Summary
Purpose
The incidence of seizures within 24 h of acute stroke has not been studied extensively. We aimed to establish the incidence of acute post-stroke seizures in a biracial cohort and to determine whether acute seizure occurrence differs by race/ethnicity, stroke subtype, and/or stroke localization.
Methods
We identified all stroke cases between July 1993 and June 1994 and in 1999 within the population of the Greater Cincinnati metropolitan region. Patients with a prior history of seizures/epilepsy were excluded from analysis.
Results
A total of 6044 strokes without a history of seizure(s) were identified; 190 (3.1%) had seizures within the first 24 h of stroke onset. Of ICH/SAH patients, 8.4% had a seizure within the first 24 h of stroke onset (p ≤ 0.0001 vs. all other stroke subtype). Of the patients with ischemic stroke, we observed higher incidence of seizures in cardioembolic versus small or large vessel ischemic (p = 0.02) strokes. Patients with seizures experienced higher mortality than patients without seizures (p < 0.001) but seizures were not an independent risk factor of mortality at 30 days after stroke. Independent risk factors for seizure development included hemorrhagic stroke, younger age, and prestroke Rankin score of ≥1. Race/ethnicity or localization of the ischemic stroke did not influence the risk for seizure development in the studied population.
Discussion
The overall incidence of acute seizures after stroke was 3.1%, with a higher incidence seen in hemorrhagic stroke, younger patients, and those presenting with higher prestroke Rankin scores. Acute seizures were associated with a higher mortality at 30 days after stroke.
Keywords: Stroke, Seizures, Acute stroke, Incidence, Epilepsy, ICH, SAH, Hemorrhage
Cerebrovascular disease has long been recognized as a risk factor for the development of epilepsy and it is considered the most common identified antecedent condition that results in symptomatic epilepsy in the elderly (Loiseau et al., 1990; Hauser et al., 1994). The reported incidence of poststroke seizures and epilepsy is dependent on study design, diagnostic criteria, duration of follow up, and population studied (Pohlmann-Eden et al., 1996; Pohlmann-Eden et al., 1997; Silverman et al., 2002; Ferro & Pinto, 2004) Poststroke seizures are often characterized as “early” and “late” with definitions varying considerably (Pohlmann-Eden et al., 1996, 1997; Silverman et al., 2002; Ferro & Pinto, 2004) Early seizures are frequently defined as seizures that occur within the first 7–14 days of stroke symptoms onset (Kotila & Waltimo, 1992; Lamy et al., 2003; Feleppa et al., 2006) but many studies examining the incidence of early poststroke seizures (ES) and epilepsy focus either on poststroke seizures occurring within the first 1–4 weeks after the stroke (Giroud et al., 1994; Arboix et al., 1996, 1997; Reith et al., 1997; Labovitz et al., 2001; Feleppa et al., 2006) or on comparing the characteristics and outcomes of early and late seizures (Gupta et al., 1988; Hornig et al., 1990; Kilpatrick et al., 1990; Sung & Chu, 1990; Kilpatrick et al., 1992; So et al., 1996; Burn et al., 1997; Berges et al., 2000; Bladin et al., 2000; Bentes et al., 2001; Dhanuka et al., 2001; Velioglu et al., 2001; Lossius et al., 2002; Afsar et al., 2003; De Reuck et al., 2005; Misirli et al., 2006). The findings from the largest seizure incidence studies in the immediate poststroke period are similar: approximately 4.2–6.1% of patients develop seizures within the first few weeks after stroke (Kilpatrick et al., 1990; Davalos et al., 1992; Giroud et al., 1994; Reith et al., 1997; Bladin et al., 2000); early seizures may be predictive of epilepsy development (Kilpatrick et al., 1992; So et al., 1996). Only one population-based study focused on seizures occurring in the first 24 h after stroke (6%) but this relatively small study examined incidence of acute seizures in predominantly white and affluent population of Rochester, Minnesota that does not reflect the population characteristics of the United States (So et al., 1996).
The Greater Cincinnati/Northern Kentucky Stroke Study (GCNKSS) is designed to investigate the differences in stroke incidence rates and case mortality in the biracial population of the greater Cincinnati metropolitan area. Our study population generally is representative of the United States with regard to the median age, percentage of black race, median household income, education level, and percent of population below the poverty level (Broderick et al., 1998). Thus, our study should provide an excellent estimate of seizure incidence in the immediate poststroke period for the U.S. population.
The main goal of our population-based study was to establish the incidence of epileptic seizures within the first 24 h of stroke onset. Secondary aims were to determine the subtype and localization of stroke most important to the seizure incidence and to evaluate whether there are any racial differences in the incidence of seizures after stroke. Our hypotheses, based on the available literature, were that patients with hemorrhagic stroke have higher incidence of seizures when compared to patients with ischemic stroke and that blacks have higher incidence of seizures than Caucasian patients.
Materials and Methods
The methodology of the GCNKSS has been previously described (Broderick et al., 1998). The study population for the GCNKSS is defined as all residents of the Cincinnati metropolitan region, which includes two southern Ohio counties and three contiguous Northern Kentucky counties that abut the Ohio River. Included in this area are 19 hospitals (18 in the 1999 data collection). Although residents of surrounding counties seek care at these hospitals, only residents of the five study area counties are included as cases. Previous studies have also documented that residents of the five counties who have a stroke exclusively seek care at these hospitals rather than more distant hospitals in the outlying region (Broderick et al., 1992a, 1992b). This study was approved by the Institutional Review Board at all participating hospitals.
Study nurses reviewed the medical records of all inpatients with primary or secondary stroke-related ICD-9 discharge diagnoses (430–436) from all acute-care hospitals in the study region. The study nurses also reviewed all autopsy cases where stroke was listed as the primary or secondary cause of death. Patients were identified as being from the study area based on their zip code of residence.
This study involved collection of all strokes that occurred in the study population between July 1, 1993 and June 30, 1994 and between January 1, 1999 and December 31, 1999. In addition to ascertaining inpatient strokes using the methodology described above, we determined out-patient strokes by monitoring all visits to all emergency departments, 5 county coroner’s offices, 16 public health clinics, 13 hospital-based outpatient clinics and family practice centers, and the neurology and medicine clinics at the VA Hospital. Additional monitoring for outpatient strokes was performed in a random sample of primary care physicians’ offices and area nursing homes.
To qualify as an incidence case, a patient must have met the criteria for one of the five stroke categories adapted from the Classification for Cerebrovascular Diseases III and from epidemiological studies of stroke in Rochester, Minnesota: cerebral ischemia, intracerebral hemorrhage (ICH), subarachnoid hemorrhage (SAH), stroke of uncertain cause, or transient ischemic attack (TIA) (Broderick et al., 1998). Cases were excluded if they had: (1) discharge/autopsy diagnosis or neuroimaging consistent with stroke but no clinical history of stroke; or (2) a clinical diagnosis of stroke and died within 24 h of symptom onset but had no focal neurological deficit and no confirmatory neuroimaging or autopsy. All excluded cases and the reasons for exclusion were recorded.
Once cases were identified, the study nurse gathered information from the chart regarding the presence or absence of seizures within the first 24 h after the onset of stroke symptoms. Data regarding the timing or duration of seizures or the clinical characteristics of seizures (focal vs. generalized) were not available in most cases and were not collected. Furthermore, information was collected regarding the stroke symptoms and physical examination findings, medical/surgical history including history of seizures prior to stroke, social history/habits, prehospital evaluation, vital signs and emergency room evaluation, diagnostic test results (including lab testing, electrocardiogram (EKG) and cardiac testing, neuroimaging of any type, etc.), treatments and outcome. Prestroke Rankin scores were determined in all patients. Classification of race/ethnicity was as self-reported in the medical administrative record. The study nurse abstracted all information and then made a determination as to whether a stroke or TIA had occurred. All borderline or possible cases were also fully abstracted for physician review.
Study physicians reviewed all abstracted charts and decided whether a stroke or TIA had occurred. Study physicians also reviewed available neuroimaging studies and characterized imaging findings. As previously, the study physician assigned stroke category and mechanism to each patient based on all available information, using definitions listed above and previously reported (Broderick et al., 1998).
For quality assurance and to assess interrater reliability, three study neurologists and two study nurse abstractors evaluated 18 medical records randomly selected from the database (both, cases and noncases). Each person decided whether the event was a stroke/TIA or not. The kappa (κ) score for these five study personnel was 0.83, indicating excellent agreement.
Statistical analyses
Data collected from 1993 to 1994 and 1999 were combined for analysis. In order to account for any potential effects of time, these time periods were included as covariates in multivariable analysis. To analyze the significance of observed differences between the patients who developed seizures and those that did not, a bivariate analysis was performed on the demographic characteristics using chi-square for categorical variables and the student’s t-test or Wilcoxon rank-sum test for continuous variables, dependent upon the distribution. To determine the independent association of early seizures on mortality and independent factors associated with acute seizure development after stroke, multivariable logistic modeling was used.
Results
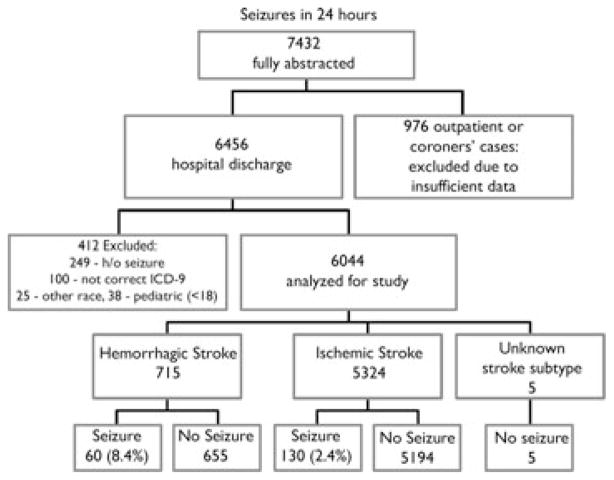
Of the 7,432 events that were fully abstracted by study nurses from both data collection periods (out of approximately 25,000 charts), and after physician review determined to meet case criteria, 6,044 met criteria for the current study (Fig. 1). Given our objective of evaluating the acute period after stoke only cases ascertained in the hospital setting were used and thus 976 were eliminated. There were 6,456 cases of hospitalized stroke. Of these, 412 patients were excluded due to previous history of seizures or epilepsy (N = 249), incorrect ICD code (N = 100), age <18 (N = 38), or race other than Caucasian or black (N = 25). Of the 6044 cases, 5,324 (88.1%) patients were determined to have an ischemic stroke or TIA, 715 (11.8%) a hemorrhagic stroke, and 5 (0.1%) a stroke of undetermined subtype.
Figure 1.
Case ascertainment for the entire study (1993, 1994, and 1999).
Epilepsia © ILAE
Our study found the overall incidence of poststroke seizures within 24 h to be 3.1% (190/6044). No differences in seizure incidence was seen when comparing patients with first-ever versus recurrent strokes (51/1494, 3.4%; p = 0.49). Patients with seizures were significantly younger, had lower initial Glasgow Coma Scale (GCS) scores, higher National Institutes of Health (NIH) Stroke Scale (NIHSS) score, higher 30-day mortality and had a higher incidence of hemorrhagic stroke (Table 1) than patients without seizures.
Table 1.
Demographic and clinical characteristics of all patients included in the study
| Variable | Seizure (n = 190)
|
No seizure (n = 5854)
|
p-value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Age (years)a | 68.0 | (15.0) | 71.7 | (13.2) | 0.001 |
| Gender (male) | 77 | 40.5% | 2556 | 43.7% | 0.39 |
| Race (black) | 33 | 17.34% | 999 | 17.1% | 0.91 |
| Hypertension | 119 | 62.6% | 3872 | 66.1% | 0.31 |
| Heart disease | 72 | 37.9% | 2374 | 40.6% | 0.46 |
| Prior stroke | 51 | 26.8% | 1443 | 24.6% | 0.49 |
| LVH | 26 | 13.7% | 642 | 11.0% | 0.24 |
| Prestroke Rankinb | 0 | (0–3) | 0 | (0–3) | 0.57 |
| NIHSSb,c | 12 | (5–22) | 6 | (3–11) | <0.0001 |
| GCSb,d | 13 | (7–15) | 15 | (14–15) | <0.0001 |
| Hemorrhagic stroke | 60 | 31.6% | 655 | 11.2% | <0.001 |
| 30-day Mortality | 61 | 32.1% | 776 | 13.3% | <0.0001 |
Data are presented as n (%).
mean (standard deviation) or
median (interquartile range).
NIHSS n’s are 143 & 5249.
GCS are 154 & 4808.
The incidence of seizures based on the stroke subtype is shown in Table 2. Patients with ischemic stroke and TIA had an overall incidence of seizures within the first 24 h after acute stroke of 2.4%. In contrast, the incidence of acute seizures in patients with hemorrhagic stroke was 8.4% (p < 0.0001) with 7.9% of ICH and 10.1% of SAH patients having acute seizures, respectively. When examining ischemic stroke mechanism, we noted an increased incidence of seizures in patients with cardioembolic strokes versus those with small or large vessel disease (p = 0.02; Table 3).
Table 2.
Incidence of seizures in patients with ischemic and hemorrhagic strokes within the first 24 h of symptom onset (p < 0.0001)
| Type of stroke | Seizure (n = 190)
|
No seizure (n = 5854)
|
Rate of seizures | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Infarct | 120 | 63.2% | 4069 | 69.5% | 2.9% |
| TIA | 10 | 5.3% | 1125 | 19.2% | 0.9% |
| ICH/IVH | 43 | 22.6% | 503 | 8.6% | 7.9% |
| SAH | 17 | 9.0% | 152 | 2.6% | 10.1% |
| Unknown | 0 | 0.0% | 5 | 0.1% | 0.0% |
Table 3.
Incidence of seizure by subtype of ischemic stroke (p = 0.01)
| Underlying Cause | Ischemic stroke and TIA
|
Rate of seizure | |||
|---|---|---|---|---|---|
| Seizure (n = 130)
|
No seizure (n = 5194)
|
||||
| N | % | N | % | ||
| Small vessel | 12 | 9.2% | 692 | 13.3% | 1.7% |
| Cardioembolic | 33 | 25.4% | 1049 | 20.2% | 3.0% |
| Large vessel | 14 | 10.8% | 805 | 15.5% | 1.7% |
| Other | 9 | 6.9% | 137 | 2.6% | 6.2% |
| Unknown | 62 | 47.7% | 2511 | 48.3% | 2.4% |
Patients who developed seizures within the first 24 h of stroke onset had a higher all-cause mortality rate at 30 days (32.1% vs. 13.3%; p < 0.0001; Table 1). To determine the independent association of seizure with mortality, and also to determine if any patient characteristics were independent predictors of seizure development in the acute setting of stroke, multivariable logistic regression analyses were performed (Table 4). Seizure was associated with a 2-fold increase in risk of 30-day mortality after controlling for age, hemorrhagic stroke, presence of heart disease, prior status as defined by Rankin scale, prior stroke, gender and race. When NIHSS and GCS were included in the multivariable logistic regression analyses, higher scores on the NIHSS (worse stroke) and lower GCS scores (poorer admission status) were associated with seizure occurrence (Table 5) with highest chance of seizures in hemorrhagic stroke patients with highest NIHSS and lowest GCS scores (Table 6). Additional multivariable logistic regression revealed that younger age, hemorrhagic stroke, and prestroke Rankin score of 1 or more were significant and independent predictors of seizure occurrence within the first 24 h after stroke (Table 7). Race/ethnicity was not significantly associated with seizure development or increased mortality.
Table 4.
Association of seizure with mortality, showing covariates
| Variable | OR | 95%CI | p-value |
|---|---|---|---|
| Seizure | 2.63 | 1.85, 3.74 | <0.0001 |
| Hemorrhage | 6.58 | 5.35, 8.09 | <0.0001 |
| Age (10 years) | 1.40 | 1.30, 1.51 | <0.0001 |
| Gender (male) | 1.00 | 0.84, 1.18 | 0.98 |
| Race (black) | 0.83 | 0.66, 1.05 | 0.12 |
| Prior stroke | 0.93 | 0.77, 1.12 | 0.46 |
| Heart disease | 1.55 | 1.31, 1.83 | <0.0001 |
| Prestroke Rankin (1 or more) | 2.13 | 1.78, 2.54 | <0.0001 |
Table 5.
Association of seizure with mortality, showing covariates including NIHSS and NIHSS/GCS
| Variable | Including NIHSS in model (741 missing)
|
Including NIHSS & GCS in model (1655 missing)
|
||
|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |
| Seizure | 1.49 | 0.94, 2.36 | 0.74 | 0.42, 1.29 |
| Hemorrhage | 4.47 | 3.39, 5.91 | 4.12 | 3.00, 5.65 |
| Age (10 years) | 1.34 | 1.25, 1.44 | 1.30 | 1.19, 1.41 |
| Gender (male) | 1.27 | 1.04, 1.55 | 1.37 | 1.08, 1.74 |
| Race (black) | 0.71 | 0.54, 0.96 | 0.74 | 0.53, 1.03 |
| Prior stroke | 0.75 | 0.60, 0.94 | 0.78 | 0.60, 1.02 |
| Heart disease | 1.52 | 1.24, 1.86 | 1.67 | 1.32, 2.11 |
| Prestroke Rankin (1 or more) | 2.06 | 1.65, 2.56 | 1.78 | 1.38, 2.30 |
| NIHSS (10–19 vs. <10) | 4.00 | 3.18, 5.02 | 2.85 | 2.14, 3.76 |
| NIHSS (20+ vs. <10) | 22.6 | 17.6, 29.2 | 9.01 | 6.41, 12.7 |
| GCS (10–14 vs. 15) | 2.24 | 1.71, 2.94 | ||
| GCS (<10 vs. 15) | 6.58 | 4.47, 9.68 | ||
Table 6.
Association of seizure with mortality, separately for Infarct/TIA and hemorrhagic stroke showing covariates
| Variable | Infarct & TIA with NIHSS in model (523 missing)
|
Hemorrhage with NIHSS & GCS in model (277 missing)
|
||
|---|---|---|---|---|
| OR | 95%CI | OR | 95%CI | |
| Seizure | 1.55 | 0.90, 2.66 | 0.85 | 0.33, 2.15 |
| Age (10 years) | 1.36 | 1.25, 1.47 | 1.30 | 1.08, 1.49 |
| Gender (male) | 1.31 | 1.05, 1.64 | 1.37 | 1.77, 2.41 |
| Race (black) | 0.64 | 0.46, 0.89 | 1.40 | 0.70, 2.81 |
| Prior stroke | 0.68 | 0.53, 0.88 | 1.06 | 0.54, 2.10 |
| Heart disease | 1.56 | 1.26, 1.94 | 1.33 | 0.68, 2.61 |
| Prestroke Rankin (1 or more) | 2.33 | 1.82, 2.97 | 1.35 | 0.74, 2.44 |
| NIHSS (10–19 vs. <10) | 4.19 | 3.26, 5.38 | 1.71 | 0.86, 3.42 |
| NIHSS (20+ vs. <10) | 23.3 | 17.5, 30.9 | 5.69 | 2.69, 12.0 |
| GCS (10–14 vs. 15) | 2.99 | 1.53, 5.84 | ||
| GCS (<10 vs. 15) | 9.92 | 4.30, 22.9 | ||
Table 7.
Risk factors for seizure development: multivariable logistic regression
| Variable | OR | 95%CI | p-value |
|---|---|---|---|
| Hemorrhage | 3.65 | 2.62, 5.10 | <0.0001 |
| Age (10 years) | 0.86 | 0.77, 0.95 | 0.004 |
| Prestroke Rankin of 1 or more | 1.51 | 1.11, 2.06 | 0.009 |
Discussion
This study examines the incidence of seizures in the first 24 h of stroke symptom onset. The incidence of seizures in patients with ischemic stroke was 2.4% while the incidence of seizures in patients with hemorrhagic stroke was significantly higher (8.4%) with the overall incidence of seizures in this population-based study of 3.1%. Further, we found that the average patient with cardioembolic stroke has higher chance of developing seizures in the first 24 h after the stroke than the average patient with ischemic stroke or TIA. Not surprisingly, seizure risk is significantly lower in patients with small or large vessel ischemic strokes which are typically subcortical and don’t affect cortical structures involved in generation of the brain’s electrical activity including seizures. The factors that predispose the patients to seizures in the first 24 h after stroke symptoms onset include younger age, hemorrhagic stroke and prestroke Rankin score of one or more. Mortality is higher in patients who develop acute-onset seizures. Other significant and independent risk factors for mortality included age, hemorrhagic stroke, and history of heart disease. Finally, we found that black race did not increase the patient’s risk for either seizure within 24 h of stroke onset or 30-day all-cause mortality after stroke.
Seizure incidence
The incidence of combined “early” and “late” seizures after stroke has been reported to be approximately 10% (5% of early seizures with peak in the first 24 h and 5% late seizures predominantly in the first 6–12 months after the stroke) (Olsen, 2001). However, this number varies widely between studies due to many factors that include differences in study design, stroke subtype(s) included in final analysis, and the duration of follow-up (Pohlmann-Eden et al., 1996, 1997; Silverman et al., 2002; Ferro & Pinto, 2004; Feleppa et al., 2006). Therefore, comparisons between studies are difficult. There have been only two studies that have specifically evaluated the incidence of seizures within the first 24 h of acute stroke. In the case series by Shinton et al. performed in Birmingham, U.K. the incidence of seizures was 5.7% out of 230 patients included in the study (Shinton et al., 1988). In this study the diagnosis of stroke was made on clinical grounds only; neuroimaging was obtained in 20% of patients included in the study hence it is not clear how many patients with is-chemic versus hemorrhagic strokes were included in this study and whether all patients had, in fact, stroke. The second, population-based study by So et al. from Rochester, Minnesota included 535 consecutive patients with stroke (4.7% had seizures during the first 24 h after infarction) admitted to the hospitals affiliated with the Mayo Clinic (So et al., 1996). Although this is the best population-based study available, the number of patients included in this report is relatively small with the study conducted in predominantly affluent white population. Hence, firm conclusions regarding the incidence of seizures in the first 24 h of stroke or the influence of race on seizure occurrence cannot be drawn from that study. Further, although not specifically mentioned, both studies appeared not to include patients with TIAs. With 6,044 subjects included (after eliminating patients with prior history of seizures), we report the incidence of seizures within the first 24 h after stroke to be 3.1%. After excluding patients with TIA and seizures attributed to TIAs, the overall incidence of seizures in our study was 3.7%, which is fairly similar to the incidence reported by So et al. (So et al., 1996) and confirms the previously reported seizure incidences on much larger and racially diverse population sample.
Stroke incidence by ischemic stroke subtype
We found that patients who were determined to have had an ischemic stroke of cardioembolic origin had a higher incidence rate of seizures compared to small or large vessel ischemic stroke or TIA, (p = 0.02). When included in multivariate analysis, this factor was not statistically significant suggesting that cardioembolic origin of stroke is not an independent risk factor for development of seizures after stroke. The available literature on this topic varies widely (Richardson and Dodge, 1954; Lesser et al., 1985; So et al., 1996; Arboix et al., 1997) because the definition of cardioembolic stroke is not uniform between the studies and the cardioembolic etiology before introduction of high-quality echocardiography and advanced imaging such as MRI, was hard to determine with certainty (Broderick et al., 1992b). One study defined cardioembolic stroke on clinical basis as a stroke of sudden onset with a neurologic deficit in “frontal or posterior cerebral regions with motor aphasia, sensory deficiency, or hemianopia”; the presence of atrial fibrillation or clear cardiac source of emboli was not required for the diagnosis of cardioembolic stroke in this large study (Giroud et al., 1994, page 960). Based on such criteria, these authors found an incidence of seizures after cardioembolic stroke to be 16.6%. A population-based study by So et al., using Rochester criteria outlined by Broderick et al. (Broderick et al., 1992b), found, similar to our study, that embolic infarcts are associated with early but not late seizure occurrence (cutoff defined as 1 week). However, this finding was not determined to be predictive of later epilepsy development in a multivariate analysis (So et al., 1996). Finally, another study found that 3/183 (1.6%) patients with cardioembolic stroke subsequently developed seizures but this was not significantly different from seizure incidence in patients other types of strokes (Arboix et al., 1997). Overall, the findings of this largest to date study are in agreement with the current literature. Cardioembolic stroke, because of the frequent cortical involvement, is associated with increased chance of seizure and epilepsy development but is not an independent risk factor for seizure occurrence.
Several studies evaluated the incidence of seizures in patients with various types of spontaneous intracranial hemorrhages (Hart et al., 1981; Sundaram & Chow, 1986; Passero et al., 2002; Vespa et al., 2003). One study evaluated the incidence of seizures within the first 72 h after primary ICH after excluding subarachnoid, traumatic, and subtentorial hemorrhages (Vespa et al., 2003). These authors reported the incidence of acute seizures at 28% but they also monitored patient’s EEGs to look for subclinical (electrographic) seizures. Therefore, it is not surprising that the incidence rate in this study was higher than ours, which recorded only clinical seizures that were documented based on clinical observation (Vespa et al., 2003). Another study evaluated seizure incidence after spontaneous intracerebral hemorrhages and found that seizures occurred in 8.1% of patients within the first 30 days after symptom onset (the incidence was 4.2% within the first 24 h) (Passero et al., 2002). Sundaram and Chow showed that the incidence of seizures in patients with spontaneous SAH was 24% with most of the seizures occurring within the first 24 h (14.5%) (Sundaram & Chow, 1986). Finally, Hart et al. found the incidence of seizures in patients with aneurysmal SAH to be 26% (with seizures occurring in 19/100 patients within the first 12 h) (Hart et al., 1981). Our incidence (8.4%) falls between the incidence reported by Passero et al. (4.2% within the first 24 h) and 28% reported by Vespa et al. (Passero et al., 2002; Vespa et al., 2003). These differences are most likely related to study design and inclusion criteria. Our incidence combines the higher risk for seizure occurrence in patients with SAH and slightly lower chance of seizure occurrence in patients with ICH and probably reflects the true incidence of acute seizures in mixed population of patients with intracranial hemorrhages based on a high number of patients with intracranial hemorrhages included in this study (N = 715).
Mortality
We found an increased risk of mortality within 30 days of stroke if seizures developed within the first 24 h after stroke onset (32.1% vs. 13.3%; p < 0.0001). In fact, after controlling for age, hemorrhagic stroke, presence of heart disease, prior status as defined by Rankin scale, prior stroke, gender and race, the presence of seizures in the first 24 h after the stroke symptom onset was associated with a 2-fold increase in risk of 30-day mortality but seizures were not an independent predictor of poor outcome (Table 5). Therefore, it appears that the presence of seizures in the immediate poststroke period is a sign of severe brain injury rather than a predictor of poor outcome.
The available epidemiology literature varies considerably in the reported mortality after stroke (Shinton et al., 1988; Kilpatrick et al., 1990; Davalos et al., 1992; Kilpatrick et al., 1992; Arboix et al., 1997; Reith et al., 1997; Bladin et al., 2000; Labovitz et al., 2001; Feleppa et al., 2006) For example, one study found an in-hospital mortality rate of 37.9% (vs. 14.4%) in patients with seizures within 48 h of acute ischemic or hemorrhagic stroke symptoms onset (Arboix et al., 1997). Another study in a mixed population of hemorrhagic and ischemic stroke patients found similar mortality up to 27 months after stroke (30.8 vs. 7.4%, respectively) with high burden of mortality within the first 24 h of stroke (Shinton et al., 1988). Vespa et al. also found a trend toward increased poor outcome in patients with posthemorrhagic seizures (27.8% vs. 15% mortality in nonseizure cohort) (Vespa et al., 2003). However, several studies found either that there were no significant differences in mortality between patients with and without seizures after stroke (Bladin et al., 2000; Velioglu et al., 2001), that surviving patients with seizures had better outcome after stroke (Reith et al., 1997), or that although the mortality in patients with seizures after stroke was higher than in patients without seizures, seizures were not an independent predictive factor of poor outcome (Davalos et al., 1992; Labovitz et al., 2001). In patients with infarct/TIA, after controlling for NIHSS, we noted a trend towards a negative effect of seizures on outcome (p = 0.09) while seizures had no effect on mortality in patients with hemorrhagic stroke after controlling for GCS and NIHSS (p = 0.73). Independent predictors of 30-day mortality following stroke included older age at the stroke symptom onset, hemorrhagic stroke, and heart disease. Hemorrhagic stroke is well known to carry a higher mortality rate. Further, it is not surprising to find that active heart disease including congestive heart failure lead to an increase in mortality as these patients have a higher chance of in-hospital complications and mortality (Broderick et al., 1992b; Kannel, 2000).
Our study is the first one to document increased mortality in patients with acute onset of seizures after stroke in a biracial population while not finding any racial differences in mortality (see below). Previous studies evaluating the impact of seizures on mortality focused on mixed but predominantly white population, hence their results are not entirely representative of the population. As our study population is biracial and largely representative of the population in the United States, our findings may impact how we view prognosis in patients with acute stroke and new onset seizures. Further, the findings of increased mortality in patients with seizures after stroke may direct further studies addressing possible seizure prevention in patients with high risk for developing seizures.
Race
In this study, we did not find difference in regard to mortality or incidence of seizures after acute stroke between black and white patients. This is despite the fact that blacks are known to have higher prevalence rates of both seizures and strokes, especially in the younger age groups (Szaflarski et al., 2006). How do we explain this discrepancy? Studies that have evaluated racial and ethnic disparities cite, as explanations, multiple barriers to care such as psychosocial factors (income, education) and cultural influences. Social factors may influence the incidence of stroke as lower income populations have poor access to care and tend to have higher rates of noncompliance. However, the development of acute seizures after stroke would not be determined by social factors but it would rather be related to the presence or absence of stroke directly observed upon admission to the hospital and be independent of clinical management, provider communication, or patient knowledge/beliefs (Szaflarski et al., 2006). Therefore, as the reason for this discrepancy is unclear, further studies are necessary. We think it is unlikely that seizures were underdiagnosed or underdocumented for black patients as compared to white patients but this study was not designed to test for this possibility.
A limitation of this study is that the population studied is biracial and does not include patients of Hispanic, Native American, or Asian ethnicity. Therefore, the numbers presented here may be an underestimate of overall incidence of seizure within 24 h of stroke onset as both elderly Hispanic males and Native Americans have been shown to have a higher incidence of epilepsy [reviewed in (Szaflarski et al., 2006)]. Other limitations include the retrospective nature of data collection and the possibility of over or underestimating the incidence of seizures as we relied on retrospective chart review and data collection rather than on direct observation.
Acknowledgments
This study was presented in part at the 125th ANA Annual Meeting, Boston, MA, October 2000 and in part at the 1st North American Regional Epilepsy Congress, San Diego, CA, December 2006. Support for this study was provided by NIH RO1 NS30678.
Conflict of interest: We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines. None of the authors has any conflicts of interest to disclose in relation to this paper.
References
- Afsar N, Kaya D, Aktan S, Sykut-Bingol C. Stroke and status epilepticus: stroke type, type of status epilepticus, and prognosis. Seizure. 2003;12:23–27. doi: 10.1016/s1059131102001437. [DOI] [PubMed] [Google Scholar]
- Arboix A, Comes E, Massons J, Garcia L, Oliveres M. Relevance of early seizures for in-hospital mortality in acute cerebrovascular disease. Neurology. 1996;47:1429–1435. doi: 10.1212/wnl.47.6.1429. [DOI] [PubMed] [Google Scholar]
- Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Comes E. Predictive factors of early seizures after acute cerebrovascular disease. Stroke. 1997;28:1590–1594. doi: 10.1161/01.str.28.8.1590. [DOI] [PubMed] [Google Scholar]
- Bentes C, Pimentel J, Ferro JM. Epileptic seizures following subcortical infarcts. Cerebrovasc Dis. 2001;12:331–334. doi: 10.1159/000047730. [DOI] [PubMed] [Google Scholar]
- Berges S, Moulin T, Berger E, Tatu L, Sablot D, Challier B, Rumbach L. Seizures and epilepsy following strokes: recurrence factors. Eur Neurol. 2000;43:3–8. doi: 10.1159/000008120. [DOI] [PubMed] [Google Scholar]
- Bladin CF, Alexandrov AV, Bellavance A, Bornstein N, Chambers B, Cote R, Lebrun L, Pirisi A, Norris JW. Seizures after stroke: a prospective multicenter study. Arch Neurol. 2000;57:1617–1622. doi: 10.1001/archneur.57.11.1617. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992a;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Phillips SJ, O’Fallon WM, Frye RL, Whisnant JP. Relationship with cardiac disease to stroke occurrence, recurrence, and mortality. Stroke. 1992b;23:1250–1256. doi: 10.1161/01.str.23.9.1250. [DOI] [PubMed] [Google Scholar]
- Broderick J, Brott T, Kothari R, Miller R, Khoury J, Pancioli A, Gebel J, Mills D, Minneci L, Shukla R. The Greater Cincinnati/Northern Kentucky Stroke Study: preliminary first-ever and total incidence rates of stroke among blacks. Stroke. 1998;29:415–421. doi: 10.1161/01.str.29.2.415. [DOI] [PubMed] [Google Scholar]
- Burn J, Dennis M, Bamford J, Sandercock P, Wade D, Warlow C. Epileptic seizures after a first stroke: the Oxfordshire Community Stroke Project. BMJ. 1997;315:1582–1587. doi: 10.1136/bmj.315.7122.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davalos A, de Cendra E, Molins A, Ferrandiz M, Lopez-Pousa S, Genis D. Epileptic seizures at the onset of stroke. Cerebrovasc Dis. 1992;2:237–331. [Google Scholar]
- De Reuck J, Goethals M, Vonck K, Van Maele G. Clinical predictors of late-onset seizures and epilepsy in patients with cerebrovascular disease. Eur Neurol. 2005;54:68–72. doi: 10.1159/000087715. [DOI] [PubMed] [Google Scholar]
- Dhanuka AK, Misra UK, Kalita J. Seizures after stroke: a prospective clinical study. Neurol India. 2001;49:33–36. [PubMed] [Google Scholar]
- Feleppa M, Di Iorio W, Saracino DM. Early poststroke seizures. Clin Exp Hypertens. 2006;28:265–270. doi: 10.1080/10641960600549215. [DOI] [PubMed] [Google Scholar]
- Ferro JM, Pinto F. Poststroke epilepsy: epidemiology, pathophysiology and management. Drugs Aging. 2004;21:639–653. doi: 10.2165/00002512-200421100-00003. [DOI] [PubMed] [Google Scholar]
- Giroud M, Gras P, Fayolle H, Andre N, Soichot P, Dumas R. Early seizures after acute stroke: a study of 1,640 cases. Epilepsia. 1994;35:959–964. doi: 10.1111/j.1528-1157.1994.tb02540.x. [DOI] [PubMed] [Google Scholar]
- Gupta SR, Naheedy MH, Elias D, Rubino FA. Postinfarction seizures. A clinical study. Stroke. 1988;19:1477–1481. doi: 10.1161/01.str.19.12.1477. [DOI] [PubMed] [Google Scholar]
- Hart RG, Byer JA, Slaughter JR, Hewett JE, Easton JD. Occurrence and implications of seizures in subarachnoid hemorrhage due to ruptured intracranial aneurysms. Neurosurgery. 1981;8:417–421. doi: 10.1227/00006123-198104000-00002. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Ramirez-Lassepas M, Rosenstein R. Risk for seizures and epilepsy following cerebrovascular insults. Epilepsia. 1994;25:666. [Google Scholar]
- Hornig CR, Buttner T, Hufnagel A, Schroder-Rosenstock K, Dorndorf W. Epileptic seizures following ischaemic cerebral infarction. Clinical picture, CT findings and prognosis. Eur Arch Psychiatry Neurol Sci. 1990;239:379–383. doi: 10.1007/BF01734546. [DOI] [PubMed] [Google Scholar]
- Kannel WB. Incidence and epidemiology of heart failure. Heart Fail Rev. 2000;5:167–173. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- Kilpatrick CJ, Davis SM, Tress BM, Rossiter SC, Hopper JL, Vandendriesen ML. Epileptic seizures in acute stroke. Arch Neurol. 1990;47:157–160. doi: 10.1001/archneur.1990.00530020053014. [DOI] [PubMed] [Google Scholar]
- Kilpatrick CJ, Davis SM, Hopper JL, Rossiter SC. Early seizures after acute stroke. Risk of late seizures. Arch Neurol. 1992;49:509–511. doi: 10.1001/archneur.1992.00530290097017. [DOI] [PubMed] [Google Scholar]
- Kotila M, Waltimo O. Epilepsy after stroke. Epilepsia. 1992;33:495–498. doi: 10.1111/j.1528-1157.1992.tb01698.x. [DOI] [PubMed] [Google Scholar]
- Labovitz DL, Hauser WA, Sacco RL. Prevalence and predictors of early seizure and status epilepticus after first stroke. Neurology. 2001;57:200–206. doi: 10.1212/wnl.57.2.200. [DOI] [PubMed] [Google Scholar]
- Lamy C, Domigo V, Semah F, Arquizan C, Trystram D, Coste J, Mas JL. Early and late seizures after cryptogenic ischemic stroke in young adults. Neurology. 2003;60:400–404. doi: 10.1212/wnl.60.3.400. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Luders H, Dinner DS, Morris HH. Epileptic seizures due to thrombotic and embolic cerebrovascular disease in older patients. Epilepsia. 1985;26:622–630. doi: 10.1111/j.1528-1157.1985.tb05702.x. [DOI] [PubMed] [Google Scholar]
- Loiseau J, Loiseau P, Duche B, Guyot M, Dartigues JF, Aublet B. A survey of epileptic disorders in southwest France: seizures in elderly patients. Ann Neurol. 1990;27:232–237. doi: 10.1002/ana.410270304. [DOI] [PubMed] [Google Scholar]
- Lossius MI, Ronning OM, Mowinckel P, Gjerstad L. Incidence and predictors for post-stroke epilepsy. A prospective controlled trial. The Akershus stroke study. Eur J Neurol. 2002;9:365–368. doi: 10.1046/j.1468-1331.2002.00415.x. [DOI] [PubMed] [Google Scholar]
- Misirli H, Ozge A, Somay G, Erdogan N, Erkal H, Erenoglu NY. Seizure development after stroke. Int J Clin Pract. 2006;60:1536–1541. doi: 10.1111/j.1742-1241.2005.00782.x. [DOI] [PubMed] [Google Scholar]
- Olsen T. Post-stroke epilepsy. Current Atherosclerosis Reports. 2001;3:340–344. doi: 10.1007/s11883-001-0029-4. [DOI] [PubMed] [Google Scholar]
- Passero S, Rocchi R, Rossi S, Ulivelli M, Vatti G. Seizures after spontaneous supratentorial intracerebral hemorrhage. Epilepsia. 2002;43:1175–1180. doi: 10.1046/j.1528-1157.2002.00302.x. [DOI] [PubMed] [Google Scholar]
- Pohlmann-Eden B, Hoch DB, Cochius J, Hennerici MG. Stroke and epilepsy: Critical review of the literature Part I. Cerebrovasc Dis. 1996;6:332–338. [Google Scholar]
- Pohlmann-Eden B, Cochius J, Hoch DB, Hennerici MG. Stroke and epilepsy: Critical review of the literature Part II. Cerebrovasc Dis. 1997;7:2–9. [Google Scholar]
- Reith J, Jorgensen HS, Nakayama H, Raaschou HO, Olsen TS. Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study. Stroke. 1997;28:1585–1589. doi: 10.1161/01.str.28.8.1585. [DOI] [PubMed] [Google Scholar]
- Richardson EP, Jr, Dodge PR. Epilepsy in cerebral vascular disease: a study of the incidence and nature of seizures in 104 consecutive autopsy-proven cases of cerebral infarction and hemorrhage. Epilepsia. 1954;3:49–74. doi: 10.1111/j.1528-1157.1954.tb03153.x. [DOI] [PubMed] [Google Scholar]
- Shinton RA, Gill JS, Melnick SC, Gupta AK, Beevers DG. The frequency, characteristics and prognosis of epileptic seizures at the onset of stroke. J Neurol Neurosurg Psychiatry. 1988;51:273–276. doi: 10.1136/jnnp.51.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman IE, Restrepo L, Mathews GC. Poststroke seizures. Arch Neurol. 2002;59:195–201. doi: 10.1001/archneur.59.2.195. [DOI] [PubMed] [Google Scholar]
- So EL, Annegers JF, Hauser WA, O’Brien PC, Whisnant JP. Population-based study of seizures disorders after cerebral infarction. Neurology. 1996;46:350–355. doi: 10.1212/wnl.46.2.350. [DOI] [PubMed] [Google Scholar]
- Sundaram MB, Chow F. Seizures associated with spontaneous subarachnoid hemorrhage. Can J Neurol Sci. 1986;13:229–231. doi: 10.1017/s0317167100036325. [DOI] [PubMed] [Google Scholar]
- Sung CY, Chu NS. Epileptic seizures in thrombotic stroke. J Neurol. 1990;237:166–170. doi: 10.1007/BF00314589. [DOI] [PubMed] [Google Scholar]
- Szaflarski M, Szaflarski JP, Privitera MD, Ficker DM, Horner RD. Racial/ethnic disparities in the treatment of epilepsy: what do we know? What do we need to know? Epilepsy Behav. 2006;9:243–264. doi: 10.1016/j.yebeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Velioglu SK, Ozmenoglu M, Boz C, Alioglu Z. Status epilepticus after stroke. Stroke. 2001;32:1169–1172. doi: 10.1161/01.str.32.5.1169. [DOI] [PubMed] [Google Scholar]
- Vespa PM, O’Phelan K, Shah M, Mirabelli J, Starkman S, Kidwell C, Saver J, Nuwer MR, Frazee JG, McArthur DA, Martin NA. Acute seizures after intracerebral hemorrhage: a factor in progressive midline shift and outcome. Neurology. 2003;60:1441–1446. doi: 10.1212/01.wnl.0000063316.47591.b4. [DOI] [PubMed] [Google Scholar]