Summary
The study of visual systems has a rich history, leading to the discovery and understanding of basic principles underlying the elaboration of neuronal connectivity. Recent work in model organisms such as fly, fish and mouse has yielded a wealth of new insights into visual system wiring. Here, we consider how axonal and dendritic patterning in columns and laminae influence synaptic partner selection in these model organisms. We highlight similarities and differences among disparate visual systems with the goal of identifying common and divergent principles for visual system wiring.
Introduction: Pre- and Post-specification of Visual System Synapses During Development
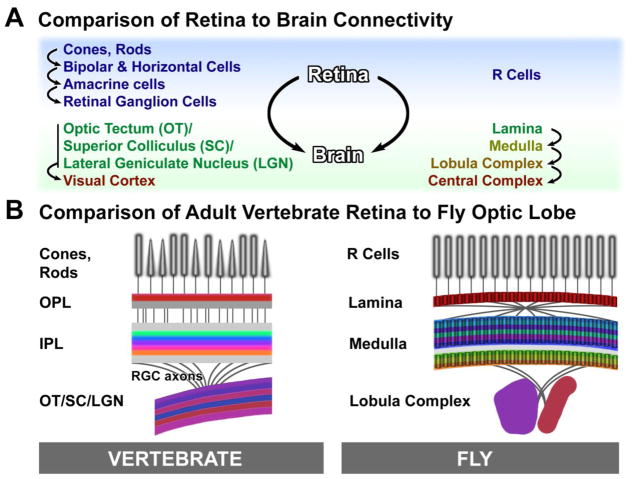
Invertebrate and vertebrate visual systems map color, motion, and feature information onto retinotopic visual maps in the brain. However, the actual anatomical structures are quite different. Fly photoreceptors (R cells) are the primary retinal output neurons that carry visual information to the first and second visual system relay stations (Fig. 1A). In contrast, visual information from photoreceptors in the vertebrate eye is extensively processed within the retina. Like R cells in flies, vertebrate retinal ganglion cells (RGCs) convey information to the first visual system relay stations in the brain, including the optic tectum/superior colliculus (OT/SC), lateral geniculate nucleus (LGN), and numerous other retinorecipient nuclei. Hence, with respect to retina output, fly R cells and vertebrate RGCs are comparable (Fig. 1A). In contrast, at the level of connectivity and visual information processing, the two synaptic plexiform layers upstream of RGCs in the vertebrate retina are comparable to brain neuropils downstream of R cells in the fly optic lobe (Fig. 1B): the vertebrate retina outer plexiform layer (OPL) to the fly lamina, and the inner plexiform layer (IPL) to the fly distal medulla [1]. These comparisons make sense in terms of circuit connectivity and function, but the actual structures and cell types are not analogous. For example, a subset of RGCs that are intrinsically photosensitive reveal that RGCs may share evolutionary origins with invertebrate photoreceptor neurons [2,3]; vertebrates may have evolved modern retinal connectivity subsequent to development of the first photosensitive cells, while connectivity in the fly lamina and medulla may have evolved independently and downstream of retinal output (Fig. 1A, B).
Fig. 1.
Adult vertebrate and fly visual system wiring. (A): Comparison of retina-to-brain connectivity based on retina output neurons and possible evolutionary relationships between vertebrate RGCs and fly R cells. (B) Comparison of vertebrate retina to the fly optic lobe based on similarities of functional connectivity.
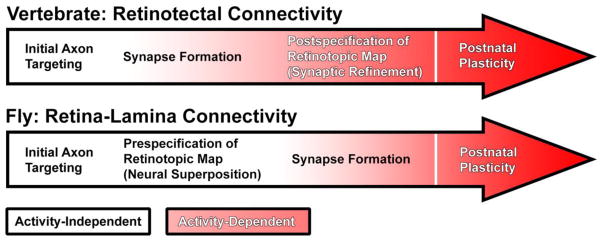
Similar design principles among disparate visual circuit ensembles may be best appreciated in the context of shared developmental processes that orchestrate iterative patterns of synaptic connectivity [4]. Synaptic specification is determined by two core processes: (1) precision of wiring prior to initial synapse formation (pre-specification); and (2) pruning and fine-tuning of connections (post-specification). In vertebrates, activity-dependent fine-tuning of synaptic specificity plays an important role in visual system connectivity, showcasing the importance of post-specification (Fig. 2) [5–7]. In contrast, visual system wiring in Drosophila appears to be predominantly determined by a genetic program, highlighting pre-specification (Fig. 2) [4,8,9]. However, in both systems pre- and post-specification likely work hand in hand: initial axonal and dendritic targeting to distinct columnar or laminar structures provides important milestones along the road to mature synaptic specificity [1,4,10,11].
Fig. 2.
Pre-specification and post-specification of synapses in vertebrate retinotectal connectivity versus fly retina-lamina connectivity
In fly and vertebrate visual systems, processing of parallel information streams is morphologically preserved in repetitive columns or mosaics of similar cell types. Orthogonal to this lateral organization is the prevalent subdivision of visual system components into layers, or laminae; these elements provide anatomically restricted regions where presumptive synaptic partners are in close proximity and facilitate synaptic partner identification, revealing common and divergent developmental principles across visual systems.
Columns and Mosaics in Synaptic Specification
During development, vertebrate cones and rods extend short axon terminals that contact horizontal cell dendrites and axons, respectively, and also rod and cone bipolar cell dendrites. Since photoreceptor projections to these interneurons are short and anatomically parallel, retinotopy is maintained in both the OPL and IPL (Fig. 3A). In contrast, during larval development fly photoreceptors extend long axons that project from the developing eye disc into the brain (Fig. 3B). Vertebrate retina output neurons, RGCs, also maintain retinotopy in their central projections to certain retinorecipient regions. Topographic mapping of RGC axons onto the tectum/superior colliculus is facilitated by orthogonal EphA/ephrin-A and EphB/ephrin-B gradients [5]. These gradients establish topographic mapping through relative, not absolute, levels of ephrin signaling to RGC axons [5,12]. Drosophila has a single Eph gene that is expressed in a gradient in the early developing medulla, so fly R cells may also respond to relative, and not absolute, levels of Eph receptor activity [13]. Therefore, Eph/ephrin signaling may contribute to synaptic pre-specification without providing an absolute synaptic address system.
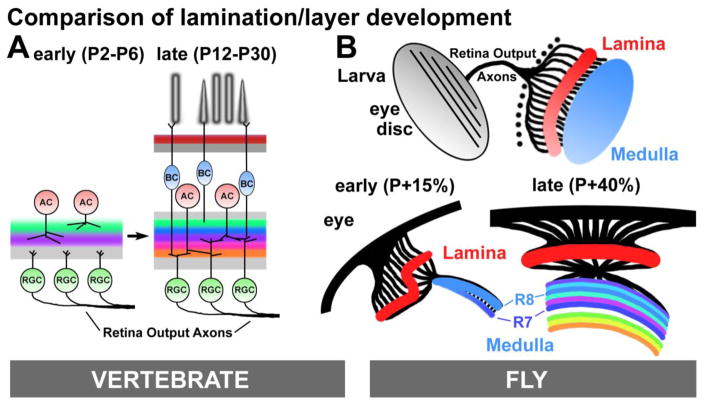
Fig. 3.
Development of layers/lamination in the vertebrate retina compared to the fly optic lobe. (A): early during vertebrate retina development, ACs start forming laminae, while RGC axons remain in a waiting layer. Between P12-P30 RGCs and BCs target pre-existing layers to form contacts. (B) During Drosophila visual system development, R1–R8 grow through the optic stalk into the lamina (R1–R6) and medulla (R7, R8) in a temporal wave. Early during puparium formation (P+15%) medulla layer development still exhibits a temporal gradient, but layer development synchronizes shortly thereafter.
In flies, adjacent columns that process information from neighboring visual fields are called ‘cartridges’ in the lamina and ‘columns’ in the medulla. Lamina cartridges exhibit an intricate wiring pattern that reflects the optical organization of the retina according to the principle of neural superposition [1,8,9]. Neural superposition is an interesting case of pre-specification. Owing to the optics of the overlying retina ommatidia, each lamina cartridge receives input from 6 R cells that each project from a different ommatidium. Though this creates an intricate wiring problem, a few simple pattern formation rules can generate correct axon sorting [14,15]. This sorting step is genetically separable from synapse formation, and in large part pre-specifies synaptic partners since the correct number of synapses form between incorrect partners when sorting is aberrant [8]. These simple rules must be executed by molecular mechanisms that ensure patterning and such mechanisms have been identified, providing support for the idea that 2-dimensional differential adhesion is achieved through the action of cell adhesion molecules such as N-cadherin and the proto-cadherin flamingo [9,16]. The sorting process synchronously organizes each column without the need for a large number of different cues to selectively label neighboring columns, demonstrating the utilization of cell adhesion molecules to establish overall patterning of connections as opposed to synapse-specific targeting cues.
In the vertebrate retina no clear columnar organization develops that maps, point-to-point, neighboring regions of the visual field to synaptic ensembles. However, in the SC columnar organization has been shown to arise in tOFF-alpha-RGCs through spontaneous activity-dependent axon segregation [17], and in the visual cortex columnar organization is morphologically well-characterized [18,19]. In contrast to flies, columnar organization in part emerges through activity-dependent mechanisms in vertebrate brain regions, whereas in the retina a stochastic distribution and mosaic spacing of retinal neurons prevails [1,20,21]. Non-random mosaic spacing is achieved through molecular cues including MEGF10 and MEGF11 [20]. Photoreceptors, postsynaptic bipolar cells (BCs) and horizontal cells (HCs) in the outer retina create cellular mosaics that obey distribution and spacing rules. Amacrine cells (ACs) in the inner nuclear layer, and displaced ACs and RGCs in the ganglion cell layer, also display a mosaic distribution [17,22]. Self-avoidance is a key principle that contributes to the elaboration of non-overlapping dendritic processes from the same neuron [23], and is most famously executed by DSCAM in flies [24] and protocadherins in vertebrates [21,25]. Tiling among neuronal process arborizations from individual neurons of the same subtype, as observed in certain RGC dendrites and bipolar cell axons and dendrites [22,26], prevents interneuronal process overlap. Together, neuronal mosaic spacing, self-avoidance, and tiling assure maximal receptive field coverage and preservation of retinotopy.
Similarities between fly and vertebrate retina development are apparent in the context of lateral cell-cell interactions. Notch-dependent lateral inhibition can create evenly distributed neuronal architecture during neuronal branch elaboration in the fly medulla [27]. Restriction of axon branches to single columns is a process akin to tiling and is observed for several columnar neurons, including L1 in medulla columns [28]. Similarly, dendritic fields of the postsynaptic Tm20 cells observe columnar restriction in the medulla. Different molecular mechanisms execute underlying non-self avoidance/repulsion: the cell-surface molecule Dscam2 autonomously in L1 [28], and R cell-derived activin non-autonomously in Tm20 [29]. In addition, intrinsic transcriptional regulation contributes to R7 axon columnar restriction [30].
In contrast to processes that obey columnar restriction, Dm8 dendrites, the main R7 postsynaptic partners, span 10–16 medulla columns [31]. Individual Dm8s exhibit variable, non-deterministic coverage patterns, raising questions about synaptic specificity [29,32–34]. Recent findings on Ig superfamily cell adhesion molecules (21 Dprs and 9 DIPs) show that different Dm8 subtypes and their specific presynaptic R7 partners express interacting molecular pairs [35,36]. Dpr11 exhibits remarkably specific expression in R7 cells of the ‘yellow’ type (yR7), and it is unclear if an identical number of Dm8s express the presumptive matching interaction partner DIP-γ. If the synaptic matchmaking code is strict, Dpr11-positive yR7 cells must either always reside in columns with a matching Dm8, or form no synapses with a resident non-matching Dm8. EM reconstruction of 7 medulla columns only showed Dm8 cells that form synapses with both R7s in the home column, and up to 15 neighboring columns [33,37] (S. Takemura, pers. comm.). A strict code would require that these synapses in neighboring columns are selective only for matching types. If the code were less strict, however, proximity of pre- and postsynaptic processes within a column could provide a simple explanation for the observed synapse distribution.
In the vertebrate visual system, synapses between ACs and RGCs in the IPL were thought to be a simple function of the degree of lateral overlap among their dendritic processes within the same lamina, a principle called Peter’s rule [38]. However, a 10-fold increase in synaptic specificity between W3B RGCs and VG3 ACs is dependent upon the homophilic Ig superfamily adhesion molecule sidekick2 [10]. Therefore, a strict application of Peter’s rule is insufficient in this case, and sidekick2 likely adds specificity to the establishment of precise connections in this crowded region of the retina. The same may apply to Dm8s in the fly medulla: Peter’s rule may facilitate synapse formation between Dm8 and R7s within its home column, while molecular interactions may further specify which synapses form or are stabilized.
In summary, the following common principles, none of which on their own are sufficient to determine synaptic specificity, underlie the development of retinotopic organization in both flies and vertebrates:
Synchronous lateral sorting and restricting neuronal processes that will subsequently define synaptic partners in a 2D plane (‘stay out of 3D’) facilitates pattern formation and limited prespecification of synaptic partners during development.
Intrinsic intra-neuronal spacing involving process self-avoidance, cell-autonomous control of branching, and neuronal outgrowth promotion or restriction sets receptive field parameters.
Extrinsically controlled inter-neuronal spacing of cell bodies to achieve mosaics, and of process overlap to achieve tiling, facilitates columnar restriction and stochastic spacing of neurons and their processes.
Pattern formation mechanisms bring correct pre- and postsynaptic processes into the same local vicinity (Peter’s rule), with molecular cues being required subsequently to increase specificity in crowded CNS regions.
The Role of Layers and Laminae for Synaptic Specification
Most of the principles discussed above are implemented synchronously in a defined, two-dimensional layer. The development of layers is a process of temporal succession; new layers emerge through relative positioning based on sequential addition of neuronal processes [39–41]. During vertebrate retina development, transitional stages of layer formation prefigure complex IPL laminar organization (Fig. 3A). Developmental and live imaging studies establish that RGCs are born early, however ACs are the first to extend neurites into the nascent IPL [41]. Very early in the establishment of mouse IPL stratification, initial domains that influence the targeting of select ACs are defined by complementary expression of the repellent semaphorin 6A (Sema6A) and its receptor plexin A4 (PlexA4) [42]. At this time only a few transitional layers are present and neural processes position themselves relative to each other. Similarly, in the fly medulla early R7 growth cones stop relative to R8 growth cones just after passing them (Fig. 3B). R7 growth cones never actively extend thereafter, forming early contacts with their main postsynaptic partners, Dm8; new layers form through intercalations between these early R8 and R7 layers [32,43] (Fig. 3B). Hence, an R7 layer is stabilized very early in what will become adult layer M6, but neither adult M6 morphology nor its molecular markers adequately predict the transitional stage.
In contrast to lateral pattern formation mechanisms (mosaic spacing, self-avoidance, and tiling), clearly defined repulsive and attractive cues for transitional target regions play critical roles in elaborating IPL lamination. In the absence of the transmembrane repellents semaphorin 5A and Sema5B, RGCs, ACs, and BCs all extend numerous processes from the IPL to ectopic locations in the outer retina, suggesting that Sema5A/5B together contribute to retinal organization and maintain the overall OPL and the IPL separation [39]. Sema6A/PlexA4-dependent development of transitional lamination generates a non-permissive zone in the inner regions of the IPL from the earliest times of IPL development that maintains the positioning of a limited number of ACs that normally project in the outermost S1 IPL region [42]. Sema6A also regulates laminar stratification of starburst amacrine cells (SACs), interneurons that project in either lamina S2 (Off SACs) or lamina S4 (On SACs) of the IPL, and Sema6A-mediated repulsion also prevents SAC dendrite mis-stratification [44]. Though many retinal neurons that project dendrites within the ON region of the IPL express Sema6A, the select expression of the Sema6A receptors PlexA4 and A2 imparts specificity to subtype-specific Sema6A-mediated laminar targeting.
Select adhesive interactions are also critical for targeting specific classes of retinal neurons to the lamina. Immunoglobulin (Ig) super family homophilic adhesion molecules with unique IPL distribution patterns identified in the chick retina play important roles in directing IPL laminar organization [45,46]. Adhesion molecules contribute to the assembly of functional retinal circuitry, as exemplified by type II cadherins and their role in wiring up direction selective circuits in the IPL [47]. Type 2 Off’ BCs express cadherin 8 (Cdh8), and ‘Type 5 On’ BCs express Cdh9. Cell-type specific Cdh8 and Cdh9 loss- and gain-of-function experiments demonstrate that these cadherins are required to direct these two BC types to their distinct laminar strata. Swapping the two cadherins between these BCs is sufficient to redirect type 2 Off BC axons to the strata normally occupied by type 5 Off BCs, and visa versa. Loss of Cdh8 compromises Off direction selective responses, whereas loss of Cdh9 affects On direction selectivity. BC axons stratify after IPL laminae are already formed by ACs and RGC dendrites. Therefore, BC axons achieve their specific laminar positioning in the context of pre-existing laminae by utilizing adhesive cues to select appropriate laminar targets.
In flies, some cell types also initially target temporary layers and later actively extend to layers that are formed different cells. For example, L3 interneuron growth cones reach their correct medulla target layer through a two-step process, suggesting similarities to vertebrate retina IPL development [39]. A combination of repulsive signaling mediated by semaphorin-1a functioning in L3 neurons and adhesive functions among L neurons mediated by more broadly expressed N-cadherin mediates initial axon targeting in the fly medulla. The role of N-cadherin is often interpreted as ‘broadly adhesive’ and functioning redundantly with other factors, in part because mutant neurons often target correctly during early development [1,9,39]. However, R7 live imaging revealed that cadN mutant fly R7 axons actively jump between correct and incorrect medulla layers, arguing against a strict role for CadN in preferential adhesion within the correct layer [43]. Subsequent to its own targeting, L3 produces the secreted attractant netrin, which influences R8 growth cones as they actively extend to this same layer [48]. Recent live imaging results show that netrin signaling stabilizes R8 terminals in the target area, but does not target R8 axons per se, similar to the stabilizing function of CadN for R7 [49]. Interestingly, R cell growth cones in turn produce a ligand (jelly belly) that is required for L3 survival, and therefore maintenance of R8 targeting [50]. These mechanisms highlight a cellular feedback loop whereby temporal succession of neuronal process extension remobilizes and then stabilizes growth cones during layer formation.
Adhesive interactions are also crucial for assembly of OPL connections, where rod and cone photoreceptors form synapses with On-rod BCs and both On- and Off-cone BCs, respectively, in stereotyped positions. On-rod BC dendrites express the mGluR6 glutamate receptor, and the transmembrane cell adhesion protein ELFN1, which is selectively expressed in rod photoreceptor axons, trans-synaptically interacts with mGluR6 [51]. Loss of ELFN1 in rods leads to a failure of rod/On-rod BC synapse formation and a loss of low light rod pathway-mediated detection. Future work will reveal additional mechanisms that establish specific connections between the ~13 BC types and rods and cones.
Select RGC axons project to specific layers in retinorecipient targets, including in the SC and the dLGN [52,53]. How important are these layers for development and adult function? Astray mutants in zebrafish lack the slit1 receptor Robo2, causing an absence of clear RGC axon lamination in the tectum; although direction selective tuning of astray RGCs is perturbed at earlier developmental stages, at late developmental stages directional RGC tuning (and underlying synaptic connectivity) is similar to wild-type, even in the absence of wild-type laminar organization [54]. These results show that lamination is not an absolute requirement for functional assembly of this circuit. Similarly, in the visual cortex of reeler mice sensory maps in barrel cortex are unaffected despite dramatic disorganization of cortical laminar organization [55,56]. The first EM ‘connectome’ of the adult mouse LGN surprisingly identified no clear anatomical subdivisions that reflect the processing of parallel visual information streams, but rather a ‘fuzzy logic’ of connectivity [57]. These studies suggest that lamination may facilitate neural development but is not absolutely required for many mature circuit functions.
Does layer-specific targeting pre-specify synaptic partners? Similar to development of lateral retinotopic organization, axons and dendrites that end up in the wrong location may form synapses there. Fly R8s that mistarget to the M6 layer in the medulla form aberrant synapses with Dm8 [58], and ectopic photoreceptors created genetically outside of the visual system synapse in brain regions far from their normal environment [59]. However, synapses in the medulla are not strictly layer-specific [37]; consequently, layer-specific targeting is unlikely to be a sufficient determinant of synaptic specificity. In the mouse retina, lamination prevents synaptogenesis with incorrect partners: some RGCs make ectopic connections with OFF bipolar cells when the correct ON bipolar cells are not present during development [7]. In contrast, mouse M1 intrinsically photosensitive RGCs (ipRGCs) maintain their association with specific ACs when both M1 RGCs and their partner ACs are misdirected to incorrect laminae [42]. Here, a short-range cell type-specific cue distinct from Sema6A is more important for synaptic connectivity than layer-specific localization. These examples highlight the importance and limitations of layers for synaptic specificity by facilitating connectivity in 2D rather than 3D. This principle includes Peter’s rule with limitations, as exemplified by the function of sidekick2 in the IPL [10].
Finally, synapse formation itself may be linked to growth cone stabilization in specific layers. In flies, the RhoGAP Syd-1 and the adapter protein liprin-alpha serve as early determinants of synapse formation [60]. Mutations affecting either gene, or the upstream cell adhesion molecule Dlar, cause R7 layer-specific targeting defects [61,62]. This may be analogous to the stabilization of mGluR6-contianing synapses between rod photoreceptors and rod-Off BC cells through trans-synaptic interactions [40]. How synapse formation stabilizes growth cones, and how growth cone dynamics influence synapse formation, remain exciting questions for the future.
In summary, the following common principles underlie the development of layered organization in both flies and vertebrates:
Temporal succession and relative positioning (e.g. through intercalation) establish new layers.
Some cell types form and define layers, while axons and dendrites of others initially wait for lamination to be established and subsequently target based on layer-specific cues.
Lamination facilitates development, but may not be an absolute requirement for connectivity and mature circuit function.
Conclusions
Taken together, this brief consideration of common and divergent principles in visual system wiring highlights how neurons of different origins adopt common mechanisms to connect circuits for the processing of parallel information streams. Both columnar and laminar organization play crucial roles in patterning neural connectivity to connect correct pre- and postsynaptic partners. In the case of columnar/lateral retinotopy development, flies and vertebrates exhibit greater anatomical divergence since there are no columns in the vertebrate retina. However, pattern formation mechanisms including mosaic spacing, self-avoidance, self/non-self recognition and tiling play strikingly similar roles in disparate visual systems, ensuring retinotopic wiring of large numbers of neurons to allow for parallel information processing. In contrast, during the development of layers/laminae, neurons utilize specific molecular cues, most notably when axons or dendrites choose among layers that have already formed. A key question remains: to what extent are synaptic partnerships pre-specified by columnar and layer patterning? Continued study of visual system development will provide critical insights into how complex neuronal organization is achieved through a balanced utilization of pattern formation rules, differential molecular adhesion and synaptic specification molecules.
Highlights.
Flies and vertebrates exhibit distinct anatomical differences in retina-brain wiring
Commonalities in connectivity underlie processing of parallel information streams
Columnar and laminar patterns bring pre- and postsynaptic partners together
Molecular cues may increase specificity, particularly in crowded CNS regions
Acknowledgments
We thank all members of the Hiesinger and Kolodkin laboratories for discussions and Ian Meinertzhagen and Shinya Takemura for sharing unpublished data. Brendan Lilley, Egemen Agi, Neset Ozel and Rebecca James provided important feedback on this manuscript. Work in the Kolodkin laboratory is supported by the NIH and the HHMI. Work in the Hiesinger laboratory is supported by the NIH (RO1EY018884, RO1EY023333), the Deutsche Forschungsgemeinschaft (SFB 958, SFB186), NeuroCure and FU Berlin. A.L. Kolodkin is an investigator with the Howard Hughes Medical Institute. P.R. Hiesinger is an investigator with the NeuroCure Cluster of Excellence Berlin.
Footnotes
Conflict of Interest Statement
We declare no conflict of interest.
Alex Kolodkin & Robin Hiesinger
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanes JR, Zipursky SL. Design principles of insect and vertebrate visual systems. Neuron. 2010;66:15–36. doi: 10.1016/j.neuron.2010.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koyanagi M, Kubokawa K, Tsukamoto H, Shichida Y, Terakita A. Cephalochordate melanopsin: evolutionary linkage between invertebrate visual cells and vertebrate photosensitive retinal ganglion cells. Curr Biol. 2005;15:1065–1069. doi: 10.1016/j.cub.2005.04.063. [DOI] [PubMed] [Google Scholar]
- 3.Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 2003;47:563–571. [PubMed] [Google Scholar]
- 4.Hassan BA, Hiesinger PR. Beyond Molecular Codes: Simple Rules to Wire Complex Brains. Cell. 2015;163:285–291. doi: 10.1016/j.cell.2015.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cang J, Feldheim DA. Developmental mechanisms of topographic map formation and alignment. Annu Rev Neurosci. 2013;36:51–77. doi: 10.1146/annurev-neuro-062012-170341. [DOI] [PubMed] [Google Scholar]
- 6.Owens MT, Feldheim DA, Stryker MP, Triplett JW. Stochastic Interaction between Neural Activity and Molecular Cues in the Formation of Topographic Maps. Neuron. 2015;87:1261–1273. doi: 10.1016/j.neuron.2015.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Okawa H, Della Santina L, Schwartz GW, Rieke F, Wong RO. Interplay of cell-autonomous and nonautonomous mechanisms tailors synaptic connectivity of converging axons in vivo. Neuron. 2014;82:125–137. doi: 10.1016/j.neuron.2014.02.016. This work demonstrates cell-autonomous and non-cell autonomous interactions in bipolar cells that constrain connections between RGC dendrites and their correct BP synaptic partners, highlighting the requirement for specific local interactions within laminae to prevent indiscriminant connectivity in their absence. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hiesinger PR, Zhai RG, Zhou Y, Koh TW, Mehta SQ, Schulze KL, Cao Y, Verstreken P, Clandinin TR, Fischbach KF, et al. Activity-independent prespecification of synaptic partners in the visual map of Drosophila. Curr Biol. 2006;16:1835–1843. doi: 10.1016/j.cub.2006.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwabe T, Neuert H, Clandinin TR. A network of cadherin-mediated interactions polarizes growth cones to determine targeting specificity. Cell. 2013;154:351–364. doi: 10.1016/j.cell.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **10.Krishnaswamy A, Yamagata M, Duan X, Hong YK, Sanes JR. Sidekick 2 directs formation of a retinal circuit that detects differential motion. Nature. 2015;524:466–470. doi: 10.1038/nature14682. A single homophilic cell adhesion molecule increases synaptic specificity ~10-fold above ‘Peter’s rule’ between distinct subtypes of amacrine and retinal ganglion cells in the mammalian retina. This work showcases how initial pattern formation and molecular specification work hand-in-hand to establish precise connectivity within crowded synaptic space. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lefebvre JL, Sanes JR, Kay JN. Development of dendritic form and function. Annu Rev Cell Dev Biol. 2015;31:741–777. doi: 10.1146/annurev-cellbio-100913-013020. [DOI] [PubMed] [Google Scholar]
- 12.Bevins N, Lemke G, Reber M. Genetic dissection of EphA receptor signaling dynamics during retinotopic mapping. J Neurosci. 2011;31:10302–10310. doi: 10.1523/JNEUROSCI.1652-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dearborn R, Jr, He Q, Kunes S, Dai Y. Eph receptor tyrosine kinase-mediated formation of a topographic map in the Drosophila visual system. J Neurosci. 2002;22:1338–1349. doi: 10.1523/JNEUROSCI.22-04-01338.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langen M, Agi E, Altschuler DJ, Wu LF, Altschuler SJ, Hiesinger PR. The Developmental Rules of Neural Superposition in Drosophila. Cell. 2015;162:120–133. doi: 10.1016/j.cell.2015.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clandinin TR, Zipursky SL. Afferent growth cone interactions control synaptic specificity in the Drosophila visual system. Neuron. 2000;28:427–436. doi: 10.1016/s0896-6273(00)00122-7. [DOI] [PubMed] [Google Scholar]
- *16.Schwabe T, Borycz JA, Meinertzhagen IA, Clandinin TR. Differential adhesion determines the organization of synaptic fascicles in the Drosophila visual system. Curr Biol. 2014;24:1304–1313. doi: 10.1016/j.cub.2014.04.047. Differential adhesion mediated by N-cadherin is required to arrange synaptic cartridges in the visual map with photoreceptors encircling postsynaptic L cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huberman AD, Manu M, Koch SM, Susman MW, Lutz AB, Ullian EM, Baccus SA, Barres BA. Architecture and activity-mediated refinement of axonal projections from a mosaic of genetically identified retinal ganglion cells. Neuron. 2008;59:425–438. doi: 10.1016/j.neuron.2008.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Reid RC. From functional architecture to functional connectomics. Neuron. 2012;75:209–217. doi: 10.1016/j.neuron.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kay JN, Chu MW, Sanes JR. MEGF10 and MEGF11 mediate homotypic interactions required for mosaic spacing of retinal neurons. Nature. 2012;483:465–469. doi: 10.1038/nature10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lefebvre JL, Kostadinov D, Chen WV, Maniatis T, Sanes JR. Protocadherins mediate dendritic self-avoidance in the mammalian nervous system. Nature. 2012;488:517–521. doi: 10.1038/nature11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wassle H. Parallel processing in the mammalian retina. Nat Rev Neurosci. 2004;5:747–757. doi: 10.1038/nrn1497. [DOI] [PubMed] [Google Scholar]
- 23.Zipursky SL, Sanes JR. Chemoaffinity revisited: dscams, protocadherins, and neural circuit assembly. Cell. 2010;143:343–353. doi: 10.1016/j.cell.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Matthews BJ, Kim ME, Flanagan JJ, Hattori D, Clemens JC, Zipursky SL, Grueber WB. Dendrite self-avoidance is controlled by Dscam. Cell. 2007;129:593–604. doi: 10.1016/j.cell.2007.04.013. [DOI] [PubMed] [Google Scholar]
- *25.Kostadinov D, Sanes JR. Protocadherin-dependent dendritic self-avoidance regulates neural connectivity and circuit function. Elife. 2015:4. doi: 10.7554/eLife.08964. Loss of protocadherin-mediated self/non-self dendritic process discrimination in SACs results in the formation of SAC autapses, reduced SAC-SAC connectivity, and reduced pruning between closely spaced SACs. These deficits result in compromised directional tuning of DS RGCs, underscoring the importance of self-avoidance, self/non-self discrimination, and synapse elimination for DS circuit assembly and function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wassle H, Puller C, Muller F, Haverkamp S. Cone contacts, mosaics, and territories of bipolar cells in the mouse retina. J Neurosci. 2009;29:106–117. doi: 10.1523/JNEUROSCI.4442-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Langen M, Koch M, Yan J, De Geest N, Erfurth ML, Pfeiffer BD, Schmucker D, Moreau Y, Hassan BA. Mutual inhibition among postmitotic neurons regulates robustness of brain wiring in Drosophila. Elife. 2013;2:e00337. doi: 10.7554/eLife.00337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Millard SS, Flanagan JJ, Pappu KS, Wu W, Zipursky SL. Dscam2 mediates axonal tiling in the Drosophila visual system. Nature. 2007;447:720–724. doi: 10.1038/nature05855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **29.Ting CY, McQueen PG, Pandya N, Lin TY, Yang M, Reddy OV, O’Connor MB, McAuliffe M, Lee CH. Photoreceptor-derived activin promotes dendritic termination and restricts the receptive fields of first-order interneurons in Drosophila. Neuron. 2014;81:830–846. doi: 10.1016/j.neuron.2013.12.012. Columnar restriction, akin to tiling, of Tm20 cells in the Drosophila medulla is mediated through activin secretion by photoreceptor axons. This study provides important insight into both principles and mechanisms underlying tiling. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kniss JS, Holbrook S, Herman TG. R7 photoreceptor axon growth is temporally controlled by the transcription factor Ttk69, which inhibits growth in part by promoting transforming growth factor-beta/activin signaling. J Neurosci. 2013;33:1509–1520. doi: 10.1523/JNEUROSCI.2023-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gao S, Takemura SY, Ting CY, Huang S, Lu Z, Luan H, Rister J, Thum AS, Yang M, Hong ST, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karuppudurai T, Lin TY, Ting CY, Pursley R, Melnattur KV, Diao F, White BH, Macpherson LJ, Gallio M, Pohida T, et al. A hard-wired glutamatergic circuit pools and relays UV signals to mediate spectral preference in Drosophila. Neuron. 2014;81:603–615. doi: 10.1016/j.neuron.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takemura SY, Bharioke A, Lu Z, Nern A, Vitaladevuni S, Rivlin PK, Katz WT, Olbris DJ, Plaza SM, Winston P, et al. A visual motion detection circuit suggested by Drosophila connectomics. Nature. 2013;500:175–181. doi: 10.1038/nature12450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nern A, Pfeiffer BD, Rubin GM. Optimized tools for multicolor stochastic labeling reveal diverse stereotyped cell arrangements in the fly visual system. Proc Natl Acad Sci U S A. 2015;112:E2967–2976. doi: 10.1073/pnas.1506763112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *35.Tan L, Zhang KX, Pecot MY, Nagarkar-Jaiswal S, Lee PT, Takemura SY, McEwen JM, Nern A, Xu S, Tadros W, et al. Ig Superfamily Ligand and Receptor Pairs Expressed in Synaptic Partners in Drosophila. Cell. 2015;163:1756–1769. doi: 10.1016/j.cell.2015.11.021. RNA profiling of cells in the fly visual systems lead to the discovery of Ig suerfamily Dpr and DIP proteins as potential molecular recognition signals in synaptically connected cells. (companion paper: Carillo et al., 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Carrillo RA, Ozkan E, Menon KP, Nagarkar-Jaiswal S, Lee PT, Jeon M, Birnbaum ME, Bellen HJ, Garcia KC, Zinn K. Control of Synaptic Connectivity by a Network of Drosophila IgSF Cell Surface Proteins. Cell. 2015;163:1770–1782. doi: 10.1016/j.cell.2015.11.022. Analysis of the Ig superfamily Dpr and DIP proteins suggests molecular recognition between synaptically connected cells and shows such a molecular pair for a subtype of R7 and Dm8 cells. (companion paper: Tan et al., 2015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takemura SY, Xu CS, Lu Z, Rivlin PK, Parag T, Olbris DJ, Plaza S, Zhao T, Katz WT, Umayam L, et al. Synaptic circuits and their variations within different columns in the visual system of Drosophila. Proc Natl Acad Sci U S A. 2015;112:13711–13716. doi: 10.1073/pnas.1509820112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peters A, Feldman ML. The projection of the lateral geniculate nucleus to area 17 of the rat cerebral cortex. I. General description. J Neurocytol. 1976;5:63–84. doi: 10.1007/BF01176183. [DOI] [PubMed] [Google Scholar]
- 39.Pecot MY, Tadros W, Nern A, Bader M, Chen Y, Zipursky SL. Multiple interactions control synaptic layer specificity in the Drosophila visual system. Neuron. 2013;77:299–310. doi: 10.1016/j.neuron.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hand RA, Kolodkin AL. Semaphorin Regulation of Neural Circuit Assembly in the Central Nervous System. In: Kumanoghoh A, editor. Semaphorins. Vol. 1 Springer; 2015. pp. 19–37. [Google Scholar]
- 41.Baier H. Synaptic laminae in the visual system: molecular mechanisms forming layers of perception. Annu Rev Cell Dev Biol. 2013;29:385–416. doi: 10.1146/annurev-cellbio-101011-155748. [DOI] [PubMed] [Google Scholar]
- 42.Matsuoka RL, Nguyen-Ba-Charvet KT, Parray A, Badea TC, Chedotal A, Kolodkin AL. Transmembrane semaphorin signalling controls laminar stratification in the mammalian retina. Nature. 2011;470:259–263. doi: 10.1038/nature09675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *43.Ozel MN, Langen M, Hassan BA, Hiesinger PR. Filopodial dynamics and growth cone stabilization in Drosophila visual circuit development. Elife. 2015:4. doi: 10.7554/eLife.10721. Layer formation in the fly medulla occurs through relative positioning and intercalation after early formation of a boundary through stabilization of R7 growth cones with N-cadherin is not required for targeting, but its loss leads to destabilization and growth cone “jumping” between correct and incorrect layersas a stabilizer, not targeting cue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun LO, Jiang Z, Rivlin-Etzion M, Hand R, Brady CM, Matsuoka RL, Yau KW, Feller MB, Kolodkin AL. On and off retinal circuit assembly by divergent molecular mechanisms. Science. 2013;342:1241974. doi: 10.1126/science.1241974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanes JR, Yamagata M. Many paths to synaptic specificity. Annu Rev Cell Dev Biol. 2009;25:161–195. doi: 10.1146/annurev.cellbio.24.110707.175402. [DOI] [PubMed] [Google Scholar]
- 46.Yamagata M, Sanes JR. Expanding the Ig superfamily code for laminar specificity in retina: expression and role of contactins. J Neurosci. 2012;32:14402–14414. doi: 10.1523/JNEUROSCI.3193-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **47.Duan X, Krishnaswamy A, De la Huerta I, Sanes JR. Type II cadherins guide assembly of a direction-selective retinal circuit. Cell. 2014;158:793–807. doi: 10.1016/j.cell.2014.06.047. The expression of cadherins 8 and 9 by two different classes of bipolar cells is shown to direct each BP cell type to different Off and On direction selective circuits in the IPL in an instructive fashion. This work highlights the role of select adhesive interactions in connecting axons to their correct laminae during later phases of IPL wiring. [DOI] [PubMed] [Google Scholar]
- 48.Timofeev K, Joly W, Hadjieconomou D, Salecker I. Localized netrins act as positional cues to control layer-specific targeting of photoreceptor axons in Drosophila. Neuron. 2012;75:80–93. doi: 10.1016/j.neuron.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Akin O, Zipursky SL. Frazzled promotes growth cone attachment at the source of a Netrin gradient in the Drosophila visual system. Elife. 2016:5. doi: 10.7554/eLife.20762. Loss of netrin signaling in Drosophila R8 neurons does not affect R8 axon extension to the correct target per se, but is instead required for stabilization of a growth cone extension in the target area. This work suggests that this textbook guidance cue may, at least in this instance, not function as a long-range targeting cue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pecot MY, Chen Y, Akin O, Chen Z, Tsui CY, Zipursky SL. Sequential axon-derived signals couple target survival and layer specificity in the Drosophila visual system. Neuron. 2014;82:320–333. doi: 10.1016/j.neuron.2014.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **51.Cao Y, Sarria I, Fehlhaber KE, Kamasawa N, Orlandi C, James KN, Hazen JL, Gardner MR, Farzan M, Lee A, et al. Mechanism for Selective Synaptic Wiring of Rod Photoreceptors into the Retinal Circuitry and Its Role in Vision. Neuron. 2015;87:1248–1260. doi: 10.1016/j.neuron.2015.09.002. Transsynaptic adhesive interactions between rods and rod bipolar cells are demonstrated in this study, identifying the first molecular cues critical for synapse assembly and function in the OPL. This work provides a first step toward understanding how complex OPL connectivity is precisely assembled and organized. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dhande OS, Stafford BK, Lim JA, Huberman AD. Contributions of retinal ganglion cells to subcortical visual processing and behaviors. Annu Rev Vis Sci. 2015:291–328. doi: 10.1146/annurev-vision-082114-035502. [DOI] [PubMed] [Google Scholar]
- 53.Robles E, Filosa A, Baier H. Precise lamination of retinal axons generates multiple parallel input pathways in the tectum. J Neurosci. 2013;33:5027–5039. doi: 10.1523/JNEUROSCI.4990-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **54.Nikolaou N, Meyer MP. Lamination Speeds the Functional Development of Visual Circuits. Neuron. 2015;88:999–1013. doi: 10.1016/j.neuron.2015.10.020. Loss of lamination in the zebrafish tectum slows down development but is ultimately not required for the establishment of a functional visual circuit, as assessed by intrinsic imaging of neuronal activity. This work poses an important question regarding the role of lamination/layering as an organizing principle in visual system wiring. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Guy J, Wagener RJ, Mock M, Staiger JF. Persistence of Functional Sensory Maps in the Absence of Cortical Layers in the Somsatosensory Cortex of Reeler Mice. Cereb Cortex. 2015;25:2517–2528. doi: 10.1093/cercor/bhu052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frost DO, Edwards MA, Sachs GM, Caviness VS., Jr Retinotectal projection in reeler mutant mice: relationships among axon trajectories, arborization patterns and cytoarchitecture. Brain Res. 1986;393:109–120. doi: 10.1016/0165-3806(86)90070-2. [DOI] [PubMed] [Google Scholar]
- 57.Morgan JL, Berger DR, Wetzel AW, Lichtman JW. The Fuzzy Logic of Network Connectivity in Mouse Visual Thalamus. Cell. 2016;165:192–206. doi: 10.1016/j.cell.2016.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kulkarni A, Ertekin D, Lee CH, Hummel T. Birth order dependent growth cone segregation determines synaptic layer identity in the Drosophila visual system. Elife. 2016:5. doi: 10.7554/eLife.13715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clements J, Lu Z, Gehring WJ, Meinertzhagen IA, Callaerts P. Central projections of photoreceptor axons originating from ectopic eyes in Drosophila. Proc Natl Acad Sci U S A. 2008;105:8968–8973. doi: 10.1073/pnas.0803254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Korner J, et al. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 2010;188:565–579. doi: 10.1083/jcb.200908055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Astigarraga S, Hofmeyer K, Farajian R, Treisman JE. Three Drosophila liprins interact to control synapse formation. J Neurosci. 2010;30:15358–15368. doi: 10.1523/JNEUROSCI.1862-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holbrook S, Finley JK, Lyons EL, Herman TG. Loss of syd-1 from R7 neurons disrupts two distinct phases of presynaptic development. J Neurosci. 2012;32:18101–18111. doi: 10.1523/JNEUROSCI.1350-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]