Abstract
The increased life expectancy of people living with HIV-1 who are taking effective anti-retroviral therapeutics is now accompanied by increased Alzheimer’s disease (AD)-like neurocognitive problems and neuropathological features such as increased levels of amyloid beta (Aβ) and phosphorylated tau proteins. Others and we have shown that HIV-1 Tat promotes the development of AD-like pathology. Indeed, HIV-1 Tat once endocytosed into neurons can alter morphological features and functions of endolysosomes as well as increase Aβ generation. Caffeine has been shown to have protective actions against AD and based on our recent findings that caffeine can inhibit endocytosis in neurons and can prevent neuronal Aβ generation, we tested the hypothesis that caffeine blocks HIV-1 Tat-induced Aβ generation and tau phosphorylation. In SH-SY5Y cells over-expressing wild-type amyloid beta precursor protein (AβPP), we demonstrated that HIV-1 Tat significantly increased secreted levels and intracellular levels of Aβ as well as cellular protein levels of phosphorylated tau. Caffeine significantly decreased levels of secreted and cellular levels of Aβ, and significantly blocked HIV-1 Tat-induced increases in secreted and cellular levels of Aβ. Caffeine also blocked HIV-1 Tat-induced increases in cellular levels of phosphorylated tau. Furthermore, caffeine blocked HIV-1 Tat-induced endolysosome dysfunction as indicated by decreased protein levels of vacuolar-ATPase and increased protein levels of cathepsin D. These results further implicate endolysosome dysfunction in the pathogenesis of AD and HAND, and by virtue of its ability to prevent and/or block neuropathological features associated with AD and HAND caffeine might find use as an effective adjunctive therapeutic agent.
Keywords: Caffeine, HIV-1 Tat, amyloid beta, tau phosphorylation, endolysosomes, BACE-1
Introduction
Human immunodeficiency virus-1 (HIV-1) continues to be a serious global health concern with more than 40 million people worldwide living with HIV-1 infection. Although effective antiretroviral therapies (ART) have increased the life span of people living with HIV-1 infection, HIV-1 infected individuals are now experiencing a family of HIV-1 associated neurocognitive disorders (HAND), the prevalence of which in the USA is greater than 50% (Ellis et al., 2010; Heaton et al., 2010). Increasingly, the incidence of Alzheimer’s disease (AD)-like clinical symptomatology and neuropathological features such as increased levels of amyloid beta (Aβ) protein (Esiri et al., 1998; Nebuloni et al., 2001; Gelman and Schuenke, 2004; Green et al., 2005; Achim et al., 2009; Clifford et al., 2009; Pulliam, 2009; Xu and Ikezu, 2009) and phosphorylated tau protein (Brew et al., 2005; Anthony et al., 2006; Patrick et al., 2011) are being noted in HIV-1 infected patients the vast majority of whom are taking ART.
HIV-1 transactivator of transcription (Tat) protein is a nonstructural transcriptional regulator essential for the replication of HIV-1. HIV-1 Tat can be transported across the blood-brain barrier from the systemic circulation (Kim et al., 2003; Banks et al., 2005), can be secreted by infected macrophages and microglia, and has been detected in brain of patients with HIV-1 associated dementia (Westendorp et al., 1995; Ellis et al., 2000; Nath, 2002). High concentrations of HIV-1 Tat levels (>4000 pg/ml) were observed in CSF of HIV infected individuals regardless of viral load (Johnson et al., 2013). HIV-1 has been shown to increase neuronal Aβ generation (Rempel and Pulliam, 2005; Giunta et al., 2009; Aksenov et al., 2010) and tau phosphorylation (Giunta et al., 2009; Fields et al., 2015). HIV-1 Tat enters neurons rapidly by receptor-mediated endocytosis with the assistance of low-density lipoprotein receptor-related protein (LRP-1) (Liu et al., 2000; Vendeville et al., 2004; King et al., 2006; Deshmane et al., 2011). Once endocytosed, HIV-1 Tat accumulates in endolysosomes (Vendeville et al., 2004) – acidic organelles where amyloidogenic processing of amyloid beta precursor protein (AβPP) to Aβ occurs in neurons (Rajendran and Annaert, 2012; Morel et al., 2013). We have shown that HIV-1 Tat contributes directly to the development of endolysosome dysfunction in neurons (Hui et al., 2012), a common pathological feature present in AD (Cataldo et al., 2000; Tate and Mathews, 2006; Boland et al., 2008) and in HAND (Gelman et al., 2005; Spector and Zhou, 2008; Zhou and Spector, 2008). Furthermore, we have shown that endolysosome dysfunction plays an important role in HIV-1 Tat-induced Aβ generation in neurons (Hui et al., 2012; Chen et al., 2013).
Caffeine, the most commonly ingested psychoactive drug in the world, has been shown to be protective against AD pathogenesis (Cao et al., 2009; Arendash and Cao, 2010; Eskelinen and Kivipelto, 2010; Wostyn et al., 2011; Cao et al., 2012; Carman et al., 2014; Flaten et al., 2014). Epidemiologically, caffeine ingestion has a reciprocal relationship with the prevalence and severity of AD (Ritchie et al., 2007; Eskelinen et al., 2009; Santos et al., 2010a; Santos et al., 2010b; Gelber et al., 2011). In animal models, caffeine has been shown to prevent AD-like features as well as reverse the features once formed (Arendash et al., 2006; Arendash et al., 2009; Espinosa et al., 2013; Han et al., 2013; Laurent et al., 2014). Although different mechanisms underlying the protective actions of caffeine have been implicated, we reported recently that caffeine blocks LDL endocytosis and that this inhibition plays an important role in caffeine’s protective effects against LDL-induced neuronal generation of Aβ (Li et al., 2015). Our findings are consistent with the notion that amyloidogenic processing of AβPP occurs predominantly within endolysosomes after AβPP is internalized (Rajendran and Annaert, 2012; Morel et al., 2013).
Because HIV-1 Tat enters neurons via receptor mediated endocytosis (Liu et al., 2000) and HIV-1 Tat accumulation in endolysosomes affects the morphology and function of these organelles including Aβ generation and tau phosphorylation (Kenessey et al., 1997; Oyama et al., 1998; Hamano et al., 2008; Wang et al., 2009; Hui et al., 2012; Chen et al., 2013; Chesser et al., 2013), and because our recent finding that caffeine blocks LDL endocytosis and LDL-induced neuronal generation of Aβ (Li et al., 2015), here we tested the hypothesis that caffeine blocks HIV-1 Tat-induced endolysosome dysfunction and AD-like pathology including Aβ generation and tau phosphorylation.
Material and Methods
Cultures of human neuroblastoma cells
Human neuroblastoma cells (SH-SY5Y) expressing wild-type AβPP were kindly supplied by Dr. Norman Haughey (Johns Hopkins, Baltimore, MD). Cells were cultured in Eagle’s minimum essential medium (MEM) supplemented with 10% fetal calf serum, penicillin/streptomycin, nonessential amino acids, and sodium pyruvate (1 mM) at 37°C in 5% CO2/95% air. For the experiments, 4 × 106 cells were seeded on 60 mm2 dishes and cultured for 48 h. Cells were treated with HIV-1 Tat1–72 or as a control a mutant form of HIV-1 Tat (TatΔ31–61) for 2 days, in the absence or presence of caffeine. Highly purified recombinant Tat1–72 and TatΔ31–61 were prepared as previously described (Ma and Nath, 1997) and were kindly provided to us by Dr. Avindra Nath (NINDS).
Quantification of Aβ levels
Secreted and intracellular Aβ levels were measured using human Aβ1–40 and Aβ1–42 ELISA kits as per the manufacturer’s protocol (Invitrogen, Carlsbad, CA). For secreted Aβ measurements, media from cultured cells was collected, diluted 1:4 with standard diluent buffer, and each sample was analyzed in duplicate. Total cellular protein levels were determined by a DC protein assay (Bio-Rad). Aβ levels were normalized to total protein content in each sample. For intracellular Aβ measurement, cells were trypsinized and collected by centrifugation at 5,000 x g and the cell pellet was homogenized thoroughly with 8-times mass of ice-cold 5 M guanidine-HCl/50 mM Tris–HCl. The samples were diluted with ice-cold reaction buffer (Dulbecco’s phosphate-buffered saline with 5% BSA and 0.03% Tween-20 supplemented with 1x protease inhibitor cocktail) and centrifuged at 16,000 x g for 20 min at 4°C. The supernatant was collected, diluted at 1:1 with standard diluent buffer, and quantified by colorimetric sandwich ELISA kits. Intracellular Aβ levels were normalized to total protein content in the samples.
Immunoblotting
Cells were lysed with RIPA buffer (Pierce) plus 10 mM NaF, 1 mM Na3VO4 and Protease Inhibitor Cocktail (Sigma). After centrifugation (14,000 x g for 10 min at 4°C), supernatants were collected and protein concentrations were determined with a DC protein assay (Bio-Rad). Proteins (10 μg) were separated by SDS-PAGE (12% gel) and following transfer to polyvinylidene difluoride membranes (Millipore), membranes were incubated overnight at 4°C with antibodies including anti-tau-5 (Abcam), anti-phospho tau (AT8, Thermo Scientific), anti-cathepsin D (Abcam), anti-LAMP-1 (Sigma), and anti-vacuolar-ATPase (Santa Cruz). GAPDH (Abcam) was used as a loading control. The immunoblots were developed with enhanced chemiluminescence, and bands were visualized and analyzed by LabWorks 4.5 software on a UVP Bioimaging System (Upland). Quantification of results was performed by densitometry and the results were analyzed as total integrated densitometric volume values (arbitrary units).
Immunoelution of Tat
HIV-1 Tat1–72 were immunoadsorbed by adding 1:100 (v:v) dilutions of mouse monoclonal antibody raised against the N-terminal portion of the Tat protein bound to protein G-coated agarose beads. Following incubation for 90 min at room temperature, samples were centrifuged at 14,000 × g for 10 min and supernatants were used as controls.
Statistical analysis
All data were expressed as means and SEM values. Statistical significance for multiple comparisons was determined by one-way ANOVA plus a Tukey post hoc test. p < 0.05 was considered to be statistically significant.
Results
Caffeine blocked HIV-1 Tat-induced increases in Aβ generation and tau phosphorylation
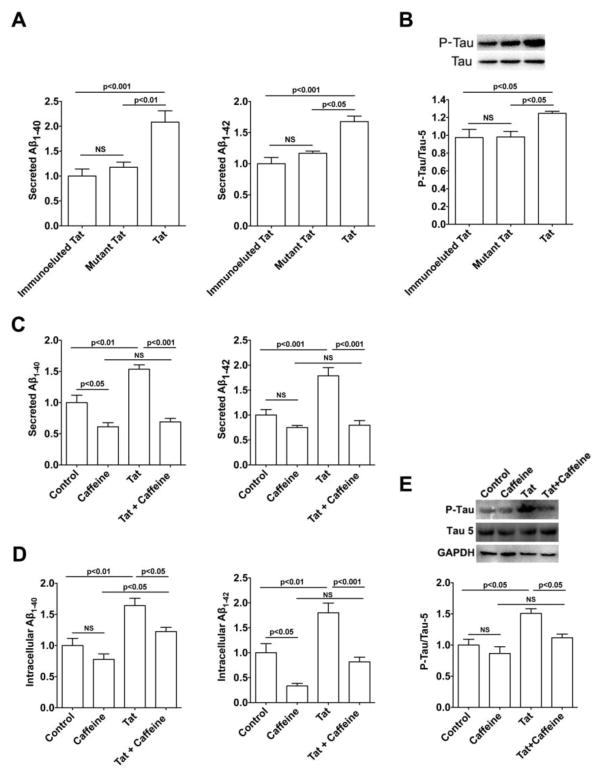
Consistent with previous findings by others and us that HIV-1 Tat increases neuronal Aβ generation (Rempel and Pulliam, 2005; Giunta et al., 2009; Aksenov et al., 2010; Chen et al., 2013) and tau phosphorylation (Giunta et al., 2009; Fields et al., 2015), we demonstrated in SH-SY5Y human neuroblastoma cells over-expressing wild-type AβPP that HIV-1 Tat treatment significantly increased levels of secreted Aβ1–40 and Aβ1–42 (Figure 1A). The HIV-1 Tat concentration used here is consistent with the Tat levels (>4000 pg/ml) measured in CSF of infected individuals regardless of viral load (Johnson et al., 2013). To test for HIV-1 Tat specificity, a mutant form of HIV-1 Tat (TatΔ31–61) that is not directly neurotoxic (Buscemi et al., 2007) and immunoeluted Tat1–72 were used as controls and neither significantly affected levels of Aβ (Figure 1A). In addition, HIV-1 Tat1–72, but not TatΔ31–61 or immunoeluted Tat1–72 significantly increased protein levels of phosphorylated tau (Figure 1B).
Figure 1. Caffeine blocked HIV-1 Tat-induced increases in Aβ generation and tau phosphorylation.
(A) HIV-1 Tat (100 nM) treatment for 2 days significantly increased secreted levels of Aβ1–40 and Aβ1–42 (n=4). Neither mutant Tat (mutant TatΔ31–61 at 100 nM for 2 days) nor immunoeluted Tat affect levels of secreted Aβ1–40 and Aβ1–42. (B) HIV-1 Tat (100 nM for 2 days), but not mutant Tat or immunoeluted Tat, increased tau phosphorylation. (C) Caffeine treatment (200 μM) not only blocked HIV-1 Tat (100 nM for 2 days) induced increases in levels of secreted Aβ1–40 and Aβ1–42 (n=4), but also blocked HIV-1 Tat-induced increases in intracellular accumulation of Aβ1–40 and Aβ1–42 (n=4). In addition, caffeine (200 μM) treatment alone significantly decreased basal levels of secreted Aβ1–40 as well as intracellular accumulation of Aβ1–42. (E) Caffeine treatment (200 μM) alone did not affect tau phosphorylation, butblocked HIV-1 Tat (100 nM for 2 days) induced increases in tau phosphorylation (n=4). One-way ANOVA with a Tukey post-hoc test was for statistical analysis.
In preliminary studies using caffeine at concentrations of 20, 200 and 2000 μM we found 200 μM to be the most effective concentration that consistently and significantly decreased Aβ levels as well as HIV-1 Tat-induced increases in levels of Aβ (data not shown). In the absence of HIV-1 Tat treatment, caffeine (200 μM) significantly decreased secreted levels of Aβ1–40 (Figure 1C) and intracellular levels of Aβ1–42 (Figure 1D). Caffeine (200 μM) blocked HIV-1 Tat-induced increases in levels of secreted Aβ1–40 and Aβ1–42 (Figure 1C) and levels of intracellular Aβ1–40 and Aβ1–42 (Figure 1D). Furthermore, while caffeine (200 μM) alone did not affect protein levels of phosphorylated tau, but it did block HIV-1 Tat-induced increases in tau phosphorylation (Figure 1E).
Caffeine blocked HIV-1 Tat-induced endolysosome dysfunction
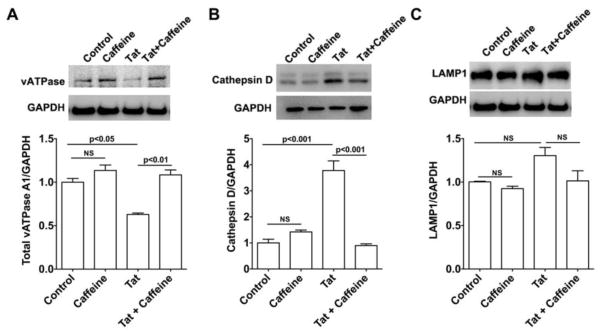
Consistent with our previous findings that HIV-1 Tat de-acidified endolysosomes (Hui et al., 2012; Chen et al., 2013), we found here that HIV-1 Tat (100 nM for 2 days) significantly decreased protein levels of vacuolar-ATPase; a major mechanism by which endolysosomes maintain their acidic environment (Figure 2A). Caffeine (200 μM) significantly blocked HIV-1 Tat-induced decreases in protein levels of vacuolar-ATPase (Figure 2A), but did not by itself affect protein levels of vacuolar-ATPase. In addition, caffeine (200 μM) alone did not affect protein levels of endolysosome enzyme cathepsin D, but did significantly block HIV-1 Tat-induced increases in protein levels of cathepsin D (Figure 2B). There appeared to be a trend that caffeine blocks Tat-induced increase in protein levels of lysosome associated membrane protein LAMP-1 (Figure 2C), although differences did not reach significant level.
Figure 2. Caffeine blocked HIV-1 Tat-induced endolysosome dysfunction.
(A) Caffeine treatment (200 μM) blocked HIV-1 Tat-induced decreases in protein levels of vacuolar-ATPase (n=4;). Caffeine treatment alone did not affect protein levels of vacuolar-ATPase. (B) Caffeine treatment (200 μM) blocked HIV-1 Tat treatment (100 nM for 2 days) induced increases in protein levels of endolysosome enzyme cathepsin D (n=4;). Caffeine treatment alone did not affect protein levels of either cathepsin D. (C) Caffeine treatment (200 μM) attenuated HIV-1 Tat treatment (100 nM for 2 days) induced increases in protein levels of lysosome associated membrane protein LAMP-1 (n=4). One-way ANOVA with a Tukey post-hoc test was for statistical analysis.
Discussion
HIV-1 virus does not infect neurons, and HIV-associated neurodegenerative pathology is not proportional to viral load (van de Bovenkamp et al., 2002). Thus, HIV-1 viral proteins and other factors have been implicated in the neurological complications associated with HIV-1 infections. Among HIV-1 viral proteins, HIV-1 Tat is present in brains of HIV-1 infected individuals and its levels stay elevated in CSF even when HIV-1 viral levels are immeasurable (Johnson et al., 2013). Others and we have shown consistently that HIV-1 Tat is neurotoxic and it continues to be linked to the pathogenesis of HAND (Nuovo et al., 1994; Nath et al., 1996; Merino et al., 2011). Increasingly, HIV-1 infection and ART treatment has been shown to contribute to the development of AD-like pathology including increases in Aβ levels (Rempel and Pulliam, 2005; Giunta et al., 2009; Aksenov et al., 2010; Chen et al., 2013; Kim et al., 2013; Fields et al., 2015). Of mechanistic significance, we have shown that HIV-1 Tat-induced endolysosome dysfunction following receptor-mediated endocytosis underlies HIV-1 Tat-induced increases in neuronal generation of Aβ (Hui et al., 2012; Chen et al., 2013). Here, we demonstrated that caffeine, a protective agent against AD, blocks HIV-1 Tat-induced increases in Aβ generation and tau phosphorylation as well as decreases in protein levels of vacuolar-ATPase. Our findings suggest that caffeine exerts it protective effects against the development of AD-like pathology, in part, by blocking HIV-1 Tat-induced endolysosome dysfunction.
Endolysosomes are acidic organelles that contain various pH-dependent lytic enzymes and vacuolar H+-ATPase helps maintain an acidic environment necessary for maintenance of protein turnover and cellular homeostasis (Appelqvist et al., 2013). Indeed, endolysosomes have been implicated in the pathogenesis of sporadic AD (Tate and Mathews, 2006; Boland et al., 2008) and HAND (Gelman et al., 2005; Spector and Zhou, 2008; Zhou and Spector, 2008; Cysique et al., 2015). Neurons, as long-lived post-mitotic cells, are especially vulnerable to perturbations of endolysosome pH and by maintaining an acidic environment endolysosomes are able to control the integrity of quality of critical proteins (Nixon and Cataldo, 1995; Bonaldo and Sandri, 2013). Endolysosome dysfunction has been implicated in the development of the two pathological hallmarks of AD; Aβ accumulation and neurofibrillary tangle formation. Following endocytosis of AβPP, a ubiquitously expressed type-I transmembrane protein with largely uncharacterized physiological functions, amyloidogenic processing of AβPP occurs (Rajendran and Annaert, 2012; Morel et al., 2013; Jiang et al., 2014) because this is where the amyloidogenic enzymes BACE-1 and γ-secretase are almost exclusively located. The acidic environment of endolysosomes is favorable for amyloidogenic metabolism of AβPP (Nixon, 2005; Rajendran et al., 2008; Shimizu et al., 2008; Sannerud et al., 2011) and once formed Aβ can either accumulate in endolysosomes or following exocytotic release it can accumulate extracellularly. Thus, Aβ accumulation can be enhanced by factors that promote AβPP internalization (Grbovic et al., 2003), that enhance protein levels and/or activities of BACE-1 and/or γ-secretase, that prevent AβPP recycling back to the cell surface (Ma et al., 2009), and that impair Aβ degradation by lysosome-resident cathepsins (Miners et al., 2011); (Torres et al., 2012). Tau is a microtubule-associated protein, and when hyperphosphorylated it aggregates and contributes to the formation of neurofibrillary tangles. Tau aggregates can be degraded by cathepsin D in autophagosomes-lysosomes (Hamano et al., 2008; Chesser et al., 2013). Thus, endolysosome dysfunction can contribute to tau aggregation and neurofibrillary tangle formation (Jo et al., 2014); (Bi and Liao, 2007), and transcriptional activation of lysosome biogenesis can clear aggregated tau (Polito et al., 2014).
HIV-1 Tat enters neurons via receptor-mediated endocytosis mainly with the assistance of LRP-1 (Liu et al., 2000; Deshmane et al., 2011). Once endocytosed, HIV-1 Tat can accumulate into and exit from endolysosomes (Vendeville et al., 2004). Further, we have shown that HIV-1 Tat can directly affect morphological and functional features of neuronal endolysosomes including endolysosome de-acidification and endolysosome enlargement (Hui et al., 2012), indicating that HIV-1 Tat has a direct effect on endolysosome structure and function. Here, we demonstrated, in AβPP over-expressing SH-SY5Y cells, that HIV-1 Tat decreased protein levels of vacuolar-ATPase, the proton pump that is essential for maintaining the acidic environment of endolysosomes. Thus, endolysosome de-acidification by HIV-1 Tat might be caused by decreased vacuolar-ATPase. The HIV-1 Tat-induced decreases in vacuolar-ATPase and endolysosome de-acidification might then cause compensatory increases in cathepsin D and LAMP-1 (Mangieri et al., 2014), as we observed in these studies. Although SH-SY5Y cells over-expressing human AβPP are human Aβ processing neuronal cell cultures that are commonly used in AD-related studies, this non-physiological over-expression of AβPP may not fully reconstitute pathogenic cascades of HAND and may lead to supplementary pathogenic effects in addition to the accumulation of Aβ. However, we did observe similar Tat-induced endolysosome dysfunction and AD-like pathology in primary cultured neurons (Hui et al., 2012; Chen et al., 2013).
Consistent with our previous findings in primary cultured neurons showing that HIV-1 Tat altered structural and functional features of endolysosomes and increased endolysosome accumulation of Aβ (Chen et al., 2013), we demonstrated here using SH-SY5Y cells over-expressing AβPP that HIV-1 Tat increased levels of secreted Aβ as well as increased accumulation of intracellular Aβ. LRP-1, a promiscuous receptor that mediates HIV-1 Tat endocytosis, has been shown by others and us to interact physically and functionally with AβPP (Waldron et al., 2006; Waldron et al., 2008; Klug et al., 2011) to increase HIV-1 Tat and AβPP internalization (Chen et al., 2013). Once internalized into acidic endosomes, AβPP can be cleaved by β- and γ-secretase to form Aβ. The increased Aβ production may also have been due to HIV-1 Tat-induced increases in protein levels of AβPP and BACE-1 and/or alternatively to HIV-1 Tat-induced endolysosome dysfunction; both are highly likely explanations because endolysosomes play a critical role in Aβ generation (Rajendran and Annaert, 2012; Morel et al., 2013; Jiang et al., 2014). HIV-1 Tat-induced endolysosome dysfunction may also contribute to increases in tau phosphorylation. Because tau and phosphorylated tau can be degraded in autophagosomes and lysosomes (Chesser et al., 2013; Jo et al., 2014), endolysosome dysfunction and impaired lysosome degradation following HIV-1 Tat endocytosis could lead to accumulations of phosphorylated tau and the development of neurofibrillary tangles.
Our findings suggest that HIV-1 Tat induced endolysosome dysfunction may play an important role in HIV-1 Tat-induced AD-like pathology. As such, attenuating endolysosome function may present an effective strategy against HIV-1 Tat-induced increases in Aβ generation and tau phosphorylation. Based on our recent findings that caffeine inhibits LDL endocytosis and LDL-induced Aβ generation in neurons (Li et al., 2015), we examined the effects of caffeine on HIV-1 Tat-induced increases in Aβ generation and tau phosphorylation. We demonstrated that caffeine blocked HIV-1 Tat induced endolysosome dysfunction as well as AD-like pathological features including increases in levels of secreted Aβ, intracellular accumulation of Aβ, and increased levels of phosphorylated tau. Our findings suggest that caffeine could prevent HIV-1 Tat-induced AD-like pathology in part by preventing HIV-1 Tat-induced endolysosome dysfunction. It is estimated that 1 cup of coffee consumption (40–100 mg of caffeine) can result in plasma concentrations of free caffeine of about 1–10 μM (Fredholm et al., 1999). However, because caffeine is highly protein bound, total plasma caffeine concentrations would be much higher (Blanchard, 1982). Caffeine concentrations used in this study (200 μM) are in the upper range of plasma concentrations of caffeine (Fredholm et al., 1999). Indeed, we confirmed in a previous study that 200 μM of caffeine does not have neurotoxic effects (Li et al., 2015).
In short, our findings add to the ever-increasing reports that caffeine provides robust protective actions against AD. Besides its protective role against the development of AD, caffeine has been shown to exert protective effect against HIV-1 pathogenesis (Daniel et al., 2005; Nunnari et al., 2005). Thus, caffeine might be used in the treatment of AD–like symptoms and neuropathology associated with HIV-1 infection.
Acknowledgments
This work was supported by the following grants received from the National Institutes of Health; P30GM103329, R01MH100972 and R01MH105329.
Footnotes
Disclosure statement
The authors have no current or potential conflicts of interest to report.
References
- Achim CL, Adame A, Dumaop W, Everall IP, Masliah E. Increased accumulation of intraneuronal amyloid beta in HIV-infected patients. J Neuroimmune Pharmacol. 2009;4:190–199. doi: 10.1007/s11481-009-9152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Mactutus CF, Booze RM. HIV-1 protein-mediated amyloidogenesis in rat hippocampal cell cultures. Neurosci Lett. 2010;475:174–178. doi: 10.1016/j.neulet.2010.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta Neuropathol. 2006;111:529–538. doi: 10.1007/s00401-006-0037-0. [DOI] [PubMed] [Google Scholar]
- Appelqvist H, Waster P, Kagedal K, Ollinger K. The lysosome: from waste bag to potential therapeutic target. J Mol Cell Biol. 2013;5:214–226. doi: 10.1093/jmcb/mjt022. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Cao C. Caffeine and coffee as therapeutics against Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 1):S117–126. doi: 10.3233/JAD-2010-091249. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Schleif W, Rezai-Zadeh K, Jackson EK, Zacharia LC, Cracchiolo JR, Shippy D, Tan J. Caffeine protects Alzheimer's mice against cognitive impairment and reduces brain beta-amyloid production. Neuroscience. 2006;142:941–952. doi: 10.1016/j.neuroscience.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Arendash GW, Mori T, Cao C, Mamcarz M, Runfeldt M, Dickson A, Rezai-Zadeh K, Tane J, Citron BA, Lin X, Echeverria V, Potter H. Caffeine reverses cognitive impairment and decreases brain amyloid-beta levels in aged Alzheimer's disease mice. J Alzheimers Dis. 2009;17:661–680. doi: 10.3233/JAD-2009-1087. [DOI] [PubMed] [Google Scholar]
- Banks WA, Robinson SM, Nath A. Permeability of the blood-brain barrier to HIV-1 Tat. Exp Neurol. 2005;193:218–227. doi: 10.1016/j.expneurol.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Bi X, Liao G. Autophagic-lysosomal dysfunction and neurodegeneration in Niemann-Pick Type C mice: lipid starvation or indigestion? Autophagy. 2007;3:646–648. doi: 10.4161/auto.5074. [DOI] [PubMed] [Google Scholar]
- Blanchard J. Protein binding of caffeine in young and elderly males. J Pharm Sci. 1982;71:1415–1418. doi: 10.1002/jps.2600711229. [DOI] [PubMed] [Google Scholar]
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. J Neurosci. 2008;28:6926–6937. doi: 10.1523/JNEUROSCI.0800-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaldo P, Sandri M. Cellular and molecular mechanisms of muscle atrophy. Dis Model Mech. 2013;6:25–39. doi: 10.1242/dmm.010389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brew BJ, Pemberton L, Blennow K, Wallin A, Hagberg L. CSF amyloid beta42 and tau levels correlate with AIDS dementia complex. Neurology. 2005;65:1490–1492. doi: 10.1212/01.wnl.0000183293.95787.b7. [DOI] [PubMed] [Google Scholar]
- Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis. 2007;26:661–670. doi: 10.1016/j.nbd.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Cirrito JR, Lin X, Wang L, Verges DK, Dickson A, Mamcarz M, Zhang C, Mori T, Arendash GW, Holtzman DM, Potter H. Caffeine suppresses amyloid-beta levels in plasma and brain of Alzheimer's disease transgenic mice. J Alzheimers Dis. 2009;17:681–697. doi: 10.3233/JAD-2009-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao C, Loewenstein DA, Lin X, Zhang C, Wang L, Duara R, Wu Y, Giannini A, Bai G, Cai J, Greig M, Schofield E, Ashok R, Small B, Potter H, Arendash GW. High Blood caffeine levels in MCI linked to lack of progression to dementia. J Alzheimers Dis. 2012;30:559–572. doi: 10.3233/JAD-2012-111781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carman AJ, Dacks PA, Lane RF, Shineman DW, Fillit HM. Current evidence for the use of coffee and caffeine to prevent age-related cognitive decline and Alzheimer's disease. J Nutr Health Aging. 2014;18:383–392. doi: 10.1007/s12603-014-0021-7. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Peterhoff CM, Troncoso JC, Gomez-Isla T, Hyman BT, Nixon RA. Endocytic pathway abnormalities precede amyloid beta deposition in sporadic Alzheimer's disease and Down syndrome: differential effects of APOE genotype and presenilin mutations. Am J Pathol. 2000;157:277–286. doi: 10.1016/s0002-9440(10)64538-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Hui L, Geiger NH, Haughey NJ, Geiger JD. Endolysosome involvement in HIV-1 transactivator protein-induced neuronal amyloid beta production. Neurobiol Aging. 2013;34:2370–2378. doi: 10.1016/j.neurobiolaging.2013.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesser AS, Pritchard SM, Johnson GV. Tau clearance mechanisms and their possible role in the pathogenesis of Alzheimer disease. Front Neurol. 2013;4:122. doi: 10.3389/fneur.2013.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, Kauwe JS. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–1987. doi: 10.1212/WNL.0b013e3181c5b445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cysique LA, Hewitt T, Croitoru-Lamoury J, Taddei K, Martins RN, Chew CS, Davies NN, Price P, Brew BJ. APOE epsilon4 moderates abnormal CSF-abeta-42 levels, while neurocognitive impairment is associated with abnormal CSF tau levels in HIV+ individuals - a cross-sectional observational study. BMC Neurol. 2015;15:51. doi: 10.1186/s12883-015-0298-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel R, Marusich E, Argyris E, Zhao RY, Skalka AM, Pomerantz RJ. Caffeine inhibits human immunodeficiency virus type 1 transduction of nondividing cells. J Virol. 2005;79:2058–2065. doi: 10.1128/JVI.79.4.2058-2065.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmane SL, Mukerjee R, Fan S, Sawaya BE. High-Performance Capillary Electrophoresis for Determining HIV-1 Tat Protein in Neurons. PLoS One. 2011;6:e16148. doi: 10.1371/journal.pone.0016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Rosario D, Clifford DB, McArthur JC, Simpson D, Alexander T, Gelman BB, Vaida F, Collier A, Marra CM, Ances B, Atkinson JH, Dworkin RH, Morgello S, Grant I. Continued high prevalence and adverse clinical impact of human immunodeficiency virus-associated sensory neuropathy in the era of combination antiretroviral therapy: the CHARTER Study. Arch Neurol. 2010;67:552–558. doi: 10.1001/archneurol.2010.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esiri MM, Biddolph SC, Morris CS. Prevalence of Alzheimer plaques in AIDS. J Neurol Neurosurg Psychiatry. 1998;65:29–33. doi: 10.1136/jnnp.65.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelinen MH, Kivipelto M. Caffeine as a protective factor in dementia and Alzheimer's disease. J Alzheimers Dis. 2010;20(Suppl 1):S167–174. doi: 10.3233/JAD-2010-1404. [DOI] [PubMed] [Google Scholar]
- Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. J Alzheimers Dis. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]
- Espinosa J, Rocha A, Nunes F, Costa MS, Schein V, Kazlauckas V, Kalinine E, Souza DO, Cunha RA, Porciuncula LO. Caffeine consumption prevents memory impairment, neuronal damage, and adenosine A2A receptors upregulation in the hippocampus of a rat model of sporadic dementia. J Alzheimers Dis. 2013;34:509–518. doi: 10.3233/JAD-111982. [DOI] [PubMed] [Google Scholar]
- Fields JA, Dumaop W, Crews L, Adame A, Spencer B, Metcalf J, He J, Rockenstein E, Masliah E. Mechanisms of HIV-1 Tat neurotoxicity via CDK5 translocation and hyper-activation: role in HIV-associated neurocognitive disorders. Curr HIV Res. 2015;13:43–54. doi: 10.2174/1570162x13666150311164201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaten V, Laurent C, Coelho JE, Sandau U, Batalha VL, Burnouf S, Hamdane M, Humez S, Boison D, Lopes LV, Buee L, Blum D. From epidemiology to pathophysiology: what about caffeine in Alzheimer's disease? Biochem Soc Trans. 2014;42:587–592. doi: 10.1042/BST20130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51:83–133. [PubMed] [Google Scholar]
- Gelber RP, Petrovitch H, Masaki KH, Ross GW, White LR. Coffee intake in midlife and risk of dementia and its neuropathologic correlates. J Alzheimers Dis. 2011;23:607–615. doi: 10.3233/JAD-2010-101428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelman BB, Schuenke K. Brain aging in acquired immunodeficiency syndrome: increased ubiquitin-protein conjugate is correlated with decreased synaptic protein but not amyloid plaque accumulation. J Neurovirol. 2004;10:98–108. doi: 10.1080/13550280490279816. [DOI] [PubMed] [Google Scholar]
- Gelman BB, Soukup VM, Holzer CE, 3rd, Fabian RH, Schuenke KW, Keherly MJ, Richey FJ, Lahart CJ. Potential role for white matter lysosome expansion in HIV-associated dementia. J Acquir Immune Defic Syndr. 2005;39:422–425. doi: 10.1097/01.qai.0000164250.41475.f2. [DOI] [PubMed] [Google Scholar]
- Giunta B, Hou H, Zhu Y, Rrapo E, Tian J, Takashi M, Commins D, Singer E, He J, Fernandez F, Tan J. HIV-1 Tat contributes to Alzheimer's disease-like pathology in PSAPP mice. Int J Clin Exp Pathol. 2009;2:433–443. [PMC free article] [PubMed] [Google Scholar]
- Grbovic OM, Mathews PM, Jiang Y, Schmidt SD, Dinakar R, Summers-Terio NB, Ceresa BP, Nixon RA, Cataldo AM. Rab5-stimulated up-regulation of the endocytic pathway increases intracellular beta-cleaved amyloid precursor protein carboxyl-terminal fragment levels and Abeta production. J Biol Chem. 2003;278:31261–31268. doi: 10.1074/jbc.M304122200. [DOI] [PubMed] [Google Scholar]
- Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–411. doi: 10.1097/01.aids.0000161770.06158.5c. [DOI] [PubMed] [Google Scholar]
- Hamano T, Gendron TF, Causevic E, Yen SH, Lin WL, Isidoro C, Deture M, Ko LW. Autophagic-lysosomal perturbation enhances tau aggregation in transfectants with induced wild-type tau expression. Eur J Neurosci. 2008;27:1119–1130. doi: 10.1111/j.1460-9568.2008.06084.x. [DOI] [PubMed] [Google Scholar]
- Han K, Jia N, Li J, Yang L, Min LQ. Chronic caffeine treatment reverses memory impairment and the expression of brain BNDF and TrkB in the PS1/APP double transgenic mouse model of Alzheimer's disease. Mol Med Rep. 2013;8:737–740. doi: 10.3892/mmr.2013.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui L, Chen X, Haughey NJ, Geiger JD. Role of endolysosomes in HIV-1 Tat-induced neurotoxicity. ASN Neuro. 2012;4:243–252. doi: 10.1042/AN20120017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Li Y, Zhang X, Bu G, Xu H, Zhang YW. Trafficking regulation of proteins in Alzheimer's disease. Mol Neurodegener. 2014;9:6. doi: 10.1186/1750-1326-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo C, Gundemir S, Pritchard S, Jin YN, Rahman I, Johnson GV. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat Commun. 2014;5:3496. doi: 10.1038/ncomms4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, Nath A. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A. 2013;110:13588–13593. doi: 10.1073/pnas.1308673110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenessey A, Nacharaju P, Ko LW, Yen SH. Degradation of tau by lysosomal enzyme cathepsin D: implication for Alzheimer neurofibrillary degeneration. J Neurochem. 1997;69:2026–2038. doi: 10.1046/j.1471-4159.1997.69052026.x. [DOI] [PubMed] [Google Scholar]
- Kim J, Yoon JH, Kim YS. HIV-1 Tat interacts with and regulates the localization and processing of amyloid precursor protein. PLoS One. 2013;8:e77972. doi: 10.1371/journal.pone.0077972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TA, Avraham HK, Koh YH, Jiang S, Park IW, Avraham S. HIV-1 Tat-mediated apoptosis in human brain microvascular endothelial cells. J Immunol. 2003;170:2629–2637. doi: 10.4049/jimmunol.170.5.2629. [DOI] [PubMed] [Google Scholar]
- King JE, Eugenin EA, Buckner CM, Berman JW. HIV tat and neurotoxicity. Microbes Infect. 2006;8:1347–1357. doi: 10.1016/j.micinf.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Klug W, Dietl A, Simon B, Sinning I, Wild K. Phosphorylation of LRP1 regulates the interaction with Fe65. FEBS Lett. 2011;585:3229–3235. doi: 10.1016/j.febslet.2011.09.028. [DOI] [PubMed] [Google Scholar]
- Laurent C, Eddarkaoui S, Derisbourg M, Leboucher A, Demeyer D, Carrier S, Schneider M, Hamdane M, Muller CE, Buee L, Blum D. Beneficial effects of caffeine in a transgenic model of Alzheimer's disease-like tau pathology. Neurobiol Aging. 2014 doi: 10.1016/j.neurobiolaging.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Li S, Geiger NH, Soliman ML, Hui L, Geiger JD, Chen X. Caffeine, Through Adenosine A3 Receptor-Mediated Actions, Suppresses Amyloid-beta Protein Precursor Internalization and Amyloid-beta Generation. J Alzheimers Dis. 2015;47:73–83. doi: 10.3233/JAD-142223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jones M, Hingtgen CM, Bu G, Laribee N, Tanzi RE, Moir RD, Nath A, He JJ. Uptake of HIV-1 tat protein mediated by low-density lipoprotein receptor-related protein disrupts the neuronal metabolic balance of the receptor ligands. Nat Med. 2000;6:1380–1387. doi: 10.1038/82199. [DOI] [PubMed] [Google Scholar]
- Ma M, Nath A. Molecular determinants for cellular uptake of Tat protein of human immunodeficiency virus type 1 in brain cells. J Virol. 1997;71:2495–2499. doi: 10.1128/jvi.71.3.2495-2499.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QL, Galasko DR, Ringman JM, Vinters HV, Edland SD, Pomakian J, Ubeda OJ, Rosario ER, Teter B, Frautschy SA, Cole GM. Reduction of SorLA/LR11, a sorting protein limiting beta-amyloid production, in Alzheimer disease cerebrospinal fluid. Arch Neurol. 2009;66:448–457. doi: 10.1001/archneurol.2009.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangieri LR, Mader BJ, Thomas CE, Taylor CA, Luker AM, Tse TE, Huisingh C, Shacka JJ. ATP6V0C knockdown in neuroblastoma cells alters autophagy-lysosome pathway function and metabolism of proteins that accumulate in neurodegenerative disease. PLoS One. 2014;9:e93257. doi: 10.1371/journal.pone.0093257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino JJ, Montes ML, Blanco A, Bustos MJ, Oreja-Guevara C, Bayon C, Cuadrado A, Lubrini G, Cambron I, Munoz A, Cebolla S, Gutierrez-Fernandez M, Bernardino JI, Arribas JR, Fiala M. HIV-1 neuropathogenesis: therapeutic strategies against neuronal loss induced by gp120/Tat glycoprotein in the central nervous system. Rev Neurol. 2011;52:101–111. [PubMed] [Google Scholar]
- Miners JS, Barua N, Kehoe PG, Gill S, Love S. Abeta-degrading enzymes: potential for treatment of Alzheimer disease. J Neuropathol Exp Neurol. 2011;70:944–959. doi: 10.1097/NEN.0b013e3182345e46. [DOI] [PubMed] [Google Scholar]
- Morel E, Chamoun Z, Lasiecka ZM, Chan RB, Williamson RL, Vetanovetz C, Dall'Armi C, Simoes S, Point Du Jour KS, McCabe BD, Small SA, Di Paolo G. Phosphatidylinositol-3-phosphate regulates sorting and processing of amyloid precursor protein through the endosomal system. Nat Commun. 2013;4:2250. doi: 10.1038/ncomms3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nath A. Human immunodeficiency virus (HIV) proteins in neuropathogenesis of HIV dementia. J Infect Dis. 2002;186(Suppl 2):S193–198. doi: 10.1086/344528. [DOI] [PubMed] [Google Scholar]
- Nath A, Psooy K, Martin C, Knudsen B, Magnuson DS, Haughey N, Geiger JD. Identification of a human immunodeficiency virus type 1 Tat epitope that is neuroexcitatory and neurotoxic. J Virol. 1996;70:1475–1480. doi: 10.1128/jvi.70.3.1475-1480.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebuloni M, Pellegrinelli A, Ferri A, Bonetto S, Boldorini R, Vago L, Grassi MP, Costanzi G. Beta amyloid precursor protein and patterns of HIV p24 immunohistochemistry in different brain areas of AIDS patients. AIDS. 2001;15:571–575. doi: 10.1097/00002030-200103300-00005. [DOI] [PubMed] [Google Scholar]
- Nixon RA. Endosome function and dysfunction in Alzheimer's disease and other neurodegenerative diseases. Neurobiol Aging. 2005;26:373–382. doi: 10.1016/j.neurobiolaging.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Nixon RA, Cataldo AM. The endosomal-lysosomal system of neurons: new roles. Trends Neurosci. 1995;18:489–496. doi: 10.1016/0166-2236(95)92772-i. [DOI] [PubMed] [Google Scholar]
- Nunnari G, Argyris E, Fang J, Mehlman KE, Pomerantz RJ, Daniel R. Inhibition of HIV-1 replication by caffeine and caffeine-related methylxanthines. Virology. 2005;335:177–184. doi: 10.1016/j.virol.2005.02.015. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ, Becker J, Burk MW, Margiotta M, Fuhrer J, Steigbigel RT. In situ detection of PCR-amplified HIV-1 nucleic acids in lymph nodes and peripheral blood in patients with asymptomatic HIV-1 infection and advanced-stage AIDS. J Acquir Immune Defic Syndr. 1994;7:916–923. [PubMed] [Google Scholar]
- Oyama F, Murakami N, Ihara Y. Chloroquine myopathy suggests that tau is degraded in lysosomes: implication for the formation of paired helical filaments in Alzheimer's disease. Neurosci Res. 1998;31:1–8. doi: 10.1016/s0168-0102(98)00020-0. [DOI] [PubMed] [Google Scholar]
- Patrick C, Crews L, Desplats P, Dumaop W, Rockenstein E, Achim CL, Everall IP, Masliah E. Increased CDK5 expression in HIV encephalitis contributes to neurodegeneration via tau phosphorylation and is reversed with Roscovitine. Am J Pathol. 2011;178:1646–1661. doi: 10.1016/j.ajpath.2010.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polito VA, Li H, Martini-Stoica H, Wang B, Yang L, Xu Y, Swartzlander DB, Palmieri M, di Ronza A, Lee VM, Sardiello M, Ballabio A, Zheng H. Selective clearance of aberrant tau proteins and rescue of neurotoxicity by transcription factor EB. EMBO Mol Med. 2014;6:1142–1160. doi: 10.15252/emmm.201303671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulliam L. HIV regulation of amyloid beta production. J Neuroimmune Pharmacol. 2009;4:213–217. doi: 10.1007/s11481-009-9151-9. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Annaert W. Membrane trafficking pathways in Alzheimer's disease. Traffic. 2012;13:759–770. doi: 10.1111/j.1600-0854.2012.01332.x. [DOI] [PubMed] [Google Scholar]
- Rajendran L, Schneider A, Schlechtingen G, Weidlich S, Ries J, Braxmeier T, Schwille P, Schulz JB, Schroeder C, Simons M, Jennings G, Knolker HJ, Simons K. Efficient inhibition of the Alzheimer's disease beta-secretase by membrane targeting. Science. 2008;320:520–523. doi: 10.1126/science.1156609. [DOI] [PubMed] [Google Scholar]
- Rempel HC, Pulliam L. HIV-1 Tat inhibits neprilysin and elevates amyloid beta. AIDS. 2005;19:127–135. doi: 10.1097/00002030-200501280-00004. [DOI] [PubMed] [Google Scholar]
- Ritchie K, Carriere I, de Mendonca A, Portet F, Dartigues JF, Rouaud O, Barberger-Gateau P, Ancelin ML. The neuroprotective effects of caffeine: a prospective population study (the Three City Study) Neurology. 2007;69:536–545. doi: 10.1212/01.wnl.0000266670.35219.0c. [DOI] [PubMed] [Google Scholar]
- Sannerud R, Declerck I, Peric A, Raemaekers T, Menendez G, Zhou L, Veerle B, Coen K, Munck S, De Strooper B, Schiavo G, Annaert W. ADP ribosylation factor 6 (ARF6) controls amyloid precursor protein (APP) processing by mediating the endosomal sorting of BACE1. Proc Natl Acad Sci U S A. 2011;108:E559–568. doi: 10.1073/pnas.1100745108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos C, Costa J, Santos J, Vaz-Carneiro A, Lunet N. Caffeine intake and dementia: systematic review and meta-analysis. J Alzheimers Dis. 2010a;20(Suppl 1):S187–204. doi: 10.3233/JAD-2010-091387. [DOI] [PubMed] [Google Scholar]
- Santos C, Lunet N, Azevedo A, de Mendonca A, Ritchie K, Barros H. Caffeine intake is associated with a lower risk of cognitive decline: a cohort study from Portugal. J Alzheimers Dis. 2010b;20(Suppl 1):S175–185. doi: 10.3233/JAD-2010-091303. [DOI] [PubMed] [Google Scholar]
- Shimizu H, Tosaki A, Kaneko K, Hisano T, Sakurai T, Nukina N. Crystal structure of an active form of BACE1, an enzyme responsible for amyloid beta protein production. Mol Cell Biol. 2008;28:3663–3671. doi: 10.1128/MCB.02185-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector SA, Zhou D. Autophagy: an overlooked mechanism of HIV-1 pathogenesis and neuroAIDS? Autophagy. 2008;4:704–706. doi: 10.4161/auto.6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tate BA, Mathews PM. Targeting the role of the endosome in the pathophysiology of Alzheimer's disease: a strategy for treatment. Sci Aging Knowledge Environ 2006. 2006:re2. doi: 10.1126/sageke.2006.10.re2. [DOI] [PubMed] [Google Scholar]
- Torres M, Jimenez S, Sanchez-Varo R, Navarro V, Trujillo-Estrada L, Sanchez-Mejias E, Carmona I, Davila JC, Vizuete M, Gutierrez A, Vitorica J. Defective lysosomal proteolysis and axonal transport are early pathogenic events that worsen with age leading to increased APP metabolism and synaptic Abeta in transgenic APP/PS1 hippocampus. Mol Neurodegener. 2012;7:59. doi: 10.1186/1750-1326-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Bovenkamp M, Nottet HS, Pereira CF. Interactions of human immunodeficiency virus-1 proteins with neurons: possible role in the development of human immunodeficiency virus-1-associated dementia. Eur J Clin Invest. 2002;32:619–627. doi: 10.1046/j.1365-2362.2002.01029.x. [DOI] [PubMed] [Google Scholar]
- Vendeville A, Rayne F, Bonhoure A, Bettache N, Montcourrier P, Beaumelle B. HIV-1 Tat enters T cells using coated pits before translocating from acidified endosomes and eliciting biological responses. Mol Biol Cell. 2004;15:2347–2360. doi: 10.1091/mbc.E03-12-0921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldron E, Jaeger S, Pietrzik CU. Functional role of the low-density lipoprotein receptor-related protein in Alzheimer's disease. Neurodegener Dis. 2006;3:233–238. doi: 10.1159/000095261. [DOI] [PubMed] [Google Scholar]
- Waldron E, Heilig C, Schweitzer A, Nadella N, Jaeger S, Martin AM, Weggen S, Brix K, Pietrzik CU. LRP1 modulates APP trafficking along early compartments of the secretory pathway. Neurobiol Dis. 2008;31:188–197. doi: 10.1016/j.nbd.2008.04.006. [DOI] [PubMed] [Google Scholar]
- Wang Y, Martinez-Vicente M, Kruger U, Kaushik S, Wong E, Mandelkow EM, Cuervo AM, Mandelkow E. Tau fragmentation, aggregation and clearance: the dual role of lysosomal processing. Hum Mol Genet. 2009;18:4153–4170. doi: 10.1093/hmg/ddp367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorp MO, Frank R, Ochsenbauer C, Stricker K, Dhein J, Walczak H, Debatin KM, Krammer PH. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- Wostyn P, Van Dam D, Audenaert K, De Deyn PP. Increased Cerebrospinal Fluid Production as a Possible Mechanism Underlying Caffeine's Protective Effect against Alzheimer's Disease. Int J Alzheimers Dis. 2011;2011:617420. doi: 10.4061/2011/617420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Ikezu T. The comorbidity of HIV-associated neurocognitive disorders and Alzheimer's disease: a foreseeable medical challenge in post-HAART era. J Neuroimmune Pharmacol. 2009;4:200–212. doi: 10.1007/s11481-008-9136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Spector SA. Human immunodeficiency virus type-1 infection inhibits autophagy. Aids. 2008;22:695–699. doi: 10.1097/QAD.0b013e3282f4a836. [DOI] [PMC free article] [PubMed] [Google Scholar]