SUMMARY
SETTING
Prevention of maternal-to-child transmission program at a tertiary care hospital in Nairobi, Kenya. The risk of acquiring Mycobacterium tuberculosis infection among peripartum human immunodeficiency virus (HIV) infected women is poorly defined.
OBJECTIVE
To determine the incidence of and co-factors for interferon-gamma release assay (IGRA) conversion among postpartum HIV-infected women using T-SPOT.TB.
DESIGN
We used data and cryopreserved peripheral blood mononuclear cells from a historical cohort of HIV-infected women enrolled at 32 weeks’ gestation and followed for 1 year postpartum between 1999 and 2005.
RESULTS
Of 89 women initially IGRA-negative during pregnancy, 11 (12.4%) became positive, 53 (59.5%) remained negative and 25 (28.1%) were indeterminate at 1 year postpartum. Mean interferon-gamma (IFN-γ) response among converters increased from ~1 to >50 spot-forming cells/well (P = 0.015). IGRA conversion was significantly associated with partner HIV infection, flush toilets, maternal illness and cough during follow-up, but not maternal CD4 count or HIV viral load.
CONCLUSION
The high rates of IGRA conversion seen among HIV-infected postpartum women in our study are similar to those of other groups at high risk for M. tuberculosis infection. This has important implications for M. tuberculosis infection screening strategies and provision of preventive therapy for the health of women and their infants.
Keywords: latent tuberculous infection, LTBI, T-SPOT.TB, maternal health, targeted LTBI testing, longitudinal assessment
ALTHOUGH THE INCREASED RISK of progression from Mycobacterium tuberculosis infection to active disease among human immunodeficiency virus (HIV) infected individuals is well described,1 data on incidence of and co-factors for M. tuberculosis infection among HIV-infected individuals,2–6 or pregnant/postpartum women are limited.7,8 Lack of a perfect test to detect M. tuberculosis infection complicates the distinction between a true new infection vs. a positive test reflecting immune response changes or test performance variation.
Unlike the tuberculin skin test (TST), interferon-gamma release assays (IGRAs) do not cross-react with bacille Calmette-Guérin (BCG) and are not boosted by previous testing; they could therefore contribute to improved estimates of M. tuberculosis infection incidence.9 Recent data suggest that IGRA conversion among HIV-infected individuals is high, even in low TB burden areas2 and among peripartum women in endemic settings.7 Pregnant/postpartum women may be at increased risk for active TB or M. tuberculosis infection due to hormonal and immunologic changes.10–12 This risk may be amplified among pregnant/postpartum HIV-infected women.
Longitudinal studies assessing IGRA conversion in HIV-infected individuals and pregnant/postpartum women would be useful in determining M. tuberculosis infection rates and co-factors that inform testing and TB prophylaxis approaches. Pregnant/postpartum HIV-infected women are a particularly relevant group due to adverse outcomes associated with maternal TB to themselves and their infants, including TB, HIV infection/progression, low birth weight, and mortality.10,13 We previously determined that positive antenatal maternal IGRA was associated with a significantly increased risk of postpartum maternal and infant TB and mortality.13 In the present study, we determined the incidence and correlates of IGRA conversion in HIV-infected postpartum Kenyan women.
METHODS
Study design and population
We used data and cryopreserved peripheral blood mononuclear cells (PBMCs) from a previously described historical cohort of HIV-infected pregnant women enrolled at 32 weeks’ gestation and followed for at least 1 year postpartum at the Kenyatta National Hospital, Nairobi, Kenya, between July 1999 and May 2005, as part of a prevention of maternal-to-child transmission study.13,14 Women received zidovudine for the prevention of vertical transmission of HIV. This study was conducted prior to widespread antiretroviral therapy (ART) availability; none of the women in this IGRA analysis received ART during study follow-up. Previously, we tested antenatal PBMCs in this cohort with T-SPOT.TB IGRA (Oxford Immunotec, Abingdon, UK) and estimated postpartum maternal and infant active TB and mortality risk associated with positive antenatal maternal IGRA;13 of the 361 women tested in pregnancy, 135 (37.4%) were positive, 170 (47.1%) were negative, and 56 (15.5%) had an indeterminate response.13
For this study, we selected women with a negative IGRA at 32 weeks’ gestation with PBMCs available at 1 year postpartum, and excluded those with clinically detected active TB during follow-up.
Written informed consent for the parent study was obtained from all participants. The University of Washington Human Subjects Division, Seattle, WA, USA, and the University of Nairobi Ethical Review Committee, Nairobi, Kenya, approved the parent and current studies.
Enrollment and follow-up procedures
Medical history and examination were conducted at enrollment during pregnancy, and follow-up visits at delivery, 2 and 4 weeks postpartum, and monthly thereafter up to 12 months.13 Blood samples were obtained at enrollment and follow-up for HIV RNA levels, CD4 cell count, and PBMC storage. CD4 cell counts were measured at the University of Nairobi using FACScan Flow Cytometer (BD, Franklin Lakes, NJ, USA). Plasma HIV RNA levels were quantified at the Fred Hutchinson Cancer Research Center, Seattle, WA, USA, using a transcription-mediated amplification assay (Gen-Probe, San Diego, CA, USA). At study visits, women were evaluated for illness symptoms as well as reports of diagnosis of upper respiratory tract infection, pneumonia, TB, malaria, or hospitalization since the previous study visit.14
T-SPOT.TB assay
Cryopreserved PBMCs were processed using previously described techniques.13 Average cell viability following overnight incubation was 62.8%. The manufacturer’s instructions for the T-SPOT.TB assay procedure and the interpretation of results were followed (Oxford Immunotec, http://www.oxfordimmunotec.com/T-SPOT_International). Developed plates were dried overnight and spots were read using an automated ELISpot reader (CTL-Immunospot® S4 Core Analyzer, Carnegie, OH, USA). Assays were considered positive if the mean spot-forming cell count per well (SFC/well) in early secreted antigenic target 6 (ESAT-6) or culture filtrate protein 10 (CFP-10) minus the negative control was ≥6 (if the mean negative control was ≤5 SFC/well), or if the mean ESAT-6 or CFP-10 SFC/well minus the negative control SFC/well was >2 times the negative control (if the mean negative control was 6–10 SFC/well). Assay results were considered negative if the above criteria were not met, and indeterminate if mean SFC/well was <20 in positive control wells or >10 in negative control wells. Assays were performed by a single technician.
Statistical analysis
Women with positive and negative IGRA at 1 year postpartum were compared on baseline characteristics using median and interquartile range (IQR) for non-normally distributed continuous variables and χ2 test (or Fisher’s exact test, if appropriate) for categorical variables. Change in CD4 cell count and HIV plasma viral load between delivery and 1 year postpartum, and interferon-gamma (IFN-γ) responses at 32 weeks’ gestation and 1 year postpartum, were compared by IGRA response status at 1 year postpartum using paired t-tests.
As an exploratory analysis, we excluded women with grey-zone results of 5–7 SFCs/well at baseline and follow-up (cut-off suggested by the T-SPOT.TB manufacturer and the US Food and Drug Administration as an indication for re-testing), and repeated the above described pair-wise IFN-γ response analysis.
RESULTS
IGRA conversion at 1 year postpartum
Of 89 HIV-infected women with a negative IGRA during pregnancy, 11 (12.4%) were positive, 53 (59.5%) were negative and 25 (28.1%) were indeterminate at 1 year postpartum (Figure). Among indeterminate assays, 16 (64%) had failed the positive control (<20 SFC/well) and 9 (36%) had failed the negative control (>10 SFC/well). Excluding indeterminate responses, 17.2% (11/64) women had an IGRA conversion.
Figure.
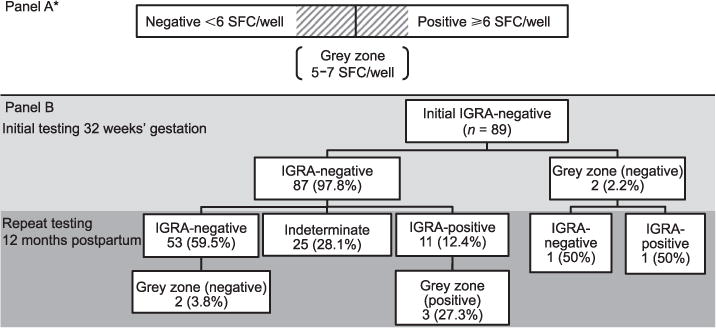
A) Cut-offs for positive and negative IGRA TB-SPOT.TB assays, including grey zone. B) Study flow of HIV-infected women with initial negative IGRA (TB-SPOT.TB) at 32 weeks’ gestation with repeated IGRA testing at 12 months postpartum. SFC = spot-forming cells; IGRA = interferon-gamma release assay; HIV = human immunodeficiency virus.
Baseline comparison of IGRA convertors vs. non-convertors
A comparison of characteristics of IGRA converters vs. non-converters is shown in Table 1. The groups had comparable age, employment status, household crowding (number of rooms, people, and people/room/household), CD4 cell count and plasma HIV viral load. A significantly greater proportion of women with IGRA conversion reported flush toilet use vs. pit latrine (63.6% vs. 28.3%, P = 0.04) and a higher proportion of HIV-positive husbands (83.3% vs. 25.0%, P = 0.05) than women without IGRA conversion. Women who reported flush toilet use were more likely to be employed, live in >1 room households, and not share a toilet than pit latrine users (data not shown).
Table 1.
Characteristics of HIV-infected women who converted from IGRA-negative at enrollment (32 weeks’ gestation) to IGRA-positive at 12 months postpartum vs. those who remained IGRA-negative
| Characteristics | IGRA-positive (n = 11) n (%) |
IGRA-negative (n = 53) n (%) |
P value |
|---|---|---|---|
| Sociodemographic | |||
| Age, years, median [IQR] | 24.0 [24.0–29.0] | 24.0 [21.0–27.0] | 0.13 |
| Ever married | |||
| Yes | 9 (81.8) | 48 (90.6) | 0.59 |
| No | 2 (18.2) | 5 (9.3) | |
| More than primary education (>8 years) | |||
| Yes | 7 (63.6) | 23 (43.4) | 0.32 |
| No | 4 (36.4) | 30 (56.6) | |
| Employed | |||
| Yes | 5 (45.5) | 18 (34.0) | 0.51 |
| No | 6 (54.5) | 35 (66.0) | |
| Living in 1 room | |||
| >1 room | 4 (36.4) | 7 (13.5) | 0.09 |
| 1 room | 7 (63.6) | 45 (86.5) | |
| Number of rooms, median [IQR] | 1.0 (1.0–2.0) | 1.0 (1.0–1.0) | 0.07 |
| Number of people/room, median [IQR] | 4.0 (2.0–4.0) | 2.0 (2.0–4.0) | 0.16 |
| Use of flush toilet | |||
| Flush toilet | 7 (63.6) | 15 (28.3) | 0.04 |
| Pit toilet | 4 (36.4) | 38 (71.7) | |
| Use of shared toilet | |||
| Yes | 9 (81.8) | 47 (88.7) | 0.62 |
| No | 2 (18.2) | 6 (11.3) | |
| Partner HIV testing and status | |||
| Husband tested for HIV | |||
| Yes | 6 (54.6) | 24 (46.1) | 0.30 |
| No | 2 (18.2) | 21 (40.4) | |
| Do not know | 3 (27.3) | 7 (13.5) | |
| Husband HIV-positive | (n = 6) | (n = 24) | |
| Yes | 5 (83.3) | 6 (25.0) | 0.05 |
| No | 0 (0.0) | 7 (29.1) | |
| Do not know | 1 (16.7) | 11 (45.8) | |
| HIV markers at enrollment during pregnancy | |||
| CD4 cell count, cell/mm3, median [IQR] | 406 [221–539] | 407 [308–616] | 0.31 |
| HIV plasma viral load, log10 copies/ml, median [IQR] | 4.65 [3.97–5.36] | 4.80 [4.09–5.26] | 0.78 |
| Maternal illness during year 1 postpartum follow-up | |||
| Average number of visits due to illness over 1 year postpartum, median [IQR]* | 6.4 [4.1–8.6] | 4.0 [3.3–4.7] | 0.008 |
| Hospitalization | |||
| Yes | 1 (9.1) | 4 (7.6) | 1.0 |
| No | 10 (90.9) | 49 (92.5) | |
| Clinical diagnosis of pneumonia | |||
| Yes | 1 (9.1) | 2 (3.8) | 0.44 |
| No | 10 (90.9) | 51 (96.2) | |
| Clinical diagnosis of URTI | |||
| Yes | 10 (90.9) | 34 (64.2) | 0.15 |
| No | 1 (9.1) | 19 (35.8) | |
| Self-report of fever | |||
| Yes | 4 (36.4) | 20 (37.7) | 1.0 |
| No | 7 (63.6) | 33 (62.3) | |
| Self-report of cough | |||
| Yes | 11 (100.0) | 37 (69.8) | 0.05 |
| No | 0 (0.0) | 16 (30.2) | |
| HIV progression markers | |||
| Change in CD4 over 1 year postpartum from delivery, cell/mm3, mean (95%CI)† | −7.1 (−16.2 to 2.0) | −8.7 (−14.0 to 3.5) | 0.78 |
| CD4 count at month 12 postpartum, cell/mm3, median [IQR] | 287.5 [190.0–683.0] | 410.0 [277.5–594.0] | 0.31 |
| CD4 % at month 12 postpartum, median [IQR] | 18.5 [16.0–27.0] | 20.0 [14.0–27.0] | 0.95 |
| Change in HIV plasma viral load over 1 year postpartum from delivery, log10 copies/ml, mean (95%CI)† | 0.03 (−0.1–0.1) | −0.02 (−0.1–0.1) | 0.48 |
| HIV plasma viral load at month 12 postpartum, log10 copies/ml, median [IQR] | 4.6 [4.4–5.1] | 4.6 [4.1–5.1] | 0.98 |
Study visit where an indicator for maternal illness has been recorded as yes.
Change in CD4 cell count and HIV plasma viral load between delivery and 1 year postpartum was calculated using paired t-tests.
HIV = human immunodeficiency virus; IGRA = interferon-gamma release assay; IQR = interquartile range; URTI = upper respiratory tract infection; CI = confidence interval.
IGRA converters had a significantly greater average number of visits with reported illnesses than non-converters (mean: 6.4 vs. 4.0, P = 0.008), and were more likely to report cough (100% vs. 69.8%, P = 0.05) (Table 1). A similar proportion in both groups were hospitalized, had clinically diagnosed pneumonia, and fever. Change in CD4 cell count and HIV viral load during follow-up did not differ between groups.
Women with and without IGRA conversion had a mean IFN-γ response at 12 months postpartum of respectively 50.9 SFC/well and 1.2 SFC/well (Table 2). Mean IFN-γ response in women who remained IGRA-negative increased slightly postpartum (0.6 vs. 1.2, paired t-test P = 0.02). The Appendix Figure A displays individual response changes between pregnancy and postpartum among IGRA converters.*
Table 2.
Pair-wise comparison of combined and antigen-specific IFN-γ response in HIV-infected women who were IGRA-negative at enrollment (32 weeks’ gestation) and IGRA-positive/negative/indeterminate at 12 months postpartum
| Antigen-specific spot count above negative control, SFC/well
|
|||
|---|---|---|---|
| Postpartum IGRA response to M. tuberculosis antigens (number of pairs) | 32 weeks’ gestation Mean (95%CI) |
12 months postpartum Mean (95%CI) |
P value* |
| Combined ESAT-6/CFP-10 response† | |||
| Positive (n = 11) | 1.23 (0.07–2.38) | 50.91 (12.93–88.89) | 0.015 |
| Negative (n = 53) | 0.57 (0.26–0.89) | 1.21 (0.78–1.63) | 0.02 |
| Indeterminate (n = 25) | 1.40 (0.42–2.37) | 9.33 (−4.97–23.64) | 0.27 |
| ESAT-6 | |||
| Positive (n = 11) | 0.59 (−0.06–1.24) | 47.0 (8.67–85.33) | 0.02 |
| Negative (n = 53) | 0.47 (0.17–0.78) | 0.92 (0.54–1.31) | 0.06 |
| Indeterminate (n = 25) | 0.44 (0.04–0.83) | 7.71 (−5.88–21.29) | 0.28 |
| CFP-10 | |||
| Positive (n = 11) | 0.95 (−0.21–2.12) | 30.73 (3.70–57.75) | 0.04 |
| Negative (n = 53) | 0.26 (0.09–0.44) | 0.57 (0.25–0.88) | 0.11 |
| Indeterminate (n = 25) | 1.15 (0.16–2.15) | 2.74 (−2.85–8.33) | 0.58 |
Paired t-test.
IGRA response to M. tuberculosis-specific antigens.
IFN-γ = interferon-gamma; HIV = human immunodeficiency virus; IGRA = interferon-gamma release assay; SFC = spot-forming cells; CI = confidence interval; ESAT-6 = early secretory antigenic 6; CFP-10 = culture filtrate protein 10.
Grey-zone analysis
Two of the 89 (2.2%) women who were IGRA-negative at baseline were within the grey zone of 5–7 spots above control; one remained negative at 1 year with an IFN-γ response below the grey zone, and one had an IGRA conversion with a strong positive response (Figure). Of the 11 IGRA conversions, three (27.3%) were within the grey zone. Of the 53 who remained IGRA-negative at 1 year, two (3.8%) were in the grey zone.
Excluding women with grey-zone responses, IGRA conversion was observed in 12.3% (7/57) at 12 months postpartum. In this subset, those with a positive postpartum IGRA had a significantly higher mean postpartum response compared to baseline (55.4 vs. 1.2 SFC/well, P = 0.03). We also observed a small IFN-γ response increase in women who remained IGRA-negative (mean response 1.1 vs. 0.5 SFC/well, P = 0.01).
DISCUSSION
In this longitudinal assessment of initially IGRA-negative HIV-infected women during pregnancy, there was a high incidence of IGRA conversions (12.4%, or 17.4% excluding indeterminate responses) at 1 year postpartum. Among IGRA converters, the median magnitude of responses increased substantially and significantly (from ~1 to >50 SFC/well) between baseline and follow-up.
To date, the majority of IGRA conversion studies in high-burden areas have focused on testing following known TB exposure. In these studies, IGRA conversion rates ranging from ~12% to 27% have been noted in household contacts in India, South Africa and the Gambia.15–17 Our estimated incidence of 12–17% IGRA conversions over the first postpartum year is similar to the estimated 14% annualized IGRA conversion among South African adolescents.18 Our study indicates that pregnant/postpartum women are at comparable risk of M. tuberculosis infection to these known high-risk groups.
In high TB burden regions, HIV-infected individuals have a high baseline prevalence of M. tuberculosis infection as detected by IGRA, suggesting that the incidence of M. tuberculosis infection in the absence of defined TB exposure is not rare.9 HIV-infected individuals may have frequent hospital/clinic visits where they could be exposed to TB patients, and are more likely to live with an HIV-TB co-infected family. Our observation of a 12–17% incidence of IGRA conversion may reflect new M. tuberculosis infections in an endemic region or detection in individuals previously negative at baseline due to imperfect test sensitivity, lower baseline antigen stimulation, or an increase in TB-specific immune responses. However, the large response change from negative to >50 spots and the plausible co-factor associations suggest that these IGRA conversions reflect new M. tuberculosis infection. This is consistent with a systematic review of TB clustering that suggested that TB cases among HIV-infected individuals in an endemic setting were more likely to be due to new infection than reactivation.19
Women with IGRA conversion were more likely to have an HIV-positive partner and use a flush toilet. HIV-infected male partners would be expected to be at greater risk of having active TB, leading to exposure of their female partners. In our previous study, flush toilet was also associated (P = 0.0001) with positive IGRAs in HIV-infected women.13 M. tuberculosis has been detected in stool specimens of individuals with active TB,20 and in soil and wastewater in high TB burden areas.21 M. tuberculosis transmission from medical waste22 and aerosolization from wound irrigation23 has been documented. While it is possible that M. tuberculosis aerosolization via flush toilets could occur,24 it is likely that flush toilet use may be a proxy for some unmeasured factor associated with acquisition of M. tuberculosis infection. In our study, flush toilets were associated with higher socio-economic status indicators. Women with IGRA conversions were significantly more likely to report cough and illnesses during the period of conversion, suggesting a subclinical syndrome at acquisition of M. tuberculosis infection, or alternatively, the development of clinically unrecognized active TB. The binary concept of latent vs. active TB is evolving to a continuum paradigm, reflecting the complexity of the interactions between host immune status, bacillary load, development of symptoms and progression to TB disease.1 Similar to other studies in HIV-infected individuals, immunosuppression measured by CD4 count was not associated with IGRA conversion.2 This is consistent with studies noting that susceptibility to TB may be related to qualitative T-cell dysfunction in addition to reduction of CD4 cells, and may partially explain why HIV-infected individuals on ART remain at higher risk of TB even after CD4 count recovery.1,25,26
Our study has important strengths and limitations. In the absence of TST data, it is difficult to speculate how many IGRA-negative women in our study would have been TST-negative. In a cross-sectional study of HIV-infected and non-HIV-infected pregnant women in India, TST and IGRA agreement was poor (κ = 0.21) in HIV-infected women.27 Our study was limited by the use of frozen and not the manufacturer-recommended fresh PBMCs, which may have resulted in a lower magnitude of IGRA responses. We observed a higher percentage of indeterminate responses (28%) than the 0.6–15% range observed in previous studies, perhaps due to the use of cryopreserved specimens.13,28–31 The validity of ELISpot responses using frozen PBMCs has been demonstrated previously.32,33 The grey-zone analysis was an approach to try to compensate for potentially lower IGRA responses associated with frozen PBMCs. Excluding women who developed clinically diagnosed active TB during follow-up could have led to underestimated rates of IGRA conversion in the case of IGRA conversion and rapid progression to active TB during follow-up. Reversions may have contributed to ‘negative’ baseline results, underestimating the prevalence of M. tuberculosis infection in pregnancy. Although a high proportion of IGRA reversions (positive to negative) has been reported in serially tested health care workers34 and HIV-infected individuals in low TB burden settings,2 reversion appears less frequent in high TB burden settings, particularly with baseline positive IGRAs well above the cut-off threshold.16,18,35,36 Our small sample size likely limited our power to detect other IGRA conversion co-factors. Women in this cohort were not systematically evaluated for active TB, as this is was not the primary aim of the parent cohort; some of the IGRA conversions could therefore reflect active TB. However, given the duration of follow-up, it is likely that the majority of women developing clinically apparent active TB were identified. Rates of IGRA conversion may be higher in our pre-ART cohort than would be seen in new ART cohorts with Option B+ (ART for life for pregnant/lactating women). The strengths of our study include frequent visits (monthly) and the relatively long duration of clinical follow-up (from 32 weeks’ gestation to 1 year postpartum).
Our study suggests that IGRA conversion in HIV-infected women between the antepartum and postpartum period is not rare. Women with HIV-infected partners have a higher risk of IGRA conversion and may be a high-risk group to target for isoniazid or other preventive therapies. While universal isoniazid preventive therapy (IPT) is currently recommended for HIV-infected individuals,37 the benefit of preventive therapy is most evident in those who are TST-positive. Although there are data to suggest that IPT is well tolerated in peripartum women in programmatic and trial settings,38,39 there are still concerns about side effects in this population; this is the subject of a current National Institutes of Health-funded trial (IMPAACT P1078). An approach of repeated M. tuberculosis infection testing followed by IPT for those with a positive test without evidence of active TB may an alternative to universal IPT. However, the feasibility and cost-effectiveness of this approach need to be examined.
Cost-effectiveness modelling in a theoretical HIV-infected cohort in a low TB burden setting suggested additional benefit for latent tuberculous infection screening during pregnancy vs. the postpartum period.40 This may not be the case in high-burden settings with ongoing risk of TB transmission. If women are screened only once during pregnancy using IGRA, our study suggests that there could be an appreciable subset of women who subsequently qualify for IPT in the postpartum period. Further longitudinal studies in HIV-infected cohorts in high TB burden regions, including pregnant/postpartum women in the era of ART and Option B+ roll-out, will be useful to contribute data on IGRA conversion rates and to inform guidelines for M. tuberculosis infection testing, diagnosis and treatment.
CONCLUSION
We found high rates of IGRA conversion among HIV-infected postpartum women, with rates similar to those seen among populations known to be at high risk for M. tuberculosis infection (known TB case contacts and adolescents in high TB-HIV burden settings). This has important implications for latent tuberculous infection testing strategies and provision of preventive therapy for the health of women and their infants.
Acknowledgments
The authors thank colleagues at the Paediatrics Research Laboratory, Kenyatta National Hospital, Nairobi, Kenya, for laboratory support for this study, and the study staff and participants. Preliminary data were presented at the 48th Annual Meeting of the Infectious Disease Society of America (poster), 21–24 October 2010, Vancouver, British Columbia, Canada.
This work was supported by the US National Institutes of Health (NIH, Bethesda, MD, USA) (R21 HD058477-01 and K24 HD054314-04, GJS, and NICHD K12-HD000850, LMC), Firland Foundation (Shoreline, WA, USA) (SJ), NIH International AIDS Training and Research Program by the Fogarty International Center (Bethesda, MD, USA) and the Office of Research on Women’s Health (D43 TW000007, to BLP, PO and EMO), STD/AIDS Research Training Grant (T32 AI007140, SML), and the Pediatric Scientist Development Program supported by the American Pediatric Society/American Academy of Pediatrics (The Woodlands, TX, USA) grant (LMC).
APPENDIX
Figure A.
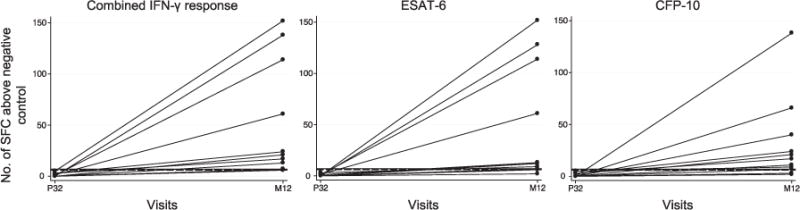
Change in magnitude of IFN-γ response in women who were negative at P32 and IGRA-positive at M12. The horizontal black dashed line represents 6 SFC/well, the manufacturer-defined cut-off point for a positive response. The horizontal black solid line represents 7 SFC/well, the upper limit for the grey zone of 5–7 SFCs/well defined by T-SPOT.TB and the US Food & Drug Administration. SFC = spot-forming cell; IFN-γ = interferon-gamma; P32 = 32 weeks’ gestation; M12 = 12 months postpartum; ESAT-6 = early secreted antigenic target 6; CFP 10 = culture filtrate protein 10.
Footnotes
The appendix is available in the online version of this article, at http://www.ingentaconnect.com/content/iuatld/ijtld/2015/00000019/00000007/art00008
Conflicts of interest: none declared.
References
- 1.Lawn SD, Wood R, Wilkinson RJ. Changing concepts of ‘latent tuberculosis infection’ in patients living with HIV infection. Clin Dev Immunol. 2011;2011:980–594. doi: 10.1155/2011/980594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aichelburg MC, Reiberger T, Breitenecker F, Mandorfer M, Makristathis A, Rieger A. Reversion and conversion of interferon-gamma release assay results in HIV-1-infected individuals. J Infect Dis. 2014;209:729–733. doi: 10.1093/infdis/jit418. [DOI] [PubMed] [Google Scholar]
- 3.French AL, Evans CT, Anastos K, et al. Incidence of tuberculin skin test conversion among HIV-infected and -uninfected women: results of a 6-year study. J Acquir Immune Defic Syndr. 2006;42:592–596. doi: 10.1097/01.qai.0000229995.25493.8b. [DOI] [PubMed] [Google Scholar]
- 4.Kirenga BJ, Worodria W, Massinga-Loembe M, et al. Tuberculin skin test conversion among HIV patients on antiretroviral therapy in Uganda. Int J Tuberc Lung Dis. 2013;17:336–341. doi: 10.5588/ijtld.12.0298. [DOI] [PubMed] [Google Scholar]
- 5.Whalen CC, Zalwango S, Chiunda A, et al. Secondary attack rate of tuberculosis in urban households in Kampala, Uganda. PLOS ONE. 2011;6:e16137. doi: 10.1371/journal.pone.0016137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fisk TL, Hon HM, Lennox JL, Fordham von Reyn C, Horsburgh CR., Jr Detection of latent tuberculosis among HIV-infected patients after initiation of highly active antiretroviral therapy. AIDS. 2003;17:1102–1104. doi: 10.1097/00002030-200305020-00027. [DOI] [PubMed] [Google Scholar]
- 7.Mathad JS, Bhosale R, Sangar V, et al. Pregnancy differentially impacts performance of latent tuberculosis diagnostics in a high-burden setting. PLOS ONE. 2014;9:e92308. doi: 10.1371/journal.pone.0092308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lighter-Fisher J, Surette AM. Performance of an interferon-gamma release assay to diagnose latent tuberculosis infection during pregnancy. Obstet Gynecol. 2012;119:1088–1095. doi: 10.1097/AOG.0b013e3182546aff. [DOI] [PubMed] [Google Scholar]
- 9.Cattamanchi A, Smith R, Steingart KR, et al. Interferon-gamma release assays for the diagnosis of latent tuberculosis infection in HIV-infected individuals: a systematic review and meta-analysis. J Acquir Immune Defic Syndr. 2011;56:230–238. doi: 10.1097/QAI.0b013e31820b07ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathad JS, Gupta A. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis. 2012;55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zenner D, Kruijshaar ME, Andrews N, Abubakar I. Risk of tuberculosis in pregnancy: a national, primary care-based cohort and self-controlled case series study. Am J Respir Crit Care Med. 2012;185:779–784. doi: 10.1164/rccm.201106-1083OC. [DOI] [PubMed] [Google Scholar]
- 12.Singh N, Perfect JR. Immune reconstitution syndrome and exacerbation of infections after pregnancy. Clin Infect Dis. 2007;45:1192–1199. doi: 10.1086/522182. [DOI] [PubMed] [Google Scholar]
- 13.Jonnalagadda S, Lohman Payne B, Brown E, et al. Latent tuberculosis detection by interferon gamma release assay during pregnancy predicts active tuberculosis and mortality in human immunodeficiency virus type 1-infected women and their children. J Infect Dis. 2010;202:1826–1835. doi: 10.1086/657411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walson JL, Brown ER, Otieno PA, et al. Morbidity among HIV-1-infected mothers in Kenya: prevalence and correlates of illness during 2-year postpartum follow-up. J Acquir Immune Defic Syndr. 2007;46:208–215. doi: 10.1097/QAI.0b013e318141fcc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill PC, Brookes RH, Fox A, et al. Longitudinal assessment of an ELISPOT test for Mycobacterium tuberculosis infection. PLOS MED. 2007;4:e192. doi: 10.1371/journal.pmed.0040192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai M, Joshi R, Dogra S, et al. T-cell assay conversions and reversions among household contacts of tuberculosis patients in rural India. Int J Tuberc Lung Dis. 2009;13:84–92. [PMC free article] [PubMed] [Google Scholar]
- 17.Shah M, Kasambira TS, Adrian PV, Madhi SA, Martinson NA, Dorman SE. Longitudinal analysis of QuantiFERON-TB Gold In-Tube in children with adult household tuberculosis contact in South Africa: a prospective cohort study. PLOS ONE. 2011;6:e26787. doi: 10.1371/journal.pone.0026787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews JR, Hatherill M, Mahomed H, et al. The dynamics of QuantiFERON®-TB Gold In-Tube conversion and reversion in a cohort of South African adolescents. Am J Respir Crit Care Med. 2015;191:584–591. doi: 10.1164/rccm.201409-1704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houben RM, Crampin AC, Ndhlovu R, et al. Human immunodeficiency virus associated tuberculosis more often due to recent infection than reactivation of latent infection. Int J Tuberc Lung Dis. 2011;15:24–31. [PubMed] [Google Scholar]
- 20.Oramasionwu GE, Heilig CM, Udomsantisuk N, et al. The utility of stool cultures for diagnosing tuberculosis in people living with the human immunodeficiency virus. Int J Tuberc Lung Dis. 2013;17:1023–1028. doi: 10.5588/ijtld.13.0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velayati AA, Farnia P, Mozafari M, et al. Identification and genotyping of Mycobacterium tuberculosis isolated from water and soil samples of a metropolitan city. Chest. 2015;147:1094–1102. doi: 10.1378/chest.14-0960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson KR, Braden CR, Cairns KL, et al. Transmission of Mycobacterium tuberculosis from medical waste. JAMA. 2000;284:1683–1688. doi: 10.1001/jama.284.13.1683. [DOI] [PubMed] [Google Scholar]
- 23.Hutton MD, Stead WW, Cauthen GM, Bloch AB, Ewing WM. Nosocomial transmission of tuberculosis associated with a draining abscess. J Infect Dis. 1990;161:286–295. doi: 10.1093/infdis/161.2.286. [DOI] [PubMed] [Google Scholar]
- 24.Johnson DL, Mead KR, Lynch RA, Hirst DV. Lifting the lid on toilet plume aerosol: a literature review with suggestions for future research. Am J Infect Control. 2013;41:254–258. doi: 10.1016/j.ajic.2012.04.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu DC, Kerr SJ, Thongpaeng P, et al. Incomplete restoration of Mycobacterium tuberculosis-specific-CD4 T-cell responses despite antiretroviral therapy. J Infect. 2014;68:344–354. doi: 10.1016/j.jinf.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 26.Lawn SD, Myer L, Bekker LG, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20:1605–1612. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 27.Mathad JS, Bhosale R, Kanade S, et al. Effect of HIV on latent TB screening of pregnant women in Pune, India; Conference on Retroviruses and Opportunistic Infections (CROI) 2014; Boston, MA, USA. 3–6 March 2014; Abstract #818. http://www.croiconference.org/sites/default/files/abstracts/818.pdf Accessed April 2015. [Google Scholar]
- 28.Leidl L, Mayanja-Kizza H, Sotgiu G, et al. Relationship of immunodiagnostic assays for tuberculosis and numbers of circulating CD4+ T-cells in HIV infection. Eur Respir J. 2010;35:619–626. doi: 10.1183/09031936.00045509. [DOI] [PubMed] [Google Scholar]
- 29.Talati NJ, Seybold U, Humphrey B, et al. Poor concordance between interferon-gamma release assays and tuberculin skin tests in diagnosis of latent tuberculosis infection among HIV-infected individuals. BMC Infect Dis. 2009;9:15. doi: 10.1186/1471-2334-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Richeldi L, Losi M, D’Amico R, et al. Performance of tests for latent tuberculosis in different groups of immunocompromised patients. Chest. 2009;136:198–204. doi: 10.1378/chest.08-2575. [DOI] [PubMed] [Google Scholar]
- 31.Mandalakas AM, Hesseling AC, Chegou NN, et al. High level of discordant IGRA results in HIV-infected adults and children. Int J Tuberc Lung Dis. 2008;12:417–423. [PubMed] [Google Scholar]
- 32.Meier T, Eulenbruch HP, Wrighton-Smith P, Enders G, Regnath T. Sensitivity of a new commercial enzyme-linked immunospot assay (T.SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis. 2005;24:529–536. doi: 10.1007/s10096-005-1377-8. [DOI] [PubMed] [Google Scholar]
- 33.Leyten EM, Arend SM, Prins C, Cobelens FG, Ottenhoff TH, van Dissel JT. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short and prolonged in vitro incubation. Clin Vaccine Immunol. 2007;14:880–885. doi: 10.1128/CVI.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zwerling A, van den Hof S, Scholten J, Cobelens F, Menzies D, Pai M. Interferon-gamma release assays for tuberculosis screening of healthcare workers: a systematic review. Thorax. 2012;67:62–70. doi: 10.1136/thx.2010.143180. [DOI] [PubMed] [Google Scholar]
- 35.Belay M, Legesse M, Dagne D, et al. QuantiFERON®-TB Gold In-Tube test conversions and reversions among tuberculosis patients and their household contacts in Addis Ababa: a one year follow-up study. BMC Infect Dis. 2014;14:654. doi: 10.1186/s12879-014-0654-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pai M, Joshi R, Dogra S, et al. Serial testing of health care workers for tuberculosis using interferon-gamma assay. Am J Respir Crit Care Med. 2006;174:349–355. doi: 10.1164/rccm.200604-472OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: WHO; 2011. http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. Accessed April 2015. [Google Scholar]
- 38.Taylor AW, Mosimaneotsile B, Mathebula U, et al. Pregnancy outcomes in HIV-infected women receiving long-term isoniazid prophylaxis for tuberculosis and antiretroviral therapy. Infect Dis Obstet Gynecol. 2013;2013:195, 637. doi: 10.1155/2013/195637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tiam A, Machekano R, Gounder CR, et al. Preventing tuberculosis among HIV-infected pregnant women in Lesotho: the case for rolling out active case finding and isoniazid preventive therapy. J Acquir Immune Defic Syndr. 2014;67:e5–e11. doi: 10.1097/QAI.0000000000000209. [DOI] [PubMed] [Google Scholar]
- 40.Kowada A. Cost effectiveness of interferon-gamma release assay for TB screening of HIV positive pregnant women in low TB incidence countries. J Infect. 2014;68:32–42. doi: 10.1016/j.jinf.2013.08.009. [DOI] [PubMed] [Google Scholar]


