Abstract
Patients undergoing maintenance hemodialysis develop both structural and functional cardiovascular abnormalities. Despite improvement of dialysis technology, cardiovascular mortality of this population remains high. The pathophysiological mechanisms of these changes are complex and not well understood. It has been postulated that several non-traditional, uremic-related risk factors, especially the long-term uremic state, which may affect the cardiovascular system. There are many cardiovascular changes that occur in chronic kidney disease including left ventricular hypertrophy, myocardial fibrosis, microvascular disease, accelerated atherosclerosis and arteriosclerosis. These structural and functional changes in patients receiving chronic dialysis make them more susceptible to myocardial ischemia. Hemodialysis itself may adversely affect the cardiovascular system due to non-physiologic fluid removal, leading to hemodynamic instability and initiation of systemic inflammation. In the past decade there has been growing awareness that pathophysiological mechanisms cause cardiovascular dysfunction in patients on chronic dialysis, and there are now pharmacological and non-pharmacological therapies that may improve the poor quality of life and high mortality rate that these patients experience.
Keywords: End stage renal disease, hemodialysis, cardiovascular complications
INTRODUCTION
In the United States there currently are more than 400,000 patients receiving maintenance hemodialysis treatment. Despite recent improvement in dialysis process, patients receiving maintenance dialysis still have high hospitalization rates, poor quality of life, and high mortality. The all-cause mortality of this patient group remains more than 20% a year and is 10 times greater than that of the general population (1). The 5-year survival rate is only about 40% irrespective of the dialysis mode, which is worse than many types of cancer (1, 2). Cardiovascular mortality accounts for 40% of all-cause mortality in this group, and the majority of deaths are due to heart failure, acute myocardial infarction, and fatal arrhythmia (1, 3, 4).
The characteristics of cardiovascular dysfunction observed in dialysis patients are distinct from those noted in the general population. Although traditional cardiovascular risk factors in patients with end stage renal disease (ESRD) are highly prevalent, they play only a partial role on the excessive cardiovascular morbidity and mortality of this population. The paradoxical association between several traditional risk factors, such as body mass index, blood pressure (BP) and serum cholesterol, and mortality have been previously reported (5). Moreover, several studies have failed to demonstrate the benefit of statin therapy on cardiovascular mortality in the dialysis population despite the fact that statin therapy has benefit on cardiovascular survival in patients with chronic kidney disease (CKD) (6–8). Moreover hemodialysis itself has been recognized to be a cause of hemodynamic instability, where intolerance is largely due to the inability to maintain effective circulating volume rather than directly from uremia. We postulate that repeated myocardial micro-injury during maintenance hemodialysis may lead to irreversible cardiac dysfunction and subsequent heart failure and death in some patients.
The objective of this review is to provide: (i) an overview of the pathological changes of the cardiovascular system in ESRD, (ii) a description of the putative pathophysiological mechanisms of hemodialysis-induced myocardial injury and comprehensive overview of the current evidence for this condition and (iii) evidence-based management strategies that may off-set these cardiovascular risks.
Cardiovascular changes in uremic patients
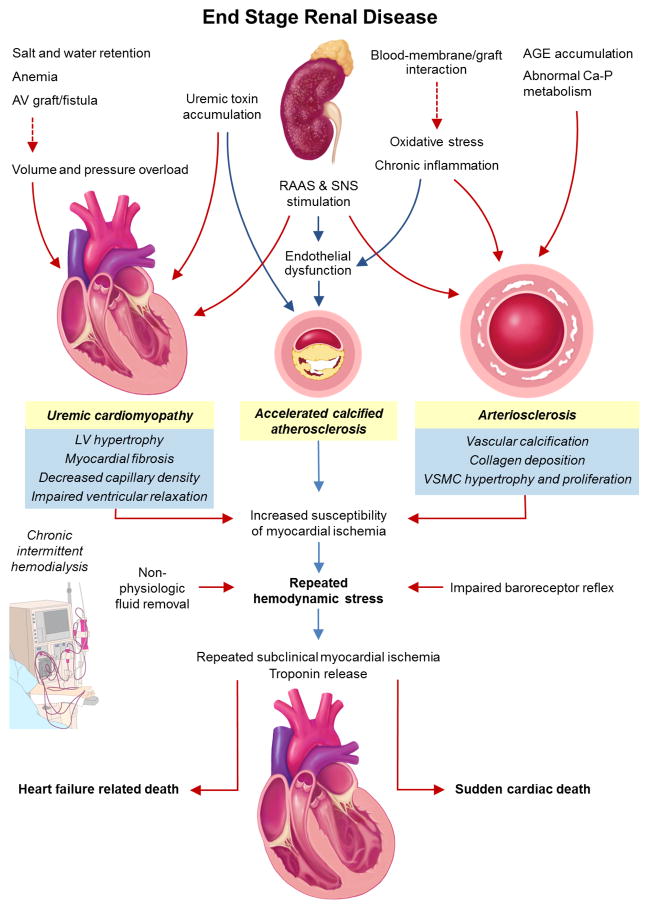
Bidirectional interactions between the cardiovascular and renal systems play a role in the maintenance of hemodynamic stability, blood volume and vascular tone. The primary dysfunction of one organ leads to progressive decline in both organ systems and is referred to as the cardiorenal system. Reno-cardiac syndrome (so-called “Type 4” cardiorenal syndrome) has been defined as the development of secondary cardiovascular dysfunction following primary kidney disease. The pathophysiological mechanisms are complex and not completely understood. Several ESRD-related factors, including activation of the renin-angiotensin-aldosterone system (RAAS), sympathetic nervous system, abnormality of calcium-phosphate metabolism, oxidative stress as well as accumulation of uremic toxin, drive the development of cardiovascular dysfunction. These changes include cardiomyopathy (uremic cardiomyopathy), Left ventricular (LV) hypertrophy, myocardial fibrosis, impaired diastolic filling, and microvascular coronary disease. Vascular changes that include atherosclerosis, vessel calcification and loss of vascular elasticity are shown in Figure 1.
Figure 1. Pathophysiology of hemodialysis-induced myocardial injury.
Abbreviations: AV, arterio-venous; AGE, advanced glycation end product; RAAS, renin angiotensin aldosterone system; SNS, sympathetic nervous system.
Uremic cardiomyopathy
Left ventricular hypertrophy
LV hypertrophy is the most common cardiac finding in dialysis patients, and it is nearly universal (9). It results from chronic volume and pressure overload, neurohormonal activation and uremic toxin accumulation (10, 11). Hypertrophy is typically a compensatory response of the left ventricle to increased afterload, and LV hypertrophy acts to maintain wall stress in the face of long-term altered loading conditions. Continuing LV overload eventually can lead to structural changes in the LV and apoptosis of cardiomyocytes (12). Hypertrophied hearts have reduced coronary blood flow reserve and are more subject to myocardial ischemia. Left atrial enlargement is universal, and atrial fibrillation is common.
Interstitial fibrosis
Diffuse interstitial cardiac fibrosis is demonstrated in uremic patients and is not totally explained by excessive non-renal hypertension (13–15). Several other factors contribute to this fibrosis, including excessive RAAS activity, hyperphosphatemia, parathyroid hormone, oxidative stresses, uremic toxins and cellular senescence (10). Interstitial collagen deposition likely contributes to ventricular diastolic dysfunction, impaired LV filling, and predisposes to atrial and ventricular arrhythmias (16). This may explain the increased risk of sudden cardiac death (SCD) in uremic patients.
Microvascular disease
At least 30% of dialysis patients with angina have only moderate epicardial coronary artery disease (CAD) (17). However, endothelial dysfunction with microvascular disease may occur. There are coronary functional changes (increase in extra-coronary resistance secondary to LV hypertrophy and endothelial dysfunction) and structural changes (wall thickening with reduced arteriolar lumen of intramyocardial arteries and reduced cardiac capillary density) (10, 15, 18–21). Myocardium-capillary mismatch is not specific to uremia and is not just a consequence of hypertension with LV hypertrophy. Microvascular coronary disease exposes cardiomyocytes to the risk of hypoxemia under the condition of high oxygen demand or low oxygen supply (15, 20). In fact, there may be ongoing ischemic myocardial injury at the microvascular level, which could explain why many of these patients have persistently elevated serum troponin levels.
Uremic vasculopathy
Atherosclerosis
In uremic patients, there are two different but overlapping macrovascular changes: atherosclerosis and arteriosclerosis. Atherosclerosis is a primarily intimal disorder of medium-sized arteries characterized by plaque formation and subsequently narrowing and occlusion of the vessels resulting in impaired conduit function (22). The unique characteristics of coronary atheroma in ESRD patients are increased medial thickness and markedly calcified plaques (23). These changes lead to chronic myocardial ischemia, and subsequently development of myocardial fibrosis, SCD and heart failure, rather than acute plaque rupture. This observation may explain the unexpected low incidence of acute myocardial infarction in this population (24).
A high atherosclerotic burden in uremic patients is well established. Although the traditional atherosclerotic risk factors are common in this setting, they only partially contribute to increased cardiovascular burden (5, 25). Several randomized controlled trials (RCTs) of statin therapy have failed to demonstrate a reduction in cardiovascular outcomes in the hemodialysis population (6, 8, 26). A plausible explanation is that non-traditional uremic-related risk factors, including chronic inflammation, oxidative stress and activation of the RAAS, may play an important role in this setting (27, 28).
Arteriosclerosis
Arteriosclerosis, a hallmark of arterial remodeling in ESRD, is characterized by diffuse calcification in combination with dilatation and increased wall thickness of the medial layer of the aorta and its main branches leading to increased arterial stiffness (29). Disturbance of calcium-phosphorus homeostasis, leading to hypocalcemia, hyperphosphatemia and secondary hyperparathyroidism, as well as uremic toxins may lead to accelerated calcification of arterial media and active osteogenic differentiation of vascular smooth muscle cells (30).
Consequences of uremic vasculopathy
The arterial system has two important functions: conduit and cushioning (29). The latter function requires a compliant arterial tree to ensure that the pulsatile flow in large arteries maintains steady continuous perfusion to peripheral organs without exposure to peak systolic pressures (31). When aortic stiffening is markedly increased as in arteriosclerosis, loss of the cushioning effect occurs resulting in loss of the ability of the aorta to accommodate the ejected blood volume from the LV. This subsequently leads to an increase in augmentation of systolic BP, whereas diastolic BP is lower due to a decrease in reservoir effect. There is an increased stroke volume run-off during systole and less blood volume to be drained during diastole (29, 32). While increasing systolic BP leads to an increase in afterload contributing LV hypertrophy and increased myocardial oxygen consumption, decreasing diastolic BP leads to decrease in diastolic coronary perfusion. These could lead to subsequent myocardial ischemia, impaired diastolic function and decreased systolic function. Moreover, increased systolic BP as well as increased pulse pressure lead to a vicious cycle and more arterial stiffness (33).
Effects of arteriovenous fistula on the cardiovascular system
Arteriovenous (AV) fistula is the preferred vascular access for long-term hemodialysis given its high blood flow rate, patency, and low infection risk (34) and association with lower all-cause and cardiovascular mortality as compared to AV graft or central venous catheter (35). However, flow-related cardiovascular complications can occur and are usually under-recognized. Creation of AV access shunts the blood from peripheral tissue, leading to instantaneous reduction in systemic vascular resistance. Circulatory compensation subsequently occurs to maintain systemic BP and peripheral perfusion by activating the RAAS and sympathetic systems, enhancing the venous return and increasing heart rate, and, in turn, leading to an increase in cardiac output and pulmonary pressure (34, 36). Cardiac output typically rises equivalent to AV access blood flow of 1–2 L/min at rest and 3–4 L/min in the setting of high flow fistula (37) and can increase as much as 10–12 L/min during exertion (38, 39).
A persistent high-output state accompanied by neurohormonal activation and increased vascular stiffness in uremia may promote progressive LV hypertrophy and LV chamber dilatation. This can occur as soon as within 2 weeks after creation of an AV fistula (40). Hemodynamic stress represented by elevation of plasma atrial and brain natriuretic peptides after creation of AV fistula has also been demonstrated in both animal experiments and in patients with CKD (38, 40). Furthermore, increased oxygen demand caused by increased LV mass in the setting of impaired coronary flow reserve, as well as decreased diastolic BP, can lead to subendocardial myocardial ischemia after formation of an AV fistula (41). High-output heart failure as defined by systemic or pulmonary venous congestion combined with high cardiac output at rest of greater than 8 L/min or a cardiac index of greater than 3.9 L/min/m2 (42) can occur in ESRD patients with AV fistula. However, the true incidence of this condition in patients on chronic dialysis has not been well described. Nevertheless, high blood flow across an AV fistula, defined by AV access blood flow of more than 2 L/min (43) or the ratio of AV access flow to cardiac output of more than 0.3 (44), has been demonstrated to be at greater risk of developing high-output heart failure. Awareness of this condition in ESRD patients is important as the vasodilatory effects of current standard neurohormonal antagonists may cause deterioration of hypotension. Additionally, interventions to reduce the vascular access blood flow, including banding of the AV fistula or revision using a distal inflow technique, can be effective in some patients with high-flow AV fistula by improving cardiac structure and hemodynamics (45, 46) and thereby reversing heart failure symptoms (47). However particular attention should be taken when considering AV access closure in patients with severe heart failure. Sudden death after surgical AV fistula ligation in a renal transplant recipient who had severe heart failure has been reported, believed to be caused by a sudden increase in systemic vascular resistance after vascular fistula closure (48).
Pathogenesis of hemodialysis-induced myocardial injury
Hemodialysis has been used for decades in patients with advanced renal failure to aid in the removal of uremic toxins from the blood and to correct metabolic disturbances. Ultrafiltration is used to maintain volume control by removal of salt and water excess. Although hemodialysis should theoretically improve cardiovascular abnormalities in patients with uremia by correcting volume overload, cardiovascular mortality remains high despite improvements in dialysis technology. Several studies of conventional hemodialysis have failed to demonstrate LV hypertrophy regression of vascular calcification or survival, suggesting inadequacy of uremic toxin clearance and failure to reduce adverse effects of hemodialysis on the cardiovascular system. Yet nocturnal or longer duration of hemodialysis has been associated with reduction in LV hypertrophy and improved survival, suggesting the way we perform hemodialysis has major implications on long-term outcomes.
Hemodialysis as Hemodynamic stressor
The rationale of thrice-weekly conventional hemodialysis is based on a combination of physiological experiments, assessment of patient acceptance, feasibility, logistics and costs (49). Over the past decade, high-flux dialyzers have been commonly used in clinical practice, and urea removal can now be achieved more rapidly. Therefore the length of a dialysis session has gradually diminished and more rapid fluid removal is necessitated. Most dialysis patients have interdialytic weight gain of more than 1.5 kg, and up to 40% gain more than 3 kg (50). Ultrafiltration may also produce rapidly non-physiological fluid removal within a limited time and may promote hemodynamic instability, either as an initiating event or a contributing insult to injury. During hemodialysis, intravascular fluid is removed directly and counterbalanced by refilling from the interstitial fluid compartment; the rapidly contracted circulating blood volume that occurs when the fluid removal rate is greater than the plasma refilling rate can be counter-productive. When cardiac preload is reduced in the setting of maladaptive cardiovascular remodeling in uremic patients, it may contribute to intradialytic hypotension and subsequently impaired myocardial perfusion and injury. Intradialytic hypotension is found in as many as 15–25% of hemodialysis sessions and is to be avoided, as it is predictive of increased mortality (51, 52).
Impaired baroreceptor sensitivity and imbalance of sympathetic-parasympathetic activities in both at rest and during exercise, and has been demonstrated in CKD patients (53–55). Autonomic function, especially the baroreceptor arc, is an important regulatory mechanism to maintain hemodynamic stability during hemodialysis, and attenuated baroreceptor sensitivity can sometimes lead to intolerable symptoms during hemodialysis (56, 57). Reduced baroreceptor sensitivity is also related to worsening outcomes in dialysis patients (58).
Activation of inflammatory response
Some investigators postulate that hemodialysis-induced transient LV systolic dysfunction may be produced by a systemic inflammatory response to hemodialysis. This response is due to the interaction between blood and the hemodialysis membrane, synthetic vascular graft or catheter, exposure to contaminated dialysate and vascular access infection (59). Inflammatory biomarkers are substantially increased in uremic patients and are associated with increased risks of all-cause and cardiac mortalities in dialysis patients (60, 61). Several studies reported increase in circulating inflammatory markers including interleukin-6 and pentraxin during single session hemodialysis (62, 63). A recent study also reported that predialysis inflammatory markers including high sensitivity C-reactive protein, and the ratio of interleukin-6 and 10 levels were independently associated with hemodialysis-induced regional LV systolic dysfunction (63). Another possibility is hemodialysis-induced systemic circulatory stress and recurrent regional ischemia of gut leading to endotoxin translocation. Endotoxin, a pro-inflammatory stimulus, has also been demonstrated to be correlated with myocardial stunning and elevated predialysis cardiac troponin T (cTnT) levels (64).
Hemodialysis-induced myocardial injury
Functional and structural abnormalities of the cardiovascular system in uremic patients may predispose the myocardium to become ischemic even in asymptomatic patients. Approximately 70% of dialysis patients with angiographically proven CAD were without angina (65, 66). Absence of ischemic symptoms may be caused by diabetic and uremic autonomic neuropathy, as well as reduction of exercise capacity (67). Subclinical myocardial ischemia during hemodialysis is not uncommon as evidenced by several studies that are summarized in Table 1.
Table 1.
Review of prospective observational studies on hemodialysis-induced myocardial injury in patients with maintenance hemodialysis
| First Author, Year (Ref #) | Study Design | Main Findings |
|---|---|---|
| Studies on dynamic ST-T changes during hemodialysis | ||
| Zuber et al., 1989 (68). | Intradialytic ECG monitoring (n = 32) | ECG changes of ischemia found in 25% with half of them were asymptomatic and ischemia occurred predominantly during the last hour of hemodialysis session |
| Kremastinos et al., 1992 (69). | Intradialytic ECG monitoring (n = 45) | ECG changes of ischemia found in 16% during and immediately after dialysis and no correlation of silent myocardial ischemia with the existence of cardiac dysfunction and angiographic proven CAD |
| Abe et al., 1996 (70). | Intradialytic ECG monitoring (n = 72) | ECG changes of ischemia found in 60%; ST depression in 43%, elevation in 11%, and T inversion in 6%. |
| Conlon et al., 1998 (71). | Intradialytic ECG monitoring (n = 70) | Asymptomatic transient ST depression developed in 23% during hemodialysis and not significantly associated with 2-year survival |
| Mohi-ud-din K et al., 2005 (72). | Intradialytic ECG monitoring (n = 70) | Asymptomatic transient ST depression developed in 22% during hemodialysis and not associated with angiographic evidenced CAD |
| Studies on reduction of MBF during hemodialysis | ||
| McIntyre et al., 2008 (74). | Intradialytic MBF assessment by H215O PET (n=4 without angiographically significant CAD) | Acutely reduction of global MBF during dialysis with progressively worsening over time and partially restored during recovery phase. Significantly greater reduction in segmental MBF in segments that developed RWMAs |
| Dasselaar et al., 2009 (75). | Intradialytic MBF assessment by 13N-NH3 PET (n=7, non-diabetic and no eventful cardiac histories) | Acutely reduction of global MBF at 30 minutes after hemodialysis started with progressively worsened over time Significantly greater reduction in segmental MBF in segments that developed RWMAs |
| Studies on development of echocardiographic LV wall motion abnormalities during hemodialysis | ||
| Burton et al., 2009 (76). | Intradialytic RWMA assessment by echocardiography (n=70) | Significant RWMAs developed in 64% during hemodialysis and independently associated with age, UF volumes, intradialytic hypotension, and predialysis cTnT level. Hemodialysis-induced myocardial stunning was significantly associated with mortality and decreased LVEF at 12 months |
| Assa et al., 2012 (78). | Intradialytic RWMA assessment by echocardiography (n=105) | Significant RWMAs developed in 27% during hemodialysis and did not associated with changes of blood volume, BP, heart rate, electrolytes, and acid–base parameters Hemodialysis induced myocardial stunning was significantly associated with mortality with adjusted HR of 4.6 (95% CI, 1.15–18.5; P=0.03) with the median duration of follow-up of 16.4 months |
| Dubin et al., 2013 (77). | Intradialytic RWMA assessment by echocardiography (n=105) | Significant RWMAs developed in 23% during hemodialysis and independently associated with history of heart failure with adjusted HR of 3.1 (95% CI, 1.1–9; P=0.04) |
Abbreviations: ECG, electrocardiogram; CAD, coronary artery disease; MBF, myocardial blood flow; PET, positron emission tomography; RWMAs, regional wall motion abnormalities; UF, ultrafiltration; cTnT, cardiac troponin T; LVEF, left ventricular ejection fraction; BP, blood pressure; HR, hazard ratio.
Electrocardiographic changes
Silent myocardial ischemia, defined by asymptomatic dynamic ST-T changes suggestive of ischemia, has been repeatedly reported with a prevalence of 16–60% (68–72). Interestingly, studies where coronary angiograms were performed found no correlation between silent ischemia and angiographic findings (69, 72). This may be explained by the existence of microcirculatory changes in the coronary system in ESRD. Some authors also raised the possibility of coronary vasospasm contributed by neurohormonal perturbations and release of vasoactive cytokines during dialysis (72). However, the frequent occurrence of abnormal electrocardiograms found in dialysis patients, especially LV hypertrophy, may make electrocardiographic interpretation difficult, and electrolyte changes during dialysis may also contribute ST changes that resemble ischemia (73).
Reduction in global and segmental myocardial blood flow
McIntyre et al. studied 4 dialysis patients (3 diabetic) without angiographically significant CAD and assessed their intradialytic myocardial blood flow (MBF) by using H215O positron emission tomography (74). Concurrent echocardiography was used to evaluate LV function and regional wall motion abnormalities (RWMAs). Global MBF was acutely reduced during the dialysis session, progressively worsened overtime and partially restored after the 30-min recovery phase. Reduction in segmental MBF was significantly greater in segments with RWMAs, and a reduction in MBF of >30% from baseline was associated with the development of RWMAs. These were confirmed by Dasselaar et al. who evaluated 7 relatively lower-risk, stable, non-diabetic patients with uneventful cardiac histories (75). Significantly reduced global MBF without new RWMAs was observed 30 minutes after starting hemodialysis; there was a small cumulative ultrafiltration volume and insignificant change in hemodynamics at that time of reduced MBF.
Segmental abnormalities of left ventricular systolic function
Burton et al. studied 70 hemodialysis patients, 40% with diabetes, using serial intradialytic echocardiography to evaluate RWMAs (76). Sixty-four percent developed new or worsening RWMAs at the fourth-hour of dialysis and partially returned towards pre-dialysis in the recovery period which may imply the development of myocardial stunning. In multivariate analysis, age, reduction in systolic BP, ultrafiltration volume and cTnT were independently associated with hemodialysis-induced RWMAs. Interestingly, the risk associated with greater fluid removal and decrease in systolic BP increased disproportionately with each additional unit of measure. Ultrafiltration volume of 1 liter was associated with 5-fold greater risk of development of hemodialysis-induced RWMAs, whereas the risk rose 26-fold for a 2-liter fluid removal. This might be related to potential hemoconcentration with subsequently increasing microcirculatory shear stress and reduced microcirculatory blood flow, a potential exacerbating cause of myocardial ischemia (7). However, another small (n=40) study did not find this association between either changes in BP, ultrafiltration volume or cTnT with the occurrence of hemodialysis-induced RWMAs, and only a history of heart failure was independently associated with this myocardial ischemia (77). Assa et al. found only 27% of 105 dialysis patients developed hemodialysis-induced regional LV systolic dysfunction, and there was no significant difference of intradialytic blood volume change between those with or without hemodialysis-induced RWMAs.(78) This corresponds with findings in the previous study regarding reduction of MBF where there was no significant change in ultrafiltration volume (75). Non-hemodynamic factors including inflammation, electrolyte shifts, acid-base shifts or dialysis-induced temperature changes may play a role (75, 79).
Long-term consequences of hemodialysis induced myocardial injury
Myocardial stunning after prolonged myocardial ischemia followed by return of myocardial perfusion has been demonstrated in patients with CAD. Repetitive myocardial ischemia and stunning may lead to irreversible LV systolic dysfunction and heart failure. Several studies have reported the association of all-cause mortality and progressive heart failure in patients with hemodialysis-induced myocardial stunning. Burton et al. observed that patients with hemodialysis-induced myocardial stunning had significantly increased mortality at 12 months.(76) Assa et al. confirmed significant increase in all-cause mortality with adjusted hazard ratio of 4.6 (78). Moreover, patients with hemodialysis-induced RWMAs who were alive at 12 months had a significantly decreased LV ejection fraction (62.1±11.4% vs. 54.7±10.1%, p<0.001), whereas the LV ejection fraction of those without hemodialysis-induced RWMAs remained unchanged (76).
Cardiac arrhythmias and sudden cardiac death
The risk of SCD increases with a progressive deterioration of kidney function (80). It has been demonstrated that when estimated glomerular filtration rate (eGFR) was less than 60 ml/min/1.73 m2, the risk of SCD increased 11% for each 10 ml/min/1.73 m2 decline in eGFR (81). SCD is accountable for 26.5% of all-cause mortality, and about half of cardiovascular death in ESRD patients is related to arrhythmias or SCD (82). The risk of SCD in hemodialysis patients is estimated to be 20- to 30-fold higher than in population with normal kidney function, especially in the first 9 months after initiating the therapy which is known to be the period of heightened SCD risk (83, 84). The incidence of SCD is higher in patients with hemodialysis when compared to the peritoneal dialysis. The pathogenesis or SCD in this population is thought to be multifactorial. Structural and functional changes of cardiovascular system in patients with ESRD, as mentioned before, play an important role in developing cardiac arrhythmias. Rapid blood volume and electrolyte shifts, especially in potassium and calcium homeostasis, may also contribute to abnormalities. These cause hemodynamic stress during hemodialysis as well as mechanical and electrical alteration of cardiac myocytes, which may lead to intra- and inter-dialytic arrhythmias and also increase the risk of SCD in patients undergoing hemodialysis, especially during the initiation of this therapy. The increased risk of SCD is related to longer dialytic intervals in patients undergoing hemodialysis three times a week which may be explained by extreme fluid and electrolyte shifts during this period (83, 85, 86). Although the incidence of SCD in this population from the national registry data seems to reduce (87), the number remains relatively high and the research studying the treatment strategy to decrease SCD and improve outcomes in this population is still limited.
CKD and ESRD patients undergoing dialysis is at risk for developing arrhythmias, especially atrial fibrillation and ventricular arrhythmias. There is limited data regarding the actual burden of arrhythmias in patients with ESRD. In the Chronic Renal Insufficiency Cohort study, atrial fibrillation was found about 18% (88). In study of non-dialysis CKD patients, the risk of atrial fibrillation increased for 1.51–2.86 times compared to subjects with normal renal function, and was associated to the degree of renal impairment (89). CKD patients with atrial fibrillation have poorer outcomes than those without atrial fibrillation, similar to the non-CKD population. Atrial fibrillation does not only increase risk of stroke of 9.8 fold in patients undergoing hemodialysis, but is also the independent risk for sudden death (90). Anticoagulation therapy to prevent the thromboembolic complications also increases hemorrhagic risk and is complicated to anticoagulation given during hemodialysis.
Therapeutic Interventions
Part of high cardiovascular morbidity and mortality in the ESRD population may be related to the fact that risk-modifying interventions are underutilized compared to the non-dialysis population. There may be potential fears of metabolic toxicity and hemodynamic instability. Most randomized clinical trials usually exclude patients with advanced renal impairment from their studies. Moreover applying the proven treatment strategies from a non-dialysis population directly to dialysis patients may be inappropriate because of the different pathophysiology and altered drug metabolism.
Pharmacological therapy
Several neurohormonal blocking agents and statins are of proven benefit in the non-CKD population, especially with heart failure and CAD. However their benefit in hemodialysis patients is still not clarified. Randomized controlled trials of these medications in hemodialysis patients are summarized in Tables 2 and 3.
Table 2.
Major randomized controlled trials of cardiovascular medications in hemodialysis patients with heart failure
| First author, year (ref #) |
Inclusion criteria |
Intervention | Duration, years |
Concomitant drugs (%) |
All-cause mortality (%) |
Cardiovascular mortality (%) |
HF hospitalization (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACEI | ARB | BB | Intervention | Placebo | HR (95% CI) |
Intervention | Placebo | HR (95% CI) |
Intervention | Placebo | HR (95% CI) |
||||
| Cice et al., 2003 (105). | HD, NYHA II-III HF, LVEF<3 5% (n=114) | Carvedilol titrated up to 25 mg twice a day | 2 | 98 | 2 | - | 51.7 | 73.2 | 0.51 (0.32–0.82) | 29.3 | 67.9 | 0.32 (0.18–0.57) | 13.8 | 57.1 | 0.19 (0.09–0.41) |
| Cice et al., 2010 (99). | HD, NYHA II-III HF, LVEF≤4 0%, on ACEI (n=332) | Telmisartan titrated up to 8o mg/day | 3 | 100 | - | 60 | 31.5 | 54.4 | 0.51 (0.32–0.82) | 30.3 | 43.7 | 0.32 (0.18–0.57) | 33.9 | 55.1 | 0.38 (0.19–0.51) |
Table 3.
Major randomized controlled trials of cardiovascular medications in hemodialysis patients
| First author, year (ref #) |
Inclusion criteria |
Intervention | Duration, years |
Composite fatal and non-fatal cardiovascular events |
All-cause mortality (%) | Cardiovascular mortality (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Placebo | HR (95%CI) |
Intervention | Placebo | HR (95%CI) |
Intervention | Placebo | HR (95%CI) |
||||
| FOSIDIAL, 2006 (95). | HD, LVH (n=397) | Fosinopril titrated up to 20 mg/day | 2 | NA | NA | 0.80 (0.59–1.1) | - | - | - | - | - | - |
| Takahashi et al., 2006 (96). | HD (n=80) | Candesartan 4–8 mg/day | 19.4 mo | 16.3 | 45.9 | 0.29 (0.12–0.70) | - | 18.9 | NA | - | - | - |
| Suzuki et al., 2008 (97). | HD, SBP >160 mmHg or >150 mmHg if receiving anti-HT drugs (n=366) | Losartan up to 100 mg, or candesartan up to 12 mg/day or valsartan up to 160 mg/day | 3 | 19 | 33 | 0.51 (0.33–0.79) | 14 | 21 | 0.64 (0.39–1.06) | - | - | - |
| OCTOPUS, 2013 (98). | HD, BP≥140/90 mmHg (n=469) | Olmesartan 10–40 mg/day until achieved target BP of <140/90 mmHg | 3.5 | 35.3 | 34 | 1.00 (0.71–1.40) | 24 | 22.2 | 0.97 (0.62–1.52) | - | - | - |
| DOHAS, 2014 (101). | HD, serum K <6.5 mEq/L (n=309) | Spironolactone 25 mg/day | 3 | 5.7 | 15.1 | 0.40 (0.20–0.81) | 6.4 | 19.7 | 0.36 (0.19–0.66) | 2.5 | 4.6 | 0.57 (.18–1.87) |
| 4D, 2005 (6). | HD, type 2 DM, LDL 90–180 mg/dL (n=1255) | Atorvastatin 20 mg/day | 4 | 37 | 38 | 0.92 (0.77–1.10) | 48 | 50 | 0.93 (0.79–1.08) | 20 | 23 | 0.81 (0.64–1.03) |
| AURORA, 2009 (26). | HD (n=2776) | Rosuvastatin 10 mg/day | 3.8 | 28.5 | 29.5 | 0.96 (0.84–1.11) | 45.8 | 47.7 | 0.96 (0.86–1.07) | 23.3 | 23.4 | 1.00 (0.85–1.16) |
| SHARP, 2011 (8). | CKD Cr≥1.7 mg/dL in men or 1.5 mg/dL in women (n = 9270) | Simvastatin 20 mg/day plus ezetimibe 10 mg/day | 4.9 | 11.3 | 13.4 | 0.83 (0.74–0.94) | 24.6 | 24.1 | 1.02 (0.94–1.1) | 5.4 | 5.9 | 0.93 (0.78–1.10) |
| Dialysis subgroup (n=3023; HD=2527, PD=496) | Simvastatin 20 mg/day plus ezetimibe 10 mg/day | 4.9 | 15 | 16.5 | 0.90 (0.75–1.08) | - | - | - | - | - | - | |
Renin-angiotensin-aldosterone system blockade
Angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs) have demonstrated benefit on reduction of LV hypertrophy and arterial stiffness of hemodialysis patients in small non-randomized studies (91–93) and also in recent meta-analyses (94). However their benefit on long-term cardiovascular mortality in patients receiving chronic hemodialysis is limited. A double-blind RCT, FOSIDIL (the Fosinopril in Dialysis study), studied 397 hemodialysis patients with LV hypertrophy who were ACEI naïve and indicated that fosinopril did not achieve statistically significant improvement of the 2-year composite outcomes of fatal first major cardiovascular events (95).
A small open-label RCT by Takahashi et al. in 80 hemodialysis patients showed candesartan was effective in improvement of survival and composite cardiovascular outcomes (96). However, two larger open-label randomized trials conducted recently by Suzuki et al. (97) and Iseki et al. (98) in hemodialysis patients demonstrated lack of efficacy of ARB therapy, as shown in Table 3.
The beneficial effects of add on ARBs therapy to standard therapy (including ACEIs) in a hemodialysis population with heart failure was demonstrated in a study by Cice et al. (99). A double-blind RCT in 322 hemodialysis patients with moderate heart failure and LV ejection fraction ≤40% indicated that the addition of telmisartan in addition to standard therapy of heart failure (100% ACEIs, 60% beta-blockers) led to a significant reduction in all-cause mortality (reduced 49%), and hospitalization due to heart failure was reduced by 81% with a mean follow-up of 2 years. However, combining ACE inhibitors and ARBs in patients with ESRD is not recommended. There are no RCT data available on add-on ARB therapy in ESRD patients without heart failure.
A recently published open-label randomized trial by Matsumoto et al. evaluated the effect of low-dose spironolactone on cardiovascular and cerebrovascular outcomes in 309 hemodialysis patients. Spironolactone was associated with a 64% reduction in 3-year all-cause mortality, and also reduced cardiovascular and cerebrovascular events. However the sample size was small and the study was not blinded. A larger, double-blind RCT, ALCHEMIST (ALdosterone Antagonist Chronic HEModialysis Interventional Survival Trial), is underway and may provide additional data on the safety and efficacy of spironolactone in hemodialysis patients (100).
There have been obvious concerns about risks of hypotension and hyperkalemia when using RAAS blockade and aldosterone antagonist in dialysis patients. In a study of add-on telmisartan to standard therapy in hemodialysis patients with heart failure (who are quite susceptible to developing hypotension), hypotension developed in 10.9% of the telmisartan group compared to 4.2% of the placebo group (p<0.005). However the beneficial effects of add-on ARB therapy on survival and cardiac function seemed to offset the risks of hypotension in this study Most studies of hemodialysis patients receiving spironolactone and/or ACEIs/ARBs demonstrated a modest rise in serum potassium with only a small number of drug discontinuations because of hyperkalemia (99, 101–103). Moreover a novel polymeric potassium binder, patiromer (RLY5016), was recently demonstrated to prevent hyperkalemia in patients with heart failure receiving standard therapy with spironolactone (104). This may provide a future strategy that will allow safer inhibition of RAAS in this population.
Beta-blockers
Beta-blockers have substantial mortality benefits in patients with acute coronary syndromes and heart failure. Because there may be subclinical myocardial ischemia in patients on hemodialysis with a high prevalence of CAD, heart failure and sympathetic over activity in the setting of ESRD, it may theoretically be possible to reduce hemodialysis-induced myocardial injury and mortality in ESRD patients. Cice et al. conducted an open-label RCT and studied the efficacy of carvedilol in 114 dialysis patients with dilated cardiomyopathy, LVEF<35% and NYHA II-III (98% on ACEIs, 2% on ARBs). Carvedilol significantly improved the 2-year cardiovascular mortality and improved LV function and morphology (105). There are no RCT data available on beta-blocker therapy in ESRD patients without heart failure. Based on inconclusive results from multiple cohort studies, the benefit of beta-blocker used in dialysis patients is still debated (106–108). Further large clinical trials would be necessary to bring clarity to this debate.
Statins
Statin therapy has been widely used to prevent cardiovascular events in the non-dialysis population. There have been two large-scale double-blind RCTs of statin therapy in patients undergoing hemodialysis, Die Deutsche Diabetes Dialyse Studie (4D) (6) and Study to Evaluate the Use of Rosuvastatin in Subjects on Regular Hemodialysis: An Assessment of Survival and Cardiovascular Events (AURORA) (26). These trials have failed to demonstrate the benefit of statin therapy. The Study of Heart and Renal Protection (SHARP) study reported the effectiveness and safety of simvastatin plus ezetimibe in significant reduction of the major atherosclerotic events in various stages of CKD (8). However, the subgroup analysis of dialysis patients in this study did not achieve success in improving the primary endpoint. The plausible explanation for the negative study in dialysis patients is likely the unique pathology and pathophysiology of cardiovascular abnormalities in this population. Moreover, the relationship between cardiovascular disease and conventional risk factors is poorly correlated (109). Lower baseline cholesterol levels in these patients may be a marker of an inflamed and malnourished state, which is associated with decreased survival (110).
Changing renal replacement therapy modality
Today, conventional hemodialysis is the most common modality used to treat ESRD patients. However, other modalities of renal replacement therapy may be more effective in removing uremic toxins, and may be gentler on volume removal and myocardial stunning. Effectiveness and impact of the different dialysis modalities and renal transplantation are shown in Table 4.
Table 4.
Effectiveness of the different dialysis modalities and renal transplantation
| Modality | Conventional hemodialysis | Peritoneal dialysis | Intensive hemodialysis | Hemodia-filtration | Renal transplantation |
|---|---|---|---|---|---|
| Uremic toxin removal | + | + | ++ | ++ | +++ |
| Hemodynamic stability | + | ++ | ++ | ++ | +++ |
| Effective fluid removal | ++ | + | +++ | +++ | +++ |
| Improved survival and cardiovascular outcomes | + | + | +/++ | ++ | +++ |
Peritoneal dialysis
Peritoneal dialysis theoretically has advantages beyond conventional hemodialysis due to continuous fluid removal with better hemodynamic stability, better preservation of residual renal function, improved clearance of medium-size uremic toxins and less systemic inflammation (111). Peritoneal dialysis may be the modality of choice, especially for patients with CAD and heart failure. Moreover, existing evidence is controversial. LV hypertrophy is more severe and more frequent in patients receiving peritoneal dialysis because of subclinical over-hydration with resultant hypertension (112). However, development of icodextrin and hypertonic dialysate solution use now allows for better control of volume status and less LV hypertrophy (113). Results of multiple, large observational cohort studies comparing the long-term outcomes of ESRD patients treated with hemodialysis or peritoneal dialysis have been inconsistent (114–117). The most recent contemporary study in the United States demonstrated insignificant differences in the 5-year survival between these two modalities (117), whereas from the French REIN registry, all-cause mortality in patients with peritoneal dialysis was greater than those treated with hemodialysis (85). The ongoing RCT in China named Comparison of the Impact of Dialysis Treatment Type on Patient Survival study (ClinicalTrials.gov identifier: NCT00510549) may provide additional data regarding this controversy.
Intensive hemodialysis
Existing evidence has demonstrated advantages of intensive hemodialysis on several surrogate outcomes including improved BP control, reduced LV mass, reduced intradialytic hypotension and improved phosphate control (49, 118). Intensive hemodialysis is defined by more frequent and/or longer duration of dialysis session. In general, intensive hemodialysis is when the duration of each dialysis session is more than 5.5 hours and/or 3 to 7 times per week (119). The RCT by Culleton et al. indicated regression of LV mass in patients receiving frequent nocturnal hemodialysis (118). The Frequent Hemodialysis Network (FHN) Daily trial also demonstrated that frequent in-center hemodialysis 6 times per week improved the composite outcomes of death, LV mass and quality of life when compared to the conventional hemodialysis, even though this strategy had more frequent interventions related to vascular access (49). However the FHN Nocturnal trial did not demonstrate that frequent nocturnal hemodialysis 5–6 times a week improved either death or LV mass, or death or quality of life (120). Table 5 summarizes major randomized clinical trials of intensive hemodialysis.
Table 5.
Major randomized controlled trials of changing hemodialysis modalities
| First author, year (ref #) | Inclusion criteria | Intervention | Duration, years | Co-primary outcomes HR (95%CI) | Number of complications related to vascular access | |||
|---|---|---|---|---|---|---|---|---|
| Death or change in LV mass | Death or change in physical-health composite score | Intervention | Placebo | HR (95%CI) | ||||
| FHN Daily trial, 2010 (49). | HD (n=245) | In-center frequent HD 6 times/week vs. conventional HD 3 times/week | 1 | 0.61 (0.46–0.82) | 0.70 (0.53–0.92) | 95 | 65 | 1.71 (1.08–2.73) |
| FHN Nocturnal trial, 2011 (120). | HD (n=87) | Frequent nocturnal HD 6 times/week vs. conventional HD 3 times/week | 1 | 0.68 (0.44–1.07) | 0.91 (0.58–1.43) | 34 | 21 | 1.88 (0.97–3.64) |
It is not known if there is a survival benefit of intensive hemodialysis. Because of the inadequate power of existing RCTs to identify a survival benefit, multiple large-scale propensity score matched cohort studies have been recently conducted (Table 6). Most studies demonstrated reduction of mortality by 13–45% in patients receiving intensive hemodialysis, while the latest study showed that patients with in-center daily hemodialysis had an increase in 1.5-year mortality with a hazard ratio of 1.6 (119, 121–123).
Table 6.
Published large-scale observational cohort studies using propensity score matching demonstrate the mortality risk reductions with intensive hemodialysis
| First author, year (ref #) | Modality | N | Mean follow-up time (years) | Mortality rate (%) | HR (95%CI) |
|---|---|---|---|---|---|
| Lacson et al., 2012 (121). | NIHD | 746 | 2 | 19 | 0.75 (0.61–0.91) |
| Nesrallah et al., 2012 (119). | DHHD | 388 | 1.8 | 13 | 0.55 (0.34–0.87) |
| Weinhandl et al., 2012 (122). | DHHD | 1873 | 1.8 | 19 | 0.87 (0.78–0.97) |
| Suri et al., 2012 (123). | DIHD | 318 | 1.5 | 20 | 1.6 (1.1–2.3) |
Abbreviations: HR, hazard ratio; CI, confidence interval; NIHD, nocturnal in-center hemodialysis; DHHD, Daily home hemodialysis; DIHD, Daily in-center hemodialysis.
Why intensive hemodialysis might improve outcomes in ESRD patients is unclear. Longer and/or more frequent hemodialysis sessions have multiple advantageous effects including effective improvement in fluid removal with reduction in the ultrafiltration rate and less intradialytic hypotension. There is also more effective clearance of middle-sized uremic toxins (such as β2-microglobulin) and phosphorus (124). These observations may help explain why intensive hemodialysis may improve cardiovascular abnormalities in uremic patients. Moreover, reduction in the ultrafiltration rate may help reduce subclinical myocardial ischemia during dialysis. McIntyre and colleagues conducted a cross-sectional study performing intradialytic echocardiography in 46 patients and demonstrated that intradialytic hypotension and RWMAs were reduced in patients receiving frequent dialysis. There was also a trend toward lower predialysis cTnT and NT-proBNP levels in the home-based dialysis groups (125).
Online hemodiafiltration
Retention of middle- to large-sized uremic toxins appears to be an important in the pathogenesis of cardiovascular dysfunction of uremic patients. Conventional hemodialysis with low-flux membranes can remove only low molecular weight molecules by diffusive transport. Despite use of high-flux membranes, which is a standard hemodialysis technique used in the United States at the present, and enables the removal of larger uremic toxins by convective transport, though the amount of convection is uncontrollable and unpredictable (126). Two large-scale RCTs did not demonstrate survival benefits of high-flow over low-flux hemodialysis (2, 127).
Hemodiafiltration, which integrates high-flux hemodialysis and the ultrafiltration of large amounts of plasma water, can increase the magnitude of convection transport. With the advanced online water treatment systems developed recently, high convection and sterile substitution volume can be achieved safely, resulting in markedly augmented removal of middle-sized uremic toxins (126). Advantages of hemodiafiltration have been documented including better control of anemia, more effective removal of phosphate, improved lipid profiles, reduced inflammation and oxidative stress, as well as lower incidence of intradialytic hypotension (128). Two recent large-scale, open-label RCTs, the Convective Transport Study (CONTRAST) (126) and the Comparison of Post-dilution Online Hemodiafiltration and Hemodialysis (Turkish OL-HDF) study (129), demonstrated a trend towards improved survival using online hemodiafiltration over low- and high-flux hemodialysis, respectively. Although these studies failed to achieve statistical significance on the mortality outcomes, their post-hoc analysis showed a 39% and 46% risk reduction in mortality in patients treated with high convection volume. The most recent RCT, the On-Line Hemodiafiltration Survival Study (ESHOL), which achieved higher convection volume than two earlier studies, demonstrated a 30% reduction in all-cause mortality of online hemodiafiltration compared to conventional high-flux hemodialysis with the number needed to treat being 8 to prevent 1 annual death (130). The mortality reduction was mainly due to significant reduction in stroke and infection-related mortality. The incidence of intradialytic hypotension was also significantly lower in the online hemodiafiltration arm. The survival benefit could be explained by more efficient removal of middle-sized and protein-bound uremic toxins which may impact on endothelial function, inflammatory state, vascular calcification, as well as have cardioprotective effects (130). Table 7 summarizes the clinical trials of online hemodiafiltration on mortality outcomes.
Table 7.
Major prospective randomized clinical trials of online hemodiafiltration and survival outcomes
| First author, year (ref #) |
Inclusion criteria |
Intervention | Duration, years |
Composite fatal and non-fatal cardiovascular events (%) |
All-cause mortality (%) | Cardiovascular mortality (%) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OL-HDF | Conventional HD |
HR (95% CI) |
OL-HDF | Conventional HD |
HR (95% CI) |
OL-HDF | Conventional HD |
HR (95% CI) |
||||
| CONSTRAST, 2012 (126). | Maintenance HD (n=714) | Online post-dilution hemodiafiltration vs. low-flux hemodialysis | 3 | 32.4 | 31.5 | 1.07 (0.83–1.39) | 36.6 | 38.8 | 0.95 (0.75–1.20) | 10.3 | 12.9 | 0.80 (0.52–1.24) |
| Turkish OL-HDF, 2013 (129).. | Maintenance HD (n=782) | Online post-dilution hemodiafiltration vs. high-flux hemodialysis | 2 | 22.4 | 25.2 | 0.82 (0.59–1.16) | 13.3 | 16.6 | 0.79 (0.55–1.14) | 8.1 | 11.2 | 0.72 (0.45–1.13) |
| ESHOL, 2013 (130). | Maintenance HD (n=906) | Online post-dilution hemodiafiltration vs. high-flux hemodialysis | 3 | NA | NA | NA | 18.6 | 27.1 | 0.70 (0.53–0.92) | 8.1 | 12.2 | 0.67 (0.44–1.02) |
Renal transplantation
Renal transplantation has been proven to have significant survival benefit beyond dialysis. Adjusted rate of all-cause mortality reduces from 6.5–7.9 fold in the dialysis population to 1–1.5 fold in renal transplant patients compared to individuals in the general population (1). Improvement of LV function and structure after renal transplant has been reported in several studies (131, 132). Interestingly, Wali et al. reported marked improvement in LV ejection fraction, as well as functional status and survival after kidney transplant in ESRD patients with systolic heart failure. Effective removal of uremic toxins, including myocardial suppressants, as well as improvement of the inflammatory state and anemia may explain some of the benefits of kidney transplantation (132).
Conclusions and Future Perspectives
Cardiovascular dysfunction in patients receiving hemodialysis impacts on global health and economic burdens. ESRD has been increasingly recognized as having a grave prognosis and lack of an evidence-based treatment strategy. Despite data indicating the benefits of neurohormonal inhibition in this condition, especially when there is heart failure, ACEIs and beta-blockers are prescribed in only 44% and 66%, respectively in US (1). Moreover, in routine clinical practice, most BP-lowering medications are frequently stopped in the morning of hemodialysis days in order to maintain hemodynamic stability throughout the hemodialysis session. Further investigations regarding how to better optimize medical therapy in this vulnerable population are much needed.
HIGHLIGHTS.
Patients undergoing maintenance hemodialysis have a mix of ischemic, metabolic, and structural changes, coupled with the stress of hemodialysis.
The “classic” heart failure manifestations of patients with end-stage renal disease (ESRD) are somewhat atypical and therapeutic options are limited.
There have been advances in dialysis technologies as well as newer insights with novel imaging techniques.
Clinicians need to better appreciate the spectrum as well as the current understanding of this unique patient population.
Acknowledgments
Funding: Dr. Tang is supported in part by grants from the National Institutes of Health (R01HL103931).
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Ishani A, et al. US Renal Data System 2013 Annual Data Report. Am J Kidney Dis. 2014;63:A7. doi: 10.1053/j.ajkd.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 2.Eknoyan G, Beck GJ, Cheung AK, Daugirdas JT, Greene T, Kusek JW, et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med. 2002;347:2010–9. doi: 10.1056/NEJMoa021583. [DOI] [PubMed] [Google Scholar]
- 3.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA. 2009;302:1782–9. doi: 10.1001/jama.2009.1488. [DOI] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Sarnak MJ. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis. 1998;32:S112–9. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Block G, Humphreys MH, Kopple JD. Reverse epidemiology of cardiovascular risk factors in maintenance dialysis patients. Kidney Int. 2003;63:793–808. doi: 10.1046/j.1523-1755.2003.00803.x. [DOI] [PubMed] [Google Scholar]
- 6.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, et al. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 7.Breidthardt T, McIntyre CW. Dialysis-induced myocardial stunning: the other side of the cardiorenal syndrome. Rev Cardiovasc Med. 2011;12:13–20. doi: 10.3909/ricm0585. [DOI] [PubMed] [Google Scholar]
- 8.Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–92. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Foley RN, Parfrey PS, Harnett JD, Kent GM, Martin CJ, Murray DC, et al. Clinical and echocardiographic disease in patients starting end-stage renal disease therapy. Kidney Int. 1995;47:186–92. doi: 10.1038/ki.1995.22. [DOI] [PubMed] [Google Scholar]
- 10.Gross ML, Ritz E. Hypertrophy and fibrosis in the cardiomyopathy of uremia--beyond coronary heart disease. Semin Dial. 2008;21:308–18. doi: 10.1111/j.1525-139X.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 11.Lekawanvijit S, Adrahtas A, Kelly DJ, Kompa AR, Wang BH, Krum H. Does indoxyl sulfate, a uraemic toxin, have direct effects on cardiac fibroblasts and myocytes? Eur Heart J. 2010;31:1771–9. doi: 10.1093/eurheartj/ehp574. [DOI] [PubMed] [Google Scholar]
- 12.Ikram H, Lynn KL, Bailey RR, Little PJ. Cardiovascular changes in chronic hemodialysis patients. Kidney Int. 1983;24:371–6. doi: 10.1038/ki.1983.169. [DOI] [PubMed] [Google Scholar]
- 13.Mall G, Huther W, Schneider J, Lundin P, Ritz E. Diffuse intermyocardiocytic fibrosis in uraemic patients. Nephrol Dial Transplant. 1990;5:39–44. doi: 10.1093/ndt/5.1.39. [DOI] [PubMed] [Google Scholar]
- 14.Mall G, Rambausek M, Neumeister A, Kollmar S, Vetterlein F, Ritz E. Myocardial interstitial fibrosis in experimental uremia--implications for cardiac compliance. Kidney Int. 1988;33:804–11. doi: 10.1038/ki.1988.71. [DOI] [PubMed] [Google Scholar]
- 15.Amann K, Breitbach M, Ritz E, Mall G. Myocyte/capillary mismatch in the heart of uremic patients. J Am Soc Nephrol. 1998;9:1018–22. doi: 10.1681/ASN.V961018. [DOI] [PubMed] [Google Scholar]
- 16.Bakth S, Arena J, Lee W, Torres R, Haider B, Patel BC, et al. Arrhythmia susceptibility and myocardial composition in diabetes. Influence of physical conditioning. J Clin Invest. 1986;77:382–95. doi: 10.1172/JCI112316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rostand SG, Kirk KA, Rutsky EA. The epidemiology of coronary artery disease in patients on maintenance hemodialysis: implications for management. Contrib Nephrol. 1986;52:34–41. doi: 10.1159/000413122. [DOI] [PubMed] [Google Scholar]
- 18.Tok D, Gullu H, Erdogan D, Topcu S, Ciftci O, Yildirim I, et al. Impaired coronary flow reserve in hemodialysis patients: a transthoracic Doppler echocardiographic study. Nephron Clin Pract. 2005;101:c200–6. doi: 10.1159/000087579. [DOI] [PubMed] [Google Scholar]
- 19.Amann K, Wiest G, Zimmer G, Gretz N, Ritz E, Mall G. Reduced capillary density in the myocardium of uremic rats--a stereological study. Kidney Int. 1992;42:1079–85. doi: 10.1038/ki.1992.390. [DOI] [PubMed] [Google Scholar]
- 20.Tyralla K, Amann K. Cardiovascular changes in renal failure. Blood Purif. 2002;20:462–5. doi: 10.1159/000063551. [DOI] [PubMed] [Google Scholar]
- 21.Meyer C, Heiss C, Drexhage C, Kehmeier ES, Balzer J, Muhlfeld A, et al. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J Am Coll Cardiol. 2010;55:454–9. doi: 10.1016/j.jacc.2009.07.068. [DOI] [PubMed] [Google Scholar]
- 22.London GM, Drueke TB. Atherosclerosis and arteriosclerosis in chronic renal failure. Kidney Int. 1997;51:1678–95. doi: 10.1038/ki.1997.233. [DOI] [PubMed] [Google Scholar]
- 23.Schwarz U, Buzello M, Ritz E, Stein G, Raabe G, Wiest G, et al. Morphology of coronary atherosclerotic lesions in patients with end-stage renal failure. Nephrol Dial Transplant. 2000;15:218–23. doi: 10.1093/ndt/15.2.218. [DOI] [PubMed] [Google Scholar]
- 24.Herzog CA. Sudden cardiac death and acute myocardial infarction in dialysis patients: perspectives of a cardiologist. Semin Nephrol. 2005;25:363–6. doi: 10.1016/j.semnephrol.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Coresh J, Longenecker JC, Miller ER, 3rd, Young HJ, Klag MJ. Epidemiology of cardiovascular risk factors in chronic renal disease. J Am Soc Nephrol. 1998;9:S24–30. [PubMed] [Google Scholar]
- 26.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, et al. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 27.Moody WE, Edwards NC, Madhani M, Chue CD, Steeds RP, Ferro CJ, et al. Endothelial dysfunction and cardiovascular disease in early-stage chronic kidney disease: cause or association? Atherosclerosis. 2012;223:86–94. doi: 10.1016/j.atherosclerosis.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 28.Drueke TB, Massy ZA. Atherosclerosis in CKD: differences from the general population. Nat Rev Nephrol. 2010;6:723–35. doi: 10.1038/nrneph.2010.143. [DOI] [PubMed] [Google Scholar]
- 29.London GM, Marchais SJ, Guerin AP. Arterial stiffness and function in end-stage renal disease. Adv Chronic Kidney Dis. 2004;11:202–9. doi: 10.1053/j.arrt.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 30.Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Heart. 2010;96:817–23. doi: 10.1136/hrt.2009.184879. [DOI] [PubMed] [Google Scholar]
- 31.Laurent S, Cockcroft J, Van Bortel L, Boutouyrie P, Giannattasio C, Hayoz D, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 32.Davies JE, Parker KH, Francis DP, Hughes AD, Mayet J. What is the role of the aorta in directing coronary blood flow? Heart. 2008;94:1545–7. doi: 10.1136/hrt.2008.144808. [DOI] [PubMed] [Google Scholar]
- 33.O’Rourke M. Mechanical principles in arterial disease. Hypertension. 1995;26:2–9. doi: 10.1161/01.hyp.26.1.2. [DOI] [PubMed] [Google Scholar]
- 34.MacRae JM, Pandeya S, Humen DP, Krivitski N, Lindsay RM. Arteriovenous fistula-associated high-output cardiac failure: a review of mechanisms. Am J Kidney Dis. 2004;43:e17–22. doi: 10.1053/j.ajkd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 35.Wasse H, Speckman RA, McClellan WM. Arteriovenous fistula use is associated with lower cardiovascular mortality compared with catheter use among ESRD patients. Semin Dial. 2008;21:483–9. doi: 10.1111/j.1525-139X.2008.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holman E. Abnormal arteriovenous communications. Great variability of effects with particular reference to delayed development of cardiac failure. Circulation. 1965;32:1001–9. doi: 10.1161/01.cir.32.6.1001. [DOI] [PubMed] [Google Scholar]
- 37.Wasse H, Singapuri MS. High-output heart failure: how to define it, when to treat it, and how to treat it. Semin Nephrol. 2012;32:551–7. doi: 10.1016/j.semnephrol.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 38.Iwashima Y, Horio T, Takami Y, Inenaga T, Nishikimi T, Takishita S, et al. Effects of the creation of arteriovenous fistula for hemodialysis on cardiac function and natriuretic peptide levels in CRF. Am J Kidney Dis. 2002;40:974–82. doi: 10.1053/ajkd.2002.36329. [DOI] [PubMed] [Google Scholar]
- 39.Bailey WB, Talley JD. High-output cardiac failure related to hemodialysis arteriovenous fistula. J Ark Med Soc. 2000;96:340–1. [PubMed] [Google Scholar]
- 40.Ori Y, Korzets A, Katz M, Perek Y, Zahavi I, Gafter U. Haemodialysis arteriovenous access--a prospective haemodynamic evaluation. Nephrol Dial Transplant. 1996;11:94–7. [PubMed] [Google Scholar]
- 41.Savage MT, Ferro CJ, Sassano A, Tomson CR. The impact of arteriovenous fistula formation on central hemodynamic pressures in chronic renal failure patients: a prospective study. Am J Kidney Dis. 2002;40:753–9. doi: 10.1053/ajkd.2002.35686. [DOI] [PubMed] [Google Scholar]
- 42.Anand IS, Florea VG. High Output Cardiac Failure. Curr Treat Options Cardiovasc Med. 2001;3:151–9. doi: 10.1007/s11936-001-0070-1. [DOI] [PubMed] [Google Scholar]
- 43.Basile C, Lomonte C, Vernaglione L, Casucci F, Antonelli M, Losurdo N. The relationship between the flow of arteriovenous fistula and cardiac output in haemodialysis patients. Nephrol Dial Transplant. 2008;23:282–7. doi: 10.1093/ndt/gfm549. [DOI] [PubMed] [Google Scholar]
- 44.Pandeya S, Lindsay RM. The relationship between cardiac output and access flow during hemodialysis. ASAIO J. 1999;45:135–8. doi: 10.1097/00002480-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 45.van Duijnhoven EC, Cheriex EC, Tordoir JH, Kooman JP, van Hooff JP. Effect of closure of the arteriovenous fistula on left ventricular dimensions in renal transplant patients. Nephrol Dial Transplant. 2001;16:368–72. doi: 10.1093/ndt/16.2.368. [DOI] [PubMed] [Google Scholar]
- 46.Movilli E, Viola BF, Brunori G, Gaggia P, Camerini C, Zubani R, et al. Long-term effects of arteriovenous fistula closure on echocardiographic functional and structural findings in hemodialysis patients: a prospective study. Am J Kidney Dis. 2010;55:682–9. doi: 10.1053/j.ajkd.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Parmar CD, Chieng G, Abraham KA, Kumar S, Torella F. Revision using distal inflow for treatment of heart failure secondary to arteriovenous fistula for hemodialysis. J Vasc Access. 2009;10:62–3. doi: 10.1177/112972980901000112. [DOI] [PubMed] [Google Scholar]
- 48.Pascual J, Martins J, Bouarich H, Galeano C, Barrios V, Marcen R, et al. Sudden death after arteriovenous fistula ligation in a renal transplant patient. Ann Vasc Surg. 2008;22:134–5. doi: 10.1016/j.avsg.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 49.Chertow GM, Levin NW, Beck GJ, Depner TA, Eggers PW, Gassman JJ, et al. In-center hemodialysis six times per week versus three times per week. N Engl J Med. 2010;363:2287–300. doi: 10.1056/NEJMoa1001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kalantar-Zadeh K, Regidor DL, Kovesdy CP, Van Wyck D, Bunnapradist S, Horwich TB, et al. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation. 2009;119:671–9. doi: 10.1161/CIRCULATIONAHA.108.807362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tisler A, Akocsi K, Borbas B, Fazakas L, Ferenczi S, Gorogh S, et al. The effect of frequent or occasional dialysis-associated hypotension on survival of patients on maintenance haemodialysis. Nephrol Dial Transplant. 2003;18:2601–5. doi: 10.1093/ndt/gfg450. [DOI] [PubMed] [Google Scholar]
- 52.Shoji T, Tsubakihara Y, Fujii M, Imai E. Hemodialysis-associated hypotension as an independent risk factor for two-year mortality in hemodialysis patients. Kidney Int. 2004;66:1212–20. doi: 10.1111/j.1523-1755.2004.00812.x. [DOI] [PubMed] [Google Scholar]
- 53.Robinson TG, Carr SJ. Cardiovascular autonomic dysfunction in uremia. Kidney Int. 2002;62:1921–32. doi: 10.1046/j.1523-1755.2002.00659.x. [DOI] [PubMed] [Google Scholar]
- 54.Chesterton LJ, Sigrist MK, Bennett T, Taal MW, McIntyre CW. Reduced baroreflex sensitivity is associated with increased vascular calcification and arterial stiffness. Nephrol Dial Transplant. 2005;20:1140–7. doi: 10.1093/ndt/gfh808. [DOI] [PubMed] [Google Scholar]
- 55.Fotbolcu H, Duman D, Ecder SA, Oduncu V, Cevik C, Tigen K, et al. Attenuated cardiovascular response to sympathetic system activation during exercise in patients with dialysis-induced hypotension. Am J Nephrol. 2011;33:491–8. doi: 10.1159/000327829. [DOI] [PubMed] [Google Scholar]
- 56.Barnas MG, Boer WH, Koomans HA. Hemodynamic patterns and spectral analysis of heart rate variability during dialysis hypotension. J Am Soc Nephrol. 1999;10:2577–84. doi: 10.1681/ASN.V10122577. [DOI] [PubMed] [Google Scholar]
- 57.Chesterton LJ, Selby NM, Burton JO, Fialova J, Chan C, McIntyre CW. Categorization of the hemodynamic response to hemodialysis: the importance of baroreflex sensitivity. Hemodial Int. 2010;14:18–28. doi: 10.1111/j.1542-4758.2009.00403.x. [DOI] [PubMed] [Google Scholar]
- 58.Fukuta H, Hayano J, Ishihara S, Sakata S, Mukai S, Ohte N, et al. Prognostic value of heart rate variability in patients with end-stage renal disease on chronic haemodialysis. Nephrol Dial Transplant. 2003;18:318–25. doi: 10.1093/ndt/18.2.318. [DOI] [PubMed] [Google Scholar]
- 59.Clementi A, Virzi GM, Goh CY, Cruz DN, Granata A, Vescovo G, et al. Cardiorenal syndrome type 4: a review. Cardiorenal Med. 2013;3:63–70. doi: 10.1159/000350397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meuwese CL, Stenvinkel P, Dekker FW, Carrero JJ. Monitoring of inflammation in patients on dialysis: forewarned is forearmed. Nat Rev Nephrol. 2011;7:166–76. doi: 10.1038/nrneph.2011.2. [DOI] [PubMed] [Google Scholar]
- 61.Zhang W, He J, Zhang F, Huang C, Wu Y, Han Y, et al. Prognostic role of C-reactive protein and interleukin-6 in dialysis patients: a systematic review and meta-analysis. J Nephrol. 2013;26:243–53. doi: 10.5301/jn.5000169. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto T, Nascimento MM, Hayashi SY, Qureshi AR, Waniewski J, Brodin LA, et al. Changes in circulating biomarkers during a single hemodialysis session. Hemodial Int. 2013;17:59–66. doi: 10.1111/j.1542-4758.2012.00720.x. [DOI] [PubMed] [Google Scholar]
- 63.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, Groen H, et al. Hemodialysis-Induced Regional Left Ventricular Systolic Dysfunction and Inflammation: A Cross-sectional Study. Am J Kidney Dis. 2013 doi: 10.1053/j.ajkd.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 64.McIntyre CW, Harrison LE, Eldehni MT, Jefferies HJ, Szeto CC, John SG, et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6:133–41. doi: 10.2215/CJN.04610510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koch M, Gradaus F, Schoebel FC, Leschke M, Grabensee B. Relevance of conventional cardiovascular risk factors for the prediction of coronary artery disease in diabetic patients on renal replacement therapy. Nephrol Dial Transplant. 1997;12:1187–91. doi: 10.1093/ndt/12.6.1187. [DOI] [PubMed] [Google Scholar]
- 66.Braun WE, Phillips DF, Vidt DG, Novick AC, Nakamoto S, Popowniak KL, et al. Coronary artery disease in 100 diabetics with end-stage renal failure. Transplant Proc. 1984;16:603–7. [PubMed] [Google Scholar]
- 67.De Vriese AS, Vandecasteele SJ, Van den Bergh B, De Geeter FW. Should we screen for coronary artery disease in asymptomatic chronic dialysis patients? Kidney Int. 2012;81:143–51. doi: 10.1038/ki.2011.340. [DOI] [PubMed] [Google Scholar]
- 68.Zuber M, Steinmann E, Huser B, Ritz R, Thiel G, Brunner F. Incidence of arrhythmias and myocardial ischaemia during haemodialysis and haemofiltration. Nephrol Dial Transplant. 1989;4:632–4. [PubMed] [Google Scholar]
- 69.Kremastinos D, Paraskevaidis I, Voudiklari S, Apostolou T, Kyriakides Z, Zirogiannis P, et al. Painless myocardial ischemia in chronic hemodialysed patients: a real event? Nephron. 1992;60:164–70. doi: 10.1159/000186733. [DOI] [PubMed] [Google Scholar]
- 70.Abe S, Yoshizawa M, Nakanishi N, Yazawa T, Yokota K, Honda M, et al. Electrocardiographic abnormalities in patients receiving hemodialysis. Am Heart J. 1996;131:1137–44. doi: 10.1016/s0002-8703(96)90088-5. [DOI] [PubMed] [Google Scholar]
- 71.Conlon PJ, Krucoff MW, Minda S, Schumm D, Schwab SJ. Incidence and long-term significance of transient ST segment deviation in hemodialysis patients. Clin Nephrol. 1998;49:236–9. [PubMed] [Google Scholar]
- 72.Mohi-ud-din K, Bali HK, Banerjee S, Sakhuja V, Jha V. Silent myocardial ischemia and high-grade ventricular arrhythmias in patients on maintenance hemodialysis. Ren Fail. 2005;27:171–5. [PubMed] [Google Scholar]
- 73.Selby NM, McIntyre CW. The acute cardiac effects of dialysis. Semin Dial. 2007;20:220–8. doi: 10.1111/j.1525-139X.2007.00281.x. [DOI] [PubMed] [Google Scholar]
- 74.McIntyre CW, Burton JO, Selby NM, Leccisotti L, Korsheed S, Baker CS, et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol. 2008;3:19–26. doi: 10.2215/CJN.03170707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dasselaar JJ, Slart RH, Knip M, Pruim J, Tio RA, McIntyre CW, et al. Haemodialysis is associated with a pronounced fall in myocardial perfusion. Nephrol Dial Transplant. 2009;24:604–10. doi: 10.1093/ndt/gfn501. [DOI] [PubMed] [Google Scholar]
- 76.Burton JO, Jefferies HJ, Selby NM, McIntyre CW. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol. 2009;4:914–20. doi: 10.2215/CJN.03900808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dubin RF, Beatty AL, Teerlink JR, Schiller NB, Bolger AF, Alokozai D, et al. Determinants of hemodialysis-induced segmental wall motion abnormalities. Hemodial Int. 2013 doi: 10.1111/hdi.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Assa S, Hummel YM, Voors AA, Kuipers J, Westerhuis R, de Jong PE, et al. Hemodialysis-induced regional left ventricular systolic dysfunction: prevalence, patient and dialysis treatment-related factors, and prognostic significance. Clin J Am Soc Nephrol. 2012;7:1615–23. doi: 10.2215/CJN.00850112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Toussaint N, Cooney P, Kerr PG. Review of dialysate calcium concentration in hemodialysis. Hemodial Int. 2006;10:326–37. doi: 10.1111/j.1542-4758.2006.00125.x. [DOI] [PubMed] [Google Scholar]
- 80.Kannel WB, Wilson PW, D’Agostino RB, Cobb J. Sudden coronary death in women. Am Heart J. 1998;136:205–12. doi: 10.1053/hj.1998.v136.90226. [DOI] [PubMed] [Google Scholar]
- 81.Pun PH, Smarz TR, Honeycutt EF, Shaw LK, Al-Khatib SM, Middleton JP. Chronic kidney disease is associated with increased risk of sudden cardiac death among patients with coronary artery disease. Kidney Int. 2009;76:652–8. doi: 10.1038/ki.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Atlas of chronic kidney disease and end-stage renal disease in the United States, national institutes of health, national institute of diabetes and digestive and kidney disease. U.S. renal data system, USRDS 2012 annual data report.
- 83.Pun PH, Middleton JP. Sudden cardiac death in hemodialysis patients: a comprehensive care approach to reduce risk. Blood Purif. 2012;33:183–9. doi: 10.1159/000334154. [DOI] [PubMed] [Google Scholar]
- 84.USRDS annual data report. Bethesda: Naitonal institutes of health, national institute of diabetes and digestive and kidney diseases; 2006. [Google Scholar]
- 85.Bleyer AJ, Hartman J, Brannon PC, Reeves-Daniel A, Satko SG, Russell G. Characteristics of sudden death in hemodialysis patients. Kidney Int. 2006;69:2268–73. doi: 10.1038/sj.ki.5000446. [DOI] [PubMed] [Google Scholar]
- 86.Bleyer AJ, Russell GB, Satko SG. Sudden and cardiac death rates in hemodialysis patients. Kidney Int. 1999;55:1553–9. doi: 10.1046/j.1523-1755.1999.00391.x. [DOI] [PubMed] [Google Scholar]
- 87.US Renal Data System USRDS 2013 Annual Data Report. National Institutes of Health, National Institute of Diabetes and Digestive and Kidnay Diseases; Bethesda: http:///www.usrds.org/2013/pdf/v2_ch4_13.pdf. [Google Scholar]
- 88.Soliman EZ, Prineas RJ, Go AS, Xie D, Lash JP, Rahman M, et al. Chronic kidney disease and prevalent atrial fibrillation: the Chronic Renal Insufficiency Cohort (CRIC) Am Heart J. 2010;159:1102–7. doi: 10.1016/j.ahj.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Baber U, Howard VJ, Halperin JL, Soliman EZ, Zhang X, McClellan W, et al. Association of chronic kidney disease with atrial fibrillation among adults in the United States: REasons for Geographic and Racial Differences in Stroke (REGARDS) Study. Circ Arrhythm Electrophysiol. 2011;4:26–32. doi: 10.1161/CIRCEP.110.957100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vazquez E, Sanchez-Perales C, Garcia-Garcia F, Castellano P, Garcia-Cortes MJ, Liebana A, et al. Atrial fibrillation in incident dialysis patients. Kidney Int. 2009;76:324–30. doi: 10.1038/ki.2009.185. [DOI] [PubMed] [Google Scholar]
- 91.London GM, Pannier B, Guerin AP, Marchais SJ, Safar ME, Cuche JL. Cardiac hypertrophy, aortic compliance, peripheral resistance, and wave reflection in end-stage renal disease. Comparative effects of ACE inhibition and calcium channel blockade. Circulation. 1994;90:2786–96. doi: 10.1161/01.cir.90.6.2786. [DOI] [PubMed] [Google Scholar]
- 92.Paoletti E, Cassottana P, Bellino D, Specchia C, Messa P, Cannella G. Left ventricular geometry and adverse cardiovascular events in chronic hemodialysis patients on prolonged therapy with ACE inhibitors. Am J Kidney Dis. 2002;40:728–36. doi: 10.1053/ajkd.2002.35680. [DOI] [PubMed] [Google Scholar]
- 93.Mitsuhashi H, Tamura K, Yamauchi J, Ozawa M, Yanagi M, Dejima T, et al. Effect of losartan on ambulatory short-term blood pressure variability and cardiovascular remodeling in hypertensive patients on hemodialysis. Atherosclerosis. 2009;207:186–90. doi: 10.1016/j.atherosclerosis.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 94.Tai DJ, Lim TW, James MT, Manns BJ, Tonelli M, Hemmelgarn BR. Cardiovascular effects of angiotensin converting enzyme inhibition or angiotensin receptor blockade in hemodialysis: a meta-analysis. Clin J Am Soc Nephrol. 2010;5:623–30. doi: 10.2215/CJN.07831109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, et al. Prevention of cardiovascular events in end-stage renal disease: results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–24. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 96.Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, et al. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis--a randomized study. Nephrol Dial Transplant. 2006;21:2507–12. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 97.Suzuki H, Kanno Y, Sugahara S, Ikeda N, Shoda J, Takenaka T, et al. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501–6. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 98.Iseki K, Arima H, Kohagura K, Komiya I, Ueda S, Tokuyama K, et al. Effects of angiotensin receptor blockade (ARB) on mortality and cardiovascular outcomes in patients with long-term haemodialysis: a randomized controlled trial. Nephrol Dial Transplant. 2013;28:1579–89. doi: 10.1093/ndt/gfs590. [DOI] [PubMed] [Google Scholar]
- 99.Cice G, Di Benedetto A, D’Isa S, D’Andrea A, Marcelli D, Gatti E, et al. Effects of telmisartan added to Angiotensin-converting enzyme inhibitors on mortality and morbidity in hemodialysis patients with chronic heart failure a double-blind, placebo-controlled trial. J Am Coll Cardiol. 2010;56:1701–8. doi: 10.1016/j.jacc.2010.03.105. [DOI] [PubMed] [Google Scholar]
- 100.Pitt B, Rossignol P. Mineralocorticoid receptor antagonists in patients with end stage renal disease (ESRD ) on chronic hemodialysis. J Am Coll Cardiol. 2014;63:537–8. doi: 10.1016/j.jacc.2013.09.057. [DOI] [PubMed] [Google Scholar]
- 101.Matsumoto Y, Mori Y, Kageyama S, Arihara K, Sugiyama T, Ohmura H, et al. Spironolactone reduces cardio- and cerebrovascular morbidity and mortality in hemodialysis patients. J Am Coll Cardiol. 2014;63:528–36. doi: 10.1016/j.jacc.2013.09.056. [DOI] [PubMed] [Google Scholar]
- 102.Matsumoto Y, Kageyama S, Yakushigawa T, Arihara K, Sugiyama T, Mori Y, et al. Long-term low-dose spironolactone therapy is safe in oligoanuric hemodialysis patients. Cardiology. 2009;114:32–8. doi: 10.1159/000210553. [DOI] [PubMed] [Google Scholar]
- 103.Hussain S, Dreyfus DE, Marcus RJ, Biederman RW, McGill RL. Is spironolactone safe for dialysis patients? Nephrol Dial Transplant. 2003;18:2364–8. doi: 10.1093/ndt/gfg413. [DOI] [PubMed] [Google Scholar]
- 104.Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double-blind, placebo-controlled study in patients with chronic heart failure (the PEARL-HF) trial. Eur Heart J. 2011;32:820–8. doi: 10.1093/eurheartj/ehq502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cice G, Ferrara L, D’Andrea A, D’Isa S, Di Benedetto A, Cittadini A, et al. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–44. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 106.Foley RN, Herzog CA, Collins AJ. Blood pressure and long-term mortality in United States hemodialysis patients: USRDS Waves 3 and 4 Study. Kidney Int. 2002;62:1784–90. doi: 10.1046/j.1523-1755.2002.00636.x. [DOI] [PubMed] [Google Scholar]
- 107.Abbott KC, Trespalacios FC, Agodoa LY, Taylor AJ, Bakris GL. Beta-blocker use in long-term dialysis patients: association with hospitalized heart failure and mortality. Arch Intern Med. 2004;164:2465–71. doi: 10.1001/archinte.164.22.2465. [DOI] [PubMed] [Google Scholar]
- 108.Kitchlu A, Clemens K, Gomes T, Hackam DG, Juurlink DN, Mamdani M, et al. Beta-blockers and cardiovascular outcomes in dialysis patients: a cohort study in Ontario, Canada. Nephrol Dial Transplant. 2012;27:1591–8. doi: 10.1093/ndt/gfr460. [DOI] [PubMed] [Google Scholar]
- 109.Baigent C, Burbury K, Wheeler D. Premature cardiovascular disease in chronic renal failure. Lancet. 2000;356:147–52. doi: 10.1016/S0140-6736(00)02456-9. [DOI] [PubMed] [Google Scholar]
- 110.Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, et al. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291:451–9. doi: 10.1001/jama.291.4.451. [DOI] [PubMed] [Google Scholar]
- 111.Sens F, Schott-Pethelaz AM, Labeeuw M, Colin C, Villar E. Survival advantage of hemodialysis relative to peritoneal dialysis in patients with end-stage renal disease and congestive heart failure. Kidney Int. 2011;80:970–7. doi: 10.1038/ki.2011.233. [DOI] [PubMed] [Google Scholar]
- 112.Enia G, Mallamaci F, Benedetto FA, Panuccio V, Parlongo S, Cutrupi S, et al. Long-term CAPD patients are volume expanded and display more severe left ventricular hypertrophy than haemodialysis patients. Nephrol Dial Transplant. 2001;16:1459–64. doi: 10.1093/ndt/16.7.1459. [DOI] [PubMed] [Google Scholar]
- 113.Konings CJ, Kooman JP, Schonck M, Gladziwa U, Wirtz J, van den Wall Bake AW, et al. Effect of icodextrin on volume status, blood pressure and echocardiographic parameters: a randomized study. Kidney Int. 2003;63:1556–63. doi: 10.1046/j.1523-1755.2003.00887.x. [DOI] [PubMed] [Google Scholar]
- 114.Liem YS, Wong JB, Hunink MG, de Charro FT, Winkelmayer WC. Comparison of hemodialysis and peritoneal dialysis survival in The Netherlands. Kidney Int. 2007;71:153–8. doi: 10.1038/sj.ki.5002014. [DOI] [PubMed] [Google Scholar]
- 115.Huang CC, Cheng KF, Wu HD. Survival analysis: comparing peritoneal dialysis and hemodialysis in Taiwan. Perit Dial Int. 2008;28(Suppl 3):S15–20. [PubMed] [Google Scholar]
- 116.McDonald SP, Marshall MR, Johnson DW, Polkinghorne KR. Relationship between dialysis modality and mortality. J Am Soc Nephrol. 2009;20:155–63. doi: 10.1681/ASN.2007111188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Mehrotra R, Chiu YW, Kalantar-Zadeh K, Bargman J, Vonesh E. Similar outcomes with hemodialysis and peritoneal dialysis in patients with end-stage renal disease. Arch Intern Med. 2011;171:110–8. doi: 10.1001/archinternmed.2010.352. [DOI] [PubMed] [Google Scholar]
- 118.Culleton BF, Walsh M, Klarenbach SW, Mortis G, Scott-Douglas N, Quinn RR, et al. Effect of frequent nocturnal hemodialysis vs conventional hemodialysis on left ventricular mass and quality of life: a randomized controlled trial. JAMA. 2007;298:1291–9. doi: 10.1001/jama.298.11.1291. [DOI] [PubMed] [Google Scholar]
- 119.Nesrallah GE, Lindsay RM, Cuerden MS, Garg AX, Port F, Austin PC, et al. Intensive hemodialysis associates with improved survival compared with conventional hemodialysis. J Am Soc Nephrol. 2012;23:696–705. doi: 10.1681/ASN.2011070676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rocco MV, Lockridge RS, Jr, Beck GJ, Eggers PW, Gassman JJ, Greene T, et al. The effects of frequent nocturnal home hemodialysis: the Frequent Hemodialysis Network Nocturnal Trial. Kidney Int. 2011;80:1080–91. doi: 10.1038/ki.2011.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lacson E, Jr, Xu J, Suri RS, Nesrallah G, Lindsay R, Garg AX, et al. Survival with three-times weekly in-center nocturnal versus conventional hemodialysis. J Am Soc Nephrol. 2012;23:687–95. doi: 10.1681/ASN.2011070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Weinhandl ED, Liu J, Gilbertson DT, Arneson TJ, Collins AJ. Survival in daily home hemodialysis and matched thrice-weekly in-center hemodialysis patients. J Am Soc Nephrol. 2012;23:895–904. doi: 10.1681/ASN.2011080761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Suri RS, Lindsay RM, Bieber BA, Pisoni RL, Garg AX, Austin PC, et al. A multinational cohort study of in-center daily hemodialysis and patient survival. Kidney Int. 2013;83:300–7. doi: 10.1038/ki.2012.329. [DOI] [PubMed] [Google Scholar]
- 124.Mehrotra R, Himmelfarb J. Dialysis in 2012: Could longer and more frequent haemodialysis improve outcomes? Nat Rev Nephrol. 2013;9:74–5. doi: 10.1038/nrneph.2012.287. [DOI] [PubMed] [Google Scholar]
- 125.Jefferies HJ, Virk B, Schiller B, Moran J, McIntyre CW. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning) Clin J Am Soc Nephrol. 2011;6:1326–32. doi: 10.2215/CJN.05200610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23:1087–96. doi: 10.1681/ASN.2011121140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Locatelli F, Martin-Malo A, Hannedouche T, Loureiro A, Papadimitriou M, Wizemann V, et al. Effect of membrane permeability on survival of hemodialysis patients. J Am Soc Nephrol. 2009;20:645–54. doi: 10.1681/ASN.2008060590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Canaud B, Bowry SK. Emerging clinical evidence on online hemodiafiltration: does volume of ultrafiltration matter? Blood Purif. 2013;35:55–62. doi: 10.1159/000345175. [DOI] [PubMed] [Google Scholar]
- 129.Ok E, Asci G, Toz H, Ok ES, Kircelli F, Yilmaz M, et al. Mortality and cardiovascular events in online haemodiafiltration (OL-HDF) compared with high-flux dialysis: results from the Turkish OL-HDF Study. Nephrol Dial Transplant. 2013;28:192–202. doi: 10.1093/ndt/gfs407. [DOI] [PubMed] [Google Scholar]
- 130.Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24:487–97. doi: 10.1681/ASN.2012080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Burt RK, Gupta-Burt S, Suki WN, Barcenas CG, Ferguson JJ, Van Buren CT. Reversal of left ventricular dysfunction after renal transplantation. Ann Intern Med. 1989;111:635–40. doi: 10.7326/0003-4819-111-8-635. [DOI] [PubMed] [Google Scholar]
- 132.Wali RK, Wang GS, Gottlieb SS, Bellumkonda L, Hansalia R, Ramos E, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45:1051–60. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]