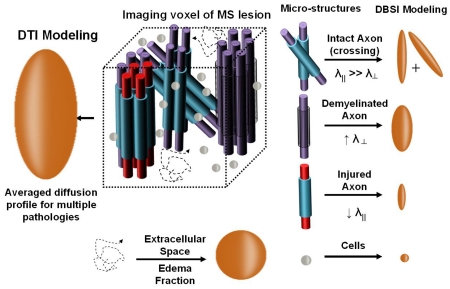
Graphical abstract
We report on a non-invasive imaging technique that can discern inflammation from axon fibers within the human CNS white matter. The healthy CNS is complex, with glial and neuronal cells, myelinated and unmyelinated axonal fibers that are typically bundled into tracts (which often cross each other), and regions of extracellular space, including cerebrospinal fluid. In pathological conditions, invading inflammatory cells and edema are also often present. Diffusion basis spectrum imaging (DBSI, a data-driven multi-tensor model) was developed at Washington University to identify and quantify these various elements within an imaging voxel. Diffusion tensor imaging (left) provides a single averaged tensor for an imaging voxel of co-existing crossing-fibers, infiltrating inflammatory cells, and extracellular space. DBSI (right) describes these components of normal anatomy and pathology using multiple tensors: free water (as in a ventricle) is represented by free isotropic diffusion tensor; cells are represented by restricted isotropic diffusion tensor; edema water is represented by hindered isotropic diffusion tensor; crossing-axons are represented by anisotropic diffusion tensors with increased radial diffusivity representing demyelination and reduced axial diffusivity reflecting axonal injury.
Keywords: multiple sclerosis, diffusion tensor imaging, inflammation
Introduction
Multiple sclerosis (MS) is a chronic disease of complex and heterogeneous pathology which involves inflammation, demyelination, remyelination and axon injury and loss. MS is common - about 2.5 million people worldwide have the disease. The pathology of MS varies between individuals and in the same individual patient over the time-course of the disease(Lucchinetti et al., 2000). The variability of the clinical course and neuropathology of MS has proven to be a barrier to the complete understanding of the disease. As biopsies of the central nervous system (CNS) are infrequent (and are usually done in atypical cases) and serial biopsies are even more rarely done, understanding of the underlying neuropathology of MS derives mainly from end-of-life autopsies and less invasive methods such as imaging and blood and cerebrospinal fluid (CSF) analyses. Methods providing a non-invasive window into the CNS to better understand the pathophysiology of progression are greatly needed.
Inflammatory demyelination of CNS white matter (WM) was formerly regarded as the main pathology of MS. Prior to the past two decades, gray matter (GM) involvement received less emphasis which is perhaps due to the difficulty to detect or measure it. However GM involvement in MS, including cortical GM damage, can be extensive and correlates better with disability measures than does WM damage (Fisher et al., 2008; Rudick et al., 2009). The primary substrate of disability is thought to be axon injury and loss (Kornek et al., 2001). Accurate measures of axon injury and loss in the CNS of MS patients are needed to better understand the pathology of MS over the course of the disease, to monitor responses to therapies, and to use as a surrogate marker in clinical trials, especially for progressive MS trials.
The advent of magnetic resonance imaging (MRI) revolutionized the diagnosis and has greatly improved the monitoring of MS. Gadolinium-enhanced MRI lesions serve as surrogate biomarkers of clinical MS relapses(Sormani et al., 2009). The latter has expedited research leading to approval of several of the thirteen disease-modifying therapies (DMTs) for relapsing forms of MS. Current DMTs work best to reduce relapse rate, but do not completely eliminate relapses, and do not work in all patients. None of the present approved DMTs are highly effective at slowing disability progression and none yet repairs axons in the CNS. Novel imaging tools that can be used to identify responses to promising new therapies, such as remyelinating agents that are in early phases (Mi et al., 2013; Wootla et al., 2013) are also needed. This paper will discuss updates on diffusion MR methods to detect and quantitate axon loss, as well as demyelination, cellular inflammation and edema in the MS-affected CNS.
Diffusion magnetic resonance imaging
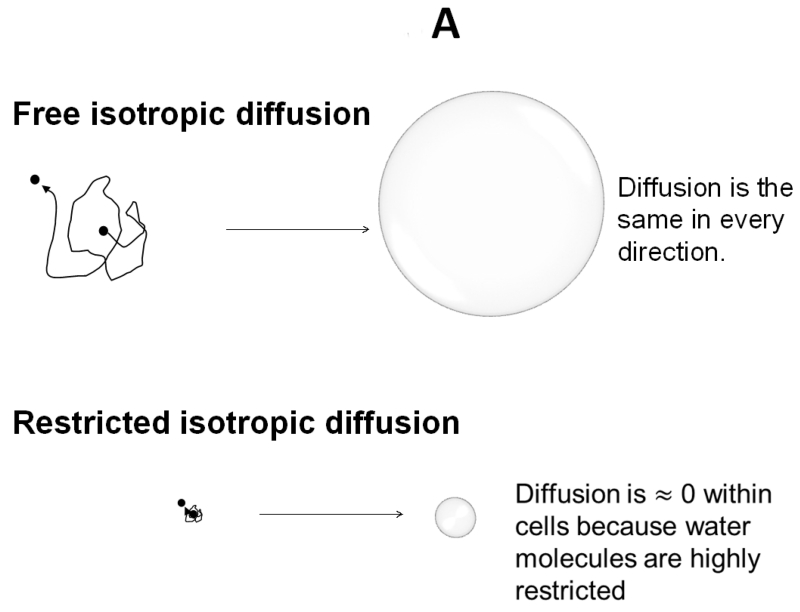
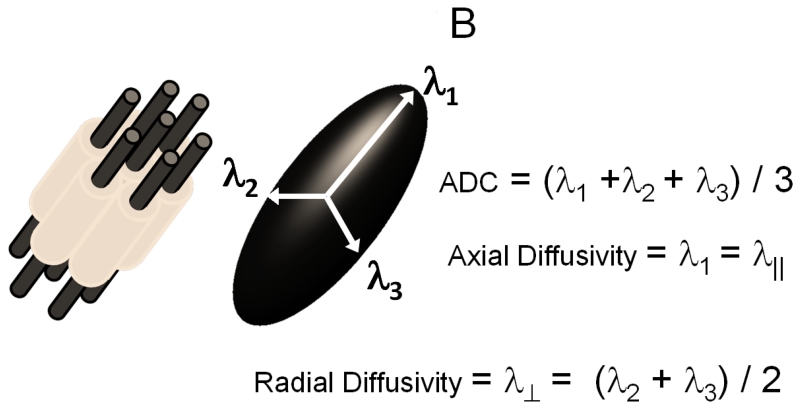
Diffusion weighted MRI measures water apparent diffusion coefficient (ADC) through the application of a pair of diffusion weighting pulsed magnetic field gradients of altering magnitude, duration, and time interval of separation between gradients; the term “b-value” derived from a landmark study(Stejskal, 1965) is used to control the extent of signal loss for quantifying ADC. Diffusion tensor imaging (DTI) employs multiple directions of diffusion weighting to quantitatively describe water diffusion in tissues (Basser et al., 1994a; Basser et al., 1994b). The displacement of cerebrospinal fluid (CSF) water molecules can be described as a sphere, i.e., isotropic diffusion. Water molecules in CSF move freely without hindrance. The ADC is the same in any direction it is measured; one-direction diffusion weighting is sufficient to describe isotropic diffusion (Fig. 1A). In contrast, the displacement of water molecules in WM tracts is an ellipsoid, i.e., anisotropic diffusion. For anisotropic diffusion, ADC varies depending on the direction of diffusion weighting gradients. A multiple-direction diffusion weighting is necessary to accurately describe water diffusion in most biological tissues (Fig. 1B).
Figure 1. Isotropic and Anisotropic diffusion depicted graphically.
A) Free and restricted isotropic diffusion. B) Anisotropic diffusion.
Diffusion Imaging in MS
Over a decade ago, we began to test if DTI-derived directional diffusivity might help understand the underlying pathology in MS. Our hypothesis was that reduced myelin would lead to an increase in radial diffusivity, i.e., the ADC perpendicular to axonal tracts, as measured by DTI. We set out to test this hypothesis using demyelinated and dysmyelinated animal models. Shiverer mice, that have a mutation in the gene encoding myelin basic protein and are therefore dysmyelinated, were examined by DTI. These mice have normal axons. The expectation was that these mice would have increased radial diffusivity due to lack of normal myelin, but normal axial diffusivity (ADC parallel to axonal tracts) indicating normal axons. Indeed, the major WM tracts of shiverer mice had axial diffusivity that was similar to that of WT with approximately 30% increase in radial diffusivity.(Song et al., 2002)
As part of a phase 2 trial of rituximab performed from 2002-2009 at Washington University, we also obtained DTI of the brains of relapsing MS patients in the study. Each enrolled patient underwent seven gadolinium-enhanced brain MRIs. We found that increased radial diffusivity at the time of a new gadolinium-enhancing lesion predicted the eventual development of a persistent black hole (PBH).(Naismith et al., 2010) As the latter is indicative of worse damage and more axon drop-out than a gadolinium-enhanced lesion that subsequently becomes isointense on T1w imaging, DTI appeared useful as an early indicator of pathology occurring at the time of lesion onset.
We also hypothesized that reduced axial diffusivity would indicate damage to the axon or even axonal transection. In another study using retinal ischemia to kill retinal ganglion cells leading to initial axon and subsequent myelin degeneration (“Wallerian degeneration”), this appeared to hold true(Sun et al., 2008). However, results obtained when applying DTI in human CNS did not always conform to expectations. In studies of transverse myelitis patients using DTI, although radial diffusivity was increased at the site of injury as expected, axial diffusivity also was increased above normal.(Naismith et al., 2013) An explanation for the increased axial diffusivity, as well as a factor expected to contribute to increased radial diffusivity, was increased extracellular space, which would be expected in regions of significant axon loss (such as in PBH). Similarly, in acute lesions with enhancement, the blood brain barrier integrity is compromised and with the entry of more free water (vasogenic edema) an increase in radial diffusivity would also be expected.
Development of diffusion Basis Spectrum imaging (DBSI)
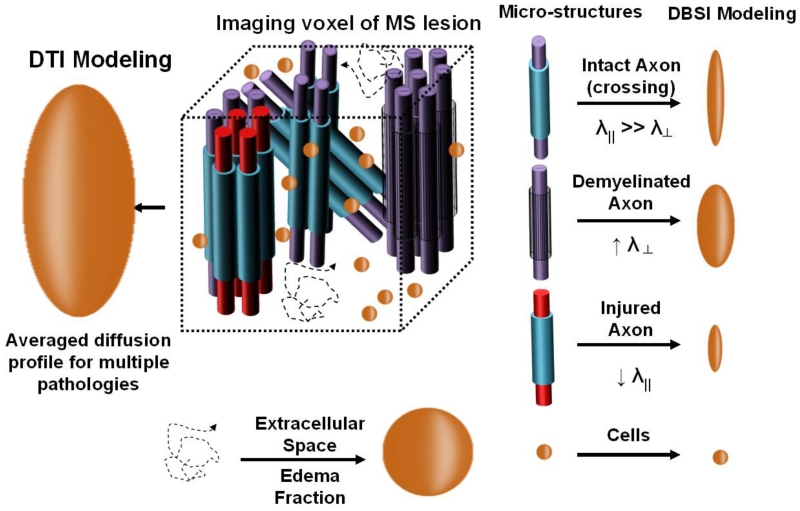
The CNS is complex, in particular the human brain. The diffusion-weighted MR signals within an image voxel represent the weighted average effect of the water molecules within multiple microstructural compartments. In the conventional DTI analysis, a single tensor is assumed and it thus also represents the averaged effects of potential multiple tensors describing each individually different microstructural compartment (Fig. 2). For example, in an MS lesion, any given voxel might contain varying amounts of normal and damaged axons which might be myelinated or demyelinated, as well as varying amounts of cells (both intrinsic and extrinsic cells) and free space that might be due to vasogenic edema in an acute lesion, or to increased extracellular space in lesions with loss of normal structure, such as in a PBH. Therefore, a method to more accurately reflect the complexities of CNS pathology in humans had to be developed.
Figure 2. Conceptual comparison between DTI and DBSI.
DTI (figuratively depicted at left) is a single averaged tensor, whereas DBSI (depicted at right) uses multiple tensors to describe structural integrity and underlying pathologies in tissues.
Diffusion basis spectrum imaging (DBSI) was developed at Washington University in an attempt to extract all those different compartments of normal anatomy and pathology that might be contained within an imaging voxel within white matter (Wang et al., 2011). Whereas in DTI the diffusion-weighted signals yield a single “averaged” tensor, DBSI extracts tensors from multiple intra-voxel components of structure and pathology (Fig. 2). For DBSI, cells have been associated with restricted isotropic diffusion tensors, reflecting microstructural barriers to diffusion (nucleus and cell membranes) that restrict water diffusion. Free water, such as within a ventricle, is represented by the isotropic diffusion tensor components with ADC of free diffusion. Isotropic diffusion tensors with intermediate diffusivity between the restricted diffusivity of cells and the free diffusivity of ventricular fluid capture diffusion profiles of the intra-voxel compartments of vasogenic edema and tissue loss (Chiang et al., 2014). We have referred to this as “hindered” diffusion.
To derive multiple tensors describing the complex realities in MS lesions, more diffusion data are needed. In our initial proof-of-concept tests, we employed a 99-direction diffusion weighting scheme, selected as prescribed in diffusion spectrum imaging where the position vectors are the entire grid points (qx, qy, qz) over the 3-D q-space under the relationship that (qx2 + qy2 + qz2) ≤ r2, where r = 3 for DBSI(Wang et al., 2013; Wang et al., 2015; Wang et al., 2011; Wedeen et al., 2005). Subsequently, we also demonstrated that fewer directions of applied gradients such as the icosahedral 25-direction sampling scheme as prescribed (Batchelor et al., 2003) with the addition of one extra non-diffusion (b = 0) weighted image can be sufficient for regions with more coherent non-crossing tracts, such as found in the spinal cord and optic nerve(Chiang et al., 2014; Murphy et al.).
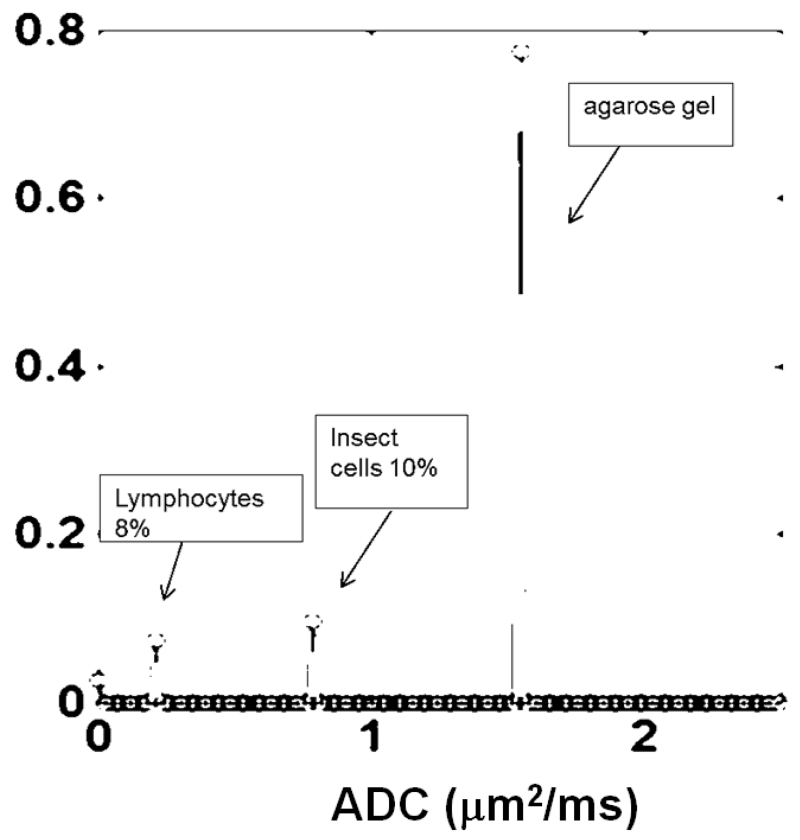
The isotropic diffusion components in DBSI detect and distinguish different-sized cells by quantifying their signal intensity and associated isotropic ADCs. We tested this by performing DBSI on a phantom containing two cell types embedded in 2% agarose: 10% by volume of 13.5-μm diameter High Five insect cells (Invitrogen) and 8% of 7-μm diameter mouse lymphocytes. As expected, the smaller lymphocytes give rise to a diffusion spectral component with ADC that is lower than that of the larger insect cells and each was detected in its correct proportion (Fig. 3). The water signal of the agarose gel was reflected by a large less restricted ADC spectral component.
Figure 3. DBSI can discern and measure cells of different diameters.
A phantom was prepared with mouse lymphocytes (diameter 7μm) and High Five insect cells (diameter 13.5μm) in 2% agarose gel. The cell types were distinguishable based on the smaller cells having lower ADC (left) vs. the larger insect cells with higher ADC (middle). DBSI correctly quantified the proportions of the two cell types. The largest peak in all three panels is the water signal of 2% agarose. ADC on the x-axis has unit of μm2/ms.
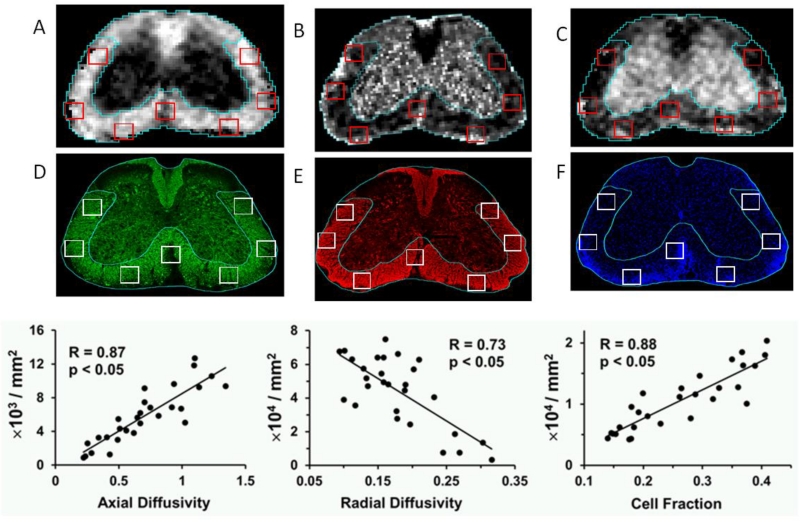
DBSI was then applied to the more complicated mouse experimental autoimmune encephalomyelitis (EAE) model for MS. EAE was induced actively in C57BL/6 mice using the MOG35-55 peptide of CNS myelin oligodendrocyte glycoprotein (MOG). DBSI was performed in living mice at four different time-points, after which at each time point mice were then anesthetized and terminally perfused to examine neuropathology. Results with in vivo DBSI findings were compared to quantitative histopathology (Fig. 4). Immunohistochemical analysis of pathology was done with SMI-31 for normal phosphorylated axons, myelin basic protein to reflect level of myelination, and DAPI stain for cell nuclei content. Moderate to strong correlations were found between the DBSI parameters of axial diffusivity, where reduction was hypothesized to reflect injured axons(R=.87, p<.05), radial diffusivity, where an increase was hypothesized to reflect demyelination(R=.73, p<.05), and restricted isotropic diffusion fraction to reflect cell content(R=.88, p<.05) and the immunohistochemical stains. These correlations were observed in data from all time-points during EAE, strongly supporting the utility of DBSI (Fig. 4).
Figure 4. DBSI performed on living EAE-affected mice correlates with axon content, myelination and cell count within mouse spinal cords.
Representative in vivo DBSI derived (A) axial and (B) radial diffusivity, and (C) cell fraction maps co-registered with immunohistochemical staining for (D) phosphorylated axons using SMI-31, (E) myelin using anti-myelin basic protein antibody, and (F) cell content using DAPI stain with identical ROI co-localized. Modeling of DBSI used in these studies was for white matter, and thus seven regions of interest within white matter were analyzed at four different time points during the course of EAE. The correlation between (G) axial diffusivity and SMI-31 positive axon counts (R=0.87, p<.05), (H) radial diffusivity and MBP positive axon counts (R=.73, p<.05), and (I) cell fraction and DAPI counts (R=0.88, p<.05) of spinal cord (at L2 level) from EAE-affected C57BL/6 mice at baseline, onset, peak, and chronic states were determined. Scale bar = 300 μm. Diffusivity = μm2/ms.
We have applied DBSI to human autopsied spinal cord from 3 deceased MS subjects, as reported (Wang et al., 2015). Regions of interest were placed randomly in the WM of cervical spinal cord sections obtained from an autopsy, the sections were scanned using DBSI, and subsequently were histologically examined for axon content using Bielschowsky’s silver stain, for myelin content using Luxol Fast Blue-PAS, and for cellularity using hematoxylin and eosin. Significant positive correlations ranging from r=0.7 to r=0.83 were found between the fiber fraction (axonal density) of each voxel and silver stained content (axon counts), and significant negative correlations ranging from − 0.42 to − 0.84 for radial diffusivity and Luxol Fast Blue stain for myelin. In two of the 3 autopsied cases, restricted isotropic fraction correlated significantly and positively with cellularity, as quantitated by H&E stain.
Future refinements to DBSI
We are currently in the midst of a multi-year study to examine the ability of DBSI to quantify white matter pathology for correlating function and predicting outcome in living MS patients. Mouse models of EAE are also employed to quantitatively examine DBSI-derived pathological metrics as a potential biomarker of axon and myelin integrity, as well as inflammation. In humans, PBHs are being used as a surrogate of axonal loss, and it is expected that fiber fractions (anisotropic diffusion tensor content) of individual voxels will be much reduced in PBH compared to MS lesions that are isointense on T1-weighted scans. Currently, DBSI quantitatively assesses the proportions of isotropic and anisotropic diffusion tensors within an imaging voxel within the white matter. By definition, the proportions must add to 100%. Thus, a “dilution effect” of a dominant pathology may lead to an inaccurate interpretation. For example, if edema (i.e., “hindered” isotropic diffusion components) is present in abundance, both cell and fiber fractions appear low, suggesting reduction of inflammation and axonal loss. This will be corrected by taking into account the “volume” of each component within the image voxel by multiplying the fraction of each component and image voxel volume. By summing the derived “fraction volume” from all voxels, cell and axon fiber volume can then be correctly represented to correlate with cell and axon counts. We are applying DBSI in animal models to determine whether axon volume may serve as a noninvasive biomarker to reflect the severity of axonal loss or whether the rate of axonal loss as determined by longitudinal quantification of axon volume can predict disease progression. It is our contention that axon volume, and to a lesser extent the other components of DBSI, can serve as a biomarker to predict outcome of DMT in MS.
Highlights.
A novel diffusion imaging called Diffusion Basis Spectrum Imaging is reviewed.
A method for separating and measuring isotropic versus anisotropic diffusion components in CNS is described.
Acknowledgements
The authors thank Drs. Yong Wang, Peng Sun, Robert Naismith, Joong Hee Kim, Tsang-Wei (William) Tu and Tsen-Huan (Abby) Lin for their critical contributions to the development and validation of diffusion basis spectrum imaging.
Funding:
This work was supported by the National Institutes of Health [grant numbers P01NS059560, U01EY025500]; the Manny & Rosalyn Rosenthal-Dr. John L. Trotter Chair in Neuroimmunology of the Barnes-Jewish Hospital Foundation (AHC).
Abbreviations
- DBSI
diffusion basis spectrum imaging
- DTI
diffusion tensor imaging
- DMT
disease modifying therapy
- ADC
apparent diffusion coefficient
- PBH
persistent black hole
- WM
white matter
- GM
gray matter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: A patent entitled “Diagnosis of Central Nervous System White Matter Pathology Using Diffusion MRI” has been filed on DBSI by Washington University. USA Patent Pending, Serial No. 61/345,367
References
- Basser PJ, Mattiello J, LeBihan D. Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B. 1994a;103:247–254. doi: 10.1006/jmrb.1994.1037. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994b;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor PG, Atkinson D, Hill DL, Calamante F, Connelly A. Anisotropic noise propagation in diffusion tensor MRI sampling schemes. Magn Reson Med. 2003;49:1143–1151. doi: 10.1002/mrm.10491. [DOI] [PubMed] [Google Scholar]
- Chiang CW, Wang Y, Sun P, Lin TH, Trinkaus K, Cross AH, Song SK. Quantifying white matter tract diffusion parameters in the presence of increased extra-fiber cellularity and vasogenic edema. Neuroimage. 2014;101:310–319. doi: 10.1016/j.neuroimage.2014.06.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher E, Lee JC, Nakamura K, Rudick RA. Gray matter atrophy in multiple sclerosis: a longitudinal study. Ann Neurol. 2008;64:255–265. doi: 10.1002/ana.21436. [DOI] [PubMed] [Google Scholar]
- Kornek B, Storch MK, Bauer J, Djamshidian A, Weissert R, Wallstroem E, Stefferl A, Zimprich F, Olsson T, Linington C, Schmidbauer M, Lassmann H. Distribution of a calcium channel subunit in dystrophic axons in multiple sclerosis and experimental autoimmune encephalomyelitis. Brain. 2001;124:1114–1124. doi: 10.1093/brain/124.6.1114. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Mi S, Blake Pepinsky R, Cadavid D. Blocking LINGO-1 as a Therapy to Promote CNS Repair: From Concept to the Clinic. CNS Drugs. 2013;27:493–503. doi: 10.1007/s40263-013-0068-8. [DOI] [PubMed] [Google Scholar]
- Murphy RK, Sun P, Xu J, Wang Y, Sullivan S, Gamble P, Wagner J, Wright NN, Dorward IG, Riew D, Santiago P, Kelly MP, Trinkaus K, Ray WZ, Song SK. Magnetic Resonance Imaging Biomarker of Axon Loss Reflects Cervical Spondylotic Myelopathy Severity. Spine (Phila Pa 1976) 41:751–756. doi: 10.1097/BRS.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Xu J, Klawiter EC, Lancia S, Tutlam NT, Wagner JM, Qian P, Trinkaus K, Song SK, Cross AH. Spinal cord tract diffusion tensor imaging reveals disability substrate in demyelinating disease. Neurology. 2013;80:2201–2209. doi: 10.1212/WNL.0b013e318296e8f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naismith RT, Xu J, Tutlam NT, Scully PT, Trinkaus K, Snyder AZ, Song SK, Cross AH. Increased diffusivity in acute multiple sclerosis lesions predicts risk of black hole. Neurology. 2010;74:1694–1701. doi: 10.1212/WNL.0b013e3181e042c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Lee JC, Nakamura K, Fisher E. Gray matter atrophy correlates with MS disability progression measured with MSFC but not EDSS. J Neurol Sci. 2009;282:106–111. doi: 10.1016/j.jns.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Sormani MP, Bonzano L, Roccatagliata L, Cutter GR, Mancardi GL, Bruzzi P. Magnetic resonance imaging as a potential surrogate for relapses in multiple sclerosis: a meta-analytic approach. Ann Neurol. 2009;65:268–275. doi: 10.1002/ana.21606. [DOI] [PubMed] [Google Scholar]
- Stejskal EO, Tanner JE. Spin diffusion measurements: Spin echoes in the presence of a time dependent field gradient. Journal of Chemical Physics. 1965;42:288. [Google Scholar]
- Sun SW, Liang HF, Cross AH, Song SK. Evolving Wallerian degeneration after transient retinal ischemia in mice characterized by diffusion tensor imaging. Neuroimage. 2008;40:1–10. doi: 10.1016/j.neuroimage.2007.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Brieland JK, Kim JH, Chen YJ, O’Neal J, O’Neil SP, Tu TW, Trinkaus K, Song SK. Diffusion tensor imaging detects treatment effects of FTY720 in experimental autoimmune encephalomyelitis mice. NMR Biomed. 2013;26:1742–1750. doi: 10.1002/nbm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Sun P, Wang Q, Trinkaus K, Schmidt RE, Naismith RT, Cross AH, Song SK. Differentiation and quantification of inflammation, demyelination and axon injury or loss in multiple sclerosis. Brain. 2015;138:1223–1238. doi: 10.1093/brain/awv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Q, Haldar JP, Yeh FC, Xie M, Sun P, Tu TW, Trinkaus K, Klein RS, Cross AH, Song SK. Quantification of increased cellularity during inflammatory demyelination. Brain. 2011;134:3590–3601. doi: 10.1093/brain/awr307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med. 2005;54:1377–1386. doi: 10.1002/mrm.20642. [DOI] [PubMed] [Google Scholar]
- Wootla B, Watzlawik JO, Denic A, Rodriguez M. The road to remyelination in demyelinating diseases: current status and prospects for clinical treatment. Expert Rev Clin Immunol. 2013;9:535–549. doi: 10.1586/eci.13.37. [DOI] [PubMed] [Google Scholar]