Abstract
The circadian clock is a transcriptional/translational feedback loop that drives the rhythmic expression of downstream mRNAs. Termed “clock-controlled genes,” these molecular outputs of the circadian clock orchestrate cellular, metabolic, and behavioral rhythms. As part of our on-going work to characterize key upstream regulators of circadian mRNA expression, we have identified a novel clock-controlled gene in Drosophila melanogaster, Achilles (Achl), which is rhythmic at the mRNA level in the brain and which represses expression of anti-microbial peptides in the immune system. Achilles knock-down in neurons dramatically elevates expression of crucial immune response genes, including IM1 (Immune induced molecule 1), Mtk (Metchnikowin), and Drs (Drosomysin). As a result, flies with knocked-down Achilles expression are resistant to bacterial challenges. Meanwhile, no significant change in core clock gene expression and locomotor activity is observed, suggesting that Achilles influences rhythmic mRNA outputs rather than directly regulating the core timekeeping mechanism. Notably, Achilles knock-down in the absence of immune challenge significantly diminishes the fly’s overall lifespan, indicating a behavioral or metabolic cost of constitutively activating this pathway. Together, our data demonstrate that (1) Achilles is a novel clock-controlled gene that (2) regulates the immune system, and (3) participates in signaling from neurons to immunological tissues.
Keywords: Circadian clock, immunity, Achilles, CG17386, Drosophila, anti-microbial peptides, gene expression, genomics, RNA-seq, bacterial infection, fat body, RNA-binding protein
1. Introduction
Circadian rhythms are internal timekeeping mechanisms that orchestrate daily oscillations of behavior, metabolism and physiology. In most living organisms, circadian rhythms play a profound role in the regulation of physiological behaviors, such as locomotor activity, sleep-wake cycle, body temperature, blood pressure, cardiovascular activity, muscle strength, feeding, glucose and lipid homeostasis, and alertness (Hastings et al., 2003). In addition, circadian rhythms regulate both adaptive and innate immunity and thereby influence resistance to infection (Scheiermann et al., 2013). Circadian rhythms are important for maintaining homeostasis by anticipating and adapting to predictable environmental changes. Consequently, disruption of circadian rhythms influences multiple pathologies, such as neurodegenerative diseases, cardiovascular diseases, obesity, diabetes, cancer, and depression (Halberg et al., 2006; Hastings et al., 2003; Klerman, 2005; Knutsson, 2003; Levi and Schibler, 2007; Wulff et al., 2010).
At the molecular level, circadian rhythms are regulated by core clock genes that underlie self-sustained 24-hour feedback loops. In flies, two transcription factors, CLOCK (CLK) and CYCLE (CYC) compose the positive branch of the feedback loop while PERIOD (PER) and TIMELESS (TIM) compose the negative branch. CLK and CYC form heterodimers and bind to E-box elements located in the promoter regions of per and Tim, promoting their expression. Once translated, PER and TIM dimerize and translocate into the nucleus, where they prevent CLK and CYC heterodimers from accessing E-box elements, thus decreasing the mRNA expression of per and Tim. The degradation of PER and TIM resets the clock and thereby starts a new round of CLK and CYC activation. Well-studied protein modifications impose appropriate delay mechanisms, thus generating a transcriptional-translational feedback loop (TTFL) that occurs about every 24 hours (Allada and Chung, 2010; Hardin, 2011; Ko and Takahashi, 2006).
In addition to promoting per and Tim expression, CLK and CYC further drive the rhythmic expression of hundreds to thousands of downstream genes. Termed “clock-controlled genes (CCGs),” these rhythmic mRNAs are not involved in the core timekeeping mechanism but instead regulate physiological processes (Hastings et al., 2003). While the core clock genes are conserved in different tissues, CCGs are highly tissue-specific. Rhythmic transcriptome profiling in 12 different mouse organs shows little overlap of CCGs between different tissues, as expected, given how diverse these different tissues are in their physiological demands (Zhang et al., 2014). The observation that CCGs are largely tissue-specific is seen in flies as well as mammals, suggesting that it is a well-conserved aspect of circadian output pathways (Ceriani et al., 2002; Panda et al., 2002; Storch et al., 2002; Xu et al., 2011). Consequently, the disruption of core clock genes causes systematic rhythmic disorders, while the disruption of CCGs is more likely to be linked to local disorders (Hughes et al., 2012; Jeyaraj et al., 2012). Since the outputs of the circadian clock are ultimately responsible for the clock’s influence on health and physiology, it is thus necessary to identify tissue-specific CCGs and to understand their regulatory mechanisms. For example, the study of cardiac specific CCGs revealed a role of rhythmic iron channels in arrhythmia development and susceptibility (Jeyaraj et al., 2012; Schroder et al., 2013). To this end, high-throughput microarray and RNA-sequencing (RNA-seq) have greatly accelerated our understanding of CCGs in diverse tissues as well as multiple cell types (Du et al., 2014; Filichkin and Mockler, 2012; Hughes et al., 2012; Keegan et al., 2007; McDonald and Rosbash, 2001; Menet et al., 2012). Furthermore, the use of these high-throughput approaches in genetically modified animals enables the understanding of their regulatory mechanisms as well as the contributions of principle oscillator and peripheral oscillators to the regulation of specific CCGs (Bugge et al., 2012; Koike et al., 2012; Meireles-Filho et al., 2014; Menet et al., 2014; Rey et al., 2011; Xu et al., 2011). Ongoing studies in our lab and others are aimed at identifying CCGs and understanding how they mediate clock output of physiological processes related to disease and therapeutics.
In animals, circadian rhythms are regulated in hierarchy. In both mammals and insects, there are neuron-based primary oscillators located in the brain. The primary oscillator in mammals resides in the suprachiasmatic nuclei (SCN), and in flies it is distributed among several diffuse clusters of neurons (Herzog, 2007; Nitabach and Taghert, 2008). In addition to the primary oscillator, there are multiple peripheral tissues that behave rhythmically. The principal oscillator integrates environmental signals and sends synchronizing cues to peripheral tissues through mechanisms that are the subject of active investigation (Hastings et al., 2003).
Circadian control of immunological defenses is one of the most dramatic examples of a pathway through which the circadian clock influences organismal health and fitness. In mammals, both principal arms of the immune system – innate and adaptive – are regulated by circadian rhythms. This is seen at both a molecular and cellular level (Curtis et al., 2015; Silver et al., 2012a; Silver et al., 2012b). High-throughput analyses have revealed rhythmicity in many genes involved in the immune response (Keller et al., 2009). In addition, cytokines and chemokines, such as IL6 (interleukin 6), TNFα (tumor necrosis factors alpha) and CXCL 12 (Chemokine (C-X-C Motif) Ligand 12) are released into the circulation in a rhythmic manner. White blood cells, including T lymphocytes, natural killer cells, macrophages, monocytes and the precursor haematopoietic stem cells are released into the circulation in a rhythmic manner and respond to stimuli rhythmically (Ella et al., 2016; Gibbs et al., 2012; Labrecque and Cermakian, 2015; Lange et al., 2010; Mendez-Ferrer et al., 2008; Scheiermann et al., 2013). Together, these molecular and cellular rhythms influence organismal immunobiology in profound ways. Mice show differential resistance against infection at different times of the day. Inflammation, immune resistance, and the severity of autoimmune diseases are also found to vary throughout the day in a rhythmic manner (Carter et al., 2016; Curtis et al., 2014; Cutolo, 2012; Gibbs and Ray, 2013). The chronic disruption of circadian rhythms, including sleep deprivation, shift work, and jet lag can precipitate disease even in healthy individuals and exacerbate existing diseases, particularly inflammatory conditions (Ranjbaran et al., 2007).
Drosophila has been widely used as a model organism to study the mechanisms of immune response due to its relative simplicity and its genetic tractability. Some exotic defense mechanisms notwithstanding (Watson et al., 2005), the humoral immune system in Drosophila is highly conserved at a molecular level with its mammalian counterpart (Muller et al., 2008). The initial discovery of an immunological role for Toll in Drosophila revolutionized the study of mammalian pattern recognition receptors (Anderson, 2000; Hoffmann, 2003; Kimbrell and Beutler, 2001). The humoral immune system of Drosophila is divided into two major pathways, Toll pathway and Imd (immune deficiency) pathway. These two pathways combat different types of bacterial and fungal infections by distinguishing the pathogen-associated molecular patterns through pattern recognition proteins, activating the downstream AMPs (anti-microbial peptides) within the immune system, particularly within the fat body. AMPs are then secreted into the heamolymph to clear the infected pathogen (Hoffmann, 2003; Imler and Hoffmann, 2000). There are seven AMP families characterized in Drosophila: Drosomycin, Metchnikowin, Cecropins, Defensin, Attacins, Diptericin and Drosocin (Hetru et al., 2003). Similar to mammals, the immune response in Drosophila is also found to be rhythmic. Genes involved in immune response are rhythmically expressed, and flies infected with pathogenic bacteria at different times of the day show a rhythmic resistance peaking during the late night (Lee and Edery, 2008; McDonald and Rosbash, 2001; Stone et al., 2012). However, it is unclear how this is regulated at both a molecular and cellular level.
Here we show that CG17386, a previously uncharacterized clock-controlled gene is highly rhythmic in the fly head. Using whole-transcriptome profiling, we find that CG17386 represses the expression of immune responsive genes. Neuron-specific CG17386 knock-down results in dramatically elevated levels of crucial immune response genes, including AMPs. As a result, flies with knocked-down CG17386 expression are more resistant to immune challenge with bacteria. Notably, CG17386 knock-down in the absence of immune challenge significantly diminishes the fly’s overall lifespan, indicating an energetic or metabolic cost of constitutively activating this pathway. Hereafter we refer to CG17386 as Achilles (Achl), in recognition that its mutant phenotype protects flies against injury and infection, while simultaneously shortening their lifespan.
2. Materials and Methods
2.1. Fly stocks and behavioral monitoring
Flies were maintained on standard food (Genesee Scientific, San Diego, California) at 25 °C in 12 hour light: 12 hour dark (LD) conditions. Humidity was maintained at roughly 50%. All fly stocks used were acquired from the Bloomington stock center: Elav-Gal4 strain: P{w[+mW.hs]=GawB}elav[C155] w[1118]; P{w[+mC]=UAS-Dcr-2.D}2. CG17386 (Achl) RNAi strain: y[1] v[1]; P{y[+t7.7] v[+t1.8]=TRiP.JF01976}attP2. RNAi control strain: y[1] v[1]; P{y[+t7.7] v[+t1.8]=UAS-GFP.VALIUM10}attP2. Canton S flies were used as wildtype controls.
Individual male flies were placed in locomotor activity monitor tubes 3–5 days after eclosion and were entrained to LD conditions for five days before being released into free-running conditions of constant darkness (DD). Automated TriKinetics (Waltham, Massachusetts) infrared beam-crossing monitor systems were used to assay locomotor activity using one minute bins.
2.2. Bacterial stocks and culture
P. aeruginosa strain PAO1 was a gift from Dr. Lon Chubiz (UMSL). S. aureus strain was acquired by Kelly O’Mara from Carolina Biological (Burlington, North Carolina). For each experiment, a frozen glycerol stock was freshly streaked onto a LB plate and grown overnight at 37 °C. A single colony was picked from this plate and grown in 1–2 ml LB media overnight. Afterwards a subculture was made in 2 ml LB at a starting OD600 nm of 0.05 or less. The culture was harvested at an OD600 nm of around 3.0. After 1X PBS wash, the bacterial was serially diluted into OD600 nm of 0.05 for P. aeruginosa (about 40 bacteria/fly) or 0.10 for S. aureus (about 100 bacteria/fly) with 1X PBS for experimental infection.
2.3. RNA Preparation
Flies of three to five days old were used for sequencing and expression analysis of Achl core clock genes. Five to ten days old flies were used for infection related qPCR analysis. For sequencing and quantitative PCR purposes, five to ten fly heads per sample were manually dissected in PBS, transferred into 100 μL of Trizol (Life Technologies, Carlsbad, California) and homogenized using RNase-free pestles (Fisher Scientific, Waltham, Massachusetts). After 5 min incubation at room temperature with an additional 400 μL of Trizol, total RNA was prepared with phase lock gels (5Prime, Gaithersburg, Maryland) and RNeasy mini kit (Qiagen, Hilden, Germany) using the manufacturer’s protocol. RNA quantity and quality were assessed using a Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, California) and a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, California).
2.4. Library preparation and RNA sequencing
RNA-seq libraries were prepared from 200ng total RNA per sample using TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, California) following the manufacturer’s protocol with 13 rounds of PCR amplification. Libraries were quantified and qualified using Qubit 2.0 Fluorometer and Agilent 2100 Bioanalyzer. Prepared libraries with unique indexes were diluted to 2.5nM and multiplexed for loading on an Illumina Miseq (UMSL Genomics Facility) for sequencing using Miseq reagent kit v2 (50 cycles). Sequencing samples with quality scores >= Q30 were over 95%.
2.5. RNA-seq alignment
The RNA-seq Unified Mapper (Grant et al., 2011) was used to align sequenced reads to the genome and transcriptome of Drosophila melanogaster (build dm3) using the following parameters:
“--strand-specific --variable-length-reads --bowtie-nu-limit 10 --nu-limit 10”. All aligned samples showed expected proportions of uniquely and non-uniquely aligned reads, and normal distribution across Drosophila chromosomes, as compared to previous studies (Hughes et al., 2012). Pearson correlation coefficient of all transcripts is no less than 0.94 between replicates.
2.6. Quantitative and reverse transcriptase PCR
Reverse transcriptase PCR was performed using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, California) following the manufacturer’s protocol with 100 nanograms to 500 nanograms of RNA. Quantitative PCR (qPCR) was performed using Brilliant III ultra-fast qPCR master mix kit (Agilent Technologies, Santa Clara, California) on an MX3005p qPCR system (Agilent Technologies, Santa Clara, California) with 10–50 ng of cDNA template. All the probes were Taqman probes labeled with FAM (fluorescein) (Applied Biosystems, Foster City, California). The Taqman probes used include: CG17386 (Achl), Dm01824077_s1; period, Dm01843684_g1; Timeless, Dm_01814247_g1; IM1, Dm02366433_s1; Mtk, Dm01821460_s1; DptB, Dm01821557_g1; PGRP-SD, Dm01840723_s1; and Rpl32: Dm_02151827_g1.
2.7. Infection and survival assay
Survival rate assay protocol was performed as described previously (Apidianakis and Rahme, 2009). Briefly, male flies 1 to 2 days after eclosion were collected, raised in the incubator on standard food (Genesee Scientific, San Diego, CA) at 25 °C in LD conditions until 5 to 6 days after eclosion. At ZT2 (zeitgeber time 2, i.e. 2 hours after lights-on), an ethanol sterilized tungsten needle that is of about 0.01 mm diameter at the tip and 0.25 mm across the needle body was injected into the thorax of five to ten flies to be coated with fly hemolymph. The needle was then dipped into the diluted bacterial solution prepared earlier and injected into the midline of the thorax of flies, as described above. Control flies were injected with 1X PBS instead of bacterial solution. Injured flies were moved into vials containing fresh food, transferred daily into new vials at 25 °C. Viability was checked every three hours between 24 hours and 48 hours post injection. For each time point and genotype, data from at least three independent experiments were pooled.
2.8. Colony forming unit assay
Colony forming unit assay protocol was adapted from the one described previously (Apidianakis and Rahme, 2009). The infection step is performed the same as in the survival rate assay described above. After 0 or 24 hours, individual flies were rinsed in 70% ethanol; homogenized in 100 ml of 1X PBS; serially diluted and spread on LB plates. These plates were incubated at 37 °C overnight before the total number of colonies was counted. For each time point and genotype, data from at least three independent experiments were pooled.
2.9. Lifespan assay
Male flies newly enclosed were collected and maintained at 25 °C in LD conditions in fresh vials with a density of 20 flies per vial. Flies were monitored for viability and transferred into new vials every five days for eight weeks. Data from three independent experiments were pooled.
2.10. Starvation assay
Seven to nine days old male flies maintained at 25 °C in LD conditions were transferred to new vials containing 1% agarose with a density of about 20 flies per vial with the same maintenance conditions. Flies were monitored for viability every three hours afterwards.
2.11. Statistical Analyses
Analysis of rhythmicity
JTK_CYCLE is an algorithm implemented in R that analyzes the rhythmicity of time-series data (Hughes et al., 2010). JTK_Cycle was performed using a period length window precisely equal to 24 hours. Lag indicates the phase of serial expression, and amplitude was calculated as previously described (Miyazaki et al., 2011).
Survival assay
Survival data were analyzed using log rank test as previously described (H. J. Motulsky, 2016).
CFU assay
The comparison of CFUs in control and RNA flies were performed using students’ T-test.
qPCR
Relative expression from qPCR data were calculated using the delta delta Ct method. Briefly, Ct data were normalized with internal control (Rpl32: Dm_02151827_g1), then normalized with either control (for gene analysis) or median (for circadian analysis) as mentioned in the Results and Legends.
RNA-Seq
GO term analysis was performed using GOrilla software available online (Eden et al., 2007; Eden et al., 2009). Two-way ANOVA was used to compare RNAi and control flies. Transcripts with averaged RPKM<1 were eliminated from further analysis. An explicit false-discovery correction (i.e. the q-value) was calculated using the method described by Benjamani-Hochberg (Benjamini and Hochberg, 1995).
Fly Behavior
Double-plotted actograms and Lomb-Scargle periodograms for assaying free-running period were generated using ActogramJ implemented in ImageJ (Schmid et al., 2011). DD statistics were measured from the first through the tenth subjective day (DD1-DD10; i.e. days 5–15 of the experiment). Only flies surviving the length of the experiment were used for statistical analysis. Individual flies were deemed to be rhythmic if their Lomb-Scargle p-value was less than 0.05 (Figure S5E). Rhythmic power was calculated by Lomb-Scargle for the averaged actograms (Figure S5A–D) as well as for each individual fly (Figure S5F). Sleep analysis was performed as described in detail previously (Kunst et al., 2014). To measure sleep, which is considered as 5 minutes of inactivity, we used a custom written MATLAB script (Parisky et al., 2008). Sleep parameters were statistically analyzed and plotted using custom written R scripts (available on request).
2.12. Data access
All raw sequencing data have been submitted to the NCBI Gene Expression Omnibus (GEO) (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE80738.
3. Results
3.1. Achl is a clock-controlled gene with rhythmic mRNA expression in the fly head
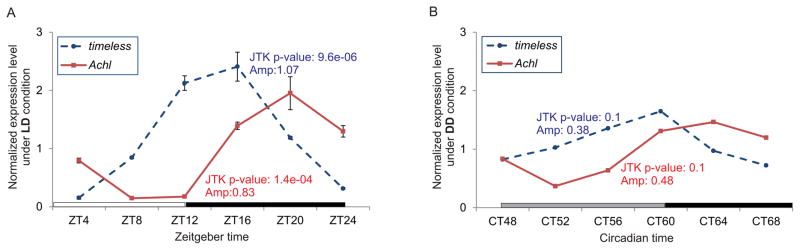
Previous high-throughput analyses revealed that Achl mRNA is rhythmically expressed in the fly head (Keegan et al., 2007) and brain (Figure S1A) (Hughes et al., 2012). We verified these observations using quantitative PCR (qPCR) assays on fly heads collected every four hours either in 12 hour light: 12 hour dark (LD) conditions or in constant darkness (DD). As expected, we found rhythmic Achl mRNA expression (Figure 1A and 1B) in both conditions, with damped amplitude under DD. Notably, the amplitude of Achl mRNA under both conditions is comparable with the amplitude of core clock genes, including Timeless. Furthermore, ChIP-seq studies from other labs suggested that CLOCK and CYCLE directly bind to the E-box regions of the Achl promoter (Abruzzi et al., 2011; Meireles-Filho et al., 2014), and Achl rhythmicity is eliminated in period mutant flies (Figure S1A) (Hughes et al., 2012); its expression is also down-regulated in Clk mutant flies (Figure S1B) (McDonald and Rosbash, 2001). Taken as a whole, these results suggest that Achl is under the direct control of the molecular clock. Larp7, a homolog of Achl in mammals, shares a conserved RNA binding domain with Achl (Figure S2B). Since Larp7 is rhythmically expressed in the mouse kidney (Figure S2A) (Zhang et al., 2014), we speculate that there may be a conserved mechanism underlying their rhythmic expression. Moreover, given the role that many RNA-binding proteins play in regulating mRNA expression, these genes may in turn regulate downstream CCGs (Morf et al., 2012; Siomi and Dreyfuss, 1997).
Figure 1. Achl is a clock-controlled gene that shows robust rhythmic mRNA expression in the fly head.
(A). qPCR assays performed on Canton S wildtype fly heads with two replicates showing that Achl is rhythmic at the mRNA level in 12hr: 12hr light:dark (LD) conditions. Timeless is a core clock gene that serves as a positive control. The bottom horizontal white and black bars represent lights on and lights off, respectively. Error bars represent +/− SEM. Data were analyzed with JTK-CYCLE to evaluate the rhythmicity (Hughes et al., 2010; Miyazaki et al., 2011). qPCR data were normalized with the median expression value of each gene.
(B). qPCR assay performed on control fly heads showing that Achl is rhythmic at the mRNA level in constant darkness (DD) conditions. The bottom horizontal gray and black bars represent subjective day and night, respectively. Data were analyzed with JTK-CYCLE to evaluate the rhythmicity (Hughes et al., 2010; Miyazaki et al., 2011). qPCR data were normalized with the median expression value of each gene.
3.2. Knocking down Achl in neurons does not affect the core clock
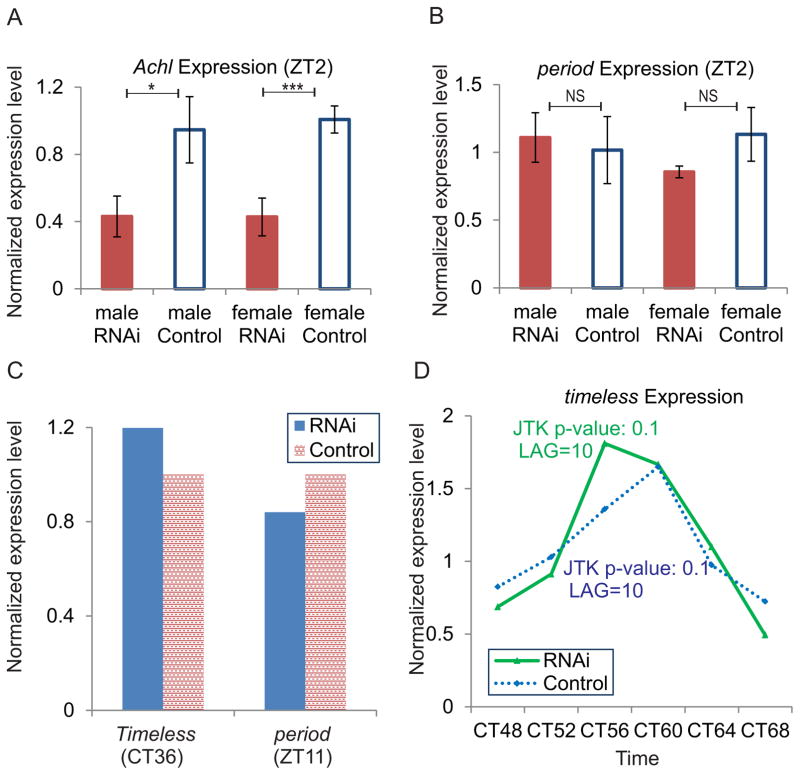
Based on the robust, high-amplitude rhythmicity of Achl in the fly head, we hypothesized that Achl is either a core clock gene or a key output gene. To test these possibilities, we generated pan-neuronal knock-down of Achl using genetically encoded RNAi constructs (i.e. Elav-Gal4; UAS-Dcr2 was crossed with TRiP UAS-Achl RNAi line). qPCR data confirmed a 60% knock down efficiency in both male and female Achl RNAi flies at ZT2 when the mRNA level of Achl is moderate (Figure 2A), compared to the negative control, an unrelated RNAi construct with the same backbone vector and genomic insertion point (UAS-GFP flies).
Figure 2. Knocking down Achl in the neurons does not affect the core clock.
(A). qPCR data from RNAi (UAS-Achl RNAi) and control (UAS-GFP RNAi) fly heads showing that that Achl expression level is significantly reduced in both males and females using genetically-encoded RNAi (i.e. Elav-Gal4; UAS-Dcr2 was crossed with TRiP UAS-Achl/GFP RNAi line) (* = p < 0.05, *** = p < 0.005; N = 3 biological replicates of 6–8 flies apiece; Error bars are +/− SEM). qPCR data were normalized with control Achl expression data of the same sex.
(B). qPCR data from RNAi and control fly heads showing that there is no significant difference in the expression of period, a core clock gene in wildtype control and Achl RNAi flies (NS = not significant, Student’s T-test; N = 3 biological replicates of 6–8 flies apiece; Error bars are +/− SEM). qPCR data were normalized with control period expression data of the same sex.
(C). qPCR data from RNAi and wildtype control fly heads showing that there is no significant difference in the expression of core clock genes (period and Timeless) at other time points. Samples collected at CT36 were females, and samples collected at ZT11 were males. qPCR data were normalized with control expression data collected at the same time.
(D). Timeless, a core clock gene, maintains its rhythmic mRNA expression in both control and RNAi fly heads, suggesting that Achl has no effect on the core clock. qPCR data were normalized with the median expression of each genotype.
The Achl RNAi line we used was generated by TRiP project and is predicted to have no off-target effects (Perkins et al., 2009). Consistent with this, we found Achl to be the only Drosophila mRNA with greater than 19 base pairs matching the RNAi construct we used (Figure S3A and S3B). Therefore, every potential off-target hit of this RNAi construct had at least 2 base pair mismatches and showed minimal knock-down in mRNA expression compared to Achl.
Achl-RNAi flies driven under a pan-neuronal driver eclosed in normal Mendalian ratios, indicating no significant developmental effects on embryonic, larval, or pupal stages. Nevertheless, we observed partially penetrant developmental defects in adult wing development. As shown in Figure S4, Achl RNAi flies have heterogeneous defects in their wing morphology, suggesting a potential role of Achl in wing development and / or wing spreading behavior after eclosion.
Two hallmarks of core circadian clock genes are (1) their effect on expression of other circadian clock genes and (2) their influence on circadian period length in constant conditions. We therefore tested whether knocking down Achl has any effect on the core clock gene expression and the overall time-keeping mechanism. As shown in Figure 2B, there is no significant difference in the expression of a core clock gene, per in Achl RNAi flies at ZT2. Since per expression peaks during the night, we performed additional qPCR analyses on samples collected at ZT11 as well as CT36 with probes targeting per and Timeless, two core clock genes, and found no difference in the expression level at these time points as well (Figure 2C), suggesting that Achl is unlikely to affect the core clock. Furthermore, we collected heads from flies maintained in DD every four hours for 24 hours and used qPCR to assess rhythmicity of core clock genes. We found no obvious defects in the period, phase, or amplitude of core clock genes, as exemplified by Timeless expression (Figure 2D).
In addition to the rhythmicity of core clock gene expression, we also examined the overall behavioral rhythmicity of Achl RNAi flies. Locomotor activity is the standard circadian behavior output that reflects the endogenous period of individual animals. We used conventional DAM (Drosophila Activity Monitoring) system monitoring to assess behavioral rhythmicity in LD and DD conditions (Pfeiffenberger et al., 2010; Schmid et al., 2011). Achl flies had normal locomotor rhythms in both LD and in DD (see averaged actograms in Figure S5A–B). The period length of these flies in constant conditions was within the experiment-to-experiment variance for negative controls (Figure S5A–D). The total number of rhythmic flies in the Achl knock-down was slightly higher than wild-type, although not statistically significant (Figure S5E). We observed that the uniformity of rhythms in Achl RNAi flies damped over time in DD (Figure S5B), and consistent with this, the power of their individual rhythms was damped on average, although not statistically significant (Figure S5F). The sleep profile of these flies (a key behavioral output of the circadian clock) was within the variance seen between different negative controls (Figure S5G–H).
Although we cannot formally exclude the possibility of a subtle behavior phenotype, based on the available data, specifically expression and rhythmicity of core clock genes, locomotor rhythms, and sleep profiles, we conclude that Achl is unlikely to play a direct role in the core clock. Instead, we favor the hypothesis that it is a clock-controlled gene influencing physiological outputs.
3.3. Knock-down of Achl results in activated expression of immune responsive genes
We then tested if Achl plays any role in the regulation of rhythmic physiological outputs. To this end, we profiled transcript expression using RNA-seq on fly heads collected from both male and female Achl RNAi and control flies to profile the genes being regulated by Achl. The samples were collected at ZT2, about 6 hours after the peak of Achl mRNA. This time point was chosen to provide sufficient time for ACHL protein to be synthesized and regulate downstream genes.
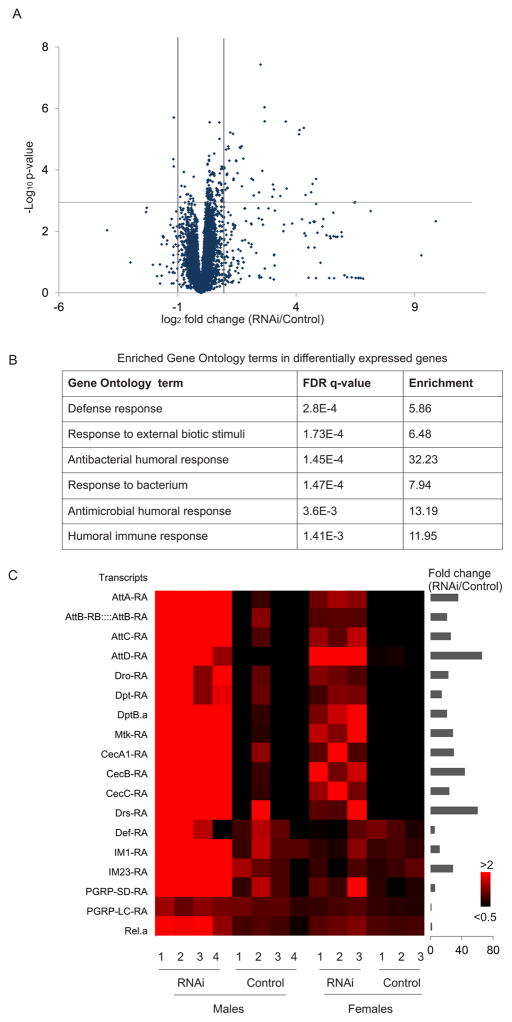
We collected 3–4 biological replicates for each genotype and sex (Table S1). RNA samples were prepared with in-line control DNAs and quality control was performed using conventional methods. For each sample, at least five million reads were obtained, roughly consistent with previous suggestions for read-depth in Drosophila RNA-seq profiling (Liu et al., 2014). The raw reads were aligned against Drosophila genome and transcriptome with RUM (RNA-seq Unified Mapper) (Grant et al., 2011). At least 95% of sequenced reads were mapped to the genome or transcriptome. We used uniquely mapped reads to calculate expression levels and disregarded ambiguous reads mapping to multiple locations. The expression values were calculated with RPKM (reads per kilobase per million mapped reads). The averaged Pearson correlation coefficient for overall transcript RPKMs was greater than 0.94 for each pairwise comparison of replicates, suggesting that the data we obtained are reproducible (Table S1). Differentially expressed genes were analyzed with a two-way ANOVA statistical analysis with factors of genotype and sex and an explicit false-discovery correction. As shown in Table 1, we found dozens to hundreds of differentially expressed transcripts under different p-value thresholds that depend on genotype in both males and females. For all further analyses, we chose to use a p-value threshold of 0.001, corresponding to a false discovery rate of q < 0.18. As shown in Figure 3A, over 90% differentially-expressed transcripts are up-regulated in Achl RNAi flies, suggesting that ACHL’s molecular function may be to repress target mRNAs.
Table 1. Differential expression statistics.
This table shows the numbers of transcripts and genes differentially expressed at different p-value thresholds as well as the number and percentage of transcripts upregulated.
| p-value | 0.0001 | 0.0005 | 0.001 | 0.005 | 0.01 | 0.05 |
|---|---|---|---|---|---|---|
| Number of transcripts | 48 | 123 | 186 | 545 | 859 | 2490 |
| Number of up-regulating transcripts (%) | 44 (91.7%) | 115 (93.5%) | 171 (91.9%) | 453 (83.1%) | 699 (81.4%) | 1775 (71.3%) |
| Number of genes | 28 | 70 | 103 | 295 | 449 | 1179 |
Figure 3. RNA-seq data showed an activation of immune responsive genes in Achl RNAi flies.
(A). Volcano plot of the RNA-seq expression data. Each dot represents a single transcript. Gray dotted lines indicate a p-value threshold of < 0.001 (corresponding to a q-value < 0.18) and fold change threshold of > 2X. For each genotype, data includes four male replicates and three female replicates.
(B). Summarized Gene Ontology analysis of differentially-expressed transcripts. Software used for analysis: Gene Ontology enrichment analysis and visualization tool (GOrilla) (Eden et al., 2007; Eden et al., 2009).
(C). Heatmap of the median-normalized expression of key immune responsive genes. Red indicates higher expression, and black indicates lower expression. Names of transcripts are marked on the left, and fold change (RNAi/Control) are marked on the right.
To determine the physiological pathways that are being affected, we performed Gene Ontology (GO) analysis for all the differentially expressed genes (Eden et al., 2007; Eden et al., 2009). As shown in Figure 3B, immune responsive pathways are dramatically enriched (see Table S2 for full list). Anti-microbial peptides (AMPs) are among the well-studied immune defensive mechanisms in insects (Hetru et al., 2003; Hoffmann, 2003). We found a striking up-regulation in their expression in Achl-RNAi flies compared to negative controls (Figure 3C). Moreover, we verified that immune defenses were up-regulated in the thorax and abdomen as well as in the fly head (Figure S6), consistent with the possibility that expression of Achl in the brain regulates systemic responses to infection.
3.4. Achl knock-down in neurons protects flies against bacterial infection
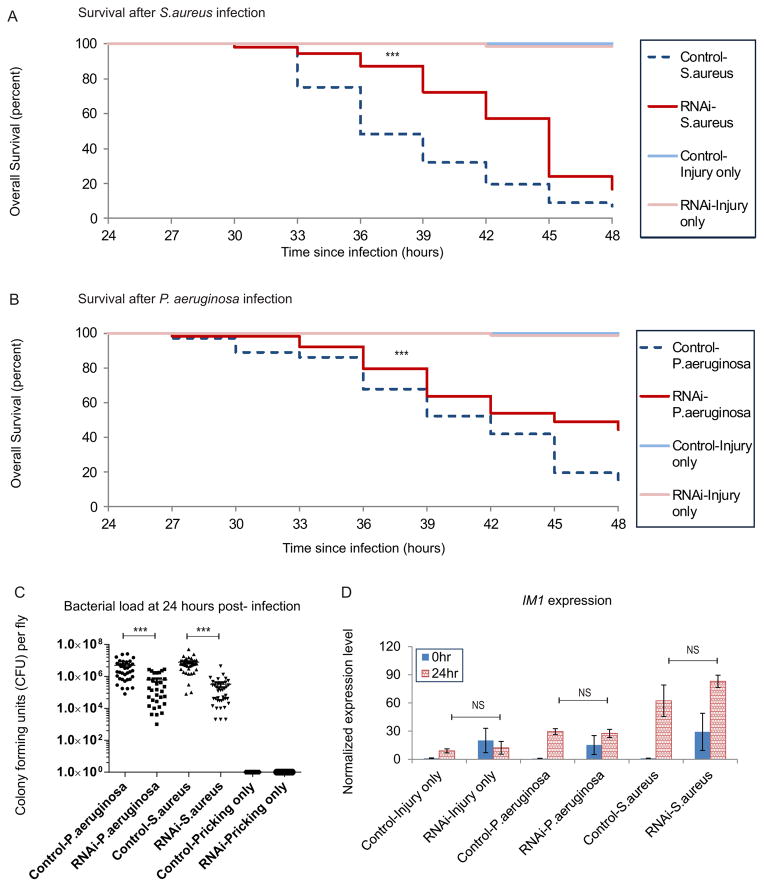
There are two strategies that hosts, including Drosophila take to defend infection: resistance and tolerance. Resistance refers to the ability to reduce bacterial growth within the host body and tolerance refers to the ability to reduce detrimental pathological effects of infection. The activated expression of immune responsive genes, which play a role in reducing bacterial growth, in Achl RNAi flies led us to hypothesize that Achl RNAi flies would be more resistant toward infection. To test this, we performed a needle inoculation assay with commonly studied and evolutionarily divergent pathogenic bacteria, P. aeruginosa and S. aureus. This needle inoculation assay is a well-characterized acute infection assay that is a conventional model for testing fly immune responses (Apidianakis and Rahme, 2009). As shown in Figure 4A and 4B, Achl RNAi flies have better overall survival compared with control flies. In addition, we examined the growth of bacteria within the fly body, and we found decreased bacterial growth after 24 hours of infection, as indicated by fewer colony forming units (CFUs) in Achl RNAi flies (Figure 4C) with the same original bacterial load (Figure S7). The fact that Achl RNAi flies had a better overall survival and less bacterial load after 24 hours of infection compared to control flies suggests that this better survival is due to increased resistance, not tolerance. Furthermore, we performed qPCR assays with four selected probes (IM1, Mtk, DptB and PGRP-SD) on flies infected for 24 hours to measure the expression of immune responsive genes. We chose to look at these four genes because they are genes with significant up-regulation in RNAi flies that could represent both pathways (Toll and Imd pathways) as well as different processes (bacterial recognition and antibacterial defense processes). As shown in Figure 4D & Figure S8, the expression of these genes after 24 hours of infection is similar for control and RNAi flies, suggesting that there is no dramatic change in the ability to respond to pathogenic bacterial infection. In addition, we found a more dramatic increased expression of these immune responsive genes in control flies than Achl RNAi flies because of the pre-activation in Achl RNAi flies. Taken together, these data suggest that Achl regulates steady-state immune responsive gene expression but does not affect peak expression after infection.
Figure 4. Achl knock-down in neurons protects flies against bacterial infection.
(A). Achl knock-down flies have a higher survival rate after S. aureus infection. Data are the combined results of at least four biological replicates. N > 50 for infected flies and > 30 for injury only flies. *** = p < 0.005 Log rank test.
(B). Achl knock-down flies have a better survival rate after P. aeruginosa infection. Data are the combined results of at least four biological repeats. N > 50 for infected flies and > 30 for injury only flies. *** = p < 0.005 Log rank test.
(C). Achl knock-down flies have decreased bacterial colony formation after bacterial infection. N > 30 for infected flies and > 20 for injury only flies. ***= p < 0.005 Students’ T-test. Mean ± SEM of Control-P. aeruginosa: 4.898e+006 ± 1.105e+006; RNAi-P. aeruginosa: 606616 ± 159290; Control-S. aureus: 7.687e+006 ± 1.301e+006; RNAi-S. aureus: 324859 ± 110479.
(D). Expression of IM1 upon infection by qPCR assay (N = 2 biological replicates of 6–8 flies apiece; Error bars are +/− SEM). 0 hour expression level is higher in RNAi than control flies; 24 hours expression level is similar in RNAi and control flies for each inoculation condition. All qPCR data were normalized with control 0 hour injury only data.
3.5. Flies with knocked-down Achl have a decreased lifespan
Altogether, these data indicate that Achl RNAi flies have a constitutively active immune system. This continuous activation is advantageous when flies are infected with bacteria, but it might cause deleterious effects in normal conditions. We therefore measured the overall lifespan of these flies and found that Achl RNAi flies have a significantly shorter lifespan than the control flies (Figure S9A). We hypothesized that this trade-off might be due to metabolic dysfunction, and therefore we examined starvation resistance of both Achl RNAi flies and control flies. To do so, flies were placed into fresh 1% agarose vials with water supply but without any source of nutrition. As shown in Figure S9B, Achl RNAi flies have a median survival time of 39 hours, while control flies have a median survival time of 48 hours. This decrease in both normal lifespan and starvation resistance suggests a behavioral or metabolic cost of constitutively activating immune pathways.
4. Discussion
The circadian clock drives tissue-specific expression of rhythmic mRNAs to regulate many different physiological processes, including the sensitivity and activity of the immune system. Here we show that Achl is expressed with 24-hour periodicity in the fly brain. Given the phase of its expression, the presence of tandem E-boxes with CLK/CYC occupancy in ChIP-seq studies, and disrupted rhythmic expression in Clock and period mutant flies, it is likely to be under the direct control of the molecular circadian clock. Since Achl encodes a protein with an RNA-binding domain, we hypothesized that it may in turn regulate the expression of downstream rhythmic genes. To test this possibility, we used whole-transcriptome RNA-seq to profile gene expression differences in the heads of wildtype flies and those with knocked-down Achl expression. Surprisingly, we found a dramatic increase in the expression of immune-response genes (Gene Ontology analysis q < 10−4; enrichment score > 5.0). Notably, most of these genes were up-regulated (Table 1 and Figure 3), with expression levels ranging from 2- to 1000-fold greater than baseline. Taken together, these data suggest that Achl directly or indirectly regulates gene expression of immune effectors.
As expected from these results, knock-down of Achl potentiates the immune system and thereby increases flies’ resistance to bacterial infection. We acknowledge that this phenotype may be due in part to changes in bacterial tolerance, although we have no data to test this possibility directly. Related to this, we also acknowledge the possibility that Achl knock-down may disrupt the microbiota in flies in a complicated and unpredictable fashion and might thereby alter normal immune function, for example, by increasing the basal expression of anti-microbial peptides. Similarly, phagocytosis is part of the rhythmic immune response in Drosophila, and its disruption might contribute to the survival phenotype we observe. Since Achl is not measurably expressed in hemocytes (Cherbas et al., 2011), any effect on phagocytosis is likely to be indirect and via a heretofore uncharacterized signaling pathway. Nevertheless, we cannot formally exclude the possibility that Achl plays a role in phagocytosis. Moreover, given the relatively minor developmental phenotype in Achl knock-downs compared to the enormous up-regulation of anti-microbial peptides, we favor the conclusion that the survival benefit of Achl knock-down is due to activation of immune response genes rather than a more elaborate mechanism. Finally, we note that Achl flies have shorter overall lifespans in the absence of infection, particularly in starvation conditions, thus indicating a metabolic or physiological cost of having a perpetually activated immune system.
Achilles’ closest mammalian homologues are La ribonucleoprotein domain family members 6 and 7 (Larp6 and Larp7). Neither gene has a known function in immunity and neither has polymorphisms that predispose human patients to auto-immune disorders in GWAS studies (Farh et al., 2015). We note that Larp7 cycles in the mouse kidney, and Larp6 cycles (albeit more weakly) in the distal colon, so both genes possess promoter and/or enhancer elements necessary for clock-driven rhythmicity. However, there is a relative paucity of circadian transcriptional profiling in immunological tissues, so it is difficult to say whether either gene is rhythmic in cells directly relevant to immunity.
Several follow-up questions emerge from these observations that are the subject of ongoing work in our laboratory. First, how does Achl regulate immune responses at a cellular level? We note that Achl expression is not normally detected in hemocytes or fat body, the conventional workhorses of the fly’s immune system. Moreover, we note that knock-down of Achl in the head affects immune gene expression in the body, indicating system-wide influences of Achl on immune function (Figure S6). We further emphasize that our experiments manipulated Achl expression strictly in neurons. Since expression of anti-microbial peptides is mainly a product of the fly fat body, we surmise that there must be a signaling mechanism downstream of Achl that conveys information from neurons to the fat body or other immunologically relevant tissues. Given the effect of Achl on the fly’s response to starvation, we speculate that signaling downstream of Achl influences both immune function and metabolic control, although more complicated mechanisms are certainly possible. Testing these possibilities and linking Achl to circadian control of both energy expenditure and immune function is a priority of ongoing studies.
Second, does Achl mRNA cycling directly contribute to functional rhythmicity in antibacterial immune defenses? We note that roughly 100–200 genes in the fly nervous system are under circadian control, and that many of them, like Achl, have never been studied in detail. Given the importance of understanding the molecular mechanisms by which circadian clocks control immune function (Edgar et al., 2016; Fortier et al., 2011; Gibbs et al., 2012; Keller et al., 2009; Rahman et al., 2015; Scheiermann et al., 2013; Silver et al., 2012b), we propose the testable hypothesis that Achl acts as a direct link between the circadian clock and immune function. This hypothesis is supported by several observations: Achl has high-amplitude mRNA rhythms that are likely driven by the clock, and it encodes an RNA-binding protein that may in turn regulate expression of downstream circadian effectors, perhaps including upstream regulators of immune activation. Arguing against this hypothesis are the observations that many clock gene mutants disrupt rather than enhance anti-bacterial immunity (Lee and Edery, 2008; Stone et al., 2012). However, we observe that Clk mutants actually confer enhanced resistance to infection (Lee and Edery, 2008), and we further note that Clk mutants have diminished Achl expression (McDonald and Rosbash, 2001). Based on this molecular phenotype, our data would predict increased expression of AMPs in Clk mutants, due to their reduced expression of Achl. This prediction is confirmed by previous microarray studies (McDonald and Rosbash, 2001). We acknowledge that there are a multitude of potential molecular mechanisms that may account for Achl’s somewhat anomalous immunological phenotype. But, given the enormous contributions flies have made as a model system to both immunity and circadian rhythms, we contend that testing this hypothesis will contribute to understanding the fundamental mechanisms linking circadian rhythms and immune function in all animals.
Supplementary Material
Figure S1. Achl is regulated by the core clock.
(A). Achl rhythmicity is disrupted in per0 flies. Data adapted from Hughes et al. (2012) Genome Research.
(B). Achl expression level is down-regulated in Clk mutant flies. Data adapted from McDonald & Rosbash. (2001) Cell.
Figure S2. Larp7, Achl’s mammalian homolog is rhythmically expressed in the mouse kidney.
(A). Larp7, a mammalian homolog of Achl, is rhythmically expressed in the mouse kidney. Data adapted from Zhang et al. (2014) PNAS.
(B). Sequence alignment of the conserved Lupus-La RNA binding domains in ACHL and its mammalian homolog, LARP7. The alignment is performed using MUSCLE (Multiple Sequence Comparison by Log- Expectation) 3.8 on EMBL-EBI (Edgar, 2004). “*” indicates positions which have a single, fully conserved residue. “:” indicates conservation between groups of strongly similar properties - scoring > 0.5 in the Gonnet PAM 250 matrix, and “.” indicates conservation between groups of weakly similar properties - scoring =< 0.5 in the Gonnet PAM 250 matrix. Different colors indicate the physicochemical properties of the residues.
Figure S3. Achl RNAi knock-down is specific.
(A). RNA-seq data demonstrate that the most likely off-target hits of the RNAi construct we use are not knocked-down. Achl is the only transcript measurably knocked-down with predicted alignment to the genetically-encoded RNAi construct we used. Control flies expressed UAS-GFP RNAi instead of UAS-Achl RNAi. RPKM: reads per kilobase per million sequenced reads.
(B). Mismatch from perfect 21-bp alignment predicted by “find OTE (off-target elements)” program (Perkins et al., 2009).
Figure S4. Achl RNAi flies have defective wing development.
Pictures captured under dissection microscope.
(A) Control flies (UAS-GFP RNAi) with normal wings.
(B) Achl RNAi flies with heterogeneous defects in their wing morphology, from slight to severe. Arrows indicate disrupted wing morphology.
Figure S5. Achl RNAi does not significantly affect behavioral rhythms or sleep.
(A & B). Averaged actograms for representative control (N = 26) (A) and Achl RNAi (N = 28) (B) male flies. Flies were placed in LD conditions and converted to DD conditions at day 5, as indicated by the arrow. These results are representative of > 5 independent experiments. The top horizontal gray and black bars represent subjective day and night.
(C & D). Lomb-Scargle periodograms corresponding to the averaged actograms in panels A and B showing the period calculated for control (C) and RNAi (D) flies. Period length differences were within the variance of negative control flies in these experiments.
(E). Percentage of individual rhythmic flies (Lomb-Scargle p < 0.05) observed. N = 26 for control and 28 for RNAi.
(F). Average power of individual rhythmic flies being analyzed; Error bars are +/− SEM. NS = p > 0.05 Students’ T-test.
(G & H). Overall sleep profile (G) and sleep amounts (H) of control (parental flies) and Achl RNAi flies showing that Achl RNAi does not dramatically alter either sleep rhythms or quantity. Error bars are +/− SEM. NS = not significant, Student’s T-test; *= p < 0.05 Students’ T-test.
Figure S6. Systemic effects of Achl knock-down.
Immune response gene IM1 is upregulated in both head and body (B) in Achl RNAi flies, even though RNAi knockdown of Achl is only observed in head (A). qPCR data is normalized with controls of the same tissue.
Figure S7. Initial bacterial load of flies in the infection assay.
(A). Achl knock-down flies and control flies have comparable initial bacterial load in bacterial infection assay. N > 50 for infected flies and > 20 for injury only flies. NS = p > 0.05 Students’ T-test. Mean ± SEM of Control-P. aeruginosa: 40.14 ± 5.408; RNAi-P. aeruginosa: 39.75 ± 4.216; Control-S. aureus: 106.8 ± 8.895; RNAi-S. aureus: 105.0 ± 9.471.
Figure S8. Expression of Immune responsive genes Mtk, DptB and PGRP-SD upon infection.
(A–C). Expression of immune responsive genes Mtk (A), DptB (B) and PGRP-SD (C) upon infection examined by qPCR assay (N = 2 biological replicates of 6–8 flies apiece; Error bars are +/− SEM). 0 hour expression level is higher in RNAi than control flies; 24 hours expression level is similar in RNAi and control flies for each inoculation condition. All qPCR data were normalized with control 0 hour injury data.
Figure S9. Achl RNAi flies have a shorter lifespan and decreased starvation resistance.
(A). Overall lifespan of Achl RNAi and control flies. Data are combined from at least three independent replicates. Total N = 60; *** = p < 0.005 Log rank test.
(B). Starvation assay performed with Achl RNAi and control flies. Data are obtained by merging at least three replicates. Total N = 90–120; *** = p < 0.005 Log rank test.
Table S1: RUM alignment statistics. This table shows the samples we used for RNA-seq, the total number of reads, and the number of uniquely- or non-uniquely aligning reads as determined by RUM.
Table S2: Full Gene Ontology enrichment table. This is a full Gene Ontology enrichment table generated by GOrilla software available online (Eden et al., 2007; Eden et al., 2009). The corresponding simplified table is shown in Figure 3B. As shown from the GOrilla website, Enrichment (N, B, n, b) is defined as follows:
N: the total number of genes; B: the total number of genes associated with a specific GO term; n: the number of genes in the top of the user’s input list or in the target set when appropriate; b: the number of genes in the intersection.
Highlights.
Achilles is a clock-controlled gene.
Achilles represses expression of immune responsive genes in Drosophila.
Neurons in the fly brain coordinate immune responses in Drosophila.
The regulation of immunity by circadian clocks is accessible to molecular genetic investigation using Drosophila.
Acknowledgments
We thank the anonymous reviewers for helpful suggestions during peer review and the members of the Hughes Lab for technical support and helpful discussions, including Camile Lugarini, Ayesha Baig, and Jason Bedwinek. We also thank Dr. Lon Chubiz for sharing the P. aeruginosa strain with us, and Kelly O’Mara for contributing the S. aureus strain, and Drs. Benjamin Schmid and Tashi Yoshii for their assistance with ActogramJ. We thank Dr. Jing Hughes for critical reading of the manuscript. We particularly thank Dr. Patty Parker and a gift from Peabody Energy for helping to establish of the UMSL Genomics Core. The Department of Biology at UMSL was instrumental in supporting the laboratory course: “Practical Next-Generation Sequencing”, which collected essential preliminary data for this project. Work in the Hughes Lab is supported by the University of Missouri-St. Louis College of Arts and Science, the University of Missouri Research Board, and the NIH (NIAMS 1R21AR069266-01A1).
Abbreviations
- Achl
Achilles
- CCG
clock-controlled gene
- RNA-seq
RNA-sequencing
- AMP
anti-microbial peptide
- CLK
CLOCK
- CYC
CYCLE
- PER
PERIOD
- TIM
TIMELESS
- TTFL
transcriptional-translational feedback loop
- SCN
suprachiasmatic nuclei
- qPCR
quantitative PCR
- LD
12 hour light: 12 hour dark
- DD
constant darkness
- ZT
zeitgeber time
- CT
circadian time
- DAM
Drosophila Activity Monitoring
- RUM
RNA-seq Unified Mapper
- RPKM
reads per kilobase per million mapped reads
- CFU
colony forming unit
- IM1
Immune induced molecule 1
- Mtk
Metchnikowin
- Drs
Drosomysin
- DptB
Diptericin B
- Imd
immune deficiency
Footnotes
Conflicts of Interest
All authors declare they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abruzzi KC, Rodriguez J, Menet JS, Desrochers J, Zadina A, Luo W, Tkachev S, Rosbash M. Drosophila CLOCK target gene characterization: implications for circadian tissue-specific gene expression. Genes & development. 2011;25:2374–2386. doi: 10.1101/gad.178079.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KV. Toll signaling pathways in the innate immune response. Current opinion in immunology. 2000;12:13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- Apidianakis Y, Rahme LG. Drosophila melanogaster as a model host for studying Pseudomonas aeruginosa infection. Nat Protoc. 2009;4:1285–1294. doi: 10.1038/nprot.2009.124. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Bugge A, Feng D, Everett LJ, Briggs ER, Mullican SE, Wang F, Jager J, Lazar MA. Rev-erbα and Rev-erbβ coordinately protect the circadian clock and normal metabolic function. Genes & development. 2012;26:657–667. doi: 10.1101/gad.186858.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SJ, Durrington HJ, Gibbs JE, Blaikley J, Loudon AS, Ray DW, Sabroe I. A matter of time: study of circadian clocks and their role in inflammation. Journal of leukocyte biology. 2016;99:549–560. doi: 10.1189/jlb.3RU1015-451R. [DOI] [PubMed] [Google Scholar]
- Ceriani MF, Hogenesch JB, Yanovsky M, Panda S, Straume M, Kay SA. Genome-wide expression analysis in Drosophila reveals genes controlling circadian behavior. J Neurosci. 2002;22:9305–9319. doi: 10.1523/JNEUROSCI.22-21-09305.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbas L, Willingham A, Zhang D, Yang L, Zou Y, Eads BD, Carlson JW, Landolin JM, Kapranov P, Dumais J, et al. The transcriptional diversity of 25 Drosophila cell lines. Genome Research. 2011;21:301–314. doi: 10.1101/gr.112961.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Fagundes CT, Yang G, Palsson-McDermott EM, Wochal P, McGettrick AF, Foley NH, Early JO, Chen L, Zhang H, et al. Circadian control of innate immunity in macrophages by miR-155 targeting Bmal1. Proceedings of the National Academy of Sciences. 2015;112:7231–7236. doi: 10.1073/pnas.1501327112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutolo M. Chronobiology and the treatment of rheumatoid arthritis. Current opinion in rheumatology. 2012;24:312–318. doi: 10.1097/BOR.0b013e3283521c78. [DOI] [PubMed] [Google Scholar]
- Du NH, Arpat AB, De Matos M, Gatfield D. MicroRNAs shape circadian hepatic gene expression on a transcriptome-wide scale. Elife. 2014;3:e02510. doi: 10.7554/eLife.02510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E, Lipson D, Yogev S, Yakhini Z. Discovering motifs in ranked lists of DNA sequences. PLoS computational biology. 2007;3:e39. doi: 10.1371/journal.pcbi.0030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eden E, Navon R, Steinfeld I, Lipson D, Yakhini Z. GOrilla: a tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinformatics. 2009;10:1–7. doi: 10.1186/1471-2105-10-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RS, Stangherlin A, Nagy AD, Nicoll MP, Efstathiou S, O’Neill JS, Reddy AB. Cell autonomous regulation of herpes and influenza virus infection by the circadian clock. Proceedings of the National Academy of Sciences. 2016;113:10085–10090. doi: 10.1073/pnas.1601895113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ella K, Csepanyi-Komi R, Kaldi K. Circadian regulation of human peripheral neutrophils. Brain, behavior, and immunity. 2016 doi: 10.1016/j.bbi.2016.04.016. [DOI] [PubMed] [Google Scholar]
- Farh KK, Marson A, Zhu J, Kleinewietfeld M, Housley WJ, Beik S, Shoresh N, Whitton H, Ryan RJ, Shishkin AA, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015;518:337–343. doi: 10.1038/nature13835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filichkin S, Mockler T. Unproductive alternative splicing and nonsense mRNAs: A widespread phenomenon among plant circadian clock genes. Biology Direct. 2012;7:1–15. doi: 10.1186/1745-6150-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortier EE, Rooney J, Dardente H, Hardy MP, Labrecque N, Cermakian N. Circadian variation of the response of T cells to antigen. Journal of immunology (Baltimore, Md : 1950) 2011;187:6291–6300. doi: 10.4049/jimmunol.1004030. [DOI] [PubMed] [Google Scholar]
- Gibbs JE, Blaikley J, Beesley S, Matthews L, Simpson KD, Boyce SH, Farrow SN, Else KJ, Singh D, Ray DW, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proceedings of the National Academy of Sciences. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs JE, Ray DW. The role of the circadian clock in rheumatoid arthritis. Arthritis research & therapy. 2013;15:205. doi: 10.1186/ar4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant GR, Farkas MH, Pizarro AD, Lahens NF, Schug J, Brunk BP, Stoeckert CJ, Hogenesch JB, Pierce EA. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM) Bioinformatics. 2011;27:2518–2528. doi: 10.1093/bioinformatics/btr427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motulsky HJ. GraphPad Statistics Guide. 2016 http://wwwgraphpadcom/guides/prism/7/statistics/indexhtm.
- Halberg F, Cornelissen G, Ulmer W, Blank M, Hrushesky W, Wood P, Singh RK, Wang Z. Cancer chronomics III. Chronomics for cancer, aging, melatonin and experimental therapeutics researchers. J Exp Ther Oncol. 2006;6:73–84. [PMC free article] [PubMed] [Google Scholar]
- Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Advances in genetics. 2011;74:141–173. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings MH, Reddy AB, Maywood ES. A clockwork web: circadian timing in brain and periphery, in health and disease. Nat Rev Neurosci. 2003;4:649–661. doi: 10.1038/nrn1177. [DOI] [PubMed] [Google Scholar]
- Herzog ED. Neurons and networks in daily rhythms. Nature reviews Neuroscience. 2007;8:790–802. doi: 10.1038/nrn2215. [DOI] [PubMed] [Google Scholar]
- Hetru C, Troxler L, Hoffmann JA. Drosophila melanogaster antimicrobial defense. The Journal of infectious diseases. 2003;187(Suppl 2):S327–334. doi: 10.1086/374758. [DOI] [PubMed] [Google Scholar]
- Hoffmann JA. The immune response of Drosophila. Nature. 2003;426:33–38. doi: 10.1038/nature02021. [DOI] [PubMed] [Google Scholar]
- Hughes ME, Grant GR, Paquin C, Qian J, Nitabach MN. Deep sequencing the circadian and diurnal transcriptome of Drosophila brain. Genome Res. 2012;22:1266–1281. doi: 10.1101/gr.128876.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes ME, Hogenesch JB, Kornacker K. JTK_CYCLE: an efficient nonparametric algorithm for detecting rhythmic components in genome-scale data sets. Journal of biological rhythms. 2010;25:372–380. doi: 10.1177/0748730410379711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler JL, Hoffmann JA. Signaling mechanisms in the antimicrobial host defense of Drosophila. Current opinion in microbiology. 2000;3:16–22. doi: 10.1016/s1369-5274(99)00045-4. [DOI] [PubMed] [Google Scholar]
- Jeyaraj D, Haldar SM, Wan X, McCauley MD, Ripperger JA, Hu K, Lu Y, Eapen BL, Sharma N, Ficker E, et al. Circadian rhythms govern cardiac repolarization and arrhythmogenesis. Nature. 2012;483:96–99. doi: 10.1038/nature10852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keegan KP, Pradhan S, Wang JP, Allada R. Meta-analysis of Drosophila circadian microarray studies identifies a novel set of rhythmically expressed genes. PLoS computational biology. 2007;3:e208. doi: 10.1371/journal.pcbi.0030208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Mazuch J, Abraham U, Eom GD, Herzog ED, Volk HD, Kramer A, Maier B. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci U S A. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimbrell DA, Beutler B. The evolution and genetics of innate immunity. Nat Rev Genet. 2001;2:256–267. doi: 10.1038/35066006. [DOI] [PubMed] [Google Scholar]
- Klerman EB. Clinical aspects of human circadian rhythms. Journal of biological rhythms. 2005;20:375–386. doi: 10.1177/0748730405278353. [DOI] [PubMed] [Google Scholar]
- Knutsson A. Health disorders of shift workers. Occupational Medicine. 2003;53:103–108. doi: 10.1093/occmed/kqg048. [DOI] [PubMed] [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet 15 Spec No. 2006;2:R271–277. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Koike N, Yoo SH, Huang HC, Kumar V, Lee C, Kim TK, Takahashi JS. Transcriptional Architecture and Chromatin Landscape of the Core Circadian Clock in Mammals. Science (New York, NY) 2012;338:349–354. doi: 10.1126/science.1226339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunst M, Hughes Michael E, Raccuglia D, Felix M, Li M, Barnett G, Duah J, Nitabach Michael N. Calcitonin Gene-Related Peptide Neurons Mediate Sleep-Specific Circadian Output inDrosophila. Current Biology. 2014;24:2652–2664. doi: 10.1016/j.cub.2014.09.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrecque N, Cermakian N. Circadian Clocks in the Immune System. Journal of biological rhythms. 2015;30:277–290. doi: 10.1177/0748730415577723. [DOI] [PubMed] [Google Scholar]
- Lange T, Dimitrov S, Born J. Effects of sleep and circadian rhythm on the human immune system. Ann N Y Acad Sci. 2010;1193:48–59. doi: 10.1111/j.1749-6632.2009.05300.x. [DOI] [PubMed] [Google Scholar]
- Lee JE, Edery I. Circadian regulation in the ability of Drosophila to combat pathogenic infections. Curr Biol. 2008;18:195–199. doi: 10.1016/j.cub.2007.12.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi F, Schibler U. Circadian rhythms: mechanisms and therapeutic implications. Annu Rev Pharmacol Toxicol. 2007;47:593–628. doi: 10.1146/annurev.pharmtox.47.120505.105208. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhou J, White KP. RNA-seq differential expression studies: more sequence or more replication? Bioinformatics. 2014;30:301–304. doi: 10.1093/bioinformatics/btt688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald MJ, Rosbash M. Microarray analysis and organization of circadian gene expression in Drosophila. Cell. 2001;107:567–578. doi: 10.1016/s0092-8674(01)00545-1. [DOI] [PubMed] [Google Scholar]
- Meireles-Filho AC, Bardet AF, Yanez-Cuna JO, Stampfel G, Stark A. cis-regulatory requirements for tissue-specific programs of the circadian clock. Curr Biol. 2014;24:1–10. doi: 10.1016/j.cub.2013.11.017. [DOI] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452:442–447. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- Menet JS, Pescatore S, Rosbash M. CLOCK:BMAL1 is a pioneer-like transcription factor. Genes & development. 2014;28:8–13. doi: 10.1101/gad.228536.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet JS, Rodriguez J, Abruzzi KC, Rosbash M. Nascent-Seq reveals novel features of mouse circadian transcriptional regulation. Elife. 2012;1:e00011. doi: 10.7554/eLife.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki M, Schroder E, Edelmann SE, Hughes ME, Kornacker K, Balke CW, Esser KA. Age-Associated Disruption of Molecular Clock Expression in Skeletal Muscle of the Spontaneously Hypertensive Rat. PLoS ONE. 2011;6:e27168. doi: 10.1371/journal.pone.0027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morf J, Rey G, Schneider K, Stratmann M, Fujita J, Naef F, Schibler U. Cold-inducible RNA-binding protein modulates circadian gene expression posttranscriptionally. Science (New York, NY) 2012;338:379–383. doi: 10.1126/science.1217726. [DOI] [PubMed] [Google Scholar]
- Muller U, Vogel P, Alber G, Schaub GA. The innate immune system of mammals and insects. Contributions to microbiology. 2008;15:21–44. doi: 10.1159/000135684. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Panda S, Antoch MP, Miller BH, Su AI, Schook AB, Straume M, Schultz PG, Kay SA, Takahashi JS, Hogenesch JB. Coordinated Transcription of Key Pathways in the Mouse by the Circadian Clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins LA, Shim HS, Perrimon N. Initial TRiP stock collection 2009 [Google Scholar]
- Pfeiffenberger C, Lear BC, Keegan KP, Allada R. Locomotor Activity Level Monitoring Using the Drosophila Activity Monitoring (DAM) System. Cold Spring Harbor Protocols. 2010;2010 doi: 10.1101/pdb.prot5518. pdb.prot5518. [DOI] [PubMed] [Google Scholar]
- Rahman SA, Castanon-Cervantes O, Scheer FA, Shea SA, Czeisler CA, Davidson AJ, Lockley SW. Endogenous circadian regulation of pro-inflammatory cytokines and chemokines in the presence of bacterial lipopolysaccharide in humans. Brain, behavior, and immunity. 2015;47:4–13. doi: 10.1016/j.bbi.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranjbaran Z, Keefer L, Stepanski E, Farhadi A, Keshavarzian A. The relevance of sleep abnormalities to chronic inflammatory conditions. Inflammation Research. 2007;56:51–57. doi: 10.1007/s00011-006-6067-1. [DOI] [PubMed] [Google Scholar]
- Rey G, Cesbron F, Rougemont J, Reinke H, Brunner M, Naef F. Genome-wide and phase-specific DNA-binding rhythms of BMAL1 control circadian output functions in mouse liver. PLoS Biol. 2011;9:e1000595. doi: 10.1371/journal.pbio.1000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiermann C, Kunisaki Y, Frenette PS. Circadian control of the immune system. Nat Rev Immunol. 2013;13:190–198. doi: 10.1038/nri3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Helfrich-Forster C, Yoshii T. A new ImageJ plug-in “ActogramJ” for chronobiological analyses. Journal of biological rhythms. 2011;26:464–467. doi: 10.1177/0748730411414264. [DOI] [PubMed] [Google Scholar]
- Schroder EA, Lefta M, Zhang X, Bartos DC, Feng HZ, Zhao Y, Patwardhan A, Jin JP, Esser KA, Delisle BP. The cardiomyocyte molecular clock, regulation of Scn5a, and arrhythmia susceptibility. Am J Physiol Cell Physiol. 2013;304:C954–965. doi: 10.1152/ajpcell.00383.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Hughes ME, Nitabach MN, Fikrig E. Circadian expression of clock genes in mouse macrophages, dendritic cells, and B cells. Brain, behavior, and immunity. 2012a;26:407–413. doi: 10.1016/j.bbi.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver AC, Arjona A, Walker WE, Fikrig E. The circadian clock controls toll-like receptor 9-mediated innate and adaptive immunity. Immunity. 2012b;36:251–261. doi: 10.1016/j.immuni.2011.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. RNA-binding proteins as regulators of gene expression. Current Opinion in Genetics & Development. 1997;7:345–353. doi: 10.1016/s0959-437x(97)80148-7. [DOI] [PubMed] [Google Scholar]
- Stone EF, Fulton BO, Ayres JS, Pham LN, Ziauddin J, Shirasu-Hiza MM. The circadian clock protein timeless regulates phagocytosis of bacteria in Drosophila. PLoS pathogens. 2012;8:e1002445. doi: 10.1371/journal.ppat.1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Watson FL, Puttmann-Holgado R, Thomas F, Lamar DL, Hughes M, Kondo M, Rebel VI, Schmucker D. Extensive diversity of Ig-superfamily proteins in the immune system of insects. Science. 2005;309:1874–1878. doi: 10.1126/science.1116887. [DOI] [PubMed] [Google Scholar]
- Wulff K, Gatti S, Wettstein JG, Foster RG. Sleep and circadian rhythm disruption in psychiatric and neurodegenerative disease. Nature reviews Neuroscience. 2010;11:589–599. doi: 10.1038/nrn2868. [DOI] [PubMed] [Google Scholar]
- Xu K, DiAngelo Justin R, Hughes Michael E, Hogenesch John B, Sehgal A. The Circadian Clock Interacts with Metabolic Physiology to Influence Reproductive Fitness. Cell Metabolism. 2011;13:639–654. doi: 10.1016/j.cmet.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Achl is regulated by the core clock.
(A). Achl rhythmicity is disrupted in per0 flies. Data adapted from Hughes et al. (2012) Genome Research.
(B). Achl expression level is down-regulated in Clk mutant flies. Data adapted from McDonald & Rosbash. (2001) Cell.
Figure S2. Larp7, Achl’s mammalian homolog is rhythmically expressed in the mouse kidney.
(A). Larp7, a mammalian homolog of Achl, is rhythmically expressed in the mouse kidney. Data adapted from Zhang et al. (2014) PNAS.
(B). Sequence alignment of the conserved Lupus-La RNA binding domains in ACHL and its mammalian homolog, LARP7. The alignment is performed using MUSCLE (Multiple Sequence Comparison by Log- Expectation) 3.8 on EMBL-EBI (Edgar, 2004). “*” indicates positions which have a single, fully conserved residue. “:” indicates conservation between groups of strongly similar properties - scoring > 0.5 in the Gonnet PAM 250 matrix, and “.” indicates conservation between groups of weakly similar properties - scoring =< 0.5 in the Gonnet PAM 250 matrix. Different colors indicate the physicochemical properties of the residues.
Figure S3. Achl RNAi knock-down is specific.
(A). RNA-seq data demonstrate that the most likely off-target hits of the RNAi construct we use are not knocked-down. Achl is the only transcript measurably knocked-down with predicted alignment to the genetically-encoded RNAi construct we used. Control flies expressed UAS-GFP RNAi instead of UAS-Achl RNAi. RPKM: reads per kilobase per million sequenced reads.
(B). Mismatch from perfect 21-bp alignment predicted by “find OTE (off-target elements)” program (Perkins et al., 2009).
Figure S4. Achl RNAi flies have defective wing development.
Pictures captured under dissection microscope.
(A) Control flies (UAS-GFP RNAi) with normal wings.
(B) Achl RNAi flies with heterogeneous defects in their wing morphology, from slight to severe. Arrows indicate disrupted wing morphology.
Figure S5. Achl RNAi does not significantly affect behavioral rhythms or sleep.
(A & B). Averaged actograms for representative control (N = 26) (A) and Achl RNAi (N = 28) (B) male flies. Flies were placed in LD conditions and converted to DD conditions at day 5, as indicated by the arrow. These results are representative of > 5 independent experiments. The top horizontal gray and black bars represent subjective day and night.
(C & D). Lomb-Scargle periodograms corresponding to the averaged actograms in panels A and B showing the period calculated for control (C) and RNAi (D) flies. Period length differences were within the variance of negative control flies in these experiments.
(E). Percentage of individual rhythmic flies (Lomb-Scargle p < 0.05) observed. N = 26 for control and 28 for RNAi.
(F). Average power of individual rhythmic flies being analyzed; Error bars are +/− SEM. NS = p > 0.05 Students’ T-test.
(G & H). Overall sleep profile (G) and sleep amounts (H) of control (parental flies) and Achl RNAi flies showing that Achl RNAi does not dramatically alter either sleep rhythms or quantity. Error bars are +/− SEM. NS = not significant, Student’s T-test; *= p < 0.05 Students’ T-test.
Figure S6. Systemic effects of Achl knock-down.
Immune response gene IM1 is upregulated in both head and body (B) in Achl RNAi flies, even though RNAi knockdown of Achl is only observed in head (A). qPCR data is normalized with controls of the same tissue.
Figure S7. Initial bacterial load of flies in the infection assay.
(A). Achl knock-down flies and control flies have comparable initial bacterial load in bacterial infection assay. N > 50 for infected flies and > 20 for injury only flies. NS = p > 0.05 Students’ T-test. Mean ± SEM of Control-P. aeruginosa: 40.14 ± 5.408; RNAi-P. aeruginosa: 39.75 ± 4.216; Control-S. aureus: 106.8 ± 8.895; RNAi-S. aureus: 105.0 ± 9.471.
Figure S8. Expression of Immune responsive genes Mtk, DptB and PGRP-SD upon infection.
(A–C). Expression of immune responsive genes Mtk (A), DptB (B) and PGRP-SD (C) upon infection examined by qPCR assay (N = 2 biological replicates of 6–8 flies apiece; Error bars are +/− SEM). 0 hour expression level is higher in RNAi than control flies; 24 hours expression level is similar in RNAi and control flies for each inoculation condition. All qPCR data were normalized with control 0 hour injury data.
Figure S9. Achl RNAi flies have a shorter lifespan and decreased starvation resistance.
(A). Overall lifespan of Achl RNAi and control flies. Data are combined from at least three independent replicates. Total N = 60; *** = p < 0.005 Log rank test.
(B). Starvation assay performed with Achl RNAi and control flies. Data are obtained by merging at least three replicates. Total N = 90–120; *** = p < 0.005 Log rank test.
Table S1: RUM alignment statistics. This table shows the samples we used for RNA-seq, the total number of reads, and the number of uniquely- or non-uniquely aligning reads as determined by RUM.
Table S2: Full Gene Ontology enrichment table. This is a full Gene Ontology enrichment table generated by GOrilla software available online (Eden et al., 2007; Eden et al., 2009). The corresponding simplified table is shown in Figure 3B. As shown from the GOrilla website, Enrichment (N, B, n, b) is defined as follows:
N: the total number of genes; B: the total number of genes associated with a specific GO term; n: the number of genes in the top of the user’s input list or in the target set when appropriate; b: the number of genes in the intersection.