells per section, A is the crude count of number of cells observed in the section, M is the thickness (in μ) of the section, and L the average length (in μ) of the cell.
Oxidative stress was visualized in adjacent slices to those used for Vactaistain above with an antibody against 8-oxo-7,8-dihydro-20-deoxyguanine (8-oxo-dG, 1:350; AMS Biotechnology, Bioggio- Lugano, Switzerland; as described in Cabungcal et al. 2013) and co-immunostained with the antibody against parvalbumin (Swant, Switzerland) followed by AlexaFluor conjugated secondary antibodies (as described Behrens et al., 2008) in the prelimbic region as well as the dorsal putamen (Bregma 1.4–0.5) and nucleus accumbens (Bregma −1.3 to −2.3). 8-oxo-dG was quantified in PV+ and non-PV+ cells in the images and values were expressed as mean fluorescence per cell as described (Kinney et al., 2006; Behrens et al., 2007; Lodge et al., 2009).
2.4 Statistical analyses
Locomotor activity and investigatory behavior data were initially analyzed by three-way analysis of variance (ANOVA), with GBR12909 and sex as the between-subjects factors and time as within subjects factors using BMDP (Statistical Solutions Inc., Saugus MA). Follow-up two-way ANOVAs were conducted on the data collapsed across sex since there were no interactions with sex. Probabilistic reversal learning data, PV cell count and 8-oxo-dG levels were analyzed by two-tailed t-test using SPSS Version 19 (IBM Corporation, Armonk, NY, USA). Posthoc comparisons among means were conducted by Tukey’s tests. The level of significance was set at 0.05.
3. Results
3.1. Adolescent GBR12909 increased locomotor activity in adulthood
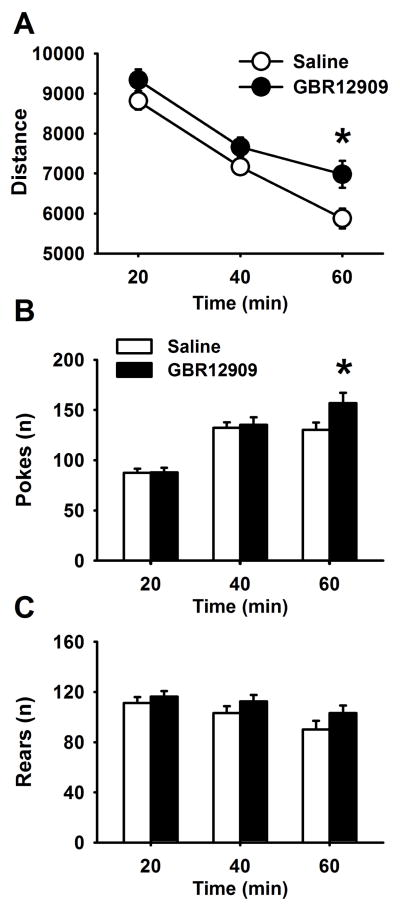
Male and female mice were allowed to explore the behavior pattern monitor (BPM) for 60 minutes. Distance travelled, pokes and rearing behavior were recorded and analyzed in 20–min intervals. Two male Saline-exposed mice were identified as outliers (distance traveled >3SD from mean) and removed from analyses. There was a main effect of sex on distance (F (1,62)=4.75, p<0.05) but there was no interaction between sex and GBR12909, so data were collapsed across sex. Adolescent GBR12909 exposure increased distance travelled across the test session (F (1,64)=4.76 p<0.05; Figure 1a). There was also a significant interaction between time and GBR12909 (F (2,128)=3.56, p<0.05). Posthoc comparisons showed that GBR12909-exposed mice were more active than saline mice during the last 20 min of the session (p<0.05), indicating less habituation to the chambers during testing. While there were no main effects of GBR12909 on hole pokes, there was a significant time x GBR12909 interaction for pokes (F (2,128)=4.97, p<0.01). As shown in Figure 1b, GBR12909-exposed mice showed higher number of hole pokes than saline-treated mice during the last 20 minutes of the session. Rearing behavior did not differ between the groups, nor were there interactions between drug exposure and time for rearing.
Figure 1.
Effects of adolescent GBR12909 exposure on locomotor activity and investigatory behavior in the behavioral pattern monitor in adult mice. Adolescent GBR exposure increased locomotor activity (A) and hole pokes (B) in the last 20 minutes of the 1 hour testing period, suggesting slower habituation than saline-exposed mice, n=18 Saline-exposed; n=20 GBR-exposed,. * p<0.05 vs saline-exposed mice. Data are presented as means ± SEM.
3.2. Adolescent GBR12909 increased premature responses in the probabilistic reversal learning task in adult mice
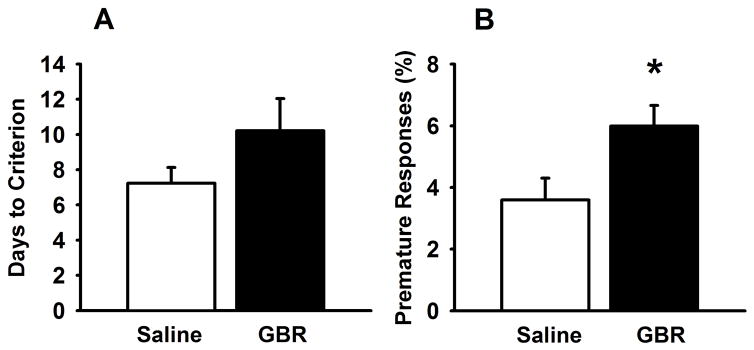
Male mice (6 months old) were tested in the probabilistic reversal learning task for 39 days (Figure 2). One Saline-exposed mouse never learned the task, thus the final number of mice were, n=14 Saline-exposed, n=15 GBR-exposed. GBR12909-exposed mice did not differ in initial learning of the task, although there was a trend for increased days to criterion in GBR12909-exposed mice but this was not statistically significantly (t (27) =1.482; p=0.149). GBR12909-exposed mice did show higher premature responses than saline-treated mice throughout the testing duration (t (27) = 2.572 p<0.05). GBR-exposed mice also showed faster responding (decreased latencies) to non-target stimuli (t(27)=2.20, p<0.05) and a trend toward faster responses to target stimuli (t (27)= 1.99, p=0.057) compared to saline-exposed mice (Table 1). Days to the first reversal, number of reversals, perseverative responses, and reward latency were not significantly different between the two adolescent exposure groups (Table 1).
Figure 2.
Effects of adolescence GBR12909 exposure on performance in probabilistic reversal learning task in adulthood. GBR-exposed mice (n=15) showed a trend toward increased trials to criterion compared to saline-exposed mice (n=14) (A). GBR-exposed mice showed increased premature responses throughout the task compared to their saline counterparts (B). * p<0.05 vs saline-exposed mice. Data are presented as means ± SEM.
Table 1.
Effects of adolescent GBR12909 exposure on additional measures in the probabilistic reversal learning task in mice tested in adulthood.
| Saline | GBR | |
|---|---|---|
| Mean Reward latency (s) | 4.71 ± 1.39 | 2.51 ± 0.22 |
| Perseverative responses | 10.95 ± 3.06 | 9.75 ± 3.12 |
| Total reversals | 3.75 ± 0.44 | 3.86 ± 0.36 |
| Days to first reversal | 11.37 ± 1.65 | 8.64 ± 0.78 |
| Mean Target Response Latency (s) | 5.27 ± 0.90 | 3.50 ± 0.31 |
| Mean Non-target Response Latency (s) | 5.37 ± 0.75 | 3.71 ± 0.29* |
Reward latencies, perseverative responses, days to first reversal and total number of reversals were not significantly different in adult mice exposed to GBR12909 in adolescence compared to saline-treated mice. GBR-exposed mice responded faster to non-target stimuli. Values are expressed as means ± SEMs.
p<0.05 vs. saline-exposed mice
3.3. Adolescent GBR12909 administration reduced PV+ cells and increased 8-oxo-dG in PFC of adult male mice
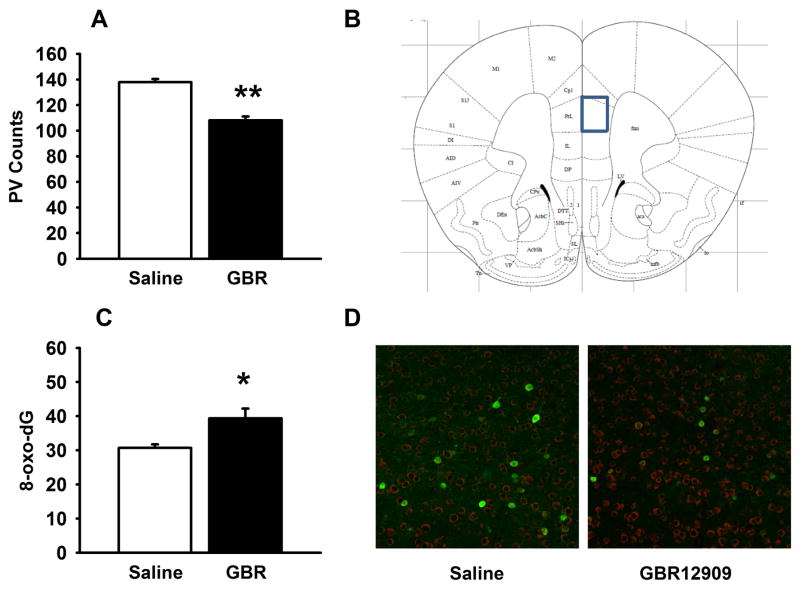
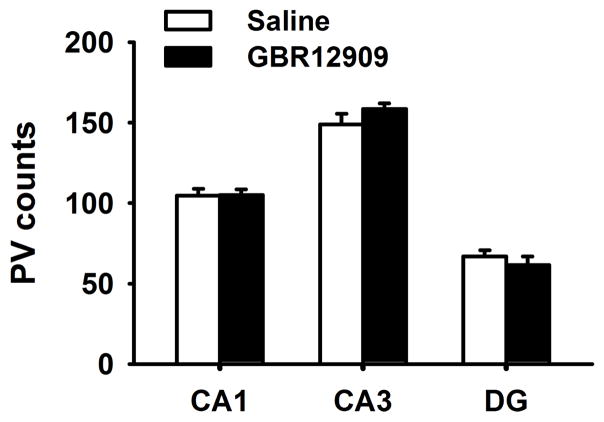
In the current set of experiments we performed immunohistochemistry in the PFC and hippocampus of male adult mice in order to quantify the loss of PV cells following exposure to GBR12909 during adolescence. The number of PV cells was decreased in GBR12909-exposed mice (t (3)=8.978, p<0.001) in PFC but not in any of the three regions of hippocampus (CA1, CA3, or dentate gyrus). We also labeled with 8-oxo-dG to determine whether administration of GBR12909 during adolescence produced increased oxidative stress in PFC. Adolescent GBR12909 exposure significantly increased 8-oxo-dG labeling in PV-positive cells in the PFC (t (3)=2.823 p<0.05), but not in non-PV cells. No differences in 8-oxo-dG were observed in the nucleus accumbens or dorsal putamen (not shown).
Figure 3.
Effects of adolescence GBR12909 exposure on PV cell count and oxidative stress in prelimbic region of prefrontal cortex of adult mice. PV cell count was significantly lower in GBR-exposed mice (A). (B) Schematic defining prelimbic region of frontal cortex for cell counting taken from (Paxinos & Franklin 2001) at Bregma 1.78. The levels of 8-oxo-dG were higher in PV+ cells in prefrontal cortex of GBR-exposed mice (C). (D) Representative image of PV (green) and 8-oxo-dG (red) labeling in prelimbic cortex from Saline-exposed (left) and GBR-exposed (right) mice. There was not a statistically significant difference in 8-oxo-dG in non-PV cells. *p<0.05, **p<0.01 vs saline-exposed mice. Data are presented as means ± SEM.
4. Discussion
In the present study adolescent exposure to GBR12909 resulted in increased oxidative stress, as measured by 8-oxo-dG, and decreased PV immunoreactivity in PFC in adulthood. Exposure to GBR12909 during adolescence also produced locomotor hyperactivity and impulsivity in a probabilistic reversal learning task. These findings indicate that the DAT inhibitor GBR12909, when administered during adolescence, has enduring and detrimental effects on brain and behavioral development. The behavioral and neurochemical effects of increased oxidative stress and loss of PV function in PFC are similar to those observed in some neurodevelopmental disorders, e.g. schizophrenia, ADHD.
Activity in a novel arena was monitored in mouse BPMs, enabling the quantification of locomotor activity and investigatory behavior. Adolescent GBR12909-exposed mice were hyperactive and showed slower habituation to the novel environment (Fig 1a) as well as increased investigatory behavior (Fig 1b). Studies using a human version of the BPM showed that bipolar disorder mania patients exhibited hyperactivity and higher object interaction (i.e. investigatory behavior) than normal controls; whereas, schizophrenia patients exhibited impaired locomotor habituation (Perry et al 2009). Previous studies have shown that pre-adolescent (PND 24–27) administration of GBR12909 leads to locomotor hyperactivity and increased rearing behavior in rats when tested in adolescence (PND 47) but not early adulthood (PND 57) (Hewitt et al 2009). In the present study, mice exhibited locomotor hyperactivity in adulthood (PND 84) when exposed to GBR12909 during adolescence (PND 35–44). GBR12909 exerts its effect by inhibiting the DAT and increased synaptic levels of DA (Heikkila et al. 1984), resulting in enhanced oxidative stress (Grima et al. 2003). The longer exposure to GBR12909 in our study (10 days), as compared to the 4-day exposure in the Hewitt et al. (2009) study, may have produced more robust effects on oxidative stress leading to sustained hyperactivity. Although there is a low density of DAT in the PFC of rodents (Sesack et al. 1998; Ciliax et al. 1995), DAT inhibition with GBR12909 does result in functional consequences. When applied locally in PFC slices, GBR12909 blocks long term depression (LTD), which can be blocked by DA D1 receptor antagonist (Bai et al. 2014). When administered systemically, GBR12909 also increases Homer 1 expression (Tomasetti et al. 2007) and induces oxidative stress (e.g. reduced GSH and increased lipid peroxidation) in frontal cortex (Queiroz et al. 2015). Additionally, cocaine produces greater c-fos activation in cortical regions compared to subcortical regions in adolescent rats (Cao et al. 2007).
In humans and experimental animals, cognitive flexibility can be assessed by reversal learning tasks (Boulougouris et al 2007; Fellows & Farah 2003). In this study we chose an operant probabilistic reversal learning task that requires animals to learn the relationship between choices and rewards, when the rewards are provided probabilistically (Zaratto et al. 2012). If the probabilities of getting a reward are relatively similar between two choices, learning is more difficult; however, if one choice is rarely rewarded and the alternate choice frequently rewarded, the response selection is easy. In a probabilistic reversal learning task, animals are required to learn the choice that has highest probability of getting reward, which results in a challenging task, more reflective of reversal learning tasks used in humans. In similar probabilistic reversal learning tasks, schizophrenia patients show deficits in reversal learning (Waltz & Gold 2007) while ADHD patients show a less flexible strategy when performing the task (Hauser et al. 2014). In the present study, we tested mice in a probabilistic reversal learning task similar to that previously used in rats (Amitai et al 2014; Bari et al 2010). Overall, GBR12909-exposed mice did not differ in their initial learning or in the reversal stage of the task (Fig 2a). Increased premature responses were observed in mice exposed to adolescent GBR12909, possibly indicating disinhibited responding in the task (Fig 2b). In addition however, GBR-exposed mice responded faster as indicated by decreased response latencies, hence their disinhibited responding could be driven by a faster temporal perception (Cope et al, 2016). GBR-exposed mice did not exhibit elevated perseverative responses nor faster latencies to choose or collect rewards, however. Hence, the elevated premature responses and increased activity of mice exposed to GBR during adolescence likely reflect motoric impulsivity and/or altered temporal perception. These findings are consistent with prior studies showing disruptions in premature responding with acute and preadolescent exposure to GBR12909. For example, previous studies have shown that acute GBR12909 increased premature responses in the five choice serial reaction task (5-CSRTT) (van Gaalen et al 2006a), and impulsive decision making in a delayed reward task (van Gaalen et al 2006b), but failed to affect reversal performance. Our data extend these findings and show that adolescent inhibition of DAT results in impulsivity in a probabilistic learning task, without affecting reversal learning.
Oxidative stress has a documented role in several neurodevelopmental disorders including schizophrenia and ADHD (Ng et al 2008). Altered oxidative status has also been shown in several other neurodevelopmental models of neuropsychiatric disease. For example, chronic perinatal administration of an NMDA receptor antagonist phencyclidine decreased GSH levels in cortical areas of rat brain (Radonjić et al 2010). Subchronic administration of ketamine leads to oxidative stress by activation of Nox2, a major source of reactive oxygen species (Behrens & Sejnowski 2009; Powell et al 2012). Maternal immune activation in mice exposed prenatally to PolyI:C also results in increased oxidative stress levels (e.g. increased protein carbonyl groups; Deslauriers et al 2014). In the current study, mice exposed to adolescent GBR12909 showed increase oxidative stress in PV+ neurons in prelimbic cortex, as measured by 8-oxo-dG levels, a marker of oxidized DNA (Fig 3a). Thus, the effect of GBR12909 on 8-oxo-dG levels appears to be specific to PV+ interneurons in the present study, although future studies should assess other subtypes of GABA interneurons more specifically. Previous studies have shown that GBR12909 decreased GSH levels and lipid peroxidation in PFC, striatum, and hippocampus acutely and up to 24h in PFC and hippocampus (Querioz et al. 2015), indicating that GBR12909 is capable of creating redox imbalance in brain. The current study also showed that PV immunoreactivity in PFC decreased in adult mice exposed to GBR12909 during adolescence (Fig 3b). PV interneurons may be more vulnerable to oxidative stress than other interneurons because of their fast spiking properties (Hasenstaub et al 2010). PV interneurons also continue to mature during adolescence, particularly their sensitivity to DA (Sullivan & O’Donnell 2012). Additionally, developmental period also plays an important role in the vulnerability of PV interneurons to oxidative stress. For example, in the neonatal ventral hippocampal lesion model, juvenile and adolescent treatment with the antioxidant N-acetyl cysteine prevented decreased PFC PV immunoreactivity typically seen in the model (Cabungcal et al 2014). Adolescence is a critical period of brain development since the brain continues to mature and synaptic pruning of PFC takes place (McCutcheon & Marinelli 2009; Laviola & Marco 2011). This late development of cortex has been suggested to be linked to certain behaviors such as impulsivity that can enhance the chances of risk taking and drug abuse in adolescence (Adriani & Laviola 2004; Benes et al 2000). Hence, oxidative stress generated by GBR12909, due to elevated extracellular DA, may interfere with cortical maturation during adolescence and produced long-lasting impairments in PV interneurons. These findings are the first to demonstrate that adolescent GBR12909 increases oxidative stress and decreases PV+ cell numbers in PFC in adulthood. Previous studies have shown that GBR12909 during adolescence increased oxidative stress and decreased PV interneurons in the anterior cingulate cortex of glutamate cysteine ligase regulatory subunit GCLM KO but not WT mice (Cabungcal et al. 2013), suggesting that lack of GCLM is required to observe the oxidative stress effects in ACC. In the present study, however, adolescent GBR12909 increased markers of oxidative stress in PV+ neurons in the prelimbic cortex in WT C57Bl/6J mice. In the present experiment, exposure to GBR12909 during adolescence led to long lasting effects on PV and 8-oxo-dG levels, however, whether these changes result immediately after the GBR12909 treatment is presently unknown. These studies are suggestive of a role of oxidative stress at the time of GBR12909 administration, but we cannot answer the question with the current design. Future studies could assess whether GBR produces the observed increase in 8-oxo-DG at the time of GBR administration and whether blocking the effect of GBR on oxidative stress prevents the alterations in behavior and PV interneurons in adulthood. There is also the possibility that behavioral testing somehow interacted with the effects of adolescent GBR12909 on PV+ interneurons. Although an interaction is unlikely, future studies could assess the effects of adolescent GBR12909 administration in mice naïve to behavioral testing.
The PFC is a forebrain region that modulates executive function, attention, working memory, behavioral inhibition, and impulsivity (Robbins 2000). PV interneurons modulate the activity of pyramidal neurons and help maintain the excitatory-inhibitory balance in PFC (Goldman-Rakic 1995). Thus deficits in PV interneurons, due to altered redox regulation during adolescence, may lead to decreased inhibitory drive in the PFC. Previously it was shown that reduced GABAergic inhibition of PFC by systemic or local administration of GABA antagonists increased locomotor activity (Matsumoto et al 2003; Pezze et al 2014) by enhancing dopamine release in striatum (Enomoto et al 2011). The reduced GABAergic inhibition in PFC may contribute to the locomotor hyperactivity in GBR12909-exposed mice. Excitotoxic lesions encompassing both dorsal and ventral sub regions of PFC lead to increased premature responses and impaired attention in the 5-choice serial reaction task (Paine et al 2011). Lesions of anterior cingulate cortex and/or prelimbic cortex impaired attention while lesions restricted to orbitofrontal cortex and/or infralimbic cortex induce impulsivity and increased premature responding (Chudasama et al 2003; Muir et al 1996; Passetti et al 2002; Pezze et al 2009). In the present study increased oxidative stress and altered PV function observed in the prelimbic area of PFC may mediate the impulsivity observed in adolescent GBR12909-exposed mice. The PFC controls the firing pattern of other subcortical structures known to be involved in attention and impulsivity such as nucleus accumbens and hippocampus (Paine et al 2011). The decrease in PV observed in prelimbic cortex with adolescent GBR12909 exposure could lead to disinhibition of its target areas. Dopamine is one of the key modulators of both excitatory and inhibitory neurotransmission in the PFC (Seemans and Yang, 2004). Dopaminergic neurons from ventral tegmental area innervates PFC during adolescence and modulation of PFC neuronal circuits by D1 and D2 receptors, responsible for excitation-inhibition balance, also changes during the course of development (Tseng and O’Donnell, 2007). In the present study, whether adolescent exposure to GBR12909 also produced alterations in DA function resulting in impulsivity remains to be tested.
Many neuropsychiatric disorders have a neurodevelopmental origin and environmental insults can lead to oxidative imbalances in susceptible individuals. The aim of this study was to provide insight into how hyperdopaminergia during adolescence can lead to dysfunctioning of fast spiking GABAergic PV interneurons via oxidative stress. Here, we show that GBR12909 administration during adolescence in mice results in hyperactivity and increased impulsivity in adulthood, although cognitive flexibility was unaffected. Adolescent GBR12909 exposure also selectively reduced PV expression in the PFC. Impairments in PFC function are known to be involved in impulsivity, which may arise from the loss of PV interneurons in frontal cortex. Our results thus add to the growing body of evidence that early developmental insults inducing oxidative stress can have long lasting effects on PV interneurons that can lead to behavioral deficits in mice. However, whether PV interneurons can be protected from oxidative stress by an antioxidant treatment remains to be established.
Figure 4.
Effects of adolescent GBR12909 exposure on PV cell count in hippocampus of adult mice. PV cell count was not significantly different between treatment groups. Data are presented as means ± SEM.
Highlights.
Adolescent GBR12909 results in locomotor hyperactivity and impulsivity in a probabilistic learning task in adult mice.
Adolescent GBR12909 decreased parvalbumin (PV) and increased oxidative stress in PV+ neurons in the prelimbic cortex.
Adolescent exposure to increased dopamine produces enduring changes in adult mice.
Acknowledgments
Supported by NIH grant R01 MH091407 and Veterans Affairs VISN 22 MIRECC. The authors would like to thank Dr. Mark Geyer for his advice and support, and Mr. Richard Sharp and Ms. Mahalah Buell for their technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–47. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Spontaneous novelty seeking and amphetamine-induced conditioning and sensitization in adult mice: evidence of dissociation as a function of age at weaning. Neuropsychopharmacology. 2002;27:225–236. doi: 10.1016/S0893-133X(02)00300-7. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Elevated levels of impulsivity and reduced place conditioning with d-amphetamine: two behavioral features of adolescence in mice. Behav Neurosci. 2003;117:695–703. doi: 10.1037/0735-7044.117.4.695. [DOI] [PubMed] [Google Scholar]
- Adriani W, Laviola G. Windows of vulnerability to psychopathology and therapeutic strategy in the adolescent rodent model. Behavioural Pharmacology. 2004;15:341–52. doi: 10.1097/00008877-200409000-00005. [DOI] [PubMed] [Google Scholar]
- Adriani W, Leo D, Greco D, Rea M, Di Porzio U, Laviola G, Perrone-Capano Methylphendidate administration to adolescent rats determines plastic changes on reward-related behavior and striatal gene expression. Neuropsychopharmacology. 2006;31:1946–1956. doi: 10.1038/sj.npp.1300962. [DOI] [PubMed] [Google Scholar]
- Akyol Ö, Herken H, Uz E, Fadillioğlu E, Ünal S, et al. The indices of endogenous oxidative and antioxidative processes in plasma from schizophrenic patients: The possible role of oxidant/antioxidant imbalance. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2002;26:995–1005. doi: 10.1016/s0278-5846(02)00220-8. [DOI] [PubMed] [Google Scholar]
- Amitai N, Young J, Higa K, Sharp R, Geyer M, Powell S. Isolation rearing effects on probabilistic learning and cognitive flexibility in rats. Cognitive, Affective, & Behavioral Neuroscience. 2014;14:388–406. doi: 10.3758/s13415-013-0204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, LeBlanc CJ, Lyss PJ. Maturational increases in c-fos expression in the ascending dopamine systems. Synapse. 2001;41:345–50. doi: 10.1002/syn.1091. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends in Neurosciences. 2008;31:183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Thompson AT, Rutstein M, Hostetter JC, Teicher MH. Dopamine receptor pruning in prefrontal cortex during the periadolescent period in rats. Synapse. 2000;37:167–9. doi: 10.1002/1098-2396(200008)37:2<167::AID-SYN11>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Bai J, Blot K, Tzavara E, Nosten-Bertrand M, Giros B, Otani S. Inhibition of Dopamine Transporter Activity Impairs Synaptic Depression in Rat Prefrontal Cortex Through Over-Stimulation of D1 Receptors. Cerebral Cortex. 2014;24:945–55. doi: 10.1093/cercor/bhs376. [DOI] [PubMed] [Google Scholar]
- Bari A, Theobald DE, Caprioli D, Mar AC, Aidoo-Micah A, et al. Serotonin Modulates Sensitivity to Reward and Negative Feedback in a Probabilistic Reversal Learning Task in Rats. Neuropsychopharmacology. 2010;35:1290–301. doi: 10.1038/npp.2009.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- Beasley CL, Reynolds GP. Parvalbumin-immunoreactive neurons are reduced in the prefrontal cortex of schizophrenics. Schizophr Res. 1997;24:349–55. doi: 10.1016/s0920-9964(96)00122-3. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–7. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- Behrens MM, Ali SS, Dugan LL. Interleukin-6 Mediates the Increase in NADPH-Oxidase in the Ketamine Model of Schizophrenia. The Journal of Neuroscience. 2008;28:13957–66. doi: 10.1523/JNEUROSCI.4457-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens MM, Sejnowski TJ. Does schizophrenia arise from oxidative dysregulation of parvalbumin-interneurons in the developing cortex? Neuropharmacology. 2009;57:193–200. doi: 10.1016/j.neuropharm.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benes FM, Berretta S. GABAergic Interneurons: Implications for Understanding Schizophrenia and Bipolar Disorder. Neuropsychopharmacology. 2001;25:1–27. doi: 10.1016/S0893-133X(01)00225-1. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham M. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: Implications for the development of psychopathology. Cerebr Cortex. 2000;10:1014–27. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Berk M, Copolov D, Dean O, Lu K, Jeavons S, et al. N-Acetyl Cysteine as a Glutathione Precursor for Schizophrenia—A Double-Blind, Randomized, Placebo-Controlled Trial. Biological Psychiatry. 2008;64:361–8. doi: 10.1016/j.biopsych.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Boulougouris V, Dalley JW, Robbins TW. Effects of orbitofrontal, infralimbic and prelimbic cortical lesions on serial spatial reversal learning in the rat. Behavioural Brain Research. 2007;179:219–28. doi: 10.1016/j.bbr.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Bulut M, Selek S, Bez Y, Cemal Kaya M, Gunes M, et al. Lipid peroxidation markers in adult attention deficit hyperactivity disorder: New findings for oxidative stress. Psychiatry Research. 2013;209:638–42. doi: 10.1016/j.psychres.2013.02.025. [DOI] [PubMed] [Google Scholar]
- Bulut M, Selek S, Gergerlioglu HS, Savas HA, Yilmaz HR, et al. Malondialdehyde levels in adult attention-deficit hyperactivity disorder. Journal of Psychiatry & Neuroscience : JPN. 2007;32:435–8. [PMC free article] [PubMed] [Google Scholar]
- Cabungcal J-H, Counotte Danielle S, Lewis EM, Tejeda Hugo A, Piantadosi P, et al. Juvenile Antioxidant Treatment Prevents Adult Deficits in a Developmental Model of Schizophrenia. Neuron. 2014;83:1073–84. doi: 10.1016/j.neuron.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabungcal J-H, Preissmann D, Delseth C, Cuenod M, Do KQ, Schenk Fo. Transitory glutathione deficit during brain development induces cognitive impairment in juvenile and adult rats: Relevance to schizophrenia. Neurobiology of Disease. 2007;26:634–45. doi: 10.1016/j.nbd.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Cabungcal J-H, Steullet P, Kraftsik R, Cuenod M, Do KQ. Early-Life Insults Impair Parvalbumin Interneurons via Oxidative Stress: Reversal by N-Acetylcysteine. Biological Psychiatry. 2013;73:574–82. doi: 10.1016/j.biopsych.2012.09.020. [DOI] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology. 2007;32:2279–89. doi: 10.1038/sj.npp.1301349. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biological Psychology. 2000;54:241–57. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Cass DK, Thomases DR, Caballero A, Tseng KY. Developmental Disruption of Gamma-Aminobutyric Acid Function in the Medial Prefrontal Cortex by Noncontingent Cocaine Exposure During Early Adolescence. Biological Psychiatry. 2013;74:490–501. doi: 10.1016/j.biopsych.2013.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceylan M, Sener S, Bayraktar AhC, Kavutcu M. Oxidative imbalance in child and adolescent patients with attention-deficit/hyperactivity disorder. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2010;34:1491–4. doi: 10.1016/j.pnpbp.2010.08.010. [DOI] [PubMed] [Google Scholar]
- Ceylan MF, Sener S, Bayraktar AC, Kavutcu M. Changes in oxidative stress and cellular immunity serum markers in attention-deficit/hyperactivity disorder. Psychiatry and Clinical Neurosciences. 2012;66:220–6. doi: 10.1111/j.1440-1819.2012.02330.x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyaya B, Di Cristo G, Higashiyama H, Knott GW, Kuhlman SJ, et al. Experience and Activity-Dependent Maturation of Perisomatic GABAergic Innervation in Primary Visual Cortex during a Postnatal Critical Period. The Journal of Neuroscience. 2004;24:9598–611. doi: 10.1523/JNEUROSCI.1851-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan A, Audhya T, Chauhan V. Brain Region-Specific Glutathione Redox Imbalance in Autism. Neurochemical Research. 2012;37:1681–9. doi: 10.1007/s11064-012-0775-4. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SEV, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behavioural Brain Research. 2003;146:105–19. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Ciliax BJ, Heilman C, Demchyshyn LL, Pristupa ZB, Ince E, Hersch SM, Niznik HB, Levey AI. The dopamine transporter: immunochemical characterization and localization in brain. J Neurosci. 1995;15:1714–23. doi: 10.1523/JNEUROSCI.15-03-01714.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cope ZA, Halberstadt AL, van Enkhuizen J, Flynn AD, Breier M, Swerdlow NR, Geyer MA, Young JW. Premature responses in the 5-choice serial reaction time task reflect rodents’ temporal strategies: Evidence from no-light and pharmacological challenges. Psychopharmacology. doi: 10.1007/s00213-016-4389-4. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadheech G, Mishra S, Gautam S, Sharma P. Oxidative stress, α-tocopherol, ascorbic acid and reduced glutathione status in schizophrenics. Indian Journal of Clinical Biochemistry. 2006;21:34–8. doi: 10.1007/BF02912908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadheech G, Mishra S, Gautam S, Sharma P. Evaluation of antioxidant deficit in schizophrenia. Indian J Psychiatry. 2008;50:16–20. doi: 10.4103/0019-5545.39753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lecea L, del Rio JA, Soriano E. Developmental expression of parvalbumin mRNA in the cerebral cortex and hippocampus of the rat. Molecular Brain Research. 1995;32:1–1. doi: 10.1016/0169-328x(95)00056-x. [DOI] [PubMed] [Google Scholar]
- del Rio JA, De Lecea L, Ferrer I, Soriano E. The development of parvalbumin-immunoreactivity in the neocortex of the mouse. Brain Res Dev Brain Res. 1994;81:247–259. doi: 10.1016/0165-3806(94)90311-5. [DOI] [PubMed] [Google Scholar]
- Deslauriers J, Racine W, Sarret P, Grignon S. Preventive effect of α-lipoic acid on prepulse inhibition deficits in a juvenile two-hit model of schizophrenia. Neuroscience. 2014;272:261–70. doi: 10.1016/j.neuroscience.2014.04.061. [DOI] [PubMed] [Google Scholar]
- Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–30. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Do KQ, Trabesinger AH, Kirsten-Krüger M, Lauer CJ, Dydak U, et al. Schizophrenia: glutathione deficit in cerebrospinal fluid and prefrontal cortex in vivo. European Journal of Neuroscience. 2000;12:3721–8. doi: 10.1046/j.1460-9568.2000.00229.x. [DOI] [PubMed] [Google Scholar]
- Dugan LL, Ali SS, Shekhtman G, Roberts AJ, Lucero J, et al. IL-6 Mediated Degeneration of Forebrain GABAergic Interneurons and Cognitive Impairment in Aged Mice through Activation of Neuronal NADPH Oxidase. PLoS One. 2009;4:e5518. doi: 10.1371/journal.pone.0005518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T, Tse MT, Floresco SB. Reducing Prefrontal Gamma-Aminobutyric Acid Activity Induces Cognitive, Behavioral, and Dopaminergic Abnormalities That Resemble Schizophrenia. Biological Psychiatry. 2011;69:432–41. doi: 10.1016/j.biopsych.2010.09.038. [DOI] [PubMed] [Google Scholar]
- Fellows LK, Farah MJ. Ventromedial frontal cortex mediates affective shifting in humans: evidence from a reversal learning paradigm. Brain. 2003;126:1830–7. doi: 10.1093/brain/awg180. [DOI] [PubMed] [Google Scholar]
- Frustaci A, Neri M, Cesario A, Adams JB, Domenici E, et al. Oxidative stress-related biomarkers in autism: Systematic review and meta-analyses. Free Radical Biology and Medicine. 2012;52:2128–41. doi: 10.1016/j.freeradbiomed.2012.03.011. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–85. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Wang Q, Burkhalter A. Multiple Distinct Subtypes of GABAergic Neurons in Mouse Visual Cortex Identified by Triple Immunostaining. Frontiers in Neuroanatomy. 2007;1:3. doi: 10.3389/neuro.05.003.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelova N, Seamans JK, Yang CR. Mechanisms of Dopamine Activation of Fast-Spiking Interneurons That Exert Inhibition in Rat Prefrontal Cortex. Journal of Neurophysiology. 2002;88:3150–66. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- Grima G, Benz B, Parpura V, Cuenod M, Do KQ. Dopamine-induced oxidative stress in neurons with glutathione deficit: implication for schizophrenia. Schizophrenia Research. 2003;62:213–24. doi: 10.1016/s0920-9964(02)00405-x. [DOI] [PubMed] [Google Scholar]
- Guney E, Cetin FH, Alisik M, Tunca H, Tas Torun Y, Iseri E, Isik Taner Y, Cayci B, Erel O. Attention Deficit Hyperactivity Disorder and oxidative stress: A short term follow up study. Psychiatry Res. 2015;229:310–7. doi: 10.1016/j.psychres.2015.07.003. [DOI] [PubMed] [Google Scholar]
- Halberstadt AL, Lehmann-Masten V, Geyer MA, Powell SB. Interactive effects of mGlu5 and 5-HT2A receptors on locomotor activity in mice. Psychopharmacology. 2011;215:81–92. doi: 10.1007/s00213-010-2115-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenstaub A, Otte S, Callaway E, Sejnowski TJ. Metabolic cost as a unifying principle governing neuronal biophysics. Proceedings of the National Academy of Sciences. 2010;107:12329–34. doi: 10.1073/pnas.0914886107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–26. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser TU, Iannaccone R, Ball J, et al. ROle of the medial prefrontal cortex in impaired decision making in juvenile attention-deficit/hyperactivity disorder. JAMA Psychiatry. 2014;71:1165–73. doi: 10.1001/jamapsychiatry.2014.1093. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L. Behavioral properties of GBR 12909, GBR 13069 and GBR 13098: Specific inhibitors of dopamine uptake. European Journal of Pharmacology. 1984;103:241–8. doi: 10.1016/0014-2999(84)90483-7. [DOI] [PubMed] [Google Scholar]
- Hewitt KN, Marsden CA, Hollis CP, Fone KCF. Behavioural characterisation of the effects of acute and repeated administration of GBR 12909 in rats: Further evaluation of a potential model of ADHD. Neuropharmacology. 2009;57:678–86. doi: 10.1016/j.neuropharm.2009.08.006. [DOI] [PubMed] [Google Scholar]
- Huang ZJ. Activity-dependent development of inhibitory synapses and innervations pattern: role of GABA signalling and beyond. J Physiology. 2009;587:1881–1888. doi: 10.1113/jphysiol.2008.168211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, Voorn P, Buijs RM, Pool CW, Uylings HBM. Development of the dopaminergic innervation in the prefrontal cortex of the rat. The Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Kann O, Papageorgiou IE, Draguhn A. Highly energized inhibitory interneurons are a central element for information processing in cortical networks. Journal of Cerebral Blood Flow & Metabolism. 2014;34:1270–82. doi: 10.1038/jcbfm.2014.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Lang CG. Effects of GBR 12909, a selective dopamine uptake inhibitor, on motor activity and operant behavior in the rat. European Journal of Pharmacology. 1989;167:385–95. doi: 10.1016/0014-2999(89)90447-0. [DOI] [PubMed] [Google Scholar]
- Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26(5):1604–15. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Adriani W, Terranova ML, Gerra G. Psychobiological risk factors for vulnerability to psychostimulants in human adolescents and animal models. Neurosci Biobehav Rev. 1999;23:993–1010. doi: 10.1016/s0149-7634(99)00032-9. [DOI] [PubMed] [Google Scholar]
- Laviola G, Macri S, Morley-Fletcher S, Adriani W. Risk-taking behavior in adolescent mice: psychobiological determinants and early epigenetic influence. Neurosci Biobehav Rev. 27:19–31. doi: 10.1016/s0149-7634(03)00006-x. [DOI] [PubMed] [Google Scholar]
- Laviola G, Marco EM. Passing the knife edge in adolescence: brain pruning and specification of individual lines of development. Neurosci Biobehav Rev. 2011;35:1631–3. doi: 10.1016/j.neubiorev.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lavoie S, Murray MM, Deppen P, Knyazeva MG, Berk M, et al. Glutathione Precursor, N-Acetyl-Cysteine, Improves Mismatch Negativity in Schizophrenia Patients. Neuropsychopharmacology. 2007;33:2187–99. doi: 10.1038/sj.npp.1301624. [DOI] [PubMed] [Google Scholar]
- Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TRE. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: stability over six years and specific associations with medication type and disorganization syndrome. Biol psychiatry. 2009;66:586–93. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo D, Adriani W, Cavaliere C, Cirillo G, Marco EM, Romano E, di Porzio U, Papa M, Perrone-Capano C, Laviola G. Methylphenidate to adolescent rats drives enduring changes of accumbal Htr7 expression: implications for impulsive behavior and neuronal morphology. Genes Brain Behav. 2009;8:356–68. doi: 10.1111/j.1601-183X.2009.00486.x. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Sheitman BB, Kinon BJ. Neurochemical sensitization in the pathophysiology of schizophrenia: deficits and dysfunction in neuronal regulation and plasticity. Neuropsychopharmacol. 1997;17:205–229. doi: 10.1016/S0893-133X(97)00045-6. [DOI] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J Neurosci. 2009;29(8):2344–54. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marco EM, Marci S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19:286–307. doi: 10.1007/s12640-010-9205-z. [DOI] [PubMed] [Google Scholar]
- Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–120. doi: 10.1038/nrn3155. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Kanno M, Togashi H, Ueno K-i, Otani H, et al. Involvement of GABAA receptors in the regulation of the prefrontal cortex on dopamine release in the rat dorsolateral striatum. European Journal of Pharmacology. 2003;482:177–84. doi: 10.1016/j.ejphar.2003.10.003. [DOI] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiser J, Weindl D, Hiller K. Complexity of dopamine metabolism. Cell Communication and Signaling : CCS. 2013;11:34. doi: 10.1186/1478-811X-11-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Beaulieu C. Quantitative aspects of synaptogenesis in the rat barrel field cortex with special reference to GABA circuitry. The Journal of Comparative Neurology. 1996;373:340–54. doi: 10.1002/(SICI)1096-9861(19960923)373:3<340::AID-CNE3>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Muir JL, Everitt BJ, Robbins TW. The Cerebral Cortex of the Rat and Visual Attentional Function: Dissociable Effects of Mediofrontal, Cingulate, Anterior Dorsolateral, and Parietal Cortex Lesions on a Five-Choice Serial Reaction Time Task. Cerebral Cortex. 1996;6:470–81. doi: 10.1093/cercor/6.3.470. [DOI] [PubMed] [Google Scholar]
- Murray GK, Cheng F, Clark L, Barnett JH, Blackwell AD, et al. Reinforcement and Reversal Learning in First-Episode Psychosis. Schizophrenia Bulletin. 2008;34:848–55. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. International Journal of Neuropsychopharmacology. 2008;11:851–76. doi: 10.1017/S1461145707008401. [DOI] [PubMed] [Google Scholar]
- Oztop D, Altun H, Baskol G, Ozsoy S. Oxidative stress in children with attention deficit hyperactivity disorder. Clin Biochem. 2012;45:745–8. doi: 10.1016/j.clinbiochem.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Paine TA, Slipp LE, Carlezon WA., Jr Schizophrenia-Like Attentional Deficits Following Blockade of Prefrontal Cortex GABAA Receptors. Neuropsychopharmacology. 2011;36:1703–13. doi: 10.1038/npp.2011.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pangratz-Fuehrer S, Hestrin S. Synaptogenesis of Electrical and GABAergic Synapses of Fast-Spiking Inhibitory Neurons in the Neocortex. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:10767–75. doi: 10.1523/JNEUROSCI.6655-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passetti F, Chudasama Y, Robbins TW. The Frontal Cortex of the Rat and Visual Attentional Performance: Dissociable Functions of Distinct Medial Prefrontal Subregions. Cerebral Cortex. 2002;12:1254–68. doi: 10.1093/cercor/12.12.1254. [DOI] [PubMed] [Google Scholar]
- Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nature reviews Neuroscience. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Paulus MP, Young JW, Kincaid MJ, et al. A reverse translational study of dysfunctional exploration in psychiatric disorders: from mice to men. Archives of general psychiatry. 2009;66:1072–80. doi: 10.1001/archgenpsychiatry.2009.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezze M, Dalley J, Robbins T. Remediation of attentional dysfunction in rats with lesions of the medial prefrontal cortex by intra-accumbens administration of the dopamine D2/3 receptor antagonist sulpiride. Psychopharmacology. 2009;202:307–13. doi: 10.1007/s00213-008-1384-4. [DOI] [PubMed] [Google Scholar]
- Pezze M, McGarrity S, Mason R, Fone KC, Bast T. Too Little and Too Much: Hypoactivation and Disinhibition of Medial Prefrontal Cortex Cause Attentional Deficits. The Journal of Neuroscience. 2014;34:7931–46. doi: 10.1523/JNEUROSCI.3450-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell SB, Sejnowski TJ, Behrens MM. Behavioral and neurochemical consequences of cortical oxidative stress on parvalbumin-interneuron maturation in rodent models of schizophrenia. Neuropharmacology. 2012;62:1322–31. doi: 10.1016/j.neuropharm.2011.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroz A, de Araújo M, da Silva Araújo T, de Souza G, Cavalcante L, et al. GBR 12909 administration as an animal model of bipolar mania: time course of behavioral, brain oxidative alterations and effect of mood stabilizing drugs. Metab Brain Dis. 2015;30:1207–15. doi: 10.1007/s11011-015-9697-6. [DOI] [PubMed] [Google Scholar]
- Radonjić NV, Knežević ID, Vilimanovich U, Kravić-Stevović T, Marina LV, et al. Decreased glutathione levels and altered antioxidant defense in an animal model of schizophrenia: Long-term effects of perinatal phencyclidine administration. Neuropharmacology. 2010;58:739–45. doi: 10.1016/j.neuropharm.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Reynolds GP, Beasley CL. GABAergic neuronal subtypes in the human frontal cortex – development and deficits in schizophrenia. Journal of Chemical Neuroanatomy. 2001;22:95–100. doi: 10.1016/s0891-0618(01)00113-2. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Progress in Brain Research. Elsevier; 2000. From arousal to cognition: the integrative position of the prefrontal cortex; pp. 469–83. [DOI] [PubMed] [Google Scholar]
- Rossignol DA, Frye RE. Melatonin in autism spectrum disorders: a systematic review and meta-analysis. Developmental Medicine & Child Neurology. 2011;53:783–92. doi: 10.1111/j.1469-8749.2011.03980.x. [DOI] [PubMed] [Google Scholar]
- Selemon LD, Zecevic N. Schizophrenia: a tale of two critical periods for prefrontal cortical development. Transl Psychiatry. 2015;5:1–11. doi: 10.1038/tp.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selten J-P, van der Ven E, Rutten BP, Cantor-Graae E. The social defeat hypothesis of schizophrenia: an update. Schizophr Bull. 2013;39:1180–1186. doi: 10.1093/schbul/sbt134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ. A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology. 2011;61:544–9. doi: 10.1016/j.neuropharm.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamford JA. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J Neurochem. 1989;52:1582–1589. doi: 10.1111/j.1471-4159.1989.tb09212.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EM, O’Donnell P. Inhibitory Interneurons, Oxidative Stress, and Schizophrenia. Schizophrenia Bulletin. 2012;38:373–6. doi: 10.1093/schbul/sbs052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarazi FI, Baldessarini RJ. Comparative postnatal development of dopamine D1, D2 and D4 receptors in rat forebrain. International Journal of Developmental Neuroscience. 2000;18:29–37. doi: 10.1016/s0736-5748(99)00108-2. [DOI] [PubMed] [Google Scholar]
- Tomasetti C, Dell’Aversano C, Iasevoli F, de Bartolomeis A. Homer splice variants modulation within cortico-subcortical regions by dopamine D2 antagonists, a partial agonist, and an indirect agonist: Implication for glutamatergic postsynaptic density in antipsychotics action. Neuroscience. 2007;150:144–58. doi: 10.1016/j.neuroscience.2007.08.022. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Post-pubertal Emergence of Prefrontal Cortical Up States Induced by D1–NMDA Co-activation. Cerebral Cortex. 2005;15:49–57. doi: 10.1093/cercor/bhh107. [DOI] [PubMed] [Google Scholar]
- Tseng K-Y, O'Donnell P. Dopamine Modulation of Prefrontal Cortical Interneurons Changes during Adolescence. Cerebral Cortex. 2007a;17:1235–40. doi: 10.1093/cercor/bhl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. D2 dopamine receptors recruit a GABA component for their attenuation of excitatory synaptic transmission in the adult rat prefrontal cortex. Synapse. 2007b;61:843–50. doi: 10.1002/syn.20432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Roux F, Rodriguez E, Rotarska-Jagiela A, Singer W. Neural synchrony and the development of cortical networks. Trends Cogn Sci. 2010;14:72–80. doi: 10.1016/j.tics.2009.12.002. [DOI] [PubMed] [Google Scholar]
- van Gaalen M, Brueggeman R, Bronius PC, Schoffelmeer AM, Vanderschuren LMJ. Behavioral disinhibition requires dopamine receptor activation. Psychopharmacology. 2006a;187:73–85. doi: 10.1007/s00213-006-0396-1. [DOI] [PubMed] [Google Scholar]
- van Gaalen MM, van Koten R, Schoffelmeer ANM, Vanderschuren LJMJ. Critical Involvement of Dopaminergic Neurotransmission in Impulsive Decision Making. Biological Psychiatry. 2006b;60:66–73. doi: 10.1016/j.biopsych.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Villagonzalo K-A, Dodd S, Dean O, Gray K, Tonge B, Berk M. Oxidative pathways as a drug target for the treatment of autism. Expert Opinion on Therapeutic Targets. 2010;14:1301–10. doi: 10.1517/14728222.2010.528394. [DOI] [PubMed] [Google Scholar]
- Volk DW, Lewis DA. Prenatal ontogeny as a susceptibility period for cortical GABA neuron disturbances in schizophrenia. Neuroscience. 2013;248:154–64. doi: 10.1016/j.neuroscience.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neuroscience & Biobehavioral Reviews. 2010;34:631–48. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Morris SE, Arrant AE, Nagel JM, Parylak S, Zhou G, Caster JM, Kuhn CM. Dopamine uptake inhibitors but not dopamine releasers induce greater increases in motor behavior and extracellular dopamine in adolescent rats than in adult male rats. J Pharmacol Exp Ther. 2010;335:124–32. doi: 10.1124/jpet.110.167320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waltz JA, Gold JM. Probabilistic reversal learning impairments in schizophrenia: Further evidence of orbitofrontal dysfunction. Schizophrenia Research. 2007;93:296–303. doi: 10.1016/j.schres.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaratto F, Laviola G, Adriani W. Choice with delayed or uncertain reinforcers in rats: influence of timeout duration and session length. Synapse. 2012;66:792–806. 7. doi: 10.1002/syn.21570. [DOI] [PubMed] [Google Scholar]