Abstract
Many parasitic protozoan diseases continue to rank among the world’s greatest global health problems, which are also common among poor populations. Currently available drugs for treatment present drawbacks, urging the need for more effective, safer, and cheaper drugs. Artemisinin (ART) and its derivatives are some of the most important classes of antimalarial agents originally derived from Artemisia annua L. However, besides the outstanding antimalarial and antischistosomal activities, ART and its derivatives also possess activities against other parasitic protozoa. In this paper, we reviewed the activities of ART and its derivatives against protozoan parasites in in vitro and in vivo, including Leishmania spp., Trypanosoma spp., Toxoplasma gondii, Neospora caninum, Eimeria tenella, Acanthamoeba castellanii, Naegleria fowleri, Cryptosporidium parvum, Giardia lamblia, and Babesia spp. We concluded that ART and its derivatives may be good alternatives for treating other non-malarial protozoan infections in developing countries, although more studies are necessary before they can be applied clinically.
Keywords: artemisinin, antiprotozoan activity, Leishmania spp, Trypanosoma spp, Toxoplasma gondii, Neospora caninum
Graphical abstract
1. Introduction
The World Health Organization recognizes 17 major parasitic and related infections as neglected tropical diseases (NTDs) that affect many countries in Africa, Asia, and Latin America. However, the lack of commercial interest in developing new pharmaceutical compounds for combating these diseases has impaired efforts to eliminate these diseases [1,2]. Therefore, discovery of new, safe, effective, and affordable active drugs is urgently needed. Artemisinin (ART) and its derivatives is one of the most important classes of antimalarial drugs. Like many other natural sesquiterpenes, ART displays a range of different biological and pharmacological properties. Except for its antimalarial and antischistosomal activities, ART has also been shown to have antimicrobial [3] and antiviral activities [4]. In addition, the antiparasitic function of ART against non-malarial parasites cannot be ignored. Here we screened the literature through extensive searches of PubMed, ResearchGate, Elsevier ScienceDirect, Wiley Online Library, and the Springer-Link Journals database using the search term “artemisinin” for publications in English with no date limits as well as manual review of some related journals. We outline the use of ART and its derivatives in treating parasitc diseases or parasitic infections caused by protozoan parasites including Leishmania spp., Trypanosoma spp., Toxoplasma gondii, Neospora caninum, Eimeria tenella, Acanthamoeba castellanii, Naegleria fowleri, Cryptosporidium parvum, Giardia lamblia, and Babesia spp.; only drugs with activities against these protozoan parasites are reported. Studies have demonstrated good efficacies of ART and its derivatives in vivo and in vitro towards some of the parasitic protozoan infections. Clinical trials using different semisynthetic and synthetic ART derivatives should be undertaken to develoop treatments for these parasitic diseases.
2. Chemical characteristics of ART and its derivatives
In 1967, under the instructions of Chairman Mao and Premier Zhou, a secret project called “Project 523” was launched to develop a new drug to combat drug-resistant malarial parasites [5,6]. In 1971, Youyou Tu and her team isolated a new anti-malarial drug, called qinghaosu (or ART), from Artemisia annua L. (Qinghao), which was shown to inhibit proliferation of Plasmodium parasites [6].
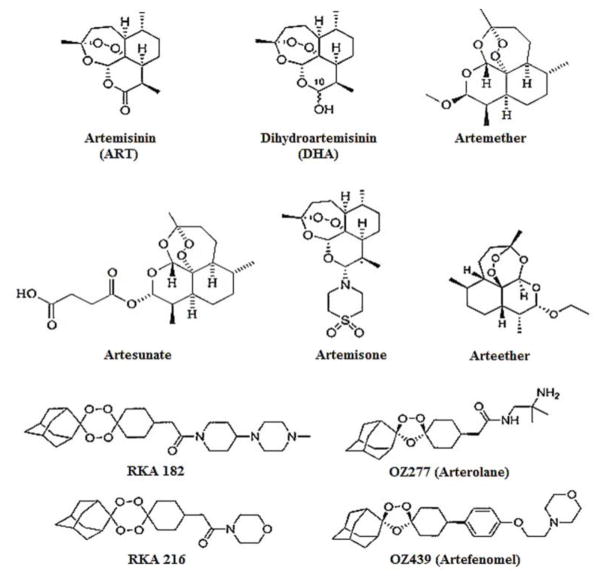
ART and its derivatives share a common structural feature called endoperoxide linkage [7]. In general, ART has poor solubility in water or oil. However, by reducing the C-10 carbonyl group of ART, a more water-soluble derivative dihydroartemisinin (DHA) can be obtained [5,8]. Artesunate is the water-soluble hemisuccinate ester of DHA [9]. According to Chaturvedi et al., by adding a methyl or ethyl ethers at the same carbonyl group, higher oil soluble compounds like artemether and arteether were obtained [7]. Artemisone was obtained by replacing the same carbonyl with amines in the ART molecule, which showed improved water solubility, better toxicity profile, and a longer half-life [8]. Due to the short in vivo half-lives and low bioavailability of ART derivatives, arterolane and artefenomel (1, 2, 4-trioxolane analogues) were created to achieve more favourable pharmacokinetics. They were synthesised by coupling symmetrical O-methyl 2-adamantanone oxime with cyclohexanones by the Griesbaum co-ozonolysis [10]. Arterolane and artefenomel have superior half-lives compared to DHA, higher bioavailability than artemether, and are comparable to artesunate [11]. However, trioxolane-like arterolane has relatively low in vivo stability [8]; that is why 1, 2, 4, 5-tetraoxanes with similar peroxide bridges, better stability and more potent anti-malarial activity such as RKA 216 and RKA182 were developed [12]. These compounds are currently under development [8]. The structures of artemisinin and its derivatives are shown in Fig 1.
Fig. 1. Chemical structures of ART and its derivatives.
ART (parent drug), DHA (active metabolite), arteether (oil-soluble derivative), artemether (oil-soluble derivative), artesunate (water soluble derivative), arterolane and artefenomel (trioxolane), and RKA 216 and 182 (tetraoxanes).
3. ART and its derivatives on Leishmania spp
Leishmaniasis affects approximately 350 million people in 98 countries around the world [13]. The disease manifests primarily as three forms, namely cutaneous leishmaniasis, mucocutaneous leishmaniasis, and visceral leishmaniasis, with the last being fatal if left untreated [14]. Current anti-leishmanial drugs include pentavalent antimony, amphotericin B, paromomycin, pentamidine, and miltefosine, with toxic effects to the liver, heart, and kidney, as well as anaemia, fever, and hypokalaemia. Therefore, usage is limited by their toxicity and adverse reactions [15, 16]. Miltefosine and paromomycin are two drugs that have been introduced more recently for the treatment of leishmaniasis [16]. However, long-term therapy with miltefosine’s long half-life (about 152 h) can promote early onset of drug resistance, and potential teratogenic and abortifacient effects limit its prescription during pregnancy [17,18]. Paromomycin is an aminoglycoside antibiotic that has shown promising results in the treatment of leishmaniasis, mainly for treating cutaneous leishmaniasis [16]. However, in vitro tests have led to the emergence of paromomycin-resistant [19], miltefosine-resistant [19], meglumine antimoniate-resistant [20], and pentamidine-resistant [21] parasites; therefore the therapy of visceral leishmaniasis is limited by resistance, toxicity, and decreased bioavailability of the existing anti-leishmanial agents [22]. The effects of ART and its derivatives on Leishmania parasites have been studied in mice and in vitro. They are efficient in inhibiting the parasite metabolism, while showing limited adverse effects on the host, indicating a higher safety index of the drugs [23,24]. A large number of in vitro or in vivo studies have shown that ART and its derivatives have activities in controlling the parasites, and the drugs shown effective against the protozoan are selected and summarized in Table 1.
Table 1.
Treatment of artemisinin and its derivatives against Leishmania spp.
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | L. major (MRHO/IR/75/ER) | Promastigotes | Artemisinin | 10, 25, 50, and 100 μg/mL. incubated for 24, 48, and 72 h | IC50 = 25 μg/mL. Apoptotic promastigotes were 12%, 25.90%, 40.52%, and 68.16%, respectively, in comparison of control that was 0.02% | Ghaffarifar et al. [28] |
| L. major (MRHO/IR/75/ER) | Promastigotes | Artemisinin | 5, 10, 25, 50, and 100 μg/mL, incubated for 24, 48, and 72 h | IC50 = 50 μg/mL. The viabilities of promastigote were ~48%, ~40%, and ~36% after 24, 48, and 72 h of treatment with 100 μg/mL | Heydari et al. [35] | |
| L. major (LV39, MRHO/SU/59/P) | Promastigotes | Artemisinin in Me2SO diluent | 100, 33.5, 9.6, 2.7, 0.75, and 0.2 μM incubated for 48 h | Estimated by the incorporation of 3H-thymidine, the viability of parasites post-treatment was ~1, 2.4, 3.4, 4.5, 4.8, and 9 cpm × 10−3, respectively. IC50 = 7.5 × 10−7 M. No surviving promastigotes were observed in 48 h cultures containing > 10−5 M artemisinin or 10−6 M artemether | Yang et al. [36] | |
| L. major (MRHO/IR/75/ER) | Promastigotes | Artemether | 5, 10, 25, 50, and 100 μg/mL, incubated for 72 h | IC50 = 25 μg/mL. Apoptotic promastigotes were 2.44%, 42.28%, and 71.95 %, respectively | Ebrahimisadr et al. [26] | |
| L. major (MRHO/IR/75/ER) | Promastigotes | Aqueous extract of Artemisia sieberi has similar component as artemisinin | 5, 10, 25, 50, and 100 μg/mL, incubated for 24, 48, and 72 h | IC50 = 25 μg/mL. After 24, 48, and 72 h of treatment with 100 μg/mL, the viabilities of promastigotes were ~34%, ~30%, and ~18%, respectively, which had higher parasite growth inhibitory effect on promastigotes vs. ~32% of treatment with artemisinin at the same concentration and duration at 72 h | Heydari et al. [35] | |
| L. major (MRHO/IR/75/ER) | Amastigotes in macrophages of BALB/c mice | Artemisinin | 5, 10, 25, 50, and 100 μg/mL, incubated for 24, 48, and 72 h | IC50 = 25 μg/mL. Apoptotic macrophages after 48 h of treatment were 0.64%, 1.43%, 1.96%, and 9.39%, respectively, in comparison of control that was 0.6% | Ghaffarifar et al. [28] | |
| L. major (MRHO/IR/75/ER) | Amastigotes in macrophages of BALB/c mice | Artemisinin | 5, 10, 25, 50, and 100 μg/mL, incubated for 24, 48, and 72 h | IC50 = 50 μg/mL, effective elimination of amastigotes from macrophages and decreasing the amastigote burden, with the cell viabilities of 2%, 1.8%, and 1.6% at 100 μg/mL, incubated for 24, 48, and 72 h, respectively. | Heydari et al. [35] | |
| L. major (LV39, MRHO/SU/59/P) | Amastigotes | Artemisinin | 123, 41, 13, 4.5, and 1.5 μM, incubated for 48 h | Estimated by the incorporation of 3H-thymidine, the viability of the parasites post-treatment was ~3, 3.5, 6, 6.1, and 7.5 cpm ×10−3. IC50 = 3 × 10−5 M. Using 10 times higher concentration to kill promastigotes than that to kill intracellular amastigotes | Yang et al. [36] | |
| L. major (MRHO/IR/75/ER) | Amastigotes in macrophages of BALB/c mice | Artemether | 5, 10, 25, 50, and 100 μg/mL, incubated for 72 h | The mean number of amastigotes per macrophage after adding artemether was 0.78, 0.64, 0.49, 0.30, and 0.21, respectively; vs. 0.96 (before adding artemether), and vs. 1.6 in control | Ebrahimisadr et al. [26] | |
| L. major (LV39, MRHO/SU/59/P) | Amastigotes | Artemether | 123, 41, 13, 4.5, and 1.5 μM, incubated for 48 h | Viability of the parasites post-treatment was ~1, 2.4, 3.4, 4.5, 4.8, 9 cpm × 10−3, and 3 × 10−6 M respectively. Using 10 times higher concentration to kill promastigotes than that to kill intracellular amastigotes. | Yang et al. [36] | |
| L. major (MRHO/IR/75/ER) | Amastigotes in macrophages of BALB/c mice | Artemisia sieberi | 5, 10, 25, 50, and 100 μg/mL, incubated for 24, 48, and 72 h | IC50 = 100 μg/mL, lower cytotoxic effects on macrophages compared to artemisinin. Effectively eliminated amastigotes from macrophages and decreased amastigote burden, with the cell viabilities of 1.2%, 0.8%, and 0.4% at 100 μg/mL, incubated for 24, 48, and 72 h, respectively | Heydari et al. [35] | |
| L. donovani (MHOM/IN/83/AG83) | Promastigotes | Artemisinin | 10 and 25 μM, incubated for 48 h | In infected macrophages, 10 μM artemisinin increased NO level to (5.52 ± 0.45) μM, and 25 μM artemisinin significantly increased NO level to (6.78 ± 0.43) μM. The NO levels achieved were comparable to that generated by uninfected macrophages, which restores its ability to eliminates the parasite | Sen et al. [27] | |
| L. donovani (MHOM/IN/83/AG83) | Promastigotes | Artemisinin | 160 μM, incubated for 24 h | Proportion of cells in sub-G0/G1 phase increased to 10.86% compared with 3.62% of controls. Nuclear DNA fragmentation as the dUTP–FITC binding after treatment for 24 h was increased from a baseline mean fluorescence intensity of 35.96 in untreated cells to 110.27 | Sen et al. [23] | |
| L. donovani (MHOM/IN/83/AG84) | Promastigotes | Artemisinin | 160 μM, incubated for 48 h | Proportion of cells in the sub-G0/G1 phase increased to 33.11% compared with 3.03% in control cells, accompanied with a decreased number of cells in the G2/M phase compared with controls, values of 27.45% vs. 41.03% at 24 h and 12.03% vs. 29.65% at 48 h | Sen et al. [23] | |
| L. donovani (NLB-065) | Promastigotes | Artemisinin | Serial dilutions: 100–0.049 μg/mL, incubated for 72 h | IC50 = (4.64 ± 0.48) μg/mL, and lower efficacy than amphotericin B (0.16 ± 0.32) μg/mL | Mutiso et al. [33] | |
| L. donovani (strain not stated) | Promastigotes | Artemisinin | Dose information not available, 72 h of drug exposure | IC50 = (30.8 ± 1.4) μM | Mishina et al. [32] | |
| L. donovani (NLB-065) | Promastigotes | Diminazene + artemisinin | Serial dilutions: 100–0.049 μg/mL, incubated for 72 h | IC50 = (2.28 ± 0.24) μg/mL, and higher efficacy than artemisinin but lower efficacy than amphotericin B | Mutiso et al. [33] | |
| L. donovani (LV9 WT) | Promastigotes | BB200 (OOH group substituted at C-10 position of fluoroartemisinin) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (2.5 ± 0.3) μM, and higher efficacy than miltefosine, DHA, and sitamaquine | Chollet et al. [30] | |
| L. donovani (LV9 WT) | Promastigotes | BB201 (paramethoxy-aniline group substituted at the C-10 position of fluoroartemisinin) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (1.5 ± 0.2) μM; most active against the parasite and higher efficacy than miltefosine, DHA, and sitamaquine | Chollet et al. [30] | |
| L. donovani (LV9 WT) | Promastigotes | BB241 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (6.25 ± 0.8) μM, and higher efficacy than DHA and sitamaquine | Chollet et al. [30] | |
| L. donovani (LV9 WT) | Promastigotes | BB242 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (5.5 ± 0.6) μM, and higher efficacy than DHA and sitamaquine | Chollet et al. [30] | |
| L. donovani (LV9 WT) | Promastigotes | DHA | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (20.2 ± 1.9) μM, and higher efficacy than sitamaquine | Chollet et al. [30] | |
| L. donovani (HePC-R, miltefosine-resistant line) | Promastigotes | BB200 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (8.2 ± 0.8) μM, and higher efficacy than sitamaquine | Chollet et al. [30] | |
| L. donovani (HePC-R) | Promastigotes | BB201 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (1.1 ± 0.1) μM, and higher efficacy than miltefosine, DHA, and sitamaquine | Chollet et al. [30] | |
| L. donovani (HePC-R) | Promastigotes | BB241 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (2.8 ± 0.3) μM, and higher efficacy than miltefosine, DHA, and sitamaquine | Chollet et al. [30] | |
| L. donovani (HePC-R) | Promastigotes | BB242 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (6.3 ± 0.7) μM, and higher efficacy than sitamaquine | Chollet et al. [30] | |
| L. donovani (HePC-R) | Promastigotes | DHA | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (4.1 ± 0.3) μM, and higher efficacy than miltefosine and sitamaquine | Chollet et al. [30] | |
| L. donovani (SITA-R, sitamaquin e-resistant line) | Promastigotes | BB200 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (12.5 ± 1.3) μM, and higher efficacy than sitamaquine | Chollet et al. [30] | |
| L. donovani (SITA-R) | Promastigotes | BB201 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (0.38 ± 0.05) μM, and higher efficacy than miltefosine, DHA, and sitamaquine | Chollet et al. [30] | |
| L. donovani (SITA-R) | Promastigotes | BB241 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (1.6 ± 0.2) μM, and higher efficacy than miltefosine, DHA, and sitamaquine | Chollet et al. [30] | |
| L. donovani (SITA-R) | Promastigotes | BB242 (fluoroartemisinin derivative) | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (12.4 ± 1.4) μM, and higher efficacy than sitamaquine | Chollet et al. [30] | |
| L. donovani (SITA-R) | Promastigotes | DHA | Serial dilutions: 2 μL of stock solution dissolved in DMSO, incubated for 3 days | IC50 = (6.0 ± 0.7) μM, and higher efficacy than sitamaquine | Chollet et al. [30] | |
| L. donovani (AG83) | Promastigotes | AAL (n-hexane fraction of A annua leaves) | Serial dilutions started from100–0 μg/mL. 100 μg AAL = 1.447 μg artemisinin, incubated for 96 h | IC50 = 14.4 μg/mL, no viable parasites were observed after 2-, 3-, or 5-day incubation with 100 μg AAL | Islamuddin et al. [37] | |
| L. donovani (AG83) | Promastigotes | AAS (n-hexane fraction of A. annua seeds) | Serial dilutions started from 100– 0 μg/mL. 100 μg AAS = 1.336 μg artemisinin, incubated for 96 h | IC50 = 14.6 μg/mL, no viable parasites were observed after 2-, 3-, or 5-day incubation with 100 μg AAS | Islamuddin et al. [37] | |
| L. donovani (strain not stated) | Promastigotes | Artemisone | Dose information not available, 72 h of drug exposure | IC50 = (12.9 ± 6.3) μM | Mishina et al. [32] | |
| L. donovani (strain not stated) | Promastigotes | 4-Fluorophenyl-artemisinin | Dose information not available, 72 h of drug exposure | IC50 = (3.0 ± 1.1) μM. Highest efficacy compared to artemisinin and artemisone, comparable to amphotericin B (IC50 = 2.8 ± 0.1 μg/mL) | Mishina et al. [32] | |
| L. donovani (MHOM/IN/83/AG83) | Amastigotes in a human leukaemia cell line (U937) | Artemisinin + iron | 0.5 mM, incubated with Fe2+ (0.2 mM) for 48 h | Increased production of reactive oxygen species following incubation of artemisinin-treated cells with Fe2+, measured using Fe2+-artemisinin 200 geometric mean fluorescence channel vs. 100 with artemisinin alone | Sen et al. [38] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | Trioxolane LC50 | At 6 different concentrations (0.90–35.93 μM), incubated for 48 h | IC50 = 9.35 μM and SI = 768.44, higher selectivity than pentamidine and milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | Trioxolane LC67 | At 6 different concentrations (14.86–445.87 μM), incubated for 48 h | IC50 = 145.98 μM and SI = 28.24 | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | Trioxolane LC95 | At 6 different concentrations (1.70–20.45 μM), incubated for 48 h | IC50 = 3.51 μM and SI = 1684.74, the highest selectivity. Higher selectivity than pentamidine, milfetosine, and amphotericin B; higher efficacy than pentamidine and milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | DHA | At 6 different concentrations (27.49–3,519.14 μM), incubated for 48 h | IC50 = 123.06 μM and SI = 2.04 | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | Deoxy-DHA | At 6 different concentrations (29.13–3,728.98 μM), incubated for 48 h | IC50 = 1249.33 μM and SI = 1.07 | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | Artesunate | At 6 different concentrations (2.03–260.29 μM), incubated for 48 h | IC50 = 40.68 μM and SI = 7.32 | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Promastigote | Deoxy-artesunate | At 6 different concentrations (21.2–2,716.06 μM), incubated for 48 h | IC50 = 747.73 μM and SI = 1.28 | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote in the human acute monocytic leukemia cell line THP-1 (ATCC TIB-202) | Trioxolane LC50 | At 3 different concentrations (11.68–1,077.82 μM), incubated for 48 h | IC50 = 79.76 μM and SI = 90.08, the highest selectivity. Higher selectivity than pentamidine, milfetosine, and amphotericin B | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote THP1 cell line | Trioxolane LC67 | At 3 different concentrations (27.87–3,566.97 μM), incubated for 48 h | IC50 = 1202.81 μM and SI = 3.43. Higher selectivity than milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote in THP1 cell line | Trioxolane LC95 | At 3 different concentrations (1.07–409.00 μM), incubated for 48 h | IC50 = 107.87 μM and SI = 54.82. Higher selectivity than pentamidine and milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote in THP1 cell line | DHA | At 3 different concentrations (27.49–219.95 μM), incubated for 48 h | IC50 = 104.06 μM and SI = 2.41. Higher selectivity than milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote in THP1 cell line | Deoxy-DHA | At 3 different concentrations (29.13–932.24 μM), incubated for 48 h | IC50 = 457.36 μM and SI = 2.91. Higher selectivity than milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote THP1 cell line | Artesunate | At 3 different concentrations (20.34–650.74 μM), incubated for 48 h | IC50 = 122.52 μM and SI = 2.43. Higher selectivity than milfetosine | Cortes et al. [29] | |
| L. infantum (MHOM/PT/88/IMT151) | Amastigote in THP1 cell line | Deoxy-artesunate | At 3 different concentrations (21.22–679.02 μM), incubated for 48 h | IC50 = 80.27 μM and SI = 11.95. Higher selectivity than pentamidine and milfetosine | Cortes et al. [29] | |
| In vivo studies | L. major (MRHO/IR/75/ER) | BALB/c mice | Artemisinin | 1 mg was dissolved in 50% ethanol and 50% distilled water, i.p., q.d. for 150 days | Decreased lesion size and increased IL-4 and IFN-γ levels. Survival rate at day 150 was ~34% | Ghaffarifar et al. [28] |
| L. major (MRHO/IR/75/ER) | BALB/c mice | Artemisinin | 0.1 mL of ointment (25 μg/mL)/mouse, t.p., b.i.d. for 150 days | Largest decreased lesion size and increased IL-4 and IFN-γ levels. Survival rate at day 150 was ~66% | Ghaffarifar et al. [28] | |
| L. major (MRHO/IR/75/ER) | BALB/c mice | Artemisinin | 1 mg was dissolved in 50% ethanol and 50% distilled water. t.p., q.d. for 150 days | Decreased lesion size and survival rate at day 150 was 0% | Ghaffarifar et al. [28] | |
| L. major (MRHO/IR/75/ER) | BALB/c mice | Artemisinin | 10 mg/kg/day, i.p. for 3 weeks | NO production was slightly restored (naive: ~13 μM/mL, without treatment: ~8 μM/mL, and artemisinin: ~9 μM/mL) | Nemati et al. [25] | |
| L. major (LV39, MRHO/SU/59/P) | BALB/c mice | Artemether | 50 mg/kg/day, intra-lesion for 15 days | Developed significantly smaller footpad lesions compared to the controls (approximately 20 mm vs. 30 mm); parasite burden (4500 cpm vs. 30520 cpm) | Yang et al. [36] | |
| L. major (LV39, MRHO/SU/59/P) | BALB/c mice | Artemether | 50 mg/kg/day, i.m. for 15 days | Developed significantly smaller lesion compared to controls; however, lesion size was not reduced further by increasing the dose of artemether | Yang et al. [36] | |
| L. major (LV39, MRHO/SU/59/P) | BALB/c mice | Artemether | 50 mg/kg/day, p.o. for 15 days | Lesion size was indistinguishable from the controls; however, the parasite burdens were lower compared to controls | Yang et al. 36] | |
| L. major (LV39, MRHO/SU/59/P) | BALB/c mice | Artemether | 200 mg/kg/day, p.o. for 15 days | Lesion size was significantly smaller compared to controls | Yang et al. [36] | |
| L. donovani (MHOM/IN/1983/AG83) | BALB/c mice | Artemisinin | 10 mg/kg, i.p. for 10 days | Reduction in hepatosplenomegaly [(53.0 ± 2.0)% in liver vs. control] | Want et al. [24] | |
| L. donovani (MHOM/IN/1983/AG83) | BALB/c mice | Artemisinin | 20 mg/kg, i.p. for 10 days | Reduction in hepatosplenomegaly [(70.3 ± 0.6)% in liver and (62.7 ± 3.7)% in spleen vs. control] | Want et al. [24] | |
| L. donovani (MHOM/IN/83/AG83) | BALB/c mice | Artemisinin | 10 mg/kg. p.o. for 2 weeks | Splenic weight decreased to (94.0 ± 7.7) mg vs. control [(107.0 ± 8.4) mg] in the beginning and [(220.0 ± 26.0) mg] at the end; final parasite removal of 82.6% | Sen et al. [27] | |
| L. donovani (MHOM/IN/83/AG83) | BALB/c mice | Artemisinin | 25 mg/kg. p.o. for 2 weeks | Splenic weight decreased to (110.0 ± 2.7) mg vs. control [(107.0 ± 8.4) mg] in the beginning and [(220.0 ± 26.0) mg] at the end; final parasite removal of 86.0% (higher efficacy) | Sen et al. [27] | |
| L. donovani (MHOM/IN/1983/AG83) | BALB/c mice | Artemisinin-loaded nanoparticles | 10 mg/kg, i.p. for 10 days | Significant reduction of hepatosplenomegaly; the percentages of parasite inhabition were (68.1 ± 2.8)% in liver and (66.3 ± 2.4)% in spleen vs. artemisinin alone | Want et al. [24] | |
| L. donovani (MHOM/IN/1983/AG83) | BALB/c mice | Artemisinin-loaded nanoparticles | 20 mg/kg, i.p. for 10 days | Significant reduction of hepatosplenomegaly; the percentages of parasite inhabition were (85.4 ± 5.4)% in liver and (82.0 ± 2.4)% in spleen vs. amphotericin B (98.6 ± 0.2)% in liver and ~90% in spleen | Want et al. [24] | |
| L. donovani (NLB-065) | BALB/c mice | Artesunate | 12.5 mg/kg. q.d. for 28 consecutive days (oral and parenteral formulas were designed; delivery route was not stated) | Parasite burden of post-treatment (~110 amastigote/500 splenic cell nuclei) vs. amphotericin B (~10 amastigotes/500 nuclei) | Mutiso et al. [33] | |
| L. donovani (NLB-065) | BALB/c mice | Diminazene + artesunate granules | 12.5 mg/kg + 12.5 mg/kg, q.d. for 28 consecutive days | Parasite burden of post-treatment (~30 amastigote/500 splenic cell nuclei) vs. amphotericin B (~10 amastigotes/500 nuclei) | Mutiso et al. [33] | |
|
| ||||||
| b.i.d.: two times per day | ||||||
|
| ||||||
| DHA: dihydroartemisinin | ||||||
|
| ||||||
| DMSO: Dimethyl sulphoxide | ||||||
IC50: concentration that causes 50% inhibition of growth
i.m.: intramuscular injection
i.p.: intraperitoneal injection
p.o.: oral administration
q.d.: one time per day
SI: selectivity index
t.p.: topical administration
The IC50 is either in μg/mL or μM as in the original papers.
In vitro studies
ART works by impairing the activation of host macrophages, preventing the production of lethal nitric oxide (NO) and restoring normal NO production in L. major-infected macrophages [25]. Similar effects have been observed for the treatment of L. major, L. infantum, L. tropica, L. braziliensis, L. mexicana, and L. amazonensis [17,26]. In addition, ART also enables externalization of phosphatidylserine and leads to the loss of mitochondrial membrane potential, cell-cycle arrest at the sub-G0/G1 phase, and programmed cell death of L. donovani promastigotes [23]. Iron in excess binds to haemoglobin and activates ART by aiding the formation of intra-parasitic heme-iron, which catalyses the cleavage of the endoperoxide ring and enables the transfer of an oxygen atom from the peroxide group to a chelated iron ion, generating a Fe(IV)O species. The resultant free radical intermediate or iron-ART adduct then effectively kill the promastigotes of Leishmania parasites by alkylation [27]. ART showed high toxicity and apoptotic effect on promastigotes and low toxicity on BALB/c macrophages [28]. Artemisinin-derived trioxanes and synthetic trioxolanes were tested against promastigotes and intramacrophage amastigotes of L. infantum, in which trioxolanes LC50 and LC95 had the best activity and safety profiles, showing potential for leishmanial therapy [29]. Fluoro-artemisinin derivatives (BB200, BB201, BB241, and BB242) are proven to have higher efficacy than DHA and sitamaquine, especially BB201 was shown to have higher efficacy than miltefosine, DHA, and sitamaquine against the parasite [30]. ART [23,28], artemether, and deoxydihydroartemisinin (Deoxy-DHA) [31] had anti-leishmanial effects in infected host cells. In another study, fluorophenyl-artemisinin was shown to have higher efficacy in inhibiting L. donovani in vitro growt than artemisinin, artemison, and comparable efficacy to amphotericin B [32].
In vivo studies
Studies have shown that the leaves and seeds of A. annua caused increased production of Th1 cytokines [interferon-gamma (IFN-γ)] and decreased Th2 cytokines [interleukin (IL)-4 and IL-10] in L. donovani-infected BALB/c mice [33]. The anti-leishmanial activity of ART was mediated by increased IL-4 and IFN-γ levels in the host cells, inducing apoptotic effects on promastigotes and reducing post-treatment lesion size in L. major-infected BALB/c mice. In addition, using topical ART ointment as a route of administration, a higher healing effect compared to other routes of administration was achieved [28]. ART-loaded nanoparticles and combination therapy of diminazene and ART have a therapeutic effect comparable to that of the first-line drug amphotericin B, leading to significant reduction in hepatomegaly and parasite burden in L. donovani-infected BALB/c mice [24,33].
Human trial
The only clinical study presently available failed to show a significant difference between the artesunate plus sulfamethoxypyrazine/pyrimethamine treatment and the placebo [34].
ART and its derivatives on Trypanosome spp
There are two distinct species of Trypanosome in humans: Trypanosoma brucei causes African trypanosomiasis or African sleeping sickness and T. cruzi causes American trypanosomiasis or Chagas’ disease [39]. The only drugs now available for Chagas disease, nifurtimox and benznidazole, are relatively toxic for adult patients, and require prolonged administration [40]. The drugs available for African sleeping sickness are pentamidine isethionate, suramin, melarsoprol, eflornithine, or nifurtimox–eflornithine combination treatment, the use of which depends on the disease stage and causative pathogen with toxicity and possible resistance [41]. However, the clinical use of the current medications is compromised due to emerging strain-specific drug resistance, serious side-effects, high toxicity levels, and lengthy parental administration [42]. There is a critical need to develop new drugs for treatment of Chagas disease and sleeping sickness. The studies of ART and its derivatives on Trypanosome spp. are summarized in Table 2.
Table 2.
Treatment of artemisinin and its derivatives against Tryponosome spp.
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | T. brucei rhodesiense (strain not stated) | Trypomastigotes, human U-937 monocytes | Artemisinin | Dose information not available, 72 h of drug exposure | IC50 = (20.4 ± 0.3) μM and IC90 = (49.1 ±1.3) μM | Mishina et al. [32] |
| T. brucei rhodesiense (strain not stated) | Trypomastigotes, human U-937 monocytes | Artemisone | Dose information not available, 72 h of drug exposure | IC50 = (22.5 ± 3.0) μM and IC90 = (44.8 ± 0.8) μM | Mishina et al. [32] | |
| T. brucei rhodesiense (strain not stated) | Trypomastigotes, human U-937 monocytes | 4-fluorophenyl-artemisinin | Dose information not available, 72 h of drug exposure | IC50 = (15.7 ± 5.6) μM and IC90 = (46.9 ± 0.6) μM | Mishina et al. [32] | |
| T. brucei rhodesiense (strain not stated) | Trypomastigotes, human U-937 monocytes | DHA | Dose information not available, 72 h of drug exposure | IC50 = (24.6 ± 2.4) μM and IC90 = (49.6 ± 1.0) μM | Mishina et al. [32] | |
| T. brucei brucei (TC221) | Trypomastigotes, human leukemia cell line (HL-60) | MeOH and CH2CL2 crude extracts of Artemisia absinthium (aerial part) | Serial dilution into 7 different conocentrations: 250–3.91 μg/mg, incubated for 48 h | IC50 = 27.90 μg/ml (MeOH) and IC50 = 27.05 μg/ml (CH2CL2) | Nibret et al. [45] | |
| T. brucei brucei (TC221) | Trypomastigotes, HL-60 | MeOH and CH2CL2 crude extracts of A. abyssinica (aerial part) | Serial dilution into 7 different conocentrations: 250–3.91 μg/mg, incubated for 48 h | IC50 = 41.76 μg/ml (MeOH) and the extracts (CH2CL2) showed the best anti-trypanosomal activity of IC50 = 19.13 μg/ml (CH2CL2) | Nibret et al. [45] | |
| T. brucei brucei (TC221) | Trypomastigotes, HL-60 | MeOH and CH2CL2 crude extracts of A. afra (leaves) | Serial dilution into 7 different conocentrations: 250–3.91 μg/mg, incubated for 48 h | IC50 = 77.54 μg/ml (MeOH) and IC50 = 25.27 μg/ml (CH2CL2). | Nibret et al. [45] | |
| T. brucei brucei (TC221) | Trypomastigotes, HL-60 | Artemisinin | Serial dilution into 7 different conocentrations: 250–3.91 μg/mg, incubated for 48 h | IC50 = 35.91 μg/ml | Nibret et al. [45] | |
| T. brucei (Lafia strain) | Peripheral blood of infected rat (parasite stage and rat species not mentioned) | Artesunate | 50 mg/mL ampoule, incubated for 300 min | 120 min of treatment: reduced motility of the trypanosome, and parasitemia reduced to moderate (++); 180 min of treatment: complete clearance of parasitaemia |
Akande et al. [43] | |
| T. brucei (Lafia strain) | Peripheral blood of infected rat | Artemether | 80 mg/mL ampoule, incubated for 300 min | 60 min of treatment: reduced motility of the trypanosome, and parasitemia reduced to moderate (++) | Akande et al. [43] | |
| T. cruzi (strain not stated) | Epimastigotes in peripheral blood of infected rat (rat species not mentioned) | Artemisinin | Dose information not available, 72 h of drug exposure (assays were performed 2–5 times with 6 drug concentrations) | IC50 = (13.4 ± 2.3) μM and IC90 = (46.5 ± 1.2) μM | Mishina et al. [32] | |
| T. cruzi (strain not stated) | Epimastigotes in peripheral blood of infected rat | Artemisone | Dose information not available, 72 h of drug exposure | IC50 = (23.3 ± 2.7) μM and IC90 = (50.5 ± 0.8) μM | Mishina et al. [32] | |
| T. cruzi (strain not stated) | Epimastigotes in peripheral blood of infected rat | 4-fluorophenyl-artemisinin | Dose information not available, 72 h of drug exposure | IC50 = (17.9 ± 3.9) μM and IC90 = (50.8 ± 0.9) μM | Mishina et al. [32] | |
| T. cruzi (strain not stated) | Epimastigotes in peripheral blood of infected rat | DHA | Dose information not available, 72 h of drug exposure | IC50 = (12.8 ± 0.3) μM and IC90 = (49.2 ± 1.5) μM | Mishina et al. [32] | |
| T. cruzi (AR-SE23C) | Epimastigotes, Vero cell (African green monkey kidney) | Artesunate | Serial dilutions: 0.01–100 μg/mL, incubated for 72 h | IC50 = 19.22 μg/mL and IC90 = 69.20 μg/mL | Olivera et al. [44] | |
| T. cruzi (Nicaragua strain) | Epimastigotes, Vero cell | Artesunate | Serial dilutions: 0.01–100 μg/mL, incubated for 72 h | IC50 = 2.35 μg/mL and IC90 = 14.34 μg/mL | Olivera et al. [44] | |
| T. cruzi (Brazil strain) | Epimastigotes, Vero cell | Artesunate | Serial dilutions: 0.01–100 μg/mL, incubated for 72 h | IC50 = 8.84 μg/mL and IC90 = 44 μg/mL | Olivera et al. [44] | |
| T. cruzi (AR-SE23C) | Amastigotes, Vero cell | Artesunate | Serial dilutions: 0.001–100 μg/mL, incubated for 72 h | IC50 = 6.63 μg/mL and IC90 = 19.25 μg/mL | Olivera et al. [44] | |
| T. cruzi (Nicaragua strain) | Amastigotes, Vero cell | Artesunate | Serial dilutions: 0.001–100 μg/mL, incubated for 72 h | IC50 = 0.05 μg/mL and IC90 = 0.70 μg/mL | Olivera et al. [44] | |
| T. cruzi (Brazil strain) | Amastigotes, Vero cell | Artesunate | Serial dilutions: 0.001–100 μg/mL, incubated for 72 h | IC50 = 2.65 μg/mL and IC90 = 3.84 μg/mL | Olivera et al. [44] | |
| T. cruzi (Brazil strain) | Trypomastigotes, Vero cell | Artesunate | Serial dilutions: 0.1–100 μg/mL, incubated for 24 h | IC50 = 56.9 μg/mL | Olivera et al. [44] | |
| T. cruzi (Brazil strain) | Trypomastigotes, from blood stream | Artesunate | Serial dilutions: 0.1–100 μg/mL, incubated for 24 h | IC50 = 31.52 μg/mL | Olivera et al. [44] | |
| In vivo studies | T. brucei brucei (Lafia strain) | Rat: Rattus novergicus | Artemether | Early-stage treatment (started 3 days before infection): 2.3 and 4.6 mg/kg, i.p., q.d., | Fluactuation of parasitaemia, and lifespan extended from 11 to 13–14 days | Oluyomi et al. [48] |
| T. brucei brucei (Lafia strain) | Rat: Rattus novergicus | Artemether | Late-stage treatment (started 10th day p.i.): 2.3 and 4.6 mg/kg, i.p., q.d., 3.2 mg/kg, i.m. on the 1st day | Reduction in parasitaemia, and lifespan extended to 14 days | Oluyomi et al. [48] | |
| T. brucei (Lafia strain) | Mice (species not mentioned) | Artemether | and 1.6 mg/kg/day i.m. for the next 4 days | 3 days post-treatment: reduction in activity of the trypanosome; 5 days post-treatment: complete clearance of the parasite | Akande et al. [43] | |
| T. brucei (Lafia strain) | Mice (species not mentioned) | Artesunate | 100 mg/kg on the 1st day and 50 mg/kg, p.o., q.d. for 5 days | 7 days post-treatment: complete clearance of the parasite | Akande et al. [43] | |
| Combination therapy | T. brucei brucei (Lafia strain) | Rat: Rattus novergicus | Artemether + halofantrine HCl | Early-stage treatment (the day parasite was first sighted in the blood): 2.3 mg/kg artemether and 7.1 mg/kg halofantrine HCl, i.p. | Maintained low parasitaemia and extended lifespan of rats to 15–16 days for prophylactic treatment vs. 11 days in control | Oluyomi et al. [48] |
| T. brucei brucei (Lafia strain) | Rat: Rattus novergicus | Artemeter + halofantrine HCI | Late-stage treatment (started 10th day p.i.): 4.6 mg/kg artemether and 14.2 mg/kg halofantrine HCl rat weight, i.p. | Parasitaemia declined drastically and extended lifespan of rats to 19 days vs. 11 days in control | Oluyomi et al. [48] | |
| T. brucei (Lafia strain) | Mice (species not mentioned) | Artesunate + diminazene aceturate | Artesunate, p.o. 50 mg/kg on the 1st day, 25 mg/kg for next 4 days, and 1.75 mg/kg later on; 3.5 mg/kg diminazene aceturate, i.m. | After 3 days of treatment, reduction in activity of the trypanosome; 5 or 7 days of reatment, complete clearance of the parasite | Akande et al. [43] | |
| T. cruzi (Brazil strain) | BALB/c mice | Artesunate + benznidazole | Artesunate (125 mg/kg/d) + benznidazole (100 mg/kg/d), p.o. for 6 consecutive days | Lower number of circulating parasites, and significantly reduced parasite density and inflammation in skeletal muscle | Olivera et al. [44] | |
| T. cruzi (Brazil strain) | BALB/c mice | Artesunate + benznidazole | Artesunate (75 mg/kg/d) + benznidazole (50 mg/kg/d), p.o. for 6 consecutive days | Lower number of circulating parasites and reduced parasite density in skeletal muscle | Olivera et al. [44] | |
|
| ||||||
| CH2CL2: dichloromethane | ||||||
DHA: dihydroartemisinin
IC50: concentration that causes 50% inhibition of growth
IC90: concentration that causes 90% inhibition of growth
i.m.: intramuscular injection
i.p.: intraperitoneal injection
MeOH: methanol
p.i.: post-infection
p.o.: oral administration
q.d.: one time per day
The IC50 is either in μg/mL or μM as in the original papers.
In vitro studies
The drugs for in vitro studies include artesunate [43], ART, artemisone, and 4-fluorophenyl-ART [32]; the cell lines or cells used include African green monkey kidney cell line (Vero) [44], leukaemia cell line (HL-60) [45], and peripheral blood of infected rat [43]. Artesunate can inhibit the replication of T. cruzi epimastigotes, amastigotes, and trypomastigotes in Vero cells in vitro [44]. Extracts (methanol extract and dichloromethane extract) from the leaves and aerial parts of four Artemisia species (Artemisia absinthium, A. abyssinica, A. afra, and A. annua) growing in Ethiopia have been tested against T. brucei brucei in vitro. The dichloromethane extract from A. abyssinica was most active (IC50 = 19.13 μg/mL), while ART from A. annua also showed anti-trypanosomal and cytotoxic activities (IC50 = 35.91 μg/mL), which may be due to lipophilic sesquiterpene lactones in the extract [45]. One of the mechanisms of trypanocidal activity is that lipophilic compounds such as lipophilic sesquiterpene lactones in the extract can increase the fluidity of the membranes leading to uncontrolled efflux of ions and metabolites resulting in cell death [45–47]. Another mechanism of trypanocidal activity is the inhibition of ATPase at the presence of calcium, e.g., the inhibition of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) by inhibiting H+/K+-ATPase could be related to the inhibition of trypanosome growth [32]. The combination of artesunate and diminazene aceturate showed a complete clearance of T. brucei on day 5 in comparison of artesunate monotherapy on day 7 [43]. Studies showed that artesunate inhibits the replication of epimastigotes and intracellular amastigotes of three strains of T. cruzi originated in different regions of Latin America in vitro [44]. In addition, artemisinin compounds inhibit T. cruzi growth in vitro and inhibit calcium-dependent ATPase activity in T. cruzi membranes [32].
In vivo studies
The animals including mice [43, 44] and rats [48] have been used in the studies. It has been reported that 2.3 mg/kg and 4.6 mg/kg of artemether are able to suppress and reduce the number of T. brucei brucei parasites, and extend the lifespan of rats at the early and late stage treatment [48]. The lifespan of infected rats was extended to 19 days using a combination of halofantrine HCl with artemether compared to 14 days with artemether monotherapy [46]. Halofantrine HCl can form complex with heme-iron [49, 50], which has lytic properties towards the parasite. The half-life of halofantrine HCl ranged from 1 to 4 days, thereby giving halofantrine HCl a high bioavailability [51], which may be one of the factors that prolongs the lifespan of T. brucei-infected rats [48,]. The reduction in parasitaemia is probably due to the ability of artemether to form complex with heme that is believed to be toxic to the parasite [52, 53]. The clinical signs of dullness and paleness of mucous membrane on T. brucei-infected mice disappeared by both artemether and artesunate treatment. However, compared with artesunate, artemether is more effective as it clears the parasite completely from the body after 5 days of post-treatment [43]. In another study, artesunate alone was not effective in reducing the parasites in T. cruzi-infected BALB/c mice and failed to cure the infection, suggesting that artesunate may be unsuitable for in vivo treatment of T. cruzi infection [44].
4. ART and its derivatives on T. gondii
T. gondii infects a large number of warm-blooded animals and is estimated to chronically infect up to one-third of the world’s human population. Toxoplasmic encephalitis in patients with AIDS is a life-threatening disease mostly due to reactivation of T. gondii cysts in the brain [54]. One of the current therapies for toxoplasmic infection is a combination of the drugs sulfadiazine and pyrimethamine [55], which cause hematological side-effects that can be controlled by the administration of folinic acid. The other treatment is the combination of trimethoprim and sulfamethoxazole (cotrimoxazole), which is well tolerated and less toxic to hematopoiesis. However, this drug can cross the placental barrier, so that its use is discouraged for pregnant women [56]. Moreover, these medicines have no effect on the tissue cysts of the parasite located predominantly in a brain and muscles [57]. It has been reported that medicinal plants can be used for toxoplasmosis as an alternative to standard drug therapy with reduced side effects. The studies of ART and its derivatives on T. gondii are analysed, and summarized in Table 3.
Table 3.
Treatment of artemisinin and its derivatives against Toxoplasma gondii
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | T. gondii (RH strain) | Tachyzoites, Human fibroblast (HFF) cells | Artemisinin | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.64 μM, TD50 ≥ 320 μM, and TI = 879. Parasites/vacuole ratio after treatment was 8, the same as the vehicle control | Hencken et al. [63] |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemisinin | 98% pure artemisinin 10–50 μM, incubated for 15–20 min | Affects Ca2+ homeostasis and signaling in the parasite, acts as an inhibitor of SERCA that was critical for intracellular survival | Nagamune et al. [60] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemisinin | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Prevented parasitic growth at 1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemisinin | Serial dilutions: 0–320 μg/mL, incubated for 94 h | IC50 = 8.0 μM, TD50 > 1,130 μM, TI ≥ 243, and >50% reduction of penetrated tachyzoites | Jones-Brando et al. [68] | |
| T. gondii (RH strain) | Tachyzoites, Vero cells (CRL 6318) | Artemisinin | 1 μg/mL, incubated for 120 h | TgPrx expression was increased as early as 30 min and reached to the plateau in 1 h by the treatment in the intracellular stage, which indicates apoptosis of the parasite | Son et al. [59] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemisinin | 0.01, 0.1, 1, and 10 μg/mL, incubated for 16 h | Reduction in plaque size at 0.1 μg/mL, and inhibited all parasite growth at 1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemisinin | 0.4, 1.3, and 4 μg/mL, incubated for 27 days | At 1.3 μg/mL, reduced T. gondii plaque size was observed at day 14 and the infection was completely eliminated at day 22 post-treatment; at 4.0 μg/mL, the parasites were completely eliminated at day 14 (comparable to pyrimethamine treatment at 10 μg/mL) | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemether | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.31 μM, TD50 ≥ 320 μM, and TI = 1814. Parasites/vacuole ratio after treatment was 9, the same as the vehicle control | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemether | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Inhibited all parasite growth at 0.1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemether | Serial dilutions: 0–320 μg/mL, incubated for 94 h | IC50 = 0.7 μM, TD50 = 220 μM, and TI = 1,100. Significant reductions in the number of penetrated parasites | Jones-Brando et al. [68] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemether | 0.01, 0.1, 1, and 10 μg/mL, incubated for 16 h | The only compound that diminished plaque size at 0.01 μg/mL; reduced plaque size and completely inhibited parasite growth at 0.1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites in peritoneal exudate cells from Swiss Webster mice | Arteether | 0.02, 0.2, 0.25, 0.5, 1.0, and 2.0 μg/mL, incubated for 21 h | > 0.5 μg/mL showed the parasite growth inhibition, higher efficacy than cycloguanil (0.5 μg/mL vs. 1.0 μg/mL), and reduction in the incorporation of 3H-uracil into the nucleic acids of tachyzoites | Holfels et al. [62] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Arteether | 0.01, 0.1, 1, and 10 μg/mL, incubated for 16 h | Reduced T.gondii plaque size and inhibited all parasite growth at 0.1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Arteether | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Greatly inhibited parasitic growth at 0.1 μg/mL (barely visible plaque) | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, epithelial cell line (LLC-MK2), derived from rhesus monkey (Macaca mulatta) kidneys | Artesunate | 0.1, 0.2, 0.4, 0.8, 1.6, 3.1, 6.25, 12.5, 25, 50, and 100 μg/mL (the duration of treatment was not available) | IC50 = 0.075 μM or 0.029 μg/mL, TD50 = 2.003 μM, and SI = 26.707, higher efficacy than cotrimoxazole, pentamidine, pyramidine, and trimethoprim against tachyzoites and inducing death of infected cells | Gomes et al. [58] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artesunate | 0.01–1.0 μM, incubated for 72 h | IC50 = 0.213 μM and IC90 = 0.368 μM | Dunay et al. [64] | |
| T. gondii (DUR strain) | Tachyzoites, THP-1 cell line | Artesunate | 0.1, 0.2, and 0.5 μg/mL, incubated for 12, 24, 48, and 96 h | Maximum parasite growth inhibition of 89% was 0.1 μg/mL at 24 h, subsequent decrease in inhibition at 96 h | Sarciron et al. [65] | |
| T. gondii (DUR strain) | Tachyzoites, THP-1 cell line | Artesunate + DHA | 0.1, 0.2, and 0.5 μg/mL, incubated for 12, 24, 48, and 96 h | Parasite growth inhibition > 82% was induced at 0.2 and 0.5 μg/mL at 12 h. Combination of the two drugs did not significantly improve the parasite growth inhibition | Sarciron et al. [65] | |
| T. gondii (DUR strain) | Tachyzoites, THP-1 cell line | DHA | 0.1, 0.2, and 0.5 μg/mL, incubated for 12, 24, 48, and 96 h | Parasite growth inhibition > 78% was induced at 0.5 μg/mL at 12 h, triggered the death of infected cells | Sarciron et al. [65] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | DHA | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Inhibited all parasite growth at 1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemisone | 0.010–1.0 μM, incubated for 72 h | IC50 = 0.12 μM and IC90 = 0.176 μM (the highest potency). The growth of tachyzoites was inhibited | Dunay et al. [64] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | 1-propyl-ether-artemisinin | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Inhibited all parasite growth at 1 μg/mL | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | 1-butyl-ether-artemisinin | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Inhibited all parasite growth at 1 μg/m | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | sec-butyl-ether-artemisinin | 0.01, 0.1, 1, and 10 μg/mL, incubated for 5 days | Greatly inhibited parasite growth at 0.1 μg/mL (barely visible plaque) | Ke et al. [67] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Artemiside | 0.010–1.0 μM, incubated for 72 h | IC50 = 0.108 μM and IC90 = 0.167 μM | Dunay et al. [64] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 1 (DART-1,3-thiazole, H substituted R1, H substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 1.1 μM, TD50 ≥ 320 μM, and TI = 511. Parasites/vacuole ratio after treatment was 2 | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3 (DART-1,3-thiazole, Me substituted R1, Me substitued R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.16 μM, TD50 = 38 μM, and TI = 240. Parasites/vacuole ratio after treatment was 2; higher efficacy than artemisinin and artemether | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 4 (DART-1,3-thiazole, H substituted R1, Me substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.37 μM, TD50 ≥ 320 μM, and TI = 1520. Parasites/vacuole ratio after treatment was 2; higher efficacy than artemisinin and artemether; higher TI than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 5 (DART-1,3-thiazole, Ph substituted R1, H substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.34 μM, TD50 ≥ 320 μM, and TI = 1654. Parasites/vacuole ratio after treatment was 2; higher efficacy than artemisinin and higher TI than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 6 (DART-1,3-thiazole, t-Bu substituted R1, H substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.25 μM, TD50 ≥ 320 μM, and TI = 2249. Parasites/vacuole ratio after treatment was 2; higher efficacy and TI than artemisinin and artemether | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 9 (DART-1,3-thiazole, p-MeOPh substituted R1, H substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.35 μM, TD50 ≥ 320 μM, and TI = 1607. Parasites/vacuole ratio after treatment was 2; higher efficacy than artemisinin and artemether, and higher TI than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 11 (DART-1,3-thiazole, p-MeSPh substituted R1, H substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.40 μM, TD50 ≥ 320 μM, and TI = 1406. Parasites/vacuole ratio after treatment was 1; higher efficacy and TI than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 12 (DART-1,3-thiazole, p-MeS(O2)Ph substituted R1, H substituted R2) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.42 μM, TD50 ≥ 320 μM, and TI = 1339. Parasites/vacuole ratio after treatment was 2; higher efficacy and TI than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 22 (DART-carboxamide, H substituted R4, p-CF3Bn substituted R5) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.34 μM, TD50 ≥ 320 μM, and TI = 1654. Parasites/vacuole ratio after treatment was 2; higher efficacy than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 24 (DART-carboxamide, H substituted R4, 3,5-F2Bn substituted R5) | 1, 5, and 10 μM, incubated for 24–26 h | IC50 = 0.36 μM, TD50 ≥ 320 μM, and TI = 1562. Parasites/vacuole ratio after treatment was 4; higher efficacy and TI than artemisinin | Hencken et al. [63] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3c (aldehyde substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 0.3 mg/L (the highest potency) TD50 = 26 mg/L, and TI = 92 (higher potency and TI than trimethoprim); significant reduction in the number of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3b (benzothiazole substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 0.4 mg/L, TD50 = 9 mg/L, and TI = 28 (higher potency and TI than trimethoprim); > 50% reduction of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3a (2-thiazole substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 0.6 mg/L, TD50 > 320 mg/L, and TI = 975 (the highest TI, higher potency and TI than trimethoprim); > 50% reduction of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3e (methyl ester substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 0.9 mg/L, TD50 = 177 mg/L, and TI = 210 (higher potency and TI than trimethoprim); > 50% reduction of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3f (ethyl amide substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 1.5 mg/L, TD50 > 72 mg/L, and TI = 52 (higher potency and TI than trimethoprim); > 50% reduction of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 3d (carboxylic acid substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 12.5 mg/L, TD50 > 320 mg/L, and TI = 60 (the least potency); > 50% reduction of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Deoxy-compound 3a (2-thiazole substituted at C-10) | Serial dilutions: 320–0.032 mg/L, incubated for 5 days | IC50 = 280.6 mg/L, TD50 = 320 mg/L, and TI = 2; >50% reduction of penetrated parasites | D’Angelo et al. [69] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 2d (Trioxane C-10 citronelyl ether) | Serial dilutions: 0–320 μg/mL, incubated for 94 h | IC50 = 1.1 μM, TD50 = 160 μM, and TI = 320 | Jones-Brando et al. [68] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 2c (Trioxane C-10 2-bromobenzoate ester) | Serial dilutions: 0–320 μg/mL, incubated for 94 h | IC50 = 1.2 μM, TD50 > 630 μM, and TI ≥ 933 | Jones-Brando et al. [68] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 2b (Trioxane C-10 benzoate ester) | Serial dilutions: 0–320 μg/mL, incubated for 94 h | IC50 = 1.4 μM, TD50 > 740 μM, and TI ≥ 933 | Jones-Brando et al. [68] | |
| T. gondii (RH strain) | Tachyzoites, HFF cells | Compound 2a (Trioxane C-10 primary alcohol) | Serial dilutions: 0–320 μg/mL, incubated for 94 h | IC50 = 8.3 μM, TD50 = 1560 μM, and TI = 190 | Jones-Brando et al. [68] | |
| In vivo studies | T. gondii (PRU strain) | CD1 mice | Artemisinin | 10 mg/kg, s.c., q.d. for 8 days | 20% of artemisinin-treated mice survived the infection at 25 days p.i., the Luc value (or parasite burden) was the highest (12 × 106) among all of the treated groups at day 9, developed higher cyst number in the brain (~90) | Dunay et al. [64] |
| T. gondii (PRU strain) | CD1 mice | Artemiside | 10 mg/kg, s.c., q.d. for 8 days | 60% of artemiside-treated mice survived the infection at 25 days p.i., the Luc value was the lowest (2 × 106) among all of the treated groups at day 9, developed the lowest cyst numbers in the brain (~10) | Dunay et al. [64] | |
| T. gondii (PRU strain) | CD1 mice | Artemisone | 10 mg/kg, s.c., q.d. for 8 days | Over 50% of artemisone-treated mice survived the infection at 25 days p.i., reduced Luc value at day 9, developed fewer cysts in the brain (~30) | Dunay et al. [64] | |
| T. gondii (DUR strain, isolated from the amniotic fluid of a pregnant woman) | OF1 mice | Artesunate + DHA | With ratio 1:1 of the two drugs, 100 mg/day p.o. in a 0.2-mL volume, t.i.d. for 5 days | No mice died during the experiment. Fewer T. gondii cysts in the brains of treated mice (267 ± 10.31) vs. control (650 ± 37.27), 75% of the cysts did not appear different from the controls after treatment. The parasite internal membranes altered with an irregular outline, and approximately 25% of the bradyzoites were damaged | Sarciron et al. [65] | |
| T. gondii (RH strain) | Kunming mice | Artemether | 200 mg/kg, q.d. for 8 days | After treatment, the intensity of glucose-6-phosphatase activity of the parasite was decresased compared with non-treated control parasites | Liang et al. [66] |
DART: dehydroartemisinin
DHA: dihydroartemisinin
IC50: concentration that causes 50% inhibition of growth
i.g.: intragastric administration
p.i.: post-infection
p.o.: oral administration,
q.d.: one time per day
s.c.: subcutaneous injection
SERCA: sarco/endoplasmic reticulum Ca2+-ATPase
SI: selectivity index
TD50: median cytotoxic dose
TI: therapeutic index
t.i.d.: three times per day
The IC50 is either in μg/mL or μM as in the original papers.
In vitro studies
RH strain of T. gondii and human foreskin fibroblast (HFF) cells were used in most of the in vitro studies. ART and its derivatives tested for their in vitro efficacy (IC50) against T. gondii and cytotoxicity (TD50) were measured to obtain the selectivity (SI) and/or therapeutic index (TI). Artesunate is proven to have much higher efficacy than current first-line drugs such as cotrimoxazole, pentamidine, pyrimethamine, and trimethoprim [58]. Intraparasitic heme activates ART and its derivatives to form reactive oxygen species (ROS) in the cytoplasm of host cells, which is then delivered across the parasitophorous vacuole membrane (PVM) to reach the tachyzoites within the PVM. ROS may damage the parasite by alkylating and poisoning one or more essential proteins and trigger the death of infected cells to control the growth of tachyzoites [59]. In addition, ART has the ability to trigger calcium-dependent secretion of microneme proteins in T. gondii parasites, affecting calcium homeostasis and signalling in the parasite, and acts as an inhibitor of SERCA [60]. Perturbation of Ca2+ intake levels not only blocks parasite microneme secretion but also prevents motility and cell invasion of the parasite [61]. Arteether is lipophilic and therefore has activity against the central nervous system infection. At concentration ≥ 0.5 μg/mL, arteether mediated reduction in incorporation of radiolabeled uracil into the nucleic acids of T. gondii tachyzoites [62] and showed higher efficacy than cycloguanil in inhibiting the parasite. Eight thiazole derivatives and two carboxamide derivatives of deoxyhydroartemisin (DART) trioxane derivatives displayed effective growth inhibitory activities of T. gondii parasites, comparable to artemether in potency and over 100 times more potent than the front-line drug trimethoprim. The thiazoles were more effective than the other derivatives in inhibiting the growth of both the extracellular and intracellular parasites. Unexpectedly, two thiazole trioxanes (named compounds 5 and 6) were parasiticidal, both inhibited parasite replication irreversibly when parasites were exposed to 10 μM of the drugs for 24 h, whereas the standard trioxane drugs (ART and artemether) were not parasiticidal [63].
In vivo studies
CD1, OF1, and Kunming mice have been used in animal studies; extensive studies have shown that ART, artemether, arteether, artesunate, DHA, artimisone, artimiside, and other derivaties are active against T. gondii. In these studies, after treatment with ART or its derivatives, the total number of cysts per brain was counted from the research subjects to evaluate the efficacy of the drugs [64–66]. Using CD1 mice-infected with the PRU-Luc-GFP type II strain of T. gondii, approximately 20% of ART-treated mice survived the infection; however 60% of the artemiside-treated mice and over 50% of the artemisone-treated mice survived the infection at 25 days p.i. In addition, compared with ART-treated mice, artemiside- and artemisone-treated mice developed significantly lower parasite burdens, e.g. luciferase (Luc) values in the brain at 9 days p.i. and significantly lower cyst numbers in the brain at 25 days p.i., indicating artemiside and artemisone have better antiparasitic efficacy on T. gondii than that of ART [64].
ART and its derivatives on N. caninum and E. tenella
Neosporosis, caused by N. caninum is a serious disease of dogs and livestock worldwide. Although no case of N. caninum infection has been reported in humans so far, the possibility of human N. caninum infection cannot be excluded due to its close phylogenetic relationship with T. gondii and its wide range of potential hosts [70]. Currently, there is no safe and effective chemotherapy for bovine neosporosis. Studies available for effective treatment of N. caninum using ART [71] and its derivatives (artemisone [72–74], artemether [75], GC007, and GC012 [74]) are summarized in Table 4.
Table 4.
Treatment of artemisinin and its derivatives against Neospora caninum and Eimeria tenella
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | N. caninum (KB-2) | Tachyzoites in mouse peritoneal macrophages (mouse strain was not stated) | Artemisinin | 0.1, 1, and 10 μg/mL, started from 12 h p.i. to 30 h | The total number of tachyzoites per 100 infected macrophages reduced with a clear dose-dependent pattern, which were about 700, 500, and 340 at 0.1, 1, and 10 μg/mL, respectively | Kim et al. [71] |
| N. caninum (KB-2) | Tachyzoites, Vero cells | Artemisinin | 0.01, 0.1, 1, and 10 μg/mL, started from 12 h p.i. to 30 h | The total number of tachyzoites per 100 infected Vero cells was reduced with a clear dose-dependent pattern, which were about 980, 570, 300, and 260 at 0.01, 0.1, 1, and 10 μg/mL, respectively | Kim et al. [71] | |
| N. caninum (KB-2) | Tachyzoites, Vero cells | Artemisinin | 0.1, 1, 10, and 20 μg/mL, started from 12 h p.i. to 12 days | 10 or 20 μg/mL eliminated all microscopic foci of N. caninum in Vero cells by 11 days (third subculture) and 1 μg/ml eliminated all microscopic foci of N.caninum by 14 days (fourth subculture) | Kim et al. [71] | |
| N. caninum (NcIS491) | Tachyzoites, Vero cells | Artemisone | 0.1, 0.5, 5.0, 10.0, or 15.0 g/mL, 5 h prior to infection 25.0 or 50.0 g/mL for | The parasite inhibitory effect was dose-dependent. Percentages of infected cells at 96 h of cultivation were 8.5%, 5.7%, 3.5%, 7.8%, 4.5%, and 1.0%, respectively. Percentages of parasite inhibition at 96 h of cultivation were 90.2%, 93.4%, 95.9%, 91.0%, 94.8%, 98.8%, and 87.3%, respectively | Mazuz et al. [72] | |
| N. caninum (NcIS491) | Tachyzoites, Vero cells | Artemisone | 24 h, added at initial 15.3% of cells infected | The parasite inhibitory effects were 50.1% and 81.9%, respectively, compared to control | Mazuz et al. [72] | |
| N. caninum-β-galactosidase expression | Tachyzoites, HFF and Vero cells | Artemisone | Pre-treatment with 5 μM for 1 or 3 h. After 2 h, either 500 nM or 5 μM of the drug was added. The one with initial 500 nM is then increased stepwise for a period of 40 days | IC50 = 3 nM. Only after 21 days of continuous treatment with 5 μM of artemisone, all tachyzoites were eliminated | Müller et al. [74] | |
| N. caninum-β-galactosidase expression | Tachyzoites, HFF and Vero cells | GC007 (artemisinin derivative) | Pre-treatment with 5 μM for 1 or 3 h. After 2 h, either 500 nM or 5 μM of the drug was added. The one with initial 500 nM is then increased stepwise for a period of 40 days | IC50 = 29 nM. Only after 21 days of continuous treatment with 5 μM of GC007, all tachyzoites were eliminated | Müller et al. [74] | |
| N. caninum-β-galactosidase expression | Tachyzoites, HFF and Vero cells | GC012 (artemisinin derivative) | Pre-treatment with 5 μM for 1 or 3 h. After 2 h, either 500 nM or 5 μM of the drug was added. The one with initial 500 nM is then increased stepwise for a period of 40 days | IC50 = 54 nM. Tachyzoites readily adapted to a gradual increase in the concentration (0.5–10 μM) within 20 days, indicating the changes in tachyzoites were less dramatic | Müller et al. [74] | |
| N. caninum-GFP expression | Tachyzoites, HFF cells | Artemether | 1, 10, 30, 40, 50, 60, 80, and 100 μg/mL for 2 h | IC50 = (1.0 ± 0.05) μg/mL, parasite growth concentration was 100 μg/mL | Qian et al. [75] | |
| In vivo studies | N. caninum (NcIS491) | Gerbils (Meriones tristrami) | Artemisone | 20 mg/kg, i.p., b.i.d. for 4 days | Among 9 treated gerbils, only 1 animal exhibited clinical signs and died 10 days p.i. vs. controls: 8 out of 9 untreated gerbils died with characteristic cerebral signs | Mazuz et al. [72] |
| N. caninum (Nc-Spain7) | BALB/c male mice | Artemisone | 50 mg/kg/day suspended in 100μL corn oil, i.g., q.d. for 6 days | Parasite burden in brains was 1–5 × 103 tachyzoites per μg DNA after treatment, lower than those of placebo (0.5–14 × 103), mefloquine (5–7.5 × 103), and artemiside (1–15 × 103) treatments; however parasite burden in lungs (1.4–2.2 × 103) after treatment, higher than those of placebo, mefloquine, and artemiside treatments | Müller et al. [73] | |
| In vivo | E. tenella (S98N3 strain) | White leghorn chicken | Pure artemisinin | 10 ppm, fed independently in food, p.o., 7 days p.i. | Significantly reduced oocyst output (0.7 × 106) vs. control (4.2 × 106) and reduced sporulation rate of oocyte (73.2% vs. 97.4%); chicken mortality rate reduced to 2.3% and lesion score reduced to 1.2 after treatment. SERCA expression in macrogamates reduced to 77.6% vs. 98.7% in control, indicating artemisinin was effective on the parasite | del Cacho et al. [78] |
| E. tenella (S98N3 strain) | White leghorn chicken | Pure artemisinin | 17 ppm in food, fed independently in food, p.o., 7 days p.i. | Significantly reduced oocyst output (0.2 × 106) vs. control (4.2 × 106), and reduced sporulation rate of oocyte (62% vs. 97.4%); the mortality rate of chickens reduced to 1.1% and lesion score reduced to 1. SERCA expression in macrogamates reduced to 66.4% vs. 98.7% in control | del Cacho et al. [78] |
b.i.d.: two times per day
IC50: concentration that causes 50% inhibition of growth
HFF: human foreskin fibroblast
i.g.: intragastric administration
i.p.: intraperitoneal injection
p.i.: post-infection
p.o.: oral administration
q.d.: one time per day
SERCA: sarco/endoplasmic reticulum Ca2+-ATPase
The IC50 is either in μg/mL or nM as in the original papers.
An in vitro study showed that at a concentration of 10 or 20 μg/mL, ART was able to reduce N. caninum and completely eliminated all microscopic foci of N. caninum in Vero cells by 11 days (third subculture) and at 1 μg/mL, ART reduced N. caninum and completely eliminated all microscopic foci of N. caninum by 14 days (fourth subculture). The morphology and growth rate of the uninfected Vero cells treated with 20 μg/mL of ART were normal, suggesting that the toxicity of ART is low. However, pretreatment of host cells or N. caninum tachyzoites with ART had no effect on intracellular replication of N. caninum tachyzoites [71]. An in vitro study showed that the parasites undergo apoptosis rather than uncontrolled cell death by long-term treatments using artemisinin derivatives including artemisone, GC007, and GC012, which further proves the activity of ART derivatives in controlling N. caninum parasites [74]. However, artemiside and artemisone did not affect the cerebral parasite burden when assessed in a chronic infection model of N. caninum-infected BALB/c mice [74]. In another in vitro study, parasite growth inhibition can be as high as 92.1% when the artemether concentration was 100 μg/mL [75].
In vivo studies
In an animal trial using gerbils (Meriones tristrami), only one out of 9 treated gerbils exhibited characteristic cerebral clinical signs and died 10 days after infection, indicating that artemisone plays a role in increasing the survivability of the infected hosts [72]. In another in vivo experiment, parasite burden in the brains of male BALB/c mice after treatment by artemisone is lower than that by placebo, mefloquine, or artemiside treatment, suggesting possible efficacy of this drug; however the parasite burden in the lungs after artemisone treatment is higher than those of the above-mentioned drugs [73]. Thus, further in vivo investigations are needed for these drugs.
Avian coccidiosis is the most significant parasitic disease in the poultry industry [76]. Chickens are affected by seven different Eimeria spp. that infect the gut and are transmitted between birds via ingestion of infective oocysts. Several drugs such as nicarbazin, amprolium, quinolone, and ionophores, alone or in combination have proven to be an effective alternative against avian coccidiosis [77]. However, the emergence of drug-resistant strains, especially after a prolonged use of a drug, is a real problem. Based on the study on ART by del Cacho et al. (2010), there are significant reduction in oocyst output, mortality, and sporulation rate with an ART dose of 17 ppm compared to 10 ppm in E. tenella-infected white leghorn chicken [78]. By using immunofluorescence technique, SERCA in macrogametes was found to be inhibited by ART. Because SERCA plays a role in Ca2+ homeostasis [79], the inhibition of SERCA by ART impairs Ca2+-dependent ATPase and causes altered secretion of the wall-forming bodies in macrogametes [78]. These findings suggested that ART can be used as an alternative anti-coccidial drug for avian coccidiosis, due to its anti-E. tenella activity on reducing intestinal lesions, oocyst output, and oocyst sporulation. The results from selected studies of ART and its derivatives on E. tenella are also summarized in Table 4.
7. ART and its derivatives on A. castellanii and N. fowleri
Acanthamoeba spp. are the causative agents of Acanthamoeba keratitis, fatal granulomatous amoebic encephalitis, and cutaneous infections [80]. Currently, no methods or single drug exists that can eliminate both cystic and trophozoite forms of Acanthamoeba. To treat the cyst form of Acanthamoeba that is highly resistant to therapy, a combination of agents is generally used [81]. An in vitro study shows that using 200 μg/mL of artemether for 24 h against A. castellanii trophozoites causes a reduction in the number of trophozoites [82]. The effect toward trophozoites was minimal with slight changes of structure at 100 μg/mL of artemether; there were a decreased number of trophozoites after 6 days of cultivation with 150 μg/mL of artemether and there were no viable trophozoites after 5 days of cultivation with 200 μg/mL of artemether. Based on the results, the amoebicidal activities of artemether against A. castelanii trophozoites were found to be time and dose-dependent. Among ART, artesunate, and DHA, DHA had the strongest amoebicidal activity against Acanthamoeba, followed by artemether [82]. The inhibition of Acanthamoeba trophozoites by artemether may be due to its ability to downregulate phosphoglycerate dehydrogenase (PGDH) and phosphoserine aminotransferase (PSAT) in trophozoites. Based on the isobaric tags for relative and absolute quantitation (iTRAQ) analysis on Acanthomoeba treated with 200 μg/mL of artemether, it was shown that the levels of PGDH and PSAT were decreased through inhibition of the L-serine biosynthesis pathway [82]. Further studies are needed to verify the efficacy and mechanisms of ART and its derivatives against Acanthamoeba infection.
N. fowleri causes a fulminating and rapidly fatal primary amoebic meningoencephalitis (PAME) in humans [83]. The major problem of curing infections involving the pathogenic free-living amoebae is the lack of effective therapeutics [84]. ART, arteether, and artesunate have been tested against experimental PAME, and the efficacy of these compounds has been compared with amphotericin B, a standard drug for this pathogen. Compared to ART and its derivatives, 2.5 mg/kg of amphotericin B is effective in treating PAME of N. fowleri-infected Swiss mice. ART and artesunate showed only slight protection at 120 mg/kg by increasing the mean survival time to 1.9 and 2.6 days, respectively. Beta-arteether showed slightly better results at 120 mg/kg with an increase of the mean survival time to 5.3 days. However, all of the mice treated with the maximum tolerated dose (180 mg/kg) of ART and its derivatives suffered from PAME. This indicates that ART can increase the mean survival time but is unable to cure PAME caused by N. fowleri in Swiss mice [85]. Therefore, more studies are needed to confirm the effect of ART towards N. fowleri. The studies of ART and its derivatives on A. castellanii and N. fowleri are summarized in Table 5
Table 5.
Treatment of artemisinin and its derivatives against A. castellanii and N. fowleri
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | A. castellanii (ATCC 30011) | Tachyzoites in peptone-yeast-glucose medium | Artemether | 100 μg/mL, incubated for 4 h | The CI is slightly changed TEM: minimal effect on the trophozoites The protein levels of PGDH and PSAT were significantly decreased by western blot analysis |
Deng et al. [82] |
| A. castellanii (ATCC 30011) | Tachyzoites in peptone-yeast-glucose medium | Artemether | 150 μg/mL, incubated for 12 h | 10 min: trophozoites rounded and detached from the bottom of the well and pseudopodia disappeared 4 h: amoebae underwent cell lysis and most visible structures including the nucleus disappeared 8 h: the CI decreased by approximately 50% 12 h: the protein levels of PGDH and PSAT enzymes were markedly downregulated by western blot analysis TEM: The nuclear condensation and mitochondrial deformation of trophozoites SEM: pseudopodium retraction and trophozoites disrupted 6 days: the numbers of viable trophozoites significantly decreased |
Deng et al. [82] | |
| A. castellanii (ATCC 30011) | Tachyzoites in peptone-yeast-glucose medium | Artemether | 200 μg/mL, incubated for 24 h | 10 min: trophozoites rounded and detached from the bottom of the well and psudopodia dissapeared 4 h: amoebae underwent cell lysis and nucleus disappeared 8 h: the CI decreased by approximately 50% 12 h: the protein levels of PGDH and PSAT enzymes markedly downregulated at 200 μg/mL of artemether, by western blot analysis 5 days: directly damaged trophozoites and no viable trophozoites were detected in the culture The necrotic changes in amoebae increased to 57.2% (PI staining). Apoptotic and necrotic cells increased from 2.77% to 8.89% and from 0.124% to 25.2%, respectively (by PI staining and annexin V-FITC double staining) The protein levels of PGDH and PSAT enzymes were significantly downregulated by the iTRAQ quantitative proteomic analysis TEM: the nuclear condensation and mitochondrial deformation of trophozoites SEM: pseudopodium retraction and trophozoites disrupted |
Deng et al. [82] | |
| In vivo studies | N. fowleri (CJ strain) | Swiss mice | Artemisinin | 60–180 mg/kg, i.m. for 5 days | Not curative and showed only slight protection as indicated by extension of the mean survival time | Gupta et al. [85] |
| N. fowleri (CJ strain) | Swiss mice | Beta-arteether | 60–180 mg/kg, i.m. for 5 days | Not curative and showed only slight protection as indicated by extension of the mean survival time | Gupta et al. [85] | |
| N. fowleri (CJ strain) | Swiss mice | Artesunate | 60–180 mg/kg, i.m. for 5 days | Not curative and showed only slight protection as indicated by extension of the mean survival time | Gupta et al. [85] |
CI: cell index
i.m.: intramuscular injection
iTRAQ: isobaric tags for relative and absolute quantitation
PGDH: phosphoglycerate dehydrogenase
PI staining: propidium iodide staining
PSAT: phosphoserine aminotransferase
SEM: scanning electron micrograph
TEM: transmission electron micrograph
8. ART and its derivatives on C. parvum and G. lamblia
Human cryptosporidiosis is mainly caused by C. hominis and C. parvum, which are responsible for most of the outbreaks of Cryptosporidium described so far [86]. However, treatment faces challenges because of the limited options of using macrolides, paramomycin, nitazoxanide, and mirazid that have partial efficacy in reducing disease severity in immunocompromised individuals [87]. The use of ART towards C. parvum infection is not effective compared to macrolides antibiotics, and ART only caused a minor decrease in the mean number of oocyts at the highest concentration (2 mg/L). Thus, the inhibitory effects of ART against C. parvum showed limited effectiveness [88]. So far there are few reports on ART and its derivatives in the treatment of Cryptosporidium spp.; further studies are needed to explore their potential in treating this disease.
Giardia lamblia (syn. G. intestinalis, G. duodenalis) is one of the most frequent human intestinal protozoa causing giardiasis and infects hundreds of millions of people every year [89]. Metronidazole, tinidazole, mebendazole, albendazole, and furazolidone are effective anti-Giardia drugs, but have severe side effects and potential toxicity. Developing more effective and less toxic drugs against this protozoan parasite is necessary. The effects of DHA (LD50 = 200 μg/mL) at different time intervals on G. lamblia in vitro were investigated by using microscopy and cytometry techniques, and it was shown that DHA induced the changes in morphology and cell cycle state in G. lamblia [90]. The studies of ART and its derivatives on C. parvum and G. lamblia are summarized in Table 6.
Table 6.
Treatment of artemisinin and its derivatives against Cryptosporidium parvum and Giardia lamblia
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and time) | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | C. parvum (solated from stools of AIDS patients) | Sporozoites, human lung carcinoma (A549 cells) | Artemisin | 0.02, 0.2, and 2 mg/L, incubated for 72 h | Minor decrease in mean numbers of oocysts (1.3%) and schizonts (1.6%) at 2 mg/L | Giacometti et al. [88] |
| In vitro | G. lamblia (isolate C2) | Trophozoites cultured in modified TYI-S-33 medium (without cells) | DHA | 12.5, 25, 50, 100 and 200 μg/mL, incubated for 12 h | G0/G1 phase: 43.70%; S phase: 0%; G2/M phase: 56.30% Mortality rate at 100 μg/mL and 200 μg/mL increased from 48.06% to 100% | Tian et al. [90] |
DHA: dihydroartemisinin
9. ART and its derivatives on Babesia spp
Human babesiosis is a zoonotic disease caused by protozoan parasites of the Babesia genus. In humans, the most prevalent are infections caused by Babesia microti and less frequently, B. divergens, B. duncani, or B. venatorum [91]. Babesiosis shares many clinical features with malaria and can be fatal, particularly in the elderly and in immunocompromised individuals. Anti-malaria drugs and some antibiotics have been used in chemotherapy of babesiosis, including atovaquone, azithromycin, clindamycin, and quinine [92]. However, atovaquone-resistant genotype of cytochrome b gene has been detected in three cases of B. gibsoni infections in Japan [93]. The investigations of ART and its derivatives on Babesia spp. are summarized in Table 7.
Table 7.
Treatment of artemisinin and its derivatives against Barbesia spp.
| Experiments | Species (strain) | Host/cell line | Drug | Treatment (dose, route, and | Effect | Reference |
|---|---|---|---|---|---|---|
| In vitro | B. bovis (Texas T2B strain from cattle) | In bovine RBCs | Artesunate | 0.26, 2.6, 26, and 260 μM, medium replaced daily, incubated for for 4 days | Less effective than atovaquone and diminazene aceturate. Growth inhibition was ≥ 2.6 μM, maintained even after withdrawal of treatment at day 4 | Goo et al. [95] |
| B. bovis (isolate 767 from naturally infected cows) | In Holstein-Friesian calf RBCs | Artemisone | 11–300 μg/mL, incubated for 24, 48, and 72 h | Parasite inhibitory effect within 24 h was dose-dependent. Significant effect was at 300 μg/mL and IC50 = 180.4 μg/mL (or 449 μM). At 72 h post-treatment, all the doses significantly inhibited parasitemia | Mazuz et al. [94] | |
| B. bigemina (isolate 871 from naturally infected cows) | In Holstein-Friesian calf RBCs | Artemisone | 11–300 μg/mL, incubated for 24, 48, and 72 h | Parasite inhibitory effect at 24 h was 61.4%–80% with IC50 = 8.9 μg/mL (22.5 μM). At 72 h post-treatment, the maximum percentage of parasite growth inhibition was 99.6% | Mazuz et al. [94] | |
| B. gibsoni (M1211 from a naturally infected Tosa dog) | In canine RBCs | Artemisinin | The concentrations for atovaquone-resistant B. gibsoni were 3–300 μM, medium replaced daily, incubated for 6 days | Growth inhibition at 300 μM and IC50 = 2210 nM | Iguchi et al. [98] | |
| B. gibsoni (M1211 from a naturally infected Tosa dog) | In canine RBCs | Artemether | The concentrations for atovaquone -resistant B. gibsoni were 1–100 μM, medium replaced daily, incubated for 6 days | Growth inhibition at 100 μM and IC50 = 4720 nM. The activity of artemether against B. gibsoni was lower than other artemisinin compounds | Iguchi et al. [98] | |
| B. gibsoni (NRCPD strain from dogs) | In canine RBCs | Artesunate | 0.26, 2.6, 26, and 260 μM, medium replaced daily, incubated for 4 days | Growth inhibition at 26 and 260 μM from day 1 post-treatment. Significant differences of test concentrations were observed at day 3 post-treatment. Withdrawal of treatment, reemergence of the parasite failed to occur at concentration ≥ 2.6 μM | Goo et al. [95] | |
| B. gibsoni (from a naturally infected Tosa dog) | In canine RBCs | Artesunate | 0.1–10 μM, medium replaced daily, incubated for 7 days | IC50 = (878.89 ± 27.13) nM | Matsuu et al. [97] | |
| B. gibsoni (from a naturally infected Tosa dog) | In canine RBCs | DHA | 0.1–10 μM, medium replaced daily, incubated for 7 days | IC50 = (937.50 ± 45.21) nM | Matsuu et al. [97] | |
| B. caballi (USDA strain) | In equine RBCs | Artesunate | 0.04–5 μg/mL, incubated for 24 h | IC50 = 0.18 μg/mL (or 468 nM) | Nagai et al. [96] | |
| B. equi (USDA strain) | In equine RBCs | Artesunate | 0.04–5 μg/mL, incubated for 24 h | IC50 = 0.1 μg/mL (or 260 nM) | Nagai et al. [96] | |
| In vivo | B. bovis (isolate 767 from naturally infected cows) | Holstein-Friesian calves | Artemisone | 5 mg/kg diluted in 5 mL of DMSO, i.p., b.i.d. for 4 consecutive days | Fever: 40.9°C to 41.3°C was recorded. In 4/5 animals, the minimum PCV was 17%, and maximum parasitemia ranged from 0.1% to 0.5%; 4/5 animals recovered, one did not response to treatment | Mazuz et al. [94] |
| B. bigemina (isolate 871 from naturally infected cows) | Holstein-Friesian calves | Artemisone | 5 mg/kg diluted in 5 mL of DMSO, i.p., b.i.d. for 4 consecutive days | No acute signs of babesiosis: no fever, the minimum PCV decreased to 27%, the parasitemia reached a maximum of 1.3%, both calves recovered, and parasitemia <0.1% during the observation period | Mazuz et al. [94] | |
| B. microti (strain not stated) | BALB/c mice | Artesunate | 1, 10, or 50 mg/kg, i.m. for 6 consecutive days, started at 2 days p.i. | Lower parasitemia was observed in 10 and 50 mg/kg groups | Goo et al. [95] | |
| B. equi (Indian strain) | Splenectomised donkeys | Artesunate | 2.5 mg/kg, i.m., q.d. for 4 days | No recrudescence, died between 16–19 days post-treatment; the mean survival time after treatment was 17 days | Kumar et al. [101] | |
| B. equi (Indian strain) | Splenectomised donkeys | Arteether | 5 mg/kg, i.m., q.d. for 3 days | No recrudescence, died between 4–6 days post-treatment; the mean survival time after treatment was 5 days | Kumar et al. [101] | |
| B. equi (Indian strain) | Splenectomised donkeys | Arteether + buparvaquone | 5 mg/kg of arteerther, i.m., q.d. for 3 days and then with 5 mg/kg of buparvaquone, i.v., q.i.d. for 4 days | Recrudescence occurred 50–54 days after treatment. Animals recovered 4 days post-treatment; the mean survival time after treatment was 66 days | Kumar et al. [101] |
b.i.d.: two times per day
DHA: dihydroartemisinin
DMSO: dimethyl sulphoxide
IC50: concentration that causes 50% inhibition of growth
i.m.: intramuscular injection
i.p.: intraperitoneal injection
i.v.: intravenous injection
PCV: packed cell volume
p.i.: post-infection
q.d.: one time per day
q.i.d.: four times per day
RBC: red blood cell
The IC50 is either in μg/mL, μM, or nM as in the original papers.
In vitro studies
Different Babesia species (B. bovis, B. bigemina, B. caballi, B. equi, and B. gibsoni) have been tested with ART and its derivatives (DHA, artemisone, artesunate, and arteether). Artemisone induced dose-dependent growth inhibition of both B. bovis and B. bigemina, of which B. bigemina appeared to be more sensitive to artemisone than B. bovis, as reflected by the different corresponding IC50 [94]. A dose- and time-dependent inhibitory effect for artesunate was also reported against B. bovis. The study showed that artesunate was less effective than atovaquone and diminazene aceturate in inhibiting the growth of B. bovis [95]. Artesunate and pamaquine were more effective in suppressing parasitemia of B. equi than that of B. cablli, but pyrimethamine was more resistant to B. equi than that of B. caballi by comparing their IC50 [96]. For B. gibsoni, artesunate was more effective in inhibiting the parasitemia than those of artemether, ART, and DHA but not atovaquone and diminazene [95, 96, 97]. In another study, it was reported that the single use of ART derivatives may only have low effectiveness, but the addition of lumefantrine to artemether may be effective for B. gibsoni infection [98]. Babesia parasites do not produce hemozoin nor contain food vacuoles [99]. The absence of these organelles in Barbesia spp. may reduce the action of ART and its derivatives compared with Plasmodium spp. The possible mechanism of ART and its derivatives against Barbesia spp. may be related to the activation of the mitochondrial electron transport system resulting in ROS production [100]. The mechanism of action is still not yet fully understood.
In vivo studies
Various Babesia species (B. bovis, B. bigemina, B. microti, and B. equi) have been tested in different experimental animal models (calves, mice, and donkeys) for the anti-babesial effects of artemisone, artesunate, and arteether. The routes of administration are mostly intramuscular [95, 101] but also intraperitoneal [94]. Calves infected with B. bigemina and B bovis were treated by artemisone (5 mg/kg) intraperitoneally and resulted in recovery of all animals, except for one B. bovis-infected calf. B. bigemina appeared to be more sensitive to artemisone than B. bovis as reflected by the differences of their IC50, although the treatment did not completely eliminate Babesia parasites. B. microti-infected BALB/c mice were intramuscularly treated by artesunate, which was not only inhibited the growth of the parasite but also delayed the increase of parasitemia, indicating that it can be a potential anti-B. microti agent [94]. Efficacy can be improved by combination with other babesiacide. It has been reported that splenectomised donkeys infected with B. equi were treated by intramuscular artesunate, arteether, or intramuscular arteether plus intravenous buparvaquone. In the artesunate-treated group, initial decrease in parasitaemia was observed, but the mean maximum parasitaemia increased dramatically between 16–19 days post-treatment; no recovery was observed in either the arteether- or buparvaquone-treated group. However, in the arteether-buparvaquone combination group, recovery was observed as parasites were cleared after 4-day treatment. Arteether and buparvaquone combination regimen also seemed to be safer than imidocarb alone in this study [101].
10. Conclusion
NTDs are an extremely important issue facing global health care, which mostly affect people living in extreme poverty. ART was discovered by Youyou Tu and her colleaques in China more than 40 years ago, which contributed greatly to treatment and control of malaria, and has led to Tu’s sharing the 2015 Nobel Prize in Physiology or Medicine. Except for its well-known anti-malarial effect, ART and its derivatives also have anti-parasitic activities against many other parasitic infections, including protozoan parasites that infect humans such as Leishmania spp., Trypanosoma spp., Acanthamoeba castellanii, Naegleria fowleri, and G. lamblia. In addition, these compounds are also active against protozoan parasites of both humans and domestic animals/livestocks such as T. gondii, C. parvum, and Babesia spp., and domestic animal/livestock protozoan parasites such as N. caninum (causing a serious disease of dogs and livestock, with the potential to infect humans) and Eimeria spp. (causing avian coccidiosis). The current first-line anti-protozoal drugs have limitions, including serious side-effects, toxicity, drug resistance, and even lack of effective or no chemotherapy. Thus, new drugs for the treatment of these protozoan infections are urgently needed. Greater emphasis should be placed on research and development of combination chemotherapies. ART or its derivatives are relaively low cost and low toxicity, and are promising anti-protozoal drugs alone or in combination with the current anti-protozoal drugs, which open new windows for combination therapies of parasitic protozoans. Arteether is a lipophilic compound that has good potential for therapies against protozoan infections involving the central nervous system such as T. gondii, Acanthamoeba, N. fowleri, and Trypanosoma spp. The emergence of drug-resistant strains of Leishmania and Trypanosoma make the current treatment face new challenges. So far there are no safe and effective chemotherapy for giardiasis and bovine neosporosis. Therefore, further efforts should focus on evaluation of the effectiveness of the compounds on these protozoan infections. Finally, the therapeutic mechanisms both in animals and in humans require more investigations. Therefore, understanding of structure-activity relationship of ART and its derivatives might lead to the development of effective drugs against parasitic protozoans.
Acknowledgments
Research reported in this publication was supported in part by the Natural Science Foundation of China (nos. 81471973 and 81273643); the Division of Intramural Research at the National Institute of Allergy and Infectious Diseases, National Institutes of Health; the Science and Technology Planning Project of Guangdong Province, China (no. 2014A020212108), and the Project 111 of the State Bureau of Foreign Experts and Ministry of Education of China (B06016); the Project on Brand Professional Construction of Sun Yat-sen University, and Three Major Construction (big team, big platform, and big project) of Sun Yat-sen University. The authors thank Cindy Clark, NIH Library Writing Center, for manuscript editing assistance.
List of abbreviations
- ART
Artemisinin
- b.i.d
two times per day
- CH2CL2
dichloromethane
- CI
cell index
- DART
dehydroartemisinin
- Deoxy-DHA
deoxydihydroartemisinin
- DHA
dihydroartemisinin
- DMSO
dimethyl sulphoxide
- HFF
human foreskin fibroblast
- IC50
concentration that causes 50% inhibition of growth
- IC90
concentration that causes 90% inhibition of growth
- i.g
intragastric administration
- i.m
intramuscular injection
- i.p
intraperitoneal injection
- iTRAQ
isobaric tags for relative and absolute quantitation
- i.v
intravenous injection
- Luc value
luciferase value
- MeOH
methanol
- NO
nitric oxide
- PCV
packed cell volume
- PGDH
phosphoglycerate dehydrogenase
- p.i
post-infection
- PI staining
propidium iodide staining
- p.o
oral administration
- PSAT
phosphoserine aminotransferase
- q.d
one time per day
- q.i.d
four times per day
- RBC
red blood cell
- s.c
subcutaneous injection
- SEM
scanning electron micrograph
- SERCA
sarco/endoplasmic reticulum Ca2+-ATPase
- SI
selectivity index
- TD50
median cytotoxic dose
- TI
therapeutic index
- TEM
transmission electron micrograph
- t.i.d
three times per day
- t.p
topical administration
Footnotes
AUTHOR CONTRIBUTIONS (need to be replaced): FL conceived, supervised, and wrote the manuscript. CSNL, NSKL, and DY participated in the writing. X-zS was writing and editing. All authors read and approved the final version of the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.World Health Organization. Working to overcome the global impact of neglected tropical diseases: First WHO report on neglected tropical diseases. Geneva: 2010. p. 172. [Google Scholar]
- 2.Trouiller P, Olliaro P, Torreele E, Orbinski J, Laing R, Ford N. Drug development for neglected diseases: a deficient market and a public-health policy failure. The Lancet. 2002;359(9324):2188–2194. doi: 10.1016/S0140-6736(02)09096-7. doi:1fr;0.1016/S0140-6736(02)09096-7. [DOI] [PubMed] [Google Scholar]
- 3.Appalasamy S, Lo KY, Ch’ng SJ, Nornadia K, Othman AS, Chan L-K. Antimicrobial activity of artemisinin and precursor derived from in vitro plantlets of Artemisia annua L. BioMed research international. 2014;2014 doi: 10.1155/2014/215872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roy S, He R, Kapoor A, Forman M, Mazzone JR, Posner GH, Arav-Boger R. Inhibition of human cytomegalovirus replication by artemisinins: effects mediated through cell cycle modulation. Antimicrobial agents and chemotherapy. 2015;59(7):3870–3879. doi: 10.1128/AAC.00262-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller LH, Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011;146(6):855–858. doi: 10.1016/j.cell.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su XZ, Miller LH. The discovery of artemisinin and the Nobel Prize in Physiology or Medicine. Science China Life Sciences. 2015;58(11):1175–1179. doi: 10.1007/s11427-015-4948-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chaturvedi D, Goswami A, Saikia PP, Barua NC, Rao PG. Artemisinin and its derivatives: a novel class of anti-malarial and anti-cancer agents. Chemical Society Reviews. 2010;39(2):435–454. doi: 10.1039/b816679j. [DOI] [PubMed] [Google Scholar]
- 8.Barnett DS, Guy RK. Antimalarials in Development in 2014. Chemical reviews. 2014;114(22):11221–11241. doi: 10.1021/cr500543f. [DOI] [PubMed] [Google Scholar]
- 9.Cui L, Su X-z. Discovery mechanisms of action and combination therapy of artemisinin. Expert review of anti-infective therapy. 2009;7(8):999–1013. doi: 10.1586/eri.09.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griesbaum K, Liu X, Kassiaris A, Scherer M. Ozonolyses of O - Alkylated Ketoximes in the Presence of Carbonyl Groups: A Facile Access to Ozonides. Liebigs Annalen. 1997;1997(7):1381–1390. doi: 10.1002/jlac.199719970715. [DOI] [Google Scholar]
- 11.Vennerstrom JL, Arbe-Barnes S, Brun R, Charman SA, Chiu FC, Chollet J, Dong Y, Dorn A, Hunziker D, Matile H. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature. 2004;430(7002):900–904. doi: 10.1038/nature02779. [DOI] [PubMed] [Google Scholar]
- 12.Opsenica I, Opsenica D, Smith KS, Milhous WK, Šolaja BA. Chemical Stability of the Peroxide Bond Enables Diversified Synthesis of Potent Tetraoxane Antimalarials (1) Journal of medicinal chemistry. 2008;51(7):2261–2266. doi: 10.1021/jm701417a. [DOI] [PubMed] [Google Scholar]
- 13.Gutiérrez V, Seabra AB, Reguera RM, Khandare J, Calderón M. New approaches from nanomedicine for treating leishmaniasis. Chemical Society Reviews. 2016;45(1):152–168. doi: 10.1039/c5cs00674k. [DOI] [PubMed] [Google Scholar]
- 14.Sharma U, Singh S. Immunobiology of leishmaniasis. Indian journal of experimental biology. 2009;47(6):412. [PubMed] [Google Scholar]
- 15.Lachaud L, Bourgeois N, Plourd M, Leproho P, Bastien P, Ouellette M. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clinical Infectious Diseases. 2009;48(2):e16–e22. doi: 10.1086/595710. [DOI] [PubMed] [Google Scholar]
- 16.Den Boer M, Davidson RN. Treatment options for visceral leishmaniasis. Expert review of anti-infective therapy. 2006;4(2):187–197. doi: 10.1586/14787210.4.2.187. [DOI] [PubMed] [Google Scholar]
- 17.Rodrigues IA, Mazotto AM, Cardoso V, Alves RL, Amaral ACF, Silva JRdA, Pinheiro AS, Vermelho AB. Natural products: insights into leishmaniasis inflammatory response. Mediators of inflammation. 2015;501:835910. doi: 10.1155/2015/835910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singh N, Kumar M, Singh RK. Leishmaniasis: current status of available drugs and new potential drug targets. Asian Pacific Journal of Tropical Medicine. 2012;5(6):485–497. doi: 10.1016/S1995-7645(12)60084-4. [DOI] [PubMed] [Google Scholar]
- 19.Hendrickx S, Boulet G, Mondelaers A, Dujardin JC, Rijal S, Lachaud L, Cos P, Delputte P, Maes L. Experimental selection of paromomycin and miltefosine resistance in intracellular amastigotes of Leishmania donovani and L. infantum. Parasitology Research. 2014;113(5):1875–81. doi: 10.1007/s00436-014-3835-7. [DOI] [PubMed] [Google Scholar]
- 20.Faraut-Gambarelli F, Piarroux R, Deniau M, Giusiano B, Marty P, Michel G, Faugère B, Dumon H. In vitro and in vivo resistance of Leishmania infantum to meglumine antimoniate: a study of 37 strains collected from patients with visceral leishmaniasis. Antimicrobial Agents and Chemotherapy. 1997;41(4):827–830. doi: 10.1128/aac.41.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coelho AC, Gentil LG, da Silveira JF, Cotrim PC. Characterization of Leishmania (Leishmania) amazonensis promastigotes resistant to pentamidine. Experimental Parasitology. 2008;120(1):98–102. doi: 10.1016/j.exppara.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 22.Islamuddin M, Chouhan G, Want MY, Ozbak HA, Hemeg HA, Afrin F. Immunotherapeutic Potential of Eugenol Emulsion in Experimental Visceral Leishmaniasis. PLoS Neglected Tropical Diseases. 2016;24(10):e0005011. doi: 10.1371/journal.pntd.0005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sen R, Bandyopadhyay S, Dutta A, Mandal G, Ganguly S, Saha P, Chatterjee M. Artemisinin triggers induction of cell-cycle arrest and apoptosis in Leishmania donovani promastigotes. Journal of medical microbiology. 2007;56(9):1213–1218. doi: 10.1099/jmm.0.47364-0. [DOI] [PubMed] [Google Scholar]
- 24.Want MY, Islamuddin M, Chouhan G, Ozbak HA, Hemeg HA, Dasgupta AK, Chattopadhyay AP, Afrin F. Therapeutic efficacy of artemisinin-loaded nanoparticles in experimental visceral leishmaniasis. Colloids and Surfaces B: Biointerfaces. 2015;130:215–221. doi: 10.1016/j.colsurfb.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Nemati S, Nahrevanian H, Haniloo A, Farahmand M. Investigation on nitric oxide and C-reactive protein involvement in antileishmanial effects of artemisinin and glucantim on cutaneous leishmaniasis. Advanced Studies in Biology. 2013;5:27–36. [Google Scholar]
- 26.Ebrahimisadr P, Ghaffarifar F, Hassan ZM. In-vitro evaluation of antileishmanial activity and toxicity of artemether with focus on its apoptotic effect. Iranian Journal of Pharmaceutical Research. 2013;12(4):903–909. [PMC free article] [PubMed] [Google Scholar]
- 27.Sen R, Ganguly S, Saha P, Chatterjee M. Efficacy of artemisinin in experimental visceral leishmaniasis. International Journal of Antimicrobial Agents. 2010;36(1):43–49. doi: 10.1016/j.ijantimicag.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Ghaffarifar F, Heydari FE, Dalimi A, Hassan ZM, Delavari M, Mikaeiloo H. Evaluation of Apoptotic and Antileishmanial Activities of Artemisinin on Promastigotes and BALB/C Mice Infected with Leishmania major. Iranian journal of parasitology. 2015;10(2):258. [PMC free article] [PubMed] [Google Scholar]
- 29.Cortes S, Albuquerque A, Cabral LI, Lopes L, Campino L, Cristiano ML. In vitro susceptibility of Leishmania infantum to Artemisinin derivatives and selected trioxolanes. Antimicrobial agents and chemotherapy. 2015;59(8):5032–5035. doi: 10.1128/AAC.00298-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chollet C, Crousse B, Bories C, Bonnet-Delpon D, Loiseau PM. In vitro antileishmanial activity of fluoro-artemisinin derivatives against Leishmania donovani. Biomedicine & Pharmacotherapy. 2008;62(7):462–465. doi: 10.1016/j.biopha.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 31.Islamuddin M, Chouhan G, Farooque A, Dwarakanath BS, Sahal D, Afrin F. Th1-biased immunomodulation and therapeutic potential of Artemisia annua in murine visceral leishmaniasis. PLoS neglected tropical diseases. 2015;9(1):e3321. doi: 10.1371/journal.pntd.0003321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mishina YV, Krishna S, Haynes RK, Meade JC. Artemisinins inhibit Trypanosoma cruzi and Trypanosoma brucei rhodesiense in vitro growth. Antimicrobial agents and chemotherapy. 2007;51(5):1852–1854. doi: 10.1128/AAC.01544-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mutiso JM, Macharia JC, Barasa M, Taracha E, Bourdichon AJ, Gicheru MM. In vitro and in vivo antileishmanial efficacy of a combination therapy of diminazene and artesunate against Leishmania donovani in BALB/c mice. Revista do Instituto de Medicina Tropical de São Paulo. 2011;53(3):129–132. doi: 10.1590/S0036-46652011000300003. [DOI] [PubMed] [Google Scholar]
- 34.Adam I, Hagelnur AA. Artesunate plus sulfamethoxypyrazine/pyrimethamine for the treatment of cutaneous leishmaniasis: a double-blind, placebo-controlled clinical trial. International journal of antimicrobial agents. 2009;34(4):380–381. doi: 10.1016/j.ijantimicag.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Heydari FE, Ghaffarifar F, Soflaei S, Dalimi A. Comparison between in vitro effects of aqueous extract of Artemisia seiberi and artemisinin on Leishmania major. Jundishapur Journal of Natural Pharmaceutical Products. 2013;8(2):70. [PMC free article] [PubMed] [Google Scholar]
- 36.Yang D, Liew F. Effects of qinghaosu (artemisinin) and its derivatives on experimental cutaneous leishmaniasis. Parasitology. 1993;106(01):7–11. doi: 10.1017/s0031182000074758. [DOI] [PubMed] [Google Scholar]
- 37.Islamuddin M, Farooque A, Dwarakanath B, Sahal D, Afrin F. Extracts of Artemisia annua leaves and seeds mediate programmed cell death in Leishmania donovani. Journal of medical microbiology. 2012;61(12):1709–1718. doi: 10.1099/jmm.0.049387-0. [DOI] [PubMed] [Google Scholar]
- 38.Sen R, Saha P, Sarkar A, Ganguly S, Chatterjee M. Iron enhances generation of free radicals by Artemisinin causing a caspase-independent. apoptotic death in Leishmania donovani promastigotes. Free Radical Research. 2010;44(11):1289–95. doi: 10.3109/10715762.2010.498475. [DOI] [PubMed] [Google Scholar]
- 39.Steverding D. The history of Chagas disease. Parasites & vectors. 2014;7(1):1. doi: 10.1186/1756-3305-7-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bermudez J, Davies C, Simonazzi A, Real JP, Palma S. Current drug therapy and pharmaceutical challenges for Chagas disease. Acta tropica. 2016;156:1–16. doi: 10.1016/j.actatropica.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Babokhov P, Sanyaolu AO, Oyibo WA, Fagbenro-Beyioku AF, Iriemenam NC. A current analysis of chemotherapy strategies for the treatment of human African trypanosomiasis. Pathogens and global health. 2013;107(5):242–252. doi: 10.1179/2047773213Y.0000000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sesti-Costa R, Carneiro ZA, Silva MC, Santos M, Silva GK, Milanezi C, da Silva RS, Silva JS. Ruthenium complex with benznidazole and nitric oxide as a new candidate for the treatment of chagas disease. PLoS Neglected Tropical Disease. 2014;8(10):e3207. doi: 10.1371/journal.pntd.0003207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Akande F, Fagbemi B. In vivo and in vitro effects of artemisinin group of drugs on trypanosomosis in mice. Journal of Natural Sciences Engineering and Technology. 2011;10(1):73–80. [Google Scholar]
- 44.Olivera GC, Postan M, González MN. Effects of artesunate against Trypanosma cruzi. Experimental parasitology. 2015;156:26–31. doi: 10.1016/j.exppara.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 45.Nibret E, Wink M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine. 2010;17(5):369–374. doi: 10.1016/j.phymed.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Bhakuni R, Jain D, Sharma R, Kumar S. Secondary metabolites of Artemisia annua and their biological activity. Current science. 2001;80:35–48. [Google Scholar]
- 47.Wink M. Evolutionary advantage and molecular modes of action of multi-component mixtures used in phytomedicine. Current Drug Metabolism. 2008;9(10):996–1009. doi: 10.2174/138920008786927794. [DOI] [PubMed] [Google Scholar]
- 48.Oluyomi SA, Justine TE, Faoziyat S. Depletion of parasitaemia by halofantrine hydrochloride and artemether in rats infected with African trypanosomes. African Journal of Pharmacy and Pharmacology. 2009;3(9):432–438. [Google Scholar]
- 49.Blauer G. Interaction of ferriprotoporphyrin IX with the antimalarials amodiaquine and halofantrine. Biochemistry international. 1988;17(4):729–734. [PubMed] [Google Scholar]
- 50.de Villiers KA, Marques HM, Egan TJ. The crystal structure of halofantrine–ferriprotoporphyrin IX and the mechanism of action of arylmethanol antimalarials. Journal of inorganic biochemistry. 2008;102(8):1660–1667. doi: 10.1016/j.jinorgbio.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Milton K, Edwards G, Ward S, Orme M, Breckenridge A. Pharmacokinetics of halofantrine in man: effects of food and dose size. British journal of clinical pharmacology. 1989;28(1):71–77. doi: 10.1111/j.1365-2125.1989.tb03507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandey AV, Tekwani BL, Singh RL, Chauhan VS. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. Journal of biological chemistry. 1999;274(27):19383–19388. doi: 10.1074/jbc.274.27.19383. [DOI] [PubMed] [Google Scholar]
- 53.Meshnick SR. Artemisinin and Its Derivatives. Antimalarial Chemotherapy. 2001;(2):191–201. doi: 10.1007/978-1-59259-111-4_10. [DOI] [Google Scholar]
- 54.Abgrall S, Rabaud C, Costagliola D. Incidence and risk factors for toxoplasmic encephalitis in human immunodeficiency virus-infected patients before and during the highly active antiretroviral therapy era. Clinical Infectious Disease. 2001;33(10):1747–1755. doi: 10.1086/322622. [DOI] [PubMed] [Google Scholar]
- 55.David HMJ, Tim GW. Toxoplasmosis: a comprehensive clinical guide, Cambridge University Press Cambridge University Press. Antitoxoplasma chemotherapy. 2001:319–359. [Google Scholar]
- 56.Masters PA, O’Bryan TA, Zurlo J, Miller DQ, Joshi N. Trimethoprim-sulfamethoxazole revisited. Archives of Internal Medicine. 2003;163(4):402–410. doi: 10.1001/archinte.163.4.402. [DOI] [PubMed] [Google Scholar]
- 57.Antczak M, Dzitko K, Długońska H. Human toxoplasmosis-Searching for novel chemotherapeutics. Biomedicine and Pharmacotherapy. 2016;82:677–84. doi: 10.1016/j.biopha.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 58.Gomes TC, Andrade HFd, Júnior, Lescano SAZ, Amato-Neto V. In vitro action of antiparasitic drugs, especially artesunate, against Toxoplasma gondii. Revista da Sociedade Brasileira de Medicina Tropical. 2012;45(4):485–490. doi: 10.1590/s0037-86822012000400014. [DOI] [PubMed] [Google Scholar]
- 59.Son E-S, Song K-J, Shin J-C, Nam H-W. Molecular cloning and characterization of peroxiredoxin from Toxoplasma gondii. The Korean journal of parasitology. 2001;39(2):133–141. doi: 10.3347/kjp.2001.39.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nagamune K, Beatty WL, Sibley LD. Artemisinin induces calcium-dependent protein secretion in the protozoan parasite Toxoplasma gondii. Eukaryotic cell. 2007;6(11):2147–2156. doi: 10.1128/EC.00262-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lovett JL, Sibley LD. Intracellular calcium stores in Toxoplasma gondii govern invasion of host cells. Journal of Cell Science. 2003;116(14):3009–3016. doi: 10.1242/jcs.00596. [DOI] [PubMed] [Google Scholar]
- 62.Holfels E, McAuley J, Mack D, Milhous WK, McLeod R. In vitro effects of artemisinin ether, cycloguanil hydrochloride (alone and in combination with sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on Toxoplasma gondii. Antimicrobial agents and chemotherapy. 1994;38(6):1392–1396. doi: 10.1128/AAC.38.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hencken CP, Jones-Brando L, Bordón C, Stohler R, Mott BT, Yolken R, Posner GH, Woodard LE. Thiazole, oxadiazole, and carboxamide derivatives of artemisinin are highly selective and potent inhibitors of Toxoplasma gondii. Journal of medicinal chemistry. 2010;53(9):3594–3601. doi: 10.1021/jm901857d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunay IR, Chan WC, Haynes RK, Sibley LD. Artemisone and artemiside control acute and reactivated toxoplasmosis in a murine model. Antimicrobial agents and chemotherapy. 2009;53(10):4450–4456. doi: 10.1128/AAC.00502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarciron ME, Saccharin C, Petavy AF, Peyron F. Effects of artesunate, dihydroartemisinin, and an artesunate-dihydroartemisinin combination against Toxoplasma gondii. The American journal of tropical medicine and hygiene. 2000;62(1):73–76. doi: 10.4269/ajtmh.2000.62.73. [DOI] [PubMed] [Google Scholar]
- 66.Liang Z, Xu L. Studies on the enzyme cytochemistry of Toxoplasma gondii and the influence of artemether. Zhongguo ji sheng chong xue yu ji sheng chong bing za zhi= Chinese journal of parasitology & parasitic diseases. 1994;13(3):182–184. [PubMed] [Google Scholar]
- 67.Ke O, Krug E, Marr J, Berens R. Inhibition of growth of Toxoplasma gondii by qinghaosu and derivatives. Antimicrobial agents and chemotherapy. 1990;34(10):1961–1965. doi: 10.1128/AAC.34.10.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jones-Brando L, D’Angelo J, Posner GH, Yolken R. In vitro inhibition of Toxoplasma gondii by four new derivatives of artemisinin. Antimicrobial agents and chemotherapy. 2006;50(12):4206–4208. doi: 10.1128/AAC.00793-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.D’Angelo JG, Bordón C, Posner GH, Yolken R, Jones-Brando L. Artemisinin derivatives inhibit Toxoplasma gondii in vitro at multiple steps in the lytic cycle. Journal of antimicrobial chemotherapy. 2009;63(1):146–150. doi: 10.1093/jac/dkn451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Petersen E, Lebech M, Jensen L, Lind P, Rask M, Bagger P, Björkman C, Uggla A. Neospora caninum infection and repeated abortions in humans. Emerging Infectious Diseases. 1999;5(2):278. doi: 10.3201/eid0502.990215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J-T, Park J-Y, Seo H-S, Oh H-G, Noh J-W, Kim J-H, Kim D-Y, Youn H-J. In vitro antiprotozoal effects of artemisinin on Neospora caninum. Veterinary parasitology. 2002;103(1):53–63. doi: 10.1016/S0304-4017(01)00580-5. [DOI] [PubMed] [Google Scholar]
- 72.Mazuz ML, Haynes R, Shkap V, Fish L, Wollkomirsky R, Leibovich B, Molad T, Savitsky I, Golenser J. Neospora caninum: in vivo and in vitro treatment with artemisone. Veterinary parasitology. 2012;187(1):99–104. doi: 10.1016/j.vetpar.2011.12.020. [DOI] [PubMed] [Google Scholar]
- 73.Müller J, Aguado-Martínez A, Manser V, Wong HN, Haynes RK, Hemphill A. Repurposing of antiparasitic drugs: the hydroxy-naphthoquinone buparvaquone inhibits vertical transmission in the pregnant neosporosis mouse model. Veterinary research. 2016;47(1):1. doi: 10.1186/s13567-016-0317-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Müller J, Balmer V, Winzer P, Rahman M, Manser V, Haynes RK, Hemphill A. In vitro effects of new artemisinin derivatives in Neospora caninum-infected human fibroblasts. International journal of antimicrobial agents. 2015;46(1):88–93. doi: 10.1016/j.ijantimicag.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 75.Qian W, Wang H, Shan D, Li B, Liu J, Liu Q. Activity of several kinds of drugs against Neospora caninum. Parasitology international. 2015;64(6):597–602. doi: 10.1016/j.parint.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Blake DP, Clark EL, Macdonald SE, Thenmozhi V, Kundu K, Garg R, Jatau ID, Ayoade S, Kawahara F, Moftah A. Population, genetic, and antigenic diversity of the apicomplexan Eimeria tenella and their relevance to vaccine development. Proceedings of the National Academy of Sciences. 2015;112(38):E5343–E5350. doi: 10.1073/pnas.1506468112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quiroz-Castañeda RE, Dantán-González E. Control of avian coccidiosis: future and present natural alternatives. BioMed research international. 2015;2015 doi: 10.1155/2015/430610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.del Cacho E, Gallego M, Francesch M, Quílez J, Sánchez-Acedo C. Effect of artemisinin on oocyst wall formation and sporulation during Eimeria tenella infection. Parasitology international. 2010;59(4):506–511. doi: 10.1016/j.parint.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Eckstein-Ludwig U, Webb R, Van Goethem I, East J, Lee A, Kimura M, O’Neill P, Bray P, Ward S, Krishna S. Artemisinins target the SERCA of Plasmodium falciparum. Nature. 2003;424(6951):957–961. doi: 10.1038/nature01813. [DOI] [PubMed] [Google Scholar]
- 80.Zamora A, Henderson H, Swiatlo E. Acanthamoeba encephalitis: a case report and review of therapy. Surgical neurology international. 2014;5:68. doi: 10.4103/2152-7806.132239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi: 10.1051/parasite/2015010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Deng Y, Ran W, Man S, Li X, Gao H, Tang W, Tachibana H, Cheng X. Artemether exhibits amoebicidal activity against Acanthamoeba castellanii through inhibition of the serine biosynthesis pathway. Antimicrobial agents and chemotherapy. 2015;59(8):4680–4688. doi: 10.1128/AAC.04758-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ramanan D, Nalaganta R, Yadav Ram KS, Ramani R. Amoebic meningo-encephalitis. Indian journal of pathology & microbiology. 2010;53(1):183–4. doi: 10.4103/0377-4929.59230. [DOI] [PubMed] [Google Scholar]
- 84.Rice CA, Colon BL, Alp M, Göker H, Boykin DW, Kyle DE. Bis-Benzimidazole Hits against Naegleria fowleri Discovered with New High-Throughput Screens. Antimicrobial agents and chemotherapy. 2015;59(4):2037–2044. doi: 10.1128/AAC.05122-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gupta S, Ghosh PK, Dutta G, Vishwakarma R. In vivo study of artemisinin and its derivatives against primary amebic meningoencephalitis caused by Naegleria fowleri. The Journal of parasitology. 1995;81(6):1012–1013. [PubMed] [Google Scholar]
- 86.Ryan U, Hijjawi N. New developments in Cryptosporidium research. International journal for parasitology. 2015;45(6):367–373. doi: 10.1016/j.ijpara.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 87.Abd-Ella OH. Diagnosis and treatment of cryptosporidiosis: an update review. Journal of the Egyptian Society of Parasitology. 2014;44(2):455–466. [PubMed] [Google Scholar]
- 88.Giacometti A, Cirioni O, Scalise G. In-vitro activity of macrolides alone and in combination with artemisin, atovaquone, dapsone, minocycline or pyrimethamine against Cryptosporidium parvum. Journal of Antimicrobial Chemotherapy. 1996;38(3):399–408. doi: 10.1093/jac/38.3.399. [DOI] [PubMed] [Google Scholar]
- 89.Centers for Disease Control and Prevention. [Last time accessed: June 27th];Parasites—Giardia. 2016 https://www.cdc.gov/parasites/giardia/
- 90.Tian XF, Shen HE, Li J, Chen Y, Yang ZH, Lu SQ. The effects of dihydroartemisinin on Giardia lamblia morphology and cell cycle in vitro. Parasitology Research. 2010;107(2):369–75. doi: 10.1007/s00436-010-1872-4. [DOI] [PubMed] [Google Scholar]
- 91.Leiby DA. Transfusion-transmitted Babesia spp.: bull’s-eye on Babesia microti. Clinical microbiology reviews. 2011;24(1):14–28. doi: 10.1128/CMR.00022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rozej-Bielicka W, Stypulkowska-Misiurewicz H, Golab E. Human babesiosis. Przeglad epidemiologiczny. 2015;69(3):489–94. 605–8. [PubMed] [Google Scholar]
- 93.Sakuma M, Fukuda K, Takayama K, Kobayashi Y, Miyama TS, Setoguchi A, Endo Y. Molecular epidemiological survey of the Babesia gibsoni cytochrome b gene in western Japan. Journal of Veterinary Medical Science. 2012;74(10):1341–1344. doi: 10.1292/jvms.12-0140. [DOI] [PubMed] [Google Scholar]
- 94.Mazuz ML, Golenser J, Fish L, Haynes RK, Wollkomirsky R, Leibovich B, Shkap V. Artemisone inhibits in vitro and in vivo propagation of Babesia bovis and B. bigemina parasites. Experimental parasitology. 2013;135(4):690–694. doi: 10.1016/j.exppara.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 95.Goo Y-K, Terkawi MA, Jia H, Aboge GO, Ooka H, Nelson B, Kim S, Sunaga F, Namikawa K, Igarashi I. Artesunate, a potential drug for treatment of Babesia infection. Parasitology international. 2010;59(3):481–486. doi: 10.1016/j.parint.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 96.Nagai A, Yokoyama N, Matsuo T, Bork S, Hirata H, Xuan X, Zhu Y, Claveria FG, Fujisaki K, Igarashi I. Growth-inhibitory effects of artesunate, pyrimethamine, and pamaquine against Babesia equi and Babesia caballi in in vitro cultures. Antimicrobial agents and chemotherapy. 2003;47(2):800–803. doi: 10.1128/AAC.47.2.800-803.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsuu A, Yamasaki M, Xuan X, Ikadai H, Hikasa Y. In vitro evaluation of the growth inhibitory activities of 15 drugs against Babesia gibsoni (Aomori strain) Veterinary parasitology. 2008;157(1):1–8. doi: 10.1016/j.vetpar.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 98.Iguchi A, Matsuu A, Matsuyama K, Hikasa Y. The efficacy of artemisinin, artemether, and lumefantrine against Babesia gibsoni in vitro. Parasitology international. 2015;64(2):190–193. doi: 10.1016/j.parint.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Kawai S, Igarashi I, Abgaandorjiin A, Miyazawa K, Ikadai H, Nagasawa H, Fujisaki K, Mikami T, Suzuki N, Matsuda H. Ultrastructural characteristics of Babesia caballi in equine erythrocytes in vitro. Parasitology research. 1999;85(10):794–799. doi: 10.1007/s004360050635. [DOI] [PubMed] [Google Scholar]
- 100.Meshnick SR, Taylor TE, Kamchonwongpaisan S. Artemisinin and the antimalarial endoperoxides: from herbal remedy to targeted chemotherapy. Microbiology Review. 1996;60(2):301–315. doi: 10.1128/mr.60.2.301-315.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kumar S, Gupta AK, Pal Y, Dwivedi SK. In-vivo therapeutic efficacy trial with artemisinin derivative, buparvaquone and imidocarb dipropionate against Babesia equi infection in donkeys. Journal of veterinary medical science. 2003;65(11):1171–1177. doi: 10.1292/jvms.65.1171. [DOI] [PubMed] [Google Scholar]