Abstract
Human birth presents an abrupt transition from intrauterine to extrauterine life. Here we introduce a novel Maturation Index (MI) that considers the relative importance of gestational age at birth and postnatal age at scan in a General Linear Model. The MI is then applied to Diffusion Tensor Imaging (DTI) in newborns for characterizing typical white matter development in neonates. DTI was performed cross-sectionally in 47 neonates (gestational age at birth=39.1±1.6 weeks [GA], postnatal age at scan=25.5±12.2 days [SA]). Radial diffusivity (RD), axial diffusivity (AD) and fractional anisotropy (FA) along 27 white matter fiber tracts were considered. The MI was used to characterize inflection in maturation at the time of birth using GLM estimated rates of change before and after birth. It is proposed that the sign (positive versus negative) of MI reflects the period of greatest maturation rate. Two general patterns emerged from the MI analysis. First, RD and AD (but not FA) had positive MI on average across the whole brain (average MIAD=0.31+/−.42, average MIRD=0.22+/−.34). Second, significant regions of negative MI in RD and FA (but not AD) were observed in the inferior corticospinal regions, areas known to myelinate early. Observations using the proposed method are consistent with proposed models of the white matter maturation process in which pre-myelination is described by changes in AD and RD due to oligodendrocyte proliferation while true myelination is characterized by changes in RD and FA due to myelin formation.
Keywords: DTI, Myelination, Non-linear, Ontology, Birth, Age, Postnatal, Gestational
Graphical abstract
1 Introduction
Human birth presents an abrupt transition from intrauterine to extrauterine life. Profound physiological changes occur with the cessation of maternal provision, including the re-organization of the neonate's cardiovascular system and the onset of pulmonary function. Within 8 minutes of delivery, oxygen saturation levels increase from roughly 60% to over 85% (Rabi et al., 2006). From the perspective of a single cell in the neonatal brain, these are dramatic changes that, based on in vitro work, may represent a shift from a local environment that promotes oligodendrocyte proliferation to one that drives oligodendrocyte maturation (Pistollato et al., 2007). Higher oxygen tension is thought to be a regulator of white matter maturation through down regulation of hypoxia-inducible factor (Yuen et al. 2014), with oxygen saturation potentially playing an important role in cystic and diffuse white matter diseases (Wellman et al. 2015). Furthermore, Toda et al. (2013) have demonstrated the initiation of barrel formation in the somatosensory cortex of mice through a reduction in serotonin signaling at birth. Birth also presents an abrupt increase in external sensory stimuli that may promote maturation through axonal signaling (Coman et al., 2005). In addition, recent work has highlighted perinatal changes in physiological dehydration, skull contraction and cerebrospinal fluid reductions as possible mediators of increased gyrification in pre-term versus fetal brains matched for postmenstrual age (Lefèvre et al., 2015). Based on these dramatic changes at the time of birth, the transition from intrauterine to extrauterine life is likely to present a significant inflection point in brain maturation, including the maturation of white matter fiber tracts. While we cannot test these mechanisms directly in this work, here, we aim to identify white matter regions with significant inflection points in maturation near the time of birth and establish a metric for characterizing the relative average rates of maturation before and after birth in a healthy population of near term born neonates.
Cross-sectional Diffusion Tensor Imaging (DTI) studies aimed at describing perinatal brain maturation trajectories typically apply univariate analyses using postmenstrual age as a predictor of microstructural maturity (Hüppi et al., 1998; Oishi et al., 2011; Ou et al., 2015; Provenzale et al., 2012; Qiu et al., 2013) or using postmenstrual age while correcting for age at scan (or gestational age) (Van Kooij et al., 2012) in a linear model. In the former case, the assumption is made that growth is linear with time from conception, despite the environmental shift that occurs when the developing brain is first exposed to the postnatal environment. Using postmenstrual age as the predictor but correcting for age at scan (or gestational age) adequately adjusts for inter-individual variation in time spent in utero. Although this may often be an appropriate choice, this approach neglects potentially interesting information about prenatal versus postnatal developmental changes when the rate of maturation may not follow a linear trajectory from conception.
Previous work describing white matter maturation has been instrumental in describing the formation, organization and maturation of white matter in early life, yet lacks a quantitative description of maturational trajectories surrounding birth. By first separating postmenstrual age (PM) into gestational age at birth (GA) and postnatal age (chronological age) at scan (SA) for regression analysis, we examine and demonstrate the ability to describe inflection points in developmental trajectories near the time of birth. Second, using tract-based statistics we propose that the newly described Maturation Index (MI) is potentially indicative of spatially asynchronous perinatal maturation in white matter microstructure.
2 Methods and Materials
2.1 Linear Regression Models, Model Comparison and the Maturation Index (MI) Defined
2.1.1 Linear Regression Models
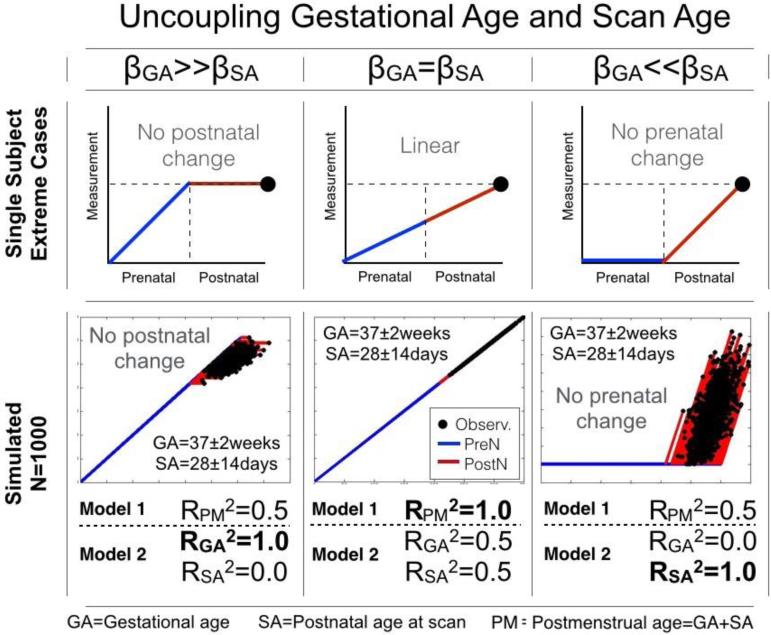
Linear regression models were implemented using Matlab (www.mathworks.com) for the examination of predictive power using two separate models. Model 1 used PM as the only indicator of participant age at the time of the MRI scan and as the predictor of diffusion measures. Model 2 used GA and SA as separate independent variables representative of age at the MRI scan and as predictors of diffusion measures. Both model 1 and model 2 included intracranial volume (ICV) as a continuous covariate and sex as a binary covariate. For this study the dependent variables were Fractional Anisotropy (FA), Radial Diffusivity (RD) or Axial Diffusivity (AD) (Y in below equations). This is shown below for the ith position along a fiber tract (models 1 and 2). In the case of linear maturation, growth rates before and after birth are equal (βi,GA = βi,SA). In this special linear case, model 2 is reduced to model 1, as PM is the sum of GA and SA (Figure 1), by definition. For this reason, the greater the difference between βi,GA and βi,SA, the greater the inflection in growth rates at the time of birth.
| Equation 1 |
| Equation 2 |
Figure 1. Schematic of post-conceptional age (PM), gestational age (GA) and age at scan definitions (SA).
PM is shown as the sum of GA and SA.
2.1.2 Model Comparison
The gain in predictive power moving from model 1 to model 2 is descriptive of the divergence of βi,GA from βi,SA and therefore reflective of whether major maturational changes occur before or after birth. In order to justify the use of model 2, the gains from using the unrestricted model (model 2) were statistically characterized using an F-distribution. Here, RSSj is the residual sum-of-squares from the respective model j, n the number of observations and pj the number of model parameters. A p-value was then calculated along the tract using an F-test, F(p2-p1,n-p2). Because model 2 always has the potential to reduce to model 1 (when βi,GA = βi,SA), RSS1 will always be larger than or equal to RSS2. A large F-statistic is driven by a difference in the residual errors between the two models and is therefore indicative of an increase in the asymmetry of maturation (βi,GA ≠ βi,SA) about the time of birth.
| Equation 3 |
2.1.3 Simulated Data Sets
Three basic data sets were simulated to demonstrate the advantages of using the unrestricted second model. The three sets each consisted of 1,000 simulated observations, Yi = βi,GA · GA + βi,SA · SA, with GA and SA randomized to a normal distribution and variance matched to our neonatal sample (GASIM=259+/−14 days; SASIM=28+/−14 days) whose dependent variable increased with GA only (set 1, in utero maturation only), GA and SA equally (set 2, linear maturation from the time of conception), or SA only (set 3, postnatal maturation only). Each set was then examined for correlations between the simulated dependent value and each of the three age variables individually: 1) GA, 2) SA and 3) PM. Explained variance of 1.0 (or 100%) occurs when the simulated data can be fully explained. The total variance explained by model 2 is given by the sum of RGA2 and RSA2.
2.1.4 Maturation Index Definition
MI values were defined using output from model 2. Specifically, MI is defined as the ratio of the difference in the squared slopes (beta value) of GA and SA, to the sum of squared slopes of GA and SA (Equation 4). The square of the slope was used to remove the sign dependence of the slope in the calculation. MI will tend towards +1 when the absolute rate of change during prenatal development is large relative to postnatal development, zero when the rates of change during pre- and postnatal development are equal and −1 when the postnatal rate of change is large relative to the rate of change during prenatal development.
| Equation 4 |
MI was plotted against the increase in explanatory power from model 2 compared to model 1 (Equations 1 and 2). A large F-Ratio coupled with a large (non-zero) MI suggests a meaningful increase in power due to accounting for the non-linearity of developmental trajectories (using GA and SA, model 2) having an inflection point at the time of birth. The MI has two principal benefits over examining the slopes individually: 1) the dimensionality of the rates of change are reduced to a single number (an index), and 2) the magnitude of the slopes are effectively normalized so that the magnitude of the inflection becomes a dimensionless parameter, facilitating whole-brain characterization.
2.2 Sample, Image Acquisition and Analysis
Infant neuroimaging was approved by the Institutional Review Board of the University of California at Irvine, and all parents provided informed, written consent. All of the 47 infants evaluated in this study were from healthy pregnancies with no major obstetric, birth or current health complications. Mother-child pairs were recruited for a research study of normative development.Sample demographics are provided in Table 1. Gestational age was determined by best obstetric estimate with a combination of last menstrual period and early uterine size, and was confirmed by obstetric ultrasonographic biometry before 15 weeks using standard clinical criteria(O'Brien et al., 1981). The mean gestational age at birth was 39.1 ± 1.6 (±SD) weeks and ranged from 34.4 to 41.9 weeks. The mean postnatal infant age at assessment was 25.5 ± 12.2 (±SD) days and ranged from 5 to 56 days. Gestational age at birth and postnatal infant age at assessment were not correlated with one another (R2=−2.8%,p=0.25).
Table 1.
Sample Demographics.
| Gestational Age at Birth (weeks) | |
| Mean (S.D.) | 39.1 (1.6) |
| Postnatal Age at Scan (days) | |
| Mean (S.D.) | 25.5 (12.2) |
| GA, SA Distribution | |
| N (%), GA>mean(GA) & SA>mean(SA) | 12 (26%) |
| N (%), GA>mean(GA) & SA<mean(SA) | 13 (28%) |
| N (%), GA<mean(GA) & SA>mean(SA) | 8 (17%) |
| N (%), GA<mean(GA) & SA<mean(SA) | 14 (29%) |
| Sex | |
| Male, N (%) | 19 (40%) |
| Birthweight (kg) | |
| Mean (S.D.) | 3.35 (0.49) |
| Birthweight for GA (percentile) | |
| Median (IQR) | 74.2 (39.5-96.1) |
| Race/Ethnicity | |
| Hispanic White N (%) | 14 (36%) |
| Hispanic of Other Race N (%) | 11 (23%) |
| Non-Hispanic White N (%) | 17 (30%) |
| Non-Hispanic of Other Race N (%) | 5 (11%) |
| Cesarean Section Deliveries | |
| Yes, N (%) | 8 (17%) |
Summary statistics are provided for the infant sample. GA=Gestational Age, SA=Postnatal Age at Scan (Chronological Age).
MRI scans were acquired during natural sleep using a 12-channel head receive coil at 3T field strength. High-resolution anatomical scans including T1-weighted (MPRAGE, TR/TE/TI=2400/3.16/1200ms, Flip Angle=8 degrees, Matrix=256×256×160, Resolution=1×1×1mm, 6m18s) and T2-weighted (TSE, TR/TE=3200/255ms, Matrix=256×256×160, Resolution=1×1×1mm, 4m18s) images were acquired. The 49-direction diffusion weighted protocol (EPI, TR/TE=8900/83ms, Matrix=256×256×75, Resolution=2×2×2mm, 42 unique directions at b=1000s/mm2, 7 at b=0) was 7 minutes and 43 seconds in duration. After feeding and soothing to the point of sleep, neonates were placed in a CIVCO beaded pillow (www.civco.com). The pillow covered the neonates’ body and head, became rigid under vacuum, and provided a comforting swaddle, motion prevention and hearing protection when used in conjunction with standard foam earplugs. A pediatric specialist observed the neonates throughout the duration of scans, monitoring for heart rate and oxygen saturation via a pulse-oximeter attached to the foot. The entire protocol included T1-weighted, T2-weighted, diffusion tensor and functional imaging of the brain.
Diffusion profile measurements were generated via the NA-MIC atlas based fiber analysis toolkit (Verde, 2014). In brief, diffusion datasets were first rigorously checked for appropriate quality. This was followed by weighted least square tensor estimation, skull stripping via prior brain mask from co-registered structural T2 weighted images, and unbiased study specific DTI atlas building. Fiber tract streamline DTI tractography (Fillard et.al. 2003) was performed via 3D Slicer (version 4.3.0) (http://www.slicer.org) in the DTI atlas space followed by fiber cleaning with FiberViewerLight (www.nitric.org/projects/fwlight). Tractography was performed for major fiber bundles: corpus callosum (genu, rostrum, tapetum, occipital, parietal), Left(L)/Right(R) cingulum, L/R fornix, L/R inferior fronto-occipital fasciculus (IFOF), L/R inferior longitudinal fasciculus (ILF), L/R superior longitudinal fasciculus (SLF), L/R uncinate fasciculus (UF), L/R motor, L/R pre-motor, L/R cortico-fugal parietal and L/R thalamo-cortical parietal and L/R optic tracts. The main body (central portion) of the corpus callosum was not analyzed as part of this study. Fiber profiles of FA, MD, AD, RD were extracted after fiber parameterization for profile analysis. For more detail please refer to Verde (2014). DTI data was visually evaluated for appropriate mapping into the DTI atlas space via slice overlay. Visual inspection and quantitative quality assessment of the extracted fiber profiles were additionally performed by computing the cross-correlation coefficient between the FA tract profiles of the DTI atlas and of each subject case (minimal r > 0.75). For most tracts, all subjects passed quantitative profile QC. Few subjects were excluded for cingulum L (5), cingulum R (7), CST L (1), fornix L (2), and uncinate R (1). Brain tissue volumes were determined for gray matter, unmyelinated white matter, myelinated white matter, and cerebral spinal fluid using an automatic, atlas-moderated expectation maximization segmentation tool as previously described(Gilmore et al., 2007; Prastawa et al., 2005). Intracranial volume defined here is the sum of the four preceding tissue classes.
2.3 MI Analyses
2.3.1 Whole Brain Analyses
A unique MI value for FA, RD and AD was calculated as described above for each point along the defined white matter fiber tracts. For the remainder of this work, each of these values will be denoted respectively as MIFA, MIRD, and MIAD. MIFA, MIRD, and MIAD values were evaluated globally to test three basic hypotheses related to the developmental timeline of white matter microstructure: 1) the rate of change of AD is greatest during pre-myelination (see discussion), a process occurring prenatally before true-myelination, therefore average MIAD is expected to be greater than MIFA and MIRD, 2) the rate of change of RD is more linear than those of AD and FA as it changes with both pre- and true-myelination (see discussion), therefore MIRD should have a mean between those of AD and FA, 3) FA changes are greatest after birth when true-myelination (see discussion) begins to increase globally and MIFA should, therefore, skew towards −1. Two-way paired t-tests between diffusion parameter MIs were used to test the above order in MIFA<MIRD, and MIRD<MIAD.
MI values along the fiber tracts used in this study were visualized in four groups: association (anterior-posterior connection), commissural (left-right connection), projection (superior-inferior connection) and limbic (those connecting limbic structures connection). Despite their anterior to posterior orientation, the cingulum and ILF (Latini 2015) were assigned to the limbic group together with the UF and fornix. The fiber groups were compared for differences within the group (paired t-tests between diffusion parameter MIs within group) and MIFA, MIRD, and MIAD were compared across groups using independent two sample t-tests. The location, size and significance of the clusters with the largest magnitude MI are reported with a focus on MIRD as justified further below.
2.3.2 Post-hoc Analyses of Projection Fibers
A post-hoc examination within a selected fiber tract was conducted in order to further elucidate the descriptive relationship between MIFA, MIRD, and MIAD. The left pre-motor tract was chosen based on two criteria: 1) the projection fibers collectively showed the greatest amount of variability, each bisected by a region of large positive MI and large negative MI, and 2) the left sided projection fibers had the most negative MI values and have been shown to myelinate before right sided fibers(Dubois et al., 2009). ROIs were defined post-hoc as having a significant F-ratio between model 2 and model 1 (Equation 3) and were applied as a mask to the tract-based data extracting a mean slope for GA and SA from the model for comparison.
A post-hoc exploration of the relationship between MIRD, MIAD, and MIFA was made using a simple colored scatter plot depicting the three metrics together.
Results
3.1 Simulated Data Sets in Support of Model 2
Three sets of simulated data were evaluated for illustrative purposes (Figure 2). The two non-linear sets (prenatal-only or postnatal-only change) had predictably marked increases in explained variance (RPM2=0.50; RGA2+RSA2=1.0) when acknowledging the separate contribution of GA and SA to PM, while the linear set (equal increase in prenatal and postnatal periods) was fully predicted (RPM2=1.0; RGA2+RSA2=1.0) by both model 1 and model 2.
Figure 2. Splitting post-conceptional age (PM) into gestational age (GA) and age at scan (SA).
Top row: Cartoon depicting three distinct paths to the same outcome. Bottom row: Three sets (βGA>>βSA; βGA=βSA; βGA<<βSA) of outcomes (N=1,000) were generated using the model Y=βGA*GA+βSA*SA with normally randomized GA (259+/−14 days) and SA (28+/−14 days). The PM age dependence (model 1) is significant in all three sets, however only the use of model 2 (GA and SA) is able to explain 100% of the variance in all three sets.
3.2 MI Analyses
3.2.1 Whole Brain Analyses
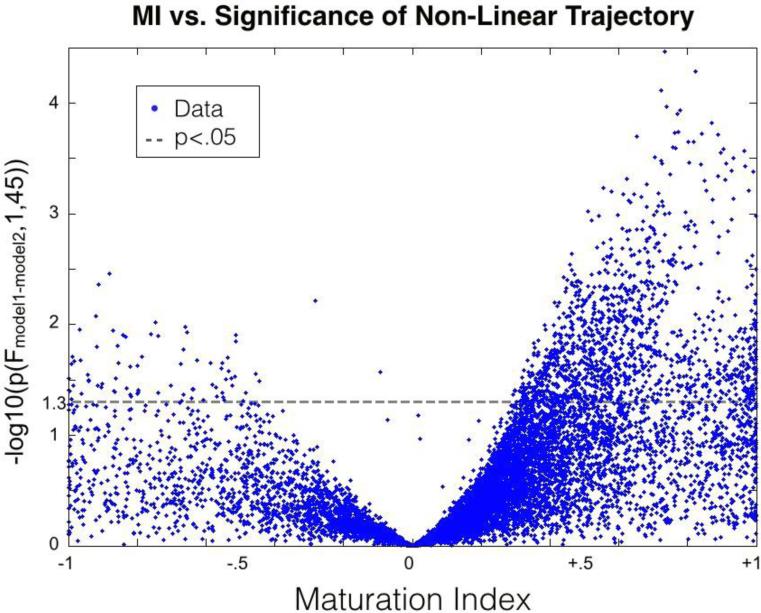
MI plotted against the F-Ratio comparing models 1 and 2 demonstrated increasing significance, and variability in significance, with increasing MI magnitude. Observations with large MI magnitude and high significance are driven by a sizeable relative difference between βi,GA and βi,SA that results in a significant increase in explanatory power. A parabolic upper limit was observed centered around an MI value of zero (the point at which βi,GA = βi,SA and model 2 reduces to model 1, hence a null increase in power) (Figure 3). The values contradicting this upper limit represent the points at which separating βi,GA and βi,SA allowed one of the other covariates (intracranial volume and/or sex) to significantly increase the explanatory power of model 2. These outlying points were limited to less than 0.1% of total observations. Points with large MI magnitude, but small F-Ratio, suggest observations that had an optimized model with βi,GA unequal to βi,SA . However, in these points, separating GA and SA resulted in little increase in explanatory power, likely due to the over-fitting of noise by the unrestricted variables GA and SA.
Figure 3. Explanatory Power Increase as a Function of Maturation Index.
The increase in explanatory power when separating PM into GA and SA is plotted against MI. MI reflects the change in estimated rates of change after birth relative to the estimated rate before birth. A large difference in slopes before and after birth results in a large magnitude MI and large increase in explanatory power when considering GA and SA as independent variables.
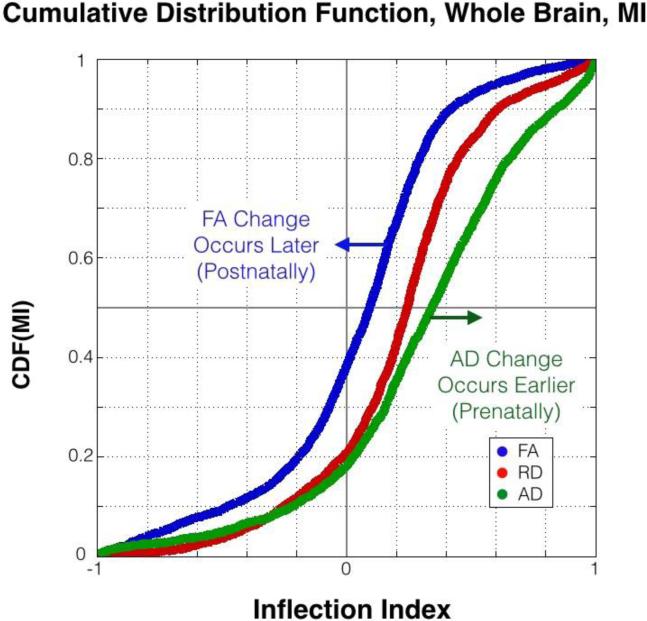
The MI across all fiber tracts revealed global differences in diffusion metrics (interquartile range, Q1/Q2/Q3: MIFA=−.12/.09/.26, MIRD=.05/.25/.40, MIAD=.09/.34/.59, Figure 4). All MI measurement distributions were skewed with long tails in the negative MI region, a region indicating more rapid post-natal compared to prenatal fiber maturation. MIAD had the most skewed distribution of the three (skew[MIFA]=−0.03, skew[MIRD]=.09, skew[MIAD]=−0.25), reflective of relatively early change in AD. The hypothesized order of MI values (MIAD>MIRD>MIFA) was confirmed (MIRD>MIFA, ΔRD-FA=.17, 95% CI [.154 .195], pRD-FA<10−10; MIAD>MIRD, ΔAD-RD=.10, 95% CI [.074 0.112], pAD-RD<10−10).
Figure 4. Cumulative Distribution Function of MIFA, MIRD, and MIAD.
MIFA was significantly greater than MIRD (ppaired<10−10) and MIRD was significantly greater than MIAD (ppaired<10−10) across the whole brain. Large MI results from a greater estimated rate of change prenatally relative to postnatally.
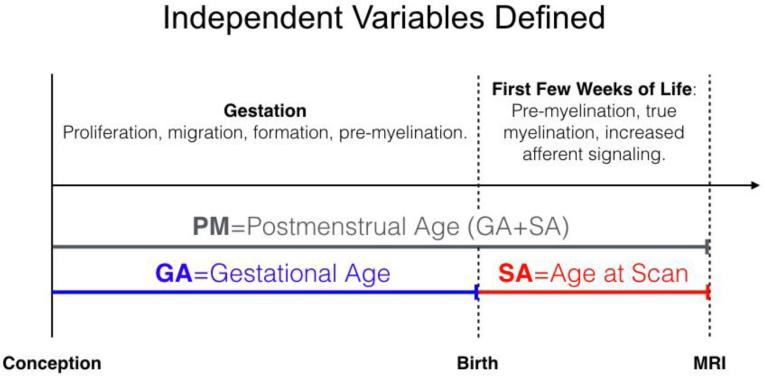
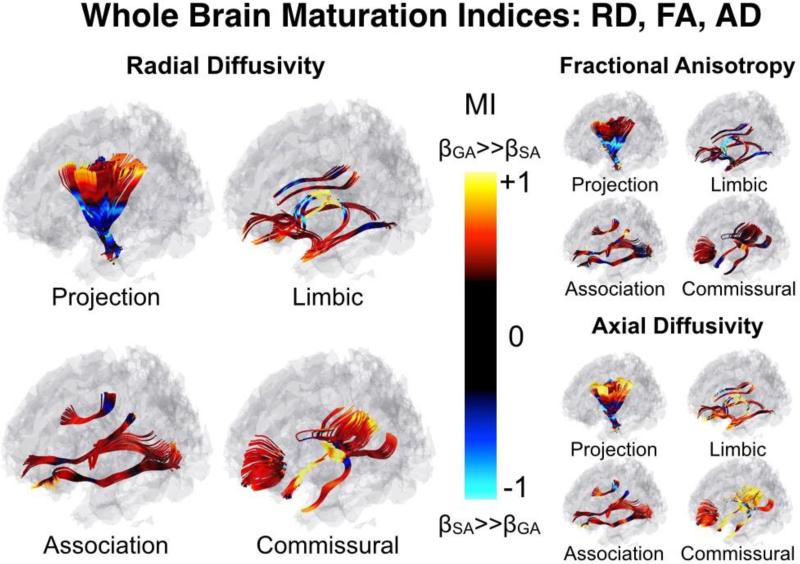
A quantitative map of MI across the brain's white matter tracts can be seen in Figure 5. In addition to the global differences in MIFA, MIRD and MIAD, there are apparent differences between fiber groups. Specifically, MIFA, MIRD and MIAD were greatest in commissural fibers, lowest in projection fibers, and with moderate values in association and limbic fibers (Table 2). While this is discussed in more detail below, these findings are reflective of large regions of negative MI bilaterally in the projection fibers, and large regions of positive MI bilaterally in the fornix and the tapetum of the corpus callosum.
Figure 5. Whole Brain Maturation Index Map.
White matter tracts are separated by group: Projection (Inferior-Superior), limbic (limbic connections), association (anterior-posterior), and commissural (left-right). Note the large negative MIRD in the inferior projection fibers (internal capsule), and large positive MIRD in the more peripheral ends of the tract. MIRD is emphasized, here, as it is predictive of both MIFA and MIRD (see figures 6 and 7). One-dimensional tract-based statistics are projected onto the three-dimensional streamlines for visualization purposes.
Table 2.
Interquartile values of MIFA, MIRD and MIAD by fiber group.
| Fiber Group | MIFA | MIRD | MIAD |
|---|---|---|---|
| Commissural** | .01/.18/.32† | .20/.34/.49 | .23/.49/.73† |
| Association* | −.04/.14/.28† | .11/.21/.33† | .11/.23/.41 |
| Limbic** | −.15/.04/.19 | .10/.25/.44† | .17/.39/.60† |
| Projection** | −.42/−.03/.20 | −.26/.03/.27 | −.18/.16/.47 |
The statistics for MI are shown using the interquartile range, 25/50/75. Commissural fibers had the largest MI and projection fibers had the lowest, with association and limbic fibers in the middle. The order of microstructural MI is retained in all fiber groups. *, ** denote fiber group retained MIFA<MIRD<MIAD within the fiber group at a minimum pairwise significance of p<.005, or p<10−10, respectively. Each microstructure MI had a pair of fiber groups that were not significantly different (two sample t-test, p>.05), these pairs are denoted with a †. All other pairs were significant at p<.05.
All 27 tracts assessed in this study had at least one cluster with significantly (p<.05, Equation 3) positive MIRD. The top ten (by significance) positive MIRD clusters are shown below (Table 3). Only 4 of the 27 tracts assessed had significant clusters of negative MIRD. All 4 of the significant clusters were located in the left sided projection fibers. At the trend level (p<.1), three of the four right sided projection fibers and the left optic tract were also significant (Table 4) suggesting left-right asymmetric development.
Table 3.
Estimated rates of change for, and coordinates of, significant clusters of positive MIRD.
| Tract | Cluster Extent (%) | Loc. | Coord. MNI | βRD,GA (%/week) | βRD,SA (%/week) | ΔβRD (%) | MIRD | p(F)min |
|---|---|---|---|---|---|---|---|---|
| L Par. Post. CF | 10 | SPG | −16,−55,56 | −5.4 | −2.5 | +54 | 0.65 | <.001 |
| CC Parietal | 8 | SPG | 13,−62,49 | −5.3 | −2.6 | +51 | 0.62 | 0.002 |
| R Premotor CT | 6 | SFG | 11,6,59 | −5.3 | −1.9 | +64 | 0.77 | <.001 |
| R Par. Post. CF | 5 | SPG | 17,−37,56 | −5.1 | −2.8 | +44 | 0.53 | <.001 |
| CC Occipital | 4 | SOG | 23,−86,31 | −5.1 | −2.4 | +53 | 0.64 | 0.002 |
| L SLF | 3 | SPG | −28,−44,61 | −4.4 | −1.5 | +67 | 0.80 | <.001 |
| R Cingulum | 3 | SPG | 12,−53,48 | −5.6 | −3.0 | +46 | 0.54 | 0.003 |
| R Fornix | 3 | ITG | 35,2,−35 | −5.0 | −2.0 | +60 | 0.78 | 0.003 |
| R ILF | 2 | STG | 49,8,−17 | −4.4 | −1.9 | +58 | 0.69 | 0.002 |
| R IFOF | 1 | MOG | 18,−101,−5 | −4.8 | −2.5 | +47 | 0.55 | 0.004 |
Top ten clusters identified as having a significant (p<.05) increase in explained variance when splitting up post-conceptional age into gestational age and age at scan. All metrics are averaged across clusters unless otherwise specified. Cluster extent is expressed in percentage of length relative to the tract. TC=Thalamocortical, CF=Corticofugal, CT=Corticothalamic, SLF=Superior Longitudinal Fasciculus, ILF=Inferior Longitudinal Fasciculus, IFOF=Inferior Fronto-Occipital Fasciculus, SPG=Superior Parietal Gyrus, SFG=Superior Frontal Gyrus, SOG=Superior Occipital Gyrus, MOG=Medial Occipital Gyrus, STG=Superior Temporal Gyrus, MTG=Medial Temporal Gyrus, ITG=Inferior Temporal Gyrus.
Table 4.
Estimated rates of change for, and coordinates of, significant clusters of negative MIRD.
| Tract | Cluster Extent (%) | Loc. | Coord. MNI | βRD,GA (%/week) | βRD,SA (%/week) | ΔβRD (%) | MIRD | p(F)min |
|---|---|---|---|---|---|---|---|---|
| L Motor CT | 13 | PLIC | −25,−15,23 | −1.6 | −2.9 | −84 | −.54 | .02 |
| L Par. Post. TC | 11 | PLIC | −25,−21,21 | −1.1 | −2.4 | −127 | −.68 | <.01 |
| L Par. Post. CF | 10 | CP | −15,−22,−12 | −1.1 | −2.7 | −142 | −.68 | .01 |
| L Premotor CT | 9 | PLIC | −22,−7,17 | −1.6 | −2.9 | −81 | −.53 | .02 |
| R Par. Post. TC | 9 | PLIC | 24,−20,17 | −1.3 | −2.0 | −62 | −.45 | .05 |
| R Motor CT | 9 | PLIC | 24,−15,17 | −1.0 | −2.0 | −102 | −.58 | .06 |
| L Optic | 3 | ST | −29,−25,−2 | −1.2 | −2.3 | −89 | −.55 | .08 |
| R Premotor CT | 3 | PLIC | 21,−6,15 | −1.0 | −2.1 | −104 | −.61 | .09 |
Four clusters were identified as having a significant (p<.05) increase in explained variance when splitting up post-conceptional age into gestational age and age at scan. Four additional clusters are listed at the trend level (p<.1). All metrics are averaged across clusters unless otherwise specified. Cluster extent is expressed in percentage of length relative to the tract. TC=Thalamocortical, CF=Corticofugal, CT=Corticothalamic, PLIC=Posterior Limb Internal Capsule, ST=Stria Terminalis, CP=Cerebral Peduncle.
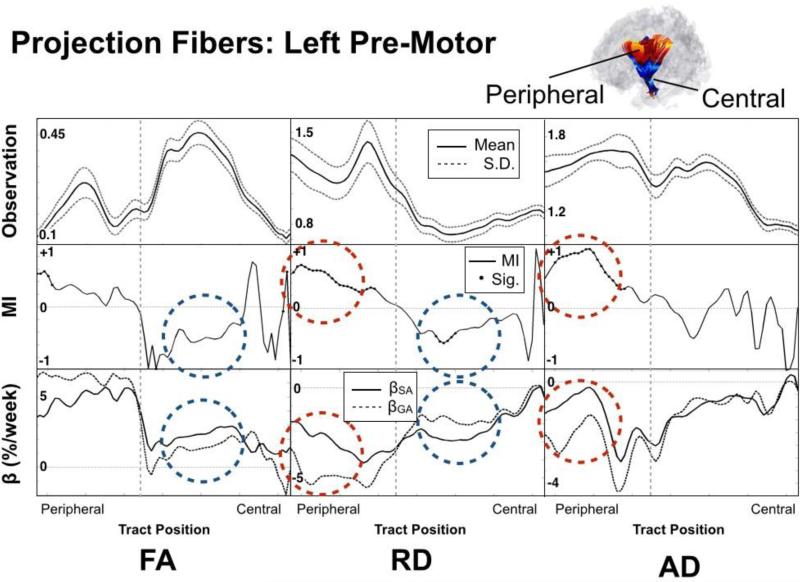
3.2.2 Post-hoc Examination of Projection Fibers
Diffusivity measures, MI and fitted slope values are shown for the left premotor corticothalamic tract in Figure 6. As seen in the figure, the tract can be considered as two distinct regions (peripheral and central) bisected by the switch in sign of MI and changing microstructural properties (FA, RD, AD), reflective of the anatomical transition into the internal capsule. The peripheral portion can be described as having: 1) small FA, large RD and moderate to high AD, 2) large positive MIAD and MIRD, and 3) large differences in AD and RD beta values with βGA>βSA. Conversely, the central portion can be qualitatively described as having: 1) large FA, small RD and moderate to low AD, 2) large negative MIFA and MIRD, and 3) large differences in beta values with βSA>βGA. Percent differences in rates of change before and after birth for FA, RD and AD in the peripheral portion were +32%, +62% and +107%, respectively. For the central portion the same changes in FA, RD and AD were −43%, −41% and −27%.
Figure 6. Detailed Features Along the Left Premotor Tract.
Top row: microstructural value (FA, RD, AD); Middle row: Maturation index; Bottom row: estimated rate of change (GA dashed line, SA solid line). Note the clear delineation between inferior and superior regions (marked by vertical dashed lines) reflected in the anatomy, MI and rate of change. Red dashed circles highlight a peripheral region with large positive MIRD and MIAD, reflective of premyelination and blue dashed circles highlight a central region with large negative MIRD and large negative MIFA reflective of true myelination. Observation axes units of measurement are relative (FA) and 10−3mm2/s (AD, RD).
Discussion
By devising a model of development that considers the rates of perinatal maturational change of white matter fiber tracts, this work has demonstrated three principal findings: 1) group level information about pre- versus post-natal developmental changes of white matter fiber tracts can be retrieved based on a cross-sectional sample of healthy newborns with similarly varying gestational age at birth and postnatal age at scan; 2) when evaluating imaging associations with age in neonates, separating postmenstrual age into gestational age and age at scan (postnatal age) results in a significantly more powerful model, as compared to using postmenstrual age as a predictor, because it accounts for an inflection in maturational changes at the time of birth; 3) the prenatal and postnatal rates of change can be cast as a unit-less index (MI), the value of which is reflective of relatively increasing or decreasing maturational processes after birth.
Two additional postulations based on adopted (Dubois et al. 2008) DTI models of myelination, the application of the proposed MI and deductive reasoning are made below: 1) shortly before birth, the developing brain is primarily in a state of pre-myelination which appears to slow at birth; and 2) more mature central regions are increasing the process of true myelination near the time of birth.
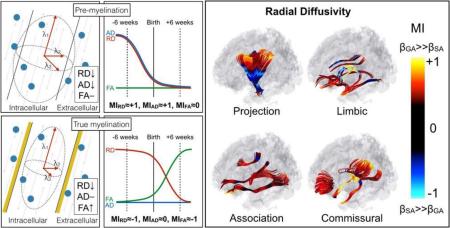
Neonatal white matter microstructural development across the brain has been characterized indirectly via DTI (Dubois et al., 2014; Dietrich et. al., 1988; Yoshida et al., 2013) measures of diffusivity (FA, AD, and RD). We are here adopting the model used by Dubois et al. (2008; Beaulieu, 2002) for inferences on myelin development based on observed DTI properties. RD measures diffusivity perpendicular to the axon and monotonically decreases during the three identified stages of axonal development: axonal organization, oligodendrocyte proliferation (premyelination) and myelin deposition (true myelination). AD measures water diffusion along the axis of the axon and increases during axonal organization as the axons become more coherently arranged along the main axis, decreases along with RD during premyelination due to isotropic reduction in water content and, unlike RD, is largely stable during true myelination as the hydrophobic myelin sheathing restricts diffusion in the perpendicular direction. FA is a relative measure of AD and RD and is therefore reflective of the difference between the two. Axonal organization is characterized by an increase in AD and decrease in RD resulting in increased FA. Premyelination has a concomitant decrease in AD and RD and therefore results in no change in FA. FA increases during true myelination are driven by a decrease in RD. While axonal organization, or fascicle coherence, will be discussed further below, it is thought that the majority of fascicles are already organized by the late prenatal stages due to an abundance of extracellular matrix and guidance molecules (Kostović and Jovanov-Milošević, 2006). Consequently, the primary mechanisms discussed here are restricted to pre-myelination (RD decrease, AD decrease, FA no change) and true myelination (RD decrease, AD no change, FA increase), the major maturational processes that occur around the time of birth.
Signs of rapid in utero premyelination (based on adopted models) followed by a decreasing rate of premyelination after birth were present throughout a relatively large percentage of the observed white matter fiber tracts (16% at p(F(+MI,RD))<.05, 25% at p<.1). All 27 of the tracts included in the analyses had at least one significant cluster suggestive of slowing premyelination at birth. Among those, commissural tracts showed the greatest propensity towards this pattern as manifested by large positive MIRD and MIAD, on average. Because the premyelination process is observed to be slowing at birth (positive MI), it is reasonable to assume it is largely being completed near term. Certainly, oligodendrocyte proliferation continues through childhood, yet this finding is consistent with typical oligodendrocyte lineage (Barateiro and Fernandes, 2014) and the window of vulnerability (23-32 postmenstrual weeks) for perinatal white matter injury (Back et al., 2001).
As expected, only a small percentage of fiber tracts in the neonatal brain suggested signs of true myelination (1% at p(F(−MI,RD))<.05, 2% at p<.1). These observations are consistent with the adopted model of true myelination (FA, RD changes) occurring postnatally (negative MI) within tightly bound regions of the tract (internal capsule). The tracts that did show evidence of true myelination at the time of birth were those that based on postmortem studies are known to be myelinated early (around the time of birth) (Kinney 2014). Specifically, the portion of corticospinal tracts running through the posterior limb of the internal capsule (PLIC) extending down into the cerebral peduncle as well as a portion of the left optic tract showed significant signs of true myelination (large negative MIRD and MIFA). Postmortem analysis in 40 postmenstrual week old infants has detected microscopic traces of myelin at birth in these regions (Kinney 2014). In addition, these sites have been identified in utero (Zanin et al., 2012) and in preterm cohorts (Kersbergen et al., 2010; Partridge et al., 2004) as early myelination sites and in in vivo neonates as being one of the earliest maturing tracts when using adults as normative measures (Dubois et. al., 2008). Finally, PLIC is the first region detectable by myelin water fraction (MWF) mapping as early as 107 postnatal days (Deoni et al., 2011).
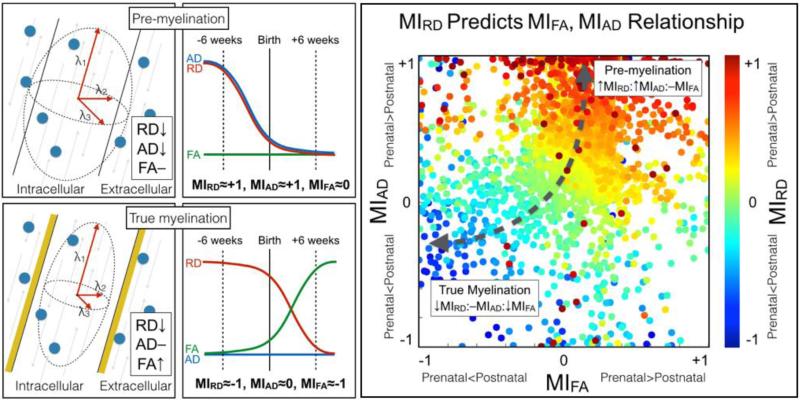
The concept that large positive MIRD is associated with large magnitude MIAD and, conversely, large negative MIRD is associated with large magnitude MIFA is introduced using a simple scatter plot. In order to further emphasize the relationship between MIFA, MIRD, and MIAD, the three metrics are shown together alongside a cartoon depiction of the hypothesized mechanisms (Figure 7, left panels) leading to the observed relationship between MIRD and the magnitude of MIAD or MIFA (Figure 7, right panels). A larger positive MIRD resulted in a large positive MIAD and null MIFA. These findings are consistent with the adopted (Dubois et al. 2008) model of pre-myelination occurring more rapidly prior to birth. Alternatively, a larger negative MIRD indicated a large negative MIFA and relatively null MIAD. These data points are consistent with the adopted model of true myelination occurring more rapidly after birth. Using this framework, MIRD is the most informative measure of diffusivity as its sign appears to be reflective of simple models of either pre-myelination or true myelination.
Figure 7. The inter-relationship bewteen MIRD, MIAD and MIFA.
Left panel set: DTI model of pre- and true myelination (far left panels) and a cartoon depiction of pre-myelination occurring before birth and true myelination occurring after birth (middle two panels). Note the process-dependence of FA, RD and AD (far left) as well as the hypothesized values for MIFA, MIRD, and MIAD (middle). Far right panel: MIRD predicts the relationship between MIFA and MIAD. As expected, the majority of data points are found to have a large positive MIRD, large positive MIAD and null MIFA. A large cluster of data points with large negative MIRD, large negative MIFA and roughly null MIAD, were found in regions known to show early signs of myelin.
One common criticism of the conventional DTI techniques applied here is the lack of specificity. Indeed, all common measures of diffusion (RD, AD, FA) may be confounded by a number of concomitant attributes including axonal diameter, compaction, water content and fiber coherence (Jones et al., 2013). While these confounds cannot and should not be dismissed in whole, we believe that because the calculation of MI is relative (comparison of rates of change before and after birth), all static confounds will be controlled for. As such, many of the inherent weaknesses of using absolute measures of FA, RD and AD are uniquely avoided using this technique. It should also be noted that alternatives to conventional diffusion imaging, while considered at the onset of the current study, were deemed to be either too long of an acquisition time (High Angular Resolution Diffusion Imaging) (Zhan et al., 2012) with the available hardware or potentially not sensitive enough to myelin (MWF) at less than 48 postmenstrual weeks (Deoni et al., 2011).
This study focused on two primary stages of axonal development: premyelination and true myelination. A third developmental stage often considered in neonates is axonal organization. During this stage, one would expect FA and AD to increase with a concomitant decrease in RD. Only one small cluster was found to have significant MI values for all three diffusion parameters in the far superior portion of the right premotor tract. While this may be a reasonable site for such a process, rates of change of both AD and RD were negative, a finding not likely in the event of increasing fiber coherence. Furthermore, the majority of axonal organization is thought to be largely concluding in the human fetus during the late preterm phase of development (Kostović and Jovanov-Milošević, 2006).
While we believe the observed MI patterns are reflective of the myelination process, it is worth considering non-myelin-specific contributions to the diffusion measurements used here. Spatial resolution is limited in the growing brain and because the brain is rapidly increasing in size, partial voluming of the fiber tracts may cause brain size dependent variation (Yoshida et al., 2013). To help account for this, intracranial volume was used in both model 1 and model 2. Similarly, the DTI acquisition methods employed in this work are susceptible to ambiguity caused by crossing fibers. While these voxels may obfuscate developmental changes, causing false negatives, it is unlikely that they would induce false positives, as the measures used here are relative, requiring the nature of the fiber crossings themselves to change as a result of birth. In addition, reductions in FA (Dubois et al., 2014) are expected when the amount, diameter or integrity of crossing fibers is increased, and such values were not observed in this study. Finally, it should be stated that the interpretation of observed effects made here is predicated on the DTI model of maturation provided by Dubois et al. (2008), and is therefore not an unequivocal observation of pre- or true-myelination patterns in the perinatal period.
While the aim of this study was to apply the MI method to perinatal diffusion data, this framework may extend to other dynamic processes with temporally well-defined events including maturation during pubertal onset, and neurodegeneration surrounding the onset of Alzheimer's disease. The MI can be generalized as the change in slope after a well-defined “event” (i.e., birth). A known “initiation” and measurement at an “observation” time point following the “event” are also required. The event, initiation and observation in the current context are birth, conception and postnatal age at MRI, respectively. The changes used in the calculation of MI are always considered using the event (birth) as the frame of reference. The variation in time separating initiation (conception) and observation (scan age) ought to be comparable in order to give equal weighting in explanatory power between the relative exposure times. While the resultant change in slope at the time of the event (birth) implies causation driven by the event, in the context of this work, the knee of an exponentially increasing or decreasing maturation curve such as those found in logistic function models of growth (Dean et al., 2014) would also drive similar results as they have a non-zero first derivative. As such, this work does not directly confirm the physiological changes occurring at birth as a mechanism for change in white matter maturation. Logistic growth has also been previously modeled as being either biphasic or monophasic linear growth and would be consistent with significant MI in this study (Ball et.al., 2013). It seems plausible from a developmental perspective that the inflection point in maturation curves be in sync with birth given the abrupt transition to the extrauterine environment. In this study, this was interpreted as rapid premyelination (large RD and FA change) before birth across large regions of the brain and rapid true myelination (large RD and AD change) in areas known to have trace amounts of myelin at birth followed by mature myelin within 28 weeks after birth (Deoni et al., 2011).
Because the developmental trajectories suggested by the MI are in concordance with our understanding of perinatal maturation of white matter fiber tracts based on histological postmortem studies, the novel MI approach used here seems to be informative about prenatal relative to postnatal maturational change using a cross-sectional design of healthy late preterm and full-term newborns. By considering three diffusion parameters and their role in different stages of white matter development, we have constructed a plausible model of the ontogeny of early life white matter. While the MI described here has been shown to be useful for characterizing average group patterns, it is not designed to probabilistically identify differences in individuals or between groups. Future efforts will focus on developing between-group tests to identify deviations from normal brain development and to better understand environmental conditions that are associated with such deviations that may be amenable to intervention.
Supplementary Material
Highlights.
DTI data were examined for dependence on gestational (GA) and postnatal age (SA).
The Maturation Index (MI) captures the dependence of DTI data on GA relative to SA.
Tract-based analysis demonstrates negative MI in regions known to myelinate early.
Positive MI was also observed and may be reflective of in-utero pre-myelination.
MI maps are presented, postulated to be indicative of perinatal brain maturation.
Acknowledgements
Support for this work was provided by National Institute of Mental Health [R01 MH091351 Buss; Fetal Programming of the Newborn and Infant Human Brain] and National Institute of Child Health and Development [R01 HD060628 Wadhwa; EMA Assessment of Biobehavioral Processes in Human Pregnancy]. We are grateful to the families for participating in the study, and to the numerous UCI staff responsible for managing recruitment and data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. The Journal of Neuroscience. 2001;21(4):1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD. Development of cortical microstructure in the preterm human brain. Proceedings of the National Academy of Sciences. 2013;110(23):9541–9546. doi: 10.1073/pnas.1301652110. DOI: 10.1073/pnas.1301652110/-/DCSupplemental/pnas.201301652SI.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barateiro A, Fernandes A. Temporal oligodendrocyte lineage progression: in vitro models of proliferation, differentiation and myelination. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research. 2014;1843(9):1917–1929. doi: 10.1016/j.bbamcr.2014.04.018. DOI: 10.1016/j.bbamcr.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system–a technical review. NMR in Biomedicine. 2002;15(7-8):435–455. doi: 10.1002/nbm.782. DOI: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Coman I, Barbin G, Charles P, Zalc B, Lubetzki C. Axonal signals in central nervous system myelination, demyelination and remyelination. Journal of the neurological sciences. 2005;233(1):67–71. doi: 10.1016/j.jns.2005.03.029. DOI: http://dx.doi.org/10.1016/j.jns.2005.03.029. [DOI] [PubMed] [Google Scholar]
- Dean DC, O'Muircheartaigh J, Dirks H, Waskiewicz N, Lehman K, Walker L, Han M, Deoni SC. Modeling healthy male white matter and myelin development: 3 through 60months of age. Neuroimage. 2014;84:742–752. doi: 10.1016/j.neuroimage.2013.09.058. DOI: 10.1016/j.neuroimage.2013.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Mercure E, Blasi A, Gasston D, Thomson A, Johnson M, WIlliams SC, Murphy DG. Mapping infant brain myelination with magnetic resonance imaging. The Journal of Neuroscience. 2011;31(2):784–791. doi: 10.1523/JNEUROSCI.2106-10.2011. DOI: 10.1523/JNEUROSCI.2106-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deoni SC, Dean DC, O'Muircheartaigh J, Dirks H, Jerskey BA. Investigating white matter development in infancy and early childhood using myelin water faction and relaxation time mapping. Neuroimage. 2012;63(3):1038–1053. doi: 10.1016/j.neuroimage.2012.07.037. DOI:10.1016/j.neuroimage.2012.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich RB, Bradley WG, Zaragoza EJ, Otto RJ, Taira RK, Wilson GH, Kangarloo H. MR evaluation of early myelination patterns in normal and developmentally delayed infants. American journal of neuroradiology. 1988;9(1):69–76. doi: 10.2214/ajr.150.4.889. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Perrin M, Mangin JF, Cointepas Y, Duchesnay E, Le Bihan D, Hertz-Pannier L. Asynchrony of the early maturation of white matter bundles in healthy infants: quantitative landmarks revealed noninvasively by diffusion tensor imaging. Human Brain Mapping. 2008;29(1):14–27. doi: 10.1002/hbm.20363. DOI: 10.1002/hbm.20363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J, Hertz-Pannier L, Cachia A, Mangin JF, Le Bihan D, Dehaene-Lambertz G. Structural asymmetries in the infant language and sensori-motor networks. Cerebral Cortex. 2009;19(2):414–423. doi: 10.1093/cercor/bhn097. DOI: 10.1093/cercor/bhn097. [DOI] [PubMed] [Google Scholar]
- Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Hüppi PS, Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. DOI: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Fillard P, Gilmore J, Lin W, Piven J, Gerig G. Quantitative analysis of white matter fiber properties along geodesic paths. Proc of MICCAI '03. 2003;1(2879):16–23. [Google Scholar]
- Gilmore JH, Lin W, Prastawa MW, Looney CB, Vetsa YSK, Knickmeyer RC, Evans DD, Smith JK, Hamer RM, Lieberman JA, Gerig G. Regional gray matter growth, sexual dimorphism, and cerebral asymmetry in the neonatal brain. The Journal of Neuroscience. 2007;27(6):1255–1260. doi: 10.1523/JNEUROSCI.3339-06.2007. DOI: 10.1523/JNEUROSCI.3339-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hüppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA, Volpe JJ. Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatric research. 1998;44(4):584–590. doi: 10.1203/00006450-199810000-00019. DOI: 10.1203/00006450-199810000-00019. [DOI] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do's and don'ts of diffusion MRI. Neuroimage. 2013;73:239–254. doi: 10.1016/j.neuroimage.2012.06.081. DOI: 10.1016/j.neuroimage.2012.06.081. [DOI] [PubMed] [Google Scholar]
- Kersbergen KJ, Leemans A, Groenendaal F, van der Aa NE, Viergever MA, de Vries LS, Benders MJ. Microstructural brain development between 30 and 40weeks corrected age in a longitudinal cohort of extremely preterm infants. Neuroimage. 2014;103:214–224. doi: 10.1016/j.neuroimage.2014.09.039. DOI: 10.1016/j.neuroimage.2014.09.039. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Kloman AS, Gilles FH. Sequence of central nervous system myelination in human infancy. II. Patterns of myelination in autopsied infants. Journal of Neuropathology & Experimental Neurology. 1988;47(3):217–234. doi: 10.1097/00005072-198805000-00003. DOI: 10.1097/00005072-198705000-00005. [DOI] [PubMed] [Google Scholar]
- Kostović I, Jovanov-Milošević N. Seminars in Fetal and Neonatal Medicine. 6. Vol. 11. WB Saunders; 2006. The development of cerebral connections during the first 20–45 weeks’ gestation. pp. 415–422. DOI: 10.1016/j.siny.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Latini F. New insights in the limbic modulation of visual inputs: The role of the inferior longitudinal fasciculus and the Li-Am bundle. Neurosurgical review. 2015;38(1):179–190. doi: 10.1007/s10143-014-0583-1. DOI: 10.1007/s10143-014-0583-1. [DOI] [PubMed] [Google Scholar]
- Lefèvre J, Germanaud D, Dubois J, Rousseau F, de Macedo Santos I, Angleys H, Mangin J, Huppi P, Girard N, De Guio F. Are Developmental Trajectories of Cortical Folding Comparable Between Cross-sectional Datasets of Fetuses and Preterm Newborns? Cerebral Cortex bhv123. 2015 doi: 10.1093/cercor/bhv123. DOI: 10.1093/cercor/bhv123. [DOI] [PubMed] [Google Scholar]
- O'Brien GD, Queenan JT, Campbell S. Assessment of gestational age in the second trimester by real-time ultrasound measurement of the femur length. Am J Obstet Gynecol. 1981;139(5):540–5. doi: 10.1016/0002-9378(81)90514-7. [DOI] [PubMed] [Google Scholar]
- Oishi K, Mori S, Donohue PK, Ernst T, Anderson L, Buchthal S, Faria A, Jiang H, Li X, Miller MI, van Zijl PC, Chang L. Multi-contrast human neonatal brain atlas: application to normal neonate development analysis. Neuroimage. 2011;56(1):8–20. doi: 10.1016/j.neuroimage.2011.01.051. DOI: 10.1016/j.neuroimage.2011.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Thakali KM, Shankar K, Andres A, Badger TM. Maternal adiposity negatively influences infant brain white matter development. Obesity. 2015;23(5):1047–1054. doi: 10.1002/oby.21055. DOI: 10.1002/oby.21055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge SC, Mukherjee P, Henry RG, Miller SP, Berman JI, Jin H, Lu Y, Glenn OA, Ferriero DM, Barkovich AJ, Vigneron DB. Diffusion tensor imaging: serial quantitation of white matter tract maturity in premature newborns. Neuroimage. 2004;22(3):1302–1314. doi: 10.1016/j.neuroimage.2004.02.038. DOI: 10.1016/j.neuroimage.2004.02.038. [DOI] [PubMed] [Google Scholar]
- Pistollato F, Chen HL, Schwartz PH, Basso G, Panchision DM. Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Molecular and Cellular Neuroscience. 2007;35(3):424–435. doi: 10.1016/j.mcn.2007.04.003. DOI: 10.1016/j.mcn.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Prastawa M, Gilmore JH, Lin W, Gerig G. Automatic segmentation of MR images of the developing newborn brain. Medical image analysis. 2005;9(5):457–466. doi: 10.1016/j.media.2005.05.007. DOI: 10.1016/j.media.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Provenzale JM, Isaacson J, Chen S. Progression of corpus callosum diffusion-tensor imaging values during a period of signal changes consistent with myelination. American Journal of Roentgenology. 2012;198(6):1403–1408. doi: 10.2214/AJR.11.7849. DOI: 10.2214/AJR.11.7849. [DOI] [PubMed] [Google Scholar]
- Qiu A, Fortier MV, Bai J, Zhang X, Chong YS, Kwek K, Saw SM, Godfrey KM, Gluckman PD, Meaney MJ. Morphology and microstructure of subcortical structures at birth: a large-scale Asian neonatal neuroimaging study. Neuroimage. 2013;65:315–323. doi: 10.1016/j.neuroimage.2012.09.032. DOI: 10.1016/j.neuroimage.2012.09.032. [DOI] [PubMed] [Google Scholar]
- Rabi Y, Yee W, Chen SY, Singhal N. Oxygen saturation trends immediately after birth. The Journal of pediatrics. 2006;148(5):590–594. doi: 10.1016/j.jpeds.2005.12.047. DOI: http://dx.doi.org/10.1016/j.jpeds.2005.12.047. [DOI] [PubMed] [Google Scholar]
- Toda T, Homma D, Tokuoka H, Hayakawa I, Sugimoto Y, Ichinose H, Kawasaki H. Birth regulates the initiation of sensory map formation through serotonin signaling. Developmental cell. 2013;27(1):32–46. doi: 10.1016/j.devcel.2013.09.002. DOI: 10.1016/j.devcel.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Van Kooij BJM, De Vries LS, Ball G, Van Haastert IC, Benders MJNL, Groenendaal F, Counsell SJ. Neonatal tract-based spatial statistics findings and outcome in preterm infants. American Journal of Neuroradiology. 2012;33(1):188–194. doi: 10.3174/ajnr.A2723. DOI: 10.3174/ajnr.A2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde AR, Budin F, Berger JB, Gupta A, Farzinfar M, Kaiser A, Ahn A, Johnson H, Matsui J, Hazlett HC, Sharma A, Goodlett C, Shi Y, Gouttard S, Vachet C, Piven J, Zhu H, Styner M. UNC-Utah NA-MIC framework for DTI fiber tract analysis. Frontiers in Neuroinformatics. 2013;7 doi: 10.3389/fninf.2013.00051. DOI: 10.3389/fninf.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmann S, Bührer C, Schmitz T. Focal necrosis and disturbed myelination in the white matter of newborn infants: a tale of too much or too little oxygen. Frontiers in Pediatrics. 2015;2:143. doi: 10.3389/fped.2014.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida S, Oishi K, Faria AV, Mori S. Diffusion tensor imaging of normal brain development. Pediatric radiology. 2013;43(1):15–27. doi: 10.1007/s00247-012-2496-x. DOI: 10.1007/s00247-012-2496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen TJ, Silbereis JC, Griveau A, Chang SM, Daneman R, Fancy SP, Zahed H, Maltepe E, Rowitch DH. Oligodendrocyte-encoded HIF function couples postnatal myelination and white matter angiogenesis. Cell. 2014;158(2):383–96. doi: 10.1016/j.cell.2014.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanin E, Ranjeva JP, Confort-Gouny S, Guye M, Denis D, Cozzone PJ, Girard N. White matter maturation of normal human fetal brain. An in vivo diffusion tensor tractography study. Brain and behavior. 2011;1(2):95–108. doi: 10.1002/brb3.17. DOI: 10.1002/brb3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan L, Jahanshad N, Ennis DB, Jin Y, Bernstein MA, Borowski BJ, Jack CR, Toga AW, Leow AD, Thompson PM. Angular versus spatial resolution trade-offs for diffusion imaging under time constraints. Human brain mapping. 2013;34(10):2688–2706. doi: 10.1002/hbm.22094. DOI: 10.1002/hbm.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.