Abstract
Obesity has been traditionally considered to protect the skeleton against osteoporosis and fracture. Recently, body fat, specifically visceral adipose tissue (VAT), has been associated with lower bone mineral density (BMD) and increased risk for some types of fractures. We studied VAT and bone microarchitecture in 710 participants (58% women, age 61.3 ± 7.7 years) from the Framingham Offspring cohort to determine whether cortical and trabecular BMD and microarchitecture differ according to the amount of VAT. VAT was measured from CT imaging of the abdomen. Cortical and trabecular BMD and microarchitecture were measured at the distal tibia and radius using high-resolution peripheral quantitative computed tomography (HR-pQCT). We focused on 10 bone parameters: cortical BMD (Ct.BMD), cortical tissue mineral density (Ct.TMD), cortical porosity (Ct.Po), cortical thickness (Ct.Th), cortical bone area fraction (Ct.A/Tt.A), trabecular density (Tb.BMD), trabecular number (Tb.N), trabecular thickness (Tb.Th), total area (Tt.Ar), and failure load (FL) from micro–finite element analysis. We assessed the association between sex-specific quartiles of VAT and BMD, microarchitecture, and strength in all participants and stratified by sex. All analyses were adjusted for age, sex, and in women, menopausal status, then repeated adjusting for body mass index (BMI) or weight. At the radius and tibia, Ct.Th, Ct.A/Tt.A, Tb.BMD, Tb.N, and FL were positively associated with VAT (all p-trend <0.05), but no other associations were statistically significant except for higher levels of cortical porosity with higher VAT in the radius. Most of these associations were only observed in women, and were no longer significant when adjusting for BMI or weight. Higher amounts of VAT are associated with greater BMD and better microstructure of the peripheral skeleton despite some suggestions of significant deleterious changes in cortical measures in the non–weight bearing radius. Associations were no longer significant after adjustment for BMI or weight, suggesting that the effects of VAT may not have a substantial effect on the skeleton independent of BMI or weight. © 2016 American Society for Bone and Mineral Research.
Keywords: GENERAL POPULATION STUDIES, OSTEOPOROSIS EPIDEMIOLOGY, BONE DENSITY, BONE MICROARCHITECTURE, VISCERAL ADIPOSITY
Introduction
Low body mass index (BMI) and low weight are associated with an increased risk of fragility fractures, and obesity has traditionally been considered to protect the skeleton against fracture because endogenous adrenal steroids are converted to estradiol, and because adipose tissue serves to attenuate skeletal loading during a fall. In fact, because adiposity protects the skeleton, the FRAX® (World Health Organization, Geneva, Switzerland/University of Sheffield, Sheffield, UK; http://www.shef.ac.uk/FRAX/reference.aspx) risk predictor depends on the gradient of risk coming from BMI when a bone mineral density (BMD) measure is not available.(1) Despite these observations, the protective effects of increased body mass on skeletal health have recently been challenged.(2) Indeed, the relation between obesity and fracture risk has been conflicting, with some studies demonstrating that obesity decreases the risk of some types of fractures but increases the risk for others,(3,4) and that the contribution of obesity to fracture risk differs by sex.(5,6)
Recently, visceral obesity has been implicated as having an inverse association with BMD,(7–9) although the possibility that the inverse association is due to the effects of obesity on measurement of DXA-derived measures of areal BMD has not been considered. Being metabolically active, visceral adipose tissue (VAT) secretes inflammatory mediators(10) and adipokines,(11) and serves to metabolize steroid hormones(12) that affect skeletal health. However, no large community-based studies in women and men have examined the relation of VAT with bone density, size, morphology, microarchitecture, and strength separately in the cortical and trabecular compartments of bone. The purpose of this study was to investigate the association between visceral adiposity and bone density, microarchitecture, and strength using state-of-the-art imaging of bone in a community based cohort study. We hypothesized that greater VAT would result in less favorable bone measures in the peripheral skeleton.
Subjects and Methods
Study design and participants
The study sample consisted of Framingham Heart Study (FHS) Offspring participants who underwent a computed tomography (CT) scan between the years 2002–2005(13,14) and then took part in the Framingham Osteoporosis Study (2012–2015). In 1948, the FHS recruited the original cohort of 5,209 participants between the ages of 28 and 62 years from the town of Framingham, MA, USA.(15) The Framingham Offspring cohort was begun in 1971 with the recruitment of 5,124 children of members of the Framingham Original cohort and the spouses of offspring, and has been examined approximately every 4 years.(16) A total of 1,418 Offspring participants (age 40 to 85 years) who were members of large families and lived near the scanning site in Framingham, MA, were recruited to participate in the FHS multidetector computed tomography (MDCT) Study to obtain trunk CT scans for vascular calcification.(17) Valid measures of VAT from the CT scans were available on 1,377 participants. Of those, 751 had bone microarchitecture assessment of the radius or tibia completed by high-resolution peripheral quantitative computed tomography (HR-pQCT) as part of the Framingham Osteoporosis Study (2012–2015). We excluded participants with diabetes (n = 41) because diabetes is associated with both VAT and bone density and architecture, and may not be completely accounted for by simple statistical adjustment. The remaining 710 participants (58% female) composed the final study sample. The protocol was reviewed and approved by the Hebrew SeniorLife and Boston University Medical Campus institutional review boards. All participants provided written informed consent.
HR-pQCT at radius and at tibia
FHS Offspring participants (n = 2,430 in total) were invited to attend an Osteoporosis Study call-back visit to have bone microarchitecture measured (2012–2015) using HR-pQCT (XtremeCT; Scanco Medical AG, Bruttisellen, Switzerland)(18) after they completed their a visit for cardiovascular assessments. The HR-pQCT device acquires CT slices with a nominal isotropic voxel size of 82 μm at the distal radius and tibia. We scanned the nondominant forearm and right tibia, unless a participant reported having a previous adult fracture or had metal in the scan region of interest, in which case the contralateral side was scanned. Participants who were pregnant or unable to hold their arm and leg still for 3 min were excluded from having an HR-pQCT scan. The region of interest scanned was identified from a 2D scout view by placing a reference line at the distal endplate of the radius or tibia. The scan region (110 slices) began 9.5 and 22.5 mm proximal to the reference line for the radius and tibia, respectively. A quality control phantom containing rods of hydroxyapatite (HA) (densities of 0, 100, 200, 400 and 800 mg HA/cm3) was scanned daily. Once weekly, a more extensive quality control procedure was performed. All scans were analyzed by one technician and reviewed for quality by the study team. Images were evaluated for movement artifact using a five-point progressive movement scale. Images with the most movement (grade = 5) were excluded, whereas images rated with some movement (grade = 4), were retained for density measures (eg, Tb.BMD, Ct.BMD) but not for architecture (eg, Tb.Th, Ct.Po).(19) Additionally, scans assessed by the study team as technically invalid were also excluded. Precision for HR-pQCT has been previously reported.(18,20)
Ten bone microarchitecture parameters at the both radius and tibia were considered. Four of the 10 were computed using the Scanco “standard analysis” (including trabecular BMD [Tb. BMD, mg HA/cm3], trabecular number [Tb.N, 1/mm], trabecular thickness [Tb.Th, mm], and total area [Tt.Ar, mm2]), whereas five cortical parameters were evaluated using the Scanco “extended cortical analysis” algorithm (including cortical BMD [Ct.BMD, mg HA/cm3], cortical tissue mineral density [Ct.TMD, mg HA/cm3], cortical porosity [Ct.Po, %], cortical thickness [Ct.Th, mm], cortical bone area fraction [Ct.A/Tt.A]), and estimated compressive strength represented as failure load, (FL, N), evaluated by micro–finite element analysis (FEA).(21,22) Axial compression conditions were applied with 1% apparent strain, and a tissue modulus of 6.829 GPa and Poisson’s ratio of 0.3.
VAT measurements
Volumetric CT scans of the spine were obtained in 2002–2005 using multidetector computed tomography (Lightspeed Ultra; General Electric Medical Systems, Milwaukee, WI), as described.(17) Scans were acquired at a tube voltage 120 kVp and had a nominal in-plane voxel size of 0.68 mm and a slice thickness of 2.5 mm. VAT (cm3) was measured from multi-detector CT abdominal imaging by manually tracing its position beneath the abdominal muscular wall, which has >0.99 interreader and intrareader correlations.(17)
Covariates
Covariate information was obtained from the Framingham Offspring Study examination closest to the VAT assessment (2002–2005) and included age (years), height (inches), weight (lbs), BMI, and menopausal status and estrogen use (women). Weight in light clothing was measured to the nearest pound using a balance beam scale. Height without shoes was measured to the nearest ¼-inch using a stadiometer. BMI (kg/m2) was calculated as weight divided by height2 after converting pounds and inches to kg and m, respectively. Estrogenic status in women was classified as “yes” for women currently taking estrogen or before menopause and “no” for women who were postmenopausal (periods stopped for at least 12 months).
Statistical analysis
Statistical analyses were conducted using Statistical Analysis Software SAS version 9.3 (SAS Institute Inc., Cary, NC, USA). We classified participants into four groups based on sex-specific quartiles of VAT measures. To quantify the association between VAT and individual HR-pQCT indices, we performed multiple linear regression analysis with individual HR-pQCT indices as the dependent variable and sex-specific VAT quartiles as the primary independent variable. In our primary analysis, we adjusted for age, sex, height, and additionally estrogenic status in women (model 1). We also performed sex-specific models adjusted for age, height, and estrogenic status (women). Based on the fitted model, we obtained the least squares means of bone microarchitecture measures. We also performed a trend test of these adjusted means across sex-specific quartile of VAT. As a secondary analysis, we additionally included BMI as a covariate in the models (model 2) and then repeated analyses adjusting for weight instead of BMI.
Results
Characteristics of study samples across sex-specific quartile of VAT
Clinical and microarchitecture characteristics of individuals according to sex-specific VAT-quartiles are shown in Table 1 and sex-specific descriptive data are shown in Supporting Table 1. In total, there were 710 participants who had both bone microarchitecture and VAT measurements. The proportions of obese men and women (BMI ≥ 30 kg/m2) were 27.9% and 27.6%, respectively. The proportion of women and the heights of individuals were similar across quartile groups, whereas the average BMI increased across VAT quartiles. The mean volume of VAT was 1966 ± 1008 cm3 with a range of 231 to 5577 cm3.
Table 1.
Clinical and Microarchitecture Characteristics of Each Sex-Specific VAT-Quartile Study Group for All Participants
| Variables | Quartile 1 (n =179) (944 ± 431 cm3; range, 231–1810) |
Quartile 2 (n =177) (1615 ± 502 cm3; range, 940–2500) |
Quartile 3 (n =178) (2174 ± 532 cm3; range, 1483–3140) |
Quartile 4 (n =176) (3168 ± 836 cm3; range, 2000–5577) |
|---|---|---|---|---|
| Women (%) | 57.5 | 57.6 | 57.9 | 57.4 |
| Age (years), mean ± SD | 58.9 ± 8.1 | 60.9 ± 8.0 | 63.2 ± 7.6 | 63.4 ± 7.2 |
| Height (inches) mean ± SD | 66.3 ± 4.1 | 65.9 ± 3.6 | 65.8 ± 3.5 | 66.0 ± 3.4 |
| BMI (kg/m2) mean ± SD | 24.1 ± 2.8 | 26.9 ± 3.6 | 28.4 ± 3.9 | 32.2 ± 4.6 |
| Postmenopausal (% of women) | 69.9 | 75.5 | 83.5 | 87.1 |
| Radius, mean ± SD | ||||
| Ct.BMD (mg HA/cm3) | 967.1 ± 56.2 | 961.1 ± 59.5 | 956.7 ± 59.4 | 943.5 ± 71.5 |
| Ct.TMD (mg HA/cm3) | 1023.5 ± 42.0 | 1017.8 ± 43.5 | 1017.9 ± 44.6 | 1009.9 ± 47.9 |
| Ct.Po (%) | 3.667 ± 1.444 | 3.747 ± 1.579 | 4.029 ± 1.585 | 4.509 ± 3.864 |
| Ct.Th (mm) | 0.858 ± 0.201 | 0.880 ± 0.224 | 0.883 ± 0.219 | 0.895 ± 0.230 |
| Ct.A/Tt.A | 0.202 ± 0.052 | 0.209 ± 0.053 | 0.207 ± 0.055 | 0.210 ± 0.057 |
| Tb.BMD (mg HA/cm3) | 161.1 ± 46.4 | 163.7 ± 46.9 | 165.3 ± 40.9 | 171.1 ± 40.7 |
| Tb.N (1/mm) | 2.025 ± 0.386 | 2.029 ± 0.375 | 2.068 ± 0.394 | 2.142 ± 0.307 |
| Tb.Th (mm) | 0.066 ± 0.012 | 0.067 ± 0.013 | 0.066 ± 0.010 | 0.067 ± 0.012 |
| Tt.Ar (mm2) | 301.0 ± 85.4 | 305.1 ± 83.2 | 306.2 ± 83.2 | 308.4 ± 76.9 |
| FL (N) | 2472 ± 831 | 2567 ± 885 | 2499 ± 739 | 2571 ± 746 |
| Tibia, mean ± SD | ||||
| Ct.BMD (mg HA/cm3) | 864.5 ± 73.1 | 861.2 ± 74.0 | 856.3 ± 86.3 | 848.2 ± 77.1 |
| Ct.TMD (mg HA/cm3) | 980.8 ± 48.5 | 982.3 ± 51.3 | 983.3 ± 46.3 | 976.7 ± 51.2 |
| Ct.Po (%) | 9.452 ± 3.111 | 9.978 ± 2.991 | 10.472 ± 5.914 | 10.644 ± 3.448 |
| Ct.Th (mm) | 1.177 ± 0.296 | 1.216 ± 0.307 | 1.207 ± 0.312 | 1.223 ± 0.299 |
| Ct.A/Tt.A | 0.163 ± 0.046 | 0.170 ± 0.045 | 0.169 ± 0.045 | 0.172 ± 0.046 |
| Tb.BMD (mg HA/cm3) | 166.7 ± 44.3 | 177.1 ± 44.8 | 177.1 ± 39.2 | 183.7 ± 36.1 |
| Tb.N (1/mm) | 1.957 ± 0.391 | 2.093 ± 0.384 | 2.087 ± 0.384 | 2.193 ± 0.388 |
| Tb.Th (mm) | 0.071 ± 0.013 | 0.071 ± 0.012 | 0.071 ± 0.012 | 0.070 ± 0.011 |
| Tt.Ar (mm2) | 775.1 ± 186.5 | 765.8 ± 153.3 | 763.3 ± 157.8 | 778.3 ± 156.3 |
| FL (N) | 6344 ± 1845 | 6499 ± 1934 | 6414 ± 1689 | 6565 ± 1554 |
VAT = visceral adipose tissue; FL = failure load.
At the radius, the unadjusted average values of Ct.BMD and Ct.TMD were lower with higher amounts of VAT, whereas Ct.Po, Ct.Th, Ct.A/Tt.A, and Tt.Ar were higher with greater amounts of VAT. Tb.BMD and Tb.N were higher with greater amounts of VAT, whereas Tb.Th was similar across quartiles of VAT. Similar patterns were observed for the tibial site (Table 1).
Adjusted bone microarchitecture across sex-specific quartile of VAT
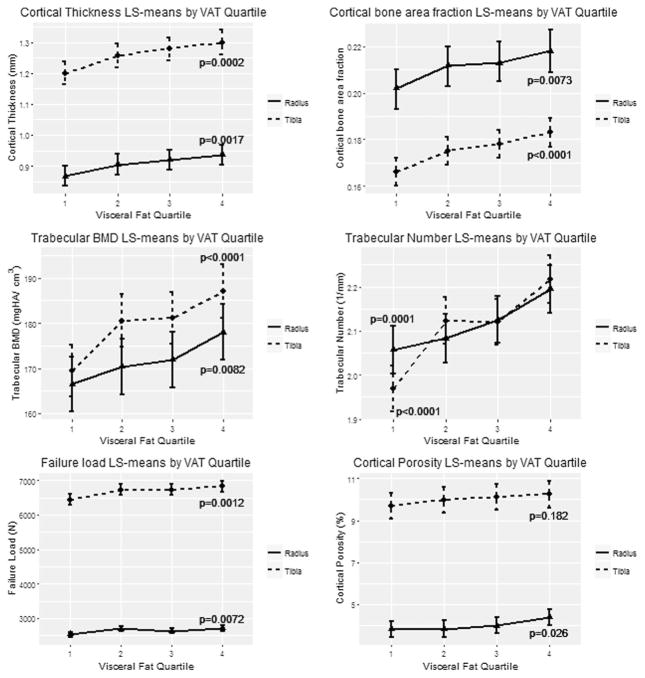
Table 2 presents results adjusted for age, height, and estrogenic status with a test for trend across sex-specific quartiles of VAT. Again, as was true for the unadjusted results, we observed similar findings at the radius and at tibia for microarchitecture measurements across sex-specific quartiles of VAT. Specifically, Ct.BMD decreased (lower in the highest VAT quartile), albeit not significantly, but Ct.Po increased significantly whereas Ct.Th and Ct.A/Tt.A significantly increased with increasing VAT. In the trabecular compartment, Tb.BMD and Tb.N increased across VAT quartiles. Failure load from micro-FEA increased significantly across VAT quartiles. Figure 1 displays the age-, sex-, and height-adjusted bone microarchitecture values for those with significant linear trend across sex-specific quartile of VAT. In secondary analyses of men and women separately, most of the significant associations were only observed in women (Supporting Tables 2 and 3).
Table 2.
Adjusted Least Squares Means Across Sex-Specific Quartiles of VAT (cm3) for All Participants
| Modela,b | Quartile 1 (n = 179) | Quartile 2 (n = 177) | Quartile 3 (n = 178) | Quartile 4 (n = 176) | p-trend | |
|---|---|---|---|---|---|---|
| Radius | ||||||
| Ct.BMD (mg HA/cm3) | Model 1 LSE | 963.1 | 962.1 | 963.5 | 953.2 | 0.181 |
| Model 2 LSE | 966.4 | 962.9 | 962.7 | 949.3 | 0.055 | |
| Ct.TMD (mg HA/cm3) | Model 1 LSE | 1021.5 | 1019.3 | 1022.9 | 1016.9 | 0.520 |
| Model 2 LSE | 1024.7 | 1020.1 | 1022.2 | 1013.1 | 0.095 | |
| Ct.Po (%) | Model 1 LSE | 3.811 | 3.822 | 3.979 | 4.384 | 0.026 |
| Model 2 LSE | 3.836 | 3.827 | 3.974 | 4.355 | 0.112 | |
| Ct.Th (mm) | Model 1 LSE | 0.867 | 0.905 | 0.921 | 0.936 | 0.002 |
| Model 2 LSE | 0.895 | 0.912 | 0.914 | 0.904 | 0.741 | |
| Ct.A/Tt.A | Model 1 LSE | 0.202 | 0.212 | 0.213 | 0.218 | 0.007 |
| Model 2 LSE | 0.207 | 0.213 | 0.212 | 0.212 | 0.538 | |
| Tb.BMD (mg HA/cm3) | Model 1 LSE | 166.5 | 170.3 | 171.9 | 178.0 | 0.008 |
| Model 2 LSE | 168.9 | 170.9 | 171.4 | 175.1 | 0.272 | |
| Tb.N (1/mm) | Model 1 LSE | 2.057 | 2.083 | 2.125 | 2.195 | <0.001 |
| Model 2 LSE | 2.085 | 2.089 | 2.119 | 2.161 | 0.094 | |
| Tb.Th (mm) | Model 1 LSE | 0.067 | 0.068 | 0.067 | 0.068 | 0.647 |
| Model 2 LSE | 0.067 | 0.068 | 0.067 | 0.068 | 0.656 | |
| Tt.Ar (mm2) | Model 1 LSE | 308.2 | 314.6 | 310.9 | 310.8 | 0.810 |
| Model 2 LSE | 314.5 | 316.3 | 309.5 | 303.2 | 0.059 | |
| FL (N) | Model 1 LSE | 2534 | 2701 | 2635 | 2706 | 0.007 |
| Model 2 LSE | 2621 | 2721 | 2614 | 2604 | 0.447 | |
| Tibia | ||||||
| Ct.BMD (mg HA/cm3) | Model 1 LSE | 863.3 | 867.2 | 872.4 | 865.8 | 0.604 |
| Model 2 LSE | 868.9 | 868.4 | 871.1 | 859.1 | 0.388 | |
| Ct.TMD (mg HA/cm3) | Model 1 LSE | 981.5 | 987.4 | 994.3 | 988.5 | 0.066 |
| Model 2 LSE | 987.1 | 988.6 | 992.9 | 981.7 | 0.538 | |
| Ct.Po (%) | Model 1 LSE | 9.681 | 9.982 | 10.128 | 10.248 | 0.182 |
| Model 2 LSE | 9.698 | 9.986 | 10.125 | 10.228 | 0.328 | |
| Ct.Th (mm) | Model 1 LSE | 1.200 | 1.257 | 1.278 | 1.299 | <0.001 |
| Model 2 LSE | 1.232 | 1.264 | 1.271 | 1.261 | 0.381 | |
| Ct.A/Tt.A | Model 1 LSE | 0.166 | 0.175 | 0.178 | 0.183 | <0.001 |
| Model 2 LSE | 0.169 | 0.176 | 0.178 | 0.179 | 0.079 | |
| Tb.BMD (mg HA/cm3) | Model 1 LSE | 169.5 | 180.6 | 181.2 | 187.1 | <0.001 |
| Model 2 LSE | 173.2 | 181.3 | 180.4 | 182.7 | 0.091 | |
| Tb.N (1/mm) | Model 1 LSE | 1.969 | 2.123 | 2.120 | 2.217 | <0.0001 |
| Model 2 LSE | 2.065 | 2.143 | 2.098 | 2.102 | 0.658 | |
| Tb.Th (mm) | Model 1 LSE | 0.071 | 0.071 | 0.071 | 0.071 | 0.837 |
| Model 2 LSE | 0.070 | 0.071 | 0.072 | 0.073 | 0.105 | |
| Tt.Ar (mm2) | Model 1 LSE | 777.2 | 772.7 | 765.9 | 773.3 | 0.568 |
| Model 2 LSE | 799.8 | 777.3 | 760.8 | 745.6 | <0.001 | |
| FL (N) | Model 1 LSE | 6444 | 6725 | 6732 | 6823 | 0.001 |
| Model 2 LSE | 6696 | 6777 | 6669 | 6519 | 0.142 | |
Bold values are significant.
VAT = visceral adipose tissue; FL = failure load.
Model 1: adjusted for age, sex, height, and additionally estrogenic status in women.
Model 2: adjusted for age, sex, height, BMI, and additionally estrogenic status in women.
Fig. 1.
LS mean and confidence levels across quartiles of VAT for various microarchitecture measurements for radius and tibia. Significant increasing linear trends were detected for cortical thickness, cortical bone area fraction, trabecular BMD, trabecular number, failure load, and cortical porosity (radius) at α = 0.05. LS = least squares; VAT = visceral adipose tissue.
In analyses combining both men and women, most of the associations between VAT and bone microarchitecture became nonsignificant after adjusting for BMI, suggesting that VAT may not have a substantial effect on the skeleton independent of BMI.” The one exception was Tt.Ar at the tibia, which was significantly smaller with greater amounts of VAT, and at the radius where the same pattern was observed with a borderline p value. In sex-specific analyses adjusting for BMI, higher Tb.BMD and Tb.N remained significantly associated with higher VAT quartiles at the radius and Tb.BMD at the tibia in women, whereas total cross-sectional area at both skeletal sites was significantly lower across VAT quartiles in men and lower for the tibia in women (Supporting Tables 2 and 3). We also performed the analysis adjusting for body weight instead of BMI and the results showed similar pattern as the analysis adjusting for BMI (results not shown).
Discussion
To our knowledge, this is the first study to assess the association of VAT with measures of bone microarchitecture in a large cohort of both men and women using HR-pQCT. At the non–weight bearing radius site, with increasing amounts of VAT, we observed a nonsignificant trend of decreasing cortical bone mineral density, significantly increased porosity, yet a greater cortical area fraction and thickness of cortical bone. Trabecular density and number were increased as VAT increased. Ultimately, despite some deleterious effects of VAT on cortical bone, the overall bone strength was greater in those with larger amounts of VAT. The observation that these associations became nonsignificant after adjusting for BMI suggests that the effect of VAT may actually be due to the mechanical loading of the skeleton in obese individuals. These results imply that if certain fractures are more common in those with visceral adiposity, fracture risk might not be due to skeletal fragility, but rather to other factors such as skeletal loading during a fracture event. At the weight bearing tibia, we did not observe any significant trends in cortical bone mineral density or porosity, but the same increases in cortical thickness and cortical area fraction that we observed in the radius were also seen at the tibia. Trabecular indices such as density and number were greater in those individuals with increasing VAT. In addition, the overall strength of the tibia increased with increasing levels of VAT. When we examined these associations in men and women separately, we noted that most of the associations were driven by results in the women. Finally, we also observed that the associations were generally in the same direction but no longer significant when we adjusted for BMI.
There have been several studies of the association between obesity and various measures of bone density and architecture, and one large study of VAT and spine bone density measured using qCT, but none of these studies have specifically examined VAT and bone microarchitecture in men and women as we have done in this investigation. Evans and colleagues(23) assessed bone microarchitecture in obese men and women who were matched by sex, age, height, and smoking status with a non-obese individual. Similar to our results they found that the obese individuals had greater cortical and trabecular indices. Our study extended these findings by focusing specifically on the visceral fat component of obesity. We did not confirm that VAT independently contributed to bone microarchitecture once BMI was considered, except for a decrease in the total cross-sectional area of bone in those with greater amounts of VAT. This was observed in both men and women at the tibia as well as at the radius in men. One could speculate that large amounts of VAT may interfere with mechanotransduction in the long bones of individuals with comparable body weight, resulting in smaller cross-sectional area. In fact, in men the failure load at the tibia was lower in the higher VAT quartiles, although the p value was borderline significant (p = 0.07). In a small study of 50 obese nondiabetic adults under age 50 years with metabolic syndrome, fat mass measured by DXA was not associated with any HR-pQCT measures, but there were no measures of VAT available.(24)
Using CT scans from a large University of Michigan health system, Zhang and colleagues(25) showed that both spinal cortical and trabecular bone density were inversely correlated with visceral adipose area even after adjusting for BMI. They concluded that the metabolically active VAT had deleterious effects on cortical and trabecular bone that oppose the seemingly positive influence of a greater mechanical loading with higher body mass. These findings of an inverse association between VAT and bone density are opposite to our findings. One explanation for this could be that visceral fat has paracrine effects such that skeletal sites near the adipose tissue may be affected differently than more remote skeletal sites. Thus, in the Zhang and colleagues(25) article, spinal BMD was negatively associated with VAT whereas in our work and in the work by Evans and colleagues,(23) distal peripheral skeletal sites were not adversely affected. Such data suggests the possibility that at these sites, the loading effect of VAT may be the predominant factor on the skeletal indices. On the other hand, a small study(2) of 35 obese men found that those with VAT higher than the median had lower trabecular density, thickness, and bone strength of the radius compared with subjects with low VAT, despite similar lumbar spine and hip BMD determined by DXA, although there were no differences in cortical density, thickness, or area. These findings of an inverse association between visceral adiposity and peripheral skeletal bone microarchitecture are opposite to our findings, and would argue in favor of visceral adiposity having systemic effects on the skeleton rather than paracrine effect on the axial skeleton. This study examined a very small sample of young obese men and did not include any non-obese controls, making it difficult to directly compare with our own results in a sample of older men and women of varying body composition.
Traditionally, obesity has been perceived as potentially protective for the skeleton because of skeletal loading effects of increased fat tissue, or even due to peripheral sex steroid production in adipose tissue. Cardiometabolic studies have clearly identified VAT as a risk for adverse health outcomes such as left ventricular concentricity, a harbinger of heart failure(26) myocardial infarction,(27) and multiple cardiovascular risk factors.(28–30) Mechanisms that have been proposed to explain the effects of visceral adiposity on end-organ disease include the portal free fatty acid theory in which VAT might alter lipoprotein metabolism by inducing an overproduction of large triglyceride-rich very low density lipoproteins,(31) and secretion of proinflammatory adipocyte-derived cytokines(10) that may have deleterious effects on the skeleton.(32,33)
There are several strengths of our study. First we are the first study using state-of-the-art imaging to measure VAT and state-of-the-art measures of bone microarchitecture in the cortical and trabecular compartments. Second, this is the largest community-based study to assess the association between VAT and bone microarchitecture in men and women. By excluding those with type 2 diabetes mellitus, which has its own distinct effects on skeletal fragility, we were able to determine visceral adiposity associations with skeletal measures independent of the effects of diabetes.
Some of the limitations of this study include the fact that we had fewer men than women, which may have reduced our power to find associations, especially in men. Second, all of the participants in this study were of European ancestry, limiting the generalization of our results to other ethnic groups. Third, although better than any other in vivo imaging technique, the resolution of the first-generation HR-pQCT scanner precludes direct analysis of trabecular thickness. Rather, trabecular thickness is derived from trabecular density and trabecular number, assuming a plate-like structure. Further, the threshold-based approach for detection of cortical porosity likely underestimated cortical porosity. In particular, although the accuracy of assessment of pores >140 μm is excellent,(34) the threshold-based cortical porosity measurement that we used may have missed very small pores and therefore underestimated the absolute value of porosity. However, given the very strong association between threshold-based porosity measurements and those from synchrotron radiation μCT (r2 = 0.94),(34) we argue that the associations between cortical porosity and VAT would likely be similar to what we report here if one were to employ imaging with improved resolution and/or use a density-based approach to assess cortical porosity. Fourth, the HR-pQCT measures were collected 10 years after VAT was measured. This 10-year gap limits our ability or power to assess the association, considering that VAT may vary over time. On the other hand, measurement of VAT 10 years before the HR-pQCT measures makes our study “quasi-prospective” in design. This design may have some advantages over a pure cross-sectional study to assess the association between VAT and bone strength. In addition, although variations in bone marrow fat could have influenced our HR-pQCT measures, the likelihood that this affected our results is low because bone marrow physiologically converts from red to yellow marrow by the age of 25 years.(35) Furthermore, although changes from yellow marrow to red marrow may occur in response to environmental or medical conditions, the likelihood of this change is so low that we expect a constant pattern of marrow adiposity in individual bones and the whole skeleton.
In conclusion, our findings suggest that changes in density and microstructure of the peripheral skeleton with increasing amounts of VAT result in mechanically stronger bone predominantly in women despite some suggestions of significant deleterious changes in cortical measures in the non–weight bearing radius. The observation that the associations were no longer significant after adjustment for BMI or weight suggests that the effects of VAT on the skeleton might be related to the loading effects of VAT rather than metabolic effects. These results imply that if the risk for fractures is increased in individuals with greater amounts of visceral adiposity, this may be due to factors other than skeletal strength of the mineralized bone tissue, such as increased loading during a fall. Future work should focus on whether visceral obesity increases the risk for incident fractures at various skeletal sites to gauge the true public health impact of visceral adiposity on skeletal health.
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of Arthritis Musculoskeletal and Skin Diseases of the National Institutes of Health under Award Number R01AR061445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Additional support was provided by Friends of HebrewSeniorLife and a research grant from the Investigator Initiated Studies Program of Merck Sharp & Dohme. The opinions expressed in this work are those of the authors and do not necessarily represent those of Merck.
Authors’ roles: Study conception and design: DPK. Acquisition of data: MLB and DPK. Analysis and interpretation of data: CTL, KEB, YZ, EL, LAC, MLB, and DPK. Drafting of the manuscript: CTL, KEB, YZ, and DPK. Critical revision and enhancing the manuscript for intellectual content: all authors. Approval of the final version: all authors.
Footnotes
Additional Supporting Information may be found in the online version of this article
Disclosures
All authors state that they have no conflicts of interest. DPK received grant support from Merck Sharp & Dohme in the form of an investigator initiated research grant but retained full independence in use and reporting of data generated from this funding.
References
- 1.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. 2008;19(4):385–97. doi: 10.1007/s00198-007-0543-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bredella MA, Lin E, Gerweck AV, et al. Determinants of bone microarchitecture and mechanical properties in obese men. J Clin Endocrinol Metab. 2012;97(11):4115–22. doi: 10.1210/jc.2012-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Compston JE, Watts NB, Chapurlat R, et al. Obesity is not protective against fracture in postmenopausal women: GLOW. Am J Med. 2011;124(11):1043–50. doi: 10.1016/j.amjmed.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gnudi S, Sitta E, Lisi L. Relationship of body mass index with main limb fragility fractures in postmenopausal women. J Bone Miner Metab. 2009;27(4):479–84. doi: 10.1007/s00774-009-0056-8. [DOI] [PubMed] [Google Scholar]
- 5.Laslett LL, Just Nee Foley SJ, Quinn SJ, Winzenberg TM, Jones G. Excess body fat is associated with higher risk of vertebral deformities in older women but not in men: a cross-sectional study. Osteoporos Int. 2012;23(1):67–74. doi: 10.1007/s00198-011-1741-8. [DOI] [PubMed] [Google Scholar]
- 6.Moayyeri A, Luben RN, Wareham NJ, Khaw KT. Body fat mass is a predictor of risk of osteoporotic fractures in women but not in men: a prospective population study. J Intern Med. 2012;271(5):472–80. doi: 10.1111/j.1365-2796.2011.02443.x. [DOI] [PubMed] [Google Scholar]
- 7.Cohen A, Dempster DW, Recker RR, et al. Abdominal fat is associated with lower bone formation and inferior bone quality in healthy premenopausal women: a transiliac bone biopsy study. J Clin Endocrinol Metab. 2013;98(6):2562–72. doi: 10.1210/jc.2013-1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katzmarzyk PT, Barreira TV, Harrington DM, Staiano AE, Heymsfield SB, Gimble JM. Relationship between abdominal fat and bone mineral density in white and African American adults. Bone. 2012;50(2):576–9. doi: 10.1016/j.bone.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell M, Mendes N, Miller KK, et al. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab. 2010;95(3):1247–55. doi: 10.1210/jc.2009-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pou KM, Massaro JM, Hoffmann U, et al. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress: the Framingham Heart Study. Circulation. 2007;116(11):1234–41. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 11.Jain SH, Massaro JM, Hoffmann U, et al. Cross-sectional associations between abdominal and thoracic adipose tissue compartments and adiponectin and resistin in the Framingham Heart Study. Diabetes Care. 2009;32(5):903–8. doi: 10.2337/dc08-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corbould AM, Bawden MJ, Lavranos TC, Rodgers RJ, Judd SJ. The effect of obesity on the ratio of type 3 17beta-hydroxysteroid dehydrogenase mRNA to cytochrome P450 aromatase mRNA in subcutaneous abdominal and intra-abdominal adipose tissue of women. Int J Obes Relat Metab Disord. 2002;26(2):165–75. doi: 10.1038/sj.ijo.0801886. [DOI] [PubMed] [Google Scholar]
- 13.Maurovich-Horvat P, Massaro J, Fox CS, Moselewski F, O’Donnell CJ, Hoffmann U. Comparison of anthropometric, area- and volume-based assessment of abdominal subcutaneous and visceral adipose tissue volumes using multi-detector computed tomography. Int J Obes (Lond) 2007;31(3):500–6. doi: 10.1038/sj.ijo.0803454. [DOI] [PubMed] [Google Scholar]
- 14.Moselewski F, O’Donnell CJ, Achenbach S, et al. Calcium concentration of individual coronary calcified plaques as measured by multidetector row computed tomography. Circulation. 2005;111(24):3236–41. doi: 10.1161/CIRCULATIONAHA.104.489781. [DOI] [PubMed] [Google Scholar]
- 15.Dawber TR, Meadors GF, Moore J, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41(3):279–86. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110(3):281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 17.Fox CS, Massaro JM, Hoffmann U, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 18.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005;90(12):6508–15. doi: 10.1210/jc.2005-1258. [DOI] [PubMed] [Google Scholar]
- 19.Pialat JB, Burghardt AJ, Sode M, Link TM, Majumdar S. Visual grading of motion induced image degradation in high resolution peripheral computed tomography: impact of image quality on measures of bone density and micro-architecture. Bone. 2012;50(1):111–8. doi: 10.1016/j.bone.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Khosla S, Riggs BL, Atkinson EJ, et al. Effects of sex and age on bone microstructure at the ultradistal radius: a population-based noninvasive in vivo assessment. J Bone Miner Res. 2006;21(1):124–31. doi: 10.1359/JBMR.050916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pistoia W, van Rietbergen B, Lochmüller EM, Lill CA, Eckstein F, Rüegsegger P. Estimation of distal radius failure load with micro-finite element analysis models based on three-dimensional peripheral quantitative computed tomography images. Bone. 2002;30(6):842–8. doi: 10.1016/s8756-3282(02)00736-6. [DOI] [PubMed] [Google Scholar]
- 22.Mueller TL, Christen D, Sandercott S, et al. Computational finite element bone mechanics accurately predicts mechanical competence in the human radius of an elderly population. Bone. 2011;48(6):1232–8. doi: 10.1016/j.bone.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 23.Evans AL, Paggiosi MA, Eastell R, Walsh JS. Bone density, microstructure and strength in obese and normal weight men and women in younger and older adulthood. J Bone Miner Res. 2015;30(5):920–8. doi: 10.1002/jbmr.2407. [DOI] [PubMed] [Google Scholar]
- 24.Madeira E, Mafort TT, Madeira M, et al. Lean mass as a predictor of bone density and microarchitecture in adult obese individuals with metabolic syndrome. Bone. 2014;59:89–92. doi: 10.1016/j.bone.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P, Peterson M, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr. 2015;101(2):337–43. doi: 10.3945/ajcn.113.081778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abbasi SA, Hundley WG, Bluemke DA, et al. Visceral adiposity and left ventricular remodeling: the Multi-Ethnic Study of Atherosclerosis. Nutr Metab Cardiovasc Dis. 2015;25(7):667–76. doi: 10.1016/j.numecd.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Diaz AA, Young TP, Kurugol S, et al. Abdominal visceral adipose tissue is associated with myocardial infarction in patients with COPD. Chronic Obstr Pulm Dis (Miami) 2015;2(1):8–16. doi: 10.15326/jcopdf.2.1.2015.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Fox CS, Hickson D, Bidulescu A, Carr JJ, Taylor HA. Fatty liver, abdominal visceral fat, and cardiometabolic risk factors: the Jackson Heart Study. Arterioscler Thromb Vasc Biol. 2011;31(11):2715–22. doi: 10.1161/ATVBAHA.111.234062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Fox CS, Hickson DA, et al. Impact of abdominal visceral and subcutaneous adipose tissue on cardiometabolic risk factors: the Jackson Heart Study. J Clin Endocrinol Metab. 2010;95(12):5419–26. doi: 10.1210/jc.2010-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosito GA, Massaro JM, Hoffmann U, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117(5):605–13. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 31.Björntorp P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10(4):493–6. [PubMed] [Google Scholar]
- 32.Sponholtz TR, Zhang X, Fontes JD, et al. Association between inflammatory biomarkers and bone mineral density in a community-based cohort of men and women. Arthritis Care Res (Hoboken) 2014;66(8):1233–40. doi: 10.1002/acr.22270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barbour KE, Lui LY, Ensrud KE, et al. Inflammatory markers and risk of hip fracture in older white women: the study of osteoporotic fractures. J Bone Miner Res. 2014;29(9):2057–64. doi: 10.1002/jbmr.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jorgenson BL, Buie HR, McErlain DD, Sandino C, Boyd SK. A comparison of methods for in vivo assessment of cortical porosity in the human appendicular skeleton. Bone. 2015;73:167–75. doi: 10.1016/j.bone.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 35.Ricci C, Cova M, Kang YS, et al. Normal age-related patterns of cellular and fatty bone marrow distribution in the axial skeleton: MR imaging study. Radiology. 1990;177(1):83–8. doi: 10.1148/radiology.177.1.2399343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.