Summary
Background
Selective BCL2 inhibition with venetoclax has substantial activity in patients with relapsed or refractory chronic lymphocytic leukaemia. Combination therapy with rituximab enhanced activity in preclinical models. The aim of this study was to assess the safety, pharmacokinetics, and activity of venetoclax in combination with rituximab.
Methods
Adult patients with relapsed or refractory chronic lymphocytic leukaemia (according to the 2008 Modified International Workshop on CLL guidelines) or small lymphocytic lymphoma were eligible for this phase 1b, dose-escalation trial. The primary outcomes were to assess the safety profile, to determine the maximum tolerated dose, and to establish the recommended phase 2 dose of venetoclax when given in combination with rituximab. Secondary outcomes were to assess the pharmacokinetic profile and analyse efficacy, including overall response, duration of response, and time to tumour progression. Minimal residual disease was a protocol-specified exploratory objective. Central review of the endpoints was not done. Venetoclax was dosed daily using a stepwise escalation to target doses (200–600 mg) and then monthly rituximab commenced (375 mg/m2 in month 1 and 500 mg/m2 in months 2–6). Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for adverse events version 4.0. Protocol-guided drug cessation was allowed for patients who achieved complete response (including complete response with incomplete marrow recovery) or negative bone marrow minimal residual disease. Analyses were done per protocol for all patients who commenced drug and included all patients who received at least one dose of venetoclax. Data were pooled across dose cohorts. Patients are still receiving therapy and follow-up is ongoing. The trial is registered at ClinicalTrials.gov, number NCT01682616.
Findings
Between Aug 6, 2012, and May 28, 2014, we enrolled 49 patients. Common grade 1–2 toxicities included upper respiratory tract infections (in 28 [57%] of 49 patients), diarrhoea (27 [55%]), and nausea (25 [51%]). Grade 3–4 adverse events occurred in 37 (76%) of 49 patients; most common were neutropenia (26 [53%]), thrombocytopenia (eight [16%]), anaemia (seven [14%]), febrile neutropenia (six [12%]), and leucopenia (six [12%]). The most common serious adverse events were pyrexia (six [12%]), febrile neutropenia (five [10%]), lower respiratory tract infection, and pneumonia (each three [6%]). Clinical tumour lysis syndrome occurred in two patients (resulting in one death) who initiated venetoclax at 50 mg. After enhancing tumour lysis syndrome prophylaxis measures and commencing venetoclax at 20 mg, clinical tumour lysis syndrome did not occur. The maximum tolerated dose was not identified; the recommended phase 2 dose of venetoclax in combination with rituximab was 400 mg. Overall, 42 (86%) of 49 patients achieved a response, including a complete response in 25 (51%) of 49 patients. 2 year estimates for progression-free survival and ongoing response were 82% (95% CI 66–91) and 89% (95% CI 72–96), respectively. Negative marrow minimal residual disease was attained in 20 (80%) of 25 complete responders and 28 (57%) of 49 patients overall. 13 responders ceased all therapy; among these all 11 minimal residual disease-negative responders remain progression-free off therapy. Two with minimal residual disease-positive complete response progressed after 24 months off therapy and re-attained response after re-initiation of venetoclax.
Interpretation
A substantial proportion of patients achieved an overall response with the combination of venetoclax and rituximab including 25 (51%) of 49 patients who achieved a complete response and 28 (57%) of 49 patients who achieved negative marrow minimal residual disease with acceptable safety. The depth and durability of responses observed with the combination offers an attractive potential treatment option for patients with relapsed or refractory chronic lymphocytic leukaemia and could allow some patients to maintain response after discontinuing therapy, a strategy that warrants further investigation in randomised studies.
Introduction
Members of the BCL2 protein family are important regulators of intrinsic apoptosis and contribute to tumour survival and therapy resistance in many cancers.1,2 BH3-mimetic BCL2 inhibitors, which bind BCL2 via the molecular site used by physiological pro-apoptotic molecules, are active against chronic lymphocytic leukaemia as single agents.3–6 Venetoclax is the first selective, potent BCL2 inhibitor.7 Monotherapy induces rapid reduction in the disease burden of chronic lymphocytic leukaemia and a high overall response of about 80% and complete response of 6–20% in patients with relapsed or refractory chronic lymphocytic leukaemia or small lymphocytic lymphoma, including disease harbouring chromosome 17p deletions (del[17p]).3,5
Research in context.
Evidence before this study
Based on preclinical data, combination therapies have the potential to enhance the activity of novel agents in the treatment of patients with relapsed or refractory chronic lymphocytic leukaemia. We searched PubMed for clinical trial reports published up to Aug 15, 2016, to identify new agents used to treat relapsed or refractory chronic lymphocytic leukaemia, using the terms “chronic lymphocytic leukemia” and “CLL”, as well as the following terms together with “CLL”: “relapsed” and “refractory”. Nearly 1450 articles were identified using these search parameters, with 279 reporting results of clinical trials. Based on recent data published within the past 5 years, several novel agents, including the B-cell receptor signalling inhibitors ibrutinib and idelalisib, and the BCL-2 inhibitor venetoclax, emerged as effective treatment options in this patient population.
Most patients treated with ibrutinib as a single agent and idelalisib in combination with rituximab (anti-CD20 antibody) achieve disease response; however, the number of complete remissions achieved is low. In addition, some patients are unable to tolerate these agents due to adverse events such as nausea, diarrhoea, colitis, elevated alanine transaminase, and elevated aspartate transaminase enzymes. Outcomes are poor for patients who must discontinue treatment due to toxicity or who progress on therapy; median overall survival after ibrutinib discontinuation ranges from 3 months for patients who have Richter’s progression to 18 months for chronic lymphocytic leukaemia progression. To date, phase 2 studies of B-cell receptor signalling inhibitors in combination with other agents have not reported clearly higher complete remission rates than ibrutinib alone and indefinite therapy is still required.
BCL-2 inhibition with venetoclax monotherapy has been shown to result in a high proportion of patients with overall responses and complete remission in two recently published studies: a first-in-human study of patients with relapsed or refractory chronic lymphocytic leukaemia (79% of patients had an overall response with 20% complete remission as determined by investigators) and a phase 2 study of patients with chronic lymphocytic leukaemia that harbours chromosome 17p deletion (79% of patients had an overall response with 8% complete remission, as determined by an independent review committee). In preclinical models of B-cell malignancy, synergy was observed when venetoclax was combined with rituximab.
Added value of the study
This phase 1b study was the first to evaluate venetoclax in any combination for the treatment of patients with relapsed or refractory chronic lymphocytic leukaemia. We hypothesised that venetoclax plus rituximab would be tolerable and increase depth of response. Our results show that venetoclax plus rituximab is highly active with an acceptable safety profile in patients with relapsed or refractory chronic lymphocytic leukaemia. A high proportion of patients achieved an overall response, with 51% of patients achieving a complete remission. Systematic evaluations of serial bone marrow biopsies revealed that 57% of all patients were negative for minimal residual disease. Patients achieving such deep responses can maintain responses without ongoing therapy for periods up to 2 years, and respond to retreatment with venetoclax at progression.
Implications of all available evidence
Venetoclax administration in combination with rituximab is a tolerable and active combination for difficult-to-treat patients with relapsed or refractory chronic lymphocytic leukaemia. The data indicate that minimal residual disease-negative complete remissions are now readily achievable in patients with relapsed chronic lymphocytic leukaemia, and that patients achieving such deep responses need not continue therapy indefinitely. Abbreviated courses of treatment with venetoclax-containing combination regimens should be explored as alternatives to long-term therapy with B-cell receptor signalling inhibitors or venetoclax monotherapy in randomised trials.
Based on preclinical synergy,7 combination therapy might have substantially enhanced clinical activity. Although rituximab has modest single-agent activity in chronic lymphocytic leukaemia and small lymphocytic lymphoma,8,9 when combined with chemotherapy, it improves the proportion of patients who achieve an overall response, progression-free survival, and overall survival.10,11 Combining rituximab with an earlier BCL2 inhibitor, navitoclax, proved tolerable and highly active in patients with relapsed or refractory lymphoid malignancies, 12 including chronic lymphocytic leukaemia,13 establishing the proof-of-principle and safety of this approach.
We hypothesised that combining venetoclax and rituximab would be well tolerated and increase depth and durability of response in patients with relapsed or refractory chronic lymphocytic leukaemia and small lymphocytic lymphoma.
Methods
Study design and participants
The M13-365 study was a phase 1b study of the combination of venetoclax plus rituximab in patients with relapsed or refractory chronic lymphocytic leukaemia or small lymphocytic lymphoma. Patients were enrolled sequentially into five dose-escalation cohorts and an expansion cohort. Adult patients (aged 18 years or older) with chronic lymphocytic leukaemia and small lymphocytic lymphoma were eligible if they had relapsed or refractory disease that required therapy by standard International Workshop for Chronic Lymphocytic Leukaemia (iwCLL) criteria,14 an Eastern Cooperative Oncology Group performance status of 1 or less, adequate marrow (neutrophil count ≥1000 cells per μL with growth factor support allowed, platelets ≥50 000 per μL, and haemoglobin ≥9·0 g/dL), renal function (calculated creatinine clearance >50 mL/min), and hepatic function (aspartate aminotransferase and alanine transaminase ≤3·0 times the upper limit of normal [ULN]; bilirubin ≤1·5 times ULN). There was no minimum estimated life expectancy mandated for study entry, provided all protocol inclusion criteria were met. Primary exclusion criteria included previous allogeneic or autologous stem-cell transplant, uncontrolled autoimmune cytopenias, and other active malignancy within 3 years. PET was not required for study entry. Tumour assessments and bone marrow biopsy were to be performed at screening within 21 days of first dose (bone marrow biopsy obtained within 12 weeks of first dose was accepted in the absence of any intervening therapies). Patients must have previously received no more than three myelosuppressive regimens, must not have received a monoclonal antibody for therapy for chronic lymphocytic leukaemia within 8 weeks before the first dose of venetoclax, other anticancer therapy within 14 days, or anti-leukaemic steroid therapy within 7 days of first dose. All patients provided written informed consent. Detailed eligibility criteria are in the appendix (p 3).
The protocol was approved by each site’s institutional review board, and done according to the Declaration of Helsinki and the International Conference on Harmonization for Good Clinical Practice.
Procedures
Oral venetoclax was given daily using a stepwise weekly escalation schedule. The starting dose and escalation schedule varied by the protocol amendment in place at enrolment; cohorts 1–2 began with 50 mg and subsequent cohorts began with 20 mg (appendix pp 10, 15). Venetoclax dose increased with weekly escalation to designated cohort doses ranging from 200 mg to 600 mg (400 mg for the expansion cohort). The first designated cohort dose was 200 mg and subsequent cohort doses were selected using the continual reassessment method15,16 to evaluate dose-limiting toxicities within the first month of combination therapy (appendix p 5). The number of patients enrolled was dependent on the toxicities observed during the dose escalation portion and up to 20 patients could be enrolled and treated at the recommended phase 2 dose and schedule in the expansion portion of the study.
In all patients, rituximab was initiated (while continuing venetoclax) 1 week after the target dose of venetoclax was achieved. The rituximab schedule varied according to the protocol amendment in place at the time of enrolment. From cohort 3 onwards (n=30), rituximab was dosed on month 1, day 1 (375 mg/m2), and months 2–6 (500 mg/m2) for six infusions in total (appendix p 15; see appendix p 5 for other rituximab schedules used in patients enrolled in earlier cohorts). Following completion of combination therapy, patients continued venetoclax monotherapy until unacceptable toxicity, disease progression, or drug cessation allowed per protocol.
Drug cessation was allowed per protocol and patients remained on study in active follow-up. Initially, venetoclax monotherapy was ceased per protocol when patients met criteria for complete response or complete response with incomplete marrow recovery at week 30, independent of whether they had minimal residual disease in the marrow. In protocol amendments 2 and 3, cessation was no longer mandated. Rather, it became an option when patients met criteria for complete response or complete response with incomplete marrow recovery and were negative for minimal residual disease in the marrow. In protocol amendment 4 (appendix p 10), patients were permitted to re-initiate venetoclax using the weekly escalation and tumour lysis syndrome prophylaxis (after reassessment of disease burden and tumour lysis syndrome risk categorisation) if disease progression meeting iwCLL criteria occurred while off therapy.
Supportive care, anti-infection prophylaxis, and myeloid growth factors were allowed according to institutional guidelines. Tumour lysis syndrome prophylaxis and management were protocol-specified, and beginning with protocol amendment 3 included tumour lysis syndrome risk categorisation based on tumour burden (lymphocytosis and lymphadenopathy) and hospitalisation for the first venetoclax dose and subsequent dose escalations for patients with bulky lymphadenopathy or peripheral absolute lymphocyte count greater than 25 × 109 cells per L or both (appendix p 6). All patients received hydration and a urate reducing agent (appendix p 5). Decisions to reduce the dose of venetoclax were made by the investigator in conjunction with the funder. Venetoclax interruption was required for febrile neutropenia and for grade 4 neutropenia that persisted for more than 1 week despite granulocytecolony stimulating factor (G-CSF) support.
Outcomes
The primary outcomes were to assess the safety profile, to determine the maximum tolerated dose, and to establish the recommended phase 2 dose of venetoclax when given in combination with rituximab. Secondary outcomes were assessment of the pharmacokinetic profile and activity of the combination, including overall response (defined as complete response plus complete response with incomplete marrow recovery plus partial response), duration of response (defined as the number of days from the date of first response [complete response, complete response with incomplete marrow recovery, nodular partial response, or partial response] by either CT scan or physical exam determination to earliest date of tumour progression or death), and time to tumour progression (defined as the number of days from the date of first dose of study drug to the date of tumour progression). An event in the time to tumour progression analyses was defined exclusively as tumour progression and excluded death. Patients who did not achieve response were not included in duration of response analyses. Minimal residual disease status was a protocol-specified exploratory objective. Central review of the endpoints was not done.
Laboratory assessments and adverse event monitoring were used to assess safety. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for adverse events version 4.0. Tumour lysis syndrome was assessed and classified as “laboratory” or “clinical” using established criteria.17 Dose-limiting toxicities were assessed during the first month of combination therapy for dose-escalation purposes. Blood was collected for pharmacokinetic analyses (appendix p 11).
Patients on therapy were assessed as per the schedule shown in the appendix (p 11). Responses were evaluated by investigators using the 2008 iwCLL criteria with the addition of computed tomography or magnetic resonance imaging (appendix p 12), or the International Working Group criteria for patients with small lymphocytic lymphoma (appendix p 13). Marrow minimal residual disease was evaluated at month 7 using local institutional methods (at least four-colour flow cytometry that did not rely on CD20 detection for identifying chronic lymphocytic leukaemia) and a sensitivity of at least 10−4.18,19
Statistical analysis
Data reported herein are as of March 4, 2016. Analyses were done per protocol and included all patients who received at least one dose of venetoclax. Data were pooled across dose cohorts and rituximab regimens, unless specified. Descriptive statistics including medians, IQRs, ranges, and SDs were calculated. Kaplan-Meier methods were used for time-to-event analyses; data for time-to-progression were censored for patients without an event at the time of last assessment or at the time of the data cutoff for patients with assessments after the data cutoff . 95% CIs based on the binomial distribution using the Clopper-Pearson exact method were calculated for overall response. Best marrow minimal residual disease status is reported; 7 month marrow minimal residual disease status was used to evaluate outcomes. Analyses were done with SAS (version 9.4). The study is registered with ClinicalTrials.gov, number NCT01682616.
Role of the funding source
The protocol was designed by the funders (AbbVie and Genentech) and investigators. Clinical data were collected by the investigators; AbbVie confirmed and compiled the data and all authors had access to the complete dataset. Two of the authors (JFS and LLL) wrote the first manuscript drafts. All authors contributed to the final manuscript and vouch for protocol adherence and data accuracy. AbbVie was involved in the decision to develop and submit the report for publication. All authors had access to the raw data. The corresponding author had full access to all of the data and the final responsibility to submit for publication.
Results
Between Aug 6, 2012, and May 28, 2014, 49 patients were enrolled (41 in the dose-escalation cohorts; eight in the safety expansion cohort) and comprised the per-protocol population for analysis. Baseline characteristics are shown in table 1. Three patients discontinued the study before rituximab commencement (one each due to adverse event of tumour lysis syndrome, consent withdrawal, and disease progression [Richter’s transformation]).
Table 1.
Patient characteristics at study entry
| Total (n=49) | |
|---|---|
| Age (years) | |
| Median (range) | 68 (50–88) |
| ≥70 years | 22 (45%) |
|
| |
| Sex | |
| Female | 19 (39%) |
| Male | 30 (61%) |
|
| |
| Diagnosis | |
| Chronic lymphocytic leukaemia | 48 (98%) |
| Small lymphocytic lymphoma | 1 (2%) |
|
| |
| Rai stage III–IV at study entry | 25 (51%) |
|
| |
| Previous therapies | |
| Median (range) | 2 (1–5) |
| Refractory to most recent therapy* | 25 (51%) |
|
| |
| Previous fludarabine-based therapy | 28 (57%) |
| Refractory to fludarabine* | 9 (18%) |
|
| |
| Previous rituximab-containing therapy | 45 (90%) |
| Refractory to rituximab* | 21 (43%) |
|
| |
| ECOG performance status | |
| Grade 0 | 25 (51%) |
| Grade 1 | 24 (49%) |
|
| |
| Peripheral blood lymphocyte count | |
| Median (range) × 109/L | 18·6 (0·3–207·1) |
| Absolute lymphocyte count >5 × 109 per L | 32 (65%) |
|
| |
| Bulky nodes | |
| >5 cm | 22 (45%) |
| >10 cm | 1 (2%) |
|
| |
| Interphase cytogenetic abnormality†‡ | |
| del(17p) | 9/47 (19%) |
| del(11q) | 20/46 (43%) |
| Neither del(17p) nor del(11q)§ | 18/49 (37%) |
| Data missing | 2 |
|
| |
| IGHV gene mutation status‡ | |
| Unmutated | 19/27 (70%) |
| Mutated | 8/27 (30%) |
| Data missing | 22 |
|
| |
| TP53 gene mutation status‡ | |
| Mutated | 10/32 (31%) |
| Unmutated | 22/32 (69%) |
Data are n (%), n/N (%), or median (range). ECOG=Eastern Cooperative Oncology Group.
Refractory includes failure to attain at least a partial response or disease progression by International Workshop for Chronic Lymphocytic Leukaemia criteria within 6 months; disease was refractory to rituximab in 21 patients and fludarabine in nine patients.
Cytogenetic abnormalities by FISH are investigator reported.
Percentages were calculated based on patients with available data.
Includes two patients with missing data for both del(17p) and del(11q) and one with missing data for del(11p) only.
31 (63%) of 49 patients remain active on study (appendix p 16). Median follow-up was 28 months (range <1–42; IQR 19–32) for all 49 enrolled patients and 29 months (range 21–42; IQR 26–40) for the 31 patients who are active on study. 18 patients discontinued the study because of disease progression (n=11), toxicity (n=3), consent withdrawal (n=3), or lost to follow-up (n=1; appendix p 16).
Treatment-emergent adverse events are summarised in table 2. The most common adverse events were grade 1–2 self-limiting gastrointestinal events (diarrhoea in 27 [55%] of 49 patients and nausea in 25 [51%] of 49 patients) and upper respiratory tract infections (28 [57%] of 49 patients). Grade 3–4 adverse events occurred in 37 (76%) of 49 patients. Peripheral blood cytopenias were the most common grade 3–4 adverse events (neutropenia in 26 [53%] of 49 patients, thrombocytopenia in eight [16%] of 49 patients, anaemia in seven [14%] of 49 patients, febrile neutropenia in six [12%] of 49 patients, and leucopenia in six [12%] of 49 patients). There was one grade 3 or higher gastrointestinal toxicity (diarrhoea). Pyrexia (six [12%] of 49, including three patients with grade 1–2 and three patients with grade 3), febrile neutropenia (five [10%] of 49), and lower respiratory tract infection and pneumonia (three [6%] of 49 each) were the most common serious adverse events.
Table 2.
Summary of treatment-emergent adverse events listed by system and class*
| Grade 1–2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Blood and lymphatic system disorders | 4 (8%) | 13 (27%) | 18 (37%) |
| Anaemia | 5 (10%) | 7 (14%) | 0 |
| Anaemia Heinz body | 0 | 1 (2%) | 0 |
| Autoimmune haemolytic anaemia | 0 | 1 (2%) | 0 |
| Febrile neutropenia | 0 | 6 (12%) | 0 |
| Histiocytosis haemophagic† | 0 | 0 | 1 (2%) |
| Immune thrombocytopenic purpura | 1 (2%) | 0 | 1 (2%) |
| Leucopenia | 2 (4%) | 5 (10%) | 1 (2%) |
| Leukocytosis | 0 | 0 | 1 (2%) |
| Lymphocytosis | 0 | 1 (2%) | 0 |
| Lymphopenia | 2 (4%) | 3 (6%) | 0 |
| Neutropenia | 1 (2%) | 11 (22%) | 15 (31%) |
| Thrombocytopenia | 3 (6%) | 3 (6%) | 5 (10%) |
|
| |||
| Endocrine disorders | 0 | 1 (2%) | 0 |
| Inappropriate antidiuretic hormone secretion | 0 | 1 (2%) | 0 |
|
| |||
| Gastrointestinal disorders | 42 (86%) | 3 (6%) | 0 |
| Abdominal pain | 5 (10%) | 0 | 0 |
| Constipation | 8 (16%) | 0 | 0 |
| Diarrhoea | 27 (55%) | 1 (2%) | 0 |
| Dyspepsia | 7 (14%) | 0 | 0 |
| Inguinal hernia | 0 | 1 (2%) | 0 |
| Mouth ulceration | 2 (4%) | 1 (2%) | 0 |
| Nausea | 25 (51%) | 0 | 0 |
| Vomiting | 10 (20%) | 0 | 0 |
|
| |||
| General disorders and administrative site conditions | 32 (65%) | 3 (6%) | 0 |
| Chills | 5 (10%) | 0 | 0 |
| Fatigue | 18 (37%) | 0 | 0 |
| Oedema peripheral | 8 (16%) | 0 | 0 |
| Pyrexia | 16 (33%)‡ | 3 (6%) | 0 |
|
| |||
| Hepatobiliary disorders | 1 (2%) | 1 (2%) | 0 |
| Hyperbilirubinaemia | 1 (2%) | 1 (2%) | 0 |
|
| |||
| Infections and infestations | 32 (65%) | 8 (16%) | 0 |
| Bronchitis | 5 (10%) | 0 | 0 |
| Clostridium difficile colitis | 0 | 1 (2%) | 0 |
| Cystitis | 0 | 1 (2%) | 0 |
| Haemophilus infection | 1 (2%) | 1 (2%) | 0 |
| Influenza | 2 (4%) | 1 (2%) | 0 |
| Lower respiratory tract infection | 2 (4%) | 3 (6%) | 0 |
| Parainfluenza virus infection | 0 | 1 (2%) | 0 |
| Pneumonia | 5 (10%) | 3 (6%) | 0 |
| Rotavirus infection | 1 (2%) | 1 (2%) | 0 |
| Sinusitis | 8 (16%) | 0 | 0 |
| Upper respiratory tract infection | 28 (57%) | 0 | 0 |
| Urinary tract infection | 10 (20%) | 0 | 0 |
|
| |||
| Injury and procedural complications | 13 (27%) | 3 (6%) | 0 |
| Contusion | 6 (12%) | 0 | 0 |
| Infusion related reaction | 5 (10%) | 2 (4%) | 0 |
| Muscle injury | 0 | 1 (2%) | 0 |
|
| |||
| Investigations | 9 (18%) | 7 (14%) | 2 (4%) |
| Alanine aminotransferase increased | 1 (2%) | 1 (2%) | 0 |
| Aspartate aminotransferase increased | 3 (6%) | 2 (4%) | 0 |
| Gamma-glutamyl transferase increased | 0 | 1 (2%) | 0 |
| Neutrophil count decreased | 1 (2%) | 1 (2%) | 2 (4%) |
| Weight increased | 1 (2%) | 1 (2%) | 0 |
| White blood cell count decreased 1 | (2%) | 1 (2%) | 0 |
|
| |||
| Metabolism and nutrition disorders | 24 (49%) | 9 (18%) | 1 (2%) |
| Decreased appetite | 8 (16%) | 0 | 0 |
| Dehydration | 2 (4%) | 1 (2%) | 0 |
| Fluid overload | 7 (14%) | 0 | 0 |
| Hyperglycaemia | 2 (4%) | 2 (4%) | 0 |
| Hyperkalaemia | 5 (10%) | 1 (2%) | 0 |
| Hyperuricaemia | 2 (4%) | 0 | 1 (2%) |
| Hypokalaemia | 5 (10%) | 1 (2%) | 0 |
| Hypomagnesaemia | 8 (16%) | 0 | 0 |
| Hyponatraemia | 0 | 1 (2%) | 0 |
| Hypophosphataemia | 5 (10%) | 2 (4%) | 0 |
| Tumour lysis syndrome | 0 | 2 (4%) | 0 |
|
| |||
| Musculoskeletal and connective tissue disorders | 27 (55%) | 2 (4%) | 0 |
| Arthralgia | 7 (14%) | 0 | 0 |
| Osteoarthritis | 1 (2%) | 1 (2%) | 0 |
| Rheumatoid arthritis | 0 | 1 (2%) | 0 |
|
| |||
| Neoplasms benign, malignant, and unspecified (including cysts and polyps) | 6 (12%) | 6 (12%) | 0 |
| Basal cell carcinoma | 2 (4%) | 1 (2%) | 0 |
| Fibrous histiocytoma | 0 | 1 (2%) | 0 |
| Lung adenocarcinoma | 0 | 1 (2%) | 0 |
| Malignant neoplasm progression | 1 (2%) | 2 (4%) | 0 |
| Squamous cell carcinoma of skin | 2 (4%) | 2 (4%) | 0 |
|
| |||
| Nervous system disorders | 26 (53%) | 0 | 0 |
| Dizziness | 7 (14%) | 0 | 0 |
| Headache | 16 (33%) | 0 | 0 |
|
| |||
| Respiratory, thoracic, and mediastinal disorders | 32 (65%) | 2 (4%) | 0 |
| Atelectasis | 0 | 1 (2%) | 0 |
| Cough | 20 (41%) | 0 | 0 |
| Nasal congestion | 9 (18%) | 0 | 0 |
| Oropharyngeal pain | 6 (12%) | 1 (2%) | 0 |
|
| |||
| Skin and subcutaneous tissue disorders | 29 (59%) | 0 | 0 |
| Pruritus | 9 (18%) | 0 | 0 |
| Rash | 6 (12%) | 0 | 0 |
|
| |||
| Vascular disorders | 8 (16%) | 2 (4%) | 0 |
| Hypertension | 2 (4%) | 2 (4%) | 0 |
Data are the total number of events; rows are listed by specific causes. Three treatment-emergent deaths were noted: one due to metabolism and nutrition disorders (reported as hyperkalaemia and tumour lysis syndrome), and two due to neoplasms (one reported as Richter’s syndrome, the other reported as malignant neoplasm progression).
Treatment-emergent adverse events in all 49 patients were reported for grade 1–2 occurring in 10% or more patients or more, and all grade 3–5 events.
Episode of fevers, rigors, and cytopenias triggered by rituximab infusion, with haemophagocytosis a prominent feature on bone marrow biopsy at the time.
Pyrexia was a grade 1–2 serious adverse event in three patients.
Overall, 26 (53%) of 49 patients experienced grade 3–4 neutropenia (15 had grade 4), of whom five (9%) entered the study on G-CSF. 24 of the 26 patients with grade 3–4 neutropenia received G-CSF support on at least one occasion and 11 of these also had at least one dose modification (venetoclax reduction or interruption in ten patients and rituximab interruption in one additional patient). The median time to first grade 3–4 neutropenia was 51 days (range 1–537). Development of grade 3–4 neutropenia was not associated with the number of previous lines of treatment or with previous exposure to fludarabine (appendix p 22).
26 (53%) of 49 patients received some form of anti-infection prophylactic medication during venetoclax treatment (against yeast in three, Pneumocystis jirovecii pneumonia in 14, herpes simplex virus in 24, and bacteria in seven). Eight (16%) of 49 patients experienced grade 3 infections (one patient experienced five episodes and one patient experienced two episodes), with pneumonia and lower respiratory tract infections most common (three patients each). The cause of five of the 13 grade 3 infection events were identified as Haemophilus influenzae, influenza A, parainfluenza 3, Clostridium difficile, and rotavirus, with the latter four infections coinciding with grade 3–4 neutropenia. No grade 4 or higher infections were observed and no patients experienced grade 3–4 opportunistic infections. The exposure-adjusted rate for grade 3 or higher infections was 0·7 per 100 patient-months.
A maximum tolerated dose was not identified; the recommended phase 2 dose of venetoclax in combination with rituximab was 400 mg. Dose-limiting toxicities occurred in seven patients (most commonly neutropenia with and without fever, n=4 [one febrile neutropenia, one neutropenia, and two reported as neutrophil count decreased]) across the dose-escalation cohorts (appendix p 19), without evident relationship to venetoclax dose. Two dose-limiting toxicities (haemophagocytosis triggered by rituximab infusion and thrombocytopenia) occurred during the dose-limiting toxicity assessment period (the first 28 days of combination therapy) and were considered for escalation decisions.
Five patients had tumour lysis syndrome; two cases were clinical, including one fatality that occurred on day 1 after the first 50 mg venetoclax dose and was considered to be a dose-limiting toxicity (appendix p 18). The protocol was then amended to implement changes, including a lower venetoclax starting dose of 20 mg and modified tumour lysis syndrome prophylaxis. Among 32 patients subsequently treated, no clinical tumour lysis syndrome per standard criteria17 was observed. After the amendment, one patient with an absolute lymphocyte count of 216 × 109 per L at screening but no bulky adenopathy experienced a grade 3 adverse event related to tumour lysis syndrome (hyperphosphataemia and hypocalcaemia that required intervention, but did not meet Howard criteria17 for tumour lysis syndrome). Two patients had biochemical evidence of laboratory-only tumour lysis syndrome (asymptomatic hyperphosphataemia and hypocalcaemia in both). Post-amendment events resolved without dose modification.
20 (41%) of 49 patients had dose reductions most commonly due to cytopenias (nine [18%]) and gastrointestinal events (four [6%]; appendix p 19). Three deaths occurred; one due to metabolism and nutrition disorders (reported as hyperkalaemia and tumour lysis syndrome), and two due to neoplasms (one reported as Richter’s syndrome, the other reported as malignant neoplasm progression).
The median time from dosing to maximum concentration in serum (Tmax) and the mean maximum concentration in serum (Cmax) for venetoclax were 6 h (range 4–8) and 1·93 μg/mL (SD 0·69), respectively, for patients receiving 400 mg venetoclax with rituximab. The log-transformed dose-normalised mean Cmax and area under the plasma concentration–time curve for venetoclax were not affected by rituximab (appendix pp 17, 18).3
Disease burden in the peripheral blood, lymph nodes, and marrow was rapidly and substantially reduced by treatment (appendix p 23). 42 (86%) of 49 patients achieved an overall response including 25 (51%) of 49 with complete response or complete response with incomplete marrow recovery; responses were observed across all doses (table 3) and prognostic subgroups (figure 1). The median time to first response was 2·9 months (range 0·7–15·7; IQR 2·79–3·02) and complete responses were attained after a median of 9·2 months (range 6·4–28·6; IQR 6·77–14·04; appendix p 24). At the completion of combination therapy, 11 patients had achieved best response of complete response. An additional 14 patients subsequently achieved complete response or complete response with incomplete marrow recovery after a median of 7 additional months (range 1·5–22; IQR 3·5–16·4) of venetoclax monotherapy (at the end of combination therapy, two of these patients had stable disease and 12 patients had achieved a partial response).
Table 3.
Response by dose level
| 200 mg (n=6) | 300 mg (n=10) | 400 mg (n=8) | 500 mg (n=7) | 600 mg (n=10) | Safety expansion, 400 mg (n=8) | Total (n=49) | |
|---|---|---|---|---|---|---|---|
| Overall response | 6 (100%) | 8 (80%) | 6 (75%) | 6 (86%) | 9 (90%) | 7 (88%) | 42 (86%), 95% CI 73–94 |
| Complete response or complete response with incomplete marrow recovery | 2 (33%) | 5 (50%) | 6 (75%) | 4 (57%) | 5 (50%) | 3 (38%) | 25 (51%), 95% CI 36–66 |
| Nodular partial response or partial response | 4 (67%) | 3 (30%) | 0 | 2 (29%) | 4 (40%) | 4 (50%) | 17 (35%), 95% CI 22–50 |
| Stable disease | 0 | 1 (10%) | 1 (13%) | 1 (14%) | 1 (10%) | 0 | 4 (8%) |
| Progressive disease | 0 | 1 (10%) | 0 | 0 | 0 | 1 (13%) | 2 (4%) |
| Not assessed* | 0 | 0 | 1 (13%) | 0 | 0 | 0 | 1 (2%) |
| Negative marrow minimal residual disease response | 3 (50%) | 3 (30%) | 6 (75%) | 4 (57%) | 7 (70%) | 5 (62%) | 28 (57%) |
Data are n (%) or n (%), 95% CI.
Patient had fatal tumour lysis syndrome on day 1.
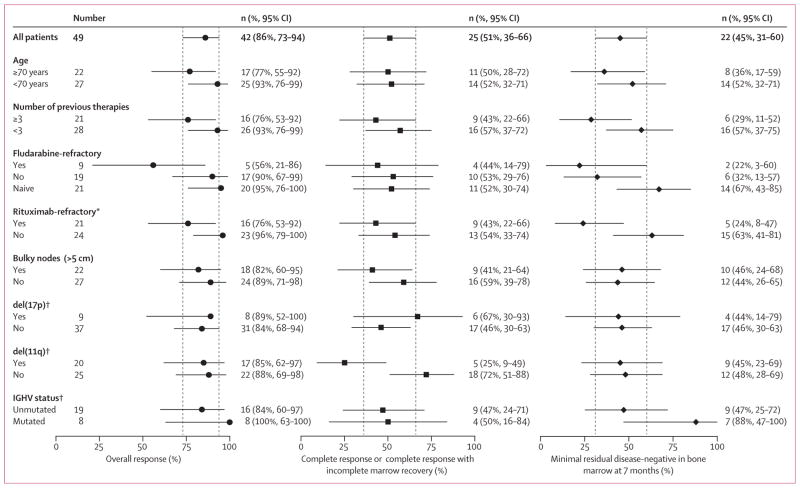
Figure 1. Overall response, complete response, and bone marrow negative minimal residual disease response.
Patients treated with venetoclax and rituximab achieved deep responses in the overall population (n=49) and in exploratory analyses of adverse prognosis subgroups. Left, overall responses; middle, complete response or complete response with incomplete marrow recovery; right, 7-month bone marrow minimal residual disease-negative. Medians (95% CIs) are shown; dashed lines indicate the 95% CIs for the overall population. *Four patients did not receive prior rituximab; see table 1. †Data not available for all patients in the FISH subgroups; see table 1.
42 patients had minimal residual disease evaluated in their bone marrow; 28 (67%) of 42 of those tested (28 [57%] of the whole cohort) had no minimal residual disease, including 20 (80%) of 25 patients with complete response or complete response with incomplete marrow recovery and eight (47%) of 17 patients with partial response as best response (all had histological complete marrow clearance). Negative marrow minimal residual disease was achieved within 7 months of therapy in 22 (79%) of 28 patients who achieved this depth of response (appendix p 24).
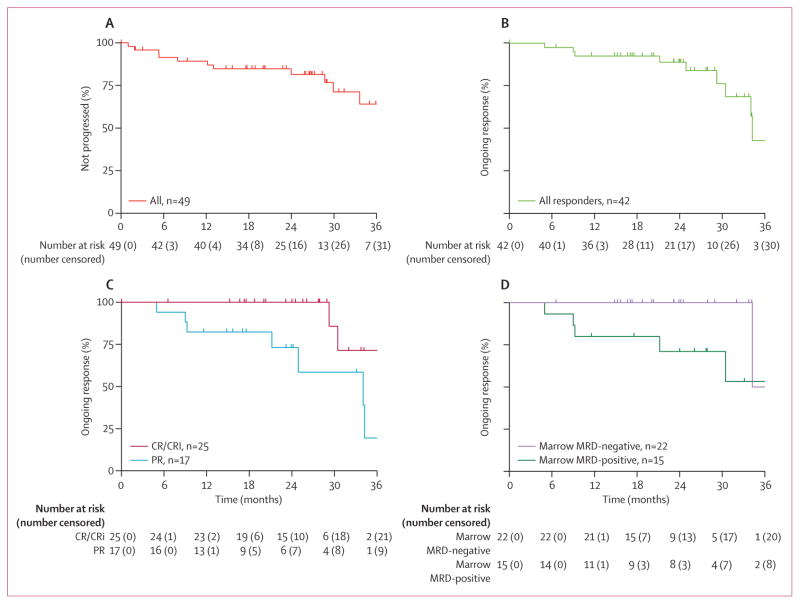
Disease progression on therapy occurred in 11 patients. Six of these events were in patients with progressive chronic lymphocytic leukaemia (between 12 and 37 months) after achieving a partial response. The remaining five patients progressed with Richter’s transformation, all no more than 9 months on study (median three previous therapies [range 2–4]); noting that PET scanning was not mandated before study entry. The median time to progression for the overall population has not been reached, with 82% (95% CI 66–91) estimated to be progression-free at 2 years (figure 2A). The 2-year actuarial overall survival and progression-free survival are reported in the appendix (p 25).
Figure 2. Durability of response.
(A) Time to progression for all 49 enrolled patients (13 patients had events). (B) Duration of response for all 42 responders (nine patients had events). (C) Duration of response by overall response category: complete response or complete response with incomplete marrow recovery (CR/CRi; n=25, two patients had events) versus partial response (PR; n=17, seven patients had events). (D) Duration of response in patients negative for minimal residual disease (MRD) at 7 months (n=22, one patient had an event) versus patients who had minimal residual disease at 7 months (n=15, five patients had events) status. Tick marks on the curves indicate patients censored for each outcome measure.
The 2-year estimate for ongoing response was 89% (95% CI 72–96; figure 2B). Deeper responses, either complete response or complete response with incomplete marrow recovery (figure 2C) or negative minimal residual disease at 7 months (figure 2D) appear more durable than other responses. The 2-year estimate for ongoing complete response or complete response with incomplete marrow recovery or overall response with negative minimal residual disease at 7 months was 100% for both (95% CI 100–100), whereas this was 73% (95% CI 42–89) for ongoing nodular partial response or partial response and 71% (95% CI 39–88) for 7-month minimal residual disease-positive overall response.
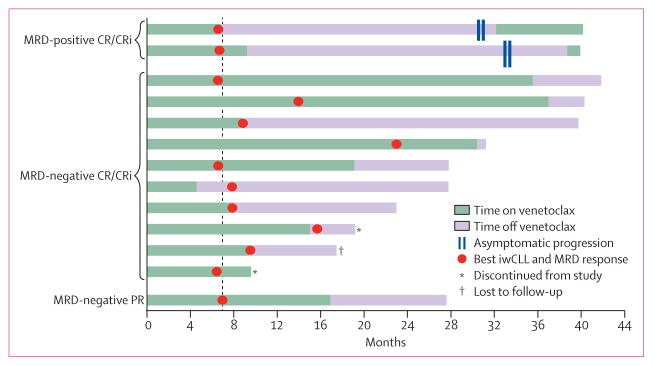
13 (26%) of 49 patients who had a deep response (complete response or complete response with incomplete marrow recovery or partial response with negative minimal residual disease) ceased venetoclax therapy after a median treatment duration of 10 months (range 4–37; IQR 8·75–19·1; figure 3). 12 patients ceased venetoclax after achieving complete response or complete response with incomplete marrow recovery. Of these, the 7-month marrow minimal residual disease status was: nine negative for minimal residual disease, one positive for minimal residual disease, one not assessed at month 7 and was positive for minimal residual disease at month 12, and one whose minimal disease status remained unknown at month 7 due to technical difficulties and who was found to be negative for minimal residual disease at month 15. One patient discontinued venetoclax treatment after they were found to be negative for minimal residual disease and achieved a partial response (residual 1·8 cm adenopathy). Two of the patients with minimal residual disease-negative complete response or complete response with incomplete marrow recovery discontinued the study without progressing, one because of an adverse event and one because they withdrew consent. Of the ten patients currently still being followed, all eight who were minimal residual disease-negative remain in ongoing remission after a median of 9·7 months (range 0·82–31; IQR 5·55–17·05) off venetoclax. The two patients with minimal residual disease-positive complete response or complete response with incomplete marrow recovery who ceased venetoclax developed clinical asymptomatic chronic lymphocytic leukaemia disease progression after 24 months off therapy (figure 3; increasing peripheral blood minimal residual disease and absolute lymphocyte count). Venetoclax was reinitiated in both patients (appendix p 15) with tumour lysis syndrome prophylaxis. Absolute lymphocyte count and peripheral blood minimal residual disease promptly decreased during the venetoclax escalation. Both achieved partial response with venetoclax alone and are continuing on therapy. Retreatment has been well tolerated with no grade 3 or higher toxicities.
Figure 3. Drug withdrawal and retreatment.
Time on and off venetoclax for 13 patients with deep response who ceased venetoclax therapy: two with marrow minimal residual disease-positive complete response or complete response with incomplete marrow recovery, ten with marrow minimal residual disease-negative complete response or complete response with incomplete marrow recovery, one with marrow minimal residual disease-negative partial response (PR). Ten patients remain in follow-up. Two (asterisks) discontinued the trial and one (dagger) was lost to follow-up without progressing. Plot shows time on venetoclax therapy (green) and time off venetoclax therapy (purple). The time of best deep response is annotated with a circle and includes International Workshop for Chronic Lymphocytic Leukaemia response and minimal residual disease status. Two patients stopped therapy for adverse events and at their next scheduled assessment achieved their best response so remained off therapy. The vertical dashed line at month 7 represents the first protocol-specified marrow assessment of minimal residual disease in the marrow. For the two patients with disease who progressed while off therapy, the timepoint of progression is indicated with hash marks and reinitiation of venetoclax is shown in green. Both have re-achieved an overall response. MRD=minimal residual disease. CR/CRi=complete response or complete response with incomplete marrow recovery. iwCLL=International Workshop for Chronic Lymphocytic Leukaemia response.
Discussion
Preclinical data strongly suggested synergistic cyototoxic activity of venetoclax in combination with anti-CD20 monoclonal antibodies, such as rituximab.7,20 However, the addition of rituximab to myelosuppressive chemotherapy regimens increases cytopenias and infections.21 In the current study, venetoclax plus rituximab did not appear to substantially alter the pharmacokinetics or the adverse event profile observed in other studies with monotherapy in chronic lymphocytic leukaemia. Grade 3–4 adverse events were observed at similar frequencies as previously reported with venetoclax monotherapy,4,5 without substantially greater incidence or severity of neutropenia or increased proportion of patients with a severe infection. Rituximab could be safely added without compromising the delivery of venetoclax at the recommended phase 2 dose of 400 mg daily. Overall, 25 (51%) of 49 patients achieved complete response after combination treatment with 28 (57%) of 49 of all patients attaining negative marrow minimal residual disease. The proportions of patients with overall responses and who were negative for minimal residual disease are unprecedented in the context of relapsed or refractory chronic lymphocytic leukaemia.3,5 High overall responses observed in this study reproduce what has been reported for venetoclax monotherapy.3,5
Venetoclax dosing in this study was done with a gradual stepwise dose-escalation, similar to the escalation in venetoclax chronic lymphocytic leukaemia monotherapy studies;3,5 rituximab was initiated after the target dose of venetoclax was achieved. After one death due to tumour lysis syndrome during the dose escalation cohorts in this study, the venetoclax dosing strategy was modified to include a more gradual escalation, patients were assigned to tumour lysis syndrome risk categories based on tumour burden, and other prophylactic and monitoring measures were implemented. No subsequent clinical tumour lysis syndrome events occurred. The introduction of rituximab after venetoclax was shown to be tolerable. Other sequencing strategies could be considered, but their safety would have to be specifically established before considering clinical use. Deep responses, including negative minimal residual disease, have been associated with longer progression-free survival and overall survival following chemoimmunotherapy.22,23 The deep responses attained with the combination of venetoclax and rituximab were durable and the high number of patients with negative minimal residual disease in this study are promising and longer follow-up will be required to assess whether this translates into prolonged survival. Durability of responses was greater in patients with either complete response or complete response with incomplete marrow recovery or negative minimal residual disease at 7 months emphasising the clinical benefit of pursuing greater degrees of disease reduction with novel therapy combinations.
13 patients who achieved deep responses ceased all therapy. Ten patients remained under observation on study and remissions were maintained for substantial periods off all therapy (median of 9 months progression-free and ongoing). Two patients who had minimal residual disease and complete response or complete response with incomplete marrow recovery progressed after 24 months off therapy; both have achieved partial response after reinitiation of venetoclax and remain on therapy. Although other recently approved agents in chronic lymphocytic leukaemia such as B-cell receptor kinase inhibitors are highly effective, it appears that ongoing therapy is required to maintain response. By contrast, the results of the current study suggest a novel therapeutic paradigm of time-limited targeted therapy for patients attaining deep responses, allowing freedom from the burdens and costs of prolonged therapy, while removing the potential clonal selection pressure of continuous drug exposure. Longer follow-up of patients maintaining remission off therapy along with consistent, safe, and effective reintroduction in the setting of disease recurrence will be necessary to firmly establish the use of this approach.
These results demonstrate that venetoclax and rituximab can be safely delivered without compromise of venetoclax dose, offering a treatment strategy that yields high rates of deep responses that can be maintained for substantial time periods when patients cease therapy.
Acknowledgments
AbbVie and Genentech provided financial support for the M13-365 study and participated in the design, study conduct, analysis, and interpretation of data, as well as the writing, review, and approval of the manuscript. Venetoclax (ABT-199/GDC-0199) is being developed in a collaboration between AbbVie and Genentech. Statistical programming support was provided by Ruiling Zhang and Srikanth Birru (both are employees of AbbVie). Editorial and technical support was provided by Evidence Scientific Solutions (Philadelphia, PA, USA), which was funded by AbbVie, Inc. Special thanks to the patients and their families, study coordinators, and support staff.
Funding AbbVie Inc and Genentech Inc.
Footnotes
See Online for appendix
Contributors
JFS, MSD, AWR, EC, SYK, RAH, and GBG were responsible for the conception and design of the study. JFS, SM, DMB, MYC, JB, MAA, AWB, STR, CST, TJK, and AWR were responsible for the provision of patients and patient care. All authors did the data analysis, data collection, and data interpretation. JFS and LLL wrote the first draft and all authors contributed to and approved the final manuscript.
Declaration of interests
JFS reports research grant funding from AbbVie and being a consultant and advisory board member for AbbVie, Roche, and Genentech. SM reports research grant funding from Genentech, AbbVie, Pharmacyclics, Novartis, Gilead, Acerta, and Xeme, and advisory board consulting and lecturing for Genentech, Pharmacyclics, and Gilead. DMB reports being a consultant for Gilead. MYC reports research grant funding from AbbVie, being an advisory board member and consultant for AbbVie, Novartis, Pharmacyclics, and Gilead, and being a member of the speakers’ bureau for Gilead. JB reports research grant funding from Gilead, Pharmacyclics, and AbbVie, and advisory board consulting for AbbVie, Gilead, Janssen, and Pharmacyclics. MSD is a consultant for AbbVie, Genentech, Infinity, Gilead, Janssen, TG Therapeutics, Celgene, and Pharmacyclics, and reports research grant funding from Infinity, TG Therapeutics, Genentech, and Pharmacyclics. MAA reports research grant funding from AbbVie, and is an employee of Walter and Eliza Hall Institute of Medical Research, which receives milestone payments related to venetoclax. CST is a consultant for AbbVie and Roche, and receives speaking fees from Roche. BP, SKA, WM, MZ, LLL, MD, EC, MV, SYK, RAH, and GBG are employees of AbbVie and could own stock. AWR reports research grant funding from AbbVie, Beigene, Janssen, Servier, Amgen, and Genentech, and is an employee of Walter and Eliza Hall Institute of Medical Research, which receives milestone payments related to venetoclax. AWB, STR, and TJK declare no competing interests.
References
- 1.Adams JM, Cory S. The Bcl-2 apoptotic switch in cancer development and therapy. Oncogene. 2007;26:1324–37. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 3.Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med. 2016;374:311–22. doi: 10.1056/NEJMoa1513257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts AW, Seymour JF, Brown JR, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–96. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stilgenbauer S, Eichhorst B, Schetelig J, et al. Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open-label, phase 2 study. Lancet Oncol. 2016;17:768–78. doi: 10.1016/S1470-2045(16)30019-5. [DOI] [PubMed] [Google Scholar]
- 6.Wilson WH, O’Connor OA, Czuczman MS, et al. Navitoclax, a targeted high-affinity inhibitor of BCL-2, in lymphoid malignancies: a phase 1 dose-escalation study of safety, pharmacokinetics, pharmacodynamics, and antitumour activity. Lancet Oncol. 2010;11:1149–59. doi: 10.1016/S1470-2045(10)70261-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Souers AJ, Leverson JD, Boghaert ER, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–08. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 8.Byrd JC, Murphy T, Howard RS, et al. Rituximab using a thrice weekly dosing schedule in B-cell chronic lymphocytic leukemia and small lymphocytic lymphoma demonstrates clinical activity and acceptable toxicity. J Clin Oncol. 2001;19:2153–64. doi: 10.1200/JCO.2001.19.8.2153. [DOI] [PubMed] [Google Scholar]
- 9.O’Brien SM, Kantarjian H, Thomas DA, et al. Rituximab dose-escalation trial in chronic lymphocytic leukemia. J Clin Oncol. 2001;19:2165–70. doi: 10.1200/JCO.2001.19.8.2165. [DOI] [PubMed] [Google Scholar]
- 10.Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376:1164–74. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 11.Robak T, Dmoszynska A, Solal-Céligny P, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol. 2010;28:1756–65. doi: 10.1200/JCO.2009.26.4556. [DOI] [PubMed] [Google Scholar]
- 12.Roberts AW, Advani RH, Kahl BS, et al. Phase 1 study of the safety, pharmacokinetics, and antitumour activity of the BCL2 inhibitor navitoclax in combination with rituximab in patients with relapsed or refractory CD20+ lymphoid malignancies. Br J Haematol. 2015;170:669–78. doi: 10.1111/bjh.13487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kipps TJ, Eradat H, Grosicki S, et al. A phase 2 study of the BH3 mimetic BCL2 inhibitor navitoclax (ABT-263) with or without rituximab, in previously untreated B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 2015;56:2826–33. doi: 10.3109/10428194.2015.1030638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hallek M, Cheson BD, Catovsky D, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–56. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O’Quigley J, Pepe M, Fisher L. Continual reassessment method: a practical design for phase I clinical trials in cancer. Biometrics. 1990;46:33–48. [PubMed] [Google Scholar]
- 16.Neuenschwander B, Branson M, Gsponer T. Critical aspects of the Bayesian approach to phase I cancer trials. Stat Med. 2008;27:2420–39. doi: 10.1002/sim.3230. [DOI] [PubMed] [Google Scholar]
- 17.Howard SC, Jones DP, Pui CH. The tumor lysis syndrome. N Engl J Med. 2011;364:1844–54. doi: 10.1056/NEJMra0904569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawstron AC, Villamor N, Ritgen M, et al. International standardized approach for flow cytometric residual disease monitoring in chronic lymphocytic leukaemia. Leukemia. 2007;21:956–64. doi: 10.1038/sj.leu.2404584. [DOI] [PubMed] [Google Scholar]
- 19.Kovacs G, Robrecht S, Fink AM, et al. Minimal residual disease assessment improves prediction of outcome in patients with chronic lymphocytic leukemia (CLL) who achieve partial response: comprehensive analysis of two phase III studies of the German CLL Study Group. J Clin Oncol. 2016;34:3758–65. doi: 10.1200/JCO.2016.67.1305. [DOI] [PubMed] [Google Scholar]
- 20.Byrd JC, Peterson BL, Morrison VA, et al. Randomized phase 2 study of fludarabine with concurrent versus sequential treatment with rituximab in symptomatic, untreated patients with B-cell chronic lymphocytic leukemia: results from Cancer and Leukemia Group B 9712 (CALGB 9712) Blood. 2003;101:6–14. doi: 10.1182/blood-2002-04-1258. [DOI] [PubMed] [Google Scholar]
- 21.Wierda W, O’Brien S, Faderl S, et al. A retrospective comparison of three sequential groups of patients with recurrent/refractory chronic lymphocytic leukemia treated with fludarabine-based regimens. Cancer. 2006;106:337–45. doi: 10.1002/cncr.21554. [DOI] [PubMed] [Google Scholar]
- 22.Badoux XC, Keating MJ, Wang X, et al. Fludarabine, cyclophosphamide, and rituximab chemoimmunotherapy is highly effective treatment for relapsed patients with CLL. Blood. 2011;117:3016–24. doi: 10.1182/blood-2010-08-304683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamanna N, Jurcic JG, Noy A, et al. Sequential therapy with fludarabine, high-dose cyclophosphamide, and rituximab in previously untreated patients with chronic lymphocytic leukemia produces high-quality responses: molecular remissions predict for durable complete responses. J Clin Oncol. 2009;27:491–507. doi: 10.1200/JCO.2008.16.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]