Abstract
The formation of primordial follicles to establish a reservoir of resting follicles and the gradual depletion of that reservoir to provide a succession of growing follicles are key to female fertility, but little is known about the regulation of these early stages of follicular development. This review summarizes the efforts of our laboratory to elucidate these critical processes in cattle. Primordial follicles first appear in fetal ovaries around the end of the first trimester of pregnancy (Day 90), during a decline in fetal ovarian production of estradiol and progesterone. In ovarian cortical pieces from 90 to 120-day-old fetuses, follicles form in vitro and estradiol or progesterone inhibits follicle formation, whereas the non-aromatizable androgen 5α-dihydrotestosterone (DHT) does not. Newly formed bovine follicles are not capable of activating within 2 days in vitro, but they can acquire the capacity to activate during a longer culture; estradiol and progesterone inhibit the acquisition of their capacity to activate. When primordial follicles first form in cattle, their oocytes are not yet in meiotic arrest and acquisition of competence to activate is correlated with their progression to meiotic arrest at the diplotene stage of first prophase. After they acquire the competence to activate, bovine primordial follicles can be stimulated to activate in vitro by insulin or kit ligand, whereas anti-Mullerian hormone (AMH) is inhibitory. Although few follicles progress to the secondary stage in vitro, addition of testosterone or vascular endothelial growth factor (VEGF) dramatically increased the incidence of that transition. Regulation of the earliest stages of follicular development is complex and far from understood; better understanding could lead to new interventions to enhance fertility.
Introduction
Mammalian ovaries have a stockpile of primordial follicles. Primordial follicles are non-growing and consist of an oocyte surrounded by a single layer of granulosa cells (Fig. 1A). Once this follicular reservoir has been formed, follicles gradually exit the resting stage to become growing, primary follicles (Fig. 1A). Establishment of the follicular reserve of primordial follicles and the gradual depletion of that resting pool to provide a steady supply of growing follicles are processes critical for female reproduction. Yet little is known about the signals that initiate follicle assembly or those that initiate follicular growth, especially in non-rodent mammalian species. Our laboratory has developed in-vitro models for studying the regulation of follicle formation, the acquisition of follicular capacity to activate (i.e. initiate growth), follicle activation, and the primary to secondary follicle transition. This review will summarize the results obtained with these models.
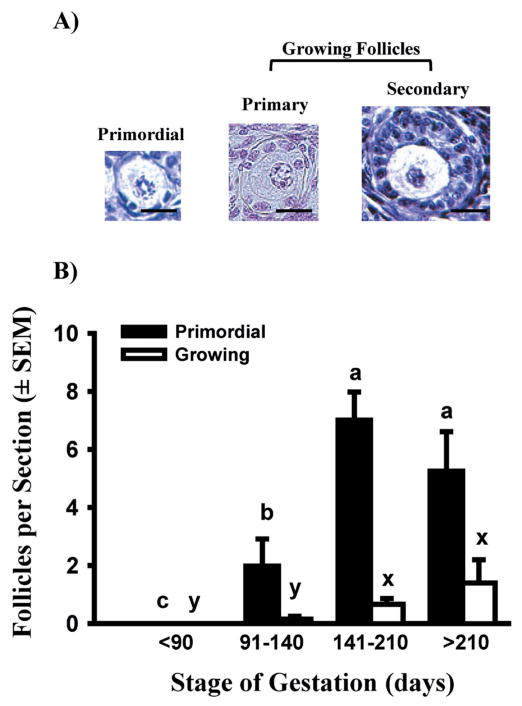
Fig. 1.
A) Photomicrographs of early stages of bovine follicular development. Bar = 20 μm. B) Numbers of primordial and growing follicles (only primary follicles before Day 211; primary plus occasional secondary follicles after Day 210) in freshly isolated bovine cortical pieces (means ± SEM). Black bars show data for primordial follicles, and white bars represent growing follicles. Means with different letters within follicle type (primordial: a, b, c; growing: x, y) across gestational stage are significantly different (P < 0.05). (Adapted from Yang & Fortune 2008, with permission.)
Summary of experimental methods
Ovaries are dissected from bovine fetuses at a nearby abattoir (Cargill Regional Beef, Wyalusing, PA) and transported to the laboratory at ambient temperature. At the laboratory, the ovarian cortex is microdissected from the underlying medulla and the cortex is cut into small pieces of about 0.5 mm3. Pieces are cultured for the desired time period on culture inserts (2 pieces/insert) in the wells of 24-well plates in 300 μl of Waymouth’s medium MB 752/1 containing ITS+ (Insulin, Transferrin, and Selenium + BSA and linoleic acid) in a humidified incubator gassed with 5% CO2:95% air at 38.5 C. Within each experimental replicate (fetus), treatments in vitro are applied to duplicate wells. Some medium (200 μl) is removed and replaced with fresh medium every other day. Cortical pieces are retrieved at the end of culture, fixed in Tousimis fixative (glutaraldehyde and formaldehyde), and processed for embedding in LR White plastic. Serial sections (2 μm) are cut with an ultramicrotome and every 20th section is analyzed for numbers and types of follicles and follicular health. Further methodological details are available in Wandji et al. (1996) and Yang & Fortune (2008). Departures from these basic methods will be noted in the sections below.
Follicle formation in cattle
In rats and mice, follicles form fairly synchronously shortly after birth. However, in ruminants and primates, follicles form during fetal life and in an apparently less synchronous fashion than in rodents. Exactly when follicles begin to form in cattle is unclear, since estimates by different laboratories vary from day 74 to day 130 of gestation, even within the same breed (Erickson 1966; Russe 1983; Dominguez et al. 1988; Tanaka et al. 2001; Garverick et al. 2010). The reason for these discrepancies is not clear, but they may be due to how crown-rump length (used to estimate gestational age) was measured and/or the number of fetal time points examined. Because of the lack of consistency in the literature, we re-examined the timing of follicular formation and the first appearance of activated (primary) follicles during bovine gestation, as a prelude to further studies. Primordial follicles have an oocyte and a single flattened layer of granulosa cells; their activation produces primary follicles with a single layer of cuboidal granulosa cells and a growing oocyte (Fig. 1A). Only occasionally were primordial follicles observed in fetal ovaries obtained before day 90 of pregnancy. Between day 91 and day 140, the number of primordial follicles increased, but then numbers appeared to level off between day 141 and day 180 (Fig. 1B). Primary follicles were observed only rarely until after day 140 (Fig. 1B).
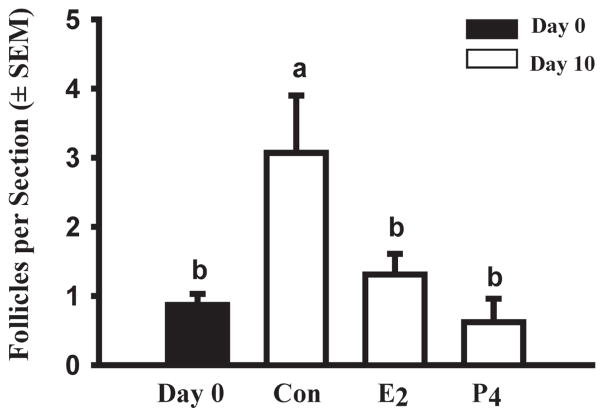
When we cultured pieces of ovarian cortex from ovaries obtained at day 91–140 of gestation in medium containing ITS+, the results indicated that follicles formed during the 10-day culture period (Fig. 2, Day 0 vs. Control) and this suggested that we could use this in-vitro model to study the regulation of follicle formation. Studies by Pepling’s laboratory have provided evidence that supports the hypothesis that follicles form shortly after birth in mice in response to withdrawal from the inhibitory effects of circulating maternal steroids (Jefferson et al. 2006; Chen et al. 2007). Experiments with newborn rat ovaries suggested that progesterone and estradiol also inhibit follicle assembly in that species (Kezele et al. 2003; Nilsson et al. 2006). There is evidence in the older literature that fetal bovine ovaries produce steroids, especially during the first trimester of pregnancy, before follicle formation commences (Shemesh et al. 1978; Dominguez et al. 1988) and the ovaries of cattle and sheep have mRNA and protein for SF-1 and steroidogenic enzymes (Quirke et al. 2001; Garverick et al. 2010). Therefore, we determined the effects of estradiol and progesterone (both at 10−6 M) on the number of follicles after 10 days of culture. Both steroids completely inhibited the increase in follicular number that occurred in control cultures (Fig. 2). Nilsson & Skinner (2009) also reported an inhibitory effect of progesterone on the assembly of bovine follicles.
Fig. 2.
Effects of steroids on bovine follicle formation in vitro. Numbers of follicles (primordial + primary) in ovarian cortical pieces from around day 100 of gestation after 0 (black bar) or 10 (white bar) days in culture with control medium (Con), estradiol (E2) or progesterone (P4; both at 10−6M). Data are means ± SEM; means with different letters (a, b) differ significantly (P < 0.05; n = 2 fetuses).
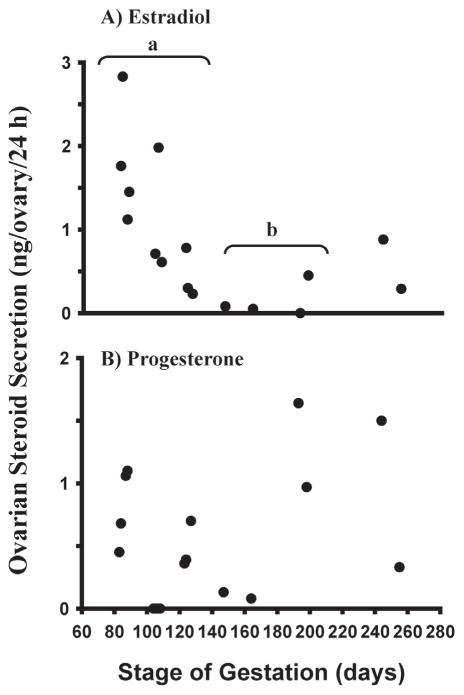
To determine whether our results in vitro are consistent with changes in fetal ovarian steroid production in vivo, we next measured secretion of estradiol and progesterone during a 24-hour culture by whole minced ovaries from different stages of gestation (see Yang & Fortune 2008 for experimental details). The results showed that estradiol production declined dramatically between day 80 to 100 of gestation, the period when follicle formation begins, and declined to even lower levels between day 100 to 140, when the first primary follicles appear (Fig. 3). Although progesterone production appeared to decline between day 80 and 140, the pattern was more erratic and the differences were not significant (Fig. 3). The higher levels of both progesterone and estradiol during later gestation are likely due to the increasing number of growing (sometimes antral) follicles at that time. In contrast to the results for estradiol and progesterone, concentrations of testosterone and androstenedione were always very low in the culture medium (Yang & Fortune 2008). It is important to note that the data in Fig. 3 are expressed per ovary and that ovarian size changes greatly over the sampling period. We are currently weighing ovaries before experiments on steroid production and indeed, the changes in ovarian steroid production appear much more pronounced expressed per wt. ovarian tissue, as recently illustrated for progesterone by Nilsson & Skinner (2009). One might wonder whether maternal steroids influence developmental events in the fetal ovary. Circulating maternal estradiol is very low during bovine pregnancy and although progesterone concentrations vary, the highest levels are not very different from levels during the luteal phase of the cycle (Senger 2003). In addition, fetal red blood cells have a powerful 20α-reductase, which converts progesterone to 20α-dihydroprogesterone (P.W. Nathanielsz, personal communication).
Fig. 3.
Estradiol and progesterone secretion (ng/ovary/24 h) in vitro by ovaries from bovine fetuses at different gestational ages (means ± SEM). Ovaries were cut into pieces (0.5 – 1 mm3). The pieces were then cultured for 24 h and steroid concentrations in the culture medium were determined by RIA. a,b: mean estradiol levels differ (P<0.05). (From Yang & Fortune 2008, with permission.)
In summary, the data support the hypothesis that estradiol and/or progesterone made by the fetal ovary inhibit follicle formation in cattle and that the decline in ovarian production of one or both steroids provides an intra-ovarian signal that initiates follicle formation. Clearly there is much more work to be done to confirm this hypothesis and to determine the steroids involved in vivo and the mechanisms of steroid action.
Acquisition by primordial follicles of the capacity to activate
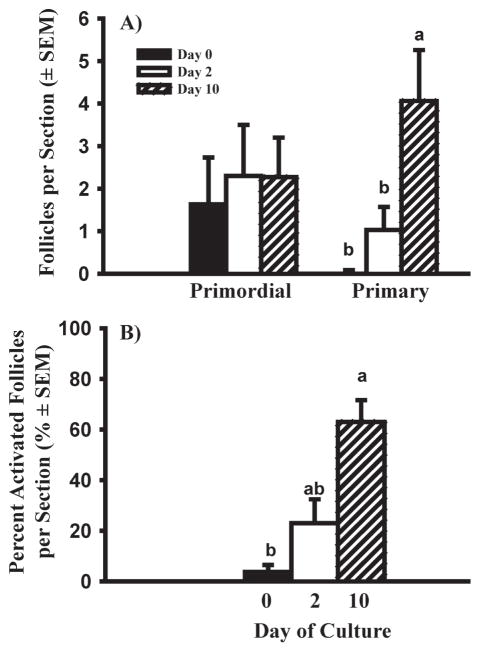
As mentioned above, newly formed primordial follicles do not proceed to primary stage (activate) in vivo until around day 141 of gestation (Fig. 1). Thus there is about a 50 day gap between follicle formation and the first observed follicle activation in vivo. There are similar gaps between these two developmental events, of 25 and 40 days, in fetal sheep and human ovaries, respectively (van Wagenen & Simpson 1965; McNatty et al. 1995). We hypothesized that the gap might occur because 1) the follicles are incapable of activating, 2) they are capable, but an activation signal is missing, or 3) they are capable, but activation is inhibited by a circulating or local factor until a certain stage of gestation. To begin to test these hypotheses, we cultured ovarian cortical pieces from ovaries at day 91–140 of gestation. Although primordial follicles in cortical pieces from fetal ovaries older than day 140, or from adult cattle, can activate within 2 days of culture (Wandji et al. 1996; BrawTal & Yossefi 1997), primordial follicles from 91–140 day-old fetuses did not (Fig. 4). However, when the culture period was extended to 10 days, about 65% of the follicles had activated by day 10 of culture (Fig. 4).
Fig. 4.
Effects of length of culture on follicle activation in bovine cortical pieces dissected from fetuses at 91–140 days of gestation. A) Number of primordial and primary follicles in fetal bovine ovarian cortex after 0, 2 and 10 days in culture. B) Percent activated (primary) follicles. Data are means ± SEM; within each panel means with no common letters (a, b) differ significantly across culture period (0, 2, and 10 days) (P < 0.05; n = 4 fetuses). (From Yang & Fortune 2008, with permission.)
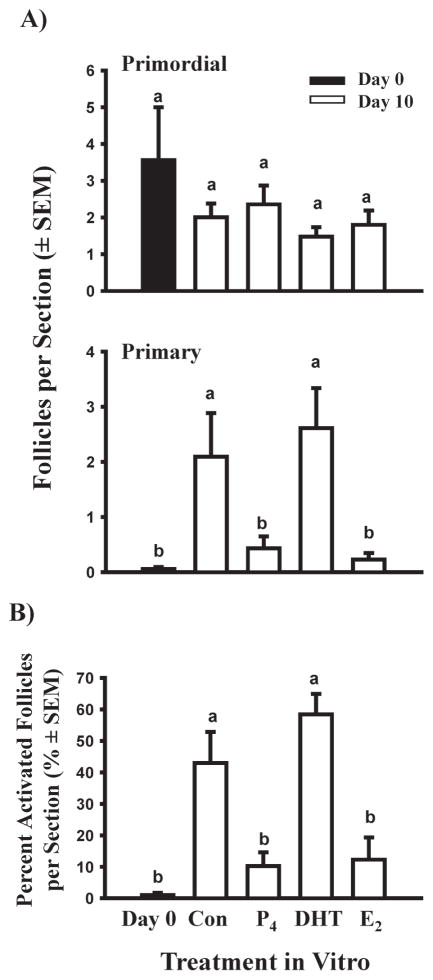
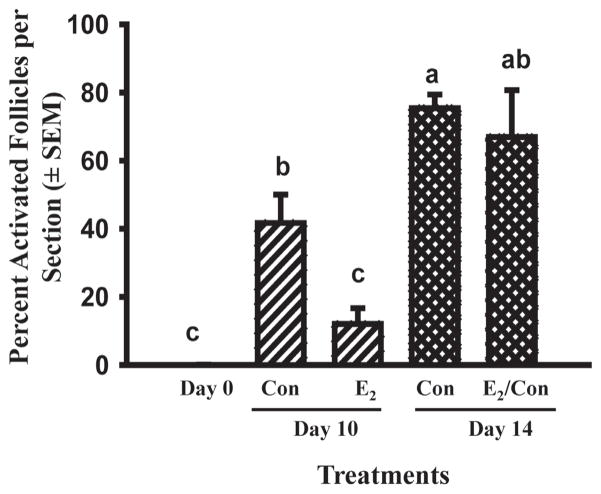
The data in Fig. 4 show that newly formed primordial follicles acquire the capacity to activate once they are removed from the milieu of the whole ovary in vivo and this suggests that in vivo there is an inhibitor(s) that prevents them from developing the capacity to activate. Based on results for newborn mouse ovaries (Jefferson et al. 2006; Chen et al. 2007) and the temporal patterns of fetal ovarian production of estradiol and progesterone in cattle (Fig. 3), the effects of adding estradiol, progesterone, or the non-aromatizable androgen DHT (10−6M) to cortical cultures derived from 91–140 day-old fetuses were tested. After 10 days of culture, about 45–60% of follicles had progressed to the primary stage (activated) in control medium (containing ITS+) or medium with DHT (Fig. 5; Yang & Fortune 2008). In contrast, in the presence of estradiol or progesterone, the number of primary follicles and the percentage of follicles that had activated were much lower and not significantly different from the day 0 control (Fig. 5). More recent experiments have shown that the effects of estradiol and progesterone are dose-dependent and that the effect of estradiol on follicle activation can be reversed when estradiol is removed from the culture medium (Fig. 6; Yang & Fortune, unpublished data).
Fig. 5.
Effects of control medium (Con), progesterone (P4), 5α-dihydrotestosterone (DHT), and estradiol (E2; all at 10−6M) on follicle activation in vitro in ovarian cortical pieces from 91 to 140-day-old fetal calves cultured for 10 days. A) Number of primordial and primary follicles in fetal bovine ovarian cortex after 0 or 10 days in culture. B) Percent activated (primary) follicles. Data are means ± SEM; within each panel means with no common letters (a, b) differ significantly (P < 0.05; n = 4 fetuses). (From Yang & Fortune 2008, with permission.)
Fig. 6.
Percentage of activated follicles in bovine cortical pieces cultured with control medium (Con) or estradiol (E2; 10−6M) for 10 or 14 days (means ± SEM). E2/Con: Day 0–10 cultured with E2 and Day 10–14 with control medium. Cortical pieces were dissected from 100 to 120-day-old fetal calves. Means with no common letters (a, b, c) differ significantly (P < 0.05; n = 2 fetuses).
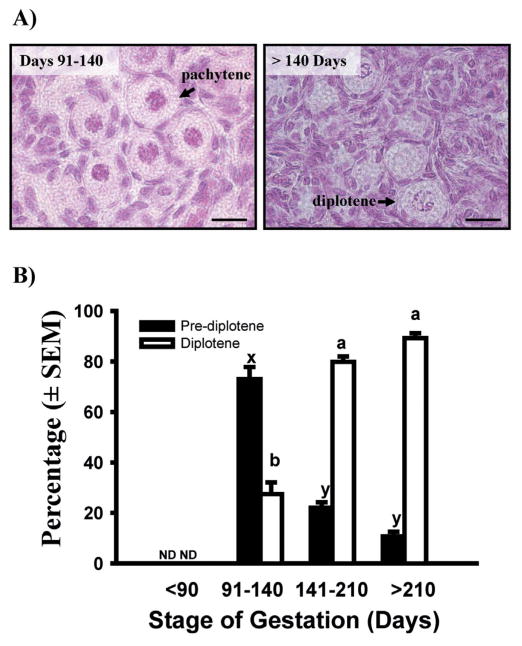
Although there is disagreement among authors about when bovine oocytes finally reach meiotic arrest in the diplotene stage of first prophase of meiosis (Henricson & Rajakoski 1959; Erickson 1966; Baker & Franchi 1967; Russe 1983), Baker & Franchi (1967) reported that bovine primordial follicles form before their oocytes have achieved meiotic arrest. Therefore, we hypothesized that the gap between the first appearance of primordial and primary follicles is necessary to allow the first meiotic prophase to progress to the diplotene stage. Ovaries obtained from fetuses at various stages of gestation were fixed in Bouin’s, embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin to allow visualization of the chromatin under light microscopy (Yang & Fortune 2008). The results showed that between 91–140 days of gestation most oocytes are at pre-diplotene stages (mostly pachytene), but after day 140 most oocytes are at the resting, diplotene stage (Fig. 7). In addition, when cortical pieces at similar fetal ages were analyzed for levels of mRNA for YBX2, a biochemical marker for diplotene oocytes, the results reflected closely the change in the proportion of diplotene vs. pre-diplotene oocytes that occurred between day 91–140 and after day 140 (Yang & Fortune 2008). The results of more recent experiments showed that when estradiol inhibits follicle activation, there is also an inhibition of the progression of first meiotic prophase and this inhibition is reversed when cortical pieces are switched to control medium (Yang & Fortune, unpublished data).
Fig. 7.
A) Photomicrographs showing bovine primordial follicles with oocytes at the pachytene or diplotene stage of first meiotic prophase. Bar = 20 μm B) Percentage of oocytes in primordial follicles at pre-diplotene (leptotene, zygotene, or pachytene) and diplotene stages of first meiotic prophase in bovine fetal ovaries collected at different stages of gestation (means ± SEM). Means with no common letters within meiotic stage across gestational stage (pre-diplotene: x, y; diplotene: a, b) are significantly different (P < 0.05; n = 3–6 fetuses per gestational stage; with 46–207 oocytes in primordial follicles examined per fetus). ND: not detected. (Adapted from Yang & Fortune 2008, with permission.)
Regulation of follicle activation after capacity to activate has been acquired
When whole newborn mouse ovaries are cultured, a subset of the newly formed follicles activates and a subset of those primary follicles proceeds to the secondary stage (with two or more layers of granulosa cells) within 8 days in vitro, roughly similar to the progression of follicular development during the same time period in vivo (Eppig & O’Brien 1996; Gigli et al. 2005). About 15 years ago, in collaboration with Dr. Eppig, our laboratory developed methods for studying bovine follicle activation in vitro. Small pieces of ovarian cortex (about the same size as newborn mouse ovaries) were isolated from fetal ovaries late in gestation (i.e., a time after primordial follicles acquire the capacity to activate) and cultured in a system similar to that used for newborn mouse ovaries (the culture system under “Summary of experimental methods” above). At the beginning of culture, most follicles were at the primordial stage, but after 2 days, the number of primordial follicles was much lower than at time 0 and there was a concomitant increase in the number of primary follicles (Wandji et al. 1996). The spontaneous wholesale activation in vitro was surprising and it did not seem to be due to the fetal nature of the cortical pieces, since Braw-Tal & Yossefi (1997) reported similar results with cortical pieces from adult cattle.
Is there an inhibitor of follicle activation in cattle?
It seemed possible that an inhibitor emanating from the medullary tissue might regulate the rate of follicle activation in vivo, so that in the absence of medullary tissue in the cortical cultures most follicles would activate. Co-culture of medullary tissue with cortical pieces had no effect on the number of activated follicles, but this result is not definitive since the inhibitor might not be present in sufficient concentrations in the co-culture situation. In 1999 Themmen’s group reported that mice null-mutant for anti-Mullerian hormone (AMH) had more growing follicles early in life, but prematurely depleted their stock of primordial follicles, compared with wild type controls (Durlinger et al. 1999). These interesting data suggested that AMH, which is secreted by ovarian follicles, is an intra-ovarian negative modulator of the rate of follicle activation. We tested the hypothesis that adding AMH to ovarian cortical cultures would decrease the rate of activation. Although AMH did not affect activation in that experiment, it significantly inhibited the growth of activated follicles (Yang et al., unpublished data). However, another experiment, in which bovine cortical pieces were transplanted beneath the chorio-allantoic membrane (CAM) of gonadectomized chick embryos, provided indirect evidence that AMH inhibits bovine follicle activation (details provided in Gigli et al. 2005).
Since activation occurs within 24 h in cortical pieces cultured with ITS+ (Fortune et al. 2000) and AMH is a large protein, it seemed possible that when cortical pieces were cultured with AMH, the hormone did not penetrate the cortical tissue fast enough to prevent the initiation of activation. To test this hypothesis, we cultured cortical pieces from fetuses at day 91–120 of gestation, a time when follicles do not activate within 2 days in vitro (Fig. 4), for 10 days with graded doses of recombinant human AMH (100, 500, or 1000 ng/ml). All three doses decreased the percentage of follicles at the primary stage and increased the percentage at the primordial stage (Yang et al., unpublished data). Immunohistochemical analysis of fetal ovaries at various ages showed staining for AMH only in secondary and small antral follicles, which appear during the last trimester (Yang et al., unpublished data). These results strongly suggest that AMH produced by secondary and later stage follicles inhibits activation and slows the growth of primary follicles.
Insulin and kit ligand stimulate follicle activation in cattle
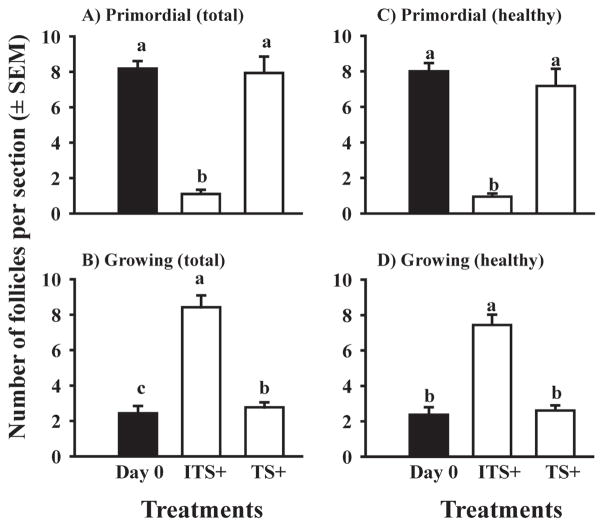
It appears likely that the activation of bovine follicles is controlled by both inhibitory and stimulatory factors. But it has been difficult to study stimulatory factors because of the high rate of what we considered “spontaneous” activation in our control medium containing ITS+. Figueiredo’s group in Brazil has tested various hormones and growth factors for effects on follicle activation in caprine ovarian tissue and they reported that several factors increased the number or percentage of growing follicles (e.g., Celestino et al. 2010). However, a high percentage of follicles activated in their control medium, which contained ITS, so the additional effects of added hormones/growth factors were very small. We tested the effects of TS+ (i.e. ITS+ without the insulin) plus graded doses of insulin and found that only the two highest doses (3.25 and 6.5 μg/ml) maximally stimulated follicle activation (Yang & Fortune, unpublished data); the highest dose used is the concentration of insulin in ITS+. Interestingly, TS+ maintained the health of the cortical tissue (Fig. 8, panels A vs. C and B vs. D), but had no effect on activation (Fig. 8; Muruvi et al., unpublished data).
Fig. 8.
Effect of insulin on the types and numbers of follicles (mean ± SEM) in ovarian cortical pieces isolated from late-term bovine fetuses. Cortical pieces were cultured for 0 (Day 0; black bar) or 4 (white bars) days in medium supplemented with ITS+ (contains insulin) or with TS+ (identical to ITS+ but without insulin). Within each panel, means (bars) with no common letters (a, b, c) differ significantly (P < 0.05; n = 5 fetuses).
The data shown in Fig. 8 are interesting because they show that what we had considered “spontaneous activation” was actually insulin-stimulated activation and thus, they have provided us with a new model for studying potential stimulators of bovine follicle activation (i.e. culture of cortical pieces in medium with TS+). We used that culture system to test the hypothesis that kit ligand is a stimulator of follicle activation in cattle. The results showed that kit ligand stimulates follicle activation in the absence of insulin and acts via binding to its receptor (Muruvi et al., unpublished data). This culture system will allow us to test other potential stimulators of bovine follicle activation.
The primary to secondary follicle transition
Although the transition from primordial to primary follicle in cattle is readily stimulated in cortical pieces in vitro by insulin or kit ligand, as discussed above, this is not the case for the primary to secondary transition. Secondary follicles are preantral follicles with two or more layers of granulosa cells. In whole newborn mouse ovaries placed in organ culture, secondary follicles are evident by day 8 of culture (Eppig & O’Brien 1996) and their numbers are not significantly different from those observed in ovaries of 8-day-old mice in vivo (Gigli et al. 2005). It is not clear whether the absence of the medulla in the bovine cortical cultures somehow prevents follicles from making the transition or whether the transition involves different signals or conditions in cattle than in mice.
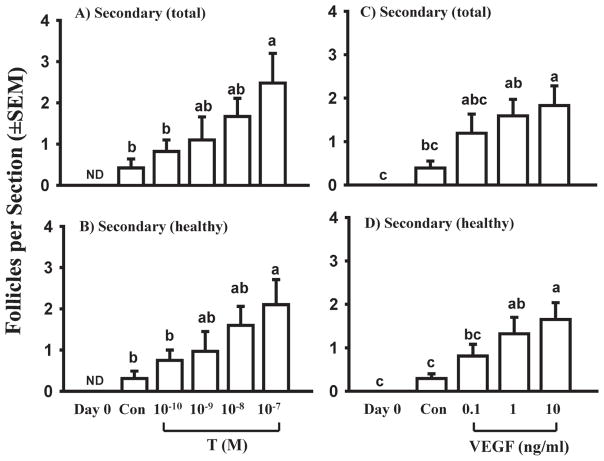
Simply extending the culture period did not increase the percentage of secondary follicles (unpublished data), nor did varying the oxygen concentration in vitro (Gigli et al. 2006). When cortical pieces were cultured with 0, 2.5, 5, or 10% fetal bovine serum (FBS) in the presence of 0, half-strength, or full-strength ITS+, the combinations most effective at increasing the numbers of secondary follicles were half-strength ITS+ with 5% or 10% FBS (Gigli et al. 2006). Because there is evidence that androgens stimulate preantral follicular growth in mice and in rhesus monkeys (Murray et al. 1998; Vendola et al. 1998; Vendola et al. 1999; Wang et al. 2001), we cultured bovine cortical pieces with testosterone for 10 days. Testosterone caused a dramatic (about 8-fold), dose-dependent increase in the number of secondary follicles compared to control cultures (Fig. 9 A, B), whereas estradiol had no effect (data not shown), suggesting that testosterone’s actions do not require conversion to estradiol (Yang & Fortune 2006). The results of further experiments suggest that androgens play a role in vivo. A specific blocker of the androgen receptor (flutamide) completely inhibited testosterone’s effects on the development of secondary follicles and immunohistochemistry showed staining for the androgen receptor in both stromal and follicular cells (see Yang & Fortune 2006 for further details). Because vascular endothelial growth factor (VEGF) is an important regulator of angiogenesis and angiogenesis plays an important role in later stages of follicular development (Zeleznik et al. 1981; Plendl 2000; Acosta et al. 2005), we treated bovine cortical cultures with graded doses of VEGF (1–100 ng/ml in one experiment and 0.1–10 ng/ml in another). The results were similar to those observed in the experiments with testosterone; there was a dose-dependent increase in the number of secondary follicles of about 6-fold compared to control cultures (Fig. 9 C, D) (Yang & Fortune 2007). Thus, we have found that both testosterone and VEGF dramatically increase the primary to secondary transition. However, in both studies most follicles were at the early secondary stage and further experiments are needed to determine how bovine follicles can be “encouraged” to develop beyond that stage.
Fig. 9.
Effects of graded doses of testosterone (T; 10−10 to 10−7 M) or vascular endothelial growth factor (VEGF; 0.1 to 10 ng/ml) on numbers of total and healthy follicles in ovarian cortical pieces isolated from late term (5–8 mo. gestation) fetuses and cultured for 10 days (white bars). Secondary follicles were not observed in freshly isolated cortical pieces (Day 0). Con: day 10 controls. Within each panel, means (bars; mean per section ± SEM) with no common letters are significantly different (P < 0.05; n = 3 fetuses). ND: not detected. (Data are from Yang & Fortune 2006 and Yang & Fortune 2007.)
Conclusions
The establishment of a pool of resting follicles and their gradual activation to begin growth are essential for female fertility. The results presented in this review suggest that steroid hormones produced by the bovine fetal ovary regulate both follicle formation and the acquisition by newly formed follicles of the capacity to activate. The progression of first prophase of meiosis in the oocytes of primordial follicles can also be regulated by steroids and achievement of meiotic arrest may be an important component of competence of follicles to activate in response to an activation signal. Once follicles have acquired the ability to activate, whether they activate or not probably depends on the balance between inhibitory and stimulatory regulators in their immediate environment. Our studies have provided evidence for AMH as an inhibitor and insulin and kit ligand as stimulators of follicle activation in vitro. The primary to secondary follicle transition is also critical for follicular development, but difficult to achieve in vitro. In our experiments, supplements to the culture medium (ITS+ and FBS), testosterone, and VEGF increased the number of secondary follicles. At this point, there are many more questions about early follicular development than there are answers. The answers seem worth pursuing, since elucidation of the regulation of early stages of follicular development may lead to new interventions to increase the size of the resting pool of follicles or to methods for enhancing the fertility of valuable domestic animals.
Acknowledgments
The authors thank Cargill Regional Beef for their generous donation of fetal bovine ovaries and Drs. S. S. Suarez and M. E. Pepling for reading the manuscript and for helpful suggestions. This research was supported by National Research Initiative Competitive Grants 2000-35203-9151 and 2003-35203-13532, by the National Institutes of Health (HD060232) and by USDA Multistate project NE-1027.
References
- Acosta TJ, Hayashi KG, Matsui M, Miyamoto A. Changes in follicular vascularity during the first follicular wave in lactating cows. Journal of Reproduction and Development. 2005;51:273–380. doi: 10.1262/jrd.16092. [DOI] [PubMed] [Google Scholar]
- Baker TG, Franchi LL. The fine structure of chromosomes in bovine primordial oocytes. Journal of Reproduction and Fertility. 1967;14:511–513. doi: 10.1530/jrf.0.0140511. [DOI] [PubMed] [Google Scholar]
- BrawTal R, Yossefi S. Studies in vivo and in vitro on the initiation of follicle growth in the bovine ovary. Journal of Reproduction and Fertility. 1997;109:165–171. doi: 10.1530/jrf.0.1090165. [DOI] [PubMed] [Google Scholar]
- Celestino JJ, Bruno JB, Lima-Verde IB, Matos MH, Saraiva MV, Chaves RN, Martins FS, Almeida AP, Cunha RM, Lima LF, Name KP, Campello CC, Silva JR, Bao SN, Figueiredo JR. Steady-state level of kit ligand mRNA in goat ovaries and the role of kit ligand in pre-antral follicle survival and growth in vitro. Molecular Reproduction and Development. 2010;77:231–240. doi: 10.1002/mrd.21138. [DOI] [PubMed] [Google Scholar]
- Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and gen-istein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–3590. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- Dominguez MM, Liptrap RM, Basrur PK. Steroi-dogenesis in fetal bovine gonads. Canadian Journal of Veterinary Research. 1988;52:401–406. [PMC free article] [PubMed] [Google Scholar]
- Durlinger ALL, Kramer P, Karels B, de Jong FH, Uilen-broek JTJ, Grootegoed JA, Themmen APN. Control of primordial follicle recruitment by antiMül-lerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, O’Brien MJ. Development in vitro of mouse oocytes from primordial follicles. Biology of Reproduction. 1996;54:197–207. doi: 10.1095/biolreprod54.1.197. [DOI] [PubMed] [Google Scholar]
- Erickson BH. Development and radio-response of the prenatal bovine ovary. Journal of Reproduction and Fertility. 1966;11:97–105. [Google Scholar]
- Fortune JE, Cushman RA, Wahl CM, Kito S. The primordial to primary follicle transition. Molecular and Cellular Endocrinology. 2000;163:53–60. doi: 10.1016/s0303-7207(99)00240-3. [DOI] [PubMed] [Google Scholar]
- Garverick HA, Juengel JL, Smith P, Heath DA, Burkhart MN, Perry GA, Smith MF, McNatty KP. Development of the ovary and ontogeny of mRNA and protein for P450 aromatase (arom) and estrogen receptors (ER) alpha and beta during early fetal life in cattle. Animal Reproduction Science. 2010;117:24–33. doi: 10.1016/j.anireprosci.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Gigli I, Byrd DD, Fortune JE. Effects of oxygen tension and supplements to the culture medium on activation and development of bovine follicles in vitro. Theriogenology. 2006;66:344–353. doi: 10.1016/j.theriogenology.2005.11.021. [DOI] [PubMed] [Google Scholar]
- Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Molecular Reproduction and Development. 2005;71:480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- Henricson B, Rajakoski E. Studies of oocytogenesis in cattle. Cornell Veterinarian. 1959;49:494–503. [PubMed] [Google Scholar]
- Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biology of Reproduction. 2006;74:161–168. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–3337. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Smith P, Hudson NL, Heath DA, Tisdall DJ, OWS, BrawTal R. Development of the sheep ovary during fetal and early neonatal life and the effect of fecundity genes. Journal of Reproduction and Fertility, Supplement. 1995;49:123–135. [PubMed] [Google Scholar]
- Murray AA, Gosden RG, Allison V, Spears N. Effect of androgens on the development of mouse follicles growing in vitro. Journal of Reproduction and Fertility. 1998;113:27–33. doi: 10.1530/jrf.0.1130027. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Progesterone regulation of primordial follicle assembly in bovine fetal ovaries. Molecular and Cellular Endocrinology. 2009;313:9–16. doi: 10.1016/j.mce.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson EE, Stanfield J, Skinner MK. Interactions between progesterone and tumor necrosis factoralpha in the regulation of primordial follicle assembly. Reproduction. 2006;132:877–886. doi: 10.1530/REP-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plendl J. Angiogenesis and vascular regression in the ovary. Anatomia, Histologia, Embryologia. 2000;29:257–266. doi: 10.1046/j.1439-0264.2000.00265.x. [DOI] [PubMed] [Google Scholar]
- Quirke LD, Juengel JL, Tisdall DJ, Lun S, Heath DA, Mc-Natty KP. Ontogeny of steroidogenesis in the fetal sheep gonad. Biology of Reproduction. 2001;65:216–228. doi: 10.1095/biolreprod65.1.216. [DOI] [PubMed] [Google Scholar]
- Russe I. Oogenesis in cattle and sheep. Bibliotheca Anatomica. 1983;24:77–92. [PubMed] [Google Scholar]
- Senger PL. Pathways to Pregnancy and Parturition. 2. Pullman, WA: Current Conceptions, Inc; 2003. [Google Scholar]
- Shemesh M, Allenberg M, Milaguir F, Ayalon N, Hansel W. Hormone secretion by cultured bovine pre and postimplantation gonads. Biology of Reproduction. 1978;19:761–767. doi: 10.1095/biolreprod19.4.761. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Nakada K, Moriyoshi M, Sawamukai Y. Appearance and number of follicles and change in the concentration of serum FSH in female bovine fetuses. Reproduction. 2001;121:777–782. [PubMed] [Google Scholar]
- van Wagenen G, Simpson ME. Embryology of the Ovary and Testis. Homo sapiens and Macaca mulatta. New Haven: Yale University Press; 1965. [Google Scholar]
- Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biology of Reproduction. 1999;61:353–357. doi: 10.1095/biolreprod61.2.353. [DOI] [PubMed] [Google Scholar]
- Vendola KA, Zhou J, Adesanya OO, Weil SJ, Bondy CA. Androgens stimulate early stages of follicular growth in the primate ovary. Journal of Clinical Investigation. 1998;101:2622–2629. doi: 10.1172/JCI2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandji SA, Srsen V, Voss AK, Eppig JJ, Fortune JE. Initiation in vitro of growth of bovine primordial follicles. Biology of Reproduction. 1996;55:942–948. doi: 10.1095/biolreprod55.5.942. [DOI] [PubMed] [Google Scholar]
- Wang H, Andoh K, Hagiwara H, Xiaowei L, Kikuchi N, Abe Y, Yamada K, Fatima R, Mizunuma H. Effect of adrenal and ovarian androgens on type 4 follicles unresponsive to FSH in immature mice. En-docrinology. 2001;142:4930–4936. doi: 10.1210/endo.142.11.8482. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Testosterone stimulates the primary to secondary follicle transition in bovine follicles in vitro. Biology of Reproduction. 2006;75:924–932. doi: 10.1095/biolreprod.106.051813. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. Vascular endothelial growth factor stimulates the primary to secondary follicle transition in bovine follicles in vitro. Molecular Reproduction and Development. 2007;74:1095–1104. doi: 10.1002/mrd.20633. [DOI] [PubMed] [Google Scholar]
- Yang MY, Fortune JE. The capacity of primordial follicles in fetal bovine ovaries to initiate growth in vitro develops during midgestation and is associated with meiotic arrest of oocytes. Biology of Reproduction. 2008;78:1153–1161. doi: 10.1095/biolreprod.107.066688. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Schuler HM, Reichert LE., Jr Gon-adotropinbinding sites in the rhesus monkey ovary: role of the vasculature in the selective distribution of human chorionic gonadotropin to the preovulatory follicle. Endocrinology. 1981;109:356–362. doi: 10.1210/endo-109-2-356. [DOI] [PubMed] [Google Scholar]