Abstract
Studies of seasonal patterns of incidence of sarcoidosis may provide a better understanding of potential environmental triggers of the disease. In this study, Olmsted County, Minnesota residents who were diagnosed with sarcoidosis between 1976 and 2013 were identified based on individual medical record review. The seasonal variation of incident sarcoidosis was then calculated. The age and sex adjusted incidence rate of sarcoidosis was lower in autumn (2.0/100,000; 95% CI 1.5–2.5) compared with winter (3.2/100,000; 95% CI 2.6–3.8), spring (2.8/100,000; 95% CI 2.2–3.4) and summer (2.9/100,000; 95% CI 2.2–3.5; p=0.024). Subgroup analysis per decade consistently showed lower incidence of sarcoidosis in the autumn.
Keywords: Sarcoidosis, Epidemiology, Seasonality
Introduction
Sarcoidosis is a systemic disorder characterized by the presence of non-caseating granuloma. It has been hypothesized that the interaction between genetic predisposition and environmental factors plays an essential role in the pathogenesis of sarcoidosis even though the exact etiology of sarcoidosis is still not known. Studies of seasonal and regional patterns of incidence of sarcoidosis may provide a better understanding of potential environmental trigger of this disease. However, previous studies on the seasonality of sarcoidosis have yielded conflicting results.1–5
Patients and Methods
Approval for this study was obtained from the Mayo Clinic and Olmsted Medical Center institutional review boards and the need for informed consent was waived. Through the resources of the Rochester Epidemiology Project (REP), all Olmsted County, Minnesota residents diagnosed with sarcoidosis between January 1, 1976 and December 31, 2013 were identified. The data linkage-system allows an essentially complete identification of all clinically recognized cases of sarcoidosis as complete access to medical records of all residents seeking for medical care (both inpatient and outpatient) for over six decades was available.6 Potential cases were identified from diagnostic codes related to sarcoidosis and non-caseating granuloma. Diagnosis of sarcoidosis was confirmed by individual medical record review which required physician diagnosis supported by presence of non-caseating granuloma, radiographic findings of intrathoracic sarcoidosis and compatible clinical presentations. Patients with evidence other granulomatous diseases such as tuberculosis were excluded. The only exception for the histopathological confirmation was stage I pulmonary sarcoidosis that required only the presence of bilateral hilar adenopathy on thoracic imaging. Cases with a diagnosis of sarcoidosis prior to residency in Olmsted County were excluded. The date of diagnosis was the date that the histopathologic confirmation was made. If biopsy was not obtained, the date of first abnormal thoracic imaging was used as the date of diagnosis.
Age and sex adjusted incidence rates were calculated using monthly population estimates for adults as the denominators. Monthly population estimates were based on decennial census counts with adjustment for estimated monthly variation in residency obtained from the REP.7 Seasonal variation was compared using quasi-Poisson regression models to account for over-dispersion.
Results
The cohort included 345 cases of incident sarcoidosis (mean age 35.4 years, 50% female, 90% Caucasian and 5% African-American). Patients in this cohort were less likely to have incident sarcoidosis in the autumn season with an age and sex adjusted rate of 2.0/100,000 (95% CI 1.5–2.5) compared with winter (3.2/100,000; 95% CI 2.6–3.8), spring (2.8/100,000; 95% CI 2.2–3.4) and summer (2.9/100,000; 95% CI 2.2–3.5; p=0.024; figure 1). Subgroup analysis per decade (1976–1985, 1986–1995, 1996–2005 and 2006–2013) revealed that the incidence of sarcoidosis was consistently lower in the autumn (rate ratios for autumn compared to spring were 0.53, 0.70, 0.64 and 0.85, respectively; figure 2), with the exception of 1996–2005 when the lowest incidence was in the summer. However, statistical power was insufficient to demonstrate statistical significance at p <.05 for the individual decades.
Figure 1.
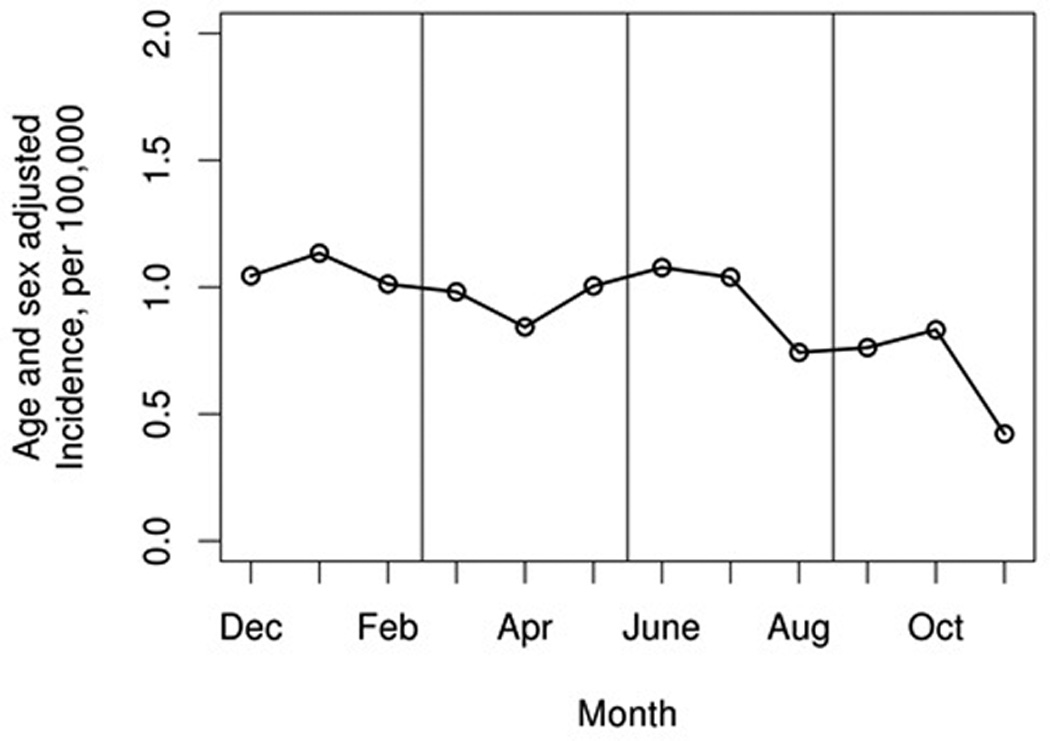
Sarcoidosis incidence rates by month among Olmsted County, Minnesota residents in 1976–2013
Figure 2.
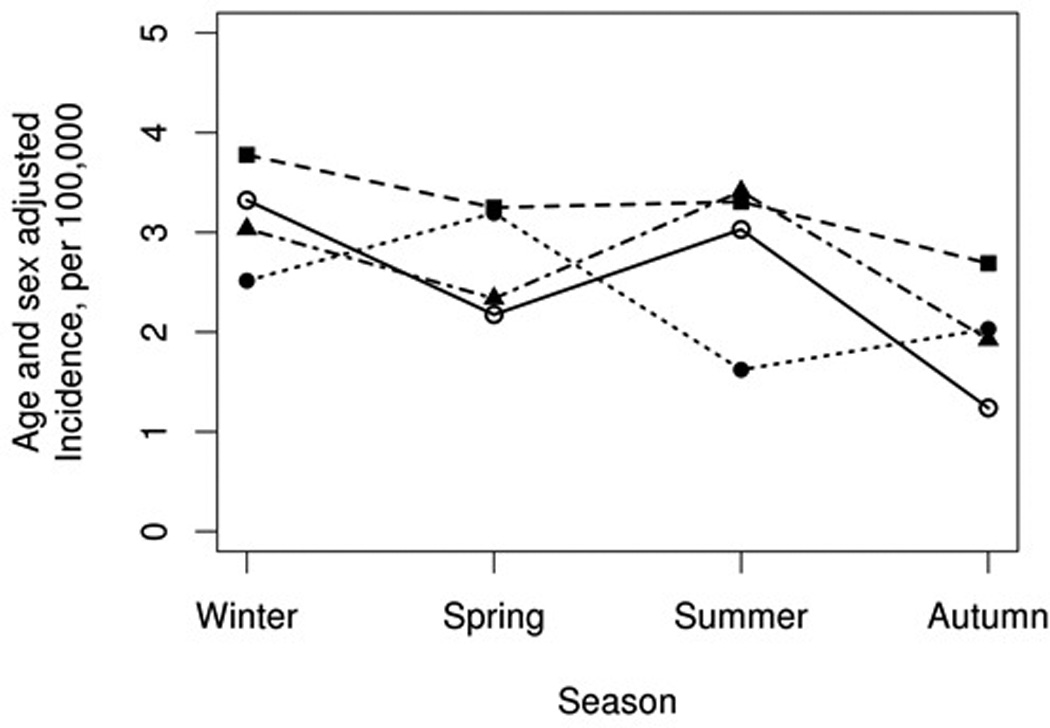
Sarcoidosis incidence rates by season among Olmsted County, Minnesota residents in 1976–2013 by decade (solid line with blank circle represents 1976–1985; dotted line with square represents 1986–1995; dotted line with filled circle represent 1996–2005; dotted line with triangle represent 2006–2013)
Discussion
This is the first study to utilize a population-based cohort to investigate the seasonality of sarcoidosis. There was a seasonal variation in the incidence of sarcoidosis with the lowest incidence observed in the autumn. This observation was consistent over 4 decades except for 1996–2005. There are several possible explanations for this seasonality.
First, several environmental exposures have been identified as potential triggers for the initiation and development of granuloma in sarcoidosis.8 Thus, it is possible that the seasonality of sarcoidosis may reflect the seasonal variation in the occurrence of environmental triggers associated with its pathogenesis.
Second, the seasonal variation of sunlight may have an influence on the observed seasonality of sarcoidosis. Hypercalcemia is well-recognized complication of sarcoidosis as a result of upregulated 1-alpha-hydroxylase activity within granulomas resulting in increased conversion of 25-hydroxy vitamin D3 to 1,25-dihydroxy vitamin D3. Exposure to sunlight is important in converting 7-dehydrocholesterol to vitamin D3 and it has been demonstrated that 1,25-dihydroxy vitamin D3 as well calcium level of patients with sarcoidosis are elevated in the summer.9 This might explain the higher incidence of sarcoidosis in the summer as some patients were diagnosed with sarcoidosis after they were found to have hypercalcemia. Of course, this is speculative, and would not explain the higher incidence in the winter compared with the autumn.
It is also possible that the apparent seasonality is actually a result of detection bias. For example, patients might undergo thoracic imaging more often in the winter and spring because of respiratory tract infections, resulting in a higher likelihood of sarcoidosis detection.
Previous studies on seasonality of sarcoidosis from different parts of the world have yielded different observations. For example, a study from Turkey2 reported the highest incidence of sarcoidosis in the spring and the lowest incidence in the summer, while a study from India3 showed the peak incidence in the summer and the lowest incidence in the winter. On the other hand, the only other study from North America, using the United States Veteran Health Administration national outpatient claim database5, did not find a significant variation in the incidence of sarcoidosis over the year. It is possible that the different patterns of seasonality between studies might reflect the different environmental stimuli in different regions/populations or it might reflect different patterns of disease presentation among different populations. For example, patients with sarcoidosis who have uveitis or Lofgren’s syndrome may seek for medical attention sooner than those with asymptomatic stage I pulmonary sarcoidosis, resulting in a shorter duration from onset of symptoms to diagnosis.
The major strengths of this study are that it is a population-based cohort that captures the true spectrum of sarcoidosis in the community, unlike referral-based studies. The risk of disease misclassification in this cohort is minimized as the diagnosis was individually verified by medical record, histopathology and radiographic study review.
The major limitations are those inherent in the retrospective nature of the study as the potential cases of sarcoidosis were not systematically evaluated and case ascertainment depends on diagnosis being made by the health-care providers. Therefore, the exact burden of undiagnosed disease remains unclear. Chronological data on the onset of symptoms are often not documented, but while detection certainly follows onset of symptoms for a variable amount of time10, case incidence is based upon when the patients came to clinical attention, reflected in the seasonality patterns reported here. Finally, the ethnic background of this cohort is predominately of Northern European ancestry with a higher proportion of health-care workers. Therefore, the observations may not be generalizable to a more diverse population.
In conclusion, seasonal variation of sarcoidosis was observed this population with the lowest incidence observed in the autumn.
Acknowledgments
Funding: This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest statement for all authors: The authors have no financial or non-financial potential conflicts of interest to declare.
Author contribution:
Patompong Ungprasert: 1. Conception and design 2. Acquisition and interpretation of data 3. Drafting of the manuscript 4. Statistical analysis
Cynthia S. Crowson: 1. Conception and design 2. Analysis and interpretation of data 3. Critical revision of the manuscript for important intellectual content 4. Statistical analysis
Eric L. Matteson: 1. Conception and design 2. Acquisition and interpretation of data 3. Critical revision of the manuscript for important intellectual content 4. Statistical analysis 5. Supervision
References
- 1.Panayeas S, Theodorakopoulos P, Bouras A, et al. Seasonal occurrence of sarcoidosis in Greece. Lancet. 1991;338:510–511. doi: 10.1016/0140-6736(91)90581-9. [DOI] [PubMed] [Google Scholar]
- 2.Demirkok SS, Basaranoglu M, Coker E, et al. Seasonality of the onset of symptoms, Tuberculin test anergy and Kveim positive reaction I a large cohort of patients with sarcoidosis. Respirology. 2007;12:591–593. doi: 10.1111/j.1440-1843.2007.01062.x. [DOI] [PubMed] [Google Scholar]
- 3.Gupta D, Agarwal R, Aggarwal AN. Seasonality of sarcoidosis: the “heat” is on…. Sarcoidosis Vasc Diffuse Lung Dis. 2013;30:241–243. [PubMed] [Google Scholar]
- 4.Alilovic M, Peros-Golubicic T, Tekavec-Trkanjec J, et al. Epidemiological characteristics of sarcoidosis patients hospitalized in the university hospital for lung disease “Jordanovac” (Zagreb, Crotia) in the 1997–2002 period. Coll Antropol. 2006;30:513–517. [PubMed] [Google Scholar]
- 5.Gerke AK, Tang F, Yang M, et al. An analysis of seasonality of sarcoidosis in the United States veteran population, 2000–2007. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:155–158. [PMC free article] [PubMed] [Google Scholar]
- 6.Rocca WA, Yawn BP, St. Sauver JL, et al. History of the Rochester Epidemiology Project: Half a century of medical records linkage in a U.S. population. Mayo Clin Proc. 2012;87:1202–1013. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, Melton LJ, 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol. 2011;173:1059–1068. doi: 10.1093/aje/kwq482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med. 2004;170:1324–1330. doi: 10.1164/rccm.200402-249OC. [DOI] [PubMed] [Google Scholar]
- 9.Bonnema SJ, Moller J, Marving J, et al. Sarcoidosis causes abnormal seasonal variation in 1,23-dihydroxy-cholecalferol. J Intern Med. 1996;239:393–398. doi: 10.1046/j.1365-2796.1996.472813000.x. [DOI] [PubMed] [Google Scholar]
- 10.Judson MA, Thompson BW, Rabin DL, et al. The diagnostic pathway to sarcoidosis. Chest. 2003;123:406–412. doi: 10.1378/chest.123.2.406. [DOI] [PubMed] [Google Scholar]


