Abstract
Since the advent of combination antiretroviral therapy (cART), pediatric HIV-1 (PHIV) has evolved from a fatal disease to a chronic disease as children perinatally infected with HIV-1 survive into adulthood. The HIV-1 transgenic (Tg) rat, which expresses 7 of the 9 HIV-1 genes constitutively throughout development, was used to model the early development of chronic neurological impairment in PHIV. Male and female Fischer HIV-1 Tg and F344N control rats, sampled from 35 litters, were repeatedly assessed during early development using multiple experimental paradigms, including somatic growth, locomotor activity, cross-modal prepulse inhibition (PPI) and gap-prepulse inhibition (gap-PPI). Later eye opening was observed in HIV-1 Tg animals relative to controls. HIV-1 Tg animals exhibited a shift in the development of locomotor activity implicating alterations in the maturation of the forebrain cholinergic inhibitory system. Alterations in the development of PPI and perceptual sharpening were observed in both auditory and visual PPI as indexed by a relative insensitivity to the dimension of time (ms for ISI; days of age for perceptual sharpening) as a function of the HIV-1 transgene. Presence of the HIV-1 transgene was diagnosed with 97.1% accuracy using auditory and visual PPI measurements from PD 17 and 21. Early selective developmental alterations observed in the HIV-1 Tg rats provide an opportunity for the development of a point-of-care screening tool, which would permit for early diagnosis of PHIV and improve the long-term outcome for children perinatally infected with HIV-1.
Keywords: Pediatric HIV-1, Neurological Deficits, Locomotor Activity, Temporal Processing, Diagnostic Screening Tool
INTRODUCTION
Worldwide, 35 million individuals are living with human immunodeficiency virus type 1 (HIV-1), including over 3.2 million children (≤15 years of age; CDC 2013). Despite the dramatic decrease in mother-to-child transmission (MTCT), the predominant source of HIV-1 infection in children (Kourtis et al. 2001), 220,000 new cases of pediatric HIV-1 (PHIV) were reported in 2014 (UNAIDS 2015). Since the advent of combination antiretroviral therapy (cART), PHIV has evolved from a fatal disease to a chronic disease as children perinatally infected with HIV-1 survive into adulthood (Smith and Wilkins 2015; Crowell et al. 2014). Chronic neurological impairment is still commonly reported in children perinatally infected with PHIV despite the advancements resulting from cART (Franklin et al. 2005; Paramesparan et al. 2010). Given the prevalence of PHIV, understanding early developmental alterations may provide an innovative clinical diagnostic screening tool for neurological impairment in HIV-1 seropositive children.
Progressive HIV-1 encephalopathy (PHE), which is often analogous to HIV-1 associated dementia (HAD) in adults, was predominantly observed prior to the advent of cART, with prevalence rates as high as 50% (Chiriboga et al. 2005; Crowell et al. 2014; Shanhbhag et al. 2005). Common neurological manifestations of PHE include microcephaly (resulting from cerebral atrophy), developmental delays, and movement disorders (Belman et al. 1985; Epstein et al. 1985; Epstein et al. 1986). Furthermore, neuroimaging analyses reveal calcification in the basal ganglia, and focal white matter lesions in children with PHE (Epstein et al. 1985; Epstein et al. 1986; Kauffman et al. 1992). Currently, in the post-cART era, the prevalence rate of PHE is between 2-15% (Chiriboga et al. 2005; Shanbhag et al. 2005).
High rates of chronic neurological impairment, including neurodevelopmental delays, continue to be reported in HIV-1 seropositive children (Franklin et al. 2005; Paramesparan et al. 2010; review, Van Rie et al. 2007). Neurological assessments, including the Bayley Scales of Infant Development and Wechsler Intelligence Scale for Children-Revised, have previously been used to assess the effect of pediatric HIV-1 on neurodevelopment (Blanchette et al. 2002; Lindsey et al. 2007; Van Rie et al. 2008; Walker et al. 2013). Despite treatment with highly active antiretroviral therapy (HAART), HIV-1 infected children exhibit significant delays in cognitive development, motor skills and language expression in both high- (Lindsey et al. 2007) and low-resource countries (Van Rie et al. 2008; Walker et al. 2013).
Neurocognitive deficits in HIV-1 seropositive children, including disease progression, are poorly understood (Crowell et al. 2014), however, there is a relative wealth of knowledge on HIV-1 associated neurocognitive disorders (HAND) in adults evidenced in both clinical and preclinical studies (i.e. Heaton et al. 2010; review, Woods et al. 2009). Neurocognitive assessments, including the Wisconsin Card Sorting Test and Stroop Color Word Test, have shown that HIV-1 seropositive individuals display significant deficits in set shifting (Carter et al. 2003) and response inhibition (Hinkin et al. 1999; Tozzi et al. 1999). Deficits in complex problem solving and abstraction have been demonstrated using the Wisconsin Card Sorting Test and Tower of London- Drexel Version neurocognitive assessment (Cattie et al. 2012; Cherner et al. 2004). Furthermore, HIV-1 seropositive individuals display a greater impulsivity than control individuals on the Iowa Gambling Test (Hardy et al. 2006; Martin et al. 2004).
The HIV-1 transgenic (Tg) rat, which expresses 7 of the 9 HIV-1 genes, has been used in preclinical studies to model neurocognitive deficits prominent in HAND and commonly observed in HIV-1 seropositive individuals (Moran et al. 2013a; Moran et al. 2013b; Moran et al. 2014a). Specifically, adult HIV-1 Tg rats exhibit significant deficits in executive functions, including attention, inhibition, and flexibility in comparison to controls (Moran et al. 2014a). Furthermore, significant alterations in temporal processing, a pre-attentive process, have been observed using cross-modal prepulse inhibition (PPI) of the auditory startle response (ASR) in the HIV-1 Tg rat (Moran et al. 2013a). On both visual and auditory prepulse trials, HIV-1 Tg rats exhibited an insensitivity to ISI duration, suggesting a lack of perceptual sharpening after the adolescent period of development (Moran et al. 2013a). There remains a critical need for longitudinal studies to understand the effect of pediatric HIV-1 infection on developmental processes (Cohen et al. 2016; Crowell et al. 2014)
Due to high rates of chronic neurocognitive deficits in both HIV-1 seropositive children and adults, there remains a basic need for accurate screening tools for the diagnosis of HAND. Early in the HIV-1 epidemic, two screening tools, the HIV Dementia Scale (HDS; Power et al. 1995) and the International HDS (IHDS; Sacktor et al. 2005), were developed to screen for HAD. However, neither the HDS or the IHDS are able to accurately screen for milder forms of neurocognitive impairment, which are more common in the post-cART era, affecting up to 40%-70% of HIV-1 infected individuals (Heaton et al. 2010; Heaton et al. 2011; Letendre et al. 2009; McArthur et al. 2010; Sacktor et al. 2005; Zipursky et al. 2013). Development of a screening tool, specifically for neurological impairment seen in PHIV has the potential for great clinical significance and may have a significant impact on the lives of HIV-1 seropositive children and adults (Zipursky et al. 2013).
Thus, the aims of the current study were twofold. First, to establish the early trajectory of developmental deficits in the HIV-1 Tg rat. The HIV-1 Tg rat, which express 7 of the 9 HIV-1 genes constitutively throughout development, provides a useful model for investigating the development of neurologic impairments in pediatric AIDS (review, Fitting et al. 2015; Peng et al. 2010; Royal et al. 2012; review, Vigorito et al. 2015). Developmental assessments, including locomotor activity, cross-modal PPI, and gap-PPI were conducted prior to weaning from postnatal day (PD) 12 to PD 21. It was hypothesized that HIV-1 Tg rats would exhibit early selective alterations in somatic growth, including body weight and eye opening, and alterations in locomotor activity, as well as preattentive processes, indexed by cross-modal PPI and gap-PPI, compared to non-transgenic F344N controls. Second, to evaluate the potential diagnostic utility of early developmental alterations. It was hypothesized that early selective developmental alterations in the HIV-1 Tg rat would be sufficiently discriminatory and robust to support their use as a point-of-care screening tool for chronic neurological impairment in PHIV. Understanding the early trajectory of developmental deficits in the HIV-1 Tg rats is vital to understanding the progression of neurocognitive deficits in children perinatally infected with HIV-1 and may provide an innovative point-of-care screening tool.
METHODS
Animals
Developmental assessments were conducted on Fischer (F344/N; Harlan Laboratories Inc., Indianapolis, IN) rats (HIV-1 Tg, n=19 litters; control, n=16 litters) prior to weaning, beginning at PD 12. All rats were tested for motor movement, assessed using locomotor activity (PD 12, 16, 20) and temporal processing deficits, assessed using cross-modal prepulse inhibition (PPI) of the auditory startle response (PD 14, 17, and 21). Gap-PPI, which also assesses temporal processing deficits, was conducted on PD 18.
Animals were delivered to the facility between PD 7 and PD 9 over the course of one year. All animals were housed with their biological dam until PD 21 when animals were weaned and separated by sex. Subsequently animals were pair- or group-housed with animals of the same sex. Rodent food (Pro-Lab Rat, Mouse, Hamster Chow #3000) and water were provided ad libitum throughout experimentation.
Animals were maintained according to the National Institute of Health (NIH) guidelines in AAALAC-accredited facilities. The targeted environmental conditions for the animal facility were 21°± 2°C, 50% ± 10% relative humidity and a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the project protocol as consistent with federal assurance (# A3049-01).
Somatic Growth
Body Weight
Body weight was assessed as a measure of somatic growth upon arrival at the facility between PD 8 and PD 10. Body weight was assessed periodically throughout early development.
Eye Opening
Eye opening was assessed as a developmental milestone from PD 13 to PD 19. Eye opening was assessed separately for the right and left eye. A scale ranging from zero to two was used with a zero score indicating a closed eye, and a score of one indicating an open eye.
Motor Development
Apparatus
Square (40 × 40 cm) (Hamilton Kinder, San Diego Instruments, San Diego, CA) activity monitors were used to assess locomotor activity. Clear Plexiglas inserts were added to convert the chambers into a round (~40 cm diameter) compartment. Free movement of animals was detected by infrared photocell (32 emitter/detector pairs) interruptions; the sensitivity of the photocells was tuned to work with the additional layer of perspex. Total locomotor activity was measured by assessing the number of photocell interruptions within a 60-minute period.
Locomotor Activity
Locomotor activity testing occurred on PD 12, 16, and 20. Testing occurred for a 60-minute period between 700 and 1200h (EST) under dim light conditions, in the absence of direct overhead lighting (<10 lux). All activity monitors were located in an isolated room.
Temporal Processing
PPI assessments in the present study employed the classic approach popularized by Ison and Hammond (1971). Specifically, the classic approach manipulates the interstimulus interval (ISI), the fundamental factor that operationally defines PPI. Thus, the present studies employed a range of ISI values to accurately assess the response amplitude curves for PPI.
Apparatus
The startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA) was enclosed in a 10 cm-thick double-walled, 81×81×116-cm isolation cabinet (external dimensions) (Industrial Acoustic Company, INC., Bronx, NY), instead of the 1.9 cm thick ABS plastic or laminate cabinets offered with this system. Sound attenuation of 30dB(A) was provided in the isolation chamber relative to the external environment. An ambient sound level of 22dB (A) was presented in the chamber without any stimuli presented. The high-frequency loudspeaker of the SR-Lab system (Radio Shack model#40-1278B), mounted inside the chamber 30 cm above the Plexiglas animal test cylinder, delivered all auditory stimuli (frequency range of 5k-16k Hz). A white LED light mounted on the wall in front of the test cylinder inside the chamber (22 lux; Light meter model #840006, Sper Scientific, Ltd, Scottsdale, AZ) was presented as the visual prepulse. The animal's response to the auditory stimulus produced deflection of the test cylinder, which was converted into analog signals by a piezoelectric accelerometer integral to the bottom of the cylinder. The response signals were digitized (12 bit A to D) and saved to a hard disk. Response sensitivities were calibrated using a SR-LAB Startle Calibration System. Sound levels were measured and calibrated with a sound level meter (Kjaer Bruel 2203) with the microphone placed inside the Plexiglas test cylinder.
Cross-modal Prepulse Inhibition
Both visual and auditory prepulse stimuli were used to test animals for PPI of the ASR on PD 14, 17 and 21. PPI was administered using a 30-min test session, beginning with a 5-min acclimation period in the dark with 70 dB (A) background white noise, followed by 6 pulse-only ASR trials with a 10s ITI. A total of 72 trials, including an equal number of visual and auditory prepulse trials, were interdigitated in an ABBA order of presentation. Trials had ISIs of 0, 30, 50, 100, 200, and 4000 msec and were presented in 6-trial blocks according to a Latin-square design. The 0 and 4000 msec ISI trials were control trials. The intertrial interval (ITI) was variable from 15 sec to 25 sec. Inside the test cylinder, the pulse stimulus intensity was 100 dB(A) (20 msec duration). The auditory prepulse was an 85 dB(A) white noise stimulus (20 msec duration); the visual prepulse of 22 lux was also of 20 msec duration. Mean peak ASR amplitude values were collected for analysis. All test sessions were conducted in the dark.
Gap-Prepulse Inhibition
Animals were tested for gap-PPI of the ASR on PD 18. Gap-PPI of the ASR was conducted with a preceding gap in background white-noise as a prestimulus. A 20-min test session began with a 5-min acclimation period in the dark with 70 dB(A) background white noise, followed by six pulse-only ASR trials, used for habituation, with a 10s intertrial interval (ITI). Thirty-six trials were presented using 6-trial blocks according to a Latin-square design. A 20-msec gap in white noise preceded a startle stimulus presented at ISIs of 30,50,100, 200 and 4000 msec. Two control trial types, using 0 and 4000 msec intervals, were included to provide a reference ASR within gap-PPI. The startle stimulus intensity was 100 dB(A) (20 msec duration) measured inside the test cylinder. Mean peak ASR amplitude values were collected for analysis. All test sessions were conducted in the dark.
Statistical Analysis
Categorical data, including eye opening, an index of somatic growth, was analyzed using a chi-squared (χ2) statistical technique. Eye opening data were assessed by individual pup. An alpha level of p≤0.05 was considered significant.
Analysis of variance (ANOVA) techniques (SPSS Statistics 20, IBM Corp., Somers, NY) were used to analyze all continuous data. To account for the nested design within the ANOVA analysis, individual observations were analyzed by using litter means and standard errors (Denenberg 1984; Wears 2002). For repeated measures factors, either orthogonal decompositions were used for those variables that classically violate compound symmetry assumptions (e.g., trials) or the Greenhouse-Geisser df correction factor was used (Greenhouse and Geisser, 1959). Tests of simple mains effects and specific linear contrasts were used, as appropriate, to evaluate age-dependent effects of the HIV-1 transgene (Winer, 1971). An alpha level of p≤0.05 was considered significant for all statistical tests.
Locomotor activity data was analyzed using a mixed factor ANOVA. Cumulative photocell interruptions were used for analysis, with genotype (HIV-1 Tg vs. control) as the between-subjects factor, and time and age as the within-subjects factor.
Cross-modal PPI data were analyzed using a mixed-factor ANOVA for both prepulse modalities (auditory, visual). Mean peak ASR amplitude for the 0-4000 msec ISIs were used for analysis, with genotype (HIV-1 Tg vs. control) as the between-subjects factor, and age, ISI, and trial as the within-subjects factors.
For gap-PPI, a mixed-factor ANOVA was performed on mean peak ASR amplitude for the 0-4000 msec ISIs, with genotype (HIV-1 Tg, n=13 litters vs. control, n=11 litters) as the between-subjects factor, and ISI and trial as the within-subjects factors.
A stepwise discriminant functional analysis was conducted to determine the diagnostic accuracy of early developmental alterations and to determine which observed measures were able to correctly identify animals in regard to their genotype (HIV-1 Tg vs. Control).
RESULTS
HIV-1 Tg animals exhibit selective alterations in somatic growth
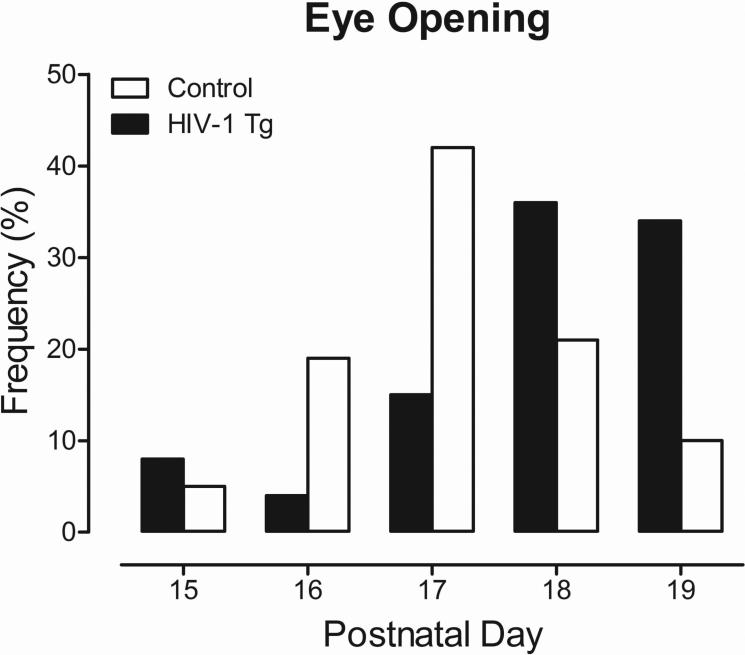
Selective alterations in somatic growth were indexed using body weight measurements and eye opening assessments. Eye opening was assessed from PD 13 to PD 19 (Figure 1). A χ2 analysis revealed a statistically significant difference between eye opening in HIV-1 Tg vs. control animals [χ2(4)=34.4, p≤0.001]. Eye opening began on PD 15 for both HIV-1 Tg and control animals. However, HIV-1 Tg animals exhibited later eye opening, indicated by a significant rightward shift, relative to control animals. On PD 17, 66% of control animals had both the right and left eye open compared to 27% of HIV-1 Tg animals. All animals, regardless of genotype, exhibited full eye opening by PD 19.
Fig. 1.
Eye opening histograms illustrating the significant shift in the distribution dependent upon genotype (HIV-1 Tg or control). HIV-1 Tg animals predominantly exhibited significantly later eye opening. Chi-squared statistical analysis, [χ2(4)=34.4, p≤0.001].
Body weight measurements, obtained upon arrival (PD 8 to PD 10), were used to assess initial somatic growth. Upon arrival, there was no significant difference in body weight between control (M=11.9g, SEM=0.7g) and HIV-1 Tg animals (M=12.5g, SEM=0.6 g). Thereafter, presence of the HIV-1 transgene results in selective alterations in somatic growth.
HIV-1 Tg animals exhibited a shift in the development of locomotor activity implicating alterations in the maturation of the forebrain cholinergic inhibitory system
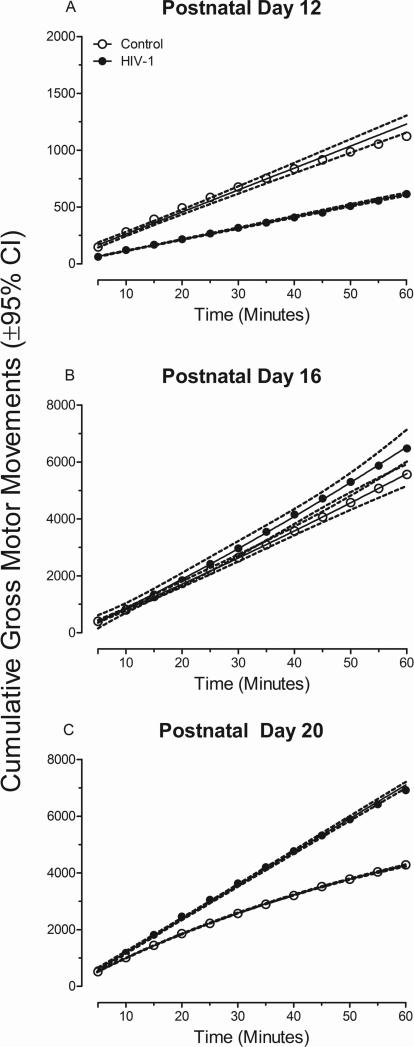
Alterations in locomotor activity in the HIV-1 Tg rat, relative to control animals, was evidenced using cumulative frequency of gross motor movements, illustrated in Figure 2a, 2b, and 2c. The overall ANOVA for cumulative frequencies of locomotor activity revealed a significant age x time x genotype interaction [F(22,726)=6.3, pGG≤0.004, ηp2=0.16] with a prominent linear-linear component [F(1,33)=16.1, p≤0.001, ηp2=0.33], age x time interaction [F(22,726)=77.4, pGG≤0.001, ηp2=0.70] with a prominent linear-linear component [F(1,33)=126.5, p≤0.001, ηp2=0.79], time x genotype interaction [F(11,363)=12.1, pGG≤0.001, ηp2=0.27] with a prominent linear component [F(1,33)=12.6, p≤0.001, ηp2=0.28], and age x genotype interaction [F(2,66)=6.9, pGG≤0.004, ηp2=0.17] with a prominent linear component [F(1,33)=19.7, p≤0.001, ηp2=0.37]. Significant main effects of genotype [F(1,33)=6.1, p≤0.02, ηp2=0.16], age [F(2,66)=103.9, pGG≤0.001, ηp2=0.76], and time [F(11,363)=607.8, pGG≤0.001, ηp2=0.95] were also observed.
Fig. 2.
Mean cumulative frequency of motor movement (±95% CI) is presented as a function of genotype (HIV-1 Tg or Control) and age. A significant time x genotype interaction at postnatal day (PD) 12 and PD 20 indicates alterations in the development of locomotor activity in the HIV-1 Tg animals.
Separate analyses at each age were conducted to determine the locus of these interactions. Analyses revealed a significant time x genotype interaction at PD 20 [F(11,363)=23.1, pGG≤0.001, ηp2=0.41], but not at PD 12 [F(11,363)=1.5, pGG≤0.237] nor PD 16 [F(11,363)=1.6, pGG≤0.217]. HIV-1 Tg animals exhibit a significantly greater number of cumulative gross motor movements on PD 20 in comparison to controls.
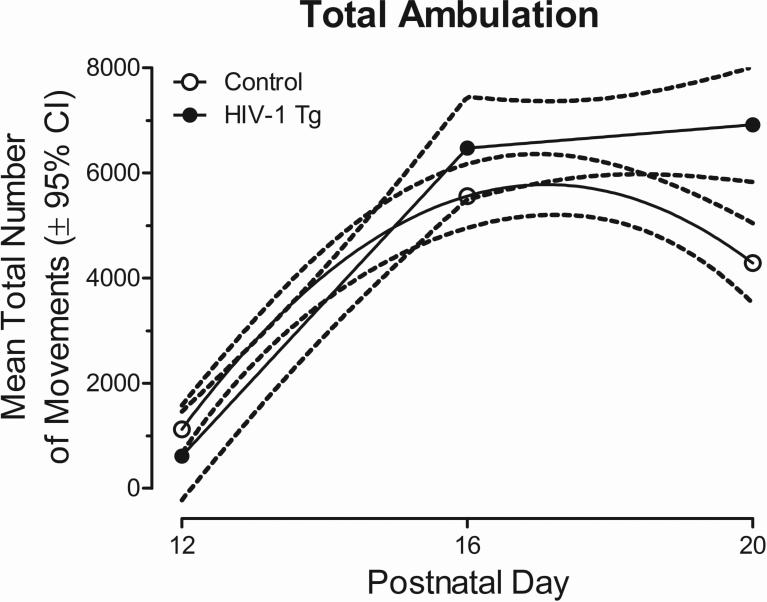
Alterations in the development of locomotor activity are further evidenced by mean total ambulation, illustrated in Figure 3. A segmented first-order polynomial was the best fit for the total ambulation in HIV-1 Tg animals, with maximal spontaneous activity exhibited on PD 20. In contrast, a second-order polynomial was the best fit for control animals, with maximal spontaneous activity exhibited on PD 16. Therefore, HIV-1 Tg animals fail to exhibit decreased levels of spontaneous activity, shown using cumulative frequencies and total ambulation, suggesting an alteration in the development of the forebrain cholinergic inhibitory system.
Fig. 3.
Mean (±95% CI) total ambulation is presented as a function of genotype (HIV-1 Tg or Control) across the three test ages. A segmented first-order polynomial was the best fit for the total ambulation in HIV-1 Tg animals, while a second-order polynomial was the best fit for control animals. The HIV-1 Tg animals fail to exhibit decreased levels of spontaneous activity on PD 20 suggesting an alteration in the development of the cholinergic system.
HIV-1 Tg animals exhibit altered temporal processing development with a visual prepulse
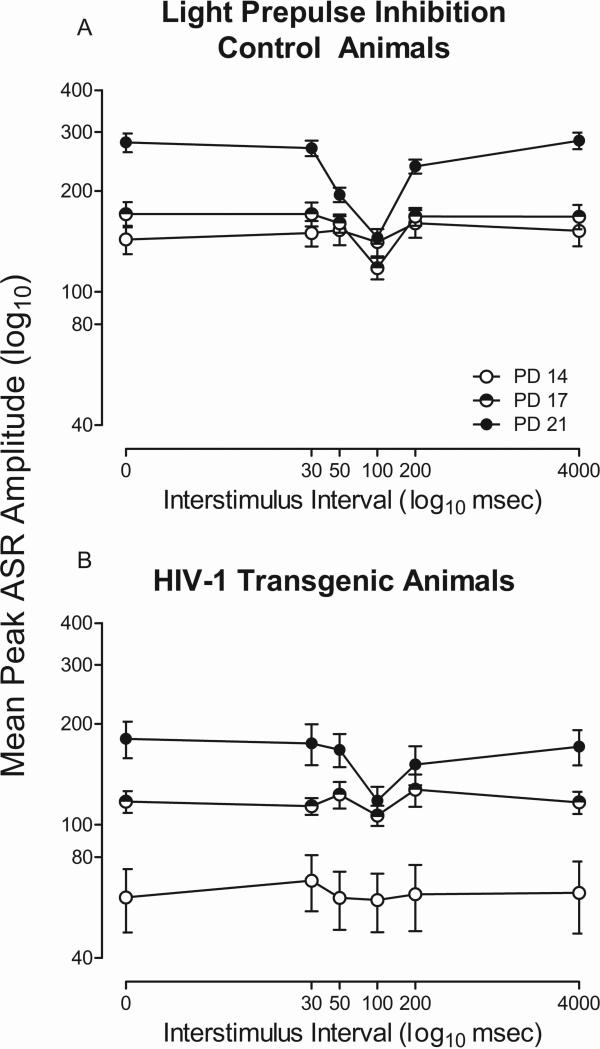
Altered development of PPI with a visual prepulse in the HIV-1 Tg rat, relative to control animals, is illustrated in Figure 4a and 4b. The overall ANOVA on mean peak ASR amplitude revealed a significant age x ISI x genotype interaction [F(10,330)=5.2, pGG≤0.001, ηp2=0.14] with a prominent linear-quadratic component [F(1,33)=19.5, p≤0.001, ηp2=0.37], a significant age x ISI interaction [F(10,330)=19.2, pGG≤0.001, ηp2=0.37] with a prominent linear-quadratic component [F(1,33)=66.8, p≤0.001, ηp2=0.67], and a significant ISI x genotype interaction [F(5,165)=12.0, pGG≤0.001, ηp2=0.27] with a prominent quadratic component [F(1,33)=21.0, p≤0.001, ηp2=0.39]. Significant main effects of genotype [F(1,33)=19.8, p≤0.001, ηp2=0.38], age [F(2,66)=48.2, pGG≤0.001, ηp2=0.60], and ISI [F(5,165)=44.6, pGG≤0.001, ηp2=0.58] were also observed. Control animals exhibited significant perceptual sharpening of the ISI function with age. In contrast, HIV-1 Tg animals fail to exhibit perceptual sharpening of the ISI function with age, evidenced by a flatter ISI function, indicating alterations in the development of the ISI function with a visual prepulse.
Fig. 4.
Mean peak ASR startle response for prepulse inhibition (PPI) with a visual prepulse is presented as a function of genotype (HIV-1 Tg or Control) and age (± SEM). A significant age x interstimulus interval (ISI) x genotype interaction was observed. HIV-1 Tg animals exhibit alterations in the development of perceptual sharpening, evidenced by their flatter ISI function.
Differences in the development of temporal processing were further examined by separate analysis of each genotype. The overall ANOVA for control animals, illustrated in Figure 4a, revealed an age x ISI interaction [F(10,140)=4.3, pGG≤0.008, ηp2=0.23] with a prominent linear-quadratic component [F(1,14)=8.9, p≤0.01, ηp2=0.39]. Main effects of age [F(2,28)=6.6, pGG≤0.009, ηp2=0.32], with a prominent linear component [F(1,14)=15.9, p≤0.001, ηp2=0.53] and ISI [F(5,70)=7.6, pGG≤0.001, ηp2=0.35], with a prominent quadratic component [F(1,14)=9.1, p≤0.009, ηp2=0.40], were also observed. In contrast, the overall ANOVA for HIV-1 Tg animals only revealed a significant main effect of age [F(2,34)=7.0, pGG≤0.004, ηp2=0.29]. The age x ISI interaction present in the control animals, but not the HIV-1 Tg animals, provides additional evidence for alterations in the development of the ISI function.
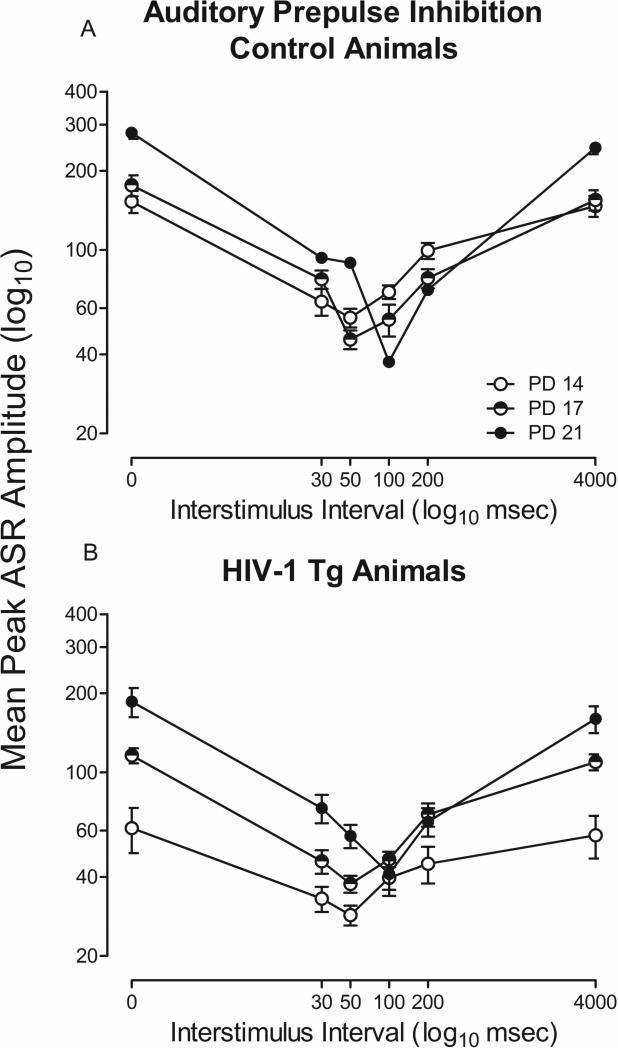
HIV-1 Tg animals exhibit altered temporal processing development with an auditory prepulse
HIV-1 Tg animals exhibit alterations in the development of PPI with an auditory prepulse, as illustrated in Figure 5a and 5b. The overall ANOVA on mean peak ASR amplitude revealed a significant age x ISI x genotype interaction [F(10,330)=3.2, pGG≤0.021, ηp2=0.09] with a prominent linear-cubic component [F(1,33)=11.1, p≤0.002, ηp2=0.25], a significant age x ISI interaction [F(10,330)=46.3, pGG≤0.001, ηp2 =0.59] with a prominent linear-quadratic component [F(1,33)=163.4, p≤0.001, ηp2=0.83], and a significant ISI x genotype interaction [F(5,165)=17.6, pGG≤0.001, ηp2=0.35] with a prominent quadratic component [F(1,33)=240.5, p≤0.001, ηp2=0.88]. Significant main effects of genotype [F(1,33)=24.2, p≤0.001, ηp2=0.42], age [F(2,66)=34.8, pGG≤0.001, ηp2=0.51], and ISI [F(5,165)=204.1, p ≤0.001, ηp2=0.86] were also observed. Both HIV-1 Tg and control animals exhibited a shift in maximal inhibition (from 30 msec to 50 msec) on postnatal day 21. However, HIV-1 Tg animals exhibited alterations in the development of the ISI function in comparison to control animals. Specifically, control animals exhibited significant perceptual sharpening with age, while HIV-1 Tg animals exhibited a flatter ISI function. Therefore, HIV-1 Tg animals also exhibited alterations in the development of the ISI function with an auditory prepulse.
Fig. 5.
Mean peak ASR startle response for prepulse inhibition (PPI) with an auditory prepulse is presented as a function of genotype (HIV-1 Tg or Control) and age (± SEM). A significant age x interstimulus interval (ISI) x genotype interaction was present, indicating an alteration in the development of ISI function in the HIV-1 Tg animals. HIV-1 Tg animals exhibit a flatter ISI function at all test ages in comparison to control animals.
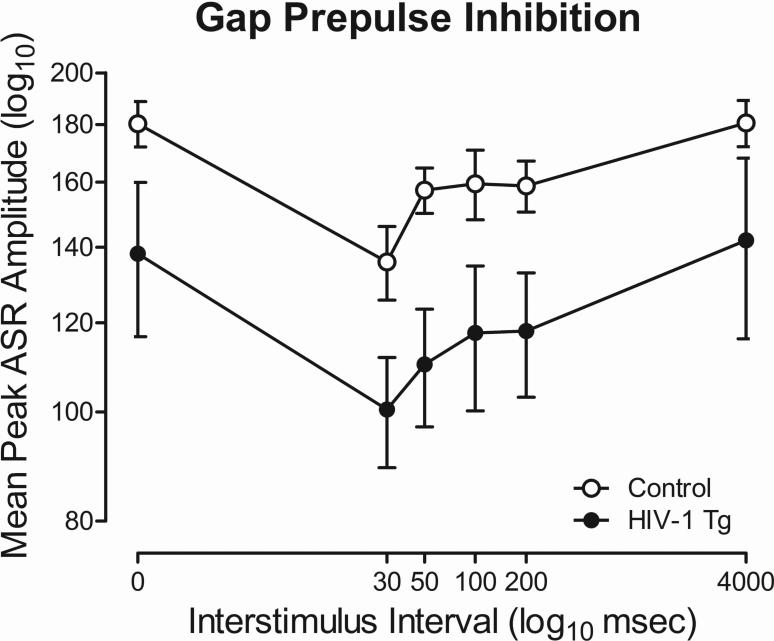
HIV-1 Tg and control animals both exhibit significant gap-PPI
Both HIV-1 Tg and control animals exhibit significant inhibition with gap-PPI, illustrated in Figure 6. The overall ANOVA conducted on mean peak ASR amplitude for gap-PPI revealed that there was no genotype x ISI interaction. A significant main effect of genotype [F(1,22)=4.7, p≤0.05, ηp2=0.18] and ISI [F(5,110)=9.5, pGG≤0.001, ηp2=0.30] were observed. HIV-1 Tg and control animals exhibited comparable peak inhibition at the 30 msec ISI in gap-PPI. HIV-1 Tg animals exhibit a downward shift in the mean peak ASR amplitude curve relative to control animals, but did not exhibit a deficit in temporal processing per se in gap-PPI.
Fig. 6.
Mean peak ASR startle response for gap-prepulse inhibition (gap-PPI) is presented as a function of genotype (HIV-1 Tg or Control; ± SEM). Although there was a main effect of genotype, there was no significant genotype x interstimulus interval (ISI) interaction present. HIV-1 Tg and control animals exhibited comparable peak inhibition at the 30 msec ISI.
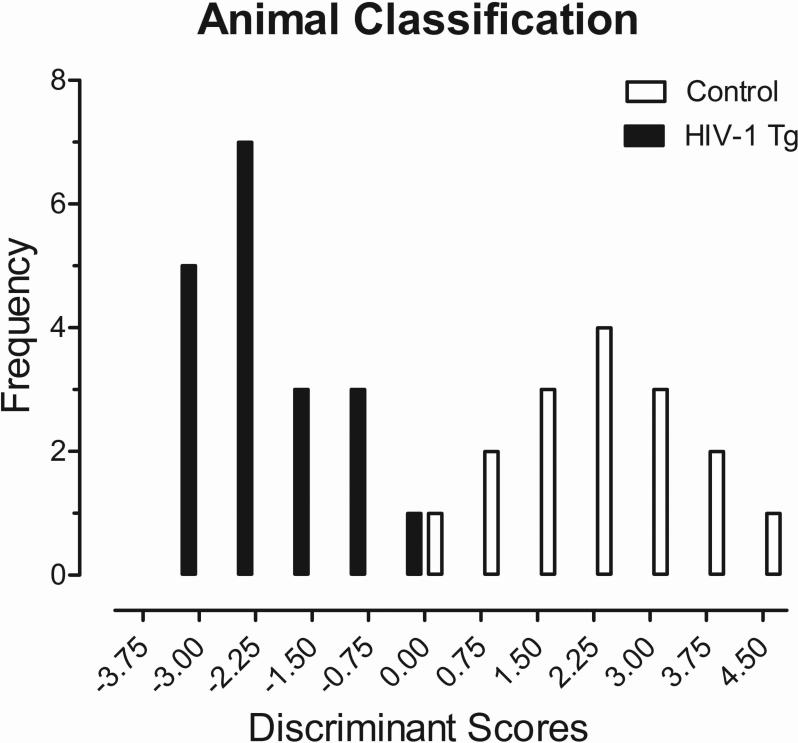
Early developmental alterations can accurately diagnose the presence of the HIV-1 Transgene
The diagnostic utility of early developmental alterations in the HIV-1 Tg rat was further analyzed using an exploratory discriminant function analysis to determine which assessments and ages were best able to identify group membership. Assessment of cross-modal PPI on PD 17 and 21 best predicted group membership, as illustrated in Figure 7. A stepwise discriminant function analysis selected six variables (Auditory Mean Peak ASR Amplitude Values at 50 msec (PD 21) and 100 msec (PD 21) and Visual Mean Peak ASR Amplitude Values at 30 msec (PD 17), 100 msec (PD 17), 200 msec (PD 17) and 50 msec (PD 21)) that maximally separated the HIV-1 Tg and control animals (canonical correlation of 0.908). Animals were classified (jackknifed) with 97.1% accuracy (F approximation of Wilks’ λ of 0.176, F (6,28) =21.9, p≤0.001).
Fig. 7.
Animal classification is illustrated as a function of the canonical variable representing the simplest linear function that best separated the HIV-1 Tg and control groups (canonical correlation 0.91) and correctly identified (jackknife classification) group membership with 97.1% accuracy (93.8% of controls, and 100% of HIV-1 Tg animals).
DISCUSSION
HIV-1 Tg rats exhibited prominent early selective developmental alterations in multiple experimental paradigms, including somatic growth, locomotor activity, cross-modal PPI, and gap-PPI. Later eye opening was observed in the HIV-1 Tg animals relative to controls. HIV-1 Tg animals failed to exhibit decreased levels of spontaneous activity on PD 20, assessed using locomotor activity, suggesting delayed maturation of the forebrain cholinergic inhibitory system. Alterations in the development of PPI and perceptual sharpening were observed in both auditory and visual PPI as indexed by a relative insensitivity to the dimension of time (ms for ISI; days of age for perceptual sharpening) as a function of the HIV-1 transgene. Presence of the HIV-1 transgene was diagnosed with 97.1% accuracy using auditory and visual PPI on PD 17 and 21. These early selective developmental alterations observed in the HIV-1 Tg rat resemble alterations observed in PHIV, providing an innovative opportunity to develop a point-of-care screening tool for the early diagnosis of chronic neurocognitive impairment observed in PHIV.
Selective alterations in somatic growth are observed in the HIV-1 Tg rat, assessed using body weight and eye opening. No statistical differences were observed in initial body weight, assessed from PD 8 to PD 10, results which are consistent with those previously reported in male Sprague-Dawley rats stereotaxically injected with Tat and/or gp120, HIV-1 viral proteins, on PD 1 (Fitting et al. 2008) and in HIV-1 infected children (Guillen et al. 2007; Parachure et al. 2015). HIV-1 Tg animals exhibited later eye opening, indicating an early selective alteration in somatic growth. Results of alterations in eye opening extend those previously reported in male Sprague-Dawley rats stereotaxically injected with HIV-1 viral proteins (Moran et al. 2014b).
HIV-1 Tg rats exhibit alterations in the development of motor movement, assessed using locomotor activity. Significant differences in the cumulative frequency of gross motor movement were evident at PD 12 and PD 20, but not at PD 16, providing evidence for alterations in the development of motor movement. Evidence for alterations in the development of the cholinergic system were suggested using total ambulation in locomotor activity. A second-order polynomial was best fit for the time course for control animals, with maximal spontaneous activity exhibited on PD 16. In contrast, a segmented first-order polynomial was the best fit for HIV-1 Tg animals, with maximal spontaneous activity exhibited on PD 20. Results of alterations in the development of motor movement in the present study extend those previously reported in adult HIV-1 Tg rat (Moran et al. 2013b), Sprague-Dawley rats stereotaxically injected with the HIV-1 viral proteins (Fitting et al. 2008), as well as in HIV-1 infected children (Ferguson and Jelsma, 2009; Foster et al. 2006; Whitehead et al. 2014).
Delayed maturation of the forebrain cholinergic inhibitory system may underlie the alterations in the development of motor movement observed in HIV-1 Tg rats. Preclinical studies have well-established the nonmonotonic development of motor activity with significant increases in spontaneous activity from PD 10 to PD 15, maximal spontaneous activity exhibited on PD 16, followed by significant decreases from PD 17 to PD 25 (Campbell et al. 1969; Campbell and Mabry 1972; Moorcroft et al. 1971). Pharmacological assessments provided evidence for the relationship between the maturation of the cholinergic-inhibitory system and measurements of spontaneous activity (Campbell et al. 1969; Fibiger et al. 1970). Injections of scopolamine hydrobromide, a muscarinic antagonist, significantly increased motor movement in animals at PD 20, but had no effect on motor movement at PD 10 and PD 15 (Campbell et al. 1969). Additionally, injections of both scopolamine hydrobromide and amphetamine produced hyperactivity in rats at PD 25, but not at PD 15, providing additional evidence for the maturation of the forebrain cholinergic inhibitory system from PD 15 to PD 25 (Fibiger et al. 1970). Thus, the decline in motor movement, observed following maximal spontaneous activity on PD 16, may be modulated, at least in part, by the maturational status of the cholinergic system.
Alterations in the development of PPI, assessed using both auditory and visual prepulses, was observed in HIV-1 Tg animals compared to control animals. In PPI with a visual prepulse, the ISI functions observed in the HIV-1 Tg and control groups were not significantly different at PD 14, but subsequently changed in different ways (i.e., PD 17 and PD 21). HIV-1 Tg animals, in comparison to controls, therefore, exhibit alterations in the development of PPI and perceptual sharpening in PPI with a visual prepulse. HIV-1 Tg animals also exhibited an alteration in the development of PPI and perceptual sharpening, indexed by a relative insensitivity to the manipulation of time, in PPI with an auditory prepulse, in comparison to control animals. Results in the present study extend those previously reported in adult HIV-1 Tg animals (Moran et al. 2013a), Sprague-Dawley rats stereotaxically injected with the HIV-1 viral proteins on PD 1 (Fitting et al. 2008; Fitting et al. 2006a; Fitting et al. 2006b), and HIV-1 seropositive adults meeting criteria for HAND (Minassian et al. 2013).
HIV-1 Tg and control animals exhibited significant inhibition in gap-PPI. Comparable peak inhibition at the 30 msec ISI was observed in both HIV-1 and control animals. A downward shift in the mean peak ASR amplitude curve was observed in HIV-1 Tg animals, however, a deficit in temporal processing per se was not observed in gap-PPI. Gap-PPI results in the present study are consistent with a preliminary longitudinal analysis conducted from PD 30 to PD 180 (McLaurin et al. 2016). Specifically, preliminary gap-PPI analyses suggest alterations in the development of temporal processing, assessed using the auditory startle response and prepulse inhibition, beginning at PD 60 (McLaurin et al. 2016).
Early selective developmental alterations observed in the HIV-1 Tg rat may provide a novel point-of-care screening tool for the diagnosis of neurocognitive deficits in children perinatally infected with HIV-1. The potential utility of early selective developmental alterations was assessed using a discriminant function analysis, which correctly identified animals in regards to their genotype (HIV-1 Tg vs. control) with 97.1% accuracy. The presence of the HIV-1 transgene was best predicted using cross-modal PPI assessments at PD 17 and PD 21; an age at which the maturation of fundamental brain development processes (e.g., synaptogenesis) are comparable to 1-3 years of age in humans (review, Semple et al. 2013).
The eyeblink startle paradigm, which measures the eyeblink component of the ASR using electromyography (EMG), provides a clinically relevant experimental paradigm for the assessment of prepulse inhibition in humans. Gap-PPI, assessed using the eyeblink startle paradigm, has been used to assess tinnitus deficits in humans, also providing critical information regarding the underlying neural circuitry (Fournier and Hebert 2013). The eyeblink startle paradigm has also been used to assess the effects of prenatal cocaine exposure (Anday et al. 1989) and chronic cocaine use (Corcoran et al. 2011; Efferen et al. 2000). Most notably, alterations in temporal processing have been observed in HIV-1 seropositive individuals with HAND using the eyeblink startle paradigm (Minassian et al., 2013). The eyeblink startle paradigm, therefore, appears to be a clinically relevant experimental paradigm for assessing temporal processing. Thus, the present study, suggests the potential clinical utility of the eyeblink startle paradigm as a point-of-care screening tool for pediatric HIV-1.
Significant evidence has been provided for alterations in the development of the dopamine (DA) system which may also underlie early selective developmental alterations observed in the HIV-1 Tg rat (review, Fitting et al. 2015). Preclinical studies in the HIV-1 Tg rat have previously implicated DA system impairments as an underlying factor in chronic neurological impairment in HIV-1 infected children (Lee et al. 2014; Moran et al. 2012; Moran et al. 2014b; Webb et al. 2010). Specifically, pharmacological assessments were used to examine alterations in the midbrain dopaminergic system in the HIV-1 Tg rat (Moran et al. 2012; Webb et al. 2010). Dopaminergic system dysfunction, assessed using Western blotting, was evidenced by alterations in phosphorylated tyrosine hydroxylase (pTH), dopamine transporter (DAT) mRNA, and/or monoamine oxidase A (MAO-A; Moran et al. 2012; Webb et al. 2010). In addition, young HIV-1 Tg rats exhibit deficits in D2/3 receptors in the dorsal striatum, assessed using [18F]fallypride positron emission tomography (PET) (Lee et al. 2014).
The DA system may be a clinically relevant target for the treatment of chronic neurological impairments observed in children perinatally infected with HIV-1. Treatment with levodopa, a DA agonist, consistently improved motor function deficits observed in children with pediatric HIV-1 (Mintz et al. 1996). Additionally, methylphenidate, which inhibits DA reuptake, has been implicated as a potential therapeutic treatment for HIV-1 seropositive adults with cognitive slowing (Hinkin et al. 2001). Although multiple neural systems may mediate the early developmental alterations observed in the HIV-1 Tg rat, HIV-1 infection often affects development of dopaminergic system function, evidenced in preclinical and clinical studies, and associated with subsequent cognitive deficits (review, Fitting et al. 2015). Thus, the DA system may serve as a key target for therapeutic intervention in children perinatally infected with HIV-1.
To date, a full understanding of the neuropathogeneis of pediatric HIV-1 has not been elucidated (Linn et al. 2015). The current literature, however, suggests striking differences in the neuropathogeneis of pediatric HIV-1, in comparison to HIV-1 seropositive adults, which may have significant implications for the development of chronic neurological impairment. Post mortem studies of HIV-1 infected children prior to the advent of cART observed infection of neural progenitor cells, which are critical for the growth and development of neurons (Schwartz et al. 2007). HIV-1 infection has also been observed in macrophages, microglial cells, and a small number of neurons in brain tissue of children perinatally infected with HIV-1 (Canto-Nogues 2005). Oxidative stress (i.e., Aksenov et al. 2006; Aksenov et al. 2001), neuroinflammation (i.e., Gao et al. 2008; Tansey and Goldberg 2010), and synaptodendritic injury (i.e., Ellis et al. 2007; Gelman and Nguyen 2010) are other potential mechanisms contributing to neurocognitive impairment in the HIV-1 Tg rat. Specifically, chronic immune activation has been previously observed in the brains of the HIV-1 Tg rat (Rao et al. 2011; Royal et al. 2012). Synaptodendritic alterations, including decreased dendritic branching complexity and shifts in dendritic spine parameters have also been observed in the HIV-1 Tg rat (Rao et al. 2011; Roscoe et al. 2014). Accordingly, the neurotoxic effects of HIV-1 viral proteins, present in the HIV-1 Tg rat, may have a significant effect on the developing brain via a variety of mechanisms (Crowell et al. 2014).
The study of early selective developmental alterations in the HIV-1 Tg rat is vital to the development of a point-of-care screening tool for chronic neurologic impairment observed in children perinatally infected with HIV-1 (review, Luzuriaga and Mofenson, 2016). HIV-1 Tg rats used in the present study are a healthier derivation of those originally described (Reid et al. 2001), exhibiting no general wasting or pathological phenotypes. HIV-1 Tg litters used in the current study displayed no significant health disparities in comparison to F344 controls (i.e., similar initial body weight, similar litter size). Despite the absence of viral replication, chronic, immune activation has been observed in the brains of HIV-1 Tg rats (Rao et al. 2011; Royal et al. 2012), suggesting the presence of a mild, neuroinflammatory microenvironment. Thus, the HIV-1 Tg rat provides a vehicle for investigating the development and underlying mechanisms involved in chronic neurologic impairments observed in PHIV (review, Vigorito et al. 2015).
HIV-1 Tg rats exhibited early selective developmental alterations, including somatic growth, motor development, and prepulse inhibition. Striking differences in the neuropathogenesis of pediatric HIV-1, in comparison to HIV-1 seropositive adults, have been observed; differences which may have significant implications for the development of chronic neurological impairment observed in the present study. Most notably, early selective developmental alterations observed in the HIV-1 Tg rats heralds an opportunity for the development of a point-of-care screening tool, which will allow for early diagnosis of PHIV and therapeutic initiation, improving long-term outcomes for children perinatally infected with HIV-1 (Edwards et al. 2015; Kitahata et al. 2009; review, Luzuriaga and Mofenson, 2016).
ACKNOWLEDGEMENTS
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392) and the interdisciplinary doctoral training program supported by the University of South Carolina Behavioral-Biomedical Interface Program.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
REFERENCES
- Anday EK, Cohen ME, Kelley NE, Leitner DS. Effect of in utero cocaine exposure on startle and its modification. Dev Pharmacol Ther. 1989;12:137–145. [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Nath A, Ray PD, Mactutus CF, Booze RM. Cocaine-mediated enhancement of Tat toxicity in rat hippocampal cell cultures: the role of oxidative stress and D1 dopamine receptor. Neurotoxicology. 2006;27:217–228. doi: 10.1016/j.neuro.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Hasselrot U, Bansal AK, Wu G, Nath A, Anderson C, Mactutus CF, Booze RM. Oxidative damage induced by the injection of HIV-1 Tat protein in the rat striatum. Neurosci Lett. 2001;305:5–8. doi: 10.1016/s0304-3940(01)01786-4. [DOI] [PubMed] [Google Scholar]
- Belman AL, Ultmann MH, Horoupian D, Novick B, Spiro AJ, Rubinstein A, Kurtzberg D, Cone-Wesson B. Neurological complications in infants and children with acquired immune deficiency syndrome. Ann Neurol. 1985;18:560–566. doi: 10.1002/ana.410180509. [DOI] [PubMed] [Google Scholar]
- Blanchette N, Smith ML, King S, Fernandes-Penney A, Read S. Cognitive development in school-age children with vertically transmitted HIV infection. Dev Neuropsychol. 2002;21:223–241. doi: 10.1207/S15326942DN2103_1. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Lytle LD, Fibiger HC. Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science. 1969;166:635–637. doi: 10.1126/science.166.3905.635. [DOI] [PubMed] [Google Scholar]
- Campbell BA, Mabry PD. Ontogeny of behavioral arousal: A comparative study. J Comp Physiol Psychol. 1972;31:253–264. doi: 10.1037/h0033694. [DOI] [PubMed] [Google Scholar]
- Cantó-Nogués C, Sánchez-Ramón S, Alvarez S, Lacruz C, Muñóz-Fernández MA. HIV-1 infection of neurons might account for progressive HIV-1-associated encephalopathy in children. J Mol Neurosci. 2005;27:79–89. doi: 10.1385/JMN:27:1:079. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rourke SB, Murji S, Shore D, Rourke BP. Cognitive complaints, depression, medical symptoms, and their association with neuropsychological functioning in HIV infection: a structural equation model analysis. Neuropsychology. 2003;17:410–419. doi: 10.1037/0894-4105.17.3.410. [DOI] [PubMed] [Google Scholar]
- Cattie JE, Doyle K, Weber E, Grant I, Woods SP, the HIV Neurobehavioral Research Program (HNRP) Group Planning deficits in HIV-associated neurocognitive disorders: Component processes, cognitive correlates, and implications for everyday functioning. J Clin Exp Neuropsyc. 2012;24:906–918. doi: 10.1080/13803395.2012.692772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Diagnosis of HIV infection in the United States and Dependent Areas (25) 2013 http://www.cdc.gov/hiv/pdf/g-l/hiv_surveillance_report _vol_25.pdf.
- Cherner M, Ellis RJ, Lazzaretto D, Young C, Mindt MR, Atkinson JH, Grant I, Heaton RK, the HNRC Group Effects of HIV-1 infection and aging on neurobehavioral functioning: preliminary findings. AIDS. 2004;18:27–34. [PubMed] [Google Scholar]
- Chiriboga CA, Fleishman S, Champion S, Gaye-Robinson L, Abrams EJ. Incidence and prevalence of HIV encephalopathy in children with HIV infection receiving highly active anti-retroviral therapy (HAART). J Pediatr. 2005;146:402–407. doi: 10.1016/j.jpeds.2004.10.021. [DOI] [PubMed] [Google Scholar]
- Cohen S, Caan MW, Mutsaerts HJ, Scherpbier HJ, Kuijpers TW, Reiss P, Majoie CB, Pajkrt D. Cerebral injury in perinatally HIV-infected children compared to matched healthy controls. Neurology. 2016;86:19–27. doi: 10.1212/WNL.0000000000002209. [DOI] [PubMed] [Google Scholar]
- Corcoran S, Norrholm SD, Cuthbert B, Sternberg M, Hollis J, Duncan E. Acoustic startle reduction in cocaine dependence persists for 1 year of abstinence. Psychopharmacology. 2011;215:93–103. doi: 10.1007/s00213-010-2114-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell CS, Malee KM, Yogev R, Muller WJ. Neurologic disease in HIV-infected children and the impact of combination antiretroviral therapy. Rev Med Virol. 2014;24:316–331. doi: 10.1002/rmv.1793. [DOI] [PubMed] [Google Scholar]
- Denenberg VH. Some statistical and experimental considerations in the use of the analysis-of-variance procedure. Am J Physiol-Reg I. 1984;246:R403–R408. doi: 10.1152/ajpregu.1984.246.4.R403. [DOI] [PubMed] [Google Scholar]
- Edwards JK, Cole SR, Westreich D, Mugavero MJ, Eron JJ, Moore RD, Mathews WC, Hunt P, Williams C. Age at entry into care, timing of antiretroviral therapy initiation, and 10-year mortality among HIV-seropositive adults in the United States. Clin Infect Dis. 2015;61:1189–1195. doi: 10.1093/cid/civ463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efferen TR, Duncan EJ, Szilagyi S, Chakravorty S, Adams JU, Gonzenbach S, Angrist B, Butler PD, Rotrosen J. Diminished acoustic startle in chronic cocaine users. Neuropsychopharmacology. 22:89–96. doi: 10.1016/S0893-133X(99)00089-5. [DOI] [PubMed] [Google Scholar]
- Ellis R, Langford D, Masliah E. HIV and antiretroviral therapy in the brain: neuronal injury and repair. Nat Rev Neurosci. 2007;8:33–44. doi: 10.1038/nrn2040. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Joshi VV, Fojas MM, Koenigsberger MR, Oleske JM. Progressive encephalopathy in children with acquired immune deficiency syndrome. Ann Neurol. 1985;17:488–496. doi: 10.1002/ana.410170512. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Sharer LR, Oleske JM, Connor EM, Goudsmit J, Bagdon L, Robert-Guroff M, Koenigsberger MR. Neurologic manifestations of human immunodeficiency virus infection in children. Pediatrics. 1986;78:678–687. [PubMed] [Google Scholar]
- Ferguson G, Jelsma J. The prevalence of motor delay among HIV infected children living in Cape Town, South Africa. Int J Rehabil Res. 2009;32:108–114. doi: 10.1097/MRR.0b013e3283013b34. [DOI] [PubMed] [Google Scholar]
- Fibiger HC, Lytle LD, Campbell BA. Cholinergic modulation of adrenergic arousal in the developing rat. J Comp Physiol Psychol. 1970;72:384–389. doi: 10.1037/h0029741. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006a;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alteration in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006b;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp12 and Tat: Differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. HIV-1 proteins, Tat and gp120, target the developing dopamine system. Curr HIV Res. 2015;13:21–42. doi: 10.2174/1570162x13666150121110731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster CJ, Biggs RL, Melvin D, Walters S, Tudor-Williams G, Lyall EGH. Neurodevelopmental outcomes in children with HIV infection under 3 years of age. Dev Med Child Neurol. 2006;48:677–682. doi: 10.1017/S0012162206001423. [DOI] [PubMed] [Google Scholar]
- Fournier P, Hebert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap? Hear Res. 2013;295:16–23. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Franklin S, Lim HJ, Rennie KM, Eastwood D, Cuene B, Havens PL. Longitudinal intellectual assessment of children with HIV infection. J Clin Psychol Med S. 2005;12:367–376. [Google Scholar]
- Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of alpha-synuclein linked to dopaminergic neurodegeneration. J Neurosci. 2008;28:7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Nguyen TP. Synaptic proteins linked to HIV-1 infection and immunoproteasome induction: proteomic analysis of human synaptosomes. J Neuroimmune Pharmacol. 2010;5:92–102. doi: 10.1007/s11481-009-9168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Guillen S, Ramos JT, Resino R, Bellon JM, Munoz MA. Impact on weight and height with the use of HAART in HIV-infected children. Pediatr Infect Dis J. 2007;26:334–338. doi: 10.1097/01.inf.0000257427.19764.ff. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH, Levine AJ, Castellon SA, Lam MN. Risky decision making assessed with the gambling task in adults with HIV. Neuropsychology. 2006;20:355–360. doi: 10.1037/0894-4105.20.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, Ellis RJ, Letendre SL, Marcotte TD, Atkinson JH, Rivera-Mindt M, Vigil OR, Taylor MJ, Collier AC, Marra CM, Gelman BB, McArthur JC, Morgello S, Simpson DM, McCutchan JA, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, Corkran SH, Duarte NA, Clifford DB, Woods SP, Collier AC, Marra CM, Morgello S, Rivera-Mindt M, Taylor MJ, Marcotte TD, Atkinson JH, Wolfson T, Gelman BB, McArthur JC, Simpson DM, Abramson I, Gamst A, Fennema-Notestine C, Jernigan TL, Wong J, Grant I, The CHARTER and HNRC Groups HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Granholm E, Siegle G. Computerized and traditional Stroop task dysfunction in HIV-1 infection. Neuropsychology. 1999;13:306–316. doi: 10.1037//0894-4105.13.2.306. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, Castellon SA, Hardy DJ, Farinpour R, Newton T, Singer E. Methylphenidate improves HIV-1-associated cognitive slowing. J Neuropsych Clin N. 2001;13:248–254. doi: 10.1176/jnp.13.2.248. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of startle reflex in rat by changes in auditory and visual environments. J Comp Physiol Psychol. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Kauffman WM, Sivit CJ, Fitz CR, Rakusan TA, Herzog K, Chandra RS. CT and MR evaluation of intracranial involvement in pediatric HIV infection: A clinical-imaging correlation. Am J Neuroradiol. 1992;13:949–957. [PMC free article] [PubMed] [Google Scholar]
- Kitahata MM, Gange SJ, Abraham AG, Merriman B, Saag MS, Justice AC, Hogg RS, Deeks SG, Eron JJ, Brooks JT, Rourke SB, Gill MJ, Bosch RJ, Martin JN, Klein MB, Jacobson LP, Rodriguez B, Sterline TR, Kirk GD, Napravnik S, Rachlis AR, Calzavara LM, Horberg MA, Silverberg MJ, Gebo KA, Goedert JJ, Benson CA, Collier AC, Van Rompaey SE, Crane HM, McKaig RG, Lau B, Freeman AM, Moore RD, NA-ACCORD Investigators Effect of early versus deferred antiretroviral therapy for HIV on survival. New Engl J Med. 2009;360:1815–1826. doi: 10.1056/NEJMoa0807252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourtis AP, Bulterys M, Nesheim SR, Lee FK. Understanding the timing of HIV transmission from mother to infant. JAMA-J Am Med Assoc. 2001;285:709–712. doi: 10.1001/jama.285.6.709. [DOI] [PubMed] [Google Scholar]
- Lee DE, Reid WC, Ibrahim WG, Peterson KL, Lentz MR, Maric D, Choyke PL, Jagoda EM Hammoud DA. Imaging dopaminergic dysfunction as a surrogate marker of neuropathology in a small-animal model of HIV. Mol Imaging. 2014;13:1–10. doi: 10.2310/7290.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Everall I, Ances BM, Bharti A, McCutchan JA. Neurologic complications of HIV disease and their treatment. Top HIV Med. 2009;17:46–56. [PMC free article] [PubMed] [Google Scholar]
- Lindsey JC, Malee KM, Brouwers P, Hughes MD. Neurodevelopmental functioning in HIV-infected infants and young children before and after the introduction of protease inhibitor-based highly active antiretroviral therapy. Pediatrics. 2007;119:681–693. doi: 10.1542/peds.2006-1145. [DOI] [PubMed] [Google Scholar]
- Linn K, Fay A, Meddles K, Isbell S, Lin PN, Thair C, Heaps J, Paul R, Mar SS. HIV- related cognitive impairment of orphans in Myanmar with vertically transmitted HIV taking antiretroviral therapy. Pediatr Neurol. 2015;53:485–491. e1. doi: 10.1016/j.pediatrneurol.2015.08.004. [DOI] [PubMed] [Google Scholar]
- Luzuriaga K, Mofenson LM. Challenges in the elimination of pediatric HIV-1 infection. New Engl J Med. 2016;374:761–770. doi: 10.1056/NEJMra1505256. [DOI] [PubMed] [Google Scholar]
- Martin EM, Pitrak DL, Weddington W, Rains NA, Nunnally G, Nixon H, Grbesic S, Vassileva J, Bechara A. Cognitive impulsivity and HIV serostatus in substance dependent males. J Int Neuropsych Soc. 2004;10:931–938. doi: 10.1017/s1355617704107054. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders mind the gap. Ann Neurol. 2010;67:699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- McLaurin KA, Booze RM, Mactutus CF. Minding the gap: Progression of temporal processing deficits in the HIV-1 transgenic rat.. To be presented at: International Society for Developmental Psychobiology; San Diego, CA.. Nov 09-11, 2016.2016. [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W, Translational Methamphetamine AIDS Research Center (TMARC) Group Prepulse inhibition in HIV-associated neurocognitive disorders. J Int Neuropsych Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz M, Hoyt L, McSherry G, Mendelson J, Oleske J. Levodopa therapy improves motor function in HIV-infected children with extrapyramidal syndromes. Neurology. 1996;47:1583–1585. doi: 10.1212/wnl.47.6.1583. [DOI] [PubMed] [Google Scholar]
- Moorcroft WH, Lytle LD, Campbell BA. Ontogeny of starvation-induced behavioral arousal in the rat. J Comp Physiol Psychol. 1971;75:59–67. doi: 10.1037/h0030670. [DOI] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: Evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: Temporal processing demands implicate perceptual and gating deficits in the HIV-1 Transgenic rat. J Neuroimmune Pharm. 2013a;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013b;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Modeling deficits in attention, inhibition, and flexibility in HAND. J Neuroimmune Pharm. 2014a;9:508–521. doi: 10.1007/s11481-014-9539-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Fitting S, Booze RM, Webb KM, Mactutus CF. Neonatal intrahippocampal HIV-1 protein Tat1-86 injection: Neurobehavioral alterations in the absence of increased inflammatory cytokine activation. Int J Dev Neurosci. 2014b;38:195–203. doi: 10.1016/j.ijdevneu.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parachure RS, Kulkarni VV, Darak TS, Mhaskar R, Miladinovic B, Emmanuel PJ. Growth patterns of HIV infected Indian children in response to ART: A clinic based cohort study. Indian J Pediatr. 2015;82:519–524. doi: 10.1007/s12098-014-1659-1. [DOI] [PubMed] [Google Scholar]
- Paramesparan Y, Garvey LJ, Ashby J, Foster CJ, Fidler S, Winston A. High rates of asymptomatic neurocognitive impairment in vertically acquired HIV-1-infected adolescents surviving into adulthood. JAIDS. 2010;55:134–136. doi: 10.1097/QAI.0b013e3181d90e8c. [DOI] [PubMed] [Google Scholar]
- Peng JS, Vigorito M, Liu XQ, Zhous DJ, Wu XW, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Power C, Selnes OA, Grim JA, McArthur JC. HIV Dementia Scale: A rapid screening test. J Acq Immune Def Synd. 1995;8:273–278. doi: 10.1097/00042560-199503010-00008. [DOI] [PubMed] [Google Scholar]
- Rao JS, Kim HW, Kellom M, Greenstein D, Chen M, Kraft AD, Harry GJ, Rapoport SI, Basselin M. Increased neuroinflammatory and arachidonic acid cascade markers, and reduced synaptic proteins, in brain of HIV-1 transgenic rats. J Neuroinflammation. 2011;8:101. doi: 10.1186/1742-2094-8-101. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, Jones O, Doodnauth D, Davis H, Sill A, O'Driscoll P, Huso D, Fouts T, Lewis G, Hill M, Kamin-Lewis R, Wei C, Ray P, Gallo RC, Reitz M, Bryant J. An HIV-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. P Natl Acad Sci USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe RF, Jr, Mactutus CF, Booze RM. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacktor NC, Wong M, Nakasujja N, Skolasky RL, Selnes OA, Musisi S, Robertson K, McArthur JC, Ronald A, Katabira E. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19:1367–1374. [PubMed] [Google Scholar]
- Schwartz L, Civitello L, Dunn-Pirio A, Ryschkewitsch S, Berry E, Cavert W, Kinzel N, Lawrence DMP, Hazra R, Major EO. Evidence of human immunodeficiency virus type 1 infection of nestin-positive neural progenitors in archival pediatric brain tissue. J Neurovirol. 2007;13:274–283. doi: 10.1080/13550280701344975. [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, Noble-Haeusslein LJ. Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol. 2013;106:1–16. doi: 10.1016/j.pneurobio.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanbhag MC, Rutstein RM, Zaoutis T, Zhao H, Chao D, Radcliffe J. Neurocognitive functioning in pediatric human immunodeficiency virus infection: Effects of combined therapy. Arch Pediatr Adolesc Med. 2005;159:651–656. doi: 10.1001/archpedi.159.7.651. [DOI] [PubMed] [Google Scholar]
- Smith R, Wilkins M. Perinatally acquired HIV infection: long-term neuropsychological consequences and challenges ahead. Child Neuropsychol. 2015;21:234–268. doi: 10.1080/09297049.2014.898744. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Goldberg MS. Neuroinflammation in Parkinson's disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol Dis. 2010;37:510–518. doi: 10.1016/j.nbd.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Galgani S, Narciso P, Ferri F, Sebastiani G,D, Amato C, Affricano C, Pigorini F, Pau FM, De Felici A, Benedetto A. Positive and sustained effects of highly active antiretroviral therapy on HIV-1-associated neurocognitive impairment. AIDS. 1999;13:1889–1897. doi: 10.1097/00002030-199910010-00011. [DOI] [PubMed] [Google Scholar]
- UNAIDS Fact sheet 2015. 2015 Retrieved from: http://www.unaids.org/en/resources/campaigns/HowAIDSchangedeverything/factsheet.
- Van Rie A, Harrington PR, Dow A, Robertson K. Neurologic and neurodevelopmental manifestations of pediatric HIV/AIDS: A global perspective. Eur J Paediatr Neurol. 2007;11:1–9. doi: 10.1016/j.ejpn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–128. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigorito M, Connaghan KP, Chang SL. The HIV-1 transgenic rat model of neuroHIV. Brain, Behav Immun. 2015;48:336–349. doi: 10.1016/j.bbi.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SY, Pierre RB, Christie CD, Chang SM. Neurocognitive function in HIV-positive children in a developing country. Int J Infect Dis. 2013;17:e862–867. doi: 10.1016/j.ijid.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wears RL. Advanced statistics: Statistical methods for analyzing cluster and cluster- randomized data. Acad Emerg Med. 2002;9:330–341. doi: 10.1111/j.1553-2712.2002.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Webb KM, Aksenov MY, Mactutus CF, Booze RM. Evidence for developmental dopaminergic alterations in the human immunodeficiency virus-1 transgenic rat. J Neurovirol. 2010;16:168–173. doi: 10.3109/13550281003690177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead N, Potterton J, Coovadia A. The neurodevelopment of HIV-infected infants on HAART compared to HIV-exposed but uninfected infants. AIDS Care. 2014;26:497–504. doi: 10.1080/09540121.2013.841828. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2nd ed. McGraw-Hill; New York: 1971. [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, Gill MJ, Rachlis A, Rosenes R, Arbess G, Marcotte T, Rourke SB. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS. 2013;27:2385–2401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]