Abstract
Objective
Membrane type-1 MMP (MT1-MMP) is elevated during thoracic aortic aneurysm (TAA) development in mouse models, and plays an important role in the activation of MMP-2 and the release of matrix bound TGF-β. This study tested the hypothesis that MT1-MMP is subject to protein kinase C (PKC)-mediated regulation, which alters intracellular trafficking and activity with TAA.
Methods
Abundance of MMP-2, native and phosphorylated-MT1-MMP, and PKC-delta was measured in aortic tissue from patients with small TAAs (<5 cm; n=8) and large TAAs (>6.5 cm; n=8), and compared to normals (n=8). Cellular localization of green fluorescent protein-tagged MT1-MMP was assessed in aortic fibroblasts isolated from control and 4-week TAA mice. Effects of PKC-mediated phosphorylation on MT1-MMP cellular localization and function (active MMP-2 versus phospo-Smad-2 abundance), was determined following treatment with a PKC activator (phorbol-12-myristate-13-acetate; 100 nM) with and without a PKC-delta-specific inhibitor (Röttlerin, 3 µM).
Results
Compared to controls, MT1-MMP abundance increased in aortas from both TAA groups. Active MMP-2 was increased only in large TAAs. Abundance of phosphorylated MT1-MMP and activated-PKC-delta was enhanced in small versus large TAAs. MT1-MMP was localized on the plasma membrane in aortic fibroblasts from control mice and in endosomes from TAA mice. PMA treatment induced MT1-MMP-GFP internalization, enhanced phospho-Smad-2, and reduced MMP-2 activation, while Röttlerin pretreatment inhibited these effects.
Conclusion
Phosphorylation of MT1-MMP mediates its activity through directing cellular localization; shifting its role from MMP-2 activation to intracellular signaling. Targeted inhibition of MT1-MMP may therefore hold therapeutic relevance as a means to attenuate TAA development.
Keywords: aneurysm, thoracic aorta, MT1-MMP, remodeling, protein kinase C
Graphical abstract
Ultramini-abstract
Disparity between abundance and activity of membrane type-I matrix metalloproteinase (MT1-MMP) in small and large clinical TAA specimens suggests that post-translational modification may regulate its activity in TAA disease. The present study demonstrates that MT1-MMP phosphorylation induces cellular internalization; shifting its role from MMP-2 activation to intracellular signaling.
INTRODUCTION
Thoracic aortic aneurysms (TAAs) constitute a potentially deadly disease which carries significant morbidity and mortality, and develops in the absence of overt symptomology.1 It is now well understood that TAAs form in response to dysregulated remodeling of the aortic extracellular matrix (ECM), tipping the homeostatic balance between matrix deposition and degradation in favor of proteolysis with subsequent fragmentation of the elastic lamellae and remodeling of the collagen matrix. These processes are mediated in part by a family of endopeptidases, the matrix metalloproteinases (MMPs).2 Key members of this family, including MMP-2 and Membrane Type-1 MMP (MT1-MMP), have played well-described roles in aneurysm development in both the abdomen and thorax.3–6 Furthermore, substantial evidence implicating altered transforming growth factor-beta (TGF-β) signaling as a primary contributor to structural remodeling also exists.5, 7, 8 However, the molecular mechanisms underlying the regulation of these processes continue to be elusive.9, 10
Previous studies from this laboratory and others have demonstrated increased MT1-MMP abundance in both human specimens and animal models of TAA, suggesting that this enzyme plays an important role in mediating the pathological remodeling process.3, 6, 11, 12 Several unique characteristics of MT1-MMP contribute to its prescribed role as a master regulator of matrix remodeling. First, MT1-MMP has a diverse portfolio of proteolytic substrates and plays an integral role in mediating peri-cellular proteolysis at the cell-matrix interface.3, 13–16 Second, MT1-MMP can activate other MMPs, such as pro-MMP-2 and proMMP-13.17, 18 thereby amplifying its proteolytic response. Third, MT1-MMP also plays an important regulatory role in releasing ECM sequestered matrikines, cytokines, and growth factors, such as transforming growth factor-beta (TGF-β).14, 19 Lastly, by virtue of being a type-I transmembrane protein, MT1-MMP cell surface expression has been shown to be regulated through an endosomal recycling process mediated by phosphorylation.20–23 Importantly, previous have suggested that MT1-MMP is phosphorylated at amino acid Threonine-567 by protein kinase C (PKC).24, 25
Together, these unique features position MT1-MMP as a turn-key mediator capable of regulating aortic structural remodeling through multiple pathways. Importantly, understanding the mechanisms governing MT1-MMP cellular function may contribute to the identification of therapeutic strategies for the treatment of aneurysm disease. The present study tested the hypothesis that MT1-MMP activity and function in TAA disease, is regulated by phosphorylation.
METHODS
Study Population
Based on previous results using an established murine model of calcium chloride-induced TAA, MT1-MMP protein abundance was elevated in TAA-induced mice, and a positive correlation between MT1-MMP activity and the time-dependent change in aortic diameter was identified.3 With this information in mind, human ascending aortic tissue specimens (n=16) were selected from a multi-institutional tissue bank, based on size intervals that bracketed the full spectrum of sizes present in the tissue bank. The rationale was based on the fact that the small aneurysm specimens represented those earlier in aneurysm development, while the large aneurysm specimens, were effectively closer to end-stage. The specimens were then stratified into one of two groups for analysis; small TAAs 4.0 – 4.9 cm (n=8), or large TAAs 6.5 – 8.0 cm (n=8). All aortic tissue specimens were obtained from patients with idiopathic degenerative aortic aneurysms with tricuspid aortic valves undergoing root replacement. All tissue specimens were collected from the widest region of the ascending aorta; no specimens were taken from the aortic sinus or proximal arch. No patients had aortic dissection, inflammatory aortic disease, or a known syndromic aortic disease, such as Marfan Syndrome, Ehlers-Danlos Syndrome, or Loeys-Dietz syndrome. Care was taken to ensure that all tissue specimens were harvested from the same region of the ascending aorta. Normal aortic specimens were harvested from heart transplant donors or recipients (n=8) with no known aortic disease. This study was approved by the Institutional Review Boards of the Medical University of South Carolina and The University of Pennsylvania. Informed consent was obtained from all patients. In addition, primary mouse aortic cell lines were isolated (n=6 independently derived, descending aortic fibroblast cell lines from normal mice; n=4, independently derived descending aortic fibroblast cell lines isolated from mice 4-weeks post-TAA induction) and were used to examine the regulation of MT1-MMP and its functional outcomes in passages 2–7. (TAAs were induced as previously described.3, 8, 26)
Aortic Sample Preparation
Aortic tissue specimens (both human and mouse) were snap frozen in dry ice/ethanol slurry immediately following resection, and stored at −80°C. Upon thawing, the tissue was transferred to cold extraction/homogenization buffer (buffer volume 1:6 w/v) containing 10mM cacodylic acid pH 5.0, 0.15M NaCl, 10mM ZnCl2, 1.5mM NaN3, and 0.01% Triton X-100 (v/v), and disrupted in a bead mill homogenizer (Tissuelyser, Qiagen Inc., Valencia, CA). Homogenates were centrifuged (800 × g, 10 min, 4°C) and the supernatants were used for immunoblotting and gelatin zymography.
Immunoblotting analysis
Ten µg of each aortic homogenate was loaded on a 4–12% bis-tris gradient gel and fractionated by electrophoresis. The proteins were transferred to nitrocellulose membranes (0.45 µm, Bio-Rad, Hercules, CA) and incubated in antisera specific for MT1-MMP (0.4 µg/ml; Cat#AB815, EMD Millipore Biosciences, Temecula, CA), MMP-2 (1:2000; Cat#04-1048, EMD Millipore), MT1-MMP (1:2000; Cat#ab38971, Abcam, Cambridge, MA), phosphorylated-Threonine (1:1000; Cat#sc-5267, Santa Cruz Biotechnology, Dallas, TX), PKC-delta (1:1000; Cat#sc-937, Santa Cruz Biotechnology), phosphorylated Smad-2 (1:2000; Cat#3104, Cell Signaling Technology, Beverly, MA), phosphorylated-PKC-delta (1:1000; Cat#9374S, Cell Signaling Technology) were diluted into 5% non-fat dry milk/PBS. A secondary peroxidase-conjugated antibody (primary antibody species-specific) was applied (1:5000, 5% non-fat dry milk/PBS) and signals were detected with a chemiluminescent substrate (Western Lighting Chemiluminescence Reagent Plus, PerkinElmer Inc., San Jose, CA) and recorded on film. Band intensity was quantified using Gel-Pro Analyzer software (v3.1.14, Media Cybernetics, Silver Spring, MD).
Gelatin Zymography
Abundance of MMP-2 was assessed by gelatin zymography.12 Homogenates (10 µg protein) were fractionated on a non-denaturing 10% polyacrylamide gel containing 0.1% (w/v) gelatin (Invitrogen Corporation, Carlsbad, CA). The gels were then equilibrated and incubated in Zymogram Developing Buffer (Invitrogen) for 18 hours at 37°C. After staining with 0.5% Coomassie Brilliant Blue (2 hours, room temperature), the gels were destained to reveal regions of gelatin clearance. The relative abundance of MMP-2 (as verified by a recombinant MMP-2 standard) was then determined by densitometry using the Gel-Pro Analyzer software.
Immunoprecipitation
Briefly, 0.15 grams of aortic tissue was suspended in 1.5mL of standard RIPA buffer (50mM Tris-HCl pH 7.4, 150 mM NaCl, 1mM PMSF, 1mM EDTA, 5ug/mL Aprotinin, 5 µg/mL Leupeptin, 1% Triton x-100, 1% Sodium Deoxycholate, 0.1% SDS) and disrupted using a bead mill homogenizer. The homogenate was centrifuged (800 × g, 10 min, 4°C) and a BCA protein assay was completed (Pierce, Cat#23225). Five hundred µg of total protein was mixed with 5.0 µg of MT1-MMP antibody (Abcam; ab38971) and allowed to incubate overnight at 4°C with gentle agitation. Fifty µL of equilibrated (RIPA buffer) Protein A/G Plus-Agarose (Cat#sc-2003; Santa Cruz Biotechnology) was added and incubated for one hour at 4°C with gentle agitation. Immunoprecipitates were collected by centrifugation (1500g, 5 minutes, 4°C), and the pellets were washed four times with PBS. The final pellet was resuspended in 60 µL of 2× Laemmli Sample Buffer and boiled for five minutes. The precipitated proteins were fractionated on 4–12% bis-tris gradient polyacrylamide gels and phospho-Threonine phosphorylated MT1-MMP was determined by Western blotting.
PKC activation/inhibition and Confocal Microscopy
Primary aortic fibroblasts from normal (Control) or 4-week TAA-induced mice were plated in glass bottom dishes and transfected with 1.5 µg of pCMV-MT1-MMP-GFP (C-terminal tag; green flourescence) using Lipofectamine2000 (Cat#11668027; ThermoFisher Scientific, Waltham, MA) according to the manufacturer’s instructions. Cells were allowed to grow for 48 h and were then fixed with 3.7% paraformaldehyde at 37°C. Nuclei were counterstained with 4’,6-diamidino-2-phenylindole (DAPI; blue fluorescence). In addition, normal aortic fibroblasts (grown either on plastic (biochemistry) or glass-bottom dishes (microscopy)) were transfected with CMV-MT1-MMP-GFP and allowed to grow for 48 h. The cells were then treated, in the absence (vehicle control, DMSO, 1:1000 dilution) or presence of phorbol 12-myristate 13-acetate (PMA, 100 nM, 30 min, 37°C), with or without Röttlerin (PKC-δ-specific inhibitor; 3 h pre-incubation plus 30 min concurrent exposure, 3 µM, 3h, 37°C). The cells were either collected for immunoblotting for phospho-Smad2 or pre-fixed (10 min, 1.5% paraformaldehyde, 2×-diluted into the growth medium) followed by normal fixation (10 min, 3.7% paraformaldehyde at 37°C) to preserve membrane localization. A second cohort of cells was treated longer (18 h) with PMA or vehicle, with or without Röttlerin, in order to determine changes in protein content (100 nM PMA 3 µM Röttlerin for 3 h, followed by 15 hour incubation in fresh drug-free medium). These cells were collected for immunoblotting for active and latent MMP-2 as well as phospho-Smad2. All cell images were captured using an Inverted Nikon A1R+ Confocal Microscope, with a 60×, 1.5NA, oil emersion objective. Representative images of at least three experiments were shown.
Data Analysis
Relative protein abundance of MT1-MMP, MMP-2 (active and total forms), PKC-delta, phospho-PKC-delta, and phospho-Smad2 were determined from densitometry of films or zymographic gels. Integrated optical densities were determined for each specimen and the results were expressed as the mean percent change of normal aorta. Prior to statistical analysis, a Shapiro-Wilk test was performed on the original data to determine normality. Differences in patient age, and differences in murine MT1-MMP abundance (Figure 3A) were compared using an unpaired two-sample t-test. For all analyses that were not normally distributed, a log transformation was performed on the original data and normality was confirmed. Using the Stata statistical package (Intercooled Stata v8.2; StataCorp LP, College Station, TX), the normally distributed data were then analyzed by one-way ANOVA with Tukey’s wholly significant difference (wsd) post hoc analysis for the separation of means. All results demonstrating a p-value less than 0.05 were considered to be statistically significant. The original data (non-transformed) were then graphically represented using box and whisker plots, including both median and mean values as different colored bars within the box, which depicts the 25th–75th interquartile range. In addition, each individual data point is represented as an independent dot overlaying the box.
RESULTS
Patient demographic information is summarized in Table 1. Patients with large TAAs were older than patients with small TAAs, and patients in both TAA groups were older than the patients utilized as normal referent controls.
Table 1.
Demographic data of Normal and Aneurysm patients
| Number | Age (yrs) (mean ± SEM) |
Range (Ascending Size; cm) |
|
|---|---|---|---|
| Normal | n=8 | 45.3±5.0 * | 2.9 92013; 3.2 |
| Small TAA | n=8 | 59.7±4.0 * | 4.0 – 4.9 |
| Large TAA | n=8 | 73.3±4.0 *# | 6.5 – 8.0 |
p<0.05 versus Normal;
p<0.05 versus Small TAA
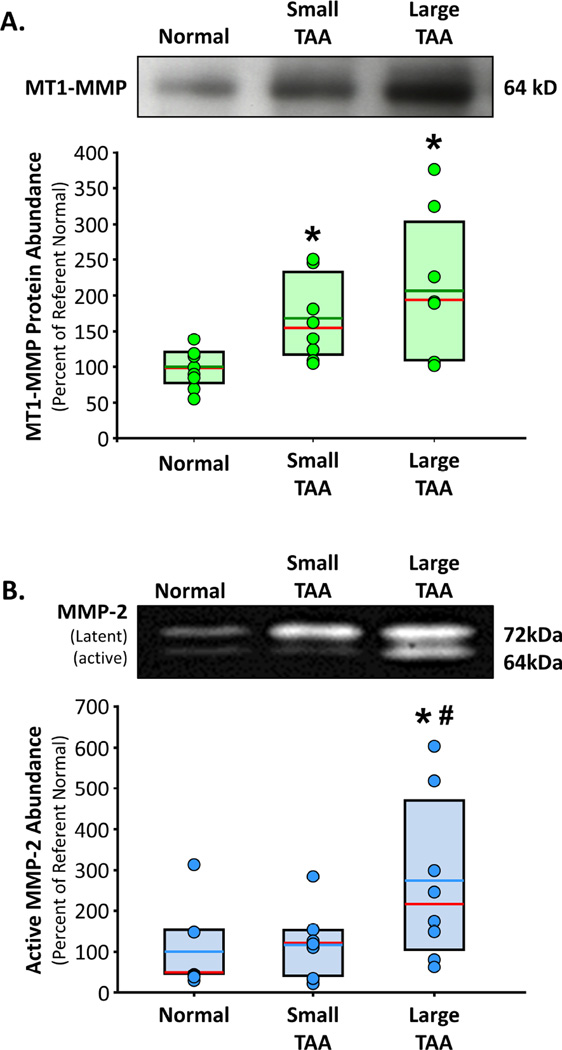
Protein abundance of MT1-MMP was significantly increased in small TAAs (168±20%, p<0.05 versus Normal) and large TAAs (206±37%, p<0.05 versus Normal), as compared to normal aorta (100±10%) (Figure 1A). The abundance of the 64 kDa active form of MMP-2 was elevated in the large TAAs only (273±70%, p<0.05 versus Normal, p<0.05 versus Small TAA), as compared to normal aorta (100±40%) and small TAAs (117±31%) (Figure 1B). No change in TIMP-2 abundance, as compared to normal aorta, was observed in either of the small or large TAA groups (data not shown).
Figure 1. MT1-MMP and MMP-2 abundance in clinical TAA specimens.
A. Top. Representative immunoblot showing MT1-MMP protein abundance in normal aorta (n=8), small TAA (4.0 – 4.9 cm; n=8), and large TAA (6.5 – 8.0 cm; n=8). Bottom. Densitometric quantitation of MT1-MMP protein abundance determined by immunoblotting. Results are expressed as a mean percent change of normal aorta. Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (green line) values are shown within the box. * p<0.05 versus Normal (ANOVA with Tukey’s wsd). B. Top. Representative zymogram showing the protein abundance of the latent form (72kD band) and active form (64kD band) of MMP-2 in normal aorta (n=7), small TAA (4.0 – 4.9 cm; n=8), and large TAA (6.5 – 8.0 cm; n=8). Bottom. Densitometric quantitation of active (64kD band) MMP-2 protein abundance determined by gelatin zymography. Results are expressed as a mean percent change of normal aorta. Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (blue line) values are shown within the box. * p<0.05 versus Normal, # p<0.05 versus Small TAA (ANOVA with Tukey’s wsd).
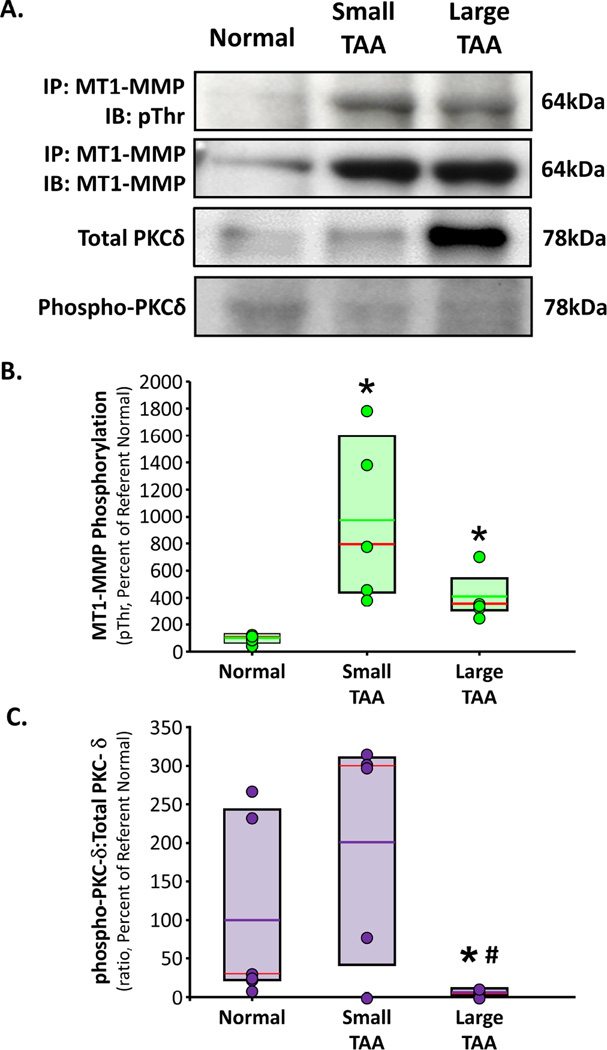
Protein homogenates from normal, small TAAs, and large TAAs, were then subjected to MT1-MMP-specific immunoprecipitation. The resulting immunoprecipitated proteins were then fractionated by gel electrophoresis and immunoblotted for MT1-MMP (to verify functional immunoprecipitation), and with an anti-phospho-Threonine antibody. The results demonstrated increased phosphorylated-MT1-MMP in both small (973±272%, p<0.05 versus Normal) and large TAAs (410±80%, p<0.05 versus Normal) as compared to Normal aorta (100±14%) (Figure 2A,B). PKC-δ-specific activation was determined by examining the ratio of phosphorylated PKC-delta (activated form) to total PKC-δ. The results demonstrated a trend toward increased PKC-δ activity in small TAAs (201±67%), and severely reduced PKC-δ activity in large TAAs (6±3%, p<0.05 versus Normal) as compared to normal aorta (100±48%) (Figure 2A,C).
Figure 2. Phosphorylation of MT1-MMP in clinical TAA specimens.
A. MT1-MMP was immunoprecipitated from normal aorta, small TAA (4.0 – 4.9 cm), and large TAA (6.5 – 8.0 cm). The immunoprecipitated protein was fractionated by electrophoresis and immunoblotted for phospho-Threonine and for MT1-MMP to confirm immunoprecipitation. Tissue homogenates from the same sample set were also immunoblotted for total PKC-δ, and phosphor-PKC-δ. Representative images are shown. B. Densitometric quantitation of MT1-MMP-specific phospho-Threonine residues in normal aorta (n=6), small TAA (4.0 – 4.9 cm; n=5), and large TAA (6.5 – 8.0 cm; n=5). Results are expressed as a mean percent change of normal aorta. Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (green line) values are shown within the box. * p<0.05 versus Normal (ANOVA with Tukey’s wsd). C. Densitometric quantitation of the ratio of phospho-PKC-δ (activated):total PKC-δ from normal aorta (n=6), small TAA (4.0 – 4.9 cm; n=5), and large TAA (6.5 – 8.0 cm; n=4). Results are expressed as a mean percent change of normal aorta. Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (purple line) values are shown within the box. * p<0.05 versus Normal, # p<0.05 versus Small TAA (ANOVA with Tukey’s wsd).
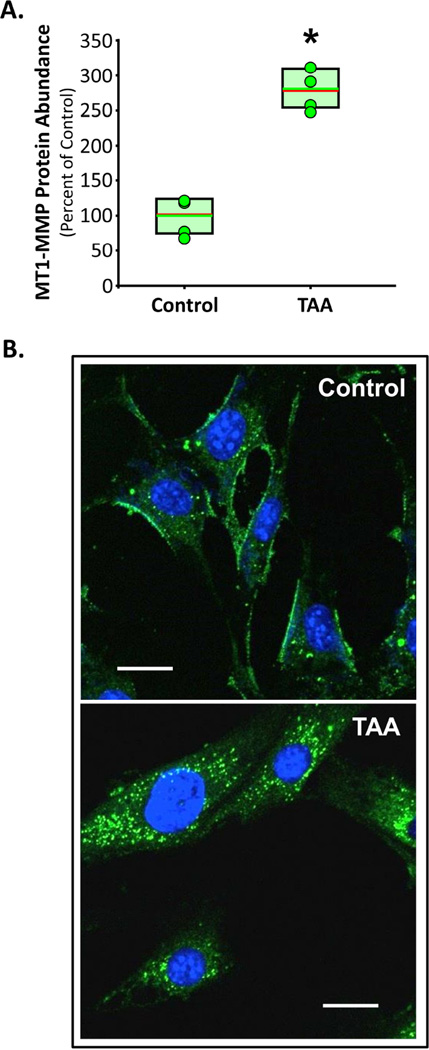
MT1-MMP abundance was examined in isolated primary normal and 4-week TAA mouse aortic fibroblast cell lines. The results were consistent with the human clinical TAA specimens, showing elevated MT1-MMP protein abundance with TAA (281±15%, p<0.05 versus Control) as compared to control fibroblasts (100±14%) (Figure 3A). To examine MT1-MMP cellular localization, both normal and 4-week TAA aortic fibroblast cell lines were transfected with MT1-MMP-GFP. MT1-MMP-GFP was predominantly localized at the plasma membrane with some MT1-MMP appearing in endosomal vesicles, at steady state in control cells (Figure 3B, top). In the 4-week TAA aortic fibroblasts however, MT1-MMP-GFP was almost exclusively localized in endosomal vesicles (Figure 3B, bottom).
Figure 3. MT1-MMP abundance and localization in primary aortic fibroblasts.
A. Protein abundance of MT1-MMP in normal and 4-week TAA aortic fibroblasts (n=4 each group). Results are expressed as a mean percent change of Control aorta. Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (green line) values are shown within the box. * p<0.05 versus Control (unpaired Student’s t-test). B. Representative images from normal and 4-week TAA aortic fibroblasts transfected with MT1-MMP-GFP. At steady-state MT1-MMP-GFP was localized predominantly at the plasma membrane in the normal cells, but was almost exclusively localized in endosomal vesicles in the 4-week TAA cells.
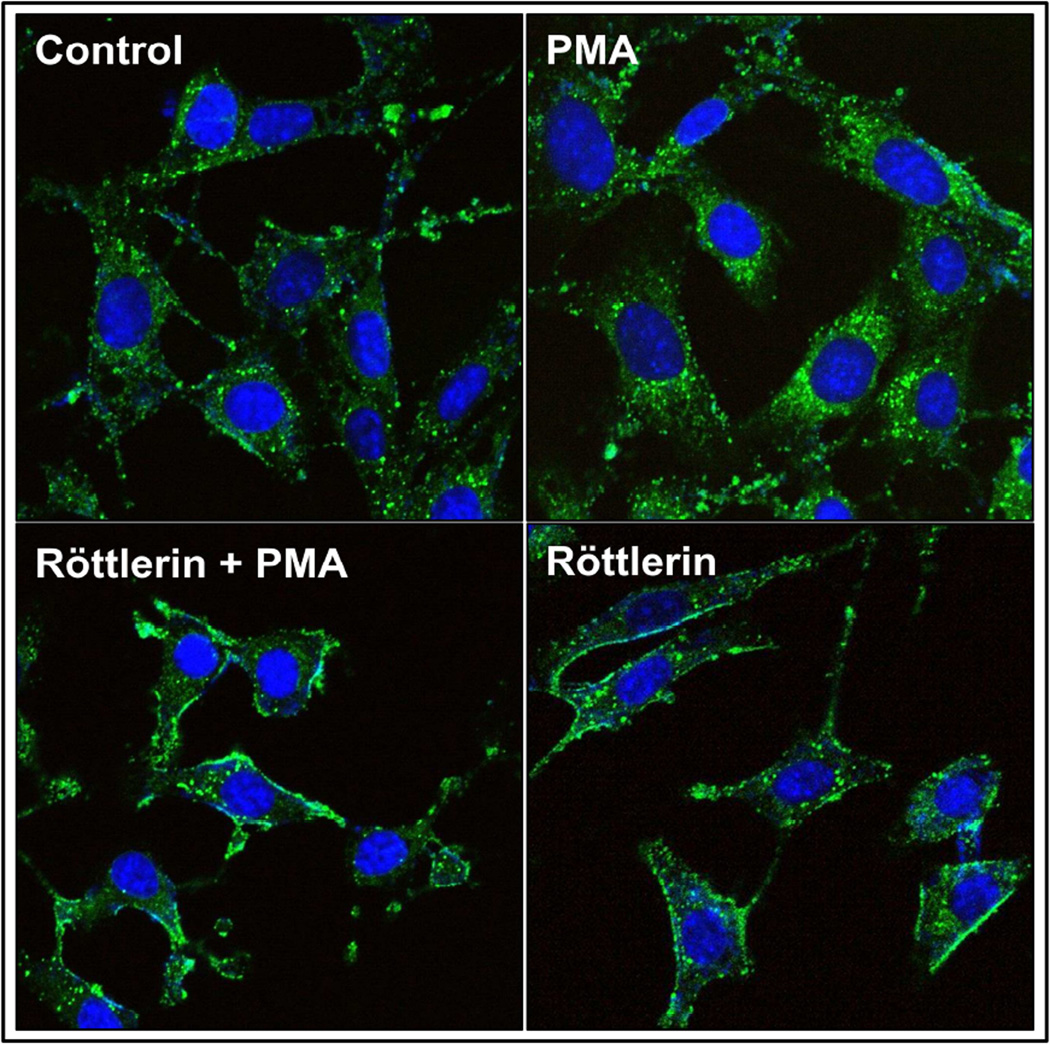
To examine the regulation of MT1-MMP cellular localization, normal aortic fibroblasts transfected with MT1-MMP-GFP were treated with PMA in the absence or presence of the PKC-δ-specific inhibitor, Röttlerin. The results demonstrated that MT1-MMP-GFP was localized predominantly at the plasma membrane in vehicle treated cells (Control; Figure 4, top-left). Upon stimulation with PMA, MT1-MMP-GFP translocated intracellularly, appearing almost exclusively in endosomal vesicles (PMA; Figure 4, top-right). When the cells were pretreated with Röttlerin, followed by PMA, MT1-MMP-GFP intracellular translocation was inhibited (Röttlerin + PMA; Figure 4, bottom-left). In fact, treatment with Röttlerin alone, appeared to be sufficient to lock MT1-MMP-GFP at the plasma membrane (Röttlerin; Figure 4, bottom-right).
Figure 4. PKC-δ-mediated MT1-MMP translocation in normal aortic fibroblasts.
Representative images of primary aortic fibroblasts transfected with MT1-MMP-GFP and treated with or without PMA, in the presence or absence of Röttlerin. Images show MT1-MMP-GFP predominantly at the plasma membrane in controls. Upon activation of PKC with PMA, MT1-MMP translocated to intracellular endosomal vesicles. When the cells were pretreated with Röttlerin, a PKC-δ-specific inhibitor, PMA-induced translocation was inhibited. Röttlerin alone was sufficient to lock MT1-MMP at the plasma membrane.
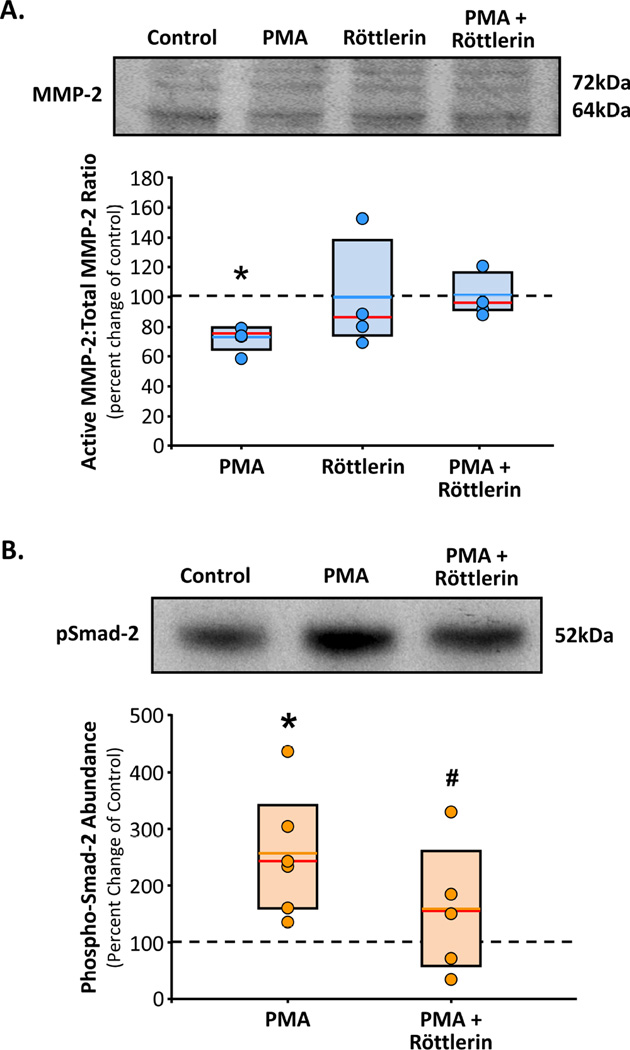
To assess the effects of MT1-MMP localization on protein function, normal aortic fibroblasts transfected with MT1-MMP-GFP were treated with PMA in the absence or presence of Röttlerin. The cells were then collected for biochemical analysis by immunoblotting for activated MMP-2 or evidence of activated TGF-β signaling. The results demonstrated that normal aortic fibroblast cell lines treated with PMA displayed reduced MMP-2 activation (73±4%, p<0.05 versus Control) (Figure 5A) and increased phosphorylation of Smad-2 (257±44%, p<0.05 versus Control) (Figure 5B), the intracellular mediator of activated TGF-β receptors. Importantly, in both circumstance, these effects were inhibited by pretreatment with Röttlerin (MMP-2: 100±4%19; pSmad2: 159±51%) (Figure 5A,B).
Figure 5. PMA induced changes in MT1-MMP function.
A. Primary aortic fibroblasts from normal mice (n=4) were treated with or without PMA, in the presence or absence of Röttlerin for 18 h (n=4 each treatment group). Cell homogenates were fractionated by gel electrophoresis and immunoblotted for latent and active MMP2. Results are expressed as a mean percent change in each cell line from its respective steady-state control (vehicle treated; dotted line). Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (blue line) values are shown within the box. * p<0.05 versus control (ANOVA with Tukey’s wsd). B. Primary aortic fibroblasts from normal mice (n=4) were treated with or without PMA (30 min), in the presence or absence of Röttlerin (3 h pre-incubation, plus 30 min concurrent; n=4 each treatment group). Cell homogenates were fractionated by gel electrophoresis and immunoblotted for phosphor-Smad-2. Results are expressed as a mean percent change in each cell line from its respective steady-state control (vehicle treated; dotted line). Individual data points in each group overlay box plots. The box defines the 25th–75th interquartile range, and both the median (red line) and mean (orange line) values are shown within the box. * p<0.05 versus control, # p<0.05 versus PMA (ANOVA with Tukey’s wsd).
DISCUSSION
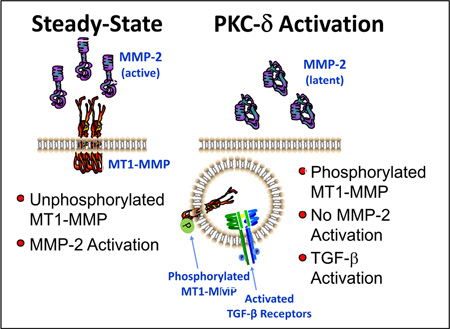
Aortic medial degeneration is a common feature of TAA, characterized by disruption of the medial elastic lamellae, alterations in collagen deposition, and changes in cellular content/phenotype. Past studies have identified and characterized a multi-functional membrane-bound protease, MT1-MMP, possessing several unique characteristics positioning it at the center of multiple mechanisms capable of regulating both ECM deposition and degradation. Multiple lines of evidence from both human and animal studies have suggested that MT1-MMP plays an essential role in aortic structural remodeling during TAA development.3, 6, 12, 27, 28 Importantly, since MT1-MMP is activated co-translationally, defining the mechanisms that govern its intracellular activity and localization during TAA development are essential for understanding its role in pathogenesis. Accordingly, the present study hypothesized that MT1-MMP activity and cellular localization is regulated by phosphorylation during TAA formation and progression. To test this hypothesis, two cohorts of TAA specimens were selected from an established aortic tissue bank (small TAAs (4.0 – 4.9 cm) and large TAAs (6.5 – 8.0 cm)) and MT1-MMP was analyzed and compared to normal aortic specimens harvested from patients without aneurysm disease. The significant findings of this study were fivefold. First, MT1-MMP protein abundance was elevated in both small and large TAA specimens, as compared to normal aortas. This increase suggests that MT1-MMP may indeed play an important role in TAA formation and progression. Second, the latent and active forms of MMP-2 were similarly measured. Interestingly, it was observed that the activated form of MMP-2 was increased only in the large TAA specimens. Because MT1-MMP plays a well-described role as a constitutive mediator of latent-MMP-2 activation, the disparity between increased MT1-MMP in the absence of increased active MMP-2 in small TAA specimens, suggested that MT1-MMP might be post-translationally regulated. Third, the observed increase in MT1-MMP-specific phospho-Threonine demonstrated, for the first time that MT1-MMP was unequivocally differentially phosphorylated during TAA development. Fourth, studies examining primary aortic fibroblast cell lines isolated from normal and 4-week TAA mice, transfected with green-fluorescent tagged MT1-MMP, demonstrated that MT1-MMP was localized predominantly at the plasma membrane surface in normal aortic fibroblasts, but was almost exclusively localized to intracellular endosomal vesicles in 4-week TAA aortic fibroblast. Last, activation of PKC could induce the translocation of MT1-MMP from the membrane surface into endosomal vesicles, and that this process could be inhibited by Röttlerin, a PKC-δ specific pharmacological inhibitor. Moreover, when MT1-MMP was localized to the plasma membrane (unphosphorylated; steady-state or in the presence of Röttlerin) it functioned to activate latent-MMP-2, however, when MT1-MMP was internalized in endosomal vesicles (phosphorylated; PMA) it functioned to activate TGF-β receptor signaling. The changes in MT1-MMP abundance and activity observed in this study, suggest MT1-MMP may function to promulgate aneurysm progression through multiple pathways. Accordingly, identifying the independent roles of MT1-MMP in small and large TAAs may reveal multiple mechanisms that could be targeted for clinical benefit.
The present study examined MT1-MMP in small and large clinical specimens and demonstrated MT1-MMP protein abundance was elevated with the advancement of TAA disease suggesting that MT1-MMP may play an important role in clinical TAA formation and progression. This is supported in a recent study by Jackson et al., who identified increased MT1-MMP mRNA expression, and increased medial immunohistochemical staining, in clinical TAA specimens associated with both bicuspid and tricuspid aortic valves.27 Furthermore, previous work from this laboratory, using an established murine model of TAA, demonstrated a positive correlation between MT1-MMP activity and the time-dependent change in aortic diameter over a 16-wk time course post-TAA induction.3
Many studies have examined MT1-MMP activity indirectly; most have used the activation of pro-MMP-2 as a surrogate indicator. Thus, the activation of latent-MMP-2 was examined in this study. A sharp increase in active MMP-2 abundance was observed in large TAAs. These results suggested that increased MT1-MMP activity serves to amplify and accelerate the proteolytic response by activating MMP-2. These results were consistent with previous animal studies in AAA that demonstrated that the MT1-MMP-dependent activation of MMP-2 was essential for aneurysm development.28, 29 Moreover, studies by Xiong and coworkers demonstrated that aortic dilatation in abdominal aortic aneurysm was inhibited in a TIMP-2-deficient mouse.30 While this was initially contrary to the expected result for the removal of an MMP inhibitor, the investigators demonstrated that the loss of TIMP-2 prevented the MT1-MMP-dependent activation of MMP-2, which was necessary for aneurysm formation. Interestingly, in the present study there was a disparity between elevated MT1-MMP and active MMP-2 in small TAAs. This was consistent with a previous study from this laboratory, which demonstrated a similar disparity between MT1-MMP and MMP-2 activation during TAA formation in an established murine model.3 This suggested, in both cases, that either the low level of MT1-MMP activity was sufficient to carry out specific activities independent of the activation of MMP-2, or that MT1-MMP may be post-translationally regulated.
By virtue of being a type-I transmembrane protein, MT1-MMP is likely subject to regulatory mechanisms that direct cellular localization through the endosomal-plasma membrane recycling process. As a result, endocytic recycling of MT1-MMP is one mechanism by which access to specific enzymatic substrates could be controlled. Previous work has shown that MT1-MMP requires a functional transmembrane domain in order to be correctly localized for latent-MMP-2 activation.20 Similarly, it was demonstrated that the cytoplasmic tail of MT1-MMP was required for endocytosis and trafficking along the micro-tubular network.22, 23 To explore potential post-translational modifications of MT1-MMP that could affect endosomal recycling, we used in silico bioinformatics to examine the C-terminal amino acid sequence for regulatory sites. Interestingly, we identified Threonine-567 (T567) as a potential PKC-mediated phosphorylation site localized within the short 20 amino acid cytoplasmic tail of MT1-MMP. Indeed, several studies have now established that MT1-MMP activity and/or cellular localization can be regulated by phosphorylation of C-terminal residues.22, 24, 25 Williams et al. demonstrated that PKC-dependent phosphorylation of T567 lead to enhanced internalization of MT1-MMP.25 Moss and colleagues, demonstrated that T567 phosphorylation resulting in altered substrate specificity and cellular localization, and suggested that PKC-δ maybe the kinase responsible.24 In the present study, immunoprecipitation of MT1-MMP, followed by immunoblotting for phosphorylated-Threonine residues, demonstrated increased MT1-MMP phospho-Threonine phosphorylation in small and large TAAs. This coincided with increased PKC-δ activation in small TAAs, and a dramatic reduction in activated PKC-δ in large TAAs.
To examine the functional consequences of MT1-MMP phosphorylation, GFP-tagged MT1-MMP was expressed in primary aortic fibroblasts isolated from normal and 4-week TAA mice. Interestingly, at steady-state in the absence of any treatment, MT1-MMP was localized predominantly at the plasma membrane with some in endosomes in the control cells, but was almost exclusively localized in endosomal vesicles in the TAA-derived cells. This suggests that the internalization of MT1-MMP likely plays an important role in mediating the cellular changes during TAA development.
Normal murine aortic fibroblasts were treated with an activator of PKC in the presence and absence of the PKC-δ-specific inhibitor Röttlerin. These studies demonstrated that inducing phosphorylation of MT1-MMP through the activation of PKC-δ, could indeed induce the translocation of MT1-MMP-GFP from the plasma membrane into endosomal vesicles, thereby altering its access to specific MT1-MMP substrates. This suggests that the reduced activation of MMP-2 in the small clinical TAAs may be the result of enhanced internalization of MT1-MMP. Furthermore, it has been well described that TGF-β is commonly sequestered within the ECM, bound by latent TGF-β binding proteins (LTBPs) and localized to the fibrillin microfibrils. Both LTBP-1 and fibrillin-1a are vital components of the elastic micro-architecture within the aortic wall, and both have been identified as substrates for MT1-MMP.31, 32 Importantly, enhanced TGF-β signaling has been implicated in aneurysm development through multiple mechanisms including the dysregulation of collagen deposition, enhanced production of ECM degradative enzymes, and the induction of changes in cellular composition and phenotype.33 Accordingly, MT1-MMP phosphorylation was then examined in relationship to the release of sequestered TGF-β and activation of the TGF-β signaling pathway as determined by the phosphorylation of Smad-2. Remarkably, when cells were treated with an activator PKC, a concomitant increase in phospho-Smad-2 was observed, in a PKC-δ-dependent fashion. These results were consistent with previous observations that TGF-β signaling could be enhanced and prolonged upon internalization of the TGF-β receptor complex.34, 35
While these novel results suggest a multifunctional role for MT1-MMP in TAA development, this study is not without limitations. First, it must be recognized that the ascending TAA specimens were selected based on aortic size alone. Accordingly, because all aortic specimens were collected when deemed surgically necessary, the clinical indications leading to early surgical intervention may have resulted in the selection of more aggressive TAAs in the small cohort. Second, because of significant differences in patient age between the normal, small, and large cohorts, age-dependent effects on MT1-MMP abundance and activity cannot be ruled out in this study. Last, while the cellular localization of MT1-MMP could not be determined in the clinical TAA specimens, it must be recognized that there are distinct differences when compared with cells from the isolated from the murine TAA model, with respect to time and progression of TAA disease. The difficulty in obtaining human aortic fibroblasts from patients without aortic disease prevented the completion of these studies using cells/tissue from only human origin.
Nevertheless, the unique findings of this study have demonstrated that MT1-MMP is elevated in clinical TAA specimens, and that phosphorylation of MT1-MMP regulates enzymatic function by altering its cellular localization; mediating access to specific substrates and shifting its functional role from MMP-2 activation to mediator of intracellular signaling. While these studies cannot differentiate the importance of the location-specific activity of MT1-MMP in relationship to advancing TAA development, these results do suggest that targeting MT1-MMP activity or protein abundance may constitute a novel therapeutic strategy for the treatment of TAA disease.
Acknowledgments
Funding: This work was supported by grants from: The National Institutes of Health (NHLBI) R01HL102121 (Ikonomidis), and the Department of Veterans Affairs (VA-ORD BLR&D Merit) I01BX000904 (Jones).
Non-standard Abbreviations and Acronyms
- TAA
thoracic aortic aneurysm
- MMP
matrix metalloproteinase
- MT1-MMP
membrane type-1-MMP
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: Both Dr. Ikonomidis and Dr. Jones have research grants to disclose. No other potential conflicts of interest exist for any of the other authors.
REFERENCES
- 1.Elefteriades JA. Natural history of thoracic aortic aneurysms: indications for surgery, and surgical versus nonsurgical risks. Ann Thorac Surg. 2002;74:S1877–S1880. doi: 10.1016/s0003-4975(02)04147-4. discussion S1892-1878. [DOI] [PubMed] [Google Scholar]
- 2.Barbour JR, Spinale FG, Ikonomidis JS. Proteinase systems and thoracic aortic aneurysm progression. J Surg Res. 2007;139:292–307. doi: 10.1016/j.jss.2006.09.020. [DOI] [PubMed] [Google Scholar]
- 3.Jones JA, Ruddy JM, Bouges S, et al. Alterations in membrane type-1 matrix metalloproteinase abundance after the induction of thoracic aortic aneurysm in a murine model. Am J Physiol Heart Circ Physiol. 2010;299:H114–H124. doi: 10.1152/ajpheart.00028.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–632. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen M, Lee J, Basu R, et al. Divergent roles of matrix metalloproteinase 2 in pathogenesis of thoracic aortic aneurysm. Arterioscler Thromb Vasc Biol. 2015;35:888–898. doi: 10.1161/ATVBAHA.114.305115. [DOI] [PubMed] [Google Scholar]
- 6.Xiong W, Knispel R, MacTaggart J, Greiner TC, Weiss SJ, Baxter BT. Membrane-type 1 matrix metalloproteinase regulates macrophage-dependent elastolytic activity and aneurysm formation in vivo. J Biol Chem. 2009;284:1765–1771. doi: 10.1074/jbc.M806239200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Habashi JP, Judge DP, Holm TM, et al. Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science. 2006;312:117–121. doi: 10.1126/science.1124287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jones JA, Barbour JR, Stroud RE, et al. Altered transforming growth factor-beta signaling in a murine model of thoracic aortic aneurysm. J Vasc Res. 2008;45:457–468. doi: 10.1159/000127437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones JA, Spinale FG, Ikonomidis JS. Transforming growth factor-beta signaling in thoracic aortic aneurysm development: a paradox in pathogenesis. J Vasc Res. 2009;46:119–137. doi: 10.1159/000151766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pyeritz RE. Recent Progress in Understanding the Natural and Clinical Histories of the Marfan Syndrome. Trends in Cardiovascular Medicine. 2016 doi: 10.1016/j.tcm.2015.12.003. X:xxxx-xxxx. [DOI] [PubMed] [Google Scholar]
- 11.Annabi B, Shedid D, Ghosn P, et al. Differential regulation of matrix metalloproteinase activities in abdominal aortic aneurysms. J Vasc Surg. 2002;35:539–546. doi: 10.1067/mva.2002.121124. [DOI] [PubMed] [Google Scholar]
- 12.Ikonomidis JS, Jones JA, Barbour JR, et al. Expression of matrix metalloproteinases and endogenous inhibitors within ascending aortic aneurysms of patients with Marfan syndrome. Circulation. 2006;114:I365–I370. doi: 10.1161/CIRCULATIONAHA.105.000810. [DOI] [PubMed] [Google Scholar]
- 13.Komiyama T, Coppola JM, Larsen MJ, et al. Inhibition of furin/proprotein convertase-catalyzed surface and intracellular processing by small molecules. J Biol Chem. 2009;284:15729–15738. doi: 10.1074/jbc.M901540200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itoh Y, Seiki M. MT1-MMP: a potent modifier of pericellular microenvironment. J Cell Physiol. 2006;206:1–8. doi: 10.1002/jcp.20431. [DOI] [PubMed] [Google Scholar]
- 15.Imai K, Ohuchi E, Aoki T, et al. Membrane-type matrix metalloproteinase 1 is a gelatinolytic enzyme and is secreted in a complex with tissue inhibitor of metalloproteinases 2. Cancer Res. 1996;56:2707–2710. [PubMed] [Google Scholar]
- 16.Ohuchi E, Imai K, Fujii Y, Sato H, Seiki M, Okada Y. Membrane type 1 matrix metalloproteinase digests interstitial collagens and other extracellular matrix macromolecules. J Biol Chem. 1997;272:2446–2451. doi: 10.1074/jbc.272.4.2446. [DOI] [PubMed] [Google Scholar]
- 17.Deryugina EI, Ratnikov B, Monosov E, et al. MT1-MMP initiates activation of pro-MMP-2 and integrin alphavbeta3 promotes maturation of MMP-2 in breast carcinoma cells. Experimental cell research. 2001;263:209–223. doi: 10.1006/excr.2000.5118. [DOI] [PubMed] [Google Scholar]
- 18.Knauper V, Will H, Lopez-Otin C, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271:17124–17131. doi: 10.1074/jbc.271.29.17124. [DOI] [PubMed] [Google Scholar]
- 19.Mu D, Cambier S, Fjellbirkeland L, et al. The integrin alpha(v)beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao J, Sato H, Takino T, Seiki M. The C-terminal region of membrane type matrix metalloproteinase is a functional transmembrane domain required for pro-gelatinase A activation. J Biol Chem. 1995;270:801–805. doi: 10.1074/jbc.270.2.801. [DOI] [PubMed] [Google Scholar]
- 21.Jiang A, Lehti K, Wang X, Weiss SJ, Keski-Oja J, Pei D. Regulation of membrane-type matrix metalloproteinase 1 activity by dynamin-mediated endocytosis. Proc Natl Acad Sci U S A. 2001;98:13693–13698. doi: 10.1073/pnas.241293698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remacle AG, Rozanov DV, Baciu PC, Chekanov AV, Golubkov VS, Strongin AY. The transmembrane domain is essential for the microtubular trafficking of membrane type-1 matrix metalloproteinase (MT1-MMP) J Cell Sci. 2005;118:4975–4984. doi: 10.1242/jcs.02610. [DOI] [PubMed] [Google Scholar]
- 23.Uekita T, Itoh Y, Yana I, Ohno H, Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss NM, Wu YI, Liu Y, Munshi HG, Stack MS. Modulation of the membrane type 1 matrix metalloproteinase cytoplasmic tail enhances tumor cell invasion and proliferation in three-dimensional collagen matrices. J Biol Chem. 2009;284:19791–19799. doi: 10.1074/jbc.M109.020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Williams KC, Coppolino MG. Phosphorylation of membrane type 1-matrix metalloproteinase (MT1-MMP) and its vesicle-associated membrane protein 7 (VAMP7)-dependent trafficking facilitate cell invasion and migration. J Biol Chem. 2011;286:43405–43416. doi: 10.1074/jbc.M111.297069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones JA, Zavadzkas JA, Chang EI, et al. Cellular phenotype transformation occurs during thoracic aortic aneurysm development. J Thorac Cardiovasc Surg. 2010;140:653–659. doi: 10.1016/j.jtcvs.2009.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson V, Olsson T, Kurtovic S, et al. Matrix metalloproteinase 14 and 19 expression is associated with thoracic aortic aneurysms. J Thorac Cardiovasc Surg. 2012;144:459–466. doi: 10.1016/j.jtcvs.2011.08.043. [DOI] [PubMed] [Google Scholar]
- 28.Sinha I, Hannawa KK, Eliason JL, et al. Early MT-1 MMP expression following elastase exposure is associated with increased cleaved MMP-2 activity in experimental rodent aortic aneurysms. Surgery. 2004;136:176–182. doi: 10.1016/j.surg.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 29.Eagleton MJ, Ballard N, Lynch E, Srivastava SD, Upchurch GR, Jr, Stanley JC. Early increased MT1-MMP expression and late MMP-2 and MMP-9 activity during Angiotensin II induced aneurysm formation. J Surg Res. 2006;135:345–351. doi: 10.1016/j.jss.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Xiong W, Knispel R, Mactaggart J, Baxter BT. Effects of tissue inhibitor of metalloproteinase 2 deficiency on aneurysm formation. J Vasc Surg. 2006;44:1061–1066. doi: 10.1016/j.jvs.2006.06.036. [DOI] [PubMed] [Google Scholar]
- 31.Ashworth JL, Murphy G, Rock MJ, et al. Fibrillin degradation by matrix metalloproteinases: implications for connective tissue remodelling. Biochem J. 1999;340(Pt 1):171–181. [PMC free article] [PubMed] [Google Scholar]
- 32.Tatti O, Vehvilainen P, Lehti K, Keski-Oja J. MT1-MMP releases latent TGF-beta1 from endothelial cell extracellular matrix via proteolytic processing of LTBP-1. Exp Cell Res. 2008;314:2501–2514. doi: 10.1016/j.yexcr.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 33.Jones JA, Beck C, Barbour JR, et al. Alterations in aortic cellular constituents during thoracic aortic aneurysm development: myofibroblast-mediated vascular remodeling. Am J Pathol. 2009;175:1746–1756. doi: 10.2353/ajpath.2009.081141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayes S, Chawla A, Corvera S. TGF beta receptor internalization into EEA1-enriched early endosomes: role in signaling to Smad2. J Cell Biol. 2002;158:1239–1249. doi: 10.1083/jcb.200204088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sigismund S, Confalonieri S, Ciliberto A, Polo S, Scita G, Di Fiore PP. Endocytosis and signaling: cell logistics shape the eukaryotic cell plan. Physiol Rev. 2012;92:273–366. doi: 10.1152/physrev.00005.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]