Abstract
Toxoplasma gondii (TOXO) is a neuroinvasive protozoan parasite that induces the formation of persistent cysts in mammalian brains. It infects approximately 1.1 million people in the United States annually. Latent TOXO infection is implicated in the etiology of psychiatric disorders, especially schizophrenia (Scz), and has been correlated with modestly impaired cognition. The acoustic startle response (ASR) is a reflex seen in all mammals. It is mediated by a simple subcortical circuit, and provides an indicator of neural function. We previously reported the association of TOXO with slowed acoustic startle latency, an index of neural processing speed, in a sample of schizophrenia and healthy control subjects. The alterations in neurobiology with TOXO latent infection may not be specific to schizophrenia. Therefore we examined TOXO in relation to acoustic startle in an urban, predominately African American, population with mixed psychiatric diagnoses, and healthy controls. Physiological and diagnostic data along with blood samples were collected from 364 outpatients treated at an inner-city hospital. TOXO status was determined with an ELISA assay for TOXO-specific IgG. A discrete titer was calculated based on standard cut-points as an indicator of seropositivity, and the TOXO-specific IgG concentration served as serointensity. A series of linear regression models were used to assess the association of TOXO seropositivity and serointensity with ASR magnitude and latency in models adjusting for demographics and psychiatric diagnoses (PTSD, major depression, schizophrenia, psychosis, substance abuse). ASR magnitude was 11.5% higher in TOXO seropositive subjects compared to seronegative individuals (p=0.01). This effect was more pronounced in models with TOXO serointensity that adjusted for sociodemographic covariates (F=7.41, p=.0068; F=10.05, p=0.0017), and remained significant when psychiatric diagnoses were stepped into the models. TOXO showed no association with startle latency (t=0.49, p=0.63) in an unadjusted model, nor was TOXO associated with latency in models that included demographic factors. After stepping in individual psychiatric disorders, we found a significant association of latency with a diagnosis of PTSD (F=5.15, p=0.024), but no other psychiatric diagnoses, such that subjects with PTSD had longer startle latency. The mechanism by which TOXO infection is associated with high startle magnitude is not known, but possible mechanisms include TOXO cyst burden in the brain, parasite recrudescence, or molecular mimicry of a host epitope by TOXO. Future studies will focus on the neurobiology underlying the effects of latent TOXO infection as a potential inroad to the development of novel treatment targets for psychiatric disease.
Keywords: Acoustic Startle Response, Toxoplasma gondii, PTSD, Mental Health, Infection, Substance Use Disorder, Schizophrenia
1. Introduction
Toxoplasma gondii (TOXO), a neurotropic protozoan parasite, can infect virtually all warm-blooded animals. Felines are the definitive host of TOXO and usually acquire the parasite through consumption of infected rodents who have latent TOXO in their muscle and brain tissue (Dubey and Jones 2008). The sexual reproductive phase of TOXO occurs in the feline intestine where highly infectious oocysts are generated and then spread by defecation to intermediate hosts. The life cycle is complete when a feline eats an intermediate host (e.g. a rodent). Humans are also intermediate hosts of TOXO, and often acquire the parasite through oral ingestion of oocysts from the soil or unwashed produce, or by eating undercooked meat or shellfish, or by vertical congenital transmission (Jones et al. 2009).
TOXO is neuroinvasive, and the majority of people infected by TOXO will harbor cysts in their brains for life. Typically, the parasite remains relatively quiescent in immunocompetent adult hosts (Suzuki 2002). However, there is substantial evidence that TOXO can cause subtle behavioral dysfunction in rodents (Webster et al. 1994) and humans (Flegr and Hrdý 1994), and probably plays an important role in serious mental health disorders (Yolken et al. 2001). This has been thoroughly reviewed by Webster and McConkey (2010), Flegr et al. (2013) and Yolken et al. (2009). This has been evaluated through studies conducted by Flegr et al. (2013) and Webster and McConkey (2010) which examined the influence of TOXO infection on rodent behavior. TOXO can alter rodent behavior by reducing their fear of cats, which provides an evolutionary advantage to TOXO, as TOXO relies on the predation of rodents by felines (Berdoy et al. 2000, Webster and McConkey 2010). From an evolutionary viewpoint, TOXO would be advantaged by any changes in an infected host that promotes passage of TOXO along its life cycle from prey to predator and back to prey.
While humans are predominately irrelevant to continuation of the TOXO life cycle in modern times, a multitude of epidemiological studies (Sutterland et al. 2015), and recent experiments in non-human primates (Poirotte et al. 2016), suggest that TOXO can also affect the human brain. TOXO's effect on the human brain is most strongly suggested by the association between TOXO and schizophrenia (Scz) as confirmed in several meta-analyses (Sutterland et al. 2015; Torrey and Yolken 2007; Torrey and Yolkin 2012). Latent TOXO infection is also associated with a heightened risk for bipolar disorder, suicidal self-directed violence, aggression, and impulsivity (Arling et al. 2009; Coccaro et al. 2016; Cook et al. 2015: Hamdani et al. 2013; Okusaga et al. 2011; Pearce et al. 2012; Pedersen et al. 2012). However, not all psychiatric diagnoses are associated with TOXO. Several large-scale studies suggest that TOXO infection is not associated with unipolar major depressive disorder (MDD) (Gale et al. 2014; Markovitz et al. 2015; Pearce et al. 2012). A meta-analysis indicated a possible connection between TOXO and substance use disorders, but potential confounds render this finding inconclusive (Sutterland et al. 2015). Two studies to date have examined the relationship between PTSD and TOXO infection, and neither detected an association (Duffy et al. 2015; Markovitz et al. 2015).
Cognition may also be affected by TOXO, as serological studies indicate that recent or persistent TOXO infection correlates with lower scores on cognitive testing, including processing speed (Dickerson et al. 2014; Havlíček et al. 2001; Hamdani et al. 2015; Mendy et al. 2015; Pearce et al. 2014) and intelligence (Flegr et al. 2003). We previously examined the association of TOXO with neural processing speed in a sample of subjects with schizophrenia and psychiatrically healthy controls (Pearce et al., 2013), because of the importance of information processing in many psychiatric disorders, and findings which indicate that a latent TOXO infection can alter neurobiology. This neurobiological alteration can be measured through the acoustic startle response (ASR). The ASR is an involuntary reflex elicited by a sudden, intense auditory stimulus, mediated by a simple subcortical circuit (Koch 1999). Latency of the ASR is the time required to generate the reflex after the stimulus, and provides an easily measured index of neural processing speed. In our previous study of subjects with schizophrenia compared to controls, latency is longer, i.e. slower, in TOXO seropositive individuals as compared to TOXO seronegative individuals in both diagnostic groups (Pearce et al. 2013).
The effects of TOXO on neural processing as measured by the ASR may not be specific to psychiatric disorders, and may also be influenced by sociodemographic factors. It is already known that the rates of TOXO infection are higher in those born outside of the United States, and in US residents who have a lower socioeconomic status, or lower level of educational attainment (Jones et al. 2001). Even so, few studies of TOXO have focused on urban US populations with high levels of socioeconomic risk factors. Therefore, the purpose of the current study was to examine the effects of latent TOXO infection on the ASR, as an index of early information processing, in an urban, predominately African American, impoverished population with high levels of trauma exposure and psychiatric morbidity.
2. Methods
2.1. Subjects
Subjects were recruited from the primary care and obstetric-gynecological outpatient medical clinics at Grady Memorial Hospital in Atlanta, Georgia for participation in the Grady Trauma Project (GTP). This is a cross-sectional study of highly traumatized minority urban population who is of low socioeconomic status with a diverse set of psychiatric diagnoses. The primary objective of the GTP is to study the neurobiology and genetics of trauma related pathology in this population (Ressler 2016). Prior to the initiation of any study procedures, the subjects gave their consent as indicated by their signature written on an informed consent approved by the Emory University Institutional Review Board and the Grady Hospital Research Oversight Committee (Nylocks et al. 2015; Kaminsky et al. 2015). Inclusion criteria were male or female sex, ages 18 and 65 years old, and sufficient proficiency in English to participate in diagnostic interviews and symptom ratings.
Sociodemographic data was collected from the subjects by means of an interview which obtained age, sex, race, employment status, education, and income. Highest education achieved was ascertained and a dichotomous variable constructed based upon whether or not the subject graduated from high school or obtained their GED. Current income was collected and a dichotomous variable was created based on income above or below the federal poverty level income of $12,000 per year (GDoC).
2.2. Symptom and diagnostic ascertainment
The presence of a current or past Axis I psychiatric disorder was determined by administration of the Structured Clinical Interview for DSM-IV (SCID-I; First et al. 2001) or the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 1998). A history of current or past substance use disorders was additionally determined by means of the Kreek-McHugh-Schluger-Kellogg scale (KMSK; Kellogg et al. 2003) and the Drug Abuse Screening Test (DAST; Skinner 1982). The data from these instruments were used to classify subjects into diagnostic categories by the construction of dichotomous variables. For each of the following diagnoses they were coded for the current or previous presence of a diagnosis of: schizophrenia (SCZ), major depression (MDD), posttraumatic stress disorder (PTSD), and any substance use disorder (including alcohol or any drug of abuse). Because of our prior published findings of low startle magnitude in cocaine addicted subjects (Efferen et al. 2000; Corcoran et al. 2011) we also created a specific dichotomous variable to define whether or not subjects had current or past history of cocaine use disorder. Finally, a composite dichotomous variable was created to define whether subjects had current or past history of any primary psychotic diagnosis on the SCID or MINI interview (including DSM-IV schizophrenia, schizoaffective disorder, schizotypal disorder, delusional disorder, brief psychotic disorder, unspecified psychosis). Those subjects without any Axis I disorder elicited by SCID or MINI interview were classified as psychiatrically healthy controls.
2.3 Venous Blood Collection and Processing
Human blood was drawn and collected into EDTA tubes. Upon collection of the sample, they were inverted 5-10 times in order to properly mix the blood and additive. The EDTA tubes were then immediately placed on ice. The EDTA plasma tubes were centrifuged at 3000 rpm for 15 minutes. The plasma was aliquoted into 1mL cryovials which were stored at -80°C.
2.4 Ascertainment of TOXO status
Venous blood was collected and assayed for TOXO IgG antibodies as per manufacturer's instructions (Bio-Rad, Catalog# 25175, Redmond, WA). Seropositivity was determined through a quotient of a weakly positive single calibrator index value and its absorbance at 405nm, multiplied by the absorbance of the sample, in order to find the sample index. An index value greater than 0.9 was indicative of TOXO seropositivity. For all seropositive subjects, a discrete titer was determined using a three-point curve of the blank, the weakly positive calibrator, and the strongly positive calibrator, as per manufacturer's instructions. TOXO serointensity was discerned from a direct calculation of absorbance against this three-point curve according to instructions provided by the manufacturer. The diagnostic criteria for TOXO positivity provided by the manufacturer was set at a value of greater than 33 IU/mL, whereas a value greater than 27 IU/mL, but less than 33 IU/mL was indicative of equivocality. A concentration less than 27 IU/mL was an indicator of negative seropositivity. For the purpose of this study both those who were positive and equivocal were grouped together given that subjects with the oldest infections have the lowest concentration of antibodies (Kodym et al. 2007) and that subjects within the equivocal group, due to the latent infection, are reported to have the most modified behavior and personality (Flegr 2013). Therefore, we decided a priori to include them with the TOXO positive subjects as compared to the TOXO negative subjects.
2.5. Acoustic startle testing
Procedures for startle testing were in accord with our previously published methods (Jovanovic et al. 2005; Jovanovic et al. 2006). The subjects were seated in a sound attenuating booth and asked to look straight ahead with their eyes open during the course of the session. Acoustic stimuli were delivered binaurally through headphones (Maico, TDH-39-P). The startle session began with 60 seconds of 70 dB white noise that persisted throughout the session. The startle probes were 108 dB, 40 ms bursts of broadband white noise with 0 s rise time. The session, designed to assess startle magnitude, startle latency, and fear potentiation of startle, began with seven startle probes. Magnitude and latency data were extracted from the session prior to the fear potentiation portion.
The eyeblink component of the acoustic startle response was recorded from two 5 mm Ag/AgCl electrodes placed over the right orbicularis oculi muscle, approximately 1 cm under the pupil and 1 cm below the lateral canthus. Electromyographic (EMG) activity of this eye-blink was recorded using the Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA). The impedances for all participants were less than 6 kilo-ohms. EMG data was sampled at 1,000 Hz and amplified using the Biopac system. The acquired EMG signal was filtered with low- and high-frequency cutoffs at 28 and 500 Hz, respectively, and then rectified and smoothed using MindWare software (MindWare Technologies, Inc., Gahanna, OH). Startle magnitude was assessed as the peak magnitude of the EMG contraction, and the latency was assessed as the time of the peak magnitude following the acoustic stimulus (Glover et al. 2012).
2.6. Statistical methods
A series of binary logistic regression models were used to evaluate the association between TOXO seropositivity and dichotomous psychiatric diagnosis variables. A secondary series of binary logistic regression models assessing the same relationship between TOXO positivity and psychiatric diagnoses was also conducted adjusting for demographic variables.
The variables startle magnitude and latency were logarithmically transformed in order to account for a non-normal distribution. T-tests were conducted to assess the association between TOXO positivity and both acoustic startle magnitude and latency. A series of linear regression models were created to assess the association between TOXO positivity and either acoustic startle magnitude or latency, adjusting for demographics and psychiatric diagnoses. All regression models were assessed via backwards elimination in a stepwise manner. All statistics and data cleaning were completed using SAS 9.4 (Cary, North Carolina). Figures were created using Microsoft Office (2013, Redmond, Washington) and GraphPad Prism (La Jolla, CA).
3. Results
3.1. Demographics
Complete data was available for 364 subjects. Demographic characteristics of the subjects are described in Table 1. 13.46% of the study participants were TOXO seropositive. The average age of TOXO positive individuals was 44.02 years (SD=11.89), whereas the average of TOXO negative individuals was 41.32 years (SD=11.46; t=1.48, p=0.14). The majority of the subjects were of African American descent (94.23%) and there were more women (69.51%) than men in the sample. Most had a monthly income of less than $1000 per month (68.13%), were unemployed (78.85%), and had either less than a 12th grade education, graduated from high school, or completed GED requirements (86.54%) (Table 1). There was no statistically significant difference in TOXO seropositivity between subjects based on these demographic variables.
Table 1.
Demographic Characteristics of a Cohort of 364 Patients from the Grady Trauma Project.
| TOXO Positive1. | TOXO Negative | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| No. | % | No. | % | X2 | p | |
|
|
|
|
||||
| Seropositivity | 49 | 13.46 | 315 | 86.54 | ||
| Gender | ||||||
| Male | 11 | 9.91 | 100 | 90.09 | 1.73 | 0.19 |
| Female | 38 | 15.02 | 215 | 84.98 | ||
| Race | ||||||
| Black | 44 | 12.83 | 299 | 87.17 | 2.05 | 0.18 |
| White or Other | 5 | 23.81 | 16 | 76.19 | ||
| Income (monthly) | ||||||
| < $1000 | 39 | 15.73 | 209 | 84.27 | 3.43 | 0.064 |
| $1000+ | 10 | 8.62 | 106 | 91.38 | ||
| Education | ||||||
| <12th Grade, or GED/ High School Graduate | 43 | 13.65 | 272 | 86.35 | 0.07 | 0.79 |
| Some or Completed: College, Technical, or Graduate School | 6 | 12.24 | 43 | 87.76 | ||
| Employment | ||||||
| Yes | 6 | 7.79 | 71 | 92.21 | 2.69 | 0.1 |
| No | 43 | 14.98 | 244 | 85.02 | ||
| Mean | SD | Mean | SD | T | P | |
| Age* | 44.0204 | 11.8857 | 41.3238 | 11.459 | 1.48 | 0.14 |
Includes subjects whose concentration of Toxoplasma was considered equivocal.
T-test conducted based on TOXO status.
Psychiatric and substance abuse characteristics of the study subjects can be seen in Table S1. Approximately half of the participants had a substance use disorder currently or by history (n=185, 50.82%), and approximately 29.4% (n=107) were had a current or past cocaine use disorder. The most common psychiatric diagnosis in the study population was PTSD (57.42%, n= 209), followed by MDD (42.03% n=153), any primary Axis 1 psychosis (16.76%, n= 57), and SCZ (8.24%, n=30). A minority of the study sample did not have any psychiatric diagnoses, including any substance use disorder, and were designated the psychiatrically healthy control group (CON, 11.81%, n= 43). There was a significant comorbidity of substance use disorders and other psychiatric diagnoses such that 38% of those with a primary Axis 1 psychiatric disorder also had a substance use disorder currently or by history.
3.2. Seroprevalence and Seropositivity
The highest level of TOXO seroprevalence was found in the CON group, while the lowest level was in the MDD group. Because this difference could be due to age or other demographic factors, we further examined the association of TOXO with these psychiatric disorders in logistic regression analysis adjusting for demographic covariates (Table 2).
Table 2.
The Association of TOXO with each psychiatric condition adjusting for demographics1.
| OR | 95% CI | aOR | 95% CI | |
|---|---|---|---|---|
|
|
|
|
|
|
| Substance Use2 | 0.54 | (0.24, 1.23) | 0.52 | (0.215, 1.252) |
| Cocaine Use | 0.67 | (0.28, 1.59) | 0.72 | (0.26, 2.00) |
| Psychosis3 | 0.36 | (0.12, 1.08) | 0.31 | (0.09, 1.06) |
| Schizophrenia4 | 0.51 | (0.14, 1.81) | 0.53 | (0.136, 2.09) |
| PTSD4 | 0.45 | (0.20, 1.02) | 0.39 | (0.16, 0.91) |
| MDD4 | 0.31 | (0.1, 0.76) | 0.23 | (0.09, 0.61) |
| CON Subjects5 | Ref |
Demographic variables include age, race, sex, education, income, and employment.
Includes any drug of abuse and/or alcohol.
Includes subjects diagnosed with a primary psychotic disorder on SCID or MINI diagnostic interview.
Includes subjects diagnosed with the disorder on SCID or MINI diagnostic interview.
Includes any subject without a psychiatric disorder and no reported substance abuse. If they were missing any one of the psychiatric illness indicators, they were considered missing.
When comparing those with a given diagnosis to the CON group in regressions adjusting for demographics, there was an inverse association with TOXO seropositivity in those with MDD, i.e. the rate of TOXO positivity was lower in MDD subjects than in CON subjects (OR=0.23, 95% CI= (0.087, 0.613)). A similar inverse association with TOXO was seen among those with PTSD (OR=0.385, 95% CI= (0.163, 0.91)). Other diagnostic variables were not significantly related to TOXO seropositivity (Table 2).
3.3. Acoustic startle magnitude and latency
An initial analysis of the distribution of startle magnitude and latency was conducted to find the relative distribution of each outcome variable. The mean magnitude was 100.85 μV (SD=140.17), with a corresponding average latency of 75.32 ms (SD=31.67) among the whole cohort. The distribution of both magnitude and latency were right skewed, and were therefor normalized via Log10 transformation prior to analysis. The range of values of serointensity are from 0.5 IU/mL to 423, and the mean serointensity for those considered seropositive was 113 IU/mL (SD= 88.54) as compared to those considered seronegative, 3.80 IU/mL (SD=4.24).
To test the association of TOXO seropositivity with acoustic startle magnitude and latency, t-tests were conducted comparing those who were seropositive to those who were seronegative (Figure 1). TOXO seropositivity, before adjusting for any covariates, was associated with acoustic startle magnitude (t=2.87, p=0.014). The mean acoustic startle magnitude was significantly higher for TOXO seropositive, as compared to seronegative subjects (Figure 1). TOXO seropositivity remained significantly and positively associated with startle magnitude in a regression model adjusted for age, race, sex, education, income, and employment (Table 3). TOXO seropositivity did not show an association with acoustic startle latency in either a t-test (t=0.49, p=0.6252), or in a regression adjusted for demographic variables.
Figure 1.
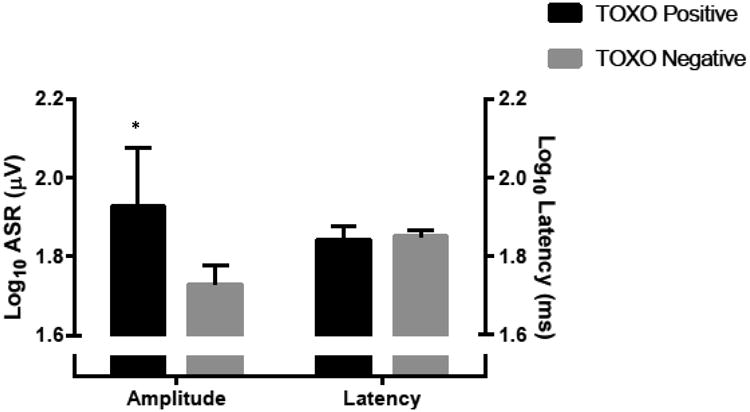
Two T-tests assessing the effect of Toxoplasma seropositivity on both Magnitude (T=2.53, p=0.014) and Latency (T=0.49, p=0.6252). * denotes significance at a level of p< 0.05
Table 3.
The association of TOXO with a Log10 (startle magnitude) outcome adjusting for demographic variables.
| Independent | N | β | SE | F | P value | |
|---|---|---|---|---|---|---|
| Dependent: Log10 (Magnitude) | Age | 364 | 0.00078 | 0.00215 | 0.13 | 0.7164 |
| Race (0=African American, 1= Other) | 0.17583 | 0.10292 | 2.92 | 0.0884 | ||
| Sex (0=Male, 1=Female) | -0.0053 | 0.05309 | 0.01 | 0.9209 | ||
| Education (0=no more than high school, 1=more than high school) | -0.065 | 0.07319 | 0.79 | 0.3753 | ||
| Income (0= <$12,000 per year, 1= ≥$12,000 per year) | 0.07823 | 0.05477 | 2.04 | 0.154 | ||
| Employment (0=No, 1=Yes) | -0.0544 | 0.06158 | 0.78 | 0.3772 | ||
| Toxoplasma (0= Negative, 1= Positive) | 0.19159 | 0.07038 | 7.41 | 0.0068 |
Given the high level of psychiatric morbidity in the sample we further examined the relationship of TOXO seropositivity with startle magnitude in a series of regression models in which each psychiatric and substance use variable (PTSD, MDD, SCZ, Psychosis, SUBST, COC), as well as CON group status were included individually in the models along with demographic factors (Tables S2-S8). The CON subjects, i.e. those without any psychiatric diagnosis, were compared in these models to those with ≥1 of the psychiatric diagnoses. In all these models, TOXO seropositivity remained significantly and independently associated with higher startle magnitude, with p values ranging from 0.004 to 0.015. There were similar effect sizes for the association of TOXO seropositivity with startle magnitude regardless of the psychiatric diagnosis that was stepped into the model (0.197 to 0.216; see Tables S2-S8). Furthermore, the association of TOXO seropositivity with startle magnitude remained significant in the regression models in which the reference group was dichotomized as mentally healthy versus having a history of any psychiatric illness (S8, p=0.0142, B=0.210). A sensitivity analysis was also conducted using a self-reported smoking variable, which was non-significant in all the models of startle magnitude. Specifically, in models with demographic covariates and TOXO seropositivity, current smoking was not associated with startle magnitude (p=0.42) but TOXO seropositivity remained a significant predictor of startle magnitude (p= 0.0335)
TOXO seropositivity was not associated with acoustic startle latency in a model with demographic factors (p=0.6305) (Table 4). The next series of analyses included the demographic covariates, and stepped in each psychiatric and substance abuse variable individually (PTSD, MDD, SSZ, substance abuse, Cocaine Use, Psychosis), as well as CON status. In contrast to our findings from similar models of startle magnitude, all of these models using startle latency with each individual psychiatric disorder found no significant association with TOXO. However, these models revealed a significant association of startle latency with the presence of PTSD (Table 5) such that latency was slower in subjects with PTSD (Beta = 0.037, p = 0.024). The rest of the psychiatric and substance use covariates all yielded p>0.05 for their respective associations with latency.
Table 4.
The association of TOXO with a Log10 (Latency) outcome adjusting for Demographic Variables.
| Independent Variables: | N | β | SE | F | P value | |
|---|---|---|---|---|---|---|
| Dependent: Log10 (Latency) | Age | 363 | 0.00073 | 0.00073 | 1.01 | 0.3165 |
| Race (0=African American, 1= Other) | -0.0314 | 0.0348 | 0.81 | 0.3678 | ||
| Sex (0=Male, 1=Female) | 0.02222 | 0.01797 | 1.53 | 0.2173 | ||
| Education (0=no more than high school, 1=more than high school) | -0.0151 | 0.02477 | 0.37 | 0.5438 | ||
| Income (0= <$12,000 per year, 1= ≥$12,000 per year) | 0.02392 | 0.0186 | 1.65 | 0.1993 | ||
| Employment (0=No, 1=Yes) | -0.0235 | 0.02085 | 1.27 | 0.2597 | ||
| Toxoplasma (0= Negative, 1= Positive) | -0.0115 | 0.0238 | 0.23 | 0.6305 | ||
Table 5.
The association of TOXO with a Log10 (Latency) outcome adjusting for Demographics adding the predictor PTSD.
| Independent | N | β | SE | F | P value | |
|---|---|---|---|---|---|---|
| Dependent: Log10 (Latency) | Age | 360 | 0.00073 | 0.00072 | 1.03 | 0.3116 |
| Race | -0.0396 | 0.03449 | 1.32 | 0.2517 | ||
| Sex | 0.01998 | 0.01789 | 1.25 | 0.2647 | ||
| Education | -0.0128 | 0.02448 | 0.27 | 0.601 | ||
| Income | 0.02306 | 0.01847 | 1.56 | 0.2128 | ||
| Employment | -0.0195 | 0.02065 | 0.9 | 0.3447 | ||
| Toxoplasma | -0.008 | 0.02359 | 0.11 | 0.7355 | ||
| PTSD (0= No PTSD, 1= PTSD) | 0.03714 | 0.01636 | 5.15 | 0.0238 |
3.4. TOXO Serointensity
Serointensity is a measure of TOXO titer and hence co-varies with dichotomized seropositivity, but provides additional information about the intensity of TOXO IgG immune response in a given serum specimen. From a subset of 341 subjects, from whom we had serointensity data, we ran similar analyses on startle magnitude and latency. In a model adjusting for demographic variables, non-African American race and TOXO serointensity were significant and independent contributors to a higher startle magnitude (Table 6.). In all subsequent analyses stepping in psychiatric covariates TOXO serointensity remained significantly associated with an increase in magnitude (S9-S15). Being of non-African American race also remained significantly associated with an increase in startle magnitude in these serointensity models except when the SCZ and CON variables were individually stepped into the models (S9-S15). Furthermore, we conducted a sensitivity analysis among the TOXO seropositive individuals only, and found a statistically non-significant positive association between serointensity and ASR (β=0.001, p=0.26, in a model adjusting for demographic covariates).
Table 6.
The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables.
| Independent | N | β | SE | F | P value | |
|---|---|---|---|---|---|---|
| Dependent: Log10 (Magnitude) | Age | 334 | 0.000736 | 0.0022 | 0.11 | 0.738 |
| Race (0=African American, 1= Other) | 0.21921 | 0.10396 | 4.45 | 0.0357 | ||
| Sex (0=Male, 1=Female) | 0.00797 | 0.05488 | 0.02 | 0.8846 | ||
| Education (0=no more than high school, 1=more than high school) | -0.04609 | 0.07399 | 0.39 | 0.5337 | ||
| Income (0= <$12,000 per year, 1= ≥$12,000 per year) | 0.08271 | 0.0564 | 2.15 | 0.1434 | ||
| Employment (0=No, 1=Yes) | -0.02794 | 0.06336 | 0.19 | 0.6595 | ||
| Serointensity (IU/mL) | 0.00151 | 0.000477 | 10.05 | 0.0017 |
In our analyses with TOXO serointensity we did not find a statistically-significant association with startle latency, and the association of PTSD with slower latency was slightly attenuated in this model compared to the model with seropositivity (S16, S17).
4. Discussion
We report a robust association between TOXO seropositivity and ASR magnitude. Although the literature includes numerous studies investigating the relationship between TOXO and psychiatric disorders, few studies have examined this involuntary startle response, which provides an index of neurocircuitry function that is less contaminated by various poorly-defined intervening variables affecting the relationship between TOXO and complex behaviors (Hinze-Selch et al. 2007; Pearce et al. 2013; Stock et al. 2014). In our study, startle magnitude was elevated in TOXO seropositive individuals even in analyses that adjusted for demographic variables and psychiatric diagnoses. Thus, the putative effect of TOXO on startle magnitude is not likely a secondary effect of TOXO on psychiatric illness, but rather more of an endophenotype that is detectable with this neurophysiological paradigm. Prior studies examining the effect of TOXO on visual motor reaction time have emphasized the potential role of socioeconomic confounders, but the current study found no significant effect of socioeconomic status on the relationship between TOXO seropositivity or serointensity and ASR magnitude (Gale et al. 2015; Pearce et al. 2014).
Several prior studies of TOXO in psychiatric illness, including SCZ and self-directed violence, have pointed to a more important role for high TOXO IgG titers (serointensity) as compared to seropositivity per se (Arling et al. 2009; Pedersen et al. 2012; Sutterland et al. 2015). Throughout the course of our analyses, TOXO serointensity and seropositivity contributed significantly to an increase in ASR magnitude. The mechanism by which a high TOXO serointensity might be correlated with this neurobehavioral outcome has not been established, but possible mechanisms include high neural cyst burden, parasite recrudescence, or molecular mimicry by TOXO of a host epitope (Pedersen et al. 2012). Hester at al. (2012) found that IgG antibodies to only a certain T. gondii antigens can identify mice with cerebral tachyzoite proliferation, which suggests that a more detailed assessment of IgG antibody specificities could elucidate mechanisms for the association between TOXO-titer and ASR in humans.
Most human studies of latent TOXO infection and behavior have relied on specialized populations such as students, military members, or hospitalized psychiatric patients. A strength of our study is that it has broader representation of an inner-city population. Our sample was predominately African American, and in some of our regression models, a significantly higher ASR magnitude was found among non-African American compared to African American race. This is in accord with our previously published finding (Hasenkamp et al. 2008). Nevertheless, TOXO remained a significant independent predictor of higher magnitude in models that included race.
Previous work focused on US veterans found a significant increase in ASR latency among those with TOXO as compared to those who were seronegative (Pearce et al. 2013). Another study conducted by Příplatová et al., found that TOXO had a significant effect on the latency of an acoustic reaction time test among those without a prepulse stimulus (Příplatová et al. 2014). The results of these two studies led us to hypothesize that TOXO seropositivity would have a significant impact on the ASR latency among members of this cohort. However, we did not detect any association between ASR latency and TOXO.
On the other hand, participants with PTSD had longer startle latency but no increase in ASR magnitude. Other studies of ASR latency and magnitude in PTSD have not reached a consensus. While the association between PTSD and longer startle latency in our population contrasts with a study conducted by Vrana et al., which found faster latency in PTSD, that study did not consider the effects of TOXO (Vrana et al. 2013). The literature on ASR magnitude in PTSD is also inconclusive. Butler et al. saw an increased ASR magnitude among PTSD patients as compared to controls, whereas Jovanovic et al. saw no association between ASR magnitude and the presence of PTSD among their population (Butler et al. 1990; Jovanovic et al. 2009). Similar to Jovanovic et al., we did not see a significant effect of PTSD on startle magnitude, even when TOXO was excluded from the model (Jovanovic et al. 2009). Thus the literature is inconclusive on the relationship between PTSD and ASR, perhaps because of ambiguities due to the unmeasured variability of the contextual threat potentially contributing to such inconsistencies (Pole 2007).
The mechanism for higher ASR magnitude among TOXO-infected participants is not known. ASR magnitude, a measure of habituation in response to repeated stimuli, has been found to be under partial control of amygdalar circuits and is potentially modulated by corticotropin releasing hormone, a component of the HPA axis (Poli and Angrilli 2015). Several authors have argued that the amygdala is targeted by TOXO, which might explain the ability of this parasitic infection in rodents to reduce fear of felines (Hari Dass and Vyas 2014; House et al. 2011; Mitra et al. 2013). Moreover, the HPA axis is activated by inflammatory cytokines such as (IL-6), tumor necrosis factor-α (TNF- α), and interferon-γ (IFN- γ), which are part of the inflammatory response to TOXO leading to the production of glucocorticoids. These glucocorticoids in turn influence neuroplasticity (Fabiani et al. 2015). TOXO directly alters neurotransmission by upregulating the production of dopamine, this upregulation of dopamine has been seen in animal models to elicit a similar effect in the magnitude of the ASR as compared to schizophrenia patients (Parlog et al. 2015; Swerdlow et al. 1986). A similar modulatory effect may derive from the ability of TOXO to induce the production of kynurenic acid, a known NMDA antagonist (Fabiani et al. 2015). However, the effects of NMDA antagonism on the ASR and pre-pulse inhibition are complex, and likely dependent upon the neuroanatomical locations involved (Koch 1999; Webster and McConkey 2010). Given the interconnectivity of both the HPA axis and amygdalar control of the fear response systems, and the way in which TOXO could affect both of these systems as well as certain neurotransmitters, more research is needed on the mechanisms by which TOXO modulates the fear response (Fabiani et al. 2015; Mitra et al. 2013; Weidenfeld et al. 2002).
Interestingly, we found an inverse association between TOXO and PTSD. This is consistent with a modulation of fear circuits by TOXO. However, we also found an inverse association of TOXO and MDD. All the while, our mentally healthy control group had the highest rate of TOXO seropositivity. The negative association found between TOXO and both PTSD and MDD was of particular interest in light of a study conducted by Markovitz et al. which is drawn from a comparable study population of inner city residents with similar demographics. Markovitz et al. found no association between TOXO seropositivity and PTSD or MDD, but found an association with general anxiety disorder (Markovitz et al. 2015). A recent study published while our current paper was under review, by Flegr and Escudero (2016), found an inverse association with TOXO seropositivity with MDD in a cohort of Eastern Europeans. While the effect in their large-scale cross-sectional study was not statistically significant for both genders, they were significant when stratified by sex. Given the larger proportion of females in our cohort, this supports our findings of an inverse association between MDD and TOXO, even though prior large-scale studies had previously found no association between TOXO and MDD (Cetinkaya et al. 2007; Hinze-Selch et al. 2007; Pearce et al. 2012; Sugden et al. 2016).
The present study is novel in that TOXO-associated differences in ASR magnitude have not been reported when assessing the acoustic startle response. While our sample size of individuals with neurophysiological response measures is large, our findings may not be generalizable to other populations in that our subject population was predominantly African American, impoverished, poorly educated, and highly traumatized. Other limitations are the lack of identified temporality in regards to both psychiatric conditions and TOXO infection, the significant comorbidity in both psychiatric and substance abuse conditions, and the cross-sectional study design. We cannot discern whether the positive association of TOXO serointensity with ASR is being driven by a long-established latent infection or a subclinical reactivation of the infection. The later possibility is suggested indirectly by the (n.s.) positive association between TOXO serointensity and ASR in analysis limited to the seropositive individuals. In the future, we would like to more effectively correlate particular PTSD psychiatric symptoms with the ASR, and account for TOXO as a potential modifier of either behavior or the ASR in this population. Furthermore, a biomolecular analysis of cytokines and other biomarkers from this population could help discern neurobehavioral mechanisms of TOXO.
Supplementary Material
Table S1. Psychiatric Diagnoses and Assessment Scores from a Cohort from the Grady Trauma Project
Table S2. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor PTSD.
Table S3. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor MDD.
Table S4. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Schizophrenia.
Table S5. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Substance Abuse.
Table S6. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Cocaine Use.
Table S7. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Psychosis.
Table S8. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Control Subjects.
Table S9. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor PTSD.
Table S10. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor MDD.
Table S11. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Schizophrenia.
Table S12. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Substance Abuse.
Table S13. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Cocaine Use.
Table S14. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Psychosis.
Table S15. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Control Subjects.
Table S16. The association of TOXO IgG serointensity with a Log10 (Latency) outcome adjusting for Demographic Variables.
Table S17. The association of TOXO IgG serointensity with a Log10 (Latency) outcome adjusting for Demographic Variables adding the predictor PTSD.
Highlights.
T. gondii seropositivity was significantly associated with elevated acoustic startle response magnitude regardless of psychiatric diagnosis.
Acoustic startle response latency was significantly longer in participants with PTSD, as compared to those without PTSD.
Those with major depressive disorder were significantly less likely to be infected with T. gondii as compared to controls adjusting for demographic variables.
Acknowledgments
Supported by the National Institutes of Mental Health (MH071537 and MH096764 to K.J.R., and R01-MH092512 to B.D.P) and the Department of Veterans Affairs Merit Review Program (CX000974-01 to E.D.). Infrastructure support from Grady Hospital; the Research and Development, Rehabilitation Research and Development, and Mental Health Service Lines, Atlanta Veterans Affairs Medical Center. We thank Dr. Kristina Butze-Mercer for assistance with demographic variables, and Dr. Adrianna Lori for assistance with data preparation. Dr. Duncan is a full time Attending Psychiatrist in the Mental Health Service Line of the Atlanta Veterans Affairs Medical Center, Decatur, Georgia. Dr. Norrholm is a full time employee of the Atlanta Veterans Affairs Medical Center. Nicholas Massa is a full time employee of the Atlanta Veterans Affairs Medical Center. The views of this paper do not necessarily reflect the views of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arling TA, Yolken RH, Lapidus M, Langenberg P, Dickerson FB, Zimmerman SA, Balis T, Cabassa JA, Scrandis DA, Tonelli LH, Postolache TT. Toxoplasma gondii antibody titers and history of suicide attempts in patients with recurrent mood disorders. The Journal of nervous and mental disease. 2009;3(12):905–908. doi: 10.1097/NMD.0b013e3181c29a23. [DOI] [PubMed] [Google Scholar]
- Berdoy M, Webster JP, Macdonald DW. Fatal attraction in rats infected with Toxoplasma gondii. Proc Biol Sci. 2000;267:1591–1594. doi: 10.1098/rspb.2000.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Archives of general psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Bremner JD. Traumatic stress: effects on the brain. Dialogues in clinical neuroscience. 2006;8(4):445. doi: 10.31887/DCNS.2006.8.4/jbremner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler RW, Braff DL, Rausch JL, Jenkins MA, Sprock J, Geyer MA. Physiological evidence of exaggerated startle response in a subgroup of Vietnam veterans with combat-related PTSD. The American journal of psychiatry. 1990;147(10):1308–12. doi: 10.1176/ajp.147.10.1308. [DOI] [PubMed] [Google Scholar]
- Cetinkaya Z, Yazar S, Gecici O, Namli MN. Anti-Toxoplasma gondii antibodies in patients with schizophrenia—preliminary findings in a Turkish sample. Schizophrenia bulletin. 2007;33(3):789–791. doi: 10.1093/schbul/sbm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccaro EF, Lee R, Groer MW, Can A, Coussons-Read M, Postolache TT. Toxoplasma gondii Infection: Relationship With Aggression in Psychiatric Subjects. The Journal of Clinical Psychiatry. 2016;77(3):334–341. doi: 10.4088/JCP.14m09621. [DOI] [PubMed] [Google Scholar]
- Cook TB, Brenner LA, Cloninger CR, Langenberg P, Igbide A, Giegling I, Hartmann AM, Konte B, Friedl M, Brundin L, Groer MW, Can A, Rujescu D, Postolache TT. “Latent” infection with Toxoplasma gondii: Association with trait aggression and impulsivity in healthy adults. Journal of psychiatric research. 2015;60:87–94. doi: 10.1016/j.jpsychires.2014.09.019. [DOI] [PubMed] [Google Scholar]
- Corcoran S, Norrholm SD, Cuthbert B, Sternberg M, Hollis J, Duncan E. Acoustic startle reduction in cocaine dependence persists for one year of abstinence. Psychopharmacology. 2011;215(1):93–103. doi: 10.1007/s00213-010-2114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson F, Stallings C, Origoni A, Katsafanas E, Schweinfurth L, Savage C, Khushalani S, Yolken R. Antibodies to Toxoplasma gondii and cognitive functioning in schizophrenia, bipolar disorder, and nonpsychiatric controls. The Journal of nervous and mental disease. 2014;202(8):589–593. doi: 10.1097/NMD.0000000000000166. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Jones JL. Toxoplasma gondii infection in humans and animals in the United States. International Journal for Parasitology. 2008;38(11):1257–78. doi: 10.1016/j.ijpara.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Duffy AR, Beckie TM, Brenner LA, Beckstead JW, Seyfang A, Postolache TT, Groer MW. Relationship Between Toxoplasma gondii and Mood Disturbance in Women Veterans. Military medicine. 2015;180(6):621–625. doi: 10.7205/MILMED-D-14-00488. [DOI] [PubMed] [Google Scholar]
- Efferen TR, Duncan EJ, Szilagyi S, Chakravorty S, Adams JU, Gonzenbach S, Angrist B, Butler P, Rotrosen J. Diminished acoustic startle in chronic cocaine users. Neuropsychopharmacol. 2000;22(1):89–96. doi: 10.1016/S0893-133X(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Fabiani S, Pinto B, Bonuccelli U, Bruschi F. Neurobiological studies on the relationship between toxoplasmosis and neuropsychiatric diseases. Journal of the Neurological Sciences. 2015;351(1–2):3–8. doi: 10.1016/j.jns.2015.02.028. doi: http://dx.doi.org/10.1016/j.jns.2015.02.028. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. Biometrics Research, New York State Psychiatric Institute; New York, NY: 2001. [Google Scholar]
- Flandreau E, et al. Cell type-specific modifications of corticotropin-releasing factor (CRF) and its type 1 receptor (CRF 1) on startle behavior and sensorimotor gating. Psychoneuroendocrinology. 2015;53:16–28. doi: 10.1016/j.psyneuen.2014.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegr J, Preiss M, Klose J, Havlicek J, Vitakova M, Kodym P. Decreased level of psychobiological factor novelty seeking and lower intelligence in men latently infected with the protozoan parasite Toxoplasma gondii Dopamine, a missing link between schizophrenia and toxoplasmosis? Biol Psychol. 2003;63(3):253–68. doi: 10.1016/s0301-0511(03)00075-9. doi:S0301051103000759[pii] [DOI] [PubMed] [Google Scholar]
- Flegr J. Influence of latent Toxoplasma infection on human personality, physiology and morphology: pros and cons of the Toxoplasma–human model in studying the manipulation hypothesis. The Journal of experimental biology. 2013;216(1):127–133. doi: 10.1242/jeb.073635. [DOI] [PubMed] [Google Scholar]
- Flegr J, Hrdý I. Influence of chronic toxoplasmosis on some human personality factors. Folia Parasitol. 1994;41:122–126. [PubMed] [Google Scholar]
- Flegr J, Escudero DQ. Impaired health status and increased incidence of diseases in Toxoplasma-seropositive subjects-an explorative cross-sectional study. Parasitology. 2016;10:1–16. doi: 10.1017/S0031182016001785. [DOI] [PubMed] [Google Scholar]
- Gale SD, Brown BL, Berrett A, Erickson LD, Hedges DW. Association between latent toxoplasmosis and major depression, generalised anxiety disorder and panic disorder in human adults. Folia parasitologica. 2014;61(4):285. [PubMed] [Google Scholar]
- Gale SD, Brown BL, Erickson LD, Berrett A, Hedges DW. Association between latent toxoplasmosis and cognition in adults: a cross-sectional study. Parasitology. 2015;142(4):557–65. doi: 10.1017/s0031182014001577. [DOI] [PubMed] [Google Scholar]
- GDoC Federal Poverty Guidelines. [Accessed April 19, 2016]; https://dch.georgia.gov/federal-poverty-guidelines-0.
- Gillespie CF, Bradley B, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma Exposure and Stress-Related Disorders in Inner City Primary Care Patients. General hospital psychiatry. 2009;31(6):505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamdani N, Daban-Huard C, Lajnef M, Gadel R, Le Corvoisier P, Delavest M, Carde S, Lépine JP, Jamain S, Houenou J, Galeh B, Richard JR, Aoki M, Charron D, Krishnamoorthy R, Yolken R, Dickerson F, Tamouza R, Leboyer M. Cognitive deterioration among bipolar disorder patients infected by Toxoplasma gondii is correlated to interleukin 6 levels. Journal of affective disorders. 2015;179:161–166. doi: 10.1016/j.jad.2015.03.038. [DOI] [PubMed] [Google Scholar]
- Hamdani N, Daban-Huard C, Lajnef M, Richard JR, Delavest M, Godin O, Le Guen E, Vederine FE, Lépine JP, Jamain S, Houenou J, Le Corvoisier P, Aoki M, Moins-Teisserenc H, Charron D, Krishnamoorthy R, Yolken R, Dickerson F, Tamouza R, Leboyer M. Relationship between Toxoplasma gondii infection and bipolar disorder in a French sample. Journal of affective disorders. 2013;148(2-3):444–8. doi: 10.1016/j.jad.2012.11.034. [DOI] [PubMed] [Google Scholar]
- Hari Dass SA, Vyas A. Toxoplasma gondii infection reduces predator aversion in rats through epigenetic modulation in the host medial amygdala. Molecular ecology. 2014;23(24):6114–22. doi: 10.1111/mec.12888. [DOI] [PubMed] [Google Scholar]
- Hasenkamp W, Norrholm SD, Green A, Lewison B, Boshoven B, Keyes M, Duncan E. Differences in startle reflex and prepulse inhibition in European-Americans and African-Americans. Psychophysiology. 2008;45(5):876–882. doi: 10.1111/j.1469-8986.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havlícek J, Gasová Z, Smith AP, Zvára K, Flegr J. Decrease of psychomotor performance in subjects with latent “asymptomatic” toxoplasmosis. Parasitology. 2001;122:515–520. doi: 10.1017/s0031182001007624. [DOI] [PubMed] [Google Scholar]
- Hester J, Mullins J, Sa Q, Payne L, Mercier C, Cesbron-Delauw M, Suzuki Y. Toxoplasma gondii antigens recognized by IgG antibodies differ between mice with and without active proliferation of tachyzoites in the brain during the chronic stage of infection. Infect Immun. 2012;80(10):3611–20. doi: 10.1128/IAI.00604-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinze-Selch D, Däubener W, Eggert L, Erdag S, Stoltenberg R, Wilms S. A controlled prospective study of Toxoplasma gondii infection in individuals with schizophrenia: beyond seroprevalence. Schizophrenia bulletin. 2007;33(3):782–788. doi: 10.1093/schbul/sbm010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House PK, Vyas A, Sapolsky R. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PLoS One. 2011;6(8):e23277. doi: 10.1371/journal.pone.0023277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JL, Dargelas V, Roberts J, Press C, Remington JS, Montoya JG. Risk factors for Toxoplasma gondii infection in the United States. Clinical Infectious Diseases. 2009;49(6):878–84. doi: 10.1086/605433. [DOI] [PubMed] [Google Scholar]
- Jones JL, Kruszon-Moran D, Wilson M, McQuillan G, Navin T, McAuley JB. Toxoplasma gondii infection in the United States: seroprevalence and risk factors. American Journal of Epidemiology. 2001;154(4):357–65. doi: 10.1093/aje/154.4.357. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear Potentiation and Fear Inhibition in a Human Fear-Potentiated Startle Paradigm. Biological Psychiatry. 2005;57(12):1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav Neurosci. 2006;120(5):995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Sakoman AJ, Esterajher S, Kozarić-Kovačić D. Altered resting psychophysiology and startle response in Croatian combat veterans with PTSD. International Journal of Psychophysiology. 2009;71(3):264–268. doi: 10.1016/j.ijpsycho.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminsky Z, et al. Epigenetic and genetic variation at SKA2 predict suicidal behavior and post-traumatic stress disorder. Translational psychiatry. 2015;5:e627. doi: 10.1038/tp.2015.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg SH, McHugh PF, Bell K, Schluger JH, Schluger RP, LaForge KS, Ho A, Kreek MJ. The Kreek-McHugh-Schluger-Kellogg scale: a new, rapid method for quantifying substance abuse and its possible applications. Drug Alcohol Depend. 2003;69(2):137–50. doi: 10.1016/s0376-8716(02)00308-3. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Progress in neurobiology. 1999;59(2):107–128. doi: 10.1016/s0301-0082(98)00098-7. doi: http://dx.doi.org/10.1016/S0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Kodym P, Machala L, Roháčová H, Širocká B, Malý M. Evaluation of a commercial IgE ELISA in comparison with IgA and IgM ELISAs, IgG avidity assay and complement fixation for the diagnosis of acute toxoplasmosis. Clinical Microbiology and Infection. 2007;13:40–47. doi: 10.1111/j.1469-0691.2006.01564.x. [DOI] [PubMed] [Google Scholar]
- Markovitz AA, Simanek AM, Yolken RH, Galea S, Koenen KC, Chen S, Aiello AE. Toxoplasma gondii and anxiety disorders in a community-based sample. Brain, behavior, and immunity. 2015;43:192–197. doi: 10.1016/j.bbi.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Mendy A, Vieira ER, Albatineh AN, Gasana J. Immediate rather than delayed memory impairment in older adults with latent toxoplasmosis. Brain, behavior, and immunity. 2015;45:36–40. doi: 10.1016/j.bbi.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Mitra R, Sapolsky RM, Vyas A. Toxoplasma gondii infection induces dendritic retraction in basolateral amygdala accompanied by reduced corticosterone secretion. Disease models & mechanisms. 2013;6(2):516–20. doi: 10.1242/dmm.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nylocks KM, Michopoulos V, Rothbaum AO, Almli L, Gillespie CF, Wingo A, Schwartz AC, Habib L, Gamwell KL, Marvar PJ, Bradley B, Ressler KJ. An angiotensin-converting enzyme (ACE) polymorphism may mitigate the effects of angiotensin-pathway medications on posttraumatic stress symptoms. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2015;168b(4):307–15. doi: 10.1002/ajmg.b.32313. [DOI] [PubMed] [Google Scholar]
- Okusaga O, Langenberg P, Sleemi A, Vaswani D, Giegling I, Hartmann AM, Konte B, Friedl M, Groer MW, Yolken RH, Rujescu D, Postolache TT. Toxoplasma gondii antibody titers and history of suicide attempts in patients with schizophrenia. Schizophrenia research. 2011;133(1):150–155. doi: 10.1016/j.schres.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Parlog A, Schluter D, Dunay IR. Toxoplasma gondii-induced neuronal alterations. Parasite immunology. 2015;37(3):159–70. doi: 10.1111/pim.12157. [DOI] [PubMed] [Google Scholar]
- Pearce BD, Hubbard S, Rivera HN, Wilkins PP, Fisch MC, Hopkins MH, Hasenkamp W, Gross R, Bliwise N, Jones JL, Duncan E. Toxoplasma gondii exposure affects neural processing speed as measured by acoustic startle latency in schizophrenia and controls. Schizophrenia research. 2013;150(1):258–261. doi: 10.1016/j.schres.2013.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD, Kruszon-Moran D, Jones JL. The Relationship Between Toxoplasma Gondii Infection and Mood Disorders in the Third National Health and Nutrition Survey. Biol Psychiatry. 2012;72:290–295. doi: 10.1016/j.biopsych.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce BD, Kruszon-Moran D, Jones JL. The association of Toxoplasma gondii infection with neurocognitive deficits in a population-based analysis. Social psychiatry and psychiatric epidemiology. 2014;49(6):1001–1010. doi: 10.1007/s00127-014-0820-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen MG, Mortensen PB, Norgaard-Pedersen B, Postolache TT. Toxoplasma gondii infection and self-directed violence in mothers. Archives of general psychiatry. 2012;69(11):1123–1130. doi: 10.1001/archgenpsychiatry.2012.668. [DOI] [PubMed] [Google Scholar]
- Poirotte C, Kappeler PM, Ngoubangoye B, Bourgeois S, Moussodji M, Charpentier MJ. Morbid attraction to leopard urine in Toxoplasma-infected chimpanzees. Current Biology. 2016;26(3):R98–R99. doi: 10.1016/j.cub.2015.12.020. [DOI] [PubMed] [Google Scholar]
- Pole N. The psychophysiology of posttraumatic stress disorder: a meta-analysis. Psychol Bull. 2007;133(5):725–46. doi: 10.1037/0033-2909.133.5.725. [DOI] [PubMed] [Google Scholar]
- Poli E, Angrilli A. Greater general startle reflex is associated with greater anxiety levels: a correlational study on 111 young women. Frontiers in behavioral neuroscience. 2015;9 doi: 10.3389/fnbeh.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Příplatová L, Šebánková B, Flegr J. Contrasting effect of prepulse signals on performance of Toxoplasma-infected and Toxoplasma-free subjects in an acoustic reaction times test. PloS one. 2014;9(11):e112771. doi: 10.1371/journal.pone.0112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Bekh Bradley, Tanja Jovanovic. Grady Trauma Project. 2016 < http://gradytraumaproject.com/project/project-overview/>.
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- Skinner HA. The Drug Abuse Screening Test. Addict Behav. 1982;7(4):363–71. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Stock AK, Heintschel von Heinegg E, Kohling HL, Beste C. Latent Toxoplasma gondii infection leads to improved action control. Brain Behav Immun. 2014;37:103–8. doi: 10.1016/j.bbi.2013.11.004. [DOI] [PubMed] [Google Scholar]
- Sugden K, Moffitt TE, Pinto L, Poulton R, Williams BS, Caspi A. Is Toxoplasma Gondii Infection Related to Brain and Behavior Impairments in Humans? Evidence from a Population-Representative Birth Cohort. PLoS One. 2016;11(2):e0148435d. doi: 10.1371/journal.pone.0148435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutterland A, et al. Acta Psychiatrica Scandinavica. 2015. Beyond the association. Toxoplasma gondii in schizophrenia, bipolar disorder, and addiction: systematic review and meta-analysis. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Host resistance in the brain against Toxoplasma gondii. Journal of Infectious Diseases 185 Suppl. 2002;1:S58–65. doi: 10.1086/337999. [DOI] [PubMed] [Google Scholar]
- Swerdlow N, Braff D, Geyer M, Koob G. Central dopamine hyperactivity in rats mimics abnormal acoustic startle response in schizophrenics. Biological psychiatry. 1986;21(1):23–33. doi: 10.1016/0006-3223(86)90005-3. [DOI] [PubMed] [Google Scholar]
- Torrey EF, Bartko JJ, Yolken RH. Toxoplasma gondii and other risk factors for schizophrenia: an update. Schizophr Bull. 2012;38:642–647. doi: 10.1093/schbul/sbs043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF, Yolken RH. Schizophrenia and toxoplasmosis. Schizophrenia bulletin. 2007;33(3):727–8. doi: 10.1093/schbul/sbm026. doi:sbm026[pii]10.1093/schbul/sbm026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana SR, Calhoun PS, McClernon FJ, Dennis MF, Lee ST, Beckham JC. Effects of smoking on the acoustic startle response and prepulse inhibition in smokers with and without posttraumatic stress disorder. Psychopharmacology. 2013;230(3):477–85d. doi: 10.1007/s00213-013-3181-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster JP, Brunton CFA, Macdonald DW. Effect of Toxoplasma gondii upon neophobic behaviour in wild brown rats, Rattus norvegicus. Parasitology. 1994;109:37–43. doi: 10.1017/s003118200007774x. [DOI] [PubMed] [Google Scholar]
- Webster JP, McConkey GA. Toxoplasma gondii-altered host behaviour: clues as to mechanism of action. Folia Parasitologica. 2010;57(2):95–104. doi: 10.14411/fp.2010.012. [DOI] [PubMed] [Google Scholar]
- Weidenfeld J, Newman ME, Itzik A, Gur E, Feldman S. The amygdala regulates the pituitary-adrenocortical response and release of hypothalamic serotonin following electrical stimulation of the dorsal raphe nucleus in the rat. Neuroendocrinology. 2002;76(2):63–69. doi: 10.1159/000064430. [DOI] [PubMed] [Google Scholar]
- Yolken RH, Bachmann S, Ruslanova I, Lillehoj E, Ford G, Torrey EF, Schroeder J. Antibodies to Toxoplasma gondii in individuals with first-episode schizophrenia. Clin Infect Dis. 2001;32:842–844. doi: 10.1086/319221. [DOI] [PubMed] [Google Scholar]
- Yolken R, Dickerson F, Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunology. 2009;31(11):706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Psychiatric Diagnoses and Assessment Scores from a Cohort from the Grady Trauma Project
Table S2. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor PTSD.
Table S3. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor MDD.
Table S4. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Schizophrenia.
Table S5. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Substance Abuse.
Table S6. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Cocaine Use.
Table S7. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Psychosis.
Table S8. The association of TOXO with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Control Subjects.
Table S9. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor PTSD.
Table S10. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor MDD.
Table S11. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Schizophrenia.
Table S12. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Substance Abuse.
Table S13. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Cocaine Use.
Table S14. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Psychosis.
Table S15. The association of TOXO IgG serointensity with a Log10 (Magnitude) outcome adjusting for Demographic Variables adding the predictor Control Subjects.
Table S16. The association of TOXO IgG serointensity with a Log10 (Latency) outcome adjusting for Demographic Variables.
Table S17. The association of TOXO IgG serointensity with a Log10 (Latency) outcome adjusting for Demographic Variables adding the predictor PTSD.


