Abstract
Microbial opsin-based optogenetic tools have been transformative for neuroscience. To extend optogenetic approaches to the immune system to remotely control immune responses with superior spatiotemporal precision, pioneering tools have recently been crafted to modulate lymphocyte trafficking, inflammasome activation, dendritic cell maturation, and antitumor immunity through the photoactivation of engineered chemokine receptors and calcium release-activated calcium channels. We highlight herein some conceptual design strategies for installing light sensitivities into the immune signaling network, and in parallel, we propose potential solutions for in vivo optogenetic applications in living organisms with near-infrared light-responsive upconversion nanomaterials. Moreover, to move beyond proof-of-concept into translational applications, we discuss future prospects for integrating personalized immunoengineering with optogenetics to overcome critical hurdles in cancer immunotherapy.
Keywords: Cancer immunotherapy, Optogenetics, Immune response, CRAC channel, Upconversion nanoparticle, genome engineering
Progress and Challenges in Cancer Immunotherapy
Cancer immunotherapy (see Glossary) has become one of the most promising therapeutic strategies to elicit durable clinical responses in patients. This paradigm-shifting therapy harnesses the power of a patient’s own immune system to attack cancer cells by targeting single or multiple steps in the “cancer-immunity cycle” (Figure 1; Key Figure) [1]. In cancer patients, tumor cells set up multiple barriers to disrupt the revolutions of this tumor-suppressive cycle. Oncogenic signals can not only suppress the intrinsic expression of chemokines to cause defective migration of dendritic cells (DCs) and impaired priming of cytotoxic T lymphocytes (CTLs) but also thwart DC differentiation and maturation or even switch anti-tumor DCs to “pro-tumor” DCs [2, 3]. Indeed, DC-based immunotherapy has proven to be disappointingly ineffective in most cancer patients, partially owing to difficulties in maintaining the maturation state of DCs and the inability of injected DCs to migrate into tumor draining lymph nodes (dLNs) [2]. Moreover, cancer cells may create a hostile immunosuppressive tumor microenvironment (TME) to prevent T cell infiltration and reduce T cell effector functions [1, 3–5].
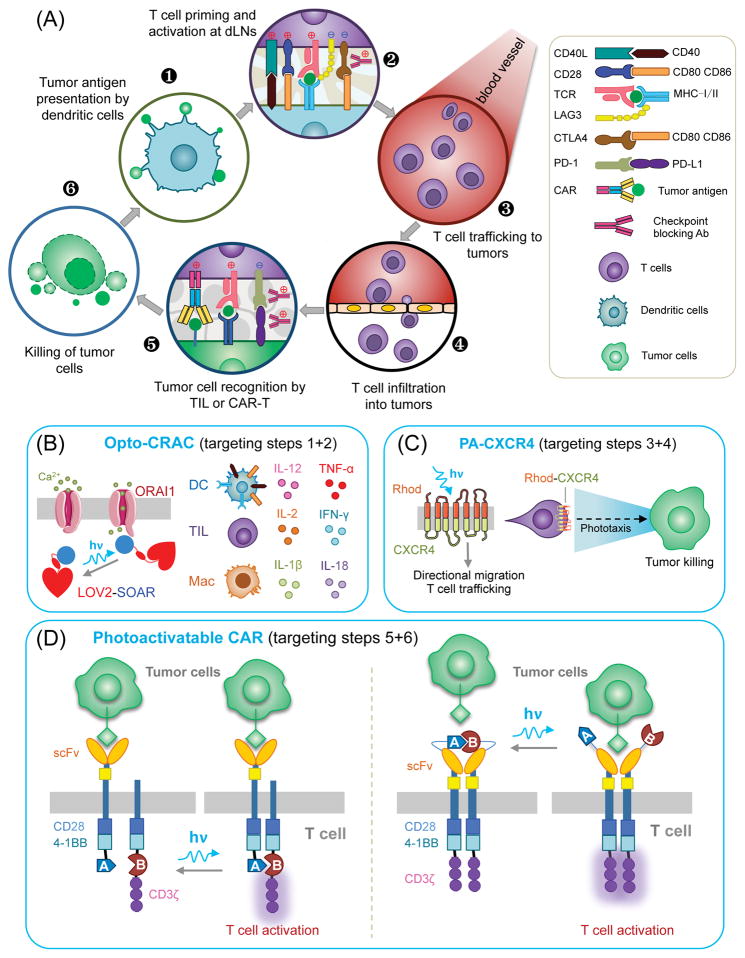
Figure 1, Key Figure. The cancer-immunity cycle and opportunities for optogenetic interventions to improve cancer immunotherapies.
(A) A typical anti-tumor immune response involves the following major steps: (1) Tumor antigens are released and captured by dendritic cells (DCs) for processing; (2) DCs present processed antigens to T cells to prime and activate TCR signaling against tumor-specific antigens in tumor draining lymph nodes (dLNs). T-cell antitumor activity is balanced by coordinated actions of both stimulatory (red-cross) and inhibitory (blue-cross) signals. (3) Activated effector T cells travel through blood vessels and (4) infiltrate into tumor sites by crossing endothelial barrier. (5) Tumor-infiltrating T cells (TILs) recognize cancer cells through the interaction between TCR and cognate antigens bound to MHC-I on tumor cells. Engineered therapeutic T cells expressing chimeric antigen receptor (CAR-T) are also used in the clinic to boost tumor killing. Immunomodulatory monoclonal antibodies can overcome local immunosuppression by blocking suppressive PD-1 receptors and (6) eventually initiate the tumor cell killing machinery. CTLA-4, cytotoxic T-lymphocyte antigen-4; PD-1, programmed cell-death 1; LAG-3, lymphocyte activation gene-3; 4-1BB, tumor necrosis factor receptor superfamily member 9.
(B) Opto-CRAC channels engineered into DCs, TILs, or macrophages (Mac) to photo-manipulate calcium-dependent events in the immune system (e.g., antigen presentation and cytokine production). LOV, light-oxygen-voltage domain from phototropin 1; SOAR/CAD, the STIM-Orai-activating domain/the CRAC activation domain.
(C) Photoactivatable rhodopsin (Rhod)-CXCR4 chimeric chemokine receptor enables T cells trafficking and phototaxis to boost tumor killing.
(D) Proposed strategies for photomanipulating engineered CAR-T cells via (left) heterodimerization of co-stimulation molecules or (right) conformational switch in the extracellular single-chain variant fragment (scFv).
Blue arrow, photoexcitation with blue light; Black arrow, reversal to the dark state.
Among all of the steps in the cancer-immunity cycle, T cell priming and activation in tumor dLNs (step 2; Figure 1a) defines the nature and strength of the ensuing antitumor immune response. T cells require two signals to mount a productive immune response: the activation signal 1 delivered through the engagement of the T cell receptor (TCR) to its cognate antigens presented by DCs; and the co-stimulatory signal 2 arising from interactions between CD28 and B7 molecules (CD80/CD86). Earlier efforts to apply synthetic biology approaches to modify T cells were mostly geared toward boosting lymphocyte activation signaling [1, 4, 5]. By combining key signaling modules from both the TCR and co-stimulatory receptors, engineered therapeutic T cells expressing chimeric antigen receptors (CARs) have been found to have curative potential for treatment of hematological malignancies [6]. A typical CAR is composed of a single-chain variable fragment (scFv) that recognizes tumor-specific antigens, a transmembrane region, the CD3ζ chain that provides the activation signal 1 to T cells, and two endodomains derived from CD28 and 4-1BB (or CD137) that provide signal 2 (Figure 1d). The application of CAR-T cell therapy, however, is limited by its stringent clinical indications and adverse effects of “on-target, off-tumor” toxicities, including cytokine-release syndrome, which arises from unconstrained cytokine release from activated T cells [5, 7]. There remains, therefore, an important unmet clinical need to develop safer CAR-T therapies by which the antitumor response can be tightly and reversibly controlled with high spatiotemporal precision.
Recent developments of anticancer therapeutics have shifted from activating T cells to revitalizing the suppressed antitumor immunity, or the combination of both [5, 8]. Following T cell activation, co-inhibitory signals mediated by cytotoxic T-lymphocyte-association protein 4 (CTLA-4) and programmed death protein 1 (PD1) are also launched to restrict T cell-mediated immune responses [5, 8]. Cancer cells capitalize on these T-cell intrinsic immune checkpoints to evade immune surveillance. Upregulation of PD-L1 in tumor cells is often induced through immune cell-driven (type I/II interferon signaling) [9] or oncogene-driven (e.g., Myc) mechanisms [10] to facilitate immune escape. By using monoclonal antibodies against immune checkpoints (ipilimumab against CTLA-4, or nivolumab and pembrolizumab against PD1), cancer immunotherapy effectively releases the tumor-induced brakes on the immune system to reinitiate the cancer-immunity cycle, which proves to be remarkably effective in the clinical management of multiple types of cancer [8]. Despite exciting progress in this area, the most ideal immunomodulatory therapy should allow precise targeting and reversible control of the immune signaling network in real time. In this way, therapists can customize the time, location, and dosage of therapeutic agents to reduce immune-related adverse events while delivering personalized therapy to cancer patients. To meet this objective, we advocate herein combining optogenetics with immunoengineering to develop smarter immunotherapies with enhanced efficacy and safety.
Optogenetics Meets Immunology
In light of its unsurpassable flexibility and spatiotemporal precision to manipulate biochemical pathways, the field of non-opsin-based optogenetics has been gaining rapid momentum in cell biology [11]. Over the past decade, we have witnessed the explosion of genetically encoded photoswitchable modules [11–13] that respond to light at varying wavelengths (Figure 2 and Table 1). One idea that has come of age is to integrate optogenetics with immunoengineering [14, 15], a nascent but rapidly growing field that requires multidisciplinary efforts from engineers, nanomaterial chemists, immunologists and cancer biologists. This approach (termed “optoimmunoengineering”) offers unique advantages that are complementary to, and also synergistic with, ongoing efforts to improve immunotherapies. First, unlike traditional genetic perturbation methods (knockout/knockdown, mutagenesis, or overexpression) that permanently perturb the spatiotemporal features of the signaling network, genetically encoded light-switches can reversibly activate or deactivate proteins or gene expression in a timely manner without eliciting long-lasting side effects. Second, optogenetic approaches enable versatile control over engineered cells with superior spatiotemporal precision. Compared with small molecule-gated CAR-T, which lacks tight spatial control [16], the design of photoactivatable CAR-T cells (Figure 1d) may allow selective activation of “living therapeutics” at desired tumor sites to minimize off-tumor cytotoxicities. When adapted to interrogate and intervene in the cancer-immunity cycle, optogenetic techniques will not only facilitate immune cells to overcome energy barriers presented by the immunosuppressive TME but also elucidate novel mechanisms underlying antitumor immunity and resistance to immunotherapy [4]. Here, we highlight several exciting examples of optoimmunoengineering that target signaling events required for T cell activation, tolerance and migration, as well as transcriptional regulation of immunomodulatory gene expression. We hope this review will stimulate further thoughts and future efforts on harnessing the power of light to control the immune system for both research and therapeutic purposes.
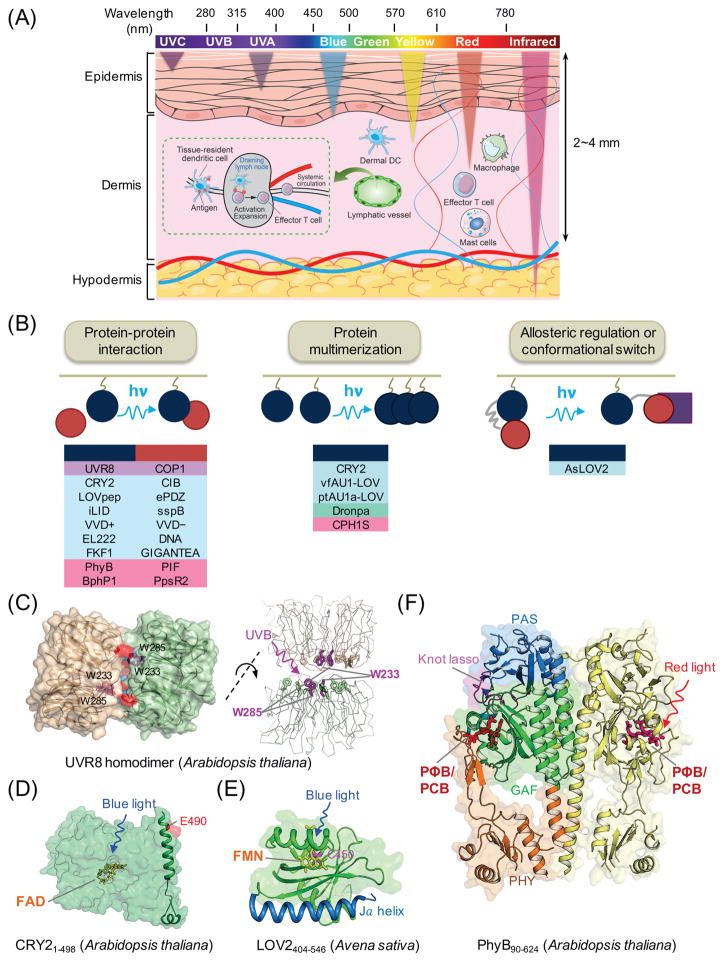
Figure 2. Genetically-encoded photoswitchable modules for optogenetic control of cellular signaling.
(A) Schematic of penetration depth of light emitting at varying wavelengths. Most existing optogenetic tools are activated by blue or yellow light that penetrates up to 0.5-1 mm in depth, with very limited effects on circulating immune cells in living tissues. Tissue-resident dendritic cells (DCs) in the dermis capture antigens (e.g., tumor antigens liberated from melanoma cells) and cross-present them to naïve T cells after migration to draining lymph nodes via lymphatics, leading to activation and expansion of T cells and subsequent trafficking to tumor sites through systemic circulation.
(B) Light-mediated control of cell signaling via photo-manipulation of protein-protein interaction (left), clustering of proteins (middle), or allosteric regulation of protein functions (right). Representative examples of photoresponsive modules are listed under each engineering strategy. UVR8, ultraviolet-B receptor 8; COP1, constitutively photomorphogenic 1; CRY2, cryptochrome-2; CIB, cryptochrome-interacting basic-helix-loop-helix protein; LOV, light-oxygen-voltage; VVD, VIVID protein with a Per-ARNT-Sim domain; FKF1: flavin-binding, kelch repeat, F-box protein; PhyB, phytochrome B; vfAU1/ptAU1, aureochrome 1 from Vaucheria frigida or Phaeodactylum tricornutum.
(C–F) 3D structures of plant-derived photoresponsive domains or proteins that are commonly used in optogenetic applications. The cofactors or chromophores that confer light sensitivities to corresponding proteins are: (C) tryptophan residues for UVR8 (PDB entry: 4DNW); (D) FAD for CRY2 (modeled from CRY1; PDB entry: 1U3D); (E) FMN for LOV2 (PDB entry: 2V0W); and (F) PΦB or PCB for PhyB (PDB entry: 4OUR). FAD, flavin adenine dinucleotide; FMN, flavin mononucleotide; PΦB, phytochromobilin; PCB, phycocyanobilin.
Table 1.
List of genetically encoded photosensitive modules used in optogenetics.
| Photoresponsive module | Cofactor | on (nm) | off (nm) | Turn-on half time | Turn-off half time | Application in the immune system | References |
|---|---|---|---|---|---|---|---|
| UVR8 | Trp | ~310 | dark | seconds | seconds | NA | [34] |
| LOV2 | FMN | 440–480 | dark | seconds | seconds | Opto-CRAC | [21, 33, 36,49, 51] |
| CRY2 | FAD | 405–488 | dark | seconds | ~5-30 min | NA | [32, 38, 43] |
| ChR2 | retinal | ~470 | dark | ms | ms | PA-CXCR4 | [23] |
| Dronpa | Cys-Tyr-Gly | 490 | 390 | seconds | seconds | NA | [60] |
| PhyB | PCB | 650 | 750 | seconds | seconds | NA | [30] |
| BphP1 | BV | 740 | 650/dark | min | min | NA | [39] |
Opto-CRAC
Ca2+ release-activated Ca2+ channels (CRAC channels), composed of the stromal interaction molecule (STIM) and ORAI (named after the keepers of Heaven’s gate in Greek mythology), are present on the surface of most immune cells to mediate Ca2+ influx and control Ca2+-dependent gene expression [17–19]. Following the engagement of immunoreceptors by antigens and subsequent Ca2+ depletion in the endoplasmic reticulum, CRAC channels are activated to trigger the nuclear translocation of the nuclear factor of activated T-cells (NFAT), and in turn, induce the expression of cytokines and other genes that are involved in maintaining effector functions of immune cells (Figure 1b). In addition to its best-established role in driving T lymphocyte activation, intracellular Ca2+ mobilization promotes inflammasome activation in macrophages, DC maturation, and antigen presentation [17–19]. By mimicking a conformational switch in STIM1 [18, 20] and thus obviating the need for physiological stimulus or store depletion to elicit Ca2+ entry, He et al. developed an Opto-CRAC system (Figure 1b) to photo-trigger Ca2+ influx and faithfully phenocopy hallmark Ca2+-dependent responses in immune cells following light stimulation [21, 22]. When introduced into therapeutic DCs in a mouse model of melanoma, engineered Opto-CRAC channels function as “photoactivatable adjuvants” to enhance DC maturation and antigen presentation (step 1; Figure 1a), thereby promoting T cell priming and activation (step 2) to facilitate the killing of melanoma and its metastases at distal sites [21]. The Opto-CRAC system might also be applied to exert precision control of Ca2+/NFAT signaling in therapeutic CAR-T cells, so that it can synergize with the upstream TCR-mediated signal 1 to boost antitumor immune response in the tumor bed.
It has been established that Ca2+ signaling patterns, in the form of transients or oscillations, dynamically control the activity of the downstream Ca2+-sensitive transcriptional factor NFAT [23–26]. However, how the Ca2+ oscillation amplitude, frequency, and duty cycle contribute to gene transcription remains unclarified. By using a light-gated G-protein coupled receptor (human melanopsin; hOPN4) as the optical actuator, along with NFAT-dependent luciferase gene expression as readout, Hannanta-anan et a.l generated a set of Ca2+ oscillation waveform inside HeLa cells and further built a mathematical model to define NFAT as an integrator of accumulative Ca2+ load, rather than a decoder that is selective to frequency [27]. One caveat in this study is that photo-stimulation of hOPN4 ultimately leads to the generation of two secondary messengers, Ca2+ and diacyl glycerol (DAG), the latter of which further activates protein kinase C and the activated protein-1 (AP-1). AP-1 subsequently cooperates with NFAT to drive gene expression in mammalian cells, a synergistic effect that might confound the interpretation of data-to-model correlations. The Opto-CRAC system, which exclusively generates Ca2+ signals and thus circumvents complications from other secondary messengers, can be exploited as a cleaner synthetic optogenetic transcription device to model and photo-tune Ca2+/NFAT-dependent transcriptional output.
In addition to Ca2+, phosphoinositide lipids also play instrumental roles in shaping the duration and strength of TCR signaling [28], as well as in controlling cell motility and chemotaxis [29, 30]. By reversibly recruiting phosphatase or kinase domains in lipid-converting enzymes to the plasma membrane (PM) with photosensitive dimerizers [31–33], the amounts of PM-resident phosphoinositides, particularly PI(4,5)P2 and PI(3,4,5)P3, can be conveniently manipulated with light to control the function of downstream effectors. The availability of these tools could add an additional layer of optogenetic control over immune cells.
PA-CXCR4
Inefficient migration and infiltration of therapeutic immune cells into the tumor site is one of the major hurdles facing the current adoptive cell transfer (ACT) therapies. By taking advantage of common structure-function relationships shared between chemokine receptors and photoresponsive rhodopsins, Xu et al. designed a photoactivatable C-X-C chemokine receptor type 4 (PA-CXCR4; Figure 1c) to optically control T cell migration and trafficking (steps 3-4; Figure 1a) [34]. PA-CXCR4-expressing CTLs exhibited light-induced directional migration or phototaxis both in vitro and in vivo. Photostimulation on tumor sites further enhanced the intratumoral infiltration of CTLs to improve the efficacy of ACT therapy [34]. Given the indispensable role of the chemokine-chemokine receptor network in driving the revolutions of the cancer-immunity cycle, this approach can be similarly adapted to confer light sensitivity to other types of chemokine receptors that are more directly involved in mediating DC homing to dLNs (CCR7) or T-cell recruitment to tumor sites (CXCR3 and CCR5) [35].
Photoactivatable CARs
A non-negligible side effect associated with current CAR-T therapies is “on-target, off-tumor” cytotoxicity. This undesirable scenario arises from the shared expression of target antigens in both normal and cancer tissues, so that CAR T cells engage on nonpathogenic tissues to cause damage. The most recent effort to mitigate this risk includes the design of a small molecule-gated chimeric T cell receptor, in which signal 1 (antigenic stimulation) and signal 2 (co-stimulation signal) can only be functionally assembled in the presence of an exogenous heterodimerizing molecule [16]. Conceivably, by replacing the chemical dimerization domains with light-inducible dimerization modules (Figure 2b), the function of these split chimeric antigen receptors may be restored following photoillumination (Figure 1d). With a similar design strategy, it may also be feasible to rewire co-inhibitory signals into activating signals through light-induced reconstitution of split chimeric receptors composed of the extracellular domain of PD1 and the endodomains from co-stimulatory molecules.
Photo-tunable gene expression systems
Another underexplored immunoengineering strategy that merits serious consideration is to photo-manipulate the expression of immunomodulatory genes. For example, the Opto-CRAC system discussed above can be repurposed to photo-induce tumor antigen-specific, Ca2+-dependent production of IL-12 driven by an NFAT-responsive promoter in tumor-targeting T cells. IL-12 is a potent inducer of antitumor immunity by augmenting the cytotoxic T and natural killer (NK) cell response [36]. Unlike the systematic administration of IL-12, which tends to induce global toxicities, light-controlled IL-12 production within the TME might hold great promise to avoid off-tumor side effects. Moreover, the recently-devised photoactivatable genome-editing and transcription systems, built upon a split Cas9 [37] or dCas9 with light-inducible dimerization modules [38], offer simple and versatile platforms to achieve genome engineering and transcriptional reprogramming at endogenous genes. Similar epigenome-editing tools can be generated through light-induced heterodimerization of catalytically inactive Cas9 [39] with epigenomic modifiers. Photo-sculpturing the mammalian (epi)genome landscape at the transcriptional or epigenetic levels might serve as a high entry point to reversibly control gene expression at user-defined loci (e.g., CTLA-4, PD1 or PDL1) without altering the genetic code. These tools will likely find broad utility in the mechanistic dissection of the causal effects of (epi)genotypes on disease phenotypes, drug response, and resistance to immunotherapies [4].
Photoresponsive modules tailored for optoimmunoengineering
To date, the optogenetic field has created a set of photosensory domains or photoreceptors that can be reversibly activated or de-activated by lights, ranging from ultraviolet (UV) irradiation to near-infrared radiation (NIR), that display varying tissue-penetrating capacities (Figure 2). The physicochemical properties of these modules (Table 1) and their applications have been extensively reviewed elsewhere [11, 12, 40]. Here, we briefly outline three general design strategies that can be tailored to manipulate the immune signaling network and construct synthetic circuits in immune cells (Figure 2b).
Light-inducible protein-protein heteromultimerization
The most widely used strategy to photomanipulate cell signaling takes advantage of conformational changes elicited by photon absorption to drive the heterodimerization of two polypeptides [33, 41–49]. Such a two-component optical system typically consists of a light-responsive protein (component A) and its effector domain (component B) that preferentially binds to component A in its lit or dark state (Figure 2b, left). Often, one component is tethered to a subcellular compartment or the plasma membrane to enable light-inducible protein translocation. By either recruiting signaling proteins to, or removing signaling proteins from, their normal sites of action, the signal transduction in immune cells can thus be rapidly initiated or terminated upon photostimulation. Ideally, this approach can be extended to engineer photoactivatable CARs by providing a light-induced “on” signal through the heteromerization of functional modules that separately deliver activation signal 1 and co-stimulatory signal 2 (Figure 1d, left). Alternatively, a light-inducible dissociation device (e.g., the LOV2 trap and release of protein, or LOVTRAP system [50]) may enable the design of smarter CARs that are therapeutically inert in the dark state (Figure 1d, right). For instance, a pair of photosensitive modules that associate in the dark can mask the antigen recognition of scFv. Upon photostimulation, the two modules dissociate to expose the tumor antigen recognition site, and engineered CAR-T cells will then be turned on to execute their effector functions. Furthermore, the incorporation of an optogenetic suicide device [51] might provide an additional optical safety switch to CAR-T therapy. For example, the pro-apoptotic protein Bax has been engineered to undergo light-dependent translocation from the cytosol toward the outer membrane of mitochondria to trigger apoptosis [51]. This strategy can be exploited to force the suicide of CAR T cells after they complete their mission in tumor sites.
Photo-inducible protein clustering
Several photosensory domains derived from plants or bacteria [52–56] undergo a monomer-to-oligomer transition following photostimulation (Figure 2b, middle). This unique feature has been exploited to induce local protein oligomerization to activate Ca2+ influx [57], Wnt signaling [22, 52], and GTPases [52] or receptor tyrosine kinases [53, 56, 58]. Compared with the optical dimerizers described above, optogenetic applications with this strategy only need a single construct. The modular nature of this approach makes it highly feasible to install light sensitivities into signaling molecules in the immunoregulatory network, particularly proteins with the caspase activation and recruitment domain that require polymerization to achieve their maximal functions [59].
Reversible photocaging through allosteric regulation
The third strategy requires combining rational design with rigorous experimentation. The design often involves the fusion of effector domains to the C-terminus of light-oxygen-voltage domain 2 (LOV2) with optimized J helix and linkers in between (Figure 2b and Figure 2e). In the dark, the activity of an effector domain is suppressed due to steric hindrance posed by the core body of LOV2. Upon illumination, the conformational change in LOV2 causes unfolding the J helix with subsequent uncaging of the coupled effector domain and functional restoration [12, 60, 61]. A similar technique was used to control the exposure of nuclear localization/export signals to modulate nuclear protein import [62] or export [63]. This tagging system can be used to sequester proteins (e.g., transcriptional factors that control the development and differentiation of immune cells) away from their sites of action to confer precise spatiotemporal control of protein activity. Nonetheless, the most desirable modular protein-caging systems that obviate multiple rounds of trial and error are yet to be developed.
Wireless optogenetics to enable immunomodulation in vivo
To move beyond proof-of-concept ex vivo studies toward addressing real-world immunological questions in vivo, we are confronted with the following challenge: how can we effectively deliver light, with minimized invasiveness, to immune cells that are constantly moving and/or deeply buried within tissues? Most of the existing optogenetic tools have activation spectra centered around 400–500 nm with extremely low tissue penetration depth (<1 mm), thus limiting their in vivo applications. Miniature head-mounted light-delivery systems, as well as subcellular-scale micro light-emitting diode (μLED; Figure 3a) implants that can be controlled by radio frequencies, have enabled in vivo wireless optogenetic manipulations in the central nervous system of freely moving mammals [64–66]. However, these devices are tailored for neuroscience and remain unrealistic for the immune system, largely owing to the high mobility and scattered distribution of immune cells across the body. Here, we present recent solutions that might pave the way for exploring future applications of optogenetic immunomodulation in mammals.
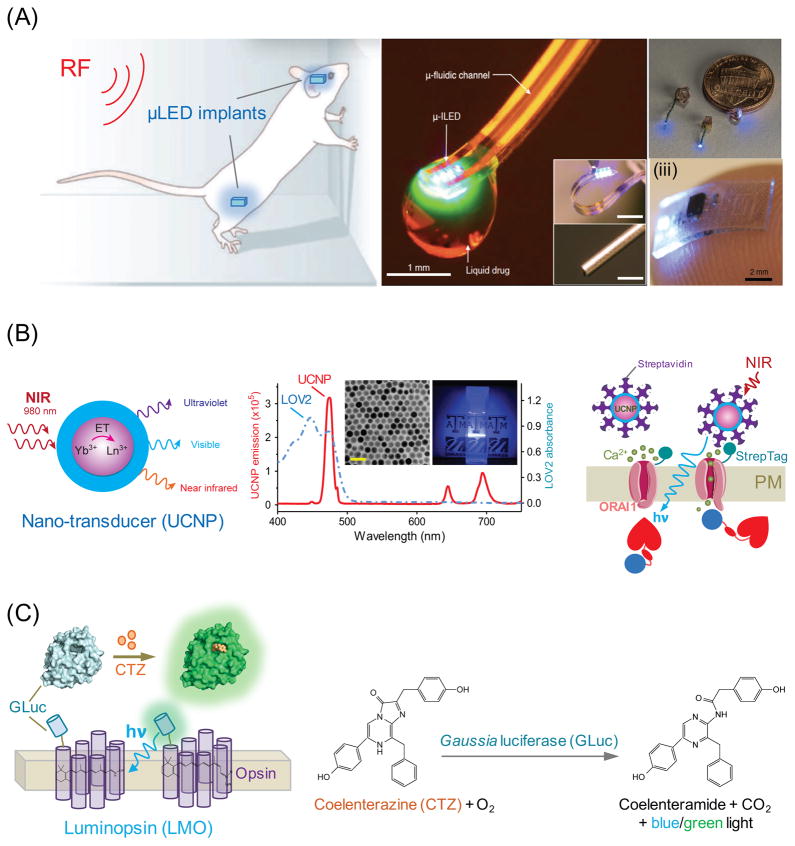
Figure 3. Strategies for in vivo optogenetic immunomodulation.
(A) Application of microLED (μLED) implants for wireless and minimally invasive control of photoactivation and drug delivery. (i) Optofluidic probes that combine microfluidic drug delivery with cellular-scale inorganic light-emitting diode (μ-ILED). Reprinted with permission from [64] (copyright 2015, Elsevier). (ii) Schematic of wireless μLED implants suitable for photoactivation of neuronal cells in peripheral nerve endings, brain, and spinal cord with size comparison with a US 1-cent coin. Reprinted with permission from [65] (copyright 2015, Nature Publishing Group). (iii) Picture of a soft, stretchable μLED device (0.7x3.8x6 mm3, 16 mg) resting on the tip of the index finger. Reprinted with permission from [66] (copyright 2015, Nature Publishing Group).
(B) Upconversion nanoparticles paired with Opto-CRAC to control calcium signaling in cells of the immune system. Left, schematic of the core/shell structure of lanthanide doped upconversion nanoparticles (UCNPs). ET, energy transfer between lanthanide ions. Middle, Superimposed spectra of LOV2 absorbance (blue) and representative NIR-to-blue UCNP emission (red). Insets showing a typical transmission electron micrograph (TEM) of synthesized UCNPs and blue light emission from the UNCP solution-containing cuvette illuminated by NIR light at 980 nm. The scale bar represents 50 nm. Right, specific targeting of streptavidin-conjugated UCNPs to cells expressing engineered ORAI1 calcium channel that contains an extracellular StrepTag.
(C) Cartoon illustrating the photoactivation of a chimeric luminopsin (LMO) receptor composed of a microbial opsin and Gaussia luciferase (GLuc) in the presence of GLuc substrate coelenterazine (CTZ). Bioluminescence is generated during the oxidation reaction of CTZ catalyzed by GLuc.
Upconversion nanoparticles
Lanthanide-doped upconversion nanoparticles (UCNPs) are capable of producing anti-Stokes luminescence by converting NIR light into shorter wavelength emissions, with additional advantages of reduced tissue scattering and phototoxicity (Figure 3b) [22, 67, 68]. UCNPs shift the light-harvesting window to the NIR region, where deep tissue penetration and remote stimulation are feasible. The emitted photons are robust enough to activate almost all existing optogenetic constructs (e.g., LOV2, CRY2 and ChR2) that utilize visible light-absorbing cofactors [22, 67, 68]. NIR-to-blue emitting UCNPs (Figure 3b), in conjugation with Opto-CRAC, have been successfully applied to boost the antitumor response to suppress tumor growth and metastasis in living animals with external NIR light [21]. Cell-specific targeting can be readily achieved through facile modifications of UCNP surfaces with user-defined antibodies or ligands, thus enabling precise spatial control of the light source. A UCNP-based NIR-stimulable optogenetic system is most compatible with adoptive cell transfer experiments or adoptive immunotherapies, which are widely used in both basic research and clinical settings. The broad adaptability of this approach with existing optical tools is anticipated to open up new possibilities to interrogate light-controllable cellular processes in whole animals, while minimally interfering with the host’s physiology.
Photoactivation with bioluminescence
The bioluminescence produced by the enzymatic reaction between Gaussia luciferase (GLuc) and its diffusible substrate coelenterazine provides a new chemogenetic method for activation or inhibition of neurons expressing luminopsins [69], in which GLuc was fused to the N- or C-terminus of microbial opsins (Figure 3c). Although this approach provides the simplest and most non-invasive way for in vivo optogenetics, quantitative analyses of the light intensity generated by enzymatic reaction remain challenging and whether it is sufficient to activate other light-sensitive proteins are yet to be determined. Like other chemogenetic techniques that rely on the injection and subsequent biodistribution of exogenous substrates, this hybrid approach lacks accurate spatial control and rapid reversibility, two limiting factors that may hinder its practical use in the immune system.
Concluding Remarks and Future Perspectives
Cancer immunotherapy has joined the conventional therapeutic modalities (i.e., surgery, radiotherapy, chemotherapy, and targeted therapy), to emerge as the fifth pillar of cancer therapy. Immunoengineering with optogenetic approaches will likely provide a non-invasive way to fine-tune the depth and breadth of antitumor response at high spatiotemporal resolution and is therefore amenable to personalize the therapeutic modality at real time. The availability of a number of photoresponsive modules has opened new exciting opportunities to install light sensitivities into the immune signaling network, thereby exerting light control on immune checkpoint blockade therapies and bestowing novel functions to therapeutic immune cells.
One fundamental roadblock that hampers the application of optogenetic tools in the immune system is their low efficiency of photoactivation within biological tissues (see Outstanding Questions). Expanding the light sensitivities toward the NIR range can push the penetration depth toward a few centimeters to enable wireless optogenetics in living mammals. Therefore, the most promising and simplest solutions to enable remote immunomodulation in vivo is either to couple the existing toolkit with NIR-stimulant nanotransducers like UCNPs [21, 22, 67, 68] or to build genetically encoded NIR light-responsive elements into the immune signaling network. The recent discovery of an NIR light-controllable optogenetic system [49], as well as the de novo design of proteins harboring NIR light-responsive cofactors [70], strongly attests to the feasibility of the latter strategy. Together, these pioneering efforts constitute a solid basis for the future development of new generations of biocompatible optogenetic systems that can be conveniently applied to living organisms without injection of exogenous factors or implantation of optical devices. If successful, we are approaching one further step toward achieving the ultimate goal of translating optoimmunoengineering technology into routine clinic use.
Outstanding Box.
Engineering strategies. The current engineering strategies to install light sensitivities into proteins of interest require multiple rounds of trial and error. How can we streamline this process and establish a light-responsive integrated framework for controlling signaling networks in the immune system?
Tissue penetration. Most of the existing optogenetic tools require visible light in the blue-green range for activation/deactivation, so they cannot penetrate deep enough to impinge on the hematopoietic and immune systems. Can we discover or devise genetically-encoded photo-sensitive modules that can be triggered by deep tissue penetrable far-red or near-infrared light to overcome this barrier?
Spectral sensitivities. The spectral overlaps between optogenetic actuators and fluorescent reporters often limit the use of optogenetic tools. How can we extend the spectral sensitivity to a wide range of wavelengths and thus expand the repertoire of optogenetic tools for simultaneous and orthogonal control over multiple cellular events?
Light sources for photoactivation. Cells of the hematopoietic and immune systems constantly circulate in the lymphatics and blood vessels. How can we efficiently and remotely deliver light to circulating cells (such as therapeutic T cells) to achieve non-invasive and precise control of cellular activities while minimally interfering with the host physiology?
In vivo applications. One of the long-term goals of optogenetic immunomodulation is to improve cancer immunotherapy by reducing systemic off-target cytotoxicity. How can we move forward to preclinical or clinical applications? If this strategy proves clinically useful, how can we integrate immunoengineering with optogenetic approaches into the current therapeutic modalities?
Trends Box.
Immunomodulatory therapies constitute a new pillar of anticancer therapy. Currently, cancer immunotherapy is associated with on-target, off-tumor cytotoxicity or immune-related adverse events. Smarter immunotherapies with enhanced safety and precise control over the anticancer immune response are needed.
Optoimmunoengineering will confer light sensitivity to the immune signaling network to enable remote and noninvasive control of both innate and adaptive immune responses with high spatiotemporal precision.
Optogenetics can be made wireless by implanting miniature light-delivery devices into peripheral lymph nodes or by using red-shifted variants of optical actuators that are capable of penetrating much deeper into biological tissues. Next-generation injectable, aqueous, soluble nano-optical systems are emerging for in vivo applications of optogenetics in the immune system.
Acknowledgments
This work was supported by the National Institutes of Health grants (R01GM112003 to Y.Z. and R01MH103133 to G.H.), the Human Frontier Science Program (to G.H.), the Welch Foundation (BE-1913 to Y.Z.) and by an allocation from the Texas A&M University Health Science Center Startup Fund (Y.Z.). We apologize to the authors of many important publications which we were unable to cite due to space constraints.
GLOSSARY
- Cancer immunotherapy
a therapy that harnesses the power of a patient’s own immune system to fight cancer, with the goal of achieving complete and long-lasting cures for cancer patients. Newly developed therapeutic options include monoclonal antibodies, cancer vaccines, immune checkpoint inhibitors, and chimeric antigen receptor based T-cell (CAR-T) therapy.
- Chimeric antigen receptor (CAR)
engineered receptors that are present on the surface of T cells to enable specific recognition of tumor antigens.
- CRAC channel
calcium release-activated calcium channel that is activated when the calcium ions are depleted from the endoplasmic reticulum of mammalian cells. A CRAC channel, composed of two major protein families called ORAI and STIM, is required for generating a productive immune response. CRAC channel dysfunction often leads to severe combined immunodeficiency.
- Immune checkpoint
key molecules hardwired into the immune signaling network that act as either co-stimulatory or inhibitory signals to modulate immune response. Checkpoint blockade therapy (e.g., anti-CTLA4 or anti-PD-1 monoclonal antibody) that targets regulatory pathways in T cells has been developed to overcome the immunosuppressive tumor microenvironment and thus to enhance antitumor immune response.
- Opto-CRAC
a photoactivatable calcium entry platform engineered from a CRAC channel by installing light sensitivities into the cytosolic domain of STIM1. The light-mediated conformational switch within engineered STIM1 molecules enables reversible opening of ORAI calcium channels on the plasma membrane.
- Optoimmunoengineering
also called optogenetic immunomouldation, combines the use of optical and genetic approaches to remotely control the activities of ion channels and/or signaling components in cells of the immune system, thereby enabling photo-tunable modulation of innate and/or adaptive immunity at high spatiotemporal resolution.
- Phototaxis
Unidirectional movement of a cell in response to light-induced activation of chemokine receptor pathways along a light gradient in a way similar to a chemokine gradient.
- Upconversion nanoparticle
a specialized type of lanthanide ion-doped nanoparticle that exhibit anti-Stokes luminescence by converting low-energy deep tissue penetrable near infrared light (NIR) into shorter wavelength emissions (e.g., NIR, visible, or UV) with higher energy. When paired with optogenetic tools based on ChR2 or LOV2, these nanoparticles can act as in situ nano-illuminators to activate light-sensitive modules with NIR light.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10. doi: 10.1016/j.immuni.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 2.Palucka K, Banchereau J. Cancer immunotherapy via dendritic cells. Nature reviews Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spranger S, et al. Melanoma-intrinsic beta-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 4.Restifo NP, et al. Acquired resistance to immunotherapy and future challenges. Nature reviews Cancer. 2016;16:121–126. doi: 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khalil DN, et al. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nature reviews. Clinical oncology. 2016 doi: 10.1038/nrclinonc.2016.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jackson HJ, et al. Driving CAR T-cells forward. Nature reviews. Clinical oncology. 2016 doi: 10.1038/nrclinonc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davila ML, et al. Efficacy and toxicity management of 19–28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Science translational medicine. 2014;6:224ra225. doi: 10.1126/scitranslmed.3008226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoos A. Development of immuno-oncology drugs - from CTLA4 to PD1 to the next generations. Nat Rev Drug Discov. 2016;15:235–247. doi: 10.1038/nrd.2015.35. [DOI] [PubMed] [Google Scholar]
- 9.Zou W, et al. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Science translational medicine. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casey SC, et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tischer D, Weiner OD. Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol. 2014;15:551–558. doi: 10.1038/nrm3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K, Cui B. Optogenetic control of intracellular signaling pathways. Trends in biotechnology. 2015;33:92–100. doi: 10.1016/j.tibtech.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glantz ST, et al. Functional and topological diversity of LOV domain photoreceptors. Proceedings of the National Academy of Sciences of the United States of America. 2016 doi: 10.1073/pnas.1509428113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swartz MA, et al. Engineering approaches to immunotherapy. Science translational medicine. 2012;4:148rv149. doi: 10.1126/scitranslmed.3003763. [DOI] [PubMed] [Google Scholar]
- 15.Jeanbart L, Swartz MA. Engineering opportunities in cancer immunotherapy. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:14467–14472. doi: 10.1073/pnas.1508516112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu CY, et al. Remote control of therapeutic T cells through a small molecule-gated chimeric receptor. Science. 2015;350:aab4077. doi: 10.1126/science.aab4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feske S, et al. Ion channels in innate and adaptive immunity. Annual review of immunology. 2015;33:291–353. doi: 10.1146/annurev-immunol-032414-112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soboloff J, et al. STIM proteins: dynamic calcium signal transducers. Nat Rev Mol Cell Biol. 2012;13:549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prakriya M, Lewis RS. Store-Operated Calcium Channels. Physiol Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma G, et al. Inside-out Ca(2+) signalling prompted by STIM1 conformational switch. Nature communications. 2015;6:7826. doi: 10.1038/ncomms8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He L, et al. Near-infrared photoactivatable control of Ca(2+) signaling and optogenetic immunomodulation. eLife. 2015;4 doi: 10.7554/eLife.10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang Y, et al. Illuminating Cell Signaling with Near-Infrared Light-Responsive Nanomaterials. ACS Nano. 2016;10:3881–3885. doi: 10.1021/acsnano.6b02284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolmetsch RE, et al. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- 24.Salazar C, et al. Decoding of calcium oscillations by phosphorylation cycles: analytic results. Biophysical journal. 2008;94:1203–1215. doi: 10.1529/biophysj.107.113084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smedler E, Uhlen P. Frequency decoding of calcium oscillations. Biochimica et biophysica acta. 2014;1840:964–969. doi: 10.1016/j.bbagen.2013.11.015. [DOI] [PubMed] [Google Scholar]
- 26.Tomida T, et al. NFAT functions as a working memory of Ca2+ signals in decoding Ca2+ oscillation. The EMBO journal. 2003;22:3825–3832. doi: 10.1093/emboj/cdg381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hannanta-Anan P, Chow BY. Optogenetic Control of Calcium Oscillation Waveform Defines NFAT as an Integrator of Calcium Load. Cell systems. 2016;2:283–288. doi: 10.1016/j.cels.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sauer K, Okkenhaug K. Editorial: Lipid Signaling in T Cell Development and Function. Frontiers in immunology. 2015;6:410. doi: 10.3389/fimmu.2015.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funamoto S, et al. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 30.Kolsch V, et al. The regulation of cell motility and chemotaxis by phospholipid signaling. Journal of cell science. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Idevall-Hagren O, et al. Optogenetic control of phosphoinositide metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:E2316–2323. doi: 10.1073/pnas.1211305109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakumoto T, Nakata T. Optogenetic control of PIP3: PIP3 is sufficient to induce the actin-based active part of growth cones and is regulated via endocytosis. PloS one. 2013;8:e70861. doi: 10.1371/journal.pone.0070861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawano F, et al. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nature communications. 2015;6:6256. doi: 10.1038/ncomms7256. [DOI] [PubMed] [Google Scholar]
- 34.Xu Y, et al. Optogenetic control of chemokine receptor signal and T-cell migration. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6371–6376. doi: 10.1073/pnas.1319296111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz O, et al. Chemokines and Chemokine Receptors in Lymphoid Tissue Dynamics. Annual review of immunology. 2016;34:203–242. doi: 10.1146/annurev-immunol-041015-055649. [DOI] [PubMed] [Google Scholar]
- 36.Tugues S, et al. New insights into IL-12-mediated tumor suppression. Cell Death Differ. 2015;22:237–246. doi: 10.1038/cdd.2014.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nihongaki Y, et al. Photoactivatable CRISPR-Cas9 for optogenetic genome editing. Nature biotechnology. 2015;33:755–760. doi: 10.1038/nbt.3245. [DOI] [PubMed] [Google Scholar]
- 38.Nihongaki Y, et al. CRISPR-Cas9-based photoactivatable transcription system. Chemistry & biology. 2015;22:169–174. doi: 10.1016/j.chembiol.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 39.Dominguez AA, et al. Beyond editing: repurposing CRISPR-Cas9 for precision genome regulation and interrogation. Nat Rev Mol Cell Biol. 2016;17:5–15. doi: 10.1038/nrm.2015.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathak GP, et al. Optogenetic control of cell function using engineered photoreceptors. Biol Cell. 2013;105:59–72. doi: 10.1111/boc.201200056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Levskaya A, et al. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yazawa M, et al. Induction of protein-protein interactions in live cells using light. Nature biotechnology. 2009;27:941–945. doi: 10.1038/nbt.1569. [DOI] [PubMed] [Google Scholar]
- 43.Kennedy MJ, et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nature methods. 2010;7:973–975. doi: 10.1038/nmeth.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strickland D, et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nature methods. 2012;9:379–384. doi: 10.1038/nmeth.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang X, et al. Photoactivated UVR8-COP1 module determines photomorphogenic UV-B signaling output in Arabidopsis. PLoS genetics. 2014;10:e1004218. doi: 10.1371/journal.pgen.1004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Motta-Mena LB, et al. An optogenetic gene expression system with rapid activation and deactivation kinetics. Nature chemical biology. 2014;10:196–202. doi: 10.1038/nchembio.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guntas G, et al. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proceedings of the National Academy of Sciences of the United States of America. 2015;112:112–117. doi: 10.1073/pnas.1417910112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taslimi A, et al. Optimized second-generation CRY2-CIB dimerizers and photoactivatable Cre recombinase. Nature chemical biology. 2016 doi: 10.1038/nchembio.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kaberniuk AA, et al. A bacterial phytochrome-based optogenetic system controllable with near-infrared light. Nature methods. 2016 doi: 10.1038/nmeth.3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H, et al. LOVTRAP: an optogenetic system for photoinduced protein dissociation. Nature methods. 2016;13:755–758. doi: 10.1038/nmeth.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hughes RM, et al. Optogenetic apoptosis: light-triggered cell death. Angewandte Chemie. 2015;54:12064–12068. doi: 10.1002/anie.201506346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bugaj LJ, et al. Optogenetic protein clustering and signaling activation in mammalian cells. Nature methods. 2013;10:249–252. doi: 10.1038/nmeth.2360. [DOI] [PubMed] [Google Scholar]
- 53.Grusch M, et al. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. The EMBO journal. 2014;33:1713–1726. doi: 10.15252/embj.201387695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taslimi A, et al. An optimized optogenetic clustering tool for probing protein interaction and function. Nature communications. 2014;5:4925. doi: 10.1038/ncomms5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heintz U, Schlichting I. Blue light-induced LOV domain dimerization enhances the affinity of Aureochrome 1a for its target DNA sequence. eLife. 2016;5:e11860. doi: 10.7554/eLife.11860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reichhart E, et al. A Phytochrome Sensory Domain Permits Receptor Activation by Red Light. Angewandte Chemie. 2016 doi: 10.1002/anie.201601736. [DOI] [PubMed] [Google Scholar]
- 57.Kyung T, et al. Optogenetic control of endogenous Ca(2+) channels in vivo. Nature biotechnology. 2015;33:1092–1096. doi: 10.1038/nbt.3350. [DOI] [PubMed] [Google Scholar]
- 58.Kim N, et al. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chemistry & biology. 2014;21:903–912. doi: 10.1016/j.chembiol.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 59.Cai X, et al. Prion-like polymerization underlies signal transduction in antiviral immune defense and inflammasome activation. Cell. 2014;156:1207–1222. doi: 10.1016/j.cell.2014.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yao X, et al. Estimation of the available free energy in a LOV2-J alpha photoswitch. Nature chemical biology. 2008;4:491–497. doi: 10.1038/nchembio.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Niopek D, et al. Engineering light-inducible nuclear localization signals for precise spatiotemporal control of protein dynamics in living cells. Nature communications. 2014;5:4404. doi: 10.1038/ncomms5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Niopek D, et al. Optogenetic control of nuclear protein export. Nature communications. 2016;7:10624. doi: 10.1038/ncomms10624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jeong JW, et al. Wireless Optofluidic Systems for Programmable In Vivo Pharmacology and Optogenetics. Cell. 2015;162:662–674. doi: 10.1016/j.cell.2015.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Montgomery KL, et al. Wirelessly powered, fully internal optogenetics for brain, spinal and peripheral circuits in mice. Nature methods. 2015;12:969–974. doi: 10.1038/nmeth.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park SI, et al. Soft, stretchable, fully implantable miniaturized optoelectronic systems for wireless optogenetics. Nature biotechnology. 2015;33:1280–1286. doi: 10.1038/nbt.3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shen J, et al. Lanthanide-doped upconverting luminescent nanoparticle platforms for optical imaging-guided drug delivery and therapy. Adv Drug Deliv Rev. 2013;65:744–755. doi: 10.1016/j.addr.2012.05.007. [DOI] [PubMed] [Google Scholar]
- 68.Chen G, et al. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem Rev. 2014;114:5161–5214. doi: 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berglund K, et al. Luminopsins integrate opto- and chemogenetics by using physical and biological light sources for opsin activation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:E358–367. doi: 10.1073/pnas.1510899113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fry HC, et al. Computational de novo design and characterization of a protein that selectively binds a highly hyperpolarizable abiological chromophore. Journal of the American Chemical Society. 2013;135:13914–13926. doi: 10.1021/ja4067404. [DOI] [PMC free article] [PubMed] [Google Scholar]