Abstract
In the vertebrate nervous system, the fast conduction of action potentials is potentiated by the myelin sheath, a multi-lamellar, lipid-rich structure that also provides vital trophic and metabolic support to axons. Myelin is elaborated by the plasma membrane of specialized glial cells, oligodendrocytes in the central nervous system (CNS) and Schwann cells (SCs) in the peripheral nervous system (PNS). The diseases that result from damage to myelin or glia, including multiple sclerosis and Charcot-Marie-Tooth disease, underscore the importance of these cells for human health. Therefore, an understanding of glial development and myelination is crucial in addressing the etiology of demyelinating diseases and developing patient therapies. In this review, we discuss new insights into the roles of mechanotransduction and cytoskeletal rearrangements as well as activity dependent myelination and axonal maintenance by glia. Together, these discoveries advance our knowledge of myelin and glia in nervous system health and plasticity throughout life.
Intrinsic factors guiding oligodendrocyte and SC development
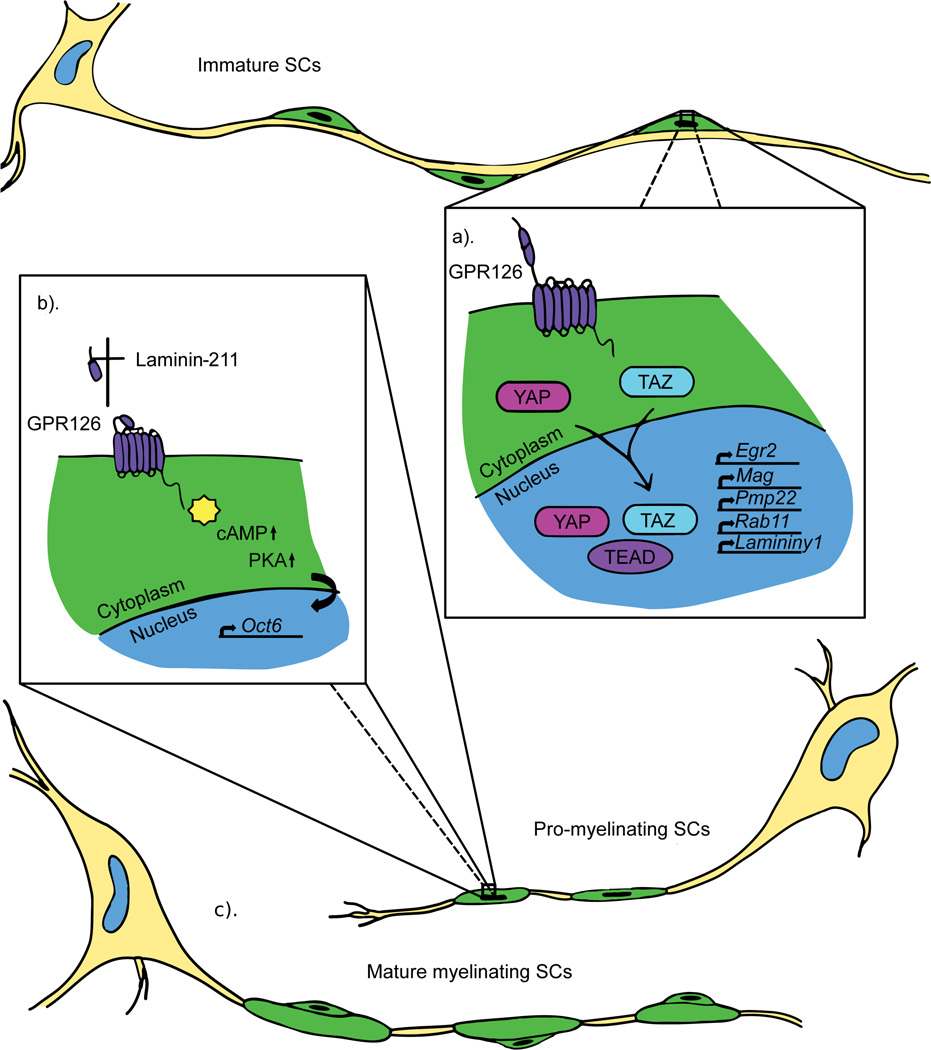
Although both cells produce myelin to insulate and support axons, oligodendrocytes and SCs differ early in their genesis. Oligodendrocytes originate from neuroepithelial precursors, whereas SCs are derived from the neural crest. Furthermore, one oligodendrocyte can myelinate multiple axon segments, but one SC myelinates only a single axon segment (Fig. 1, Fig. 2). This is achieved through a process called radial sorting in which cytoplasmic processes from immature SCs extend into axon bundles and “select” an axon segment [1]. SC development is mediated by a host of transcription factors and signaling molecules, including Sox10, which persists throughout development and differentiation, activating other transcription factors [1]. In pro-myelinating SCs, which have radially sorted axons and wrapped 1–1.5 turns around an axon, the G protein-coupled receptor (GPCR) GPR126/ADGRG6 elevates cAMP to promote expression of the transcription factor Oct6/Pou3f1 [1]. Oct6 and Sox10, along with other factors, activate the master regulator of PNS myelination, Krox-20/Egr2, which is essential for expression of critical myelin genes, including Myelin basic protein (Mbp) [1].
Figure 1.
Mechanotransduction plays a critical role in SC development and differentiation. Immature SCs migrate and divide along growing axons. The forces associated with migration are thought to activate the mechanotransducers YAP/TAZ in SC cytoplasm, which then translocate to the nucleus where they interact with the TEAD family transcription factors to drive expression of important myelin genes (a). After SCs have formed a “1:1” relationship with axons in the pro-myelinating stage, maturation of the basal lamina and subsequent polymerization of Laminin-211 is thought to activate GPR126, which initiates a transcriptional cascade activating Oct6 and promoting myelination (b). Eventually, SCs wrap myelin around axon segments to form internodes (c).
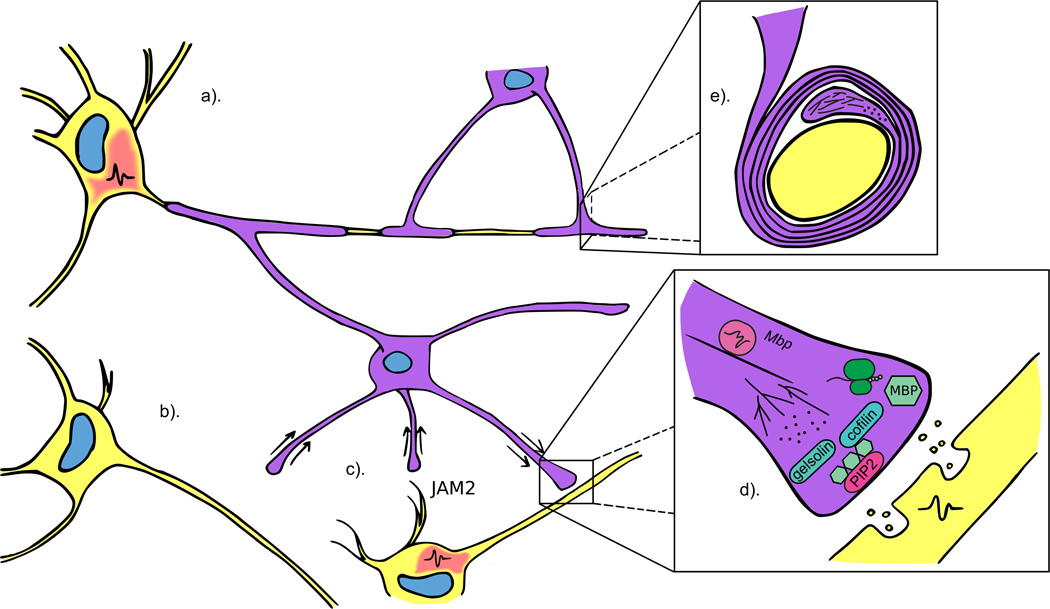
Figure 2.
Multiple factors fine tune the myelination potential of oligodendrocytes. Oligodendrocytes preferentially myelinate electrically active axons (a) and retract processes from inactive axons (b). Furthermore, the intrinsic myelination program is moderated by negative regulators, such as JAM2, which are expressed on dendrites (c). Vesicular release from active axons initiates a cascade of events, one of which is the translation of locally transported Mbp mRNA. MBP then competes with the factors gelsolin and cofilin for binding to PIP2 on the inner oligodendrocyte membrane, resulting in release of the two proteins and subsequent actin disassembly (d). During wrapping, filamentous actin is located at the leading edge of the inner tongue and is proposed to propel the membrane forward by actin disassembly (e) (image adapted from Nawaz et al. 2015).
Proliferative and migratory oligodendrocyte precursor cells (OPCs) extend and retract numerous processes during development [2]. Recent work has found that OPCs can migrate along blood vessels in a Wnt-dependent manner involving the receptor-ligand pair Cxcr4-Cxcl12, which are expressed on OPCs and endothelial cells, respectively [3]. Oligodendrocyte differentiation requires some shared SC factors, including Sox10 and Yin yang 1 (Yy1), in addition to the oligodendrocyte specific regulators Olig1, Olig2, Nkx2.2 [2] and Myelin regulatory factor, Myrf, which plays an analogous role to Krox-20 [4]. Recent work in SCs and oligodendrocytes has identified novel roles for signaling molecules, including a suite of GPCRs, GPR17, GPR56 and GPR37 in the CNS [5][6][7][8] and GPR44 and the zinc finger Zeb2 in the PNS [9][10][11]. While new myelin regulators remain to be uncovered, elucidating the function of known molecules and pathways is key to understanding myelination in development and repair.
Mechanical regulation of myelinating glia during development and differentiation
A unique signaling mechanism in SCs occurs via the basal lamina (BL), and recent evidence points to the molecular mechanisms by which this structure mechanically regulates myelination. In SCs, GPR126 can interact with axonally-derived Prion protein (PrPc)[12] as well as two SC-derived components of the BL, collagen IV and Laminin-211 [13][14]. Laminin-211 polymerization was proposed to activate GPR126 mechanically, initiating SC myelination (Fig. 1)[13], and SCs respond to mechanical properties of the BL with intracellular molecules such as Focal adhesion kinase (FAK)[15]. Recently, two Hippo pathway signaling molecules, YAP and TAZ (YAP/TAZ), have been implicated as mediators of mechanotransduction during SC development. YAP/TAZ respond to mechanical or chemical stimuli and translocate to the nucleus to regulate gene transcription. In vitro culture experiments found nuclear localized YAP/TAZ during SC spreading, plating on stiffer surfaces, plating on Laminin-211, and experimentally applied stretching (Fig. 1). Analysis of mouse mutants demonstrated that YAP/TAZ signaling is required for radial sorting and myelination [16]. YAP also has a role in modulating internode length during development and disease. [17]. In concert with TEAD transcription factors, nuclear YAP activates genes involved in the myelination program, including Krox-20/Egr2 and Myelin associated glycoprotein (MAG), Rab11, and Laminin ϒ1. The polarity protein Crb3 inhibits YAP nuclear translocation and knock-down of Crb3 increases the length of SC myelin segments [17]. Crb3 is therefore thought to modulate YAP activity to temper internode length. Interestingly, a dystrophic mouse model of peripheral neuropathy exhibited reduced nuclear YAP with shorter internodes, a phenotype that could be rescued by manual sciatic nerve elongation via femoral distraction to increase nuclear YAP [17]. These data suggest that migration of SCs along axons and/or longitudinal nerve growth could activate YAP/TAZ signaling during development. Perhaps physical maturation of the BL and GPR126 activation is similarly linked to developmental YAP/TAZ signaling, as GPCRs are known upstream regulators of this pathway [18]. Downstream of YAP/TAZ signaling, TEAD1 directly regulates Peripheral myelin protein 22 (Pmp22), mis-regulation of which causes Charcot-Marie-Tooth disease [19].
While a role for YAP/TAZ signaling in oligodendrocytes has not been described, these cells are also responsive to mechanical stimuli. OPC proliferation and migration can be altered by plating on substrates of varying stiffness [20], resulting in differentiation in a density-dependent manner. Plating at high density with polystyrene beads promoted OPC differentiation, demonstrating that this process is mediated by physical space limitations, rather than by extracellular signals [21]. How might external forces drive oligodendrocyte development? A recent report demonstrates that mechanical stimuli interact with the nucleus via the Linker of Nucleoskeleton and Cytoskeleton complex (LINC). One LINC complex component in particular, SYNE1, which binds the nuclear envelope and actin, was shown to link extracellular stimuli, including high density plating with beads and mechanical force using a cell-compression device, to nuclear changes [22]. The switch from primarily euchromatin to heterochromatin is a hallmark of differentiation in oligodendrocytes [23] and requires SYNE1 [22]. Histone modifying complexes, specifically HDAC1 and HDAC2, affect nuclear reorganization by altering chromatin configuration and are essential for oligodendrocyte and SC differentiation. Epigenetic regulation of oligodendrocytes and SCs during development and myelination is reviewed in greater detail elsewhere [1][4].
Producing the myelin sheath
In a feat of cellular morphogenesis, glial cells massively upregulate production of their plasma membrane and spiral it around an axon segment. These dramatic shape changes require extensive cytoskeletal rearrangements, and great inroads have been made in understanding how such rearrangements drive myelin sheath formation. Using zebrafish in vivo imaging and 3D electron microscopic reconstruction, Snaidero and colleagues demonstrated that the plasma membrane inner tongue maintains contact with the axon segment as it wraps and progressively spreads out to form the myelin internode. Initial inner tongue movement is aided by the transport of critical material, including mRNA and protein, through nanometer wide channels [24]. How is the inner tongue propelled around the axon? Two elegant studies suggest actin dynamics as a driving force. Nawaz et al. used zebrafish live imaging to determine that F-actin is initially localized to the leading edge, but later excluded from the developing membrane. Culture experiments demonstrated that F-actin depolymerization by drug treatment increased cell spreading, leading to a model in which the force of actin filament disassembly propels the membrane forward (Fig. 2). Interestingly, Zuchero and colleagues found that actin disassembly is driven in part by competition of MBP protein for binding to PI(4,5)P2, which then releases the actin disassembly factors gelsolin and cofilin (Fig. 2). The dynamic interplay between actin assembly during development and disassembly during myelination highlights a potential form of temporal control. Because actin assembly is necessary for OPC development [25], the timing of disassembly must be tightly regulated. What factors could influence timing? One possibility is axonal activity. In vitro, vesicular glutamate release from axons in response to electrical stimulation phosphorylates Fyn kinase at the oligodendrocyte membrane, leading to local translation of Mbp [26]. Together, these discoveries implicate axons in temporally influencing myelination via actin disassembly.
A role for actin dynamics has similarly been described in the PNS. Inhibition of F-actin formation resulted in delayed SC differentiation [27] and SC-specific deletion of neural Wiskott-Aldrich syndrome protein (N-WASp), a mechanical transducer that remodels actin via Arp2/3, inhibits myelination and causes motor deficits [28][29]. Unlike oligodendrocytes, SCs must sort axons prior to myelination. Radial sorting, like myelination, requires dramatic cell shape changes that are mediated by proteins regulating the cytoskeleton, including the Rho family GTPases Rac1 and Cdc42 [30][31]. Although these studies point to the importance of cytoskeletal rearrangements in SC development, less is known about the forces driving myelination. Interestingly, both oligodendrocytes and SCs transport Mbp along microtubules to sites of membrane elaboration [32][33]. Whether actin disassembly and local translation of Mbp in SCs have roles in driving myelination remains to be determined.
Activity-dependent control of myelination and myelin maintenance
Oligodendrocytes have intrinsic myelinating capacity and can myelinate fixed axons in addition to synthetic nanofibers and micropillars [21][34][35]. What prevents oligodendrocytes from myelinating dendrites or other cells in the CNS? Using a candidate approach, Redmond et al. identified the transmembrane protein JAM2 as a negative regulator of oligodendrocyte myelination (Fig. 2). Overexpression of JAM2 attenuated the ability of plated oligodendrocytes to myelinate micropillars, and loss of Jam2 in a mouse model caused an increase in myelinated neuronal cell bodies, implicating repulsive cues in modulating myelination [36]. Another study indicates that a component of intrinsic myelination may be hardwired in oligodendrocytes. When plated on nanofibers, spinal cord oligodendrocytes produced more myelin than cortex-derived oligodendrocytes [37]. Are these regional differences due to environmental cues or other factors? One possibility is that there are specific subtypes of oligodendrocytes with distinct myelinating capacities. To this end, single-cell RNA sequencing was used to characterize cell types in the murine hippocampus and cortex. Interestingly, findings from these experiments suggested seven distinct subtypes of oligodendrocytes, including OPCs [38]. Furthermore, a recent study using the same technique to probe oligodendrocyte heterogeneity in more detail proposed 13 distinct populations of oligodendrocytes in the mouse brain [39].
While negative regulators prevent aberrant myelination in the CNS, variation in myelin distribution along single axons of the developing cortex suggests a fine-tuning of myelination capacity beyond an intrinsic program [40]. Indeed, early work implicated electrical signaling as an instructive cue in oligodendrocyte development and myelination [41][42]. How might activity influence myelination? Previous work demonstrated that neurons form functional synapses on OPCs [43]. Recent research suggests, however, that while oligodendrocytes are more likely to myelinate electrically active axons, this occurs independently of synapse formation, instead relying on vesicular release of glutamate and ATP [44]. A critical role for vesicle transport in myelination was confirmed in vivo using zebrafish. Mensch and colleagues used tetanus toxin to inhibit vesicular release, resulting in fewer sheaths, while increasing activity led to more sheaths per oligodendrocyte [45]. In a complementary study, Hines et al. found that initial oligodendrocyte axon ensheathment is activity independent, but preferential contact is maintained on axons releasing vesicles. Processes are either retracted from inactive axons or produce shorter myelin sheaths (Fig. 2) [46]. However, the necessity of vesicular release is differentially regulated in the CNS. This cue is required for myelination by reticulospinal neurons but not by commissural primary ascending (CoPA) neurons [47]. Why there are different regulatory mechanisms depending on neuronal subtype is an area of future investigation.
Rather than a simple static insulator deposited during development, myelin is now recognized as a player in nervous system plasticity. Myelination during development and in adulthood is modulated by an animal’s social experience [48][49] and myelin remodeling occurs throughout life [50]. Furthermore, learning new skills, such as juggling and language acquisition, results in changes to myelin [51][52]. How do myelin alterations occur and how do they affect nervous system plasticity? One possibility is that activity stimulates formation of new oligodendrocytes. To this end, it was shown that differentiation of oligodendrocytes from precursors is necessary for mice to learn a new skill effectively [53], and that neuronal activity promotes oligodendrogenesis and concomitant behavior changes [54]. What is the role of new oligodendrocytes? A recent paper examined the timing of oligodendrogenesis in response to learning and found significant formation of new oligodendrocytes in mice learning to navigate a complex wheel within the first 2.5 hours. Furthermore, mice unable to form new oligodendrocytes exhibit learning deficits as early as 2–3 hours after first encountering the wheel. This early necessity for new oligodendrocytes in the learning process indicates a level of active involvement [55]. Whether this occurs through modifying circuits, providing metabolic support or an as yet undetermined mechanism is an area of future investigation.
Myelin and metabolism
In addition to promoting efficient action potential propagation, myelin is also critical for trophic and metabolic support of axons [56]. To provide metabolites to axons accurately, glia must “know” the metabolic requirements of axons. Could electrical activity by axons function as a means of communication? NMDA glutamate receptors are present on oligodendrocytes [57][58], but were thought to be dispensable for oligodendrocyte development, myelination, and injury response [59][60]. However, recent work has implicated these receptors in mediating calcium influxes in mature oligodendrocytes [61]. Furthermore, NMDA receptors have been shown to link electrical activity in axons to the production of lactate by oligodendrocytes, a critical energy source for axons. By “learning” via NMDA receptor signaling which axons are fast spiking, oligodendrocytes are able to vary lactate production. Loss of NMDA receptors specifically in oligodendrocytes, while not critical during development, causes eventual neurodegeneration from reduced metabolism [62]. Lactate production and metabolic support of axons by SCs is also critical in the PNS [63][64]. The lactate transporter that is used by oligodendrocytes, MCT1, is present in SCs and mediates axonal health [65][66]. However, these studies did not address a role for electrical activity in SC regulation of axonal metabolism. Interestingly, a recent report found that ATP release by electrically active axons mediates mitochondrial signaling to promote energy production in SCs and disruption of this signaling pathway resulted in hypomyelination [67].
Conclusion and Outlook
From static insulating factor to dynamic structure critical in enabling nervous system plasticity, our conceptions about myelin have changed dramatically in recent years. However, although both SCs and oligodendrocytes produce myelin, the mechanisms by which they do so are distinct (Fig. 3). Oligodendrocytes possess an intrinsic ability to myelinate that is fine-tuned by environmental cues, such as mechanical stimulation and electrical activity from axons. New studies suggest the existence of distinct subsets of oligodendrocytes, raising the possibility that such heterogeneity could contribute to differences in innate myelination and re-myelination abilities. It will be exciting to uncover the extent to which interplay between the extracellular environment and oligodendrocyte heterogeneity influences myelination during development and repair. Advances in cellular techniques, including 3D electron microscopic reconstructions and live imaging, have contributed to a better understanding of the physical process of myelination by oligodendrocytes, including a surprising role for actin dynamics. Further research into the cytoskeletal and architectural reorganization of membrane during myelination will help us better understand this feat of morphogenesis and elucidate how to promote re-myelination in disease or injury.
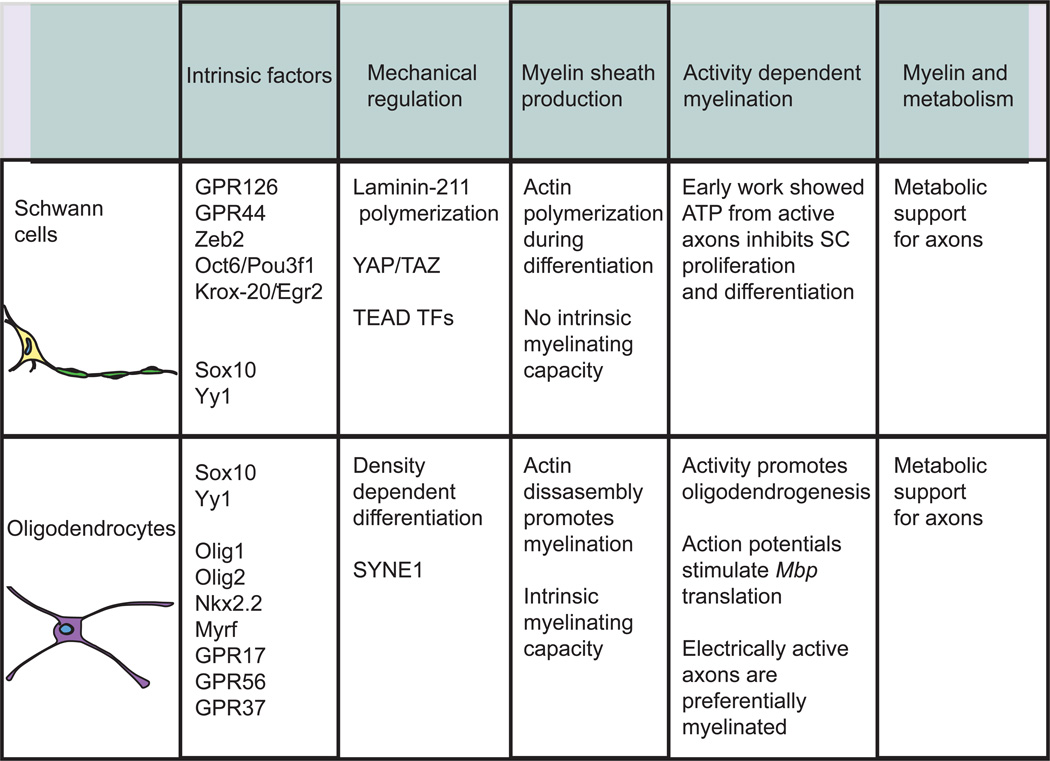
Figure 3.
Comparing and contrasting SC and oligodendrocyte development and differentiation. Although both SCs and oligodendrocytes produce the myelin critical for nervous system function, there are important differences in the mechanisms by which they generate myelin. The similarities and differences between SCs and oligodendrocytes discussed in this review are summarized in the table above.
SCs are incapable of myelinating inert structures [37], relying instead on instructive cues. PNS myelination also appears to be less finely tuned compared to the CNS, with stricter correlations between axon diameter and myelin thickness. Whether PNS myelin undergoes dynamic changes similar to CNS myelin has not been well studied. While early work demonstrated a role for axonal activity in modulating SC development and myelination [68], this area of research has lagged behind progress made in the CNS. The mechanisms by which SCs elaborate a myelin sheath are similarly mysterious. One pertinent question is whether actin dynamics, which are vital during CNS myelination, play an analogous role in SCs. A current focus in SCs is on mechanotransduction, and advances in this area are already guiding therapeutic developments through techniques such as optimal matrices for acellular nerve allografts [69].
In summary, the studies highlighted in this review demonstrate that myelination in the CNS and PNS is distinct while sharing some similar processes. Future work would benefit from comparing and contrasting these systems to clarify common or unique aspects of development and myelination. Therapeutic advances will be realized through continued investigation into the mechanisms and controls of myelination from genesis through maturity.
Highlights.
Schwann cell development is mediated by mechanical signals.
Some mechanical signals in Schwann cells activate the Hippo signaling pathway.
Intrinsic oligodendrocyte myelinating capacity is fine-tuned by the environment.
Lactate made by myelinating glia is critical for axonal metabolism and health.
Acknowledgments
We thank members of the Monk lab for helpful discussions and gratefully acknowledge Matt McCoy for help with figures and editing the text. We apologize to our colleagues whose primary work we were unable to cite due to space limitations. A.L.H. is supported by the Philip and Sima Needleman Foundation and by an NIH fellowship (F31 NS096814). Work in the Monk lab is supported by grants from the NIH (R01 NS079445, R01 HD80601), the Muscular Dystrophy Association (MDA 293295), the Missouri Spinal Cord Injury/Disease Research Program (16-03), and K.R.M. is a Harry Weaver Neuroscience Scholar of the National Multiple Sclerosis Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Monk KR, Feltri ML, Taveggia C. New insights on schwann cell development. Glia. 2015;63:1376–1393. doi: 10.1002/glia.22852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simons M, Armin-Nave K. Oligodendrocytes: myelination and axonal support. Cold Spring Harb. Perspect. Biol. 2015;8:a020479. doi: 10.1101/cshperspect.a020479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tsai H, Niu J, Munji R, Davalos D, Chang J, Zhang H, Tien A, Kuo CJ, Chan JR, Daneman R, Fancy SJ. Oligodendrocyte precursors migrate along vasculature in the developing nervous system. Science. 2016;351:379–384. doi: 10.1126/science.aad3839. **OPCs were shown to migrate along blood vessels via Cxcr4 on OPCs and Cxcl12 on endothelial cells.
- 4.Emery B, Lu QR. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harb. Perspect. Biol. 2015;7:a020461. doi: 10.1101/cshperspect.a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Wu H, Wang S, Koito H, Li J, Ye F, Hoang J, Escobar SS, Gow A, Arnett HA, et al. The oligodendrocyte-specific G protein-coupled receptor GPR17 is a cell-intrinsic timer of myelination. Nat. Neurosci. 2009;12:1398–1406. doi: 10.1038/nn.2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ackerman SD, Garcia C, Piao X, Gutmann DH, Monk KR. The adhesion GPCR Gpr56 regulates oligodendrocyte development via interactions with Gα12/13 and RhoA. Nat. Commun. 2015;6:6122. doi: 10.1038/ncomms7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giera S, Deng Y, Luo R, Ackerman SD, Mogha A, Monk KR, Ying Y, Jeong S-J, Makinodan M, Bialas AR, et al. The adhesion G protein-coupled receptor GPR56 is a cell-autonomous regulator of oligodendrocyte development. Nat. Commun. 2015;6:6121. doi: 10.1038/ncomms7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang H-J, Vainshtein A, Maik-Rachline G, Peles E. G protein-coupled receptor 37 is a negative regulator of oligodendrocyte differentiation and myelination. Nat. Commun. 2016;7:10884. doi: 10.1038/ncomms10884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Trimarco A, Forese MG, Alfieri V, Lucente A, Brambilla P, Dina G, Pieragostino D, Sacchetta P, Urade Y, Boizet-Bonhoure B, et al. Prostaglandin D2 synthase/GPR44: a signaling axis in PNS myelination. Nat. Neurosci. 2014;17:1682–1692. doi: 10.1038/nn.3857. [DOI] [PubMed] [Google Scholar]
- 10.Wu LMN, Wang J, Conidi A, Zhao C, Wang H, Ford Z, Zhang L, Zweier C, Ayee BG, Maurel P, et al. Zeb2 recruits HDAC–NuRD to inhibit Notch and controls Schwann cell differentiation and remyelination. Nat. Neurosci. 2016;19:1060–1072. doi: 10.1038/nn.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintes S, Brinkmann BG, Ebert M, Fröb F, Kungl T, Arlt FA, Tarabykin V, Huylebroeck D, Meijer D, Suter U, et al. Zeb2 is essential for Schwann cell differentiation, myelination and nerve repair. Nat. Neurosci. 2016;19:1050–1059. doi: 10.1038/nn.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Küffer A, Lakkaraju AKK, Mogha A, Petersen SC, Airich K, Doucerain C, Marpakwar R, Bakirci P, Senatore A, Monnard A, et al. The prion protein is an agonistic ligand of the G protein-coupled receptor Adgrg6. Nature. 2016;536:464–468. doi: 10.1038/nature19312. *This manuscript described an unexpected axonally-derived binding partner for GPR126.
- 13. Petersen SC, Luo R, Liebscher I, Giera S, Jeong SJ, Mogha A, Ghidinelli M, Feltri ML, Schöneberg T, Piao X, Monk KR. The adhesion GPCR GPR126 has distinct, domain-dependent functions in Schwann cell development mediated by interaction with Laminin-211. Neuron. 2015;85:755–769. doi: 10.1016/j.neuron.2014.12.057. *GPR126, which is necessary for SC myelination, was proposed to be activated mechanically in part by maturation of the basal lamina, specifically through interaction with Laminin-211.
- 14. Paavola KJ, Sidik H, Zuchero JB, Eckart M, Talbot WS. Type IV collagen is an activating ligand for the adhesion G protein-coupled receptor GPR126. Sci. Signal. 2014;7:1–9. doi: 10.1126/scisignal.2005347. *This manuscript defined collagen IV as the first known binding partner for the adhesion GPCR GPR126.
- 15.Grove M, Brophy PJ. FAK Is required for Schwann cell spreading on immature basal lamina to coordinate the radial sorting of peripheral axons with myelination. J. Neurosci. 2014;34:13422–13434. doi: 10.1523/JNEUROSCI.1764-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poitelon Y, Lopez-Anido C, Catignas K, Berti C, Palmisano M, Williamson C, Ameroso D, Abiko K, Hwang Y, Gregorieff A, et al. YAP and TAZ control peripheral myelination and the expression of laminin receptors in Schwann cells. Nat. Neurosci. 2016;19:879–887. doi: 10.1038/nn.4316. ** The classical signaling molecules YAP and TAZ were shown to respond to mechanical stimuli and relocate to the nucleus to influence SC development and myelination. With references 17 and 19, this study defined a key role for the Hippo pathway in SC development.
- 17. Fernando RN, Cotter L, Perrin-Tricaud C, Berthelot J, Bartolami S, Pereira JA, Gonzalez S, Suter U, Tricaud N. Optimal myelin elongation relies on YAP activation by axonal growth and inhibition by Crb3/Hippo pathway. Nat. Commun. 2016;7:1–14. doi: 10.1038/ncomms12186. *YAP nuclear translocation, mediated by Crb3, was proposed to regulate internode length. Shorter internodes in a dystrophic mouse model were rescued by increased nuclear YAP. With references 16 and 19, this study defined a key role for the Hippo pathway in SC development.
- 18.Varelas X. The Hippo pathway effectors TAZ and YAP in development, homeostasis and disease. Development. 2014;141:1614–1626. doi: 10.1242/dev.102376. [DOI] [PubMed] [Google Scholar]
- 19. Lopez-Anido C, Poitelon Y, Gopinath C, Moran JJ, Ma KH, Law WD, Antonellis A, Feltri ML, Svaren J. Tead1 regulates the expression of Peripheral Myelin Protein 22 during Schwann cell development. Hum. Mol. Genet. 2016;0:1–15. doi: 10.1093/hmg/ddw158. *This study demonstrates how Hippo signaling promotes expression of key myelin genes in SCs. With references 16 and 18, this study defined a key role for the Hippo pathway in SC development.
- 20.Jagielska A, Norman AL, Whyte G, Van Vliet KJ, Guck J, Franklin RJM. Mechanical environment modulates biological properties of oligodendrocyte progenitor cells. Stem Cells Dev. 2012;21:2905–2914. doi: 10.1089/scd.2012.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg SS, Kelland EE, Tokar E, De La Torre AR, Chan JR. The geometric and spatial constraints of the microenvironment induce oligodendrocyte differentiation. PNAS. 2008;105:14662–14667. doi: 10.1073/pnas.0805640105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Magri L, Zhang F, Marsh NO, Albrecht S, Huynh JL, Kaur J, Kuhlmann T, Zhang W, Slesinger PA, Cassacia P. Chromatin landscape defined by repressive histone methylation during oligodendrocyte differentiation. J. Neurosci. 2015;35:352–365. doi: 10.1523/JNEUROSCI.2606-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hernandez M, Patzig J, Mayoral SR, Costa KD, Chan JR, Casaccia P. Mechanostimulation promotes nuclear and epigenetic changes in oligodendrocytes. J. Neurosci. 2016;36:806–813. doi: 10.1523/JNEUROSCI.2873-15.2016. *Members of the LINC complex, including SYNE1, were shown to play a role in relaying signals from the periphery to the nucleus, allowing oligodendrocytes to respond to mechanical stimuli by altering the chromatin landscape.
- 24.Snaidero N, Möbius W, Czopka T, Hekking LHP, Mathisen C, Verkleij D, Goebbels S, Edgar J, Merkler D, Lyons DA, et al. Myelin membrane wrapping of CNS axons by PI(3,4,5)P3-dependent polarized growth at the inner tongue. Cell. 2014;156:277–290. doi: 10.1016/j.cell.2013.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim H, Dibernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 Is required for oligodendrocyte morphogenesis and normal CNS myelination. J. Neurosci. 2006;26:5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wake H, Lee PR, Fields RD. Control of local protein synthesis and initial events in myelination by action potentials. Science. 2011;333:1647–1651. doi: 10.1126/science.1206998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Valle C, Gorman D, Gomez AM, Bunge MB. Actin plays a role in both changes in cell shape and gene-expression associated with Schwann cell myelination. J. Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin F, Dong B, Georgiou J, Jiang Q, Zhang J, Bharioke A, Qiu F, Lommel S, Feltri ML, Wrabetz L, et al. N-WASp is required for Schwann cell cytoskeletal dynamics, normal myelin gene expression and peripheral nerve myelination. Development. 2011;1337:1329–1337. doi: 10.1242/dev.058677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Novak N, Bar V, Sabanay H, Frechter S, Jaegle M, Snapper SB, Meijer D, Peles E. N-WASP is required for membrane wrapping and myelination by Schwann cells. J. Cell Biol. 2011;192:243–250. doi: 10.1083/jcb.201010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Armin-Nave K, Franklin RJM, Meijer D, et al. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system development. J. Cell Biol. 2007;177:1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nodari A, Zambroni D, Quattrini A, Court FA, Urso AD, Recchia A, Tybulewicz VLJ, Wrabetz L, Feltri ML. β1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J. Cell Biol. 2007;177:1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Colman DR, Kreibich G, Frey AB, Sabatini DD. Synthesis and incorporation of myelin polypeptides into CNS myelin. J. Cell Biol. 1982;95:598–608. doi: 10.1083/jcb.95.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Trapp BD, Moench T, Pulley M, Barbosa E, Tennekoon G, Griffin J. Spatial segregation of mRNA encoding myelin-specific proteins. 1987;84:7773–7777. doi: 10.1073/pnas.84.21.7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee S, Leach MK, Redmond SA, Chong SYC, Mellon SH, Tuck SJ, Feng Z-Q, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nat. Methods. 2012;9:917–922. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mei F, Fancy SPJ, Shen YA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, et al. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nat. Med. 2014;20:954–960. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Redmond SA, Mei F, Eshed-Eisenbach Y, Osso LA, Leshkowitz D, Shen YA, Kay JN, Aurrand-Lions M, Lyons DA, Peles E, Chan JR. Somatodendritic expression of JAM2 inhibits oligodendrocyte myelination. Neuron. 2016;91:1–13. doi: 10.1016/j.neuron.2016.07.021. **The transmembrane protein JAM2 was found to be a negative regulator of oligodendrocyte myelination, preventing these cells from aberrantly myelinating dendrites or other CNS cell types.
- 37. Bechler ME, Byrne L, ffrench-Constant C. CNS myelin sheath lengths are an intrinsic property of oligodendrocytes. Curr. Biol. 2015;25:2411–2416. doi: 10.1016/j.cub.2015.07.056. *Oligodendrocytes taken from the cortex and spinal cord were shown to possess different innate abilities to myelinate innert microfibers. This differential capacity was maintained even when the diameter of the microfibers was altered.
- 38.Zeisel A, Muñoz-Manchado AB, Codeluppi S, Lönnerberg P, La Manno G, Jureus A, Marques S, Munguba H, He L, Betsholtz C, et al. Cell types in the mouse cortex and hippocampus revealed by single-cell RNA-seq. Science. 2015;347:1138–1142. doi: 10.1126/science.aaa1934. [DOI] [PubMed] [Google Scholar]
- 39. Marques S, Zeisel A, Codeluppi S, Bruggen D Van, Falcão AM, Xiao L, Li H, Häring M, Hochgerner H, Romanov RA, et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science. 2016;352:1326–1329. doi: 10.1126/science.aaf6463. **RNA-seq was used to profile oligodendrocyte progenitors throughout development and differentiation, proposing distinct oligodendrocyte populations in the adult mouse.
- 40.Tomassy GS, Berger DR, Chen H-H, Kasthuri N, Hayworth KJ, Vercelli A, Seung HS, Lichtman JW, Arlotta P. Distinct profiles of myelin distribution along single axons of pyramidal neurons in the neocortex. Science. 2014;344:319–324. doi: 10.1126/science.1249766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shrager P, Novakovic SD. Control of myelination, axonal growth, and synapse formation in spinal cord explants by ion channels and electrical activity. Dev. Brain Res. 1995;88:68–78. doi: 10.1016/0165-3806(95)00081-n. [DOI] [PubMed] [Google Scholar]
- 42.Demerens C, Stankoff B, Logak M, Anglade P, Allinquant B, Couraud F, Zalc B, Lubetzki C. Induction of myelination in the central nervous system by electrical activity. PNAS. 1996;93:9887–9892. doi: 10.1073/pnas.93.18.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin S, Bergles DE. Synaptic signaling between GABAergic interneurons and oligodendrocyte precursor cells in the hippocampus. Nat. Neurosci. 2004;7:24–32. doi: 10.1038/nn1162. [DOI] [PubMed] [Google Scholar]
- 44.Wake H, Ortiz FC, Woo DH, Lee PR, Angulo MC, Fields RD. Nonsynaptic junctions on myelinating glia promote preferential myelination of electrically active axons. Nat. Commun. 2015;6:7844. doi: 10.1038/ncomms8844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mensch S, Baraban M, Almeida R, Czopka T, Ausborn J, El Manira A, Lyons DA. Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 2015;18:6–10. doi: 10.1038/nn.3991. * In vivo zebrafish work determined that vesicular release by axons regulates the number of myelin sheaths per oligodendrocyte. Conversely, sheath number increases in response to increased vesicular release.
- 46. Hines JH, Ravanelli AM, Schwindt R, Scott EK, Appel B. Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 2015;18:683–689. doi: 10.1038/nn.3992. *Vesicular release by electrically active axons in live zebrafish biases oligodendrocyte process selection. Although electrical activity was not observed to be necessary for initial axon ensheathment by oligodendrocyte processes, inhibiting activity of specific axons results in more frequent process retraction and overall shorter myelin sheaths.
- 47. Koudelka S, Voas MG, Almeida RG, Baraban M, Soetaert J, Meyer MP, Talbot WS, Lyons DA. Individual neuronal subtypes exhibit diversity in CNS myelination mediated by synaptic vesicle release. Curr. Biol. 2016;26:1447–1455. doi: 10.1016/j.cub.2016.03.070. **Although vesicular release by reticulospinal axons was shown to be important in mediating myelination by oligodendrocytes, inhibiting vesicular release in CoPA neurons did not have the same effect. This indicates that there may be differential regulatory mechanisms between neuronal subtypes.
- 48.Makinodan M, Rosen KM, Ito S, Corfas G. A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science. 2012;337:1357–1360. doi: 10.1126/science.1220845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu J, Dietz K, Deloyht JM, Pedre X, Kelkar D, Kaur J, Vialou V, Lobo MK, Dietz DM, Nestler EJ, et al. Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 2012;15:1621–1624. doi: 10.1038/nn.3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young KM, Psachoulia K, Tripathi RB, Dunn S, Cossell L, Attwell D, Tohyama K, Richardson WD. Oligodendrocyte dynamics in the healthy adult CNS : evidence for myelin remodeling. Neuron. 2013;77:873–885. doi: 10.1016/j.neuron.2013.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Scholz J, Klein MC, Behrens TEJ, Johansen-Berg H. Training induces changes in white matter architecture. Nat. Neurosci. 2010;12:1370–1371. doi: 10.1038/nn.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. J. Cogn. Neurosci. 2012;24:1664–1670. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- 53.McKenzie IA, Ohayon D, Li H, de Faria JP, Emery B, Tohyama K, Richardson WD. Motor skill learning requires active central myelination. Science. 2014;346:318–322. doi: 10.1126/science.1254960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gibson EM, Purger D, Mount CW, Goldstein AK, Lin GL, Wood LS, Inema I, Miller SE, Bieri G, Zuchero JB, et al. Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science. 2014;344:480–481. doi: 10.1126/science.1252304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Xiao L, Ohayon D, McKenzie IA, Sinclair-Wilson A, Wright JL, Fudge AD, Emery B, Li H, Richardson WD. Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 2016;19:1210–1217. doi: 10.1038/nn.4351. **Differentiation of new oligodendrocytes was found to occur very shortly after mice were exposed to a complex wheel to model learning. An inability to produce new oligodendrocytes similarly led to early deficits in learning, implicating generation of new oligodendrocytes as having an active role in this process.
- 56.Armin-Nave K. Myelination and the trophic support of long axons. Nat. Rev. Neurosci. 2010;11:275–283. doi: 10.1038/nrn2797. [DOI] [PubMed] [Google Scholar]
- 57.Káradóttir R, Cavelier P, Bergersen LH, Attwell D. NMDA receptors are expressed in oligodendrocytes and activated in ischaemia. Nature. 2005;438:1162–1166. doi: 10.1038/nature04302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salter MG, Fern R. NMDA receptors are expressed in developing oligodendrocyte processes and mediate injury. Nature. 2005;438:1167–1171. doi: 10.1038/nature04301. [DOI] [PubMed] [Google Scholar]
- 59.De Biase LM, Kang SH, Baxi EG, Fukaya M, Pucak ML, Mishina M, Calabresi PA, Bergles DE. NMDA receptor signaling in oligodendrocyte progenitors is not required for oligodendrogenesis and myelination. J. Neurosci. 2011;31:12650–12662. doi: 10.1523/JNEUROSCI.2455-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guo F, Maeda Y, Ko EM, Delgado M, Horiuchi M, Soulika A, Miers L, Burns T, Itoh T, Shen H, et al. Disruption of NMDA receptors in oligodendroglial lineage cells does not alter their susceptibility to experimental autoimmune encephalomyelitis or their normal development. J. Neurosci. 2012;32:639–645. doi: 10.1523/JNEUROSCI.4073-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Micu I, Plemel JR, Lachance C, Proft J, Jansen AJ, Cummins K, van Minnen J, Stys PK. The molecular physiology of the axo-myelinic synapse. Exp. Neurol. 2016;276:41–50. doi: 10.1016/j.expneurol.2015.10.006. [DOI] [PubMed] [Google Scholar]
- 62. Saab A, Tzvetavona ID, Trevisiol A, Baltan S, Dibaj P, Kusch K, Möbius W, Goetze B, Jahn HM, Huang W, et al. Oligodendroglial NMDA receptors regulate glucose import and axonal energy metabolism. Neuron. 2016;91:119–132. doi: 10.1016/j.neuron.2016.05.016. **NMDA receptors on oligodendrocytes control lactate production in response to axonal activity, which can then be used by axons for energetic support. Furthermore, oligodendrocyte specific NMDA receptor subunit mutant mice have eventual deficits in axon health, most likely resulting from unmet metabolic requirements.
- 63.Beirowski B, Babetto E, Golden JP, Chen Y-J, Yang K, Gross RW, Patti GJ, Milbrandt J. Metabolic regulator LKB1 is crucial for Schwann cell–mediated axon maintenance. Nat. Neurosci. 2014;17:1351–1361. doi: 10.1038/nn.3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pooya S, Liu X, Kumar VB, Anderson J, Imai F, Zhang W, Ciraolo G, Ratner N, Setchell KD, Yoshida Y, et al. The tumour suppressor LKB1 regulates myelination through mitochondrial metabolism. Nat. Commun. 2015;5:1–15. doi: 10.1038/ncomms5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang P, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012;487:443–448. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Domènech-Estévez E, Baloui H, Repond C, Rosafio K, Médard J-J, Tricaud N, Pellerin L, Chrast R. Distribution of monocarboxylate transporters in the peripheral nervous system suggests putative roles in lactate shuttling and myelination. J. Neurosci. 2015;35:4151–4156. doi: 10.1523/JNEUROSCI.3534-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ino D, Sagara H, Suzuki J, Kanemaru K, Okubo Y, Iino M. Neuronal regulation of Schwann cell mitochondrial report neuronal regulation of Schwann cell mitochondrial Ca2+ signaling during myelination. Cell Rep. 2015;12:1951–1959. doi: 10.1016/j.celrep.2015.08.039. [DOI] [PubMed] [Google Scholar]
- 68.Stevens B, Fields RD. Response of Schwann cells to action potentials in development. Science. 2000;287:2267–2271. doi: 10.1126/science.287.5461.2267. [DOI] [PubMed] [Google Scholar]
- 69.Jesuraj NJ, Santosa KB, Macewan MR, Moore AM, Kasukurthi R, Ray WZ, Flagg ER, Hunter DA, Borschel GH, Johnson PJ, et al. Schwann cells seeded in acellular nerve grafts improve functional recovery. Muscle Nerve. 2014;49:267–276. doi: 10.1002/mus.23885. [DOI] [PMC free article] [PubMed] [Google Scholar]