Abstract
Background & Aims
Contrary to conventional wisdom, the rectoanal gradient during evacuation is negative in many healthy people, undermining the utility of anorectal high resolution manometry (HRM) for diagnosing defecatory disorders. We aimed to compare HRM and magnetic resonance imaging (MRI) for assessing rectal evacuation and structural abnormalities.
Methods
We performed a retrospective analysis of 118 patients (all female; 51 with constipation, 48 with fecal incontinence, and 19 with rectal prolapse; age, 53±1 years) assessed by HRM, the rectal balloon expulsion test (BET), and MRI at Mayo Clinic, Rochester, Minnesota, from February 2011 through March 2013. Thirty healthy asymptomatic women (age, 37±2 years) served as controls. We used principal components analysis of HRM variables to identify rectoanal pressure patterns associated with rectal prolapse and phenotypes of patients with prolapse.
Results
Compared to patients with normal findings from the rectal BET, patients with an abnormal BET had lower median rectal pressure (36 vs 22 mmHg, P=.002), a more negative median rectoanal gradient (−6 vs −29 mmHg, P=.006) during evacuation, and a lower proportion of evacuation, based on MRI analysis (median of 40% vs 80%, P<.0001). A score derived from rectal pressure and anorectal descent during evacuation and a patulous anal canal was associated (P=.005) with large rectoceles (3 cm or larger). A PC logistic model discriminated between patients with and without prolapse with 96% accuracy. Among patients with prolapse, there were 2 phenotypes – characterized by high (PC1) or low (PC2) anal pressures at rest and squeeze along with higher rectal and anal pressures (PC1) or a higher rectoanal gradient during evacuation (PC2).
Conclusions
In a retrospective analysis of patients assessed by HRM, measurements of rectal evacuation by anorectal HRM, BET, and MRI were correlated. HRM alone, and together with anorectal descent during evacuation, may identify rectal prolapse and large rectoceles, respectively, and also identify unique phenotypes of rectal prolapse.
Keywords: anismus, balloon expulsion, diagnosis, pelvic floor dysfunction
BACKGROUND
In addition to symptoms of constipation, direct assessments (barium or magnetic resonance imaging [MRI] defecography, rectal balloon expulsion test [BET]) or indirect assessments (i.e., manometry, anal surface electromyography) are required to diagnose defecatory disorders.1 In the United States, manometry and a BET are initially recommended, followed by barium or MR defecography if necessary.2, 3
Conceptually, rectal pressure should exceed anal pressure (i.e., a positive rectoanal pressure gradient) for defecation to occur. However, in many asymptomatic people – 87% in 1 study – assessed with high-resolution manometry (HRM), anal pressure exceeds rectal pressure during evacuation (i.e., rectoanal dyssynergia), which is regarded as an abnormal finding. Hence, the utility of HRM for diagnosing defecatory disorders is unclear.4 Similarly, defecography reveals classical features of pelvic floor dysfunction even in some asymptomatic people.5 However, most studies evaluating defecography for diagnosing defecatory disorders have been uncontrolled and used semi-quantitative rather than quantitative criteria to identify dyssynergia.6–8 For example, in one study, normal defecation was defined, among other criteria, by “evacuation of >60% of the contrast in <60 seconds,” while the normal value in another study was >35% in 2.5 minutes.6, 9 Other criteria (e.g., “adequate pelvic floor descent,” “opening of the anorectal angle during defecation,” and “absence of contraction of the puborectalis muscle and the external anal sphincter during evacuation”) were not precisely defined.6
Adding to this confusion, the results of these tests are often discordant; dyssynergia during defecography may occur in conjunction with a normal anorectal manometry or rectal BET.7 The agreement between balloon expulsion and barium defecography is fair and between water perfused anorectal manometry and defecography is poor.10 Prompted by these observations, the Rome IV criteria recommend at least two abnormal anorectal tests for diagnosing defecatory disorders in constipated patients.1 These findings also potentially undermine the diagnostic utility of these tests. However, comparing two continuous metrics (e.g., rectal evacuation and BET) expressed as dichotomous (i.e., normal or abnormal) measures can be misleading11 and erroneously imply that the two measures are unrelated. Hence, it may be preferable to compare these metrics expressed on a continuous scale.
In most centers, HRM and the BET are performed before defecography. Therefore, it would be useful to identify HRM patterns that predict structural abnormalities (e.g., a rectocele or rectal prolapse) and guide the need for defecography.8, 12 Intuitively, it would seem that increased rectal pressure would predispose to rectal prolapse and rectocele, which reflect rectal outpouching internally and into the vagina, respectively. While HRM patterns may identify structural rectoanal outlet obstruction, the data are conflicting.8, 13 Few studies have evaluated the pathophysiology of rectoceles; greater perineal descent, a greater rectovaginal pressure gradient, and impaired rectal emptying are postulated risk factors.5, 14–16 Finally, most descriptions of MR defecography (MRI) in defecatory disorders do not describe bladder and uterine descent that are also visualized with MRI.5, 8, 17, 18
Towards the overall objective of appraising the utility of HRM in patients with defecatory disorders, the specific aims of this study were to: (i) compare findings from HRM and MRI, and separately BET versus MRI; (ii) compare rectoanal variables (i.e., rectal pressure and anorectal descent during evacuation, patulous anal canal) in patients with and without rectoceles; and (iii) identify rectoanal pressure patterns associated with rectal prolapse and phenotypes in patients with prolapse.
METHODS
Study Subjects
Between February 2011 and March 2013, 160 patients had HRM, BET and MRI defecography at Mayo Clinic in Rochester, MN. After excluding 25 men, 9 patients in whom the indication was not constipation, fecal incontinence (FI), or rectal prolapse, 5 patients with a history of left colon or major rectal surgery, and 3 patients with incomplete data, 118 female patients (age 53±1 years [mean+SEM]) were included in this study. Thirty healthy asymptomatic women (age 37±2 years) served as controls. The Institutional Review Board at Mayo Clinic approved this study.
Procedures
Anorectal Manometry and Rectal Balloon Expulsion Test
Rectoanal pressures were measured at rest, during squeeze, and simulated evacuation with a HRM catheter (Sierra Scientific Instruments; Los Angeles, CA).19, 20 During simulated evacuation, which was assessed over 20 seconds, the program identified the most positive (or least negative) difference between rectal and anal (Rectal – Anal) pressure over 3 seconds.
Rectal BET was evaluated with validated techniques.21 The BET was performed in the left lateral position in 114 of 118 patients; 4 patients had a latex allergy. Due to a change in local practice during the study period, BET was performed in the seated position in all 30 healthy controls. There is significant agreement between these techniques for evaluating rectal balloon expulsion in healthy people and in constipated patients.21 For the seated test, patients expelled a balloon filled with 50 mL water in the seated position. Based on data from 62 asymptomatic women, more than 60 seconds is considered abnormal,21 which is comparable to the normal value at some other centers.22 In the left lateral position, patients were asked to expel a balloon filled with 50 mL of water and connected to a series of weights. The test was considered abnormal if more than 200 gm of external traction was required to aid expulsion.5, 23
MRI
Established methods were used to image the anal sphincters with an endorectal (MRInnervu®, Medrad, Inc, Indianola, PA) and torso phased-array coil in 130 participants (30 controls and 100 patients) followed by MR defecography in all participants (30 controls and 118 patients).5, 24, 25 Anorectal images were acquired at rest, during squeeze, and defecation in the supine position with a four-element phased-array coil placed around the pelvis and using a volumetric three-dimensional fast spin-echo parasagittal sequence followed by two-dimensional fast imaging employing a steady-state acquisition (FIESTA) sequence for dynamic real-time imaging (field of view 36 cm, slice thickness 5 mm, TE minimum, flip angle 60).
An experienced radiologist (JG) reviewed all images and characterized the external (EAS) and internal anal sphincter (IAS) appearance as normal or abnormal. Anorectal motion and structural abnormalities were also documented.5, 24–26 For the analysis, rectoceles ≥3 cm were considered to be large. Pelvic floor dysfunction was defined by the presence of 2 or more features during evacuation8: anorectal angle increase less than 8°, anorectal descent of less than 2.1 cm or greater than 4.2 cm, and rectal evacuation less than 25%. These values are less than the 25th or greater than the 75th percentile values (perineal descent of 4.2 cm during evacuation) in a cohort of 119 healthy people with an average age of 52 years assessed at Mayo Clinic in Rochester, MN (unpublished data). The radiologist also recorded if the anal canal was patulous or co-apted at rest.
Statistical Analysis
The Wilcoxon Rank Sum Test and Mann-Whitney U Test were used respectively for overall and between group (i.e., healthy people versus constipation, versus FI, versus prolapse, and constipation versus FI) comparisons. Relationships among anorectal parameters during evacuation were evaluated with Spearman correlation coefficients. There were 4 comparisons; hence an adjusted P value of < .0125 was considered significant for these analyses. Multiple variable linear regression models were used to predict rank transformed rectal evacuation during MRI from manometry parameters, anorectal motion measured with MRI, and a combination thereof.
A rectocele score was computed from rectoanal parameters in each participant. For this score, rectal pressure and anorectal descent were scored 0 or 1 when their values were respectively less than or greater than the median values for these parameters in patients, i.e., 33.6 mmHg and 3.1 cm, respectively. For a patulous anal canal, the score was 0 or 1 if the canal was absent or present, respectively. This score was calculated as follows: rectal pressure during evacuation + anorectal descent during evacuation - patulous anal canal. The score ranged from −1 to 2.
Two separate principal components (PC) analyses were used to identify anorectal parameters that (i) discriminated between asymptomatic controls and patients with prolapse and (ii) identified phenotypes among patients with rectal prolapse. As detailed elsewhere, these PC were comprised of weighted linear combinations of anorectal variables that best explained the variance among subjects.5, 20, 27 Thus, the first PC score (or PC1) was a weighted linear combination of the 5 HRM variables that accounted for the maximum variation between subjects. The second linear combination (PC2) explained the maximum possible remaining variation and was uncorrelated with PC1. The correlations between PC scores and the original HRM variables were computed.28 A multiple variable logistic regression model using age and PC1 from a model including both healthy controls and rectal prolapse patients was used to discriminate between healthy controls and rectal prolapse. Except where stated otherwise, an α of <0.05 was considered significant.
RESULTS
Clinical Features
The primary symptoms were constipation (51 patients, 43%), FI (48 patients, 41%), and rectal prolapse without constipation or FI (19 patients, 16%) (Table 1). The age distribution was associated (P<.001) with group status; patients with constipation, FI, and rectal prolapse were older than healthy people. Patients with constipation (43%), FI (40%) or prolapse (42%) were more likely than healthy individuals (13%) to have undergone a hysterectomy (χ P<.05).
Table 1.
Distribution of Rectoanal Parameters in Groups a
| Healthy People | Constipation | Fecal Incontinence | Rectal prolapse | P b | |
|---|---|---|---|---|---|
| N | 30 | 51 | 48 | 19 | |
| Age, y | 35 (28, 47) | 47 (34,59) c | 60 (55, 67) d e | 61 (44, 69) d | <.0001 |
| BMI, kg/m2 | 26 (23, 28) | 26 (22, 30) | 26 (22, 29) | 24 (22 – 32) | NS |
| Anal Resting Pressure, mmHg | 95 (78, 101) | 71 (57, 90) d | 38 (26, 57) d e | 58 (39 – 67) d | <.0001 |
| Anal Squeeze Pressure Increment, mmHg | 119 (75, 143) | 53 (26, 93) d | 34 (15, 73) d | 39 (22, 82) d | <.0001 |
| Rectoanal Gradient – SE, mmHg | − 42 (−57, −30) | −13 (−29, 1) d | 2 (−7, 10) d e | −3 (−15, 7) d | <.0001 |
| Abnormal Balloon Expulsion Test, n (%) | 1 (3%) | 17 (33%) d | 3 (6%) | 0 (0%) | <.0001 |
| During Squeeze | |||||
| Change in Rectoanal Angle from Rest to Squeeze (°) | −26 (−34, −20) | −17 (−24, −11) d | −15 (−23, −8) d | −17 (−33, −11) | .002 |
| Perineal Ascent from Rest to Squeeze (cm) f | −1.4 (−2.2, −1.1) | −1.2 (−1.5, −0.8) | −0.9 (−1.9, −0.6) | −1.3 (−1.6, −0.8) | NS |
| During Defecation | |||||
| Change in Rectoanal Angle from Rest to Defecation (°) | 16 (8, 35) | 15 (7, 33) | 11 (−3, 27) | 13 (1, 22) | NS |
| Perineal Descent from Rest to Defecation (cm) f | 2.6 (1.8, 3.9) | 3.5 (2.4, 5.0) | 2.6 (1.5, 3.5) d | 3.2 (1.9, 5.0) | .0339 |
Abbreviations: BMI, body mass index; SE, simulated evacuation
Values are Median and IQR unless stated.
Test for association with group status by Wilcoxon Rank Sum Testing. Pairwise comparisons were performed with Mann-Whitney U Test.
P <.01 and
P < .001 relative to controls.
P < .001 relative to constipation.
Positive and negative values represent descent (ie, during defecation) and ascent (ie, during squeeze), respectively.
Anorectal Pressures
Anal resting pressure and the squeeze pressure increment were lower in constipation, FI, and rectal prolapse than controls (Table 1). The resting pressure was lower (P<.001) in FI than in constipation. The rectoanal gradient during evacuation was more negative (P<.001) in healthy than in all patient cohorts and in FI than in constipation. The BET was abnormal in a greater (P<.001) proportion (17/51, 33%) of constipated patients than controls (1/30, 3%).
The mean (10th, 90th percentile values) for the rectoanal gradient during evacuation in healthy women was −41 (−7, −78) mmHg, This gradient was lower (i.e., more negative than −78 mmHg) in only 2 of 51 constipated women.
Anorectal Motion
Compared to rest, the rectoanal angle decreased and increased (P<.001) respectively during squeeze and evacuation in healthy participants (Table 1). During squeeze, narrowing of the anorectal angle was smaller (P<.001) in constipation and FI than in healthy women. During evacuation, perineal descent was greater (P<.01) in constipated patients than in healthy women or those with FI.
Relationship between HRM and MRI Parameters and BET
Compared to a normal rectal BET, patients with an abnormal test had reduced (P≤.006) median rectal pressure and a more negative rectoanal gradient during simulated evacuation and evacuated less during MRI (Table 2).
Table 2.
Comparison of MRI and Manometry Parameters in Participants With Normal and Abnormal Balloon Expulsion Tests a
| All Patients and Controls | Normal BET | Abnormal BET | P for Diff b |
|---|---|---|---|
| Manometry | |||
| Resting Anal Pressure (mmHg) | 61 (36, 86) | 67 (57, 91) | 0.16 |
| Residual Anal Pressure (mmHg) | 46 (32, 70) | 53 (37, 73) | 0.63 |
| Rectal Pressure SE (mmHg) | 36 (23, 54) | 22 (17, 33) | 0.002 |
| Rectoanal Gradient – SE (mmHg) | −6 (−26, 4) | −29 (−55, −9) | 0.006 |
| MRI | |||
| Rectal Evacuation (%) | 80 (70, 95) | 40 (0, 68) | <0.0001 |
| Change in Rectoanal Angle from Rest to Defecation (°) | 14 (5, 31) | 5 (−5, 22) | 0.11 |
| Change in Anorectal Junction Location from Rest to Defecation (cm) | 2.9 (1.9, 4.7) | 3.3 (1.6, 4.6) | 0. 99 |
Abbreviations: BET, balloon expulsion test; MRI, magnetic resonance imaging; SE, simulated evacuation.
Values are medians and interquartile ranges.
Kruskal-Wallis for tests of difference.
Relationship between HRM and MRI Parameters during Rectal Evacuation
Rectal evacuation during MRI was inversely correlated with anal resting pressure (r= −0.29, P=.0004) and anal pressure during evacuation (r=−0.24, P=.0034) but directly correlated with rectal pressure (r=0.27, P=.001) and the rectoanal gradient (r=0.48, P<.0001) during evacuation.
During MR defecography, 41 of 148 participants (28%) had pelvic floor dysfunction, which was more common (P=.016) in patients with an abnormal (52%) than a normal BET (24%). An abnormal BET had sensitivity of 27%, specificity of 90%, positive predictive value of 52%, and a negative predictive value of 76% for identifying pelvic floor dysfunction by MRI.
In a multivariate model based on our data, 21%, 31%, and 36% of the variance in rectal evacuation can be explained respectively by HRM, MRI, or a combination of the two tests, respectively (Table 3). In the MRI model, a patulous anal canal was the strongest predictor of variance. In the combined model, HRM and MRI parameters were independent predictors of rectal evacuation.
Table 3.
Multiple Variable Models of Rectal Evacuation measured with MRI y
| Parameter | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| Parameter estimate | R2 * | Parameter estimate | R2 * | Parameter estimate | R2 * | |
| Manometry | ||||||
| Anal resting pressure | −0.09 | 0.005 | ||||
| Rectal pressure – SE | 0.45 | 0.138 a | 0.42 | 0.14 a | ||
| Anal pressure – SE | −0.44 | 0.092 a | −0.40 | 0.09 a | ||
| Recto-anal gradient – SE | ||||||
| MRI | ||||||
| Anorectal angle at rest | −0.07 | 0.002 | ||||
| Change in anorectal angle from rest to defecation | 0.27 | 0.037 b | ||||
| Location of anorectal junction at rest | 5.73 | 0.063 c | ||||
| Anorectal descent from rest to defecation | 6.13 | 0.127a | 6.03 | 0.18 a | ||
| Patulous anal canal | 24.49 | 0.148 a | 15.82 | 0.07c | ||
| Total variance explained (%) | 21 a | 31 a | 36 a | |||
Abbreviations: MRI, magnetic resonance imaging; SE, simulated evacuation
Rectal evacuation (%) measured with MRI was the dependent variable in all models
Squared partial correlation coefficient
P<0.001
P<0.05
P<0.01
Pelvic Organ Prolapse
Twenty-three patients (15%) and 6 controls (17%) had large rectoceles. Enteroceles (20 patients [17%] versus 0 controls, P=.01) and large (>4 cm) cystoceles (24 patients [20%] versus 0 controls [0%], P<.01) were more common in patients than controls. However, moderate-sized (2–4 cm) cystoceles (53 patients [45%] versus 12 controls [40%]), peritoneoceles (12 patients [10%] versus 1 control [3%]), and sigmoidoceles (7 patients [6%] versus 1 control [3%]) were not. Among patients who had a MRI and a BET, 20 of 22 (91%) with a large rectocele had a normal BET. Likewise, 29 of 31 patients (94%) with an enterocele, peritoneocele, and/or sigmoidocele had a normal BET. Of note, while the average age of all controls was 35 years, women with a cystocele were older, i.e., their average age was 42 years, but only 2 (of 13) had a hysterectomy. Four of 6 controls with a large rectocele had increased perineal descent during defecation; 2 had a hysterectomy.
Relationship between a Rectocele and other Rectoanal Parameters
The IAS, EAS, and puborectalis were normal in 26 healthy people (87%) and 37 patients (37%). Among controls, 3 (10%) had IAS injury, 3 (10%) had EAS injury, and 2 (6.7%) had puborectalis injury. Fifty patients (42%) had IAS injury, 32 (27%) had EAS injury, and 32 (27%) had puborectalis injury. Neither EAS nor puborectalis injury were associated with large rectoceles (data not shown).
Large rectoceles were associated with rectal pressure (P=.1, data not shown) and anorectal descent during evacuation (P=.0005, data not shown). In contrast, 26 of 123 (21%) patients without but only 3 of 46 patients (7%) with a patulous canal had a large rectocele (P<.01). The rectocele score, which combined these 3 variables, was associated (P=.005) with a rectocele measuring 3 cm or larger. Thus, 1 in 17 participants (6%) with a score of -1, 3 of 40 (8%) with a score of 0, 13 of 59 (22%) with a score of 1, and 12 of 31 (39%) with a score of 2 had a rectocele 3 cm or larger (Figure 1).
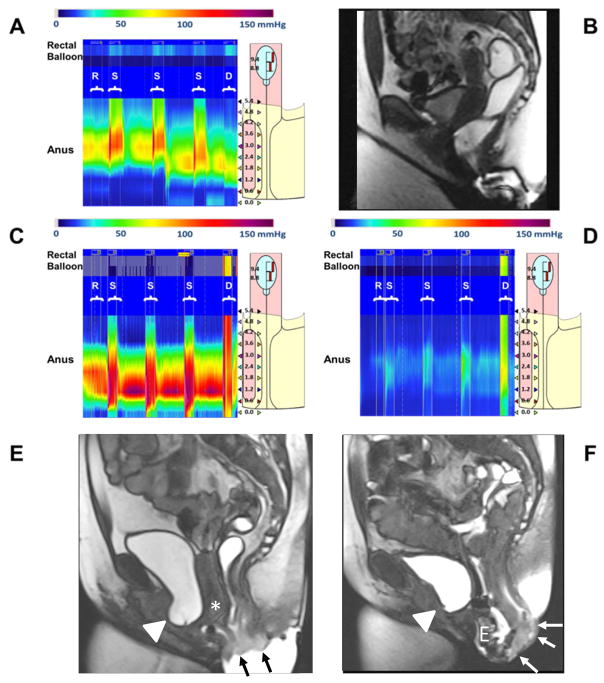
Figure 1.
High resolution manometry (HRM) and magnetic resonance imaging (MRI) findings in rectal prolapse. Panels A (HRM) and B (MRI) of a patient with a high PC1 score in the all participant model, which is associated with a lower risk of prolapse. Anal pressures were normal at rest (R) and during squeeze (S). Defecation (D) is associated with increased rectal pressure, anal relaxation, and rectal evacuation. Panels C–F show HRM and MRI features of two rectal prolapse phenotypes; high PC1 (Panels C and E) and high PC2 (Panels D and F). In contrast to Panel A, observe increased pressures during defecation (D) throughout rectum and anus in Panels C and D. Panels E and F: MRI shows small (Panel E) and large (Panel F) rectal prolapse (arrows), a cystocele (arrowhead), enterocele (E), and uterine prolapse (asterisk).
HRM in Rectal Prolapse
Among all participants, 3 composite scores (or PCs) explained 53%, 31%, and 12%, respectively (total 96%) of the total inter-subject variation in the 5 anorectal variables (Table 4). In a logistic model, the PC1 score adjusted for age discriminated between controls and rectal prolapse with an accuracy (area under the ROC curve) of 96%. In this model, a greater PC1 score was associated with a lower risk (Odds ratio 0.091 [95% CI: 0.027 – 0.31]) of rectal prolapse. Based on the correlations between PC1 and rectoanal parameters (Table 4), rectal prolapse is associated with lower anal pressures at rest, during squeeze, and during simulated evacuation and a greater rectoanal gradient during evacuation.
Table 4.
Correlation Coefficients for Principal Component (PC) Scores Versus Anorectal Variables From Controls Versus Prolapsed Patients and for Prolapsed Patients Alone. a
| Variable | Controls b and Prolapse (N=67) | Prolapse alone (N=39) | |||||
|---|---|---|---|---|---|---|---|
| PC1 (53%) | PC2 (31%) | PC3 (12%) | PC1 (48%) | PC2 (31%) | PC3 (13%) | PC4 (7%) | |
| Anal resting pressure | 0.91c | −0.21 | 0.18 | 0.67c | −0.68c | −0.02 | 0.46d |
| Anal squeeze pressure | 0.85c | −0.10 | 0.47c | 0.75c | −0.61c | 0.37e | −0.29 |
| Rectal pressure during bear down | 0.12 | 0.94c | 0.25e | 0.75c | 0.25 | 0.18 | −0.17 |
| Rectoanal gradient | −0.72c | 0.56c | 0.36e | 0.02 | 0.71c | 0.57c | 0.04 |
| Anal pressure during bear down | 0.83c | 0.17 | −0.26e | 0.85c | −0.16 | −0.13 | −0.17 |
| R2 | 53% | 31% | 12% | 48% | 31% | 13% | 7% |
All values except for the last row are Spearman correlation coefficients
Only healthy controls without prolapse
P<.001
P <.01
P <.05
Values in bold indicate correlations that were statistically significant; Values in parentheses indicate % of total variance explained
Among patients with rectal prolapse, PC1 and PC2 explained 48% and 31% of the variance, respectively (Table 4, Figure 2). PC1 was correlated with higher anal pressures at rest and squeeze and higher rectal and anal pressures during evacuation. In contrast, PC2 was inversely correlated with anal pressures at rest and during squeeze; PC2 was correlated with a greater rectoanal pressure gradient during evacuation. PC3 and PC4 explained the residual variance (Table 4).
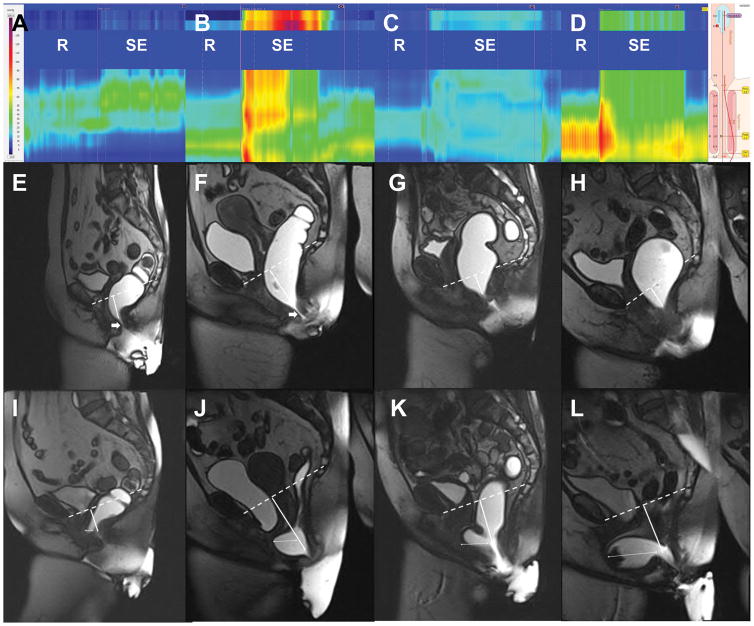
Figure 2.
High resolution manometry (upper panel) and magnetic resonance imaging at rest (middle panel) and during evacuation (lower panel) in 3 patients with different combinations of perineal descent and rectal pressure. The scoring system is described in the Statistical Analysis. Panels E and F demonstrate a mildly patulous canal at rest (thick arrows). Panels I–L show rectoceles (thin arrows) which are smallest in Panel I and largest in the patient with greater rectal pressure and perineal descent (Panel L). Abbreviations: R, Rest; SE, Simulated Evacuation.
DISCUSSION
Because a majority of healthy people have a negative rectoanal gradient during evacuation, the utility of HRM for diagnosing defecatory disorders has been questioned.4, 10 Taken at the extreme, these observations undermine the concept that rectoanal discoordination is responsible for defecatory disorders. Offsetting those concerns, rectal evacuation assessments with anorectal HRM, BET, and MRI were significantly correlated in this study, which substantiates the criterion validity of these tests. Although these tests were performed on different days, in different positions, and with (MRI) or without (BET) rectal filling, rectal and anal pressures during evacuation independently explained rectal evacuation measured with MRI and the BET. Taken together, HRM and MRI parameters only explained 36% of the variance in rectal evacuation, which suggests that these parameters do not completely capture the pelvic floor motion factors involved in successful defecation.
A prolonged BET was very specific (90%) for identifying reduced rectal evacuation measured with MRI. This confirms a comparison of BET versus barium defecography6 and suggests that an abnormal BET almost always suggests a defecatory disorder. Similar to a previous earlier study,5 the sensitivity of a BET versus MRI was only 27%, compared to 88% versus barium defecography.6 This suggests that a normal BET may not always exclude a defecatory disorder. Perhaps these differences between the sensitivity of barium and MRI are related to differences in techniques and the criteria for abnormal evacuation between studies. In that study, barium defecography was not performed in all patients.6
As previously observed,29 during evacuation, the rectoanal gradient measured with high resolution manometry is negative even in asymptomatic women. Indeed, the mean (10th–90th percentile values) for the rectoanal gradient measured with HRM in asymptomatic women aged less than 50 years was −41 (−7, −78) mmHg, which is very similar to the corresponding values (−41 mmHg [−1, −74]) in a different cohort of healthy women aged less than 50 years.30 In the present study, this gradient was not useful for diagnosing defecatory disorders because it was lower (i.e., more negative) than −78 mmHg in only 2 of 51 constipated women. However, this inference is subject to the limitation that in this study, the healthy women were younger than the constipated women; the gradient is greater (i.e., less negative) in older than younger healthy women.30 With larger datasets, the confidence intervals for the rectoanal gradient during evacuation in healthy women may be narrower, thereby increasing its utility for identifying defecatory disorders.
Enterocoeles and large cystoceles, but not large rectoceles, were more common in patients than controls. Four of 6 women (67%) with a large rectocele had increased perineal descent. Of note, 91% of patients with a rectocele larger than 3 cm and 94% of patients with an enterocele, a peritoneocele, and/or a sigmoidocele had a normal BET. Taken together, these findings support the current recommendation, which was rated as “moderate” strength and supported by “moderate quality of evidence” to proceed to defecography with barium or MRI in patients with clinically-suspected defecatory disorders and a normal BET.3
Confirming previous studies,5, 14 large rectoceles were associated with increased anorectal descent during evacuation. By contrast, large rectoceles were less common in patients with a patulous anal canal, probably because a patulous canal allows the rectum to empty. Indeed, of the MRI variables, a patulous anal canal was the strongest independent predictor of rectal evacuation. Contrary to a previous study,31 rectoceles were not associated with sphincter or puborectalis injury in this study. Because perineal descent can be estimated by digital rectal examination,5 it is conceivable that the risk of a large rectocele can be assessed with rectal examination and an anal manometry, thereby guiding the decision to proceed with defecography.
HRM predicted rectal prolapse and identified phenotypes among patients with prolapse. PC1 discriminated between patients with and without rectal prolapse with an accuracy of 96%. Among patients with prolapse, the analysis uncovered 2 PCs (PC1 and PC2), which were characterized by high and low anal pressures, respectively. Because the individual PCs were not, by design, correlated with each other, it is conceivable that the PCs represented distinct (i.e., independent) underlying pathophysiological mechanisms. Because anal resting pressure was lower in patients with more severe prolapse, likely because the prolapse stretched the anal sphincters,32 high anal pressure (PC1) reflected early while low anal pressure (PC2) reflected late stage rectal prolapse. Also, among patients with prolapse, PC1 was characterized by greater rectal and anal pressures during evacuation, which may indicate dyssnergia and/or excessive straining, which has been implicated to be a risk factor for rectal prolapse.33
The limitations of this study are as follows. Only patients who were referred for MR defecography in clinical practice were included. Neither anorectal manometry nor MRI were performed in the seated position. However, the agreement between supine MRI and seated barium defecography for detecting rectoceles and between seated and supine MRI defecography for detecting clinically relevant pelvic floor abnormalities is good.34, 35 While the rectal BET was conducted in the seated position in controls and in the left lateral position in patients, there is significant agreement between the results of these tests.36
In summary, measurements of rectal evacuation with anorectal HRM, BET, and MRI were significantly correlated, which substantiates the criterion validity of these techniques. Additionally, HRM is useful for predicting rectal prolapse and large rectoceles and for identifying phenotypes in patients with rectal prolapse.
Acknowledgments
Financial support: This project was supported by USPHS NIH Grant R01 DK078924
Abbreviations
- BET
Balloon Expulsion Test
- EAS
external anal sphincter
- FI
fecal incontinence
- HRM
high-resolution anorectal manometry
- IAS
internal anal sphincter
- MRI
magnetic resonance imaging
- PFD
Pelvic Floor Dysfunction
- SSFSE
single-shot, fast spin-echo
Footnotes
Disclosures: None of the authors have conflicts of interests.
Author Contributions:
DP - planning and conducting the study, interpreting data, and drafting the manuscript
THL and GP - analyzing data
JGF - collecting and interpreting data
ARZ – statistical analysis
AEB - planning and conducting the study, collecting and interpreting data, and drafting the manuscript
All authors approved the final draft submitted.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rao S, Bharucha AE, Chiarioni G, et al. Functional anorectal disorders. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bharucha AE, Locke GR, Pemberton JH. American Gastroenterological Association technical review on constipation. Gastroenterology. 2013;144:218–238. doi: 10.1053/j.gastro.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wald A, Bharucha AE, Cosman BC, et al. ACG clinical guideline: management of benign anorectal disorders. Am J Gastroenterol. 2014;109:1141–1157. doi: 10.1038/ajg.2014.190. [DOI] [PubMed] [Google Scholar]
- 4.Grossi U, Carrington EV, Bharucha AE, et al. Diagnostic accuracy study of anorectal manometry for diagnosis of dyssynergic defecation. Gut. 2016;65:447–455. doi: 10.1136/gutjnl-2014-308835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bharucha AE, Fletcher JG, Seide B, et al. Phenotypic variation in functional disorders of defecation. Gastroenterology. 2005;128:1199–1210. doi: 10.1053/j.gastro.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 6.Minguez M, Herreros B, Sanchiz V, et al. Predictive value of the balloon expulsion test for excluding the diagnosis of pelvic floor dyssynergia in constipation. Gastroenterology. 2004;126:57–62. doi: 10.1053/j.gastro.2003.10.044. [DOI] [PubMed] [Google Scholar]
- 7.Bordeianou L, Savitt L, Dursun A. Measurements of pelvic floor dyssynergia: which test result matters? Dis Colon Rectum. 2011;54:60–65. doi: 10.1007/DCR.0b013e3181fd2373. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich H, Sauter M, Fox M, et al. Assessment of Obstructive Defecation by High-Resolution Anorectal Manometry Compared With Magnetic Resonance Defecography. Clin Gastroenterol Hepatol. 2015;13:1310–1317. e1311. doi: 10.1016/j.cgh.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 9.Palit S, Bhan C, Lunniss PJ, et al. Evacuation proctography: a reappraisal of normal variability. Colorectal Dis. 2014;16:538–546. doi: 10.1111/codi.12595. [DOI] [PubMed] [Google Scholar]
- 10.Palit S, Thin N, Knowles CH, et al. Diagnostic disagreement between tests of evacuatory function: a prospective study of 100 constipated patients. Neurogastroenterol Motil. 2016:S. doi: 10.1111/nmo.12859. [DOI] [PubMed] [Google Scholar]
- 11.Altman DG, Royston P. The cost of dichotomising continuous variables. BMJ. 2006;332:1080. doi: 10.1136/bmj.332.7549.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiarioni G, Kim SM, Vantini I, et al. Validation of the balloon evacuation test: reproducibility and agreement with findings from anorectal manometry and electromyography. Clin Gastroenterol Hepatol. 2014;12:2049–2054. doi: 10.1016/j.cgh.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Jodorkovsky D, Macura KJ, Gearhart SL, et al. High-resolution anorectal manometry and dynamic pelvic magnetic resonance imaging are complementary technologies. J Gastroenterol Hepatol. 2015;30:71–74. doi: 10.1111/jgh.12697. [DOI] [PubMed] [Google Scholar]
- 14.Yoshioka K, Matsui Y, Yamada O, et al. Physiologic and anatomic assessment of patients with rectocele. Dis Colon Rectum. 1991;34:704–708. doi: 10.1007/BF02050355. [DOI] [PubMed] [Google Scholar]
- 15.Siproudhis L, Dautreme S, Ropert A, et al. Dyschezia and rectocele--a marriage of convenience? Physiologic evaluation of the rectocele in a group of 52 women complaining of difficulty in evacuation. Dis Colon Rectum. 1993;36:1030–1036. doi: 10.1007/BF02047295. [DOI] [PubMed] [Google Scholar]
- 16.Shafik A, El-Sibai O, Shafik AA, et al. On the pathogenesis of rectocele: the concept of the rectovaginal pressure gradient. Int Urogynecol J Pelvic Floor Dysfunct. 2003;14:310–315. doi: 10.1007/s00192-003-1087-7. discussion 315. [DOI] [PubMed] [Google Scholar]
- 17.Klingele CJ, Bharucha AE, Fletcher JG, et al. Pelvic organ prolapse in defecatory disorders. Obstet Gynecol. 2005;106:315–320. doi: 10.1097/01.AOG.0000171104.72972.34. [DOI] [PubMed] [Google Scholar]
- 18.Reiner CS, Tutuian R, Solopova AE, et al. MR defecography in patients with dyssynergic defecation: spectrum of imaging findings and diagnostic value. Br J Radiol. 2011;84:136–144. doi: 10.1259/bjr/28989463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ratuapli S, Bharucha AE, Noelting J, et al. Phenotypic identification and classification of functional defecatory disorders using high-resolution anorectal manometry. Gastroenterology. 2013;144:314–322. doi: 10.1053/j.gastro.2012.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratuapli S, Bharucha AE, Harvey D, et al. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil. 2013;25:e813–820. doi: 10.1111/nmo.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tantiphlachiva K, Rao P, Attaluri A, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8:955–960. doi: 10.1016/j.cgh.2010.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Pezim ME, Pemberton JH, Levin KE, et al. Parameters of anorectal and colonic motility in health and in severe constipation. Dis Colon Rectum. 1993;36:484–491. doi: 10.1007/BF02050015. [DOI] [PubMed] [Google Scholar]
- 24.Bharucha AE, Fletcher JG, Harper CM, et al. Relationship between symptoms and disordered continence mechanisms in women with idiopathic fecal incontinence. Gut. 2005;54:546–555. doi: 10.1136/gut.2004.047696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prichard D, Harvey DM, Fletcher JG, et al. Relationship Among Anal Sphincter Injury, Patulous Anal Canal, and Anal Pressures in Patients With Anorectal Disorders. Clin Gastroenterol Hepatol. 2015;13:1793–1800. doi: 10.1016/j.cgh.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bharucha AE, Fletcher JG, Melton LJ, 3rd, et al. Obstetric trauma, pelvic floor injury and fecal incontinence: a population-based case-control study. Am J Gastroenterol. 2012;107:902–911. doi: 10.1038/ajg.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ravi K, Bharucha AE, Camilleri M, et al. Phenotypic variation of colonic motor functions in chronic constipation. Gastroenterology. 2010;138:89–97. doi: 10.1053/j.gastro.2009.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morrison DF. The structure of multivariate observations: I. Principal Components. In: Blackwell D, Solomon H, editors. Multivariate statistical methods. 2. New York: McGraw-Hill Book Company; 1976. pp. 266–301. [Google Scholar]
- 29.Lee TH, Bharucha AE. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J Neurogastroenterol Motil. 2016;22:46–59. doi: 10.5056/jnm15168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noelting J, Ratuapli SK, Bharucha AE, et al. Normal values for high-resolution anorectal manometry in healthy women: effects of age and significance of rectoanal gradient. Am J Gastroenterol. 2012;107:1530–1536. doi: 10.1038/ajg.2012.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regadas FSP, Murad-Regadas SM, Wexner SD, et al. Anorectal three-dimensional endosonography and anal manometry in assessing anterior rectocele in women: a new pathogenesis concept and the basic surgical principle. Colorectal Dis. 2007;9:80–85. doi: 10.1111/j.1463-1318.2006.01088.x. [DOI] [PubMed] [Google Scholar]
- 32.Harmston C, Jones OM, Cunningham C, et al. The relationship between internal rectal prolapse and internal anal sphincter function. Colorectal Dis. 2011;13:791–795. doi: 10.1111/j.1463-1318.2010.02266.x. [DOI] [PubMed] [Google Scholar]
- 33.Porter NH. A physiological study of the pelvic floor in rectal prolapse. Ann R Coll Surg Engl. 1962;31:379–404. [PMC free article] [PubMed] [Google Scholar]
- 34.Bertschinger KM, Hetzer FH, Roos JE, et al. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223:501–508. doi: 10.1148/radiol.2232010665. [DOI] [PubMed] [Google Scholar]
- 35.Vitton V, Vignally P, Barthet M, et al. Dynamic anal endosonography and MRI defecography in diagnosis of pelvic floor disorders: comparison with conventional defecography. Dis Colon Rectum. 2011;54:1398–1404. doi: 10.1097/DCR.0b013e31822e89bc. [DOI] [PubMed] [Google Scholar]
- 36.Ratuapli S, Bharucha AE, Harvey D, et al. Comparison of rectal balloon expulsion test in seated and left lateral positions. Neurogastroenterol Motil. 2013;25:e813–820. doi: 10.1111/nmo.12208. [DOI] [PMC free article] [PubMed] [Google Scholar]