Summary
Canonical non-homologous end joining (c-NHEJ) repairs DNA double-strand breaks (DSBs) in G1 cells with biphasic kinetics. We show that DSBs repaired with slow kinetics, including those localizing to heterochromatic regions or harboring additional lesions at the DSB site, undergo resection prior to repair by c-NHEJ and not alt-NHEJ. Resection-dependent c-NHEJ represents an inducible process during which Plk3 phosphorylates CtIP, mediating its interaction with Brca1 and promoting the initiation of resection. Mre11 exonuclease, EXD2, and Exo1 execute resection, and Artemis endonuclease functions to complete the process. If resection does not commence, then repair can ensue by c-NHEJ, but when executed, Artemis is essential to complete resection-dependent c-NHEJ. Additionally, Mre11 endonuclease activity is dispensable for resection in G1. Thus, resection in G1 differs from the process in G2 that leads to homologous recombination. Resection-dependent c-NHEJ significantly contributes to the formation of deletions and translocations in G1, which represent important initiating events in carcinogenesis.
Keywords: DNA double-strand breaks, non-homologous end joining, resection, nucleases
Graphical Abstract
Highlights
-
•
DSBs in G1 are repaired by resection-independent or resection-dependent c-NHEJ
-
•
Resection-dependent c-NHEJ is induced by Plk3-dependent CtIP interaction with Brca1
-
•
G1 resection is executed from DSB ends by Mre11 exo, EXD2, and Exo1, but not Mre11 endo
-
•
Artemis completes resection in G1, forming deletions and, potentially, translocations
Biehs et al. identify and characterize a resection-dependent canonical non-homologous end joining process that operates in G1 phase cells. Resection in G2 leads to homologous recombination. Resection in G1 is distinct from that because the same toolbox of resection factors is orchestrated in a way that is compatible with an end-joining process.
Introduction
DNA double-strand breaks (DSBs) are repaired by two major pathways: canonical non-homologous end joining (c-NHEJ) or homologous recombination (HR) (Jackson and Bartek, 2009, Lukas and Lukas, 2013). c-NHEJ rejoins DSBs using little or no sequence homology and functions throughout the cell cycle. Key players are the Ku70/80 heterodimer and the DNA-dependent protein kinase catalytic subunit (DNA-PKcs), which is recruited to DNA-bound Ku70/80, generating the DNA-PK holoenzyme (Jette and Lees-Miller, 2015). DNA ligase IV (Lig4), XRCC4, XRCC4-like factor (XLF)/Cernunnos, and parolog of XRCC4 and XLF (PAXX) operate during later stages of c-NHEJ (Ochi et al., 2015). HR is initiated by CtBP-interacting protein (CtIP)-dependent resection, creating 3′ single-stranded DNA (ssDNA) overhangs at DSB ends (Huertas and Jackson, 2009, Sartori et al., 2007). HR exerts its major role at stalled or collapsed replication forks in S phase but also contributes to DSB repair during G2 (Moynahan and Jasin, 2010).
DSB repair can occur by alternative NHEJ mechanisms, termed alt-NHEJ (Nussenzweig and Nussenzweig, 2007). alt-NHEJ involves CtIP-dependent resection, poly-(ADP-ribose)-polymerases (PARPs), Lig1 or 3 instead of Lig4, and XRCC1 (Lee-Theilen et al., 2011, Wang et al., 2005). CtIP-dependent end joining occurs in G1 cells (Yun and Hiom, 2009) and contributes to translocation formation at restriction enzyme- or ionizing radiation (IR)-induced DSBs (Zhang and Jasin, 2011, Barton et al., 2014). Polo-like kinase 3 (Plk3) phosphorylates CtIP in G1, promoting limited end resection and enhancing translocations (Barton et al., 2014). Thus, various lines of evidence demonstrate that end resection occurs in G1, although it is unclear whether the pathway(s) utilizing resected ends are restricted to alt-NHEJ or include a c-NHEJ process.
IR-induced DSBs are repaired with biphasic kinetics involving a fast and a slow process (DiBiase et al., 2000, Riballo et al., 2004). In G1, cells defective in c-NHEJ fail to repair DSBs by either process (Riballo et al., 2004). In G2, c-NHEJ deficiency affects only the fast process, whereas the slow process represents HR (Beucher et al., 2009). The slow processes in G1 and G2 repair heterochromatic DSBs (about 15%–20% of all DSBs) (Goodarzi et al., 2008, Riballo et al., 2004) and require the Artemis nuclease, suggesting involvement of end-processing steps (Riballo et al., 2004).
Loss of downstream HR factors (e.g., Brca1, Brca2, Rad51, and Rad54) diminishes HR, causing unrepaired DSBs in the slow component in G2 (Beucher et al., 2009, Shibata et al., 2011). Loss of CtIP also abolishes HR in G2 because resection is not initiated. However, it does not result in unrepaired DSBs because c-NHEJ, which is normally restricted to the fast component in G2, can repair unresected breaks (Shibata et al., 2011). Thus, a repair factor may function in the repair process even though its loss does not confer a repair defect (e.g., CtIP in G2). This is important because CtIP depletion does not cause a repair defect in G1 but strongly diminishes chromosome translocations (Barton et al., 2014). Therefore, we considered the possibility that the slow process in G1 represents a resection-dependent process (as in G2) and that preventing resection by CtIP depletion causes a pathway switch from resection-dependent to resection-independent c-NHEJ, explaining the lack of a repair defect and reduced chromosome translocations (making the assumption that resected DSBs are more prone to undergo mis-rejoining than unresected DSBs).
We clarify this provocative possibility and verify that the slow repair component in G1 represents a resection-dependent rejoining process. The process utilizes c-NHEJ and not alt-NHEJ factors. Thus, the two main repair processes in G1 human cells are resection-independent and resection-dependent c-NHEJ. Resection-dependent c-NHEJ avidly forms IR-induced translocations, highlighting its physiological relevance. We then investigated the factors regulating resection-dependent c-NHEJ and identified striking differences to the resection process in G2. First, resection-dependent c-NHEJ is initiated after DSB induction by Plk3, which phosphorylates CtIP at Ser327 to mediate CtIP-Brca1 interaction. Thus, in contrast to G2, where cyclin-dependent kinases (CDKs) constitutively phosphorylate CtIP, the initiation of resection in G1 is inducible. Following initiation by Plk3/CtIP/Brca1, Mre11 exonuclease, EXD2, and Exo1 execute resection, and Artemis completes the process. Mre11 endonuclease activity is dispensable for resection in G1, suggesting that resection commences from the DSB end and not internally as in G2 (Shibata et al., 2014). Our findings reveal differences in resection between G1 and G2, enhancing our understanding of DSB repair in human cells and facilitating the design of approaches to reactivate HR in G1 for gene targeting (Orthwein et al., 2015).
Results
Slow Artemis-Dependent c-NHEJ Promotes Translocation Formation in G1
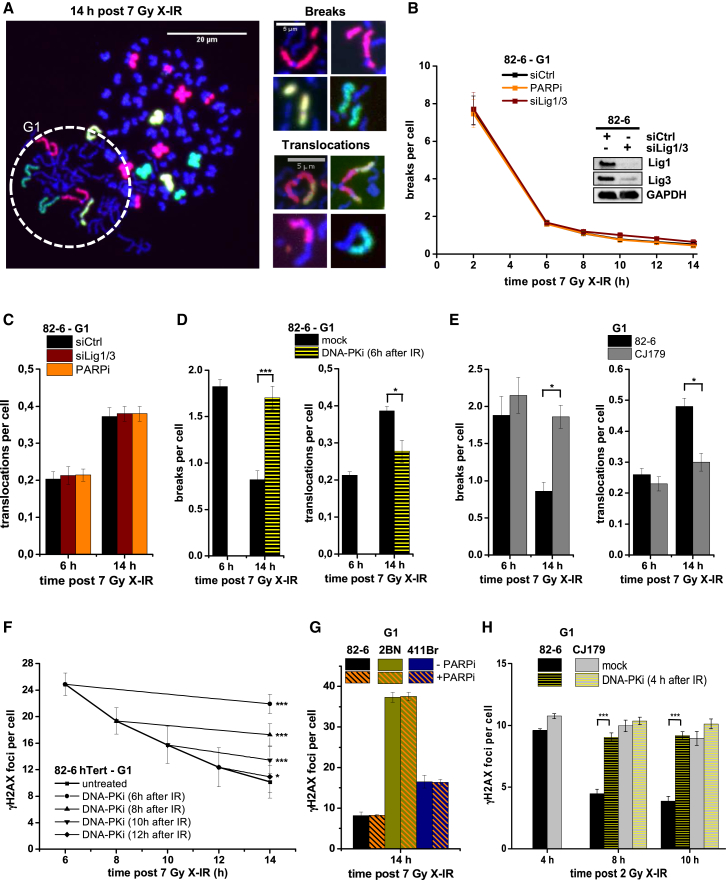
We enumerated translocations forming in G1 human fibroblasts by premature chromosome condensation (PCC) combined with fluorescence in situ hybridization (FISH). We harvested asynchronous 82-6 fibroblasts at defined times after X-ray IR (X-IR) and fused them with mitotic HeLa cells to promote PCC of fibroblast chromosomes. G1 PCC spreads were distinct from G2 spreads and from mitotic HeLa cells by their one-chromatid morphology and the G1-specific marker CDT1 (Nishitani et al., 2001). PCC spreads from S phase cells displayed massive chromosome breakage and were also excluded from the analysis (Gotoh and Durante, 2006). Nocodazole was added to prevent G2 cells from entering G1, and control experiments confirmed that cells were irradiated and maintained in G1 (Figures S1 and S2A–S2C). We stained chromosomes 1, 2, and 4 by FISH and identified chromosome breaks as stained fragments and translocations by the appearance of chromosomes with color junctions (Figure 1A). Chromosome breaks were rejoined with biphasic kinetics (Figure 1B), consistent with DSB rejoining assessed by γH2AX analysis or pulsed field gel electrophoresis (Riballo et al., 2004). Some translocations formed within 6 hr after X-IR, when most chromosome breaks were repaired. However, between 6 and 14 hr after X-IR, translocations doubled, but few breaks were repaired, demonstrating that the slow process is particularly error-prone (Figure 1C; Barton et al., 2014).
Figure 1.
A Slow Artemis-Dependent c-NHEJ Process Promotes Translocation Formation in G1
(A) Left: FISH-stained G1 PCC spread from 82-6 control cells (dashed circle) fused with mitotic HeLa cells. Right: chromosome breaks and translocations in G1 PCC spreads.
(B and C) Chromosome breaks (B) and translocations (C) in G1 82-6 cells treated with siLig1/3 or PARP inhibitor (PARPi). Data are mean ± SEM.
(D) Chromosome breaks and translocations in G1 82-6 cells treated with DNA-PK inhibitor (DNA-PKi) 6 hr after X-IR. Data are mean ± SEM.
(E) Chromosome breaks and translocations in G1 82-6 and Artemis-deficient CJ179 cells. Data are mean ± SEM.
(F) γH2AX foci in G1 82-6 cells treated with DNA-PKi at various times after X-IR. Data are mean ± SEM.
(G) γH2AX foci in G1 82-6, XLF-deficient 2BN and Lig4-mutated 411Br cells treated with PARPi. Data are mean ± SEM.
(H) γH2AX foci in G1 82-6 and CJ179 cells treated with DNA-PKi 4 hr after X-IR. Data are mean ± SEM.
See also Figures S1 and S2.
To investigate the process causing translocations, we treated cells with a PARP inhibitor or small interfering RNA (siRNA) Lig1/3 (siLig1/3) but found no effect on chromosome break repair or translocation formation (Figures 1B and 1C), demonstrating that X-IR-induced translocations in G1 human cells do not form by alt-NHEJ. Next, we added a DNA-PK inhibitor 6 hr after X-IR (when the fast repair component is completed) and observed elevated chromosome breaks and diminished translocations 14 hr after X-IR compared with untreated cells (Figure 1D). Indeed, translocation levels after DNA-PK inhibitor addition at 6 hr are only slightly increased compared with translocations arising in the first 6 hr when DNA-PKcs was active. This suggests that a slow c-NHEJ process involving DNA-PKcs substantially contributes to X-IR-induced chromosome translocation formation.
Because Artemis is essential for slow DSB repair, we next carried out combined PCC/FISH analysis in Artemis-deficient fibroblasts. We observed elevated unrepaired breaks and diminished translocations 14 but not 6 hr after X-IR (Figure 1E). Thus, Artemis deficiency specifically affects the slow component of translocation formation, confirming that such translocations arise from the slow DSB repair process.
To confirm that the slow Artemis-dependent translocations arise from c-NHEJ, we employed a semi-automated microscopic approach that assesses repair kinetics by γH2AX focus analysis in defined cell-cycle phases (Figure S2D). First, we investigated DNA-PK involvement during the slow repair process in G1. Using 7 Gy, the same dose used for translocation measurements, we added the DNA-PK inhibitor 6, 8, 10, and 12 hr after X-IR and analyzed γH2AX foci at 14 hr in G1 cells. Inhibitor addition at all time points strongly impaired DSB repair, consistent with the notion that DNA-PK is bound to break ends throughout the slow repair process (Figure 1F). Furthermore, PARP inhibition did not affect γH2AX focus levels 14 hr after X-IR in control, XLF-deficient or Lig4-mutated fibroblasts (Figure 1G), although it increased focus numbers in HeLa cells treated with siKu80 (Figure S2E).
To confirm that DNA-PK and Artemis operate in the same slow repair process, we added the DNA-PK inhibitor to G1 phase Artemis-deficient and control fibroblasts. Using 2 Gy, we added the inhibitor 4 hr after X-IR (when the fast DSB repair process had completed) and scored γH2AX foci 8 and 10 hr after X-IR. Of note, DNA-PK inhibition did not affect the focus level of Artemis-deficient cells but increased focus numbers in control cells to that of Artemis-deficient cells, demonstrating that DNA-PK and Artemis function during slow DSB repair (Figure 1H). Collectively, we show that the slow Artemis-dependent component of translocation formation and DSB repair represents a c-NHEJ process and that human cells do not employ alt-NHEJ as long as Ku is present.
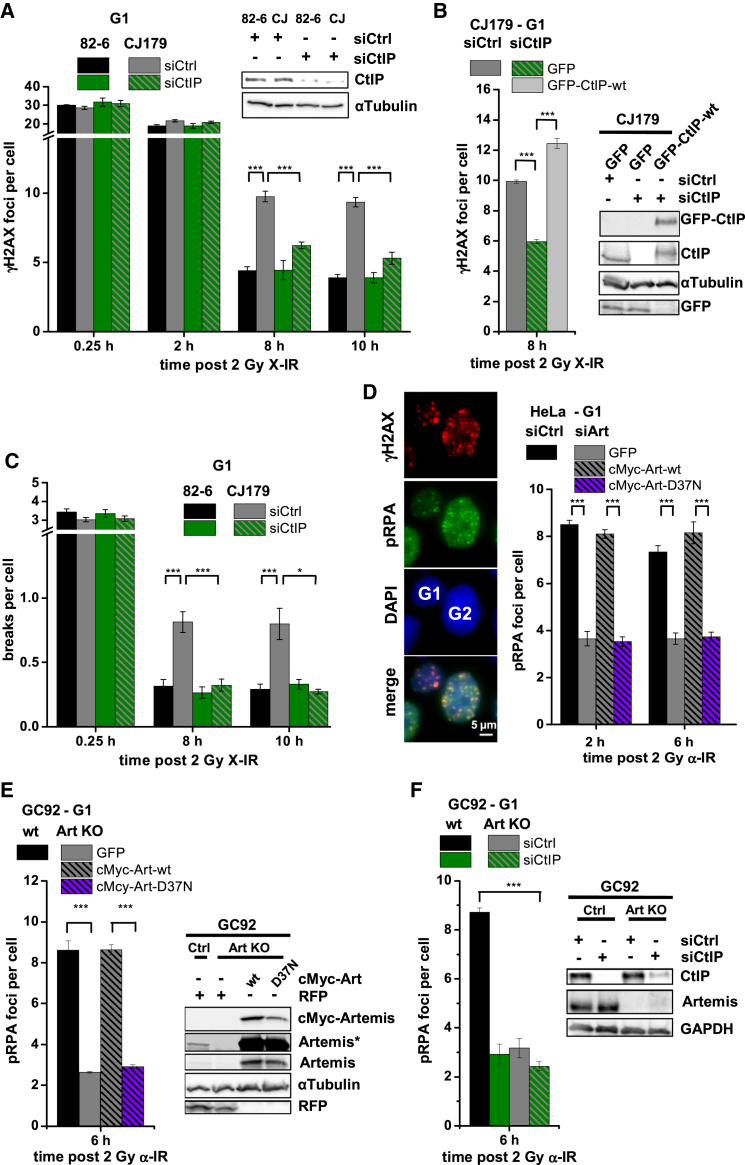
Artemis and CtIP Function during Slow DSB Repair in G1
The generation of X-IR-induced translocations in human cells by a c-NHEJ process rather than alt-NHEJ is consistent with results employing designer nucleases (Ghezraoui et al., 2014). We observed previously that CtIP contributes to the slow component of X-IR-induced translocations in G1 human cells, although the underlying repair pathway was not examined (Barton et al., 2014). This raised the possibility that the slow c-NHEJ process in G1 involves Artemis and CtIP. Therefore, we measured the kinetics of DSB repair in siCtIP-depleted G1 fibroblasts. In wild-type (WT) cells, we observed similar γH2AX focus numbers at all times, analyzed with or without siCtIP (Figure 2A; Barton et al., 2014). In G2, siCtIP abolishes resection and HR but does not cause a repair defect because c-NHEJ can be used if resection is not initiated. Consequently, co-depletion of CtIP/Brca1 or CtIP/Brca2 relieves the repair defect caused by loss of Brca1 or Brca2, respectively (Kakarougkas et al., 2013, Shibata et al., 2011).
Figure 2.
Artemis and CtIP Function during Slow DSB Repair and Promote Resection in G1
(A) γH2AX foci in G1 82-6 and CJ179 cells treated with siCtIP. Data are mean ± SEM.
(B) γH2AX foci in CJ179 cells treated with siCtIP. Cells were transfected with GFP or GFP-CtIP-WT constructs, and γH2AX foci were analyzed in GFP+ G1 cells. Data are mean ± SEM.
(C) Chromosome breaks in G1 82-6 and CJ179 cells treated with siCtIP. Data are mean ± SEM.
(D) pRPA foci in G1 HeLa cells treated with siArtemis. Cells were transfected with GFP or cMyc-Artemis plasmids, and foci were analyzed in GFP/cMyc+ G1 cells. Data are mean ± SEM.
(E) pRPA foci in G1 GC92 WT and CRISPR/Cas9-generated Artemis KO cells. Cells were transfected with GFP or cMyc-Artemis constructs, and pRPA foci were analyzed in GFP/cMyc+ G1 cells. Data are mean ± SEM.
(F) pRPA foci in G1 GC92 WT and Artemis KO cells treated with siCtIP. Data are mean ± SEM.
See also Figure S3.
Thus, we considered that siCtIP might similarly cause a switch from a resection-dependent to a resection-independent process in G1. Because Artemis is essential for the slow repair process in G1, as are Brca1 and Brca2 in G2, we examined whether siCtIP affects repair in Artemis mutants in G1. As expected, we observed the same level of γH2AX foci in Artemis-deficient and control fibroblasts at 15 min and 2 hr but higher levels in Artemis-deficient fibroblasts 8 and 10 hr after X-IR (Figure 2A). Strikingly, siCtIP rescued the repair defect of G1 Artemis mutants (Figure 2A), and overexpression of siRNA-resistant GFP-tagged CtIP in siCtIP-treated Artemis mutants restored the repair defect (Figure 2B). A similar rescue by siCtIP was observed in another cell system (Figure S3A). We conclude that Artemis and CtIP function during slow DSB repair in G1. The finding that Artemis but not CtIP deficiency confers a repair defect strongly suggests that Artemis functions downstream of CtIP, reflective of the situation in G2, where several factors (e.g., Brca1 and Brca2) function downstream of CtIP to promote HR and that their combined depletion with CtIP rescues their repair defects. This was confirmed by investigating chromosome breaks in G1 cells using PCC/FISH. We observed similar initial breakage levels for all conditions, a pronounced repair defect in Artemis-deficient fibroblasts 8 and 10 hr after X-IR, and rescue by siCtIP (Figure 2C). In conclusion, Artemis and CtIP function during slow DSB repair, which confirms our translocation measurements and shows that this DSB repair process causes slow translocation formation.
Artemis and CtIP Promote Resection in G1
To gain direct evidence for resection during slow DSB repair in G1, we examined phospho-replication protein A (pRPA) foci by immunofluorescence using our semi-automated microscopic approach (Figure S2D). Because pRPA foci are difficult to detect after X-IR in G1, we exploited α particle IR (α-IR), which induces multiple damages in close proximity, creating complex DSBs. DSB end complexity impedes NHEJ and slows DSB repair, which promotes resection, pRPA focus formation, and HR usage in G2 (Barton et al., 2014, Shibata et al., 2011).
Indeed, pRPA foci are readily observed in G1 2 and 6 hr after α-IR and require CtIP (Barton et al., 2014). siArtemis reduced pRPA focus numbers (Figure 2D), demonstrating that Artemis and CtIP promote resection in G1. We transfected Artemis-depleted cells with siRNA-resistant cMyc-tagged Artemis constructs and enumerated pRPA foci in cMyc+ G1 cells. WT but not nuclease-deficient Artemis restored the resection defect conferred by siArtemis (Figure 2D). We also generated an Artemis knockout (KO) cell line by CRISPR/Cas9 technology and observed fewer pRPA foci in G1 Artemis KO cells than in control cells. The diminished focus level was restored by WT but not nuclease-defective Artemis (Figure 2E). The resection defect in Artemis KO cells was similar to that of siCtIP cells, and siCtIP in Artemis KO cells caused no further defect (Figure 2F). Finally, assessment of ssDNA in G1 cells by enumerating bromodeoxyuridine (BrdU) foci confirmed that Artemis is required for resection (Figure S3B). To confirm that resection after α-IR represents the same Artemis/CtIP-dependent slow c-NHEJ pathway uncovered in the Artemis rescue experiments, we examined γH2AX foci after α-IR in G1. We observed delayed repair kinetics compared with X-IR and a requirement for Artemis and CtIP (Figure S3C; Barton et al., 2014). Moreover, DNA-PK but not PARP inhibition conferred a repair defect (Figure S3C). Given the slow kinetics, Artemis requirement, and PARP independence, the repair of α-IR-induced DSBs appears to represent the same c-NHEJ pathway that repairs ∼15%–20% of DSBs after X-rays.
We also measured pRPA foci after 20-Gy X-rays, a dose giving similar focus numbers as 2-Gy α-IR. siArtemis and siCtIP reduced pRPA levels as for α-IR (Figure S3D). We then examined whether Ku was retained at resected DSBs by co-staining against pRPA and Ku80. Strikingly, although the samples showed significant Ku80 background staining, nearly every G1 pRPA focus co-localized with a Ku80 focus (Figure S3E). As a control, we co-stained Ku80 and Rad51 in G2 cells using the same conditions (20 Gy, 4 hr) plus a lower dose at a later time point (4 Gy, 8 hr). We rarely observed co-localization of Ku80 and Rad51, demonstrating antibody specificity (Figure S3F). Importantly, a recent paper showed that Ku is removed from resected DSBs in G2 concomitant with Rad51 loading (Chanut et al., 2016). We conclude that Artemis nuclease, together with CtIP, promotes DSB resection in G1, although the extent of resection is more limited than in G2 because detecting pRPA requires high doses or complex DSBs. We further propose that Ku remains bound during resection in G1.
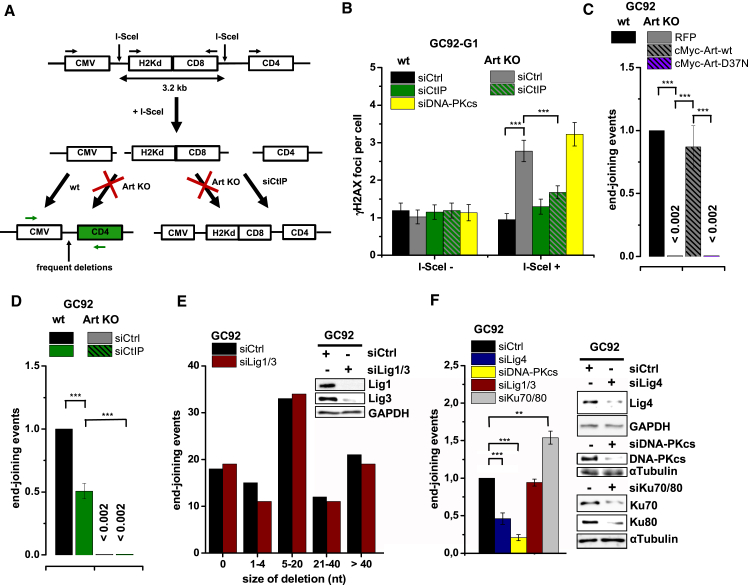
Molecular Characterization of G1 Resection
To molecularly characterize the Artemis- and CtIP-dependent resection process, we employed a reporter assay containing two I-SceI restriction sites located 3.2 kilobase pairs (kbp) apart. Joining of the distant DSB ends causing loss of the intervening fragment was monitored (Figure 3A). The joining events arise in G1 (Barton et al., 2014) and require CtIP (Rass et al., 2009), suggesting that they necessitate some level of resection. Thus, we examined whether this assay selectively monitors the resection-dependent slow repair process. We first enumerated γH2AX foci that arise following I-SceI transfection. Control cells showed no significant focus induction over background, whereas siDNA-PKcs or usage of Artemis KO cells increased focus numbers (Figure 3B), suggesting that repair of these I-SceI-induced DSBs requires Artemis and DNA-PKcs. Notably, siCtIP had no effect in control cells but reduced the elevated focus numbers observed in Artemis KO cells (Figure 3B). This recapitulates our findings after X-IR, suggesting that this assay monitors the slow DSB repair process. Next, we used the Artemis KO cells containing the reporter and observed a complete reduction of end joining events involving loss of the 3.2-kbp fragment, which was restored by WT but not nuclease-deficient Artemis (Figure 3C). siCtIP reduced end joining events in control but not in Artemis KO cells (Figure 3D). Collectively, these findings show that the diminished end joining events in Artemis KO cells arise because of unrepaired DSBs, whereas siCtIP reduces end joining involving loss of the intervening fragment at the expense of events that escape detection in the assay (Figure 3A). Sequence analysis of the repair junctions in control cells revealed that loss of the intervening fragment is often associated with additional deletions (consistent with the notion that this assay monitors resection-dependent end joining) and frequently involves micro-homology usage (Figure 3E; Table S1).
Figure 3.
Molecular Characterization of G1 Resection
(A) Schematic of the NHEJ reporter assay. The repair of two I-SceI-induced DSBs can result in loss of the intervening fragment, which is detected by a CD4+ signal (Rass et al., 2009). CD4+ clones were amplified by PCR (green arrows) across the repair site and sequenced. Repair of the two DSBs can also occur without loss of the intervening fragment, which escapes detection.
(B) γH2AX foci in GC92 WT and Artemis KO cells treated with siDNA-PKcs or siCtIP. Cells were transfected with I-SceI, and foci were scored in I-SceI+ and I-SceI− cells (identified by immunofluorescence [IF] against I-SceI). Data are mean ± SEM.
(C) End joining events in GC92 WT and Artemis KO cells containing the NHEJ reporter substrate. Cells were transfected with RFP or cMyc-Artemis constructs. Events were quantified by the fraction of CD4+ and RFP/cMyc+cells relative to all RFP/cMyc+cells, and results were normalized to WT cells. Data are mean ± SEM.
(D) End joining events in GC92 WT and Artemis KO cells treated with siCtIP. Data are mean ± SEM.
(E) Distribution of deletion sizes obtained from the sequence analysis of GC92 WT and siLig1/3-treated cells. nt, nucleotide.
(F) End joining events in GC92 cells treated with siKu70/80, siLig4, siLig1/3, or siDNA-PKcs. Data are mean ± SEM.
See also Table S1.
We also investigated the role of alt-NHEJ and c-NHEJ in this reporter assay. Depletion of Ku increased the frequency of events (Figure 3F), consistent with the observation that alt-NHEJ can effect rejoining without Ku (Guirouilh-Barbat et al., 2004). In contrast, siDNA-PKcs and siLig4 substantially reduced end joining, whereas siLig1/3 had no significant effect on rejoining frequency, deletion size, or micro-homology usage (Figures 3E and 3F; Table S1). These data demonstrate that this reporter assay monitors a c-NHEJ process and that alt-NHEJ has no significant role in Ku-proficient cells. Thus, the reporter assay confirms the results obtained from the analysis of DSB repair pathway usage after IR.
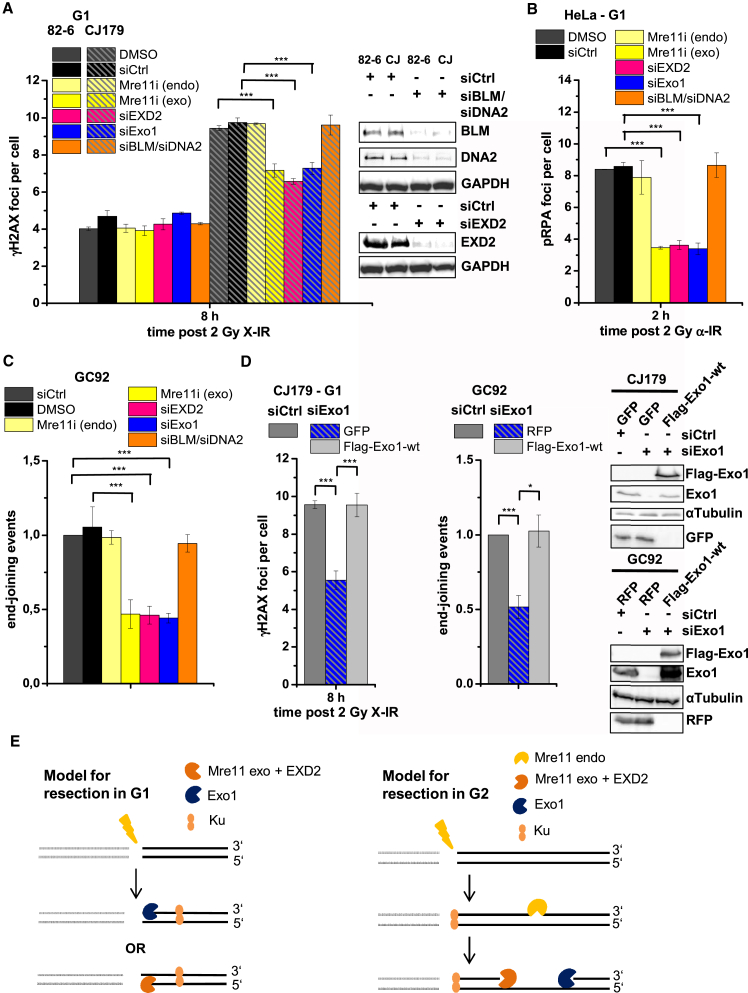
Similar and Distinct Nuclease Requirements for Resection in G1 versus G2
Having established that Artemis and CtIP promote a resection-dependent slow NHEJ process in G1, we examined whether resection proceeds similar to that in G2. First, we asked which additional nucleases execute resection in G1 and investigated Mre11, EXD2, Exo1, and Bloom syndrome mutated protein (BLM)/DNA2. We applied the three approaches described in Figures 2 and 3 to monitor resection-dependent slow NHEJ, assessing rescue of the Artemis repair defect in G1, pRPA focus formation in G1, and G1-specific end joining events in the reporter assay. Because Mre11 is required to activate ataxia telangiectasia mutated (ATM), its loss causes a repair defect in this process (Riballo et al., 2004), precluding analysis by siRNA. We therefore inhibited Mre11 nuclease activities by small-molecule inhibitors that selectively target its endo- or exonuclease activities (Shibata et al., 2014) without affecting ATM activation (Figure S4A). Inhibition of Mre11’s endonuclease activity did not affect the γH2AX focus or chromosome break level of Artemis-deficient cells (Figure 4A; Figure S4B), pRPA levels (Figure 4B; Figure S3D), and the frequency of end joining in the reporter assay (Figure 4C). In contrast, inhibition of Mre11’s exonuclease activity partially rescued the repair defect of Artemis-deficient cells (Figure 4A; Figure S4B), diminished the pRPA focus level (Figure 4B; Figure S3D), and reduced the frequency of end joining in the reporter assay (Figure 4C). These data suggest that Mre11 functions during resection in G1 as an exonuclease, whereas its endonuclease activity is dispensable. This differs from G2, where Mre11 endonuclease inhibition abolishes HR in a reporter assay (Figure S4C) and rescues the repair defect of Artemis-deficient cells (Figure S4D).
Figure 4.
Similar and Distinct Nuclease Requirements for Resection in G1 versus G2
(A–C) γH2AX foci in G1 82-6 and CJ179 cells (A), pRPA foci in G1 HeLa cells (B), and end-joining events in GC92 cells (C). Cells were treated with an Mre11 endo- or exonuclease inhibitor, siEXD2, siExo1, or siBLM/siDNA2. Data are mean ± SEM.
(D) γH2AX foci in G1 CJ179 and end joining events in GC92 cells. Cells were treated with siExo1 and transfected with GFP, RFP, or FLAG-Exo1-WT constructs, and GFP+, RFP+ or FLAG+ cells were analyzed. Data are mean ± SEM.
(E) Model for DSB end resection in G1 and G2. DNA-PKcs binding to Ku was omitted for clarity.
See also Figure S4.
Examining Exo1’s function, we obtained results nearly identical to Mre11 exonuclease inhibition. Specifically, siExo1 partially rescued the repair defect of Artemis-deficient cells (Figure 4A; Figure S4B), diminished the pRPA focus level (Figure 4B; Figure S3D), and reduced end joining in the reporter assay (Figure 4C). Expression of siRNA-resistant FLAG-tagged Exo1 restored the defect in Artemis-deficient cells treated with siExo1 and end joining in siExo1-treated cells in the reporter assay (Figure 4D). Additionally, siEXD2 had the same effect in our three assays as siExo1 or Mre11 exonuclease inhibition, whereas combined depletion of BLM and DNA2 was without effect (Figures 4A–4C). Collectively, these data show that the 5′-3′ exonuclease activity of Exo1 (Lee and Wilson, 1999) and the 3′-5′ exonuclease activities of Mre11 (Paull and Gellert, 1998) and EXD2 (Broderick et al., 2016) promote limited resection at slowly repairing DSBs in G1. Given that DNA-PKcs inhibition blocks the slow repair process and that Ku is required for DNA-PKcs binding, we propose that Ku70/80 remains bound to DSBs during resection, moving away from the break ends to expose DNA ends for nuclease access while limiting the extent of resected DNA (Figure 4E). This model is consistent with our analysis of Ku foci in G1 (Figure S3E). This suggests that resection in G1 is distinct from G2, where Mre11’s endonuclease activity is proposed to initiate resection internal to the break end, followed by resection toward and away from the end by Mre11 exonuclease and Exo1, respectively (Figure 4E; Shibata et al., 2014). Consistent with this model, loss of Mre11 exonuclease activity or Exo1 causes a repair defect in G2 because the incompletely resected DSBs cannot be repaired by HR or NHEJ (Figure S4D).
Brca1 and 53BP1 Together Promote Resection-Dependent Slow DSB Repair in G1
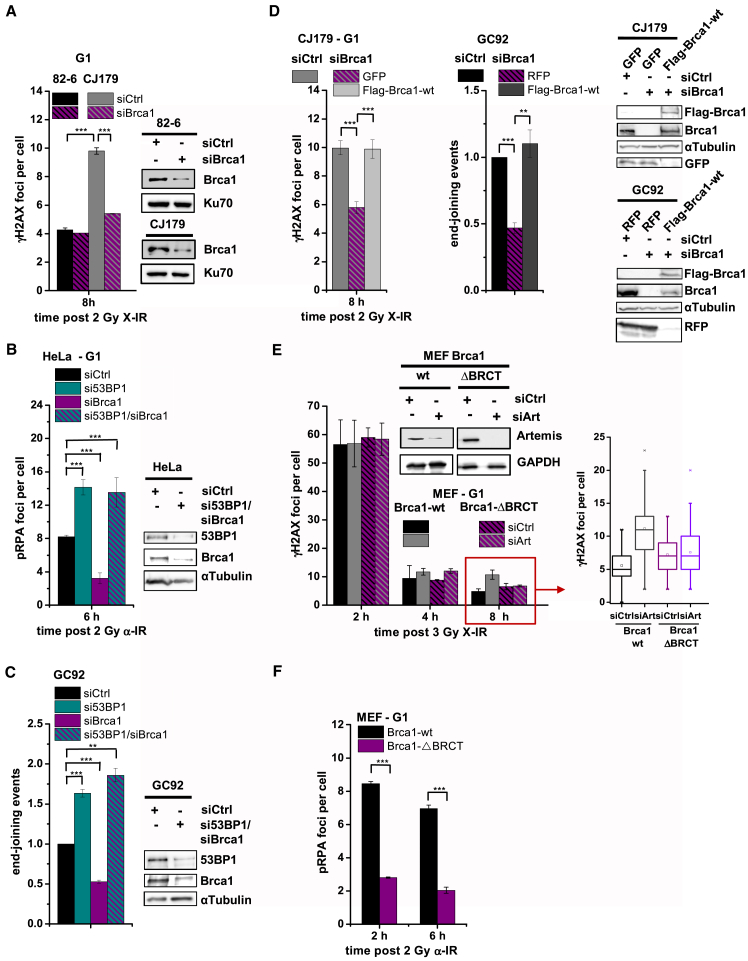
Because Brca1 promotes resection in G2, we investigated whether it is also required for resection in G1 by applying our three assays. Using human fibroblasts, we observed that siBrca1 did not cause a repair defect in control cells but substantially rescued the defect of Artemis-deficient cells (Figure 5A; Figure S4B). We also observed diminished pRPA foci after siBrca1 (Figure 5B; Figure S3D) and reduced end joining in the reporter assay (Figure 5C). Expression of siRNA-resistant FLAG-tagged Brca1 restored the repair defect in siBrca1-treated Artemis-deficient cells and end joining in siBrca1-treated cells in the reporter assay (Figure 5D). Because loss of 53BP1 relieves the repair defect of Brca1 mutants in G2, we asked whether si53BP1 affects resection following siBrca1. Of note, combined si53BP1 and siBrca1 treatment increased pRPA focus numbers (Figure 5B) and end joining in the reporter assay (Figure 5C) to the level conferred by si53BP1 alone. This shows that Brca1 functions during resection-dependent c-NHEJ by counteracting 53BP1, similar to its described function during HR. Interestingly, si53BP1 led to increased pRPA foci numbers (Figure 5B) and elevated end joining events in the reporter assay (Figure 5C) compared with control cells, which were reduced to control levels after expression of siRNA-resistant HA-tagged 53BP1 (Figure S5A). This suggests that resection in the absence of 53BP1 is less restricted than in control cells. Significantly, depletion of Lig1/3 in si53BP1-treated cells nearly completely abolished end joining events in the reporter assay (Figure S5B), arguing that the repair process in 53BP1-defective cells differs from the resection-dependent c-NHEJ pathway described here. This is reminiscent of the situation in G2, where loss of 53BP1 channels DSB repair from gene conversion to single-strand annealing (Ochs et al., 2016). Thus, 53BP1 promotes resection-dependent c-NHEJ by regulating the extent of resection.
Figure 5.
Brca1 and 53BP1 Together Promote Resection-Dependent Slow DSB Repair in G1
(A) γH2AX foci in G1 82-6 and CJ179 cells treated with siBrca1. Data are mean ± SEM.
(B) pRPA foci in G1 HeLa cells treated with si53BP1 and/or siBrca1. Data are mean ± SEM.
(C) End joining events in GC92 cells treated with si53BP1 and/or siBrca1. Data are mean ± SEM.
(D) γH2AX foci in G1 CJ179 and end joining events in GC92 cells. Cells were treated with siBrca1 and transfected with GFP, RFP or FLAG-Brca1-WT constructs, and GFP+, RFP+ or FLAG+ cells were analyzed. Data are mean ± SEM.
(E) γH2AX foci in G1 Brca1-WT and Brca1-ΔBRCT MEFs treated with siArtemis. Data are mean ± SEM.
(F) pRPA foci in G1 Brca1-WT and Brca1-ΔBRCT MEFs. Data are mean ± SEM.
See also Figure S5.
We next examined mouse embryonic fibroblasts (MEFs) carrying either Brca1-WT or Brca1-ΔBRCT, which lacks the interaction site with CtIP (Kakarougkas et al., 2013). siArtemis caused a repair defect in Brca1-WT but not Brca1-ΔBRCT MEFs (Figure 5E), which is rescued by siRNA-resistant cMyc-tagged Artemis (Figure S5C). We also observed diminished pRPA foci in Brca1-ΔBRCT compared with Brca1-WT MEFs (Figure 5F). To consolidate these functional studies, we measured Brca1 focus formation. We confirmed that Brca1 accumulation at DSBs after X-IR is visible but weaker in G1 compared with G2 (Figure S5D; Chapman et al., 2013, Escribano-Díaz et al., 2013, Feng et al., 2013). However, both G1 and G2 cells showed robust Brca1 accumulation at α-IR-induced DSBs (Figure S5E). Collectively, this suggests that Brca1 functions during resection-dependent slow DSB repair in G1 in a manner requiring its BRCA1 C-terminal (BRCT) domain. Furthermore, loss of Brca1 in G1 (unlike in G2) does not cause a DSB repair defect, demonstrating that it determines pathway choice in G1 but functions downstream of that step in G2.
Plk3 Promotes Resection-Dependent Slow DSB Repair in G1
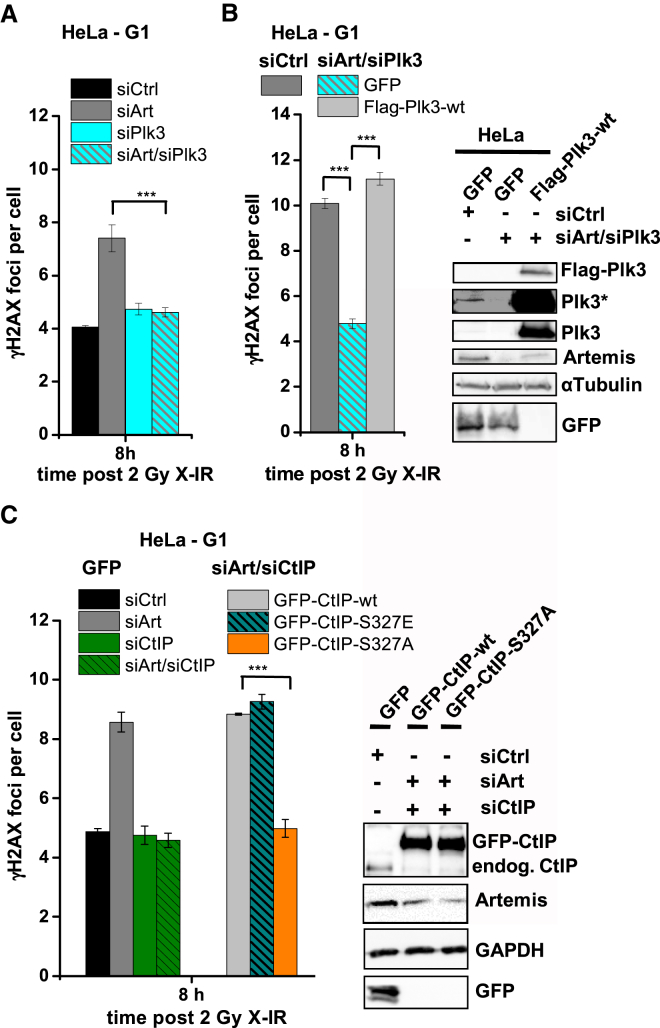
Because Plk3 regulates CtIP in G1 (Barton et al., 2014), we investigated its role during slow repair in G1. We observed that, like siCtIP, siPlk3 does not cause a DSB repair defect but rescues the defect of siArtemis-treated HeLa cells (Figure 6A). Expression of siRNA-resistant FLAG-tagged Plk3 restored the repair defect in Artemis/Plk3-depleted cells (Figure 6B). siPlk3 in G2, where Plk3 is dispensable for CtIP regulation, did not rescue the defect of siArtemis-treated cells (Figure S6A). We confirmed these results with fibroblasts using siPlk3 and a Plk3 inhibitor (Plki) (Lansing et al., 2007; Figure S6B). Because Plk3 phosphorylates CtIP in G1 at Ser327 (Barton et al., 2014), we asked whether this phosphorylation event is required during resection-dependent repair. We co-depleted Artemis and CtIP in HeLa cells, transfected them with siRNA-resistant GFP-CtIP constructs, and enumerated γH2AX foci in G1. Notably, GFP-CtIP-WT and a phospho-mimic substitution at Ser327 (GFP-CtIP-S327E), but not a non-phosphorylatable mutant (GFP-CtIP-S327A), restored the Artemis repair defect in Artemis/CtIP-depleted cells (Figure 6C). The same result was obtained with fibroblasts (Figure S6C), confirming that CtIP phosphorylation at Ser327 is necessary for resection-dependent c-NHEJ.
Figure 6.
Plk3 Is Required for Resection-Dependent Slow DSB Repair in G1
(A) γH2AX foci in G1 HeLa cells treated with siArtemis and/or siPlk3. Data are mean ± SEM.
(B) γH2AX foci in G1 HeLa cells treated with siArtemis/siPlk3 and transfected with GFP or FLAG-Plk3-WT constructs, and GFP+ or FLAG+ G1 cells were analyzed. Data are mean ± SEM.
(C) γH2AX foci in G1 HeLa cells treated with siArtemis and/or siCtIP. Cells were transfected with GFP or GFP-CtIP constructs, and GFP+ G1-phase cells were analyzed. Data are mean ± SEM.
See also Figure S6.
CtIP Phosphorylation at Ser327 by Plk3 Mediates Interaction with Brca1 in G1
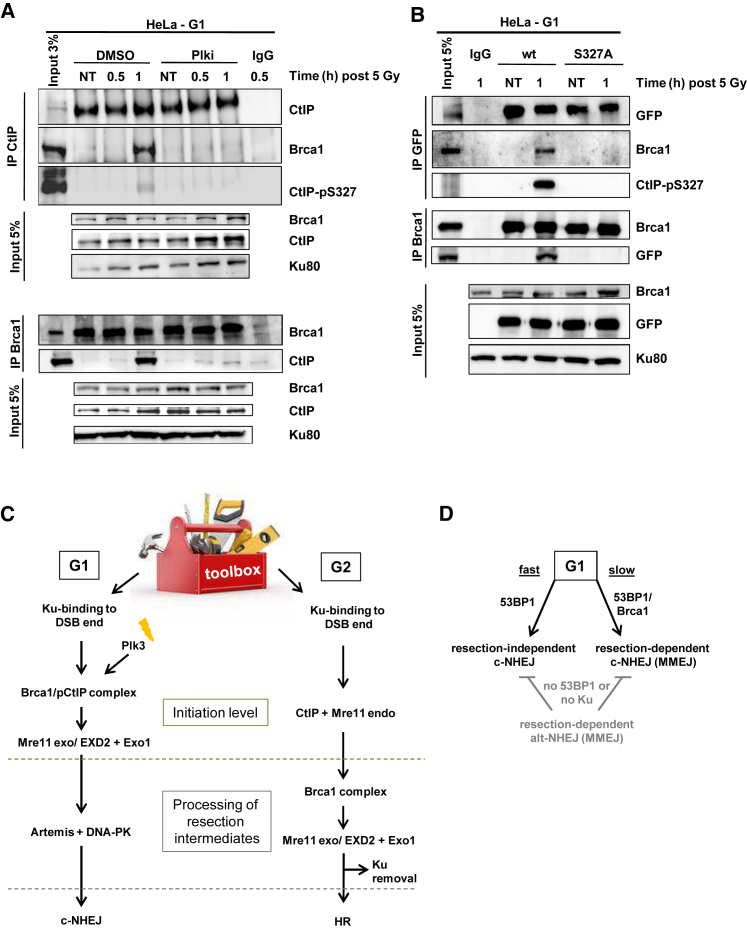
CtIP phosphorylation at Ser327 by CDKs in G2 mediates its interaction with Brca1 (Yu and Chen, 2004). Although this phosphorylation occurs constitutively in undamaged G2 cells, CtIP phosphorylation by Plk3 in G1 is only observed after IR (Barton et al., 2014). Because CtIP phosphorylation at Ser327 and Brca1’s BRCT domain, which encompasses the CtIP interaction site, are required for slow repair in G1, we examined whether Brca1 and CtIP physically interact in G1 using co-immunoprecipitation analysis in synchronized G1 HeLa cells (Figure S7A). We confirmed that CtIP phosphorylation at Ser327 is only observed after IR and that Plki abolished the IR-induced signal (Figure 7A; Barton et al., 2014). Notably, Brca1 co-immunoprecipitated with CtIP closely followed the CtIP phosphorylation signal at Ser327; that is, it was absent in unirradiated samples, appeared 1 hr after IR, and was absent when the samples were treated with Plki (Figure 7A). In the reverse experiment, we observed strong levels of CtIP co-immunoprecipitated with Brca1 1 hr after IR only in non-Plki treated samples (Figure 7A). This analysis shows that CtIP and Brca1 physically interact in G1 in a damage-inducible manner that requires Plk3.
Figure 7.
CtIP Phosphorylation at Ser327 by Plk3 Mediates Interaction with Brca1 in G1
(A) Interaction of CtIP and Brca1 in synchronized G1 HeLa cells treated with Plki.
(B) Interaction of CtIP and Brca1 in G1 HeLa cells transfected with GFP-CtIP-WT or GFP-CtIP-S327A. Brca1 or GFP/CtIP was immunoprecipitated from cell extracts, and protein levels were analyzed.
(C) Model summarizing the hierarchy of investigated factors involved in resection-dependent c-NHEJ in G1 in comparison with HR in G2.
(D) Model for DSB repair pathway choice in G1 human cells.
See also Figure S7.
We next examined whether the damage-inducible CtIP-Brca1 interaction in G1 depends on CtIP phosphorylation at Ser327. We transfected HeLa cells with GFP-CtIP-WT or non-phosphorylatable GFP-CtIP-S327A, irradiated them or not, and immunoprecipitated GFP. The transfection stress caused >90% of the GFP-positive HeLa cells to arrest in G1 (Figure S7B). In cells transfected with GFP-CtIP-WT, we observed pronounced CtIP phosphorylation at Ser327 1 hr after IR but not without IR (Figure 7B). Brca1 did not co-immunoprecipitate with GFP-CtIP in unirradiated cells, but a robust signal was observed 1 hr after IR. Cells transfected with GFP-CtIP-S327A showed no CtIP phosphorylation at Ser327 and no detectable Brca1 (Figure 7B). Conversely, we detected a strong signal for GFP-CtIP co-immunoprecipitated with Brca1 in the irradiated GFP-CtIP-WT but not the GFP-CtIP-S327A sample (Figure 7B). These data show that CtIP and Brca1 physically interact in G1 in a damage-inducible manner dependent on CtIP phosphorylation at Ser327.
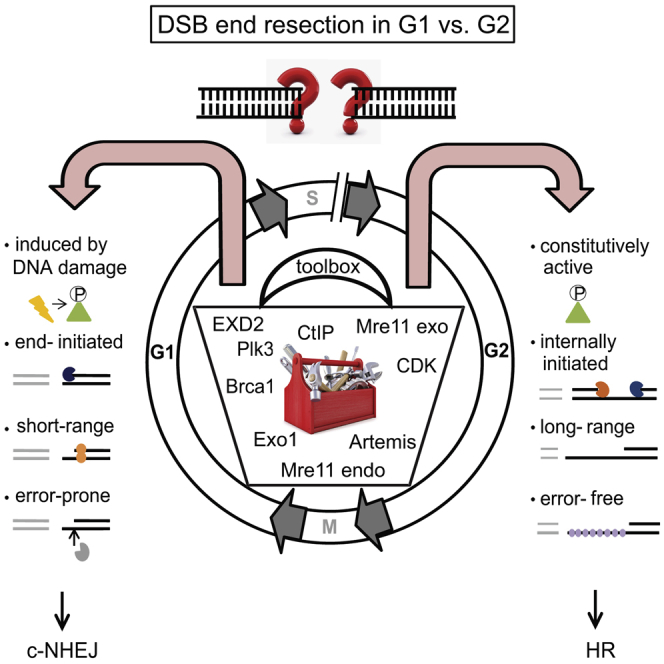
Discussion
DSB resection can arise in G1 as well as S and G2, and Rad51 binding to extended ssDNA regions can occur in genetically manipulated G1 cells (Orthwein et al., 2015). However, Rad51 loading to ssDNA does not normally occur in G1, and resection is too limited to detect RPA binding microscopically, limiting our ability to study resection in G1.
Our study was initiated by the finding that CtIP depletion rescues the repair defect of Artemis mutants. Artemis is required for the slow DSB repair process that, after X-IR, repairs DSBs localizing to heterochromatic DNA regions. This has provided a readout to probe the role of additional factors for G1 resection. We reasoned that resection occurs in heterochromatin because repair is delayed (Goodarzi et al., 2008). Additionally, to study resection in G1, we utilized α-IR, which induces complex DSBs that are repaired with slow kinetics and undergo resection in G2 (Shibata et al., 2011). In G1, DSBs induced by α-IR give rise to CtIP-dependent pRPA foci (Barton et al., 2014). This second approach fully consolidated the findings obtained with the Artemis rescue experiments.
As a third approach to study resection in G1, we used a reporter assay containing two I-SceI restriction sites that monitors end joining of the two distant ends with loss of the intervening fragment (Rass et al., 2009). We considered that such events represent slow DSB repair, whereas fast repair may promote end joining events without loss of the intervening fragment that escape detection in the assay. Because slow DSB repair involves resection, we reasoned that this assay might specifically monitor resection-dependent end joining. Thus, we exploited three independent methods to study resection-dependent slow DSB repair in G1. The reporter assay additionally revealed that resection-dependent end joining is associated with nucleotide losses of 5–20 bp, although the extent of resection might be larger for α-IR-induced DSBs.
Using these three approaches, we characterized the resection process in G1, revealing differences from G2 resection (Figure 7C). First, we showed that (as in G2 phase) the slow component of DSB repair in G1 represents a resection-dependent repair process. This is significant because previous work has established that slow DSB repair involves the c-NHEJ factors Lig4, XRCC4, and XLF (Beucher et al., 2009, Riballo et al., 2004). Here we show that DNA-PKcs inhibition at later times (when the fast repair process is completed) stops repair in G1, suggesting that DNA-PK is required for the slow process. This is distinct from the situation in G2, where DNA-PK is removed during resection. Thus, the slow component of DSB repair involves resection in G1 and G2, but, in G1, repair occurs via c-NHEJ, whereas, in G2, HR effects repair.
A second difference between resection in G1 versus G2 likely explains how DNA-PK binding to resected DSBs is maintained in G1 but prevented in G2. Although Mre11 initiates resection in G2 as an endonuclease internal to the DSB, this function of Mre11 is dispensable for resection in G1. Thus, it is likely that resection in G1 initiates from the DSB end by the exonuclease functions of Mre11, EXD2, and Exo1, which, because of their different polarity, can resect one or the other DNA strand. An interesting model is that Ku remains bound to the DSBs but moves away from the ends to allow nuclease access (i.e., translocates inward, a feature well described in biochemical studies; Turchi et al., 2000). In G2, in contrast, nucleolytic incision on the 5′ strand internal to the DSB is followed by resection toward and away from the DSB by the exonuclease functions of Mre11/EXD2 and Exo1, respectively. After removal of DNA-PK by still unknown processes, the large region of ssDNA likely prevents DNA-PK re-binding. Notably, maintained DNA-PK binding to DSBs during resection in G1 but not G2 (Figure 1F; Figure S3E) could also explain the more limited resection in G1.
The third difference between resection in G1 and G2 concerns the initiation step. In G2, CtIP is constitutively phosphorylated by CDKs at Ser327, mediating interaction with Brca1 and promoting HR (Yu et al., 2006, Yun and Hiom, 2009), although the latter notion has been challenged (Reczek et al., 2013). Brca1 counteracts the anti-resection functions of 53BP1 and Rif1 (Chapman et al., 2013, Escribano-Díaz et al., 2013, Feng et al., 2013). In G1, IR activates Plk3, which phosphorylates CtIP at Ser327 (Barton et al., 2014). Hence, CtIP interacts with Brca1 in G1 only after damage induction. Because the CtIP interaction domain of Brca1 is required for resection, Brca1’s role in promoting resection may be transiently kept in check to allow resection-independent c-NHEJ before activating the more error-prone resection-dependent c-NHEJ process.
Our findings also reveal distinctions in the commitment step to resection-dependent repair between G1 and G2 (Figure 7C). Depletion of Brca1 (and also Exo1 and Mre11 exonuclease) causes a repair defect in G2 because Brca1 lies downstream of CtIP-dependent initiation of resection (Kakarougkas et al., 2013). In contrast, Brca1 depletion does not cause a defect in G1, suggesting that it is required for the initiation process, which, if prevented, allows rejoining without resection. This might also explain the controversy concerning Brca1’s role in G1 (because the assays used may or may not be specific for the described resection-dependent process) (Wu et al., 2010). Interestingly, our results show that Brca1 relieves a 53BP1 barrier to resection, defining a hitherto undescribed role for Brca1 in G1. Significantly, although 53BP1 creates a block to all resection and its loss allows unregulated resection and alt-NHEJ, the interplay between BRCA1 and 53BP1 promotes resection-dependent c-NHEJ (Figure 7D).
An important distinction between factors that initiate resection versus Artemis is that X-ray-induced DSBs are repaired without the initiating factors but remain unrepaired without Artemis. Thus, we propose that Artemis does not process the primary IR-induced DSBs as hypothesized previously (Riballo et al., 2004) but, rather, resolves intermediate structures that arise following resection by Exo1/EXD2 or Mre11 exonuclease, respectively. An interesting (although not the only) model is that 5′ or 3′ ssDNA overhangs are captured by a channel in DNA-PKcs, identified by structural studies, that is of the required size to allow passage of ssDNA but not double-stranded DNA (dsDNA) (Leuther et al., 1999, Williams et al., 2008, Williams et al., 2014). This could create a hairpin-like end necessitating Artemis for cleavage (Figure S7C). Our observation that Artemis is required for pRPA focus formation suggests that RPA binding only occurs after such cleavage. This model is appealing because it explains the absolute requirement for Artemis in removing trapped resection intermediates and reflects its role in cleaving hairpin intermediates during V(D)J recombination (Ma et al., 2002). The model is consistent with biochemical studies and explains why Ku and DNA-PKcs are required for efficient Artemis activity (Chang et al., 2015). Moreover, Artemis’s endonucleolytic function downstream of initiation is consistent with the observed loss of nucleotides during resection-dependent c-NHEJ.
Micro-homology mediated end-joining (MMEJ) is a DSB rejoining process defined by short micro-homology usage (McVey and Lee, 2008). MMEJ is often taken to be synonymous with alt-NHEJ. However, the rejoining step of MMEJ could occur by c-NHEJ or alt-NHEJ. Our findings suggest that resection-dependent c-NHEJ represents MMEJ because alt-NHEJ does not substantially contribute to DSB rejoining or translocation formation in G1 human cells, functioning only in the absence of Ku or 53BP1 (Figure 7D). Alt-NHEJ has a greater function in rodent cells, where it contributes to translocations, potentially because of lower DNA-PK levels (Ghezraoui et al., 2014). Importantly, resection-dependent c-NHEJ significantly contributes to IR-induced translocations in human cells, consistent with the contribution of CtIP after DSB induction by restriction enzymes (Zhang and Jasin, 2011). Thus, our finding that CtIP (which was hitherto believed to promote alt-NHEJ) functions during c-NHEJ unifies these apparently contradicting notions. Thus, we propose that the slow component of DSB repair in G0/G1 phase human cells can result in MMEJ, with rejoining involving c-NHEJ and not alt-NHEJ.
In summary, we have identified and characterized a resection-dependent c-NHEJ process and revealed distinctions from the resection process during HR. Resection is activated in G1 by Plk3 which phosphorylates CtIP at Ser327, mediating its binding to Brca1, and is then executed by Exo1, EXD2, and Mre11 exonuclease. Mre11’s endonuclease function, which initiates resection during HR in G2, is not involved. Finally, Artemis functions as an endonuclease downstream of the executing exonucleases to complete the process. DNA-PK coordinates the completion of repair by c-NHEJ. Thus, resection-dependent c-NHEJ uses the same toolbox of resection factors involved in HR but orchestrates them to be compatible with an end-joining process (Figure 7C).
STAR★Methods
Key Resources Table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit-anti-53BP1 | Bethyl | A300-272A |
| Mouse-anti-53BP1 (clone BP13) | Millipore | #05-726 |
| Rabbit-anti-Artemis | Novus Biologicals | NB100-542 |
| Rabbit-anti-Artemis | GenTex | GTX100128 |
| Rabbit-anti-Artemis | Abcam | ab35649 |
| Rabbit-anti-BLM | Abcam | ab2179 |
| Mouse-anti-Brca1 (D-9) | Santa Cruz | sc-6954 |
| Rabbit-anti-Brca1 (C-20) | Santa Cruz | sc-642 |
| Mouse-anti-Brca1_MS13 | Abcam | ab16781 |
| Mouse-anti-BrdU (3D4) | BD PharMingen | 555627 |
| Mouse-anti-CD4-FITC | Biolegend | 100510 |
| Mouse-anti-CtIP (E-2) | Santa Cruz | sc-48415 |
| Rabbit-anti-CtIP | Bethyl | A300-488A |
| Mouse-anti-CtIP (D-4) | Santa Cruz | sc-271339 |
| Rabbit-anti-pCtIP (Ser327) | Phosphosolutions | N/A |
| Rabbit-anti-CDT1 | Abcam | ab202067 |
| Rabbit-anti-DNA2 | Abcam | ab 96488 |
| Rabbit-anti-DNA-PKcs | Novus Biologicals | NB100-658 |
| Rabbit-anti-EXD2 | Sigma | HPA005848 |
| Mouse-anti-Exo1 | Abcam | ab3307 |
| Mouse-anti-Flag (M2) | Sigma | F3165 |
| Rabbit-anti-GAPDH (FL-335) | Santa Cruz | sc-25778 |
| Mouse-anti-GFP | Roche | 11 814 460 001 |
| Rabbit-anti-GFP | Santa Cruz | sc-8334 |
| Mouse-anti-phospho-Histone H2A.X (Ser139) | Millipore | # 05-636 |
| Rabbit-anti-phospho-Histone H2A.X (Ser139) | Abcam | ab81299 |
| Mouse-anti-HA tag (HA.C5) | Abcam | ab18181 |
| Mouse-anti-Ku70 (A-9) | Santa Cruz | sc-5309 |
| Mouse-anti-Ku80 (111) | Abcam | ab79220 |
| Mouse-anti-Lig1 (1A9) | Santa Cruz | sc-47703 |
| Mouse-anti-Lig3 | Santa Cruz | sc-56089 |
| Rabbit-anti-Lig4 | Acris | SP1275 |
| Mouse-anti-cMyc (9E10) | Santa Cruz | sc-40 |
| Rabbit-anti-Plk3 | Abcam | ab33119 |
| Rabbit-anti-tRFP | Evrogen | AB233 |
| Rabbit-anti-RPA32/RPA2 (phosphoT21) | Abcam | ab109394 |
| Rabbit-anti-I-SceI (FL-86) | Santa Cruz | sc-98269 |
| Mouse-anti-alpha-Tubulin (TU-02) | Santa Cruz | sc- 8035 |
| Goat-anti-mouse IgG-HRP | Santa Cruz | sc-2031 |
| Goat-anti-rabbit IgG-HRP | Santa Cruz | sc-2030 |
| Goat-anti-mouse IgG AlexaFluor 488 | Molecular Probes | A11001 |
| Goat-anti-mouse IgG AlexaFluor 594 | Molecular Probes | A11005 |
| Goat-anti-rabbit IgG AlexaFluor 488 | Molecular Probes | A11008 |
| Goat-anti-rabbit IgG AlexaFluor 594 | Molecular Probes | A11012 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Plk inhibitor GW 843682X | Tocris Bioscience | 2977 |
| DNA-PK inhibitor Nu7441 | Tocris Bioscience | 3712 |
| EdU | baseclick | BCN-001 |
| BrdU | BD Bioscience | 550891 |
| DAPI | Sigma-Aldrich | D9542 |
| PARP inhibitor PJ34 | Calbiochem | 528151 |
| Mre11 (endo) inhibitor (PFM01) | Shibata et al., 2014 | N/A |
| Mre11 (exo) inhibitor (PFM39) | Shibata et al., 2014 | N/A |
| Dynabeads Protein G | Thermo Scientific | 10004D |
| Anti-rat IgG MicroBeads | Miltenyi Biotec | 130-048-501 |
| Nocodazole | Sigma-Aldrich | M1404 |
| KaryoMAX Colcemid | GIBCO | 15212012 |
| Polyethylenglycol (PEG) | Roche | 10783641001 |
| RNase-A | Sigma-Aldrich | R4875 |
| Thymidine | Sigma-Aldrich | T1895-1G |
| KOD Hot Start DNA Polymerase | Novagen | 71086-4 |
| Critical Commercial Assays | ||
| Effectene Transfection Reagent | QIAGEN | 301425 |
| jetPEI Transfection Reagent | Polyplus | 13-101-10 |
| HiPerFect Transfection Reagent | QIAGEN | 301707 |
| PEI | Sigma-Aldrich | 408727-7 |
| EdU-Click Kit (Cy5) | baseclick | BCK-EDU-647-1 |
| peqGOLD Xchange Plasmid maxi-EF Kit | peqlab | 12-7404-01 |
| MACS separation column | Miltenyi Biotec | 130-042-201 |
| MasterPure Complete DNA & RNA Purification | Epicenter | MC85200 |
| PureLink Genomic DNA Mini Kit | Thermo Scientific | K1820-01 |
| FISH XCP Mix | MetaSystems | D-0328-200-MC |
| LumiLight Western Blotting Substrate | Roche | 12015200001 |
| WesternBright Quantum | Advansta | 541015 |
| WesternBright Sirius | Advansta | 541021 |
| Experimental Models: Cell Lines | ||
| Human: 82-6 hTert | Riballo et al., 2004 | N/A |
| Human: CJ179 hTert | Riballo et al., 2004 | N/A |
| Human: HeLa-S3 | ATCC | ATCC-CCL-2.2 |
| Human: GC92 | Rass et al., 2009 | N/A |
| Mouse: MEF Brca1-wt | Shakya et al., 2011 | N/A |
| Mouse: MEF Brca1-ΔBRCT | Shakya et al., 2011 | N/A |
| Human: 2BN hTert | Shibata et al., 2011 | N/A |
| Human: 411BR hTert | Jeggo Lab (Sussex) | N/A |
| Human: HeLa pGC | Barton et al., 2014 | N/A |
| Experimental Models: Organisms/Strains | ||
| DH5α E. coli | This paper | N/A |
| Recombinant DNA | ||
| pGEM-T Easy Vector | Promega | A1360 |
| pUC19 | New England Biolabs | N3041S |
| pEGFP_C1 | Clontech | 632470 |
| tRFP | Jeggo Lab (Sussex) | N/A |
| siRNA-resistant pEGFP-CtIP | Barton et al., 2014 | N/A |
| siRNA-resistant pClneo-cMyc-Artemis | Beucher et al., 2009 | N/A |
| siRNA-resistant Flag-Plk3 | Barton et al., 2014 | N/A |
| siRNA-resistant Flag-Brca1 | Shakya et al., 2011 | N/A |
| siRNA-resistant HA-53BP1 | Jeggo Lab (Sussex) | N/A |
| siRNA-resistant Flag-Exo1 | Jeggo Lab (Sussex) | N/A |
| Sequence-Based Reagents | ||
| see Table S2 | ||
| Software and Algorithms | ||
| Metafer | MetaSystems | N/A |
| LAS AF Lite | Leica | N/A |
| AxioVision V4.6.3.0 | Zeiss Imaging Solutions | N/A |
| ImageJ | Open Source | N/A |
| ChemiCapt | Vilber Lourmat | N/A |
| FusionCapt Advance FX7 | Vilber Lourmat | N/A |
| Other | ||
| X-ray tube: MCN 165/796704 | Philips | N/A |
| Microscope: Axiovert 200M | Zeiss | N/A |
| Microscope: Image Z.2 | Zeiss | N/A |
| Confocal laser scanning microscope: TCS SP5 II | Leica | N/A |
| Chemiluminescence detection: ChemiSmart 5000 | Vilber Lourmat | N/A |
| Chemiluminescence detection: Fusion FX | Vilber Lourmat | N/A |
Contact for Reagent and Resource Sharing
Further information and requests for reagents may be directed to, and will be fulfilled by the Lead Contact, Markus Löbrich (lobrich@bio.tu-darmstadt.de).
Experimental Model and Subject Details
Cell Lines and Cell Culture
Cell lines used were control 82-6 hTert (Riballo et al., 2004), Artemis-deficient CJ179 hTert (Riballo et al., 2004), HeLa (ATCC), GC92 (Rass et al., 2009), Brca1-wt and Brca1-ΔBRCT MEFs (Shakya et al., 2011), XLF-deficient 2BN hTert (Shibata et al., 2011), hypomorphic Lig4-mutated 411Br hTert (Jeggo Lab), and HeLa pGC (Barton et al., 2014). Cells were tested for mycoplasm contamination by PCR, HeLa cells were authenticated by ATCC. HeLa, GC92 cells and MEFs were cultured in DMEM with 10% FCS and 1% NEAA, 82-6, CJ179, 411Br and 2BN cells in MEM with 20% FCS, 1% NEAA. All cells were maintained at 37°C in a 5% CO2 incubator.
Bacterial Strains
Competent DH5α E.coli were used for transformations.
Method Details
Generation of Artemis KO Cells with CRISPR/Cas9
Vectors encoding Artemis guide RNAs (see Key Resources Table) were used. GC92 cells were transfected with the Artemis gRNA plasmids, a Cas9 and a EGFP plasmid using PEI following the manufacturer’s instructions. Single GFP-positive cells were sorted into 96 well plates and tested for knockdown on a protein level using immunoblotting. Genomic DNA was extracted from potential KO cells with PureLink Genomic DNA Mini Kit and PCR was performed with KOD Hot Start DNA Polymerase to amplify the targeted regions. PCR products were cloned into pGEM-T Easy Vector and transformed into DH5α competent E. coli. Isolated plasmid DNA of at least 10 colonies from each transformation were sent for sequencing to ensure frameshift mutation in the targeted region.
RNA Interference and Plasmid Transfection
SiRNA transfection of HeLa, 82-6, CJ179, and GC92 cells and MEFs was carried out using HiPerFect Transfection Reagent following the manufacturer’s instructions. 53BP1 (25nM), Artemis (15 nM), BLM (50nM), Brca1 (25 nM), CtIP (50 nM), DNA2 (20nM), DNA-PKcs (15 nM), EXD2 (25nM), Exo1 (20 nM), Ku70 (25 nM), Ku80 (25 nM), Lig1 (25 nM), Lig3 (25 nM), Lig4 (20 nM), and Plk3 (25 nM) siRNAs were used (target sequences are listed in the Key Resources Table). Experiments were either performed 48 hr after transfection or after 72 hr with an additional siRNA transfection after 24 hr. In complementation studies, the endogenous protein was depleted by siRNA in HeLa, MEF, 82-6, CJ179 or GC92 cells and 24 hr after siRNA transfection, cells were transfected with various plasmids (see Key Resources Table) using Effectene or jetPEI following the manufacturer’s instructions. HeLa cells for immunoprecipitation experiments were transfected with PEI following the manufacturer’s instructions. For I-SceI-transfection, GC92 or HeLa pGC cells were transfected using jetPEI following the manufacturer’s instructions.
IR and Chemical Treatment
X-IR was performed at 90 kV and 19 mA. A 241Am source was used for α-IR. Chemical inhibitors were added 1 hr prior to IR and maintained during repair incubation. The Plk inhibitor GW 843682X (IC50 values of 2.2 and 9.1 nM for Plk1 and Plk3, respectively), the DNA-PK inhibitor Nu7441, the PARP inhibitor PJ34, the Mre11 (endo) inhibitor and the Mre11 (exo) inhibitor (Shibata et al., 2014) were used at concentrations of 0.5, 7.5, 15, 50, and 300 μM.
Chromosomal Analysis
For the analysis of translocations and chromosome breaks in G1, exponentially growing or confluent 82-6 or CJ179 fibroblasts were irradiated. To prevent progression of G2-irradiated cells into G1 during repair incubation, cells were treated with nocodazole (100 ng/ml) prior to IR. After repair incubation, cells were mixed at a ratio of 1:1 with mitotic HeLa cells (enriched by treatment with colcemid for 20 h). Cell fusion was mediated by Polyethylenglycol (PEG). For FISH experiments whole chromosome probes 1, 2 and 4 were used and the staining was performed following the manufacturer’s protocol (MetaSystems). Pictures of the chromosomes were acquired by using an Axioplan2 microscope with an EC Plan Neofluar (63x) (Zeiss) and Metafer software (MetaSystems). Only the stained chromosomes were analyzed.
Protein Extracts, Immunoprecipitation, and Immunoblotting
Knockdown efficiencies and expression of exogenous plasmids were confirmed by immunoblotting. For immunoprecipitation, 2 μg antibodies (see Key Resource Table) were linked to Dynabeads Protein G, washed three times in 0.1% BSA/PBS and then incubated with the cell extract at 4°C for 2 hr. After immunoblotting, the membrane was blocked in 5% low fat milk or 5% BSA in TBS/0.1% Tween20. Immunoblotting was carried out in TBS/0.1% Tween20/1% low fat milk or 5% BSA over night at 4°C, followed by HRP-conjugated secondary antibody incubation in PBS/0.1% Tween20/1% low fat milk or 5% BSA for 1 hr. Immunoblots were developed using LumiLight immunoblotting substrate or WesternBright Quantum or Sirius. Signal detection was performed with ChemiSmart5000 or Fusion FX. Antibodies are listed in the Key Resources Table.
Immunofluorescence
Cells were grown on glass coverslips for X-IR and on Mylar foil for α-IR. The thymidine analog EdU and nocodazole (100 ng/μl) were added 30 min prior to IR and cells were fixed and stained. EdU incorporation was detected with an EdU-Click kit. For BrdU foci analysis, cells were pre-extracted for 10 min with 0.5% Triton X-100. Cells were examined with a Zeiss microscope and Metafer software. For each foci counting experiment at least 40 cells were evaluated. For Ku80 foci staining, cells were pre-extracted and stained as previously described (Chanut et al., 2016). Additionally, after the secondary antibody staining, cells were fixed with 2% PFA for 10 min. For image acquisition Z stacks were obtained with a confocal laser scanning microscope using a 100x immersion objective. Co-localization of Ku80 and pRPA or Rad51 foci was analyzed using LAS AF Lite software (dx < 20 nm for co-localization of signal intensity in line profile). Antibodies are listed in the Key Resources Table.
Reporter Assays
HeLa pGC cells containing an HR substrate were transfected with a I-SceI-plasmid 24 hr after seeding and treated with inhibitors. GC92 cells containing an NHEJ substrate were transfected with siRNA. For complementation studies, cells were transfected with constructs 24 hr after seeding. 48 hr after seeding, cells were transfected with a I-SceI-expressing plasmid and either treated with an inhibitor or transfected again with siRNA. After 72 hr the cells were fixed and stained. Up to 10,000 cells were analyzed per sample for either GFP (HeLa pGC cells) or CD4 (for GC92) positive cells with a microscope (Axiovert 200M) and Metafer software. Antibodies are listed in the Key Resources Table.
Sequence Analysis in NHEJ Reporter Assay
GC92 cells were dissociated with 50 mM EDTA in PBS and stained with 1.5 μg rat-α-CD4-FITC antibody for 30 min at 4°C. After washing, cells were incubated 15 min at 4°C with goat-α-rat microbeads. Then, the CD4-positive GC92 cells were separated and enriched by using a miniMACS column. After purification of DNA with MasterPure Complete DNA & RNA Purification, PCR was performed using the primers listed in the Key Resources Table. To isolate the individual clones, PCR products were cloned into pUC19 and sequenced (MWG Eurofins).
Anti-pSer327 Antibody Preparation
Phospho-specific antibodies were produced in rabbits against CtIP-pSer327 (custom antibody service from Phosphosolutions). The antigens were synthetic phospho-peptides corresponding to amino acids surrounding the phosphorylated Ser327 in the human CtIP sequence. The resulting solution of purified antibody was stored at −20°C.
Cell Synchronization and Flow Cytometry
Proliferating HeLa cells were treated with 2 mM thymidine for 16 hr, released in thymidine-free medium for 10 hr and again pulse-treated with thymidine for 14 hr. Cells were again released in fresh medium without thymidine for 18 hr to obtain G1 cells. Cell synchronization was controlled by propidium iodide and BrdU flow cytometry analysis.
Quantification and Statistical Analysis
All data were derived from at least n = 3 replicates for foci analysis and chromosomal studies, or from at least n = 4 biological replicates for the NHEJ and HR reporter assays. Column plots show the mean value and boxplots were created with SigmaPlot12.0. Background foci/chromosome breaks/chromosome translocations were subtracted from the mean values. The error bars in the column plots show the SEM between the experiments. p values in column and line plots originate from Student’s t test. They compare all cells analyzed in foci and chromosomal experiments and compare the mean data in the NHEJ and HR reporter assay (∗, p < 0.05; ∗∗, p < 0.01; ∗∗∗, p < 0.001). p values in boxplots originate from Mann-Whitney U test analyzed with SigmaPlot12.0.
Author Contributions
R.B., M.S., and O.B. performed most experiments and interpreted the data. S.J., J.K., J.S., and A.S. participated in the experiments. A.S., P.A.J., and M.L. conceived the experiments. M.L. wrote the paper with help from P.A.J.
Acknowledgments
We thank Tanya Paull for providing phospho-specific antibodies; Susan Lees-Miller for helpful discussions; and Junjie Chen, Zihua Gong, Hemmo Meyer, and Markus Wolf for experimental input. We thank Ratna Weimer, Bettina Basso, Christel Braun, and Cornelia Schmitt for technical assistance and Amira Elbakry for help with manuscript editing. Work in the M.L. laboratory is supported by the Deutsche Forschungsgemeinschaft (GRK1657), the European Union (grant RISK-IR), and the Bundesministerium für Bildung und Forschung (02NUK034A/037C and 02NUK016D/017E). P.A.J. was supported by the Medical Research Council (G000050 and G0500897).
Published: January 26, 2017
Footnotes
Supplemental Information includes seven figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.molcel.2016.12.016.
Contributor Information
Atsushi Shibata, Email: shibata.at@gunma-u.ac.jp.
Penny A. Jeggo, Email: p.a.jeggo@sussex.ac.uk.
Markus Löbrich, Email: lobrich@bio.tu-darmstadt.de.
Supplemental Information
References
- Barton O., Naumann S.C., Diemer-Biehs R., Künzel J., Steinlage M., Conrad S., Makharashvili N., Wang J., Feng L., Lopez B.S. Polo-like kinase 3 regulates CtIP during DNA double-strand break repair in G1. J. Cell Biol. 2014;206:877–894. doi: 10.1083/jcb.201401146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beucher A., Birraux J., Tchouandong L., Barton O., Shibata A., Conrad S., Goodarzi A.A., Krempler A., Jeggo P.A., Löbrich M. ATM and Artemis promote homologous recombination of radiation-induced DNA double-strand breaks in G2. EMBO J. 2009;28:3413–3427. doi: 10.1038/emboj.2009.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick R., Nieminuszczy J., Baddock H.T., Deshpande R.A., Gileadi O., Paull T.T., McHugh P.J., Niedzwiedz W. EXD2 promotes homologous recombination by facilitating DNA end resection. Nat. Cell Biol. 2016;18:271–280. doi: 10.1038/ncb3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.H., Watanabe G., Lieber M.R. Unifying the DNA end-processing roles of the artemis nuclease: Ku-dependent artemis resection at blunt DNA ends. J. Biol. Chem. 2015;290:24036–24050. doi: 10.1074/jbc.M115.680900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanut P., Britton S., Coates J., Jackson S.P., Calsou P. Coordinated nuclease activities counteract Ku at single-ended DNA double-strand breaks. Nat. Commun. 2016;7:12889. doi: 10.1038/ncomms12889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman J.R., Barral P., Vannier J.B., Borel V., Steger M., Tomas-Loba A., Sartori A.A., Adams I.R., Batista F.D., Boulton S.J. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol. Cell. 2013;49:858–871. doi: 10.1016/j.molcel.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBiase S.J., Zeng Z.C., Chen R., Hyslop T., Curran W.J., Jr., Iliakis G. DNA-dependent protein kinase stimulates an independently active, nonhomologous, end-joining apparatus. Cancer Res. 2000;60:1245–1253. [PubMed] [Google Scholar]
- Escribano-Díaz C., Orthwein A., Fradet-Turcotte A., Xing M., Young J.T., Tkáč J., Cook M.A., Rosebrock A.P., Munro M., Canny M.D. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol. Cell. 2013;49:872–883. doi: 10.1016/j.molcel.2013.01.001. [DOI] [PubMed] [Google Scholar]
- Feng L., Fong K.W., Wang J., Wang W., Chen J. RIF1 counteracts BRCA1-mediated end resection during DNA repair. J. Biol. Chem. 2013;288:11135–11143. doi: 10.1074/jbc.M113.457440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezraoui H., Piganeau M., Renouf B., Renaud J.B., Sallmyr A., Ruis B., Oh S., Tomkinson A.E., Hendrickson E.A., Giovannangeli C. Chromosomal translocations in human cells are generated by canonical nonhomologous end-joining. Mol. Cell. 2014;55:829–842. doi: 10.1016/j.molcel.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi A.A., Noon A.T., Deckbar D., Ziv Y., Shiloh Y., Löbrich M., Jeggo P.A. ATM signaling facilitates repair of DNA double-strand breaks associated with heterochromatin. Mol. Cell. 2008;31:167–177. doi: 10.1016/j.molcel.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Gotoh E., Durante M. Chromosome condensation outside of mitosis: mechanisms and new tools. J. Cell. Physiol. 2006;209:297–304. doi: 10.1002/jcp.20720. [DOI] [PubMed] [Google Scholar]
- Guirouilh-Barbat J., Huck S., Bertrand P., Pirzio L., Desmaze C., Sabatier L., Lopez B.S. Impact of the KU80 pathway on NHEJ-induced genome rearrangements in mammalian cells. Mol. Cell. 2004;14:611–623. doi: 10.1016/j.molcel.2004.05.008. [DOI] [PubMed] [Google Scholar]
- Huertas P., Jackson S.P. Human CtIP mediates cell cycle control of DNA end resection and double strand break repair. J. Biol. Chem. 2009;284:9558–9565. doi: 10.1074/jbc.M808906200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S.P., Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jette N., Lees-Miller S.P. The DNA-dependent protein kinase: A multifunctional protein kinase with roles in DNA double strand break repair and mitosis. Prog. Biophys. Mol. Biol. 2015;117:194–205. doi: 10.1016/j.pbiomolbio.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakarougkas A., Ismail A., Katsuki Y., Freire R., Shibata A., Jeggo P.A. Co-operation of BRCA1 and POH1 relieves the barriers posed by 53BP1 and RAP80 to resection. Nucleic Acids Res. 2013;41:10298–10311. doi: 10.1093/nar/gkt802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing T.J., McConnell R.T., Duckett D.R., Spehar G.M., Knick V.B., Hassler D.F., Noro N., Furuta M., Emmitte K.A., Gilmer T.M. In vitro biological activity of a novel small-molecule inhibitor of polo-like kinase 1. Mol. Cancer Ther. 2007;6:450–459. doi: 10.1158/1535-7163.MCT-06-0543. [DOI] [PubMed] [Google Scholar]
- Lee B.I., Wilson D.M., 3rd The RAD2 domain of human exonuclease 1 exhibits 5′ to 3′ exonuclease and flap structure-specific endonuclease activities. J. Biol. Chem. 1999;274:37763–37769. doi: 10.1074/jbc.274.53.37763. [DOI] [PubMed] [Google Scholar]
- Lee-Theilen M., Matthews A.J., Kelly D., Zheng S., Chaudhuri J. CtIP promotes microhomology-mediated alternative end joining during class-switch recombination. Nat. Struct. Mol. Biol. 2011;18:75–79. doi: 10.1038/nsmb.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuther K.K., Hammarsten O., Kornberg R.D., Chu G. Structure of DNA-dependent protein kinase: implications for its regulation by DNA. EMBO J. 1999;18:1114–1123. doi: 10.1093/emboj/18.5.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J., Lukas C. Molecular biology. Shielding broken DNA for a quick fix. Science. 2013;339:652–653. doi: 10.1126/science.1234602. [DOI] [PubMed] [Google Scholar]
- Ma Y., Pannicke U., Schwarz K., Lieber M.R. Hairpin opening and overhang processing by an Artemis/DNA-dependent protein kinase complex in nonhomologous end joining and V(D)J recombination. Cell. 2002;108:781–794. doi: 10.1016/s0092-8674(02)00671-2. [DOI] [PubMed] [Google Scholar]
- McVey M., Lee S.E. MMEJ repair of double-strand breaks (director’s cut): deleted sequences and alternative endings. Trends Genet. 2008;24:529–538. doi: 10.1016/j.tig.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynahan M.E., Jasin M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 2010;11:196–207. doi: 10.1038/nrm2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani H., Taraviras S., Lygerou Z., Nishimoto T. The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J. Biol. Chem. 2001;276:44905–44911. doi: 10.1074/jbc.M105406200. [DOI] [PubMed] [Google Scholar]
- Nussenzweig A., Nussenzweig M.C. A backup DNA repair pathway moves to the forefront. Cell. 2007;131:223–225. doi: 10.1016/j.cell.2007.10.005. [DOI] [PubMed] [Google Scholar]
- Ochi T., Blackford A.N., Coates J., Jhujh S., Mehmood S., Tamura N., Travers J., Wu Q., Draviam V.M., Robinson C.V. DNA repair. PAXX, a paralog of XRCC4 and XLF, interacts with Ku to promote DNA double-strand break repair. Science. 2015;347:185–188. doi: 10.1126/science.1261971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochs F., Somyajit K., Altmeyer M., Rask M.B., Lukas J., Lukas C. 53BP1 fosters fidelity of homology-directed DNA repair. Nat. Struct. Mol. Biol. 2016;23:714–721. doi: 10.1038/nsmb.3251. [DOI] [PubMed] [Google Scholar]
- Orthwein A., Noordermeer S.M., Wilson M.D., Landry S., Enchev R.I., Sherker A., Munro M., Pinder J., Salsman J., Dellaire G. A mechanism for the suppression of homologous recombination in G1 cells. Nature. 2015;528:422–426. doi: 10.1038/nature16142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull T.T., Gellert M. The 3′ to 5′ exonuclease activity of Mre 11 facilitates repair of DNA double-strand breaks. Mol. Cell. 1998;1:969–979. doi: 10.1016/s1097-2765(00)80097-0. [DOI] [PubMed] [Google Scholar]
- Rass E., Grabarz A., Plo I., Gautier J., Bertrand P., Lopez B.S. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat. Struct. Mol. Biol. 2009;16:819–824. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- Reczek C.R., Szabolcs M., Stark J.M., Ludwig T., Baer R. The interaction between CtIP and BRCA1 is not essential for resection-mediated DNA repair or tumor suppression. J. Cell Biol. 2013;201:693–707. doi: 10.1083/jcb.201302145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riballo E., Kühne M., Rief N., Doherty A., Smith G.C., Recio M.J., Reis C., Dahm K., Fricke A., Krempler A. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to gamma-H2AX foci. Mol. Cell. 2004;16:715–724. doi: 10.1016/j.molcel.2004.10.029. [DOI] [PubMed] [Google Scholar]
- Sartori A.A., Lukas C., Coates J., Mistrik M., Fu S., Bartek J., Baer R., Lukas J., Jackson S.P. Human CtIP promotes DNA end resection. Nature. 2007;450:509–514. doi: 10.1038/nature06337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakya R., Reid L.J., Reczek C.R., Cole F., Egli D., Lin C.S., deRooij D.G., Hirsch S., Ravi K., Hicks J.B. BRCA1 tumor suppression depends on BRCT phosphoprotein binding, but not its E3 ligase activity. Science. 2011;334:525–528. doi: 10.1126/science.1209909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A., Conrad S., Birraux J., Geuting V., Barton O., Ismail A., Kakarougkas A., Meek K., Taucher-Scholz G., Löbrich M., Jeggo P.A. Factors determining DNA double-strand break repair pathway choice in G2 phase. EMBO J. 2011;30:1079–1092. doi: 10.1038/emboj.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata A., Moiani D., Arvai A.S., Perry J., Harding S.M., Genois M.M., Maity R., van Rossum-Fikkert S., Kertokalio A., Romoli F. DNA double-strand break repair pathway choice is directed by distinct MRE11 nuclease activities. Mol. Cell. 2014;53:7–18. doi: 10.1016/j.molcel.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turchi J.J., Henkels K.M., Zhou Y. Cisplatin-DNA adducts inhibit translocation of the Ku subunits of DNA-PK. Nucleic Acids Res. 2000;28:4634–4641. doi: 10.1093/nar/28.23.4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Rosidi B., Perrault R., Wang M., Zhang L., Windhofer F., Iliakis G. DNA ligase III as a candidate component of backup pathways of nonhomologous end joining. Cancer Res. 2005;65:4020–4030. doi: 10.1158/0008-5472.CAN-04-3055. [DOI] [PubMed] [Google Scholar]
- Williams D.R., Lee K.J., Shi J., Chen D.J., Stewart P.L. Cryo-EM structure of the DNA-dependent protein kinase catalytic subunit at subnanometer resolution reveals alpha helices and insight into DNA binding. Structure. 2008;16:468–477. doi: 10.1016/j.str.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G.J., Hammel M., Radhakrishnan S.K., Ramsden D., Lees-Miller S.P., Tainer J.A. Structural insights into NHEJ: building up an integrated picture of the dynamic DSB repair super complex, one component and interaction at a time. DNA Repair (Amst.) 2014;17:110–120. doi: 10.1016/j.dnarep.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J., Lu L.Y., Yu X. The role of BRCA1 in DNA damage response. Protein Cell. 2010;1:117–123. doi: 10.1007/s13238-010-0010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Chen J. DNA damage-induced cell cycle checkpoint control requires CtIP, a phosphorylation-dependent binding partner of BRCA1 C-terminal domains. Mol. Cell. Biol. 2004;24:9478–9486. doi: 10.1128/MCB.24.21.9478-9486.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Fu S., Lai M., Baer R., Chen J. BRCA1 ubiquitinates its phosphorylation-dependent binding partner CtIP. Genes Dev. 2006;20:1721–1726. doi: 10.1101/gad.1431006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun M.H., Hiom K. CtIP-BRCA1 modulates the choice of DNA double-strand-break repair pathway throughout the cell cycle. Nature. 2009;459:460–463. doi: 10.1038/nature07955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Jasin M. An essential role for CtIP in chromosomal translocation formation through an alternative end-joining pathway. Nat. Struct. Mol. Biol. 2011;18:80–84. doi: 10.1038/nsmb.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.