Abstract
Background
The interhemispheric competition hypothesis attributes the distribution of selective attention to a balance of mutual inhibition between homotopic, interhemispheric connections in parietal cortex [1,2]. In support of this hypothesis, repetitive inhibitory TMS over right parietal cortex in healthy individuals rapidly induces interhemispheric imbalance in cortical activity that spreads beyond the site of stimulation [3]. Behaviorally, the impacts of inhibitory rTMS may be long delayed from the onset of stimulation, as much as 30 minutes [4,5].
Objective
In this study, we examine the temporal dynamics of inhibitory rTMS on cortical network integrity that supports sustained visual attention.
Methods
Healthy individuals received 15 min of 1Hz offline, inhibitory rTMS (or sham) over left parietal cortex, and then immediately engaged in a bilateral visual tracking task while we recorded brain activity with fMRI. We computed functional connectivity (FC) between three nodes of the attention network engaged by visual tracking: the intraparietal sulcus (IPS), frontal eye fields (FEF) and human MT+ (hMT+).
Results
FC immediately and significantly decreased between the stimulation site (left IPS) and all other regions, then recovered to normal levels within 30 minutes. rTMS increased FC between left and right FEF at approximately 36 min following stimulation, and between sites in the unstimulated hemisphere approximately 48 min after stimulation.
Conclusions
These findings demonstrate large-scale changes in cortical organization following inhibitory rTMS. The immediate impact of rTMS on connectivity to the stimulation site dovetails with the putative role of interhemispheric balance for bilateral visual sustained attention. The delayed, compensatory increases in functional connectivity have implications for models of dynamic reorganization in networks supporting spatial and nonspatial selective attention, and compensatory mechanisms within these networks that may be stabilized in chronic stroke.
Keywords: visual attention, functional connectivity, intraparietal sulcus, rTMS, fMRI
Introduction
The interhemispheric competition hypothesis is the leading proposal for cortical control of visual spatial attention. This hypothesis attributes the spatial distribution of selective attention to a balance of mutual inhibition between homotopic, interhemispheric connections in parietal cortex [6,7,1]. Disruptions in interhemispheric balance are increasingly linked to impaired visual attention in patients, such as hemispatial neglect and extinction [8]. Indeed, more severe hemispatial neglect is correlated with increased interhemispheric imbalances in cortical activity and decreased interhemispheric functional connectivity in the parietal components of the dorsal attention network [9–11]. In healthy individuals, asymmetric mutual inhibition is believed to underlie pseudoneglect and pseudoextinction [12–14].
Interhemispheric imbalance is implicated specifically in the ability to attend to rapidly changing competing sensory inputs, such as in visual tracking. Bilateral visual tracking is a task that requires the coordination of spatiotemporal attention to selected targets while suppressing signals for irrelevant distractors in both visual fields [15,2,4]. In healthy individuals, the load and spatial attention demands imposed by visual tracking are correlated with neural activity in the superior parietal lobule (specifically in the intraparietal sulcus, IPS) and the frontal eye fields (FEF) [16,17], the same neural circuits implicated by the interhemispheric competition hypothesis [1,8,18].
Two causal studies link the interhemispheric balance in parietal cortex to tracking abilities. Chronic right parietal patients with impaired visual tracking can improve their tracking scores following 1Hz offline inhibitory rTMS to the healthy left parietal cortex [4]. And in healthy individuals, rTMS over left IPS impairs tracking, with the severity of impaired performance correlated with the impact of rTMS on BOLD activity [3]. In addition to changes in performance overall, both of these studies reported a delay in the peak rTMS impact on behavior and cortical activity. In the right parietal patients, the peak improvement in tracking performance was observed 30 minutes after stimulation. In the healthy individuals, the correlation between individual subject performance and the magnitude of the univariate BOLD response emerged 25 minutes following stimulation. Delayed impact of rTMS is difficult to explain with current models of TMS intervention but is not without precedent, and has been linked to long-term depression/potentiation mechanisms [19,20].
In this study, we investigate changes in functional connectivity during visual tracking, specifically measuring the influence of rTMS interventions on interhemispheric relationships. Functional connectivity is a metric of network integrity that reveals the structure of neural pathways independent of the magnitude of BOLD activity levels [21,22]. Using the protocol of Plow et al. [3], we examine the time dependent changes in cortico-cortical functional connectivity altered by rTMS, and compare its impact to visual tracking scores in healthy individuals. In this analysis we examine connectivity in a simplified model of three brain regions in the dorsal attention network engaged by visual tracking: the intraparietal sulcus (IPS), frontal eye fields (FEF) and human MT+ (hMT+). These three brain regions were selected because they are linked to tracking abilities in healthy individuals [2,17,3] and the interhemispheric weights within these regions predicts individual bias in spatial allocation of attention [23]. Importantly, understanding the dynamic nature of connectivity within these mechanisms, and how their dynamic interplay changes across time when disrupted, could help determine the extent to which these neural circuits may be amenable to therapeutic interventions to promote long-term plasticity.
Methods
Participants
Nine healthy subjects (mean age ± SD 27.72 ± 5.99 years, 7 males) participated in the experiment. Two subjects were excluded from analysis due to gradient artifacts in the MR data. All subjects had normal or corrected-to-normal vision. All participants met all TMS [24] and MRI screening criteria and provided written informed consent in accordance with the Institutional Review Board of the Beth Israel Deaconess Medical Center, Boston, MA.
Stimuli & Procedure
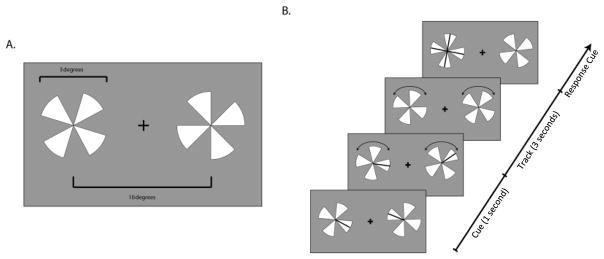
Data used in this analysis was previously published in Plow et al. [3]. Briefly, subjects participated in a total of two experimental sessions in which they engaged in a visual tracking task following offline, inhibitory rTMS or sham (conducted on 2 separate days in a counterbalanced order). In this task, subjects monitored high-contrast pairs of four-spokes-pinwheels displayed on either side of a central fixation. At the beginning of each trial two spokes, one for each pinwheel, were flickered briefly, indicating the targets. Both pinwheels then rotated at a fixed rate for 3 seconds. At the end of the trial all spokes on the target pinwheel appeared as probes and subjects were asked to indicate on a four alternative forced choice procedure which spoke was the target (top, bottom, right or left, Figure 1) [2,25]. Subjects were briefly tested before each fMRI session where we psychophysically measured the speed threshold at which they could report the target spoke at 85% accuracy, using a staircase procedure. We then used that same individually estimated threshold speed throughout the entire fMRI session.
Figure 1. Bilateral Visual Tracking Task.
A. Two pinwheels were presented. Cartoon of one frame of the task, where sizes of the pinwheels and distances are reported. B. One spoke for each pinwheel was cued (a black line flashed briefly). Once the black lines disappeared, the pinwheels began to rotate in randomly different directions. Once they stopped, one pinwheel was highlighted, and using a four-alternative forced-choice procedure, subjects indicated which spoke was the one cued at the beginning of the trial. The side of the target was unpredictable across trials.
Stimuli were generated in MATLAB using the Psychophysics Toolbox [26,27] and displayed on a PC laptop with a 17″ monitor screen projected with a rear-view mirror attached to the head coil in the scanner. Subjects completed a total of 48 trials of tracking over four scans (twelve per scan) with the trials randomly and evenly split across the two hemifields. Data for tracking in the left and right hemifields were pooled in the subsequent functional connectivity analysis.
rTMS
Transcranial magnetic stimulation was applied using a MagStim device (MagStim, Whitland, Wales, UK) with a 70-mm figure-of-eight coil guided by neuronavigation (BrainsightTM, Rogue Research Inc., Montreal, QC, Canada). Low-frequency 1 Hz rTMS was applied for 15-min at 75% of the maximum stimulator output, targeting the left intraparietal sulcus (IPS), identified using frameless stereotaxic image guidance co-registered with the individual subject’s anatomical images [2]. Specifically, all subjects participated in a previous TMS experiment where we anatomically and functionally defined the left posterior IPS individually (average Talairach (mean ± SD): X = −23.37 ± 5.24; Y = −67.60 ± 4.25 and Z = 52.88 ± 2.47 mm) [2]. The TMS coil was held with the handle pointing posteriorly at an angle of 45° to the inter-hemispheric fissure, at an orientation that aligned it perpendicular to the left IPS. For the sham condition we placed the edge of the coil at an angle perpendicular to the head, while stimulation was delivered at the same intensity as in the rTMS session. The fMRI data collection was initiated within four minutes from completion of rTMS/sham.
fMRI procedure and analysis
Brain imaging was conducted with a whole-body 3T Phillips scanner equipped a standard birdcage headcoil. We acquired high-resolution T1-weighted MPRAGE images for each subject that reconstructed the individual structural brain anatomy (1 × 1 × 1.2 mm saggital images with no gap between slices, 170 slices). Subjects participated in four gradient-echo planar imaging (EPI) scans (TR = 2 s, TE = 55 ms, flip angle = 90°, TE = 30 ms, FOV = 23 cm and 96 × 96 matrix, final voxel size of 2.4 × 2.4 × 4 mm and a gap of 0.5mm, 20 axial slices acquired interleaved) in which 366 volumes were collected in each 12:12 min scan.
fMRI data collection was initiated within four minutes following the rTMS/sham. The first three EPI scans were collected successively, and the fourth and final scan was completed following a twelve minute interval during which subjects relaxed and viewed a popular cinema movie.
All functional scans were corrected for slice acquisition timing and for movement within and across the volumes. We removed the linear trends and slow temporal fluctuations (3 cycles per scan high pass filter) from each voxel. Functional data was then registered to standardized Talaraich space [28] and minimally spatially smoothed with a 3 mm FWHM filter.
We computed functional connectivity between the left and right corresponding regions for three bilateral regions of interest (ROIs): the intraparietal sulcus (IPS), human middle temporal complex (hMT+) and the frontal eye fields (FEF). These regions were identified in individual subjects, using a contrast of visual tracking versus fixation, and included only voxels significantly correlated with visual tracking with a Bonferroni family-wise error correction of p < .0001). Scans that were collected following TMS and sham conditions were included in the localization mapping. The ROIs were further localized in individual subjects using the following anatomical landmarks: the dorsal ridge of the intraparietal sulcus (IPS), the fundus of the descending branch of the inferior occipital sulcus, and the anterior wall and fundus of the precentral sulcus (FEF). All ROIs were constructed as a sphere with 10mm radius centered on the peak voxels of activation and anatomical landmark. The group mean Talairach coordinates for the centroid of each region (right and left hemispheres) are shown in Table 1.
Table 1.
Group mean Talairach X, Y and Z coordinates for the centroid of each region (left and right hemispheres).
| Left hemisphere | Right hemisphere | |||||
|---|---|---|---|---|---|---|
| X | Y | Z | X | Y | Z | |
|
|
||||||
| Intraparietal sulcus | −25.6 (6.2) | −60.9 (4.0) | 53.9 (5.1) | 22.3 (4.8) | −62.6 (2.1) | 52.4 (2.4) |
| human MT+ | −46.7 (2.1) | −66.7 (3.6) | 3.7 (1.8) | 43.7 (2.4) | −65.6 (0.5) | 5.1 (3.3) |
| Frontal eye field | −28.9 (3.4) | −10.1 (3.0) | 52.0 (2.7) | 31.3 (2.5) | −11.1 (4.6) | 53.1 (2.9) |
Functional connectivity was computed as the Pearson’s r correlation coefficient for the z-score normalized timeseries of BOLD activity from each ROI. The ROI timeseries were constructed from the normalized (z-scored) BOLD for the entire 12 minute scans, which included intervals of visual tracking interspersed with fixation (rest). Correlation coefficients were Fisher-z transformed and the effect of rTMS on functional connectivity was estimated as FC = FCTMS − FCSHAM. This was computed separately for each 12 min scan then averaged across subjects.
Statistical significance was assessed by a bootstrap Monte Carlo procedure that computed the expected difference in correlation coefficients expected by chance. Bootstrap timeseries were constructed from timelocked pairs of timepoints selected from two ROIs, sampled with replacement from the TMS and sham conditions. This process was repeated 1,000 times to generate a distribution of samples that test the null hypothesis that the TMS and sham functional connectivity scores originate from the same population distribution. Those correlation coefficients with scores that exceeded two standard deviations of the simulated null population distribution were deemed statistically significant.
The recovery from the impact of TMS on functional connectivity was estimated by computing the slope of a linear fit to the FC scores from the first three sequential scans. The slope and intercept of those fits gives an estimate of the impact of the rTMS on the individual subjects. We also compared these metrics of rTMS impact to individual subject tracking scores to determine the strength of the relationship between the rTMS induced reorganization of cortical connectivity and behavior.
Results
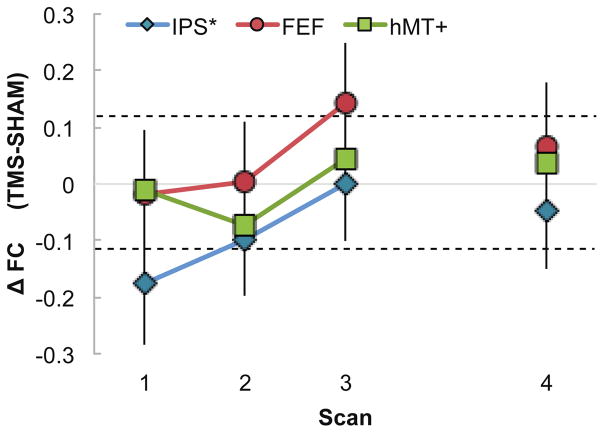
Figures 2 and 3 show how functional connectivity evolved in the approximately one hour following stimulation over the left IPS, with connectivity scores normalized by connectivity following sham. Positive scores that exceed the dashed line indicate significantly stronger functional connectivity following rTMS as compared to sham, while negative scores (below the dashed line) indicate rTMS significantly reduced functional connectivity. Significance was assessed via a bootstrap procedure (see methods).
Figure 2. Interhemispheric FC across time.
Functional connectivity difference between homotopic areas in the left (stimulated) and right hemisphere (Sham FC was subtracted from TMS FC). Colored symbols indicate the three areas: IPS (Intraparietal Sulcus, blue diamonds), FEF (Frontal Eye Field, red circles) and hMT+ (human Middle Temporal Area, green squares). While the first three runs were collected in close succession (Scans 1, 2 and 3 on the x-axis), scan 4 was collected approximately 48 minutes from the end of stimulation, following an intermediate run during which subjects rested while watching a video clip of a popular movie. Values above the dashed lines indicate statistically significant difference (between Sham and TMS), determined using a the bootstrap Monte Carlo procedure. FC between left and right IPS significantly decreased during Scan 1 and slowly recovered in Scan 2. FC between left and right FEF significantly increased during Scan 3, over 30 minutes after the end of stimulation.
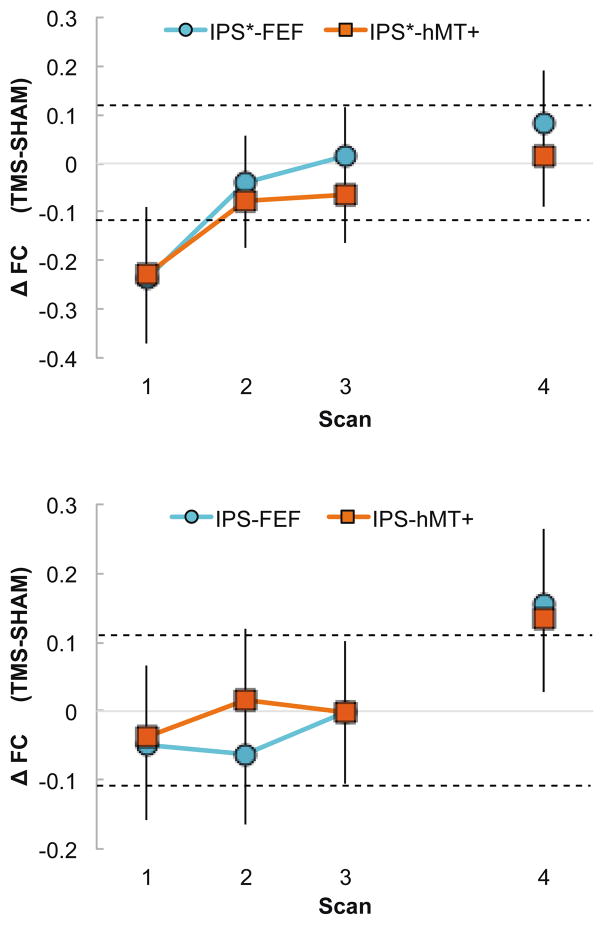
Figure 3. Intrahemispheric FC across time.
Intrahemispheric impact of rTMS on FC within the left hemisphere (a. stimulated) and the right hemisphere (b. unstimulated). FC scores reflect the change in functional connectivity in the TMS condition as compared to Sham. A) FC between the left IPS and the left FEF (blue circles) and between the left IPS and left hMT+ (red squares) immediately and significantly decreased during Scan 1 (dotted line indicates significant difference) and recovered starting from Scan 2, 3 and 4. B) FC between the right IPS and the right FEF (blue circles) as well as between the right IPS and right hMT+ (red squares) significantly increased around 48 minutes after the end of stimulation, during Scan 4.
Figure 2 shows functional connectivity between the right and left regions of interest (interhemispheric homotopic connections). Functional connectivity between the stimulation site and the homologous right IPS decreased significantly immediately following stimulation (scan 1), and recovered to within normal levels by approximately 30 minutes following the rTMS (scan 3). This timecourse of normalized functional connectivity is consistent with the duration over which rTMS influences behavioral performance in healthy individuals as reported in a wide range of cognitive and attention tasks [29,30].
We also examined functional connectivity in two regions distal and functionally connected to the stimulation site. The influence of rTMS on the functional connectivity between left and right FEF was delayed and brief, with increased connectivity approximately 36 min following stimulation that returned to levels consistent with sham within 60 min. The late increase in functional connectivity in the FEF was unexpected, however it is consistent with a previous report of delayed impact of rTMS on the bilateral FEF BOLD activity following theta burst stimulation over right FEF [31] and may relate to potential compensatory effects observed in Plow et al. [3] rTMS had no impact on interhemispheric connectivity between the left and right hMT+.
We next looked at the impact of rTMS on inter-regional functional connectivity, and found that the influence depended on hemisphere and time. In the stimulated left hemisphere, FEF and hMT+ connectivity to the stimulation site (left IPS) decreased immediately following rTMS, then recovered to normal levels (Figure 3a). This pattern of connectivity has the same timing as the IPS interhemispheric connections.
In the unstimulated right hemisphere, FEF and hMT+ connectivity increased only in the fourth and final scan, approximately 50 minutes following stimulation and after a period of free-viewing the movie. These delayed and remote effects of rTMS on functional connectivity in the unstimulated hemisphere were unanticipated.
In previous reports using the same paradigm, the extent to which rTMS shifts the cortical imbalance in neural activity between homotopic regions in parietal cortex is linked to individual subject tracking scores [3]. We therefore investigated a possible brain-behavior link in functional connectivity changes over time by examining the correlation between recovery in functional connectivity and visual tracking.
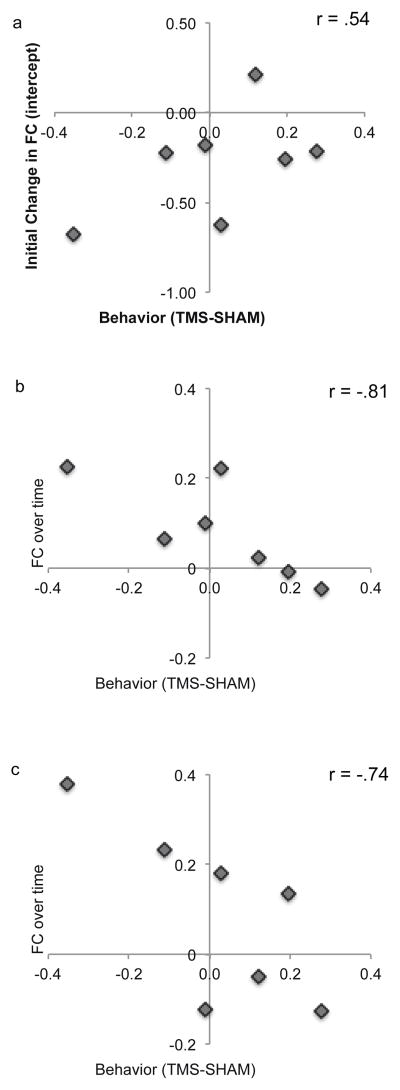
To quantify the temporal dynamics of functional connectivity recovery following rTMS, we modeled the functional connectivity scores using linear regression for the first 40 min following stimulation. Whereas the initial impact of rTMS (as indicated by the intercept) had a marginal relationship with functional connectivity to the stimulation site (r = .54, p>.05), it was the recovery of the functional connectivity that most strongly correlated with tracking performance (Table 2 and Figure 4). Dynamic changes in the left and right IPS functional connectivity, the left and right hMT+ connectivity, and the connections between the stimulated IPS and hMT+ were all significantly correlated with tracking scores (r = −.78, r = −.87, and r = −.74, respectively; all p < .05; Table 2). Those subjects that experienced the greatest impact of rTMS on functional connectivity also made the most tracking errors. We found no relationship between the impact of rTMS on functional connectivity in the unstimulated hemisphere.
Table 2. Brain-behavior correlation scores.
Correlations between rTMS induced changes in visual tracking in each visual field (contra-stimulation, ipsi-stimulation, and overall performance) and dynamics of functional connectivity, broken down by initial impact (intercept) and dynamic recovery (slope).
| Intercepts | Slopes | ||||||
|---|---|---|---|---|---|---|---|
| Contra | Ipsi | Overall | Contra | Ipsi | Overall | ||
| Homotopic | IPS*-IPS | r=.38 | r=.64 | r=.54 | r=−.67 | r=−.81† | r=−.78† |
| FEF-FEF | r=.30 | r=.00 | r=.16 | r=−.53 | r=−.35 | r=−.46 | |
| hMT+-hMT+ | r=.72 | r=.57 | r=.66 | r=−.87† | r=−.78† | r=−.87† | |
| Stimulated Hemisphere | IPS*-FEF | r=.44 | r=.36 | r=.42 | r=−.56 | r=−.50 | r=−.55 |
| IPS*-hMT+ | r=.61 | r=.57 | r=.62 | r=−.74† | r=−.67 | r=−.74† | |
| Unstimulated Hemisphere | IPS-FEF | r=−.12 | r=−.36 | r=−.25 | r=−.24 | r=−.08 | r=−.17 |
| IPS-hMT+ | r=−.10 | r=.14 | r=.02 | r=−.68 | r=−.63 | r=−.65 | |
Significance at p < .05 (two-tailed).
Figure 4. Relationship between tracking performance and impact of rTMS.
(A) Change in tracking accuracy vs the initial impact of rTMS on homotopic IPS connectivity. Behavioral scores reflect the change in visual tracking following rTMS as compared to sham. Initial impact of rTMS on functional connectivity is computed as the intercept of the best linear fit for functional connectivity following rTMS vs sham (see methods). Those subjects most impacted by rTMS (on this connection, and many others) also have the most recovery in FC over time. (B) Change in tracking accuracy vs recovery (slope) of the homotopic IPS functional connectivity. These are the subjects where the rTMS really changed FC. (C) Change in tracking accuracy vs recovery (slope) of the left (stimulated) hemisphere IPS-hMT+ functional connectivity.
Discussion
In a normally functioning system, visual orienting is controlled by bilateral cortical mechanisms that direct attention to contralateral space, and the functional integrity of this attention network is critical for healthy visual attention. In this study, we measured the temporal dynamics of functional connectivity for 50 minutes during a visual tracking task, with and without 1 Hz repetitive TMS. This study is motivated by the observation that inhibitory TMS over the IPS impairs bilateral visual tracking in healthy individuals [2] and improves bilateral tracking when applied to the healthy (left) parietal cortex in right parietal patients [4]. Both of these findings are consistent with the interhemispheric competition hypothesis for bilateral control of visual orienting.
Inhibitory rTMS over left IPS induced widespread changes in the functional integrity of the dorsal attention network. These changes occurred both inter- and intra-hemispherically, with immediate and delayed timing, respectively. The immediate changes manifested as a decreased connectivity between the homotopic regions at the IPS stimulation site, and decreased inter-regional connectivity in stimulated hemisphere (FEF and hMT+ to the stimulation site). These changes in connectivity normalized within 36 minutes following stimulation. Delayed effects of the rTMS included increasing connectivity between homologous regions of the FEF approximately 36 min following stimulation, and increased interregional connectivity very late in the unstimulated hemisphere.
That rTMS can induce rapid changes in cortical activity directly under the site of stimulation and downstream from the stimulation site is well known [32]. Low frequency inhibitory rTMS decreases metabolic activity local to the stimulation site as measured by PET, and is often accompanied by compensatory increases in neural activity in functionally connected regions in the normal population [3,33–36].
Whereas at least four studies have documented large-scale shifts in interhemispheric balance within the dorsal attention network following rTMS to the parietal cortex [4,3,23,37], ours is the first to consider changes in functional connectivity during a sustained attention task. Functional connectivity measures are dominated by low frequency fluctuations on the order of .1 Hz or slower [38,39], reflecting phase-locked covariations of functionally connected regions. Our study demonstrates rTMS will disrupt the spontaneous connections between these regions for time extending beyond the period of stimulation, creating a wave of compensatory activity that alters neural coupling for extended durations remote from the region of stimulation.
The timing of recovery we observed for connectivity to the stimulation site, approximately 36 minutes, is consistent with the observed interval of impaired behavior on a wide range of attention tasks following rTMS [29]. We were also able to link the impact and recovery of functional connectivity to the stimulation site with individual subject tracking errors, establishing a causal relationship between the impact of rTMS on functional connectivity and the ability of individual subjects to engage in visual tracking.
The rTMS stimulation also induced delayed increases in functional connectivity distal to the stimulation site. We observed an increase in connectivity in the unstimulated right hemisphere, between the right IPS and right FEF, and right IPS and right hMT+. These findings were unpredicted, but not without precedent. Agosta et al., [4] observed peak improvement in tracking performance 20–30 minutes following inhibitory rTMS applied to the healthy, contralesional parietal cortex in stroke patients. Likewise, Nyffler et al. [40] observed delayed peak impact of theta burst rTMS over FEF on saccadic latency. A subsequent study using the same paradigm documented delayed (20–35 min) peak decrease BOLD activity in the stimulated FEF and, to a lesser extent, in functionally connected regions [31]. The timescale of these changes is consistent with that of long-term depression/potentiation [19,41] and there is some evidence that the magnitude of these delayed effects may depend on the level of exertion engaged during stimulation [42].
That downstream changes in functional connectivity would be observed within the dorsal attention network is not surprising. The intraparietal sulcus and frontal eye field are strongly interconnected via the superior longitudinal fasciculi [43–45] and both are important for the implementation of voluntary directed attention to task-relevant features [see 46 for a recent review]. Focal TMS over IPS and FEF both disrupt selective attention in visual search tasks, albeit with different timing, in which targets are defined by conjunctions of features and thus require feature binding [47–49].
Posterior parietal cortex and the FEF are also linked to sustained awareness of sensory events, and to maintaining sustained cortical modulations in sensory cortex during encoding of task-relevant events [50–52]. Single pulses of TMS over posterior parietal cortex decreases the perceptual sensitivity for targets that are being monitored in the contralateral visual field [53] and shortens dominance intervals during binocular rivalry [54]. Patients with damage to posterior parietal cortex experience the perceptual fading of visual events more quickly that typical individuals [55]. The implication is that connectivity between posterior parietal cortex, frontal eye fields and sensory cortex are essential for sustained salience of attended features [56]. Our study demonstrates that acute disruptions to connectivity between these regions are followed by an interval dynamic stabilization within this highly connected network.
These findings also have implications for stroke models that implicate imbalanced network activity, as damage to one hemisphere leads to disinhibition in the competing, unaffected hemisphere [57]. This up-regulated activity in the unaffected hemisphere thus leads to excessive inhibition in the affected hemisphere. That is, not only does the lesioned hemisphere suffer the frank damage from the stroke, it is also further suppressed by “unabated” inhibition from the unaffected hemisphere. The improvement seen in the patients specifically implicates the ability of rTMS to restructure the balance of cortical activity through functional connectivity. In our study we found that it is the interplay and strength of inter- and intra-hemispheric connections among the cortical areas within the dorsal attention network that determine the efficiency of the sustained attention system [58].
Highlights.
Offline inhibitory rTMS over the left intraparietal sulcus has a local, immediate and brief impact on the network integrity to functionally connected cortical regions in the dorsal attention network
Those individuals with more disruptions in local functional connectivity are most impaired on sustained visual attention tasks, which dovetails with the hypothesized role of interhemispheric balance for bilateral visual sustained attention
rTMS over left intraparietal sulcus creates remote and delayed increases in interregional functional connectivity in distal brain sites, which may have implications for models of compensatory mechanisms that may be stabilized in chronic stroke
Acknowledgments
Ela B. Plow was supported by NIH career development award (1K01HD069504). This study was supported in part by the “Harvard Catalyst” and the Harvard-Thorndike Clinical Research Center at Beth Israel Deaconess Medical Center (UL1 RR025758 – NCRR – NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Lorella Battelli was supported by the Autonomous Province of Trento, Call “Grandi Progetti 2012,” project “Characterizing and improving brain mechanisms of attention – ATTEND”. We thank A. Bifone for comments on an earlier version of the manuscript.
Footnotes
Conflict of interest statement: The authors declare no competing financial interests
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kinsbourne M. Hemineglect and hemisphere rivalry. Adv Neurol. 1977;18:41–9. [PubMed] [Google Scholar]
- 2.Battelli L, Alvarez GA, Carlson T, Pascual-Leone A. The role of the parietal lobe in visual extinction studied with transcranial magnetic stimulation. J Cogn Neurosci. 2009;21:1946–55. doi: 10.1162/jocn.2008.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plow EB, Cattaneo Z, Carlson TA, Alvarez GA, Pascual-Leone A, Battelli L. The compensatory dynamic of inter-hemispheric interactions in visuospatial attention revealed using rTMS and fMRI. Front Hum Neurosci. 2014;8:226. doi: 10.3389/fnhum.2014.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Agosta S, Herpich F, Miceli G, Ferraro F, Battelli L. Contralesional rTMS relieves visual extinction in chronic stroke. Neuropsychologia. 2014;62:269–76. doi: 10.1016/j.neuropsychologia.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 5.Hubl D, Nyffeler T, Wurtz P, Chaves S, Pflugshaupt T, Lüthi M, et al. Time course of blood oxygenation level–dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience. 2008;151:921–8. doi: 10.1016/j.neuroscience.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 6.Mesulam M-M. Spatial attention and neglect: parietal, frontal and cingulate contributions to the mental representation and attentional targeting of salient extrapersonal events. Philos Trans R Soc Lond B Biol Sci. 1999;354:1325–46. doi: 10.1098/rstb.1999.0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reuter-Lorenz P, Kinsbourne M, Moscovitch M. Hemispheric control of spatial attention. Brain Cogn. 1990;12:240–66. doi: 10.1016/0278-2626(90)90018-j. [DOI] [PubMed] [Google Scholar]
- 8.Corbetta M, Shulman GL. Spatial Neglect and Attention Networks. Annu Rev Neurosci. 2011;34:569–99. doi: 10.1146/annurev-neuro-061010-113731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbetta M, Kincade MJ, Lewis C, Snyder AZ, Sapir A. Neural basis and recovery of spatial attention deficits in spatial neglect. Nat Neurosci. 2005;8:1603–10. doi: 10.1038/nn1574. [DOI] [PubMed] [Google Scholar]
- 10.Baldassarre A, Ramsey L, Hacker CL, Callejas A, Astafiev SV, Metcalf NV, et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain. 2014;137:3267–83. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of Functional Connectivity in Frontoparietal Networks Underlies Behavioral Deficits in Spatial Neglect. Neuron. 2007;53:905–18. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Benwell CSY, Harvey M, Thut G. On the neural origin of pseudoneglect: EEG-correlates of shifts in line bisection performance with manipulation of line length. NeuroImage. 2014;86:370–80. doi: 10.1016/j.neuroimage.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodbourn PT, Holcombe AO. “Pseudoextinction”: Asymmetries in simultaneous attentional selection. J Exp Psychol Hum Percept Perform. 2015;41:364–84. doi: 10.1037/a0038734. [DOI] [PubMed] [Google Scholar]
- 14.Jewell G, McCourt ME. Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia. 2000;38:93–110. doi: 10.1016/s0028-3932(99)00045-7. [DOI] [PubMed] [Google Scholar]
- 15.Battelli L, Cavanagh P, Intriligator J, Tramo MJ, Hénaff M-A, Michèl F, et al. Unilateral right parietal damage leads to bilateral deficit for high-level motion. Neuron. 2001;32:985–95. doi: 10.1016/s0896-6273(01)00536-0. [DOI] [PubMed] [Google Scholar]
- 16.Culham J. Timing in the visual hierarchy. Trends Cogn Sci. 1998;2:473. doi: 10.1016/s1364-6613(98)01264-9. [DOI] [PubMed] [Google Scholar]
- 17.Shim WM, Alvarez GA, Vickery TJ, Jiang YV. The Number of Attentional Foci and Their Precision Are Dissociated in the Posterior Parietal Cortex. Cereb Cortex. 2009:bhp197. doi: 10.1093/cercor/bhp197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Somers DC, Sheremata SL. Attention maps in the brain. Wiley Interdiscip Rev Cogn Sci. 2013;4:327–40. doi: 10.1002/wcs.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hallett M. Transcranial Magnetic Stimulation: A Primer. Neuron. 2007;55:187–99. doi: 10.1016/j.neuron.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 20.Thickbroom GW. Transcranial magnetic stimulation and synaptic plasticity: experimental framework and human models. Exp Brain Res. 2007;180:583–93. doi: 10.1007/s00221-007-0991-3. [DOI] [PubMed] [Google Scholar]
- 21.Biswal BB, Ulmer JL. Blind source separation of multiple signal sources of fMRI data sets using independent component analysis. J Comput Assist Tomogr. 1999;23:265–71. doi: 10.1097/00004728-199903000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- 23.Szczepanski SM, Kastner S. Shifting Attentional Priorities: Control of Spatial Attention through Hemispheric Competition. J Neurosci. 2013;33:5411–21. doi: 10.1523/JNEUROSCI.4089-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rossi S, Hallett M, Rossini PM, Pascual-Leone A. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson TA, Alvarez GA, Cavanagh P. Quadrantic deficit reveals anatomical constraints on selection. Proc Natl Acad Sci. 2007;104:13496–500. doi: 10.1073/pnas.0702685104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brainard DH. The Psychophysics Toolbox. Spat Vis. 1997;10:433–6. [PubMed] [Google Scholar]
- 27.Pelli DG. The VideoToolbox software for visual psychophysics: transforming numbers into movies. Spat Vis. 1997;10:437–42. [PubMed] [Google Scholar]
- 28.Talairach J, Tournoux P. 3-Dimensional proportional system: an approach to cerebral imaging. Thieme; 1988. Co-planar stereotaxic atlas of the human brain. [Google Scholar]
- 29.Hilgetag CC, Théoret H, Pascual-Leone A. Enhanced visual spatial attention ipsilateral to rTMS-induced ‘virtual lesions’ of human parietal cortex. Nat Neurosci. 2001;4:953–7. doi: 10.1038/nn0901-953. [DOI] [PubMed] [Google Scholar]
- 30.Marshall TR, O’Shea J, Jensen O, Bergmann TO. Frontal eye fields control attentional modulation of alpha and gamma oscillations in contralateral occipitoparietal cortex. J Neurosci Off J Soc Neurosci. 2015;35:1638–47. doi: 10.1523/JNEUROSCI.3116-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubl D, Nyffeler T, Wurtz P, Chaves S, Pflugshaupt T, Lüthi M, et al. Time course of blood oxygenation level–dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience. 2008;151:921–8. doi: 10.1016/j.neuroscience.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 32.Bergmann TO, Karabanov A, Hartwigsen G, Thielscher A, Siebner HR. Combining non-invasive transcranial brain stimulation with neuroimaging and electrophysiology: Current approaches and future perspectives. NeuroImage. 2016 doi: 10.1016/j.neuroimage.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 33.Paus T, Jech R, Thompson CJ, Comeau R, Peters T, Evans AC. Transcranial magnetic stimulation during positron emission tomography: a new method for studying connectivity of the human cerebral cortex. J Neurosci. 1997;17:3178–84. doi: 10.1523/JNEUROSCI.17-09-03178.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee TG, D’Esposito M. The Dynamic Nature of Top-Down Signals Originating from Prefrontal Cortex: A Combined fMRI–TMS Study. J Neurosci. 2012;32:15458–66. doi: 10.1523/JNEUROSCI.0627-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bestmann S. The physiological basis of transcranial magnetic stimulation. Trends Cogn Sci. 2008;12:81–3. doi: 10.1016/j.tics.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 36.Grefkes C, Nowak DA, Wang LE, Dafotakis M, Eickhoff SB, Fink GR. Modulating cortical connectivity in stroke patients by rTMS assessed with fMRI and dynamic causal modeling. NeuroImage. 2010;50:233–42. doi: 10.1016/j.neuroimage.2009.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol. 1997;77:3386–90. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- 38.Biswal B, Zerrin Yetkin F, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar mri. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 39.Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. Am J Neuroradiol. 2001;22:1326–33. [PMC free article] [PubMed] [Google Scholar]
- 40.Nyffeler T, Wurtz P, Lüscher H-R, Hess CW, Senn W, Pflugshaupt T, et al. Extending lifetime of plastic changes in the human brain. Eur J Neurosci. 2006;24:2961–6. doi: 10.1111/j.1460-9568.2006.05154.x. [DOI] [PubMed] [Google Scholar]
- 41.Huang Y-Z, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta Burst Stimulation of the Human Motor Cortex. Neuron. 2005;45:201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 42.Todd G, Rogasch NC, Flavel SC, Ridding MC. Voluntary movement and repetitive transcranial magnetic stimulation over human motor cortex. J Appl Physiol. 2009;106:1593–603. doi: 10.1152/japplphysiol.91364.2008. [DOI] [PubMed] [Google Scholar]
- 43.Pestilli F, Yeatman JD, Rokem A, Kay KN, Wandell BA. Evaluation and statistical inference for human connectomes. Nat Methods. 2014;11:1058–63. doi: 10.1038/nmeth.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rushworth MFS, Behrens TEJ, Johansen-Berg H. Connection Patterns Distinguish 3 Regions of Human Parietal Cortex. Cereb Cortex. 2006;16:1418–30. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- 45.Thiebaut de Schotten M, ffytche DH, Bizzi A, Dell’Acqua F, Allin M, Walshe M, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage. 2011;54:49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- 46.Buschman TJ, Kastner S. From Behavior to Neural Dynamics: An Integrated Theory of Attention. Neuron. 2015;88:127–44. doi: 10.1016/j.neuron.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalla R, Muggleton NG, Juan C-H, Cowey A, Walsh V. The timing of the involvement of the frontal eye fields and posterior parietal cortex in visual search. Neuroreport. 2008;19:1067–71. doi: 10.1097/WNR.0b013e328304d9c4. [DOI] [PubMed] [Google Scholar]
- 48.O’Shea J, Muggleton NG, Cowey A, Walsh V. Timing of Target Discrimination in Human Frontal Eye Fields. J Cogn Neurosci. 2004;16:1060–7. doi: 10.1162/0898929041502634. [DOI] [PubMed] [Google Scholar]
- 49.Muggleton NG, Kalla R, Juan C-H, Walsh V. Dissociating the contributions of human frontal eye fields and posterior parietal cortex to visual search. J Neurophysiol. 2011;105:2891–6. doi: 10.1152/jn.01149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ekstrom LB, Roelfsema PR, Arsenault JT, Bonmassar G, Vanduffel W. Bottom-Up Dependent Gating of Frontal Signals in Early Visual Cortex. Science. 2008;321:414–7. doi: 10.1126/science.1153276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-Down Control of Human Visual Cortex by Frontal and Parietal Cortex in Anticipatory Visual Spatial Attention. J Neurosci. 2008;28:10056–61. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blankenburg F, Ruff CC, Bestmann S, Bjoertomt O, Josephs O, Deichmann R, et al. Studying the Role of Human Parietal Cortex in Visuospatial Attention with Concurrent TMS–fMRI. Cereb Cortex. 2010;20:2702–11. doi: 10.1093/cercor/bhq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kanai R, Muggleton NG, Walsh V. TMS Over the Intraparietal Sulcus Induces Perceptual Fading. J Neurophysiol. 2008;100:3343–50. doi: 10.1152/jn.90885.2008. [DOI] [PubMed] [Google Scholar]
- 54.Carmel D, Walsh V, Lavie N, Rees G. Right parietal TMS shortens dominance durations in binocular rivalry. Curr Biol. 2010;20:R799–800. doi: 10.1016/j.cub.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 55.Mennemeier MS, Chatterjee A, Watson RT, Wertman E, Carter LP, Heilman KM. Contributions of the parietal and frontal lobes to sustained attention and habituation. Neuropsychologia. 1994;32:703–16. doi: 10.1016/0028-3932(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 56.Taylor PCJ, Nobre AC, Rushworth MFS. FEF TMS Affects Visual Cortical Activity. Cereb Cortex. 2007;17:391–9. doi: 10.1093/cercor/bhj156. [DOI] [PubMed] [Google Scholar]
- 57.Pelled G, Bergstrom DA, Tierney PL, Conroy RS, Chuang K-H, Yu D, et al. Ipsilateral cortical fMRI responses after peripheral nerve damage in rats reflect increased interneuron activity. Proc Natl Acad Sci. 2009;106:14114–9. doi: 10.1073/pnas.0903153106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Strens LH, Oliviero A, Bloem BR, Gerschlager W, Rothwell JC, Brown P. The effects of subthreshold 1 Hz repetitive TMS on cortico-cortical and interhemispheric coherence. Clin Neurophysiol. 2002;113:1279–85. doi: 10.1016/s1388-2457(02)00151-7. [DOI] [PubMed] [Google Scholar]