Abstract
Approximately 50% of HIV-1 seropositive individuals develop HIV-1 associated neurocognitive disorders (HAND), which commonly include alterations in executive functions, such as inhibition, set shifting, and complex problem solving. Executive function deficits in HIV-1 are fairly well characterized, however, relatively few studies have explored the elemental dimensions of neurocognitive impairment in HIV-1. Deficits in temporal processing, caused by HIV-1, may underlie the symptoms of impairment in higher level cognitive processes. Translational measures of temporal processing, including cross-modal prepulse inhibition (PPI), gap-prepulse inhibition (gap-PPI), and gap threshold detection, were studied in mature ovariectomized female HIV-1 transgenic (Tg) rats, which express 7 of the 9 HIV-1 genes constitutively throughout development. Cross-modal PPI revealed a relative insensitivity to the manipulation of interstimulus interval (ISI) in HIV-1 Tg animals in comparison to control animals, extending previously reported temporal processing deficits in HIV-1 Tg rats to a more advanced age, suggesting the permanence of temporal processing deficits. In gap-PPI, HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI in comparison to control animals. In gap-threshold detection, HIV-1 Tg animals displayed a profound differential sensitivity to the manipulation of gap duration. Presence of the HIV-1 transgene was diagnosed with 91.1% accuracy using gap threshold detection measures. Understanding the generality and permanence of temporal processing deficits in the HIV-1 Tg rat is vital to modeling neurocognitive deficits observed in HAND and provides a key target for the development of a diagnostic screening tool.
Keywords: Temporal Processing, Prepulse Inhibition, HIV-1 Associated Neurocognitive Disorders, Diagnostic Screening Tool
Introduction
Worldwide, approximately 36 million individuals are currently living with human immunodeficiency virus type 1 (HIV-1), including over 1.2 million individuals in the United States (UNAIDS 2015). Since the advent of combination antiretroviral therapy (cART), the prevalence of the most severe forms of neurocognitive impairment, including HIV-1 associated dementia (HAD), have dramatically decreased (Ances & Ellis 2007). Antiretrovirals alone, however, are not sufficient to protect the brain as HAND remains as prevalent as before cART, afflicting up to 40%-70% of HIV-1 infected individuals (Heaton et al., 2010; Letendre et al. 2010; McArthur et al. 2010); which is also true in long-standing aviremic patients (Woods et al. 2009; Winston et al. 2013; Alfahad & Nath 2013). Asymptomatic neurocognitive impairment (ANI) conveys a 2-6× risk for progression to symptomatic HAND (Grant et al. 2014). HIV-1 seropositive individuals with HAND commonly display deficits in attention and executive function (Heaton et al. 2010; 2011). Although executive function deficits in HIV-1 are fairly well characterized, relatively few studies have explored the elemental dimensions of neurocognitive impairment in HIV-1.
Temporal processing deficits, which may underlie the symptoms of higher level cognitive deficits, have been studied through the use of the auditory system using amplitude and latency measures of auditory evoked potentials (AEPs), both before (Castello et al. 1998; Fein et al. 1995; Gil et al. 1992; Koralnik et al. 1990) and after the advent of cART (Chao et al. 2004; Matas et al. 2010). Translational experimental paradigms, including prepulse inhibition (PPI) of the auditory startle response (ASR), have commonly been used to study temporal processing (Hoffman & Searle 1965; Ison & Hammond 1971). The PPI experimental paradigm introduces a punctate prestimulus (i.e., a light or tone) prior to a startle stimulus; when the time interval, or interstimulus interval (ISI), between the prepulse and startling stimulus is between 30 to 500 msec, ASR is dramatically reduced (Hoffman & Ison 1980). Utilization of the PPI experimental paradigm provides a functional approach to understand more complex neurological processes (Hoffman & Ison 1980).
Cross-modal PPI has previously been used to assess temporal processing deficits in the HIV-1 Tg rat (Moran et al. 2013a). HIV-1 Tg and control animals between two and six months were tested for PPI of the ASR using both auditory and visual prepulses. Alterations in the development of both auditory and visual PPI, evidenced by an insensitivity to the manipulation of ISI and a lack of perceptual sharpening with age, were observed in HIV-1 Tg animals in comparison to controls (Moran et al. 2013a). Temporal processing deficits, indexed using auditory PPI, have also been observed in male Sprague-Dawley rats stereotaxically injected with the HIV-1 viral proteins Tat and gp120, revealing a differential sensitivity to the manipulation of ISI (Fitting et al. 2006 a,b, 2008). HIV-1 seropositive individuals meeting criteria for HAND exhibited significant temporal processing deficits, assessed using eyeblink startle response (Minassian et al. 2013). Thus, although prior research clearly suggests that HIV-1 individuals exhibit basic abnormalities in auditory processing, few studies have focused on the generality and translational aspects of temporal processing. One notable exception is the use of cross-modal PPI in the HIV-1 Tg rat (Moran et al. 2013a,b); the present study extends these observations.
Gap prepulse inhibition (gap-PPI), based on the modification of PPI, is another translational experimental paradigm commonly utilized to assess temporal processing (Ison et al. 1998; Leitner et al. 1993). In gap-PPI, the absence of a background (i.e., gap) serves as a punctate prestimulus, in comparison to the presentation of an added stimulus in cross-modal PPI. When the ISI is manipulated in gap-PPI, significant decreases in the startle amplitude are observed when the gap in background noise occurs between 30 to 200 msec prior to the startling stimulus (Ison et al. 1998). Manipulations in the gap duration, as in gap threshold detection, consistently suggest that as gap duration increases, greater decreases in startle amplitude are observed (Ison et al. 2005; Ison & Bowen, 2000). Additionally, prior research has established an inverse relationship between gap duration & ISI; as ISI increases, shorter gap durations produce significant inhibition (Ison 1982; Ison et al. 1991; Leitner et al. 1993).
The present study utilized cross-modal PPI, gap-PPI and gap threshold detection to further our understanding of temporal processing deficits, with a focus on the generality and permanence of these deficits. The HIV-1 Tg rat, which expresses 7 of the 9 HIV-1 genes, was presently tested at a more advanced age, however, prior to any documented signs of neurological or clinical wasting (Royal et al. 2012; Peng et al. 2010). The potential utility of gap threshold detection as a diagnostic screening tool for HAND was assessed using a discriminant function analysis. Understanding the generality and permanence of temporal processing deficits in the HIV-1 Tg rats is vital to modeling the neurocognitive deficits in HAND and may provide a key target for the development of a diagnostic screening tool.
Methods
Animals
A cross-sectional study was conducted using ovariectomized female Fischer (F344/N; Harlan Laboratories Inc., Indianapolis, IN) rats (HIV-1 Tg, n=21; control, n=25) between 9 and 10 months of age. One control animal was removed from the study between gap-PPI and gap threshold detection assessments because of an invasive tumor. All animals were pair- or group-housed throughout experimentation. Rodent food (2020X Teklad Global Extruded Rodent Diet (Soy Protein-Free)) and water were available ad libitum throughout the experiment. Animals were maintained according to the National Institutes of Health (NIH) guidelines in AAALAC-accredited facilities. The targeted environmental conditions for the animal facility were 21° ± 2° C, 50% ± 10% relative humidity, and a 12-h light:12-h dark cycle with lights on at 0700 h (EST). The Institutional Animal Care and Use Committee (IACUC) of the University of South Carolina approved the project under federal assurance (# A3049-01).
Apparatus
The startle platform (SR-Lab Startle Reflex System, San Diego Instruments, Inc., San Diego, CA) was enclosed in an isolation cabinet (10 cm-thick double-walled, 81×81×116-cm, Industrial Acoustic Company, Inc., Bronx, NY), which provided 30db(A) of sound attenuation relative to the external environment. The high-frequency loudspeaker of the SR-Lab system (model#40-1278B, Radio Shack, Fort Worth, TX), mounted inside the chamber 30 cm above the Plexiglas animal test cylinder, delivered all auditory stimuli (frequency range of 5k-16k Hz). The auditory startle stimulus intensity was 100 dB(A)) measured inside the test cylinder (model#2203, Bruel & Kjaer, Norcross, GA). Visual prepulses were presented using a white LED light (22 lux; Light meter model #840006, Sper Scientific, Ltd, Scottsdale, AZ) which was mounted on the wall in front of the test cylinder inside the chamber. The animal's ballistic response to the auditory stimulus produced deflection of the test cylinder, which provided an analog signal via a piezoelectric accelerometer integral to the bottom of the cylinder. The response signals were digitized (12 bit A to D) and saved to a hard disk. Response sensitivities were calibrated using a SR-LAB Startle Calibration System.
Procedure
Habituation
Habituation was assessed with a 36-trial auditory startle test session, beginning with a 5-min acclimation period in the dark with 70 dB(A) background white noise. Thirty-six trials of a 100 dB(A) white noise stimulus (20 msec duration) were presented with a fixed intertrial interval (ITI) of 10 sec.
Cross-modal Prepulse Inhibition Test
Cross-modal prepulse inhibition was conducted similar to our prior publication (Moran et al. 2013a). In brief, both visual and auditory prepulse stimuli were used to test animals for PPI of the ASR. PPI was administered using a 30-min test session, beginning with a 5-min acclimation period in the dark with 70 dB(A) background white noise, followed by 6 pulse-only ASR trials with a fixed 10-sec ITI. A total of 72 trials, including an equal number of visual and auditory prepulse trials, were interdigitated in an ABBA counterbalanced order of presentation. Trials employed interstimulus intervals (ISIs) of 0, 30, 50, 100, 200, and 4000 msec and were presented in 6-trial blocks according to a Latin-square design. The 0 and 4000 msec ISI trials served as control trials. The ITI was variable from 15 - 25 sec. Mean peak ASR amplitude values were collected for analysis.
Auditory Gap-Prepulse Inhibition Test
Animals were tested for gap-PPI of the ASR with a preceding gap in background white-noise as the prepulse stimulus. A 20-min test session began with a 5-min acclimation period in the dark with 70 dB(A) background white noise, followed by 6 pulse-only ASR trials, used for habituation, with a fixed 10-sec ITI. Thirty-six trials were presented using 6-trial blocks using a Latin Square design. The ITI was variable from 15 - 25 sec. Animals were tested with a 20-msec gap in white noise preceding a startle stimulus presented at ISIs of 0, 30, 50, 100, 200, and 4000 msec. Two control trials, the 0 and 4000 msec ISI trials, were included to provide a reference ASR within gap-PPI. Mean peak ASR amplitude values were collected for analysis.
Auditory Gap Threshold Detection
Animals were tested for auditory gap threshold detection by manipulating the duration of a preceding gap in background white-noise. A 20-min test session, began with a 5-min acclimation period in the dark with 70 dB(A) background white noise, followed by 6 pulse-only ASR trials with a fixed 10-sec ITI. A total of 36 gap prepulse trials were presented with a 50-msec ISI. The intertrial interval (ITI) was variable from 15 - 25 sec. Trials had a gap duration of 5, 10, 20, 30, 40, and 50 msec and were presented according to a Latin Square design. Mean peak ASR amplitude values were collected for analysis.
Statistical Analyses
All data were analyzed with analysis of variance (ANOVA) statistical techniques (SPSS Statistics 23, IBM Corp., Somers, NY). More specifically, a mixed-factor ANOVA was used with genotype (HIV-1 Tg vs. control) as a between-subjects factor, while ISI, gap duration, and trials served as the within-subjects factors, as appropriate. For the repeated-measures factors, either orthogonal decompositions were used for those variables that classically violate compound symmetry assumptions (e.g., trials) or the Greenhouse–Geisser df correction factor was used (Greenhouse & Geisser,1959). Tests of simple main effects and specific linear contrasts were also used to evaluate age-dependent and trial-dependent effects of the HIV-1 transgene (Winer 1971). For gap threshold detection, data were log transformed in accordance with Steven's Power Law, which relates stimulus intensity (duration) to sensation magnitude and generalizes to all sense modalities (Stevens 1970). Regression analyses and graphs utilized GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA). A discriminant function analysis (DFA) was conducted to determine the potential diagnostic utility of gap threshold detection. Variables for the DFA were selected on the basis of the percentage of total variance explained. Two variables were entered into the equation, including gap threshold detection at 5 msec and the rate of change in threshold sensitivity (slope). The alpha level was set at a p≤0.05.
Results
HIV-1 Tg animals and control animals exhibit significant ASR intrasession habituation
Both control and HIV-1 Tg animals exhibited a linear decrease in mean peak ASR amplitude during habituation. There was no significant difference between groups (HIV-1 Tg: β=-2.47±2.5 (95% CI); Control: β=-4.06±4.05 (95% CI)) in rate at which mean peak ASR amplitudes decreased [F(1,68)=1.68, p≤0.20]. Thus, both HIV-1 Tg and control animals displayed significant ASR intrasession habituation.
HIV-1 Tg animals exhibit a relative insensitivity to ISI in both auditory and visual PPI
In auditory PPI, HIV-1 Tg rats displayed a differential sensitivity to the manipulation of ISI, indexed by a rightward shift in peak inhibition (Figure 1a). Control animals exhibited peak inhibition at 30 msec, while HIV-1 Tg animals exhibited maximal peak inhibition at 100 msec. The overall ANOVA conducted on mean peak ASR amplitude during auditory PPI confirmed these observations, revealing a significant genotype × ISI interaction [F(5,220)=38.2, p GG ≤0.001, ηp2=.465] with a prominent quadratic component [F(1,44)=46.1, p ≤0.001, ηp2=0.512]. Main effects of ISI [F(5,220)=137.8, p GG ≤0.001, ηp2=0.758] and genotype [F(1,44)=37.6, p ≤0.001, ηp2=0.809] were also noted. The significant rightward shift in peak inhibition observed in the HIV-1 Tg animals provided evidence for alterations in sensitivity to the manipulation of ISI.
Fig. 1.
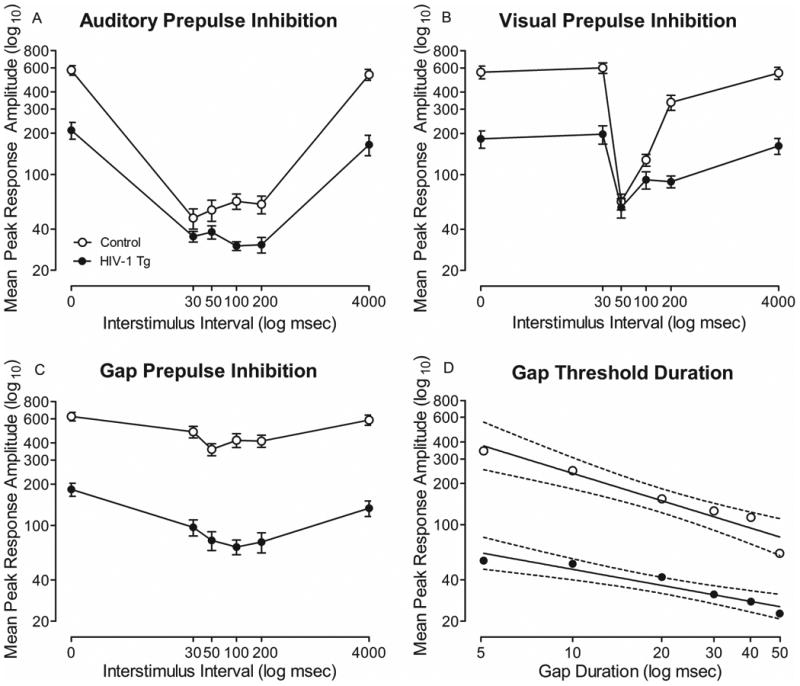
Mean peak ASR startle response is presented as a function of genotype (HIV-1 Tg or Control; ± SEM or ± 95% CI). (a) In auditory PPI, control animals exhibited maximum peak inhibition at 30 msec, however, HIV-1 Tg animals exhibited a rightward shift to maximum peak inhibition at 100 msec, indicating a differential sensitivity to the manipulation of ISI. (b) In visual PPI, HIV-1 Tg animals exhibited a significantly flatter ISI function, indicating a relative insensitivity to the manipulation of ISI. (c) In gap-PPI, control animals exhibited peak inhibition at 50 msec, however HIV-1 Tg animals exhibited a rightward shift to maximum peak inhibition at 100 msec, providing additional evidence for a differential sensitivity to the manipulation of ISI. (d) Auditory gap threshold detection revealed a significant difference between groups in rate at which mean peak ASR amplitude decreased. Mean peak ASR amplitude decreased significantly more slowly for HIV-1 Tg animals (β=-2.51±0.40) in comparison to control animals (β=-4.46±0.65).
In visual PPI, HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI in comparison to control animals (Figure 1b). Both HIV-1 Tg and control animals displayed peak inhibition at 50 msec ISI. However, HIV-1 Tg animals exhibited a significantly flatter ISI function. The overall ANOVA conducted on mean peak ASR amplitude during visual PPI confirmed these observations, revealing a significant genotype × ISI interaction [F(5,220)=28.9, p GG ≤0.001, ηp2=0.397] with a prominent quadratic component [F(1,44)=37.2, p ≤0.001, ηp2=0.458]. Additionally, main effects of ISI [F(5,220)=74.1, p GG ≤0.001, ηp2=0.627] and genotype [F(1,44)=35.7, p≤0.001, ηp2=0.448] were observed. The genotype × ISI interaction indicated a relative insensitivity to the manipulation of ISI in HIV-1 Tg animals. Thus, regardless of modality, HIV-1 Tg animals exhibited a significant insensitivity to the manipulation of ISI, suggesting a general and permanent deficit in temporal processing.
HIV-1 Tg animals exhibit a differential sensitivity to ISI during gap-PPI
HIV-1 Tg rats displayed a rightward shift in peak inhibition during gap-PPI, indicative of a differential sensitivity to the manipulation of ISI, illustrated in Figure 1c. Control animals displayed peak inhibition at 50 msec, while HIV-1 Tg animals exhibited peak inhibition at 100 msec. The overall ANOVA conducted on mean peak ASR amplitude during gap-PPI confirmed these observations, revealing a significant ISI × genotype interaction, [F(5,220)=5.0, pGG≤0.001, ηp2=0.101] with a prominent quadratic component [F(1,44)=17.1, p ≤0.001, ηp2=0.280]. Significant main effects of ISI [F(5,220)=24.8, pGG≤0.001, ηp2=0.361] and genotype [F(1,44)=74.9, p ≤0.001, ηp2=0.630] were also observed. Thus, the rightward shift in peak inhibition, observed in HIV-1 Tg rats, provided additional evidence for temporal processing deficits.
HIV-1 Tg rats display a relative insensitivity to gap duration in auditory gap threshold detection
A relative insensitivity to the manipulation of gap duration was observed in HIV-1 Tg rats (Figure 1d). As gap duration increased, both control and HIV-1 Tg animals exhibited a linear decrease in mean peak ASR amplitude. However, there was a significant difference between groups in rate at which mean peak ASR amplitudes decreased [F(1,8)=6.1, p≤0.04]. The mean peak ASR amplitude decreased significantly more slowly for HIV-1 Tg animals (β=-2.51±0.40) in comparison to control animals (β=-4.46±0.65), suggesting a relative insensitivity to the manipulation of gap duration. The overall ANOVA conducted on mean peak ASR amplitude during gap detection confirmed these observations, revealing a significant duration × genotype interaction [F(5,215)=5.4, pGG≤0.001, ηp2=0.112] with a prominent linear component [F(1,43)=50.6, p ≤0.001, ηp2=0.541]. A main effect of duration [F(5,215)=66.4, p GG ≤0.001, ηp2=0.607], and genotype [F(1,43)=77.5, p ≤0.001, ηp2=0.643] were also observed.
Auditory gap threshold detection can accurately diagnose the presence of the HIV-1 Transgene
The potential utility of gap threshold detection as a diagnostic tool for HAND was analyzed using a discriminant function analysis. A discriminant score, comprised of a weighted linear sum of the log mean peak ASR amplitude for gap threshold detection at 5 msec, the shortest empirically evaluated duration, as well as the slope, the rate of change in threshold sensitivity, maximally separated the HIV-1 Tg and control animals (canonical correlation of 0.84), as illustrated in Figure 2. Animals were classified with 91.1% accuracy (F approximation of Wilks' λ of 0.290, χ2(2, N=45)=52.0, p≤0.001) suggesting the potential clinical utility of gap threshold detection.
Figure 2.
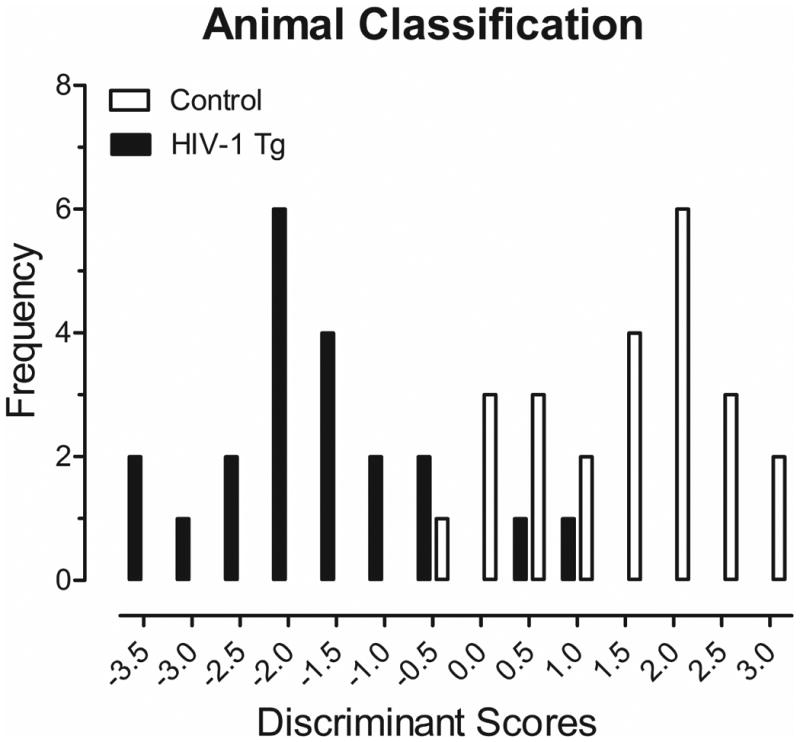
A discriminant function analysis was conducted to determine the potential utility of gap threshold detection as a diagnostic screening tool for HAND. Two variables were chosen (log mean peak ASR amplitude for gap threshold detection at 5 msec, slope) which represented the simplest linear function that best separated the HIV-1 Tg and control groups (canonical correlation 0.84) and correctly identified (jackknife classification) group membership with 91.1% accuracy (95.8% of controls, and 85.7% of HIV-1 Tg animals).
Discussion
HIV-1 Tg animals exhibited significant temporal processing deficits, assessed using multiple translational experimental paradigms, including cross-modal PPI, gap-PPI, and gap threshold detection. HIV-1 Tg animals displayed an insensitivity to the manipulation of ISI in cross-modal PPI, extending temporal processing deficits previously observed in the HIV-1 Tg rat to a more advanced age (Moran et al. 2013a, b), suggesting the permanence of temporal processing deficits. The generality of temporal processing deficits in the HIV-1 Tg rat was examined using the gap-PPI and auditory gap threshold duration experimental paradigms. In gap-PPI, HIV-1 Tg animals exhibited a differential sensitivity to the manipulation of ISI, evidenced by a shift in the point of maximal inhibition. More profoundly, in auditory gap threshold detection, HIV-1 Tg animals, in comparison to F344/N controls, exhibited a relative insensitivity to the manipulation of gap duration. The presence of the HIV-1 transgene was diagnosed with 91.1% accuracy using gap threshold detection measures. Temporal processing deficits, characteristic of individuals with HAND, can be modeled using the HIV-1 Tg rat, providing a key target for the development of a diagnostic screening tool for HAND.
Cross-modal PPI revealed an insensitivity to the manipulation of ISI in HIV-1 Tg rats in comparison to control animals. In auditory PPI, HIV-1 Tg animals exhibited a differential sensitivity to the manipulation of ISI, evidenced by a shift in the point of maximal inhibition. Control animals exhibited peak inhibition at 30 msec, while HIV-1 Tg animals exhibited a rightward shift to maximal peak inhibition at 100 msec. Temporal processing deficits in auditory PPI extend those previously observed in Sprague-Dawley rats stereotaxically injected with the HIV-1 viral proteins Tat and gp120 (Fitting et al., 2006a, b, 2008). In visual PPI, HIV-1 Tg animals exhibited a relative insensitivity to the manipulation of ISI, indicated by a significantly flatter ISI function. Alterations in cross-modal PPI extend those previously reported in the HIV-1 Tg rat (Moran et al. 2013a) to a more advanced age, suggesting the permanence of temporal processing deficits.
Gap-PPI and gap threshold detection, translational experimental paradigms, were used to extend our knowledge and assess the generality of temporal processing deficits in the HIV-1 Tg rat. Gap-PPI revealed a differential sensitivity to the manipulation of ISI in HIV-1 Tg animals compared to controls, evidenced by a rightward shift in maximum peak inhibition. Control animals exhibited maximal inhibition at the 50 msec ISI, while HIV-1 Tg animals exhibited maximal inhibition at the 100 msec ISI. Auditory gap threshold detection revealed a relative insensitivity to the manipulation of gap duration in HIV-1 Tg animals compared to control animals. As gap duration increased, both HIV-1 Tg and control animals exhibited a linear decrease in mean peak ASR amplitude. However, HIV-1 Tg animals exhibited a significantly slower rate of linear decline as duration increased in comparison to controls. Auditory gap threshold detection results of the present study resemble alterations in olfactory threshold scores previously reported in HIV-1 seropositive adults with neurocognitive impairment (Razani et al. 1996). To our knowledge, the present study is the first to employ gap-PPI and gap threshold detection in the HIV-1 Tg rat, indicating the generality of temporal processing deficits.
Temporal processing deficits may provide a key target for the development of a diagnostic screening tool. Presence of the HIV-1 transgene was diagnosed with 91.1% accuracy using two auditory gap threshold detection measurements, suggesting the potential utility of auditory gap threshold detection as a potential diagnostic screening tool for HAND. Alterations in temporal processing have previously been implicated as a diagnostic screening tool for tinnitus (Dehmel et al. 2012; Fournier & Hébert, 2013; Sun et al. 2014). Gap-PPI assessments were utilized in preclinical studies in rats with noise over-expression-induced tinnitus (Dehmel et al. 2012) or salicylate-induced tinnitus (Sun et al. 2014). Subsequent clinical studies employed the gap-PPI experimental paradigm in adults with tinnitus using the eyeblink startle reflex, replicating preclinical studies and proposing a potential neural mechanism for tinnitus (Fournier & Hébert, 2013). Thus, alterations in gap threshold detection, observed in the HIV-1 Tg rat may provide an innovative diagnostic screening tool for HAND.
Temporal processing deficits observed in the HIV-1 Tg rat may result from dopamine (DA) system alterations in the brain neural circuitry mediating PPI, which has been established using lesioning (e.g., Leitner & Cohen 1985) and electrical stimulation studies (e.g., Li & Yeomans 2000). The serial circuit mediating PPI begins with auditory input relayed to the inferior colliculus. Visual and tactile input, in contrast, are relayed to the superior colliculus (SC). Sensory input, regardless of modality, is then sent from the SC to the pedunculopontine tegmental nucleus (PPTg), triggering a cholinergic projection to the caudal pontine reticular nucleus (PnC; Fendt et al. 2001; Koch & Schnitzler 1997). Activation of the PnC is relayed to motor neurons causing a startle response. In the serial circuit mediating PPI, the nucleus accumbens receives DA inputs from the ventral tegmental area (VTA). which subsequently triggers the nucleus accumbens, PPTg, and finally PnC, altering the startle response (Koch 1999).
The role of DA in the serial circuit mediating PPI has been reported in previous behavioral and pharmacological studies, providing evidence for dysfunction in the modulation of PPI by the DA system (review, Geyer et al. 2001; Zhang et al. 2000). Administration of apomorphine, a DA agonist, disrupts sensorimotor gating (Geyer et al. 2001), similar to disruptions seen in schizophrenic patients, which has been measured using event-evoked potentials (Adler et al. 1982) and the eyeblink response (Braff et al. 1978). Schizophrenic patients, in comparison to healthy controls, display an insensitivity to the manipulation of ISI. Administration of apomorphine in Sprague-Dawley rats neonatally injected with gp120 (Fitting et al. 2006b) revealed an insensitivity to the manipulation of ISI; results which are comparable to those observed in the HIV-1 Tg rat. Although temporal processing deficits observed in the HIV-1 Tg rat may be caused by dysfunction of multiple neural systems, HIV-1 infection often causes DA system dysfunction and is further associated with neurocognitive deficits (Chang et al. 2008; di Rocco et al. 2000; Kumar et al. 2011; Wang et al. 2004).
The classic approach to analysis of PPI manipulated ISI, as was performed in the present study. The ISI approach, popularized by Ison & Hammond (1971), employs a range of ISI values to accurately assess the response amplitude curves for PPI. A range of ISI values has previously been used to establish temporal processing deficits in the HIV-1 Tg rat (Moran et al. 2013a) and in male Sprague-Dawley rats stereotaxically injected with the HIV-1 viral proteins Tat and gp120 (Fitting et al. 2006 a,b, 2008). Additionally, plotting the startle amplitude scores (e.g., as in the log-log plots portrayed in Figures 1a,b,c) allows researchers to examine increases (sharper curve inflection) or decreases (flattening of the response amplitude curve; Figure 1b) in PPI, as well as shifts in the peak response inhibition (i.e., Figure 1a,c). In contrast, the contemporary procedure for the analysis of PPI, often used in pharmacological studies, commonly employs a single ISI, as has been popularized and recommended in several protocols (e.g., Curzon et al. 2009; Geyer & Swerdlow, 2001). The contemporary approach suggests presenting PPI as percent inhibition, calculated as follows: 100 × {[(startle response amplitude during control trials)-(startle response amplitude during prepulse + pulse trials)] / (startle response amplitude during control trials)}. Use of the contemporary approach for the analysis of PPI precludes observations of increases (sharper curve inflection) or decreases (flattening of the response amplitude curve) in the shape of the PPI response amplitude curve as well as shifts in the point of peak response inhibition (i.e., they are not all temporally bound at 100 msec), and like any percent of control measure, fails to disambiguate changes in PPI from changes in the baseline startle response. Results in Table 1 show the use of percent PPI for the 100 msec ISI for the present study, illustrating the caveats present in the contemporary methodology for PPI analysis. For example, the results for visual PPI suggest that HIV-1 Tg animals fail to exhibit significant inhibition. Figure 1b, which visually illustrates visual PPI utilizing the classic ISI approach, however, provides evidence that the differences observed in percent PPI are not due to a failure to inhibit, but are due to changes in baseline responses. Thus, the use of the classic approach affords an undeniable opportunity to assess the temporal processes inherent in PPI, which may be a critical underlying dimension for the cognitive impairments observed in HAND.
Table 1. Mean Percent Prepulse Inhibition (PPI) at the 100 msec ISI (± 95% CI).
| HIV-1 Tg | Control | |
|---|---|---|
| Auditory PPI | 79.5±4.6 | 88.2± 2.5 |
| Visual PPI | 40.8±12.7 | 75.1±4.1 |
| Auditory Gap PPI | 51.1±10.7 | 31.1±11.8 |
The study of temporal processing deficits in the HIV-1 Tg rat is vital for understanding the generality and permanence of temporal processing deficits, and may provide a key target for the development of a diagnostic screening tool. The HIV-1 Tg rat used in the present study, which resembles HIV-1 seropositive individuals on cART, displayed no significant health disparities in comparison to F344/N controls (i.e. similar growth rates), consistent with our previous studies (Moran et al. 2012, 2013a; Roscoe et al. 2014). HIV-1 Tg animals used in the current study are a healthier derivation of those originally described by Reid et al. (2001), which displayed severe phenotypical alterations, and wasting at a relatively young age (5-9 months). In contrast, no general wasting or pathological phenotypes (e.g., hindlimb paralysis) were observed in the HIV-1 Tg animals used in the current study through 10 months of age. Both HIV-1 Tg and control animals displayed similar inhibition in visual PPI confirming that, despite the presence of cataracts in the HIV-1 Tg group, and their mature age, animals were able to detect the 20 msec visual stimulus presented. Thus, the current HIV-1 Tg rat displays a moderate phenotype closely resembling HAND, suggesting its utility for subsequent studies of the progression of HAND.
Temporal processing deficits observed in mature HIV-1 Tg rats resemble sensorimotor gating deficits commonly exhibited in HIV-1 seropositive individuals. To our knowledge, the present study is the first to examine temporal processing deficits in the HIV-1 Tg rat using gap-PPI and auditory gap threshold detection. Alterations in threshold sensitivity, assessed using gap threshold detection, may provide an innovative diagnostic screening tool for HAND, which may be of clinical interest in the post-cART era (Chan & Brew, 2014; Zipursky et al. 2013). The present study provides significant evidence for the generality, and potential permanence, of temporal processing deficits as a function of the chronic expression of the HIV-1 transgene.
Acknowledgments
This work was supported in part by grants from NIH (National Institute on Drug Abuse, DA013137; National Institute of Child Health and Human Development, HD043680; National Institute of Mental Health, MH106392) and the interdisciplinary research training program supported by the University of South Carolina Behavioral-Biomedical Interface Program.
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
References
- Adler LE, Pachtman E, Franks RD, Pecevich M, Waldo MC, Freedman R. Neurophysiological evidence for a defect in neuronal mechanisms involved in sensory gating in schizophrenia. Biol Psychiat. 1982;17:639–654. [PubMed] [Google Scholar]
- Alfahad TB, Nath A. Update on HIV-1 associated neurocognitive disorders. Current Neurol Neurosci. 2013;13:387. doi: 10.1007/s11910-013-0387-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normal and schizophrenics. Psychophysiology. 1978;15:339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Castello E, Baroni N, Pallestrini E. Neurotological and auditory brain stem response findings in human immunodeficiency virus-positive patients without neurologic manifestations. Ann Oto Rhinol Laryn. 1998;107:1054–1060. doi: 10.1177/000348949810701210. [DOI] [PubMed] [Google Scholar]
- Chan P, Brew BJ. HIV associated neurocognitive disorders in the modern antiviral treatment era: Prevalence, characteristics, biomarkers, and effects of treatment. Curr HIV/AIDS Rep. 2014;11:317–324. doi: 10.1007/s11904-014-0221-0. [DOI] [PubMed] [Google Scholar]
- Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, Fowler JS. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42:869–878. doi: 10.1016/j.neuroimage.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Lindgren JA, Flenniken DL, Weiner MW. ERP evidence of impaired central nervous system function in virally suppressed HIV patients on antiretroviral therapy. Clin Neurophysiol. 2004;115:1583–1591. doi: 10.1016/j.clinph.2004.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curzon P, Zhang M, Radek RJ, Fox GB. The behavioral assessment of sensorimotor processes in the mouse: Acoustic startle, sensory gating, locomotor activity, rotarod, and beam walking. In: Buccafusco J, editor. Methods of behavior analysis in neuroscience. 2. Boca Raton, FL: 2009. Chapter 8. [PubMed] [Google Scholar]
- Dehmel S, Eisinger D, Shore SE. Gap prepulse inhibition and auditory brainstem-evoked potentials as objective measures for tinnitus in guinea pigs. Front Systems Neurosci. 2012;6:1–15. doi: 10.3389/fnsys.2012.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rocco A, Bottiglieri T, Dorfman D, Werner P, Morrison C, Simpson D. Decreased homovanilic acid in cerebrospinal fluid correlates with impaired neuropsychologic function in HIV-1-infected patients. Clin Neuropharmacol. 2000;23:190–194. doi: 10.1097/00002826-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Fein G, Biggins CA, Mackay S. Delayed latency of the event-related brain potential P3A component in HIV disease: progressive effects with increasing cognitive impairment. Arch Neurol-Chicago. 1995;52:1109–1118. doi: 10.1001/archneur.1995.00540350103022. [DOI] [PubMed] [Google Scholar]
- Fendt M, Li L, Yeomans JS. Brain stem circuits mediating prepulse inhibition of the startle reflex. Psychopharmacology. 2001;156:216–224. doi: 10.1007/s002130100794. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal hippocampal Tat injections: developmental effects on prepulse inhibition (PPI) of the auditory startle response. Int J Dev Neurosci. 2006a;24:275–283. doi: 10.1016/j.ijdevneu.2006.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal glycoprotein 120 injection: the role of dopaminergic alteration in prepulse inhibition in adult rats. J Pharmacol Exp Ther. 2006b;318:1352–1358. doi: 10.1124/jpet.106.105742. [DOI] [PubMed] [Google Scholar]
- Fitting S, Booze RM, Mactutus CF. Neonatal intrahippocampal injection of the HIV-1 proteins gp12 and Tat: Differential effects on behavior and the relationship to stereological hippocampal measures. Brain Res. 2008;1232:139–154. doi: 10.1016/j.brainres.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier P, Hébert S. Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap? Hear Res. 2013;295:16–23. doi: 10.1016/j.heares.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Krebs-Thomson K, Braff DL, Swerdlow NR. Pharmacological studies of prepulse inhibition models of sensorimotor gating deficits in schizophrenia: a decade in review. Psychopharmacology. 2001;156:117–154. doi: 10.1007/s002130100811. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr Protoc Neurosci. 2001:8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Gil R, Breux JP, Neu JP, Becq-Giraudon B. Cognitive evoked potentials and HIV infection. Clin Neurophysiol. 1992;22:385–391. doi: 10.1016/s0987-7053(05)80096-6. [DOI] [PubMed] [Google Scholar]
- Grant I, Franklin DR, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014;83:2055–2062. doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychometrika. 1959;24:95–112. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy CHARTER study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychology Review. 1980;87:175–189. [PubMed] [Google Scholar]
- Hoffman HS, Searle JL. Acoustic variables in modification of startle reaction in rat. J Comp Physiol Psychol. 1965;60:53–58. doi: 10.1037/h0022325. [DOI] [PubMed] [Google Scholar]
- Ison JR. Temporal acuity in auditory function in the rat: Reflex inhibition by brief gaps in noise. J Comp Physiol Psychol. 1982;96:945–954. [PubMed] [Google Scholar]
- Ison JR, Agrawal P, Pak J, Vaughn WJ. Changes in temporal acuity with age and with hearing impairment in the mouse: a study of the acoustic startle reflex and its inhibition by brief decrements in noise level. J Acoust Soc Am. 1998;104:1696–1704. doi: 10.1121/1.424382. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, Rivoli PJ, Moore JT. The behavioral response of mice to gaps in noise depends on its spectral components and its bandwidth. J Acoust Soc Am. 2005;117:3944–3951. doi: 10.1121/1.1904387. [DOI] [PubMed] [Google Scholar]
- Ison JR, Bowen PG. Scopolamine reduces sensitivity to auditory gaps in the rat, suggesting a cholinergic contribution to temporal acuity. Hear Res. 2000;145:169–176. doi: 10.1016/s0378-5955(00)00088-5. [DOI] [PubMed] [Google Scholar]
- Ison JR, Hammond GR. Modification of startle reflex in rat by changes in auditory and visual environments. J Comp Physiol Psychol. 1971;75:435–452. doi: 10.1037/h0030934. [DOI] [PubMed] [Google Scholar]
- Ison JR, O'Connor K, Bowen GP, Bocirnea A. Temporal resolution of gaps in noise by the rat is lost with functional decortication. Behav Neurosci. 1991;105:33–40. doi: 10.1037//0735-7044.105.1.33. [DOI] [PubMed] [Google Scholar]
- Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–128. doi: 10.1016/s0301-0082(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Koch M, Schnitzler HU. The acoustic startle response in rats: Circuits mediating evocation, inhibition and potentiation. Behav Brain Res. 1997;89:35–49. doi: 10.1016/s0166-4328(97)02296-1. [DOI] [PubMed] [Google Scholar]
- Koralnik IJ, Beaumanoir A, Hausler R, Kohler A, Safran AB, Delacoux R, et al. A controlled-study of early neurologic abnormalities in men with asymptomatic human- immunodeficiency-virus infection. New Engl J Med. 1990;323:864–870. doi: 10.1056/NEJM199009273231303. [DOI] [PubMed] [Google Scholar]
- Kumar AM, Ownby RL, Waldrop-Valverde D, Fernandez B, Kumar M. Human immunodeficiency virus infection in the CNS and decreased dopamine availability: relationship with neuropsychological performance. J Neurovirol. 2011;17:26–40. doi: 10.1007/s13365-010-0003-4. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Cohen ME. Role of the inferior colliculus in the inhibition of acoustic startle in the rat. Physiol Behav. 1985;34:65–70. doi: 10.1016/0031-9384(85)90079-4. [DOI] [PubMed] [Google Scholar]
- Leitner DS, Hammond GR, Springer CP, Ingham KM, Mekilo AM, Bodison PR, et al. Parameters affecting gap detection in the rat. Percept Psychophys. 1993;54:395–405. doi: 10.3758/bf03205275. [DOI] [PubMed] [Google Scholar]
- Letendre SL, Ellis RJ, Ances BM, McCutchan JA. Neurologic complications of HIV disease and their treatment. Topics HIV Med. 2010;18:45–55. [PMC free article] [PubMed] [Google Scholar]
- Li L, Yeomans JS. Using intracranial electrical stimulation to study the timing of prepulse inhibition of the startle reflex. Brain Res Protoc. 2000;5:67–74. doi: 10.1016/s1385-299x(99)00056-2. [DOI] [PubMed] [Google Scholar]
- Matas CG, Silva SM, Marcon Bde A, Goncalves IC. Electrophysiological manifestations in adults with HIV/AIDS submitted and not submitted to antiretroviral therapy. Pro Fono. 2010;22:107–113. doi: 10.1590/s0104-56872010000200007. [DOI] [PubMed] [Google Scholar]
- McArthur JC, Steiner J, Sacktor N, Nath A. Human immunodeficiency virus-associated neurocognitive disorders mind the gap. Ann of Neurol. 2010;67(6):699–714. doi: 10.1002/ana.22053. [DOI] [PubMed] [Google Scholar]
- Minassian A, Henry BL, Woods SP, Vaida F, Grant I, Geyer MA, Perry W. Prepulse inhibition in HIV-Associated Neurocognitive Disorders. J Int Neuropsychol Soc. 2013;19:709–717. doi: 10.1017/S1355617713000301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Aksenov MY, Booze RM, Webb KM, Mactutus CF. Adolescent HIV-1 transgenic rats: evidence for dopaminergic alterations in behavior and neurochemistry revealed by methamphetamine challenge. Curr HIV Res. 2012;10:415–424. doi: 10.2174/157016212802138788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Mactutus CF. Time and time again: Temporal processing demands implicate perceptual and gating deficits in the HIV-1 Transgenic rat. J Neuroimmune Pharm. 2013a;8:988–997. doi: 10.1007/s11481-013-9472-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran LM, Booze RM, Webb KM, Mactutus CF. Neurobehavioral alterations in HIV-1 transgenic rats: evidence for dopaminergic dysfunction. Exp Neurol. 2013b;239:139–147. doi: 10.1016/j.expneurol.2012.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng JS, Vigorito M, Liu XQ, Zhous DJ, Wu XW, Chang SL. The HIV-1 transgenic rat as a model for HIV-1 infected individuals on HAART. J Neuroimmunol. 2010;218:94–101. doi: 10.1016/j.jneuroim.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Razani J, Murphy C, Davidson TM, Grant I, McCutchan A. Odor sensitivity is impaired in HIV-positive cognitively impaired patients. Physiol Behav. 1996;59:877–881. doi: 10.1016/0031-9384(95)02163-9. [DOI] [PubMed] [Google Scholar]
- Reid W, Sadowska M, Denaro F, Rao S, Foulke J, Hayes N, et al. An Hiv-1 transgenic rat that develops HIV-related pathology and immunologic dysfunction. P Natl Acad Sci USA. 2001;98:9271–9276. doi: 10.1073/pnas.161290298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roscoe RF, Jr, Mactutus CF, Booze RM. HIV-1 transgenic female rat: Synaptodendritic alterations of medium spiny neurons in the nucleus accumbens. J Neuroimmune Pharmacol. 2014;9:642–653. doi: 10.1007/s11481-014-9555-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royal W, Zhang L, Guo M, Jones O, Davis H, Bryant JL. Immune activation, viral gene product expression and neurotoxicity in the HIV-1 transgenic rat. J Neuroimmunol. 2012;247:16–24. doi: 10.1016/j.jneuroim.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens SS. Neural events and the psychophysical law. Science. 1970;170:1043–1050. doi: 10.1126/science.170.3962.1043. [DOI] [PubMed] [Google Scholar]
- Sun W, Doolittle L, Flowers E, Zhang C, Wang Q. High doses of salicylate causes prepulse facilitation of onset-gap induced acoustic startle response. Behav Brain Res. 2014;258:187–192. doi: 10.1016/j.bbr.2013.10.024. [DOI] [PubMed] [Google Scholar]
- UNAIDS. Aids Info: Indicators. [22 March 2016];2015 http://aidsinfo.unaids.org/
- Wang GJ, Change L, Volkow ND, Telang F, Logan J, Ernst T, Fowler JS. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain. 2004;127:2452–2458. doi: 10.1093/brain/awh269. [DOI] [PubMed] [Google Scholar]
- Winer BJ. Statistical Principles in Experimental Design. 2nd. New York: 1971. [Google Scholar]
- Winston A, Arenas-Pinto A, Stohr W, Fisher M, Orkin CM, Aderogba K, et al. Neurocognitive function in HIV infected patients on antiretroviral therapy. PLoS One. 2013;8:e61949. doi: 10.1371/journal.pone.0061949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev. 2009;19:152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipursky AR, Gogolishvili D, Rueda S, Brunetta J, Carvalhal A, McCombe JA, Gill MJ, Rachlis A, Rosenes R, Arbess G, Marcotte T, Rourke SB. Evaluation of brief screening tools for neurocognitive impairment in HIV/AIDS: a systematic review of the literature. AIDS. 2013;27:2385–2401. doi: 10.1097/QAD.0b013e328363bf56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Forkstam C, Engel JA, Svensson L. Role of dopamine in prepulse inhibition of acoustic startle. Psychopharmacology. 2000;149:181–188. doi: 10.1007/s002130000369. [DOI] [PubMed] [Google Scholar]


