Abstract
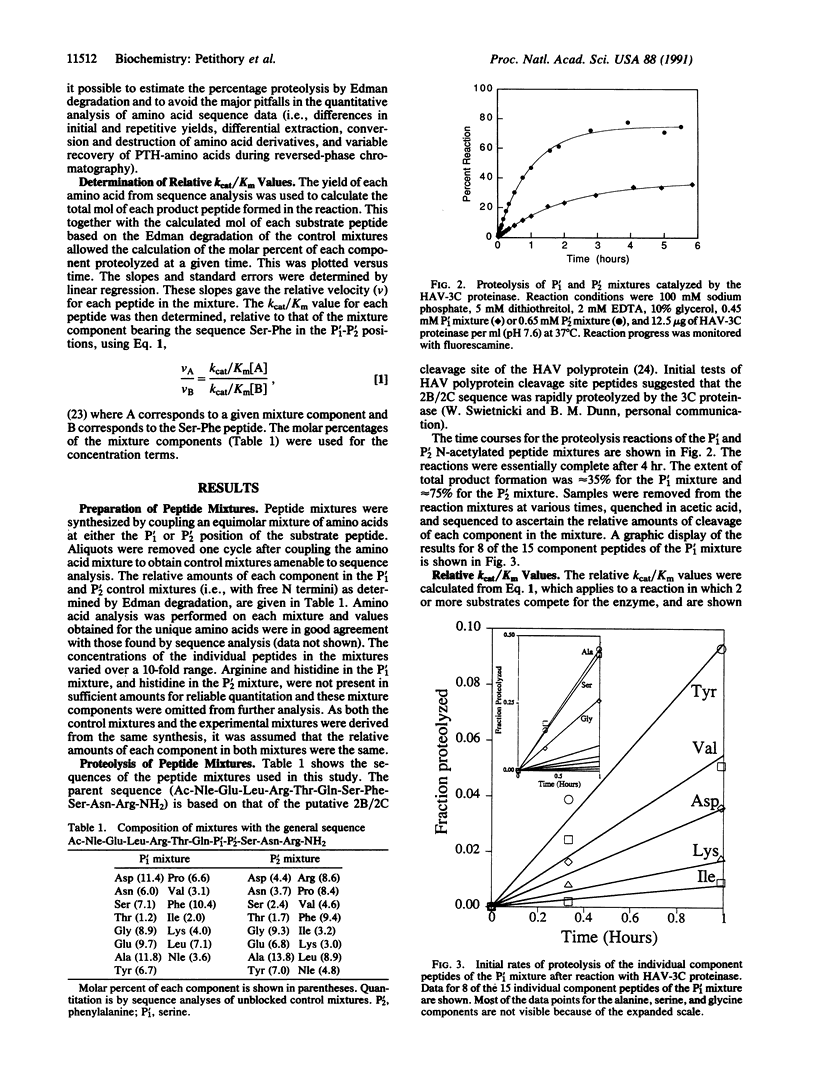
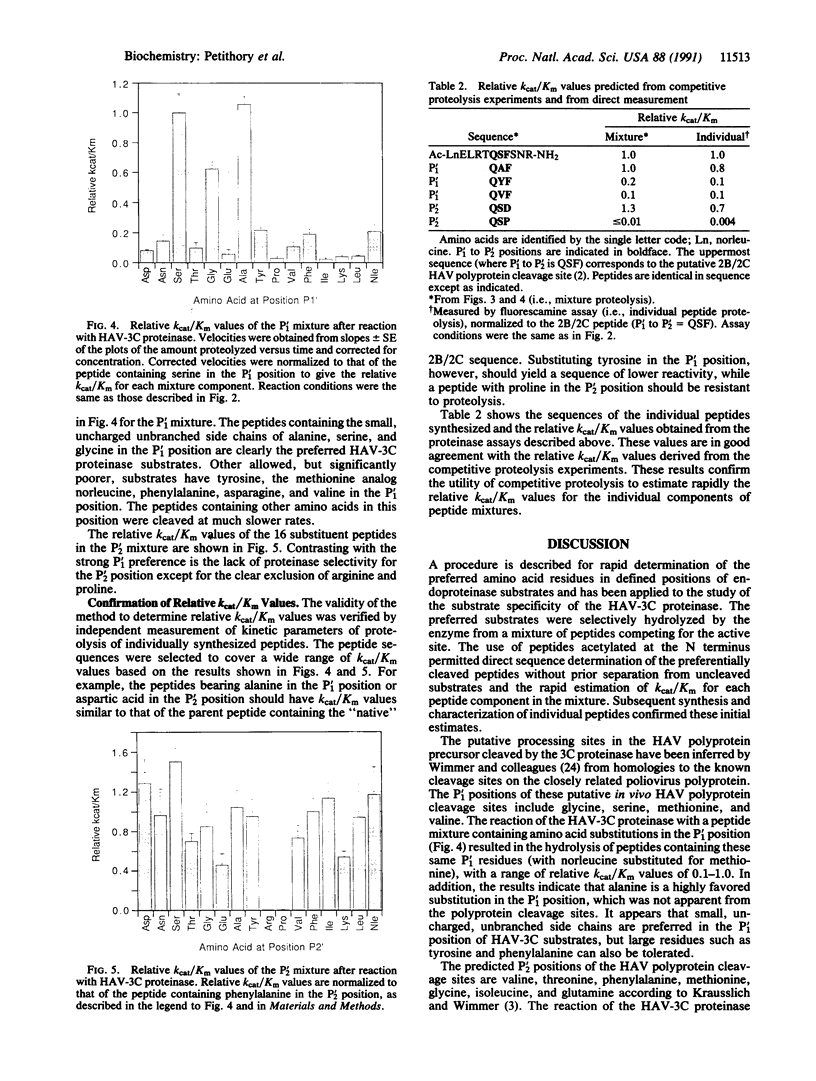
The preferred amino acid residues at the P'1 and P'2 positions of peptide substrates of the 3C proteinase from hepatitis A virus (HAV-3C) have been determined by a rapid screening method. The enzyme was presented with two separate mixtures of N-terminal acetylated peptides, which were identical in sequence except for the amino acids at the P'1 or P'2 positions, where a set of 15 or 16 amino acids was introduced. Enzyme-catalyzed hydrolysis of the peptide mixtures generated free amino termini, which allowed direct sequence analysis by Edman degradation. The relative yield of each amino acid product in the appropriate sequencing cycle gave the amount of each substrate mixture component hydrolyzed. This allowed the simultaneous evaluation of the relative kcat/Km values for each component in the mixture. The peptide substrates preferred by the HAV-3C proteinase in the P'1 mixture were glycine, alanine, and serine. The enzyme has little specificity at P'2; only arginine and proline peptides were excluded as substrates. This method provides a rapid determination of the preferred residues for a peptide substrate and should be applicable to other endoproteinases.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold E., Luo M., Vriend G., Rossmann M. G., Palmenberg A. C., Parks G. D., Nicklin M. J., Wimmer E. Implications of the picornavirus capsid structure for polyprotein processing. Proc Natl Acad Sci U S A. 1987 Jan;84(1):21–25. doi: 10.1073/pnas.84.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A., Schechter I. Mapping the active site of papain with the aid of peptide substrates and inhibitors. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):249–264. doi: 10.1098/rstb.1970.0024. [DOI] [PubMed] [Google Scholar]
- Birkett A. J., Soler D. F., Wolz R. L., Bond J. S., Wiseman J., Berman J., Harris R. B. Determination of enzyme specificity in a complex mixture of peptide substrates by N-terminal sequence analysis. Anal Biochem. 1991 Jul;196(1):137–143. doi: 10.1016/0003-2697(91)90129-h. [DOI] [PubMed] [Google Scholar]
- Blair W. S., Hwang S. S., Ypma-Wong M. F., Semler B. L. A mutant poliovirus containing a novel proteolytic cleavage site in VP3 is altered in viral maturation. J Virol. 1990 Apr;64(4):1784–1793. doi: 10.1128/jvi.64.4.1784-1793.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordingley M. G., Callahan P. L., Sardana V. V., Garsky V. M., Colonno R. J. Substrate requirements of human rhinovirus 3C protease for peptide cleavage in vitro. J Biol Chem. 1990 Jun 5;265(16):9062–9065. [PubMed] [Google Scholar]
- Cordingley M. G., Register R. B., Callahan P. L., Garsky V. M., Colonno R. J. Cleavage of small peptides in vitro by human rhinovirus 14 3C protease expressed in Escherichia coli. J Virol. 1989 Dec;63(12):5037–5045. doi: 10.1128/jvi.63.12.5037-5045.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewalt P. G., Lawson M. A., Colonno R. J., Semler B. L. Chimeric picornavirus polyproteins demonstrate a common 3C proteinase substrate specificity. J Virol. 1989 Aug;63(8):3444–3452. doi: 10.1128/jvi.63.8.3444-3452.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerle T., Hellen C. U., Wimmer E. Site-directed mutagenesis of the putative catalytic triad of poliovirus 3C proteinase. J Biol Chem. 1991 Mar 25;266(9):5412–5416. [PubMed] [Google Scholar]
- Ivanoff L. A., Towatari T., Ray J., Korant B. D., Petteway S. R., Jr Expression and site-specific mutagenesis of the poliovirus 3C protease in Escherichia coli. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5392–5396. doi: 10.1073/pnas.83.15.5392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay J., Dunn B. M. Viral proteinases: weakness in strength. Biochim Biophys Acta. 1990 Jan 30;1048(1):1–18. doi: 10.1016/0167-4781(90)90015-t. [DOI] [PubMed] [Google Scholar]
- Korant B. D. Viral proteases: an emerging therapeutic target. Crit Rev Biotechnol. 1988;8(2):149–157. doi: 10.3109/07388558809150543. [DOI] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Lawson M. A., Semler B. L. Picornavirus protein processing--enzymes, substrates, and genetic regulation. Curr Top Microbiol Immunol. 1990;161:49–87. [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr D. C., Long A. C., Kay J., Dunn B. M., Cameron J. M. Hydrolysis of a series of synthetic peptide substrates by the human rhinovirus 14 3C proteinase, cloned and expressed in Escherichia coli. J Gen Virol. 1989 Nov;70(Pt 11):2931–2942. doi: 10.1099/0022-1317-70-11-2931. [DOI] [PubMed] [Google Scholar]
- Pallai P. V., Burkhardt F., Skoog M., Schreiner K., Bax P., Cohen K. A., Hansen G., Palladino D. E., Harris K. S., Nicklin M. J. Cleavage of synthetic peptides by purified poliovirus 3C proteinase. J Biol Chem. 1989 Jun 15;264(17):9738–9741. [PubMed] [Google Scholar]
- Parks G. D., Baker J. C., Palmenberg A. C. Proteolytic cleavage of encephalomyocarditis virus capsid region substrates by precursors to the 3C enzyme. J Virol. 1989 Mar;63(3):1054–1058. doi: 10.1128/jvi.63.3.1054-1058.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Duke G. M., Palmenberg A. C. Encephalomyocarditis virus 3C protease: efficient cell-free expression from clones which link viral 5' noncoding sequences to the P3 region. J Virol. 1986 Nov;60(2):376–384. doi: 10.1128/jvi.60.2.376-384.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks G. D., Palmenberg A. C. Site-specific mutations at a picornavirus VP3/VP1 cleavage site disrupt in vitro processing and assembly of capsid precursors. J Virol. 1987 Dec;61(12):3680–3687. doi: 10.1128/jvi.61.12.3680-3687.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Translation of encephalomyocarditis virus RNA in vitro yields an active proteolytic processing enzyme. Eur J Biochem. 1978 Apr 17;85(2):457–462. doi: 10.1111/j.1432-1033.1978.tb12260.x. [DOI] [PubMed] [Google Scholar]
- Strop P., Konvalinka J., Stys D., Pavlickova L., Blaha I., Velek J., Travnicek M., Kostka V., Sedlacek J. Specificity studies on retroviral proteinase from myeloblastosis-associated virus. Biochemistry. 1991 Apr 9;30(14):3437–3443. doi: 10.1021/bi00228a013. [DOI] [PubMed] [Google Scholar]
- Vakharia V. N., Devaney M. A., Moore D. M., Dunn J. J., Grubman M. J. Proteolytic processing of foot-and-mouth disease virus polyproteins expressed in a cell-free system from clone-derived transcripts. J Virol. 1987 Oct;61(10):3199–3207. doi: 10.1128/jvi.61.10.3199-3207.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner J. R., Dunn B. M. Development of synthetic peptide substrates for the poliovirus 3C proteinase. Arch Biochem Biophys. 1991 May 1;286(2):402–408. doi: 10.1016/0003-9861(91)90058-q. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Filman D. J., Hogle J. M., Semler B. L. Structural domains of the poliovirus polyprotein are major determinants for proteolytic cleavage at Gln-Gly pairs. J Biol Chem. 1988 Nov 25;263(33):17846–17856. [PubMed] [Google Scholar]