Abstract
Obsessive-compulsive disorder (OCD) is characterized by excessive absorption with internally-generated distressing thoughts and urges, with difficulty incorporating external information running counter to their fears and concerns. In the present study, we experimentally probed this core feature of OCD through the use of a novel attention switching task that investigates transitions between internally focused (IF) and externally focused (EF) attentional states. Eighteen OCD patients and 18 controls imagined positive and negative personal event scenarios (IF state) or performed a color-word Stroop task (EF state). The IF/EF states were followed by a target detection (TD) task requiring responses to external stimuli. Compared to controls, OCD patients made significantly more errors and showed reduced activation of superior and inferior occipital cortex, thalamus, and putamen during TD following negative IF, with the inferior occipital hypoactivation being significantly greater for TD following negative IF compared to TD following the other conditions. Patients showed stronger functional connectivity between the inferior occipital region and dorsomedial prefrontal cortex. These findings point to an OCD-related impairment in the visual processing of external stimuli specifically when they follow a period of negative internal focus, and suggest that future treatments may wish to target the transition between attentional states.
Keywords: Default mode, Rumination, Negative thought, Task positive network, Occipital
1. Introduction
Obsessive-compulsive disorder (OCD) is characterized by persistent engagement with negative and intrusive thoughts, images, or ideas. Though experienced as intrusive, these obsessions are internally generated and often take the form of imagined scenarios of harm or bad events. Obsessions elicit extensive efforts to counteract the anxiety that they produce, yet one of the more perplexing features of OCD is that patients appear unable to use available external information to counteract their negative thoughts and fears. Neuroscientifically, this impairment could indicate an imbalance between brain systems that subserve internally focused (IF) and externally focused (EF) attentional states (Stern and Taylor, 2014). Specifically, persistent obsessions and repetitive information seeking could result from excessive activation of brain systems that generate internal (imagined) fears of negative events, and/or an inability to appropriately recruit systems that process externally observable evidence indicating that feared events did or will not occur. Although the hypothesis of an imbalance between IF and EF attention in OCD may seem intuitive from a clinical perspective, it has not been directly examined using brain imaging where its underlying neural mechanisms can be identified.
Results from neuroimaging studies indicate that IF and EF cognitive processes recruit dissociable large-scale networks reflecting these different “modes of processing”. IF cognition, including mental simulations and imagination of future events (“future thinking”), autobiographical memory, and self-referential processing, are associated with activation of the “default mode network” (DMN), a large-scale network comprised of ventromedial and anterior dorsomedial prefrontal cortex, posterior cingulate cortex, and hippocampus (Andrews-Hanna et al., 2014; Buckner et al., 2008; D’Argembeau et al., 2008; Spreng et al., 2009). In healthy individuals, the DMN decreases in activation (or deactivates) when attention is directed to information in the environment (EF cognition), which may reflect the suspension of IF processes in order to respond efficiently to external information (Andrews-Hanna et al., 2010; 2014; Buckner et al., 2008; Gusnard et al., 2001; McKiernan et al., 2003; Shulman et al., 1997). The two networks most consistently associated with EF cognition are the dorsal attention network linked to visuospatial attention and motor planning and the fronto-parietal control network involved in higher-order functions such as working memory, conflict detection, and response inhibition (Buchsbaum et al., 2005; Corbetta and Shulman, 2002; Spreng et al., 2010; Vincent et al., 2008). Together, these networks have been referred to as the “task-positive” network (TPN) – so named because it positively activates in many tasks of EF cognition historically used in fMRI research (Power et al., 2011; Yarkoni et al., 2010). The TPN is predominantly composed of dorsal anterior cingulate cortex (ACC) and supplementary motor area, dorsolateral prefrontal cortex (DLPFC), precentral gyrus, anterior insula, lateral parietal cortex, occipital cortex, striatum, and thalamus (Buchsbaum et al., 2005; Corbetta and Shulman, 2002; Fox et al., 2005; Spreng et al., 2010; Vincent et al., 2008).
Previous research has found abnormal functioning of DMN and TPN in OCD. Patients show hyperactivation of DMN regions during error detection (Fitzgerald et al., 2005; Stern et al., 2011) and both economic (Stern et al., 2013) and moral (Harrison et al., 2012) decision making, but exhibit hypoactivation during fear extinction (Milad et al., 2013). Patients also show altered functional connectivity between DMN and TPN at rest (Fitzgerald et al., 2010; Stern et al., 2012), indicating that the intrinsic relationship between IF and EF brain systems is disrupted in OCD. Prior work examining OCD patients during switching between two external tasks (focusing on the color or shape of stimuli on the screen) found altered switch-related activity in VMPFC, DLPFC, ACC, and lateral parietal cortex, findings that were interpreted as reflecting an imbalance between ventral “affective” and dorsal “ cognitive” fronto-striatal circuitry (Gu et al., 2008). However, no studies of OCD have investigated the effects of internal absorption on the subsequent neural processing of external information, despite clear relevance of such an investigation to the clinical phenomenology of the disorder. Using an attentional state switching task in healthy individuals (Stern et al., 2015), we have shown differential TPN activity and connectivity during a target detection task based on whether it was preceded by an IF event imagination task or an EF working memory task, indicating that prior attentional state affects subsequent neural functioning. Here, we take the novel approach of investigating behavior, brain activation, and functional connectivity to test the hypothesis that OCD patients will show altered neural processing of external information (in a target detection task) compared to controls specifically when previously engaged in an internally focused task. Such an investigation may help determine the neural mechanisms associated with OCD patients’ characteristic inability to disengage from obsessional thinking, which could identify biological targets for the treatment of this core behavior.
2. Methods
2.1. Subjects
Data were analyzed from 18 patients with OCD and 18 healthy controls (HC). Patients met DSM-IV criteria for OCD, excluding primary hoarding subtypes, and were excluded for bipolar disorder or psychosis, and current post-traumatic stress disorder, panic disorder, tic disorder, or eating disorder. Eight patients had current Axis I comorbidities and 15 patients were taking serotonin reuptake inhibitors (SRIs) (see Supplement for details). Fluoxetine equivalences were calculated for use in post-hoc analyses examining medication effects. HC were free of psychiatric and neurological diagnoses as well as psychotropic medication.
Diagnoses were made using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al., 1998). Symptoms of general anxiety and depression were quantified using Beck Anxiety and Depression Inventories (Beck et al., 1988; 1961). Obsessive-compulsive symptom severity was measured in the OCD group using the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS; Goodman et al., 1989). OCD patients did not differ from HC in age, years of education, or gender (Table 1). All subjects provided informed written consent approved by the Mount Sinai Institutional Review Board.
Table 1.
Demographic, clinical, and behavioral information.
| OCD (n=18) |
HC (n=18) |
|||
|---|---|---|---|---|
| mean | sd | mean | sd | |
| Demographic and clinical data | ||||
| Age (years) | 28.2 | 7.1 | 27.2 | 6.5 |
| Education (years) | 17.4 | 1.9 | 16.6 | 1.5 |
| Sex | 11F/7M | 10F/8M | ||
| BAI* | 21.4 | 12.8 | 3.0 | 6.1 |
| BDI* | 11.8 | 7.4 | 1.4 | 1.9 |
| Y-BOCS | 19.9 | 5.5 | ||
| Behavioral data | ||||
| Percent errors | ||||
| TD-negative IF* | 3.1 | 1.6 | 1.1 | 1.2 |
| TD-positive IF | 2.8 | 1.9 | 1.9 | 1.8 |
| TD-EF | 2.6 | 2.0 | 2.4 | 1.7 |
| TD-rest* | 3.2 | 2.2 | 1.6 | 1.4 |
| Reaction time (ms) | ||||
| TD-negative IF | 517.9 | 33.3 | 511.8 | 38.6 |
| TD-positive IF | 523.3 | 41.2 | 512.1 | 34.5 |
| TD-EF | 524.6 | 35.7 | 509.3 | 34.7 |
| TD-rest | 521.0 | 37.0 | 506.6 | 33.0 |
| Ratings of ease (1–5) | ||||
| Negative IF | 3.8 | 0.69 | 3.9 | 0.66 |
| Ppositive IF | 4.0 | 0.51 | 4.1 | 0.67 |
| EF | 3.9 | 0.74 | 4.2 | 0.78 |
| Rest | 3.8 | 0.85 | 4.2 | 0.88 |
| Ratings of emotion (1–5) | ||||
| Negative IF | 1.7 | 0.46 | 1.9 | 0.44 |
| Positive IF | 4.3 | 0.40 | 4.4 | 0.49 |
| EF | 3.0 | 0.35 | 3.1 | 0.30 |
| Rest | 3.1 | 0.42 | 3.3 | 0.57 |
OCD=obsessive-compulsive disorder, HC=healthy controls, sd=standard deviation.
There were no group differences in age, years of education, or sex. OCD patients showed significantly greater scores on the Beck Anxiety Inventory (BAI) and Beck Depression Inventory (BDI) than HC. For behavioral data, reaction times are for correct responses on TD blocks; ratings of ease were made on 5-point Likert scales where 1=“very difficult”, 3=“neutral”, and 5=“very easy”; ratings of emotion were made on 5-point Likert scales where 1=“very negative”, 3=“neutral”, and 5=“very positive”.
Difference between OCD and HC groups significant at p < 0.05 using two-sample t-tests.
2.2. Task overview
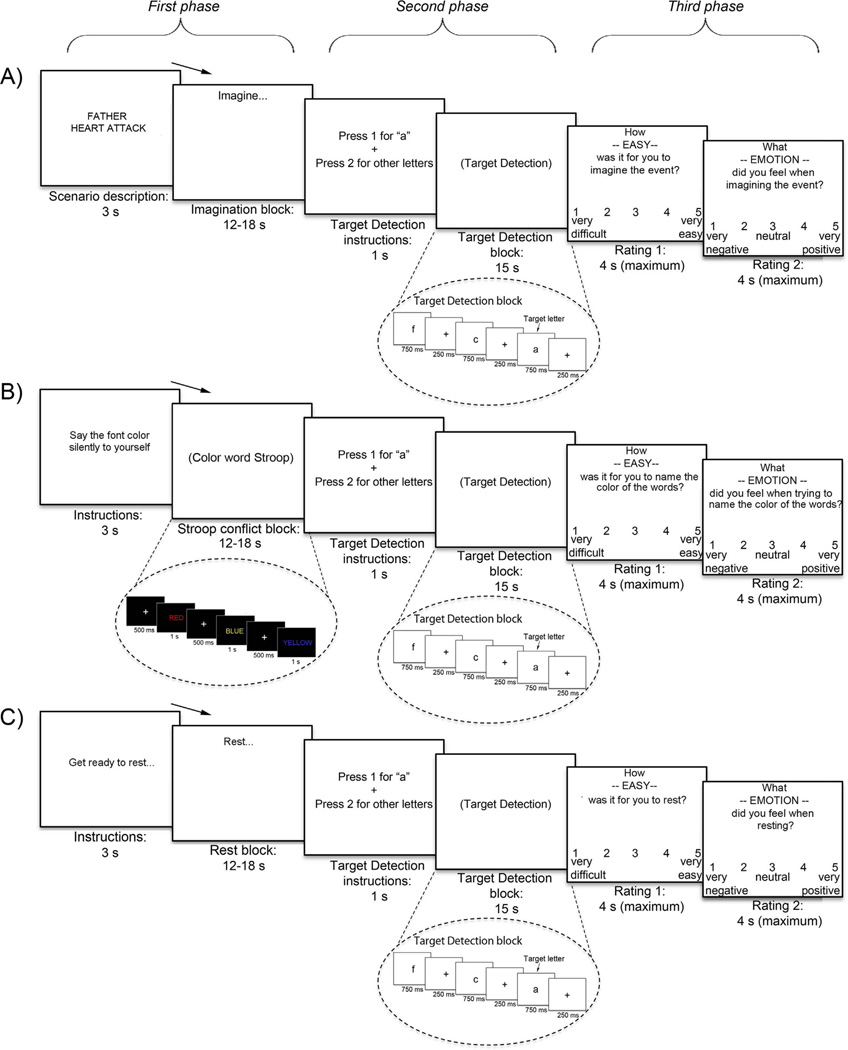
The attentional state switching task (Fig. 1) used a block design with 48 sequences, each with three phases (see Supplemental methods and Stern et al., (2015)). In the first phase of a sequence, subjects performed one of three “initial” tasks to set the attentional state: internally focused personal event imagination (IF block, including both negative events [negative IF] and positive events [positive IF]), externally focused Stroop conflict task (EF block), or eyes-open rest. After observing a 3 s instruction screen, the duration of each task was 15 s on average, jittered in 1.5 s increments between 12 and 18 s. Following each of these initial tasks, subjects switched to an externally focused target detection task (TD block) for 15 s (second phase). Critically, TD blocks were identical regardless of whether they followed negative IF, positive IF, EF, or rest. In the third phase, following the TD block, subjects rated how easy it was to perform the initial (first phase) task (e.g., to imagine the events, perform the Stroop, or rest, from very difficult to very easy) and also rated their emotion (from very negative to very positive) using 5-point Likert scales (maximum 4 s per rating) (see Supplemental methods for details on ratings). Following a fixation cross (jittered between 0–8 s), a new sequence began. Each sequence type (negative IF, positive IF, EF, and rest) was presented 12 times over 6 runs. Subjects were instructed to keep their eyes open and looking at the screen during the entire task, which was confirmed by a camera focused on the eyes. Following the task, subjects answered debriefing questions where they rated the difficulty of the different task components (see Supplement).
Fig. 1.
Subjects performed one of three initial tasks prior to switching to target detection (TD): A) internally focused (IF) event imagination (half negative and half positive events), B) externally focused (EF) Stroop conflict task, and C) rest. After TD, subjects rated the ease and emotion associated with the initial tasks.
2.3. Task details
2.3.1. Internal focus (IF) block (first phase)
During IF sequences, subjects imagined different event scenarios prior to switching to perform target detection. Each IF block started with a 2–4 word cue describing the event to be imagined, followed by the word “Imagine” at the top of the screen for the duration of the block. Event scenarios were personalized for each subject. During a screening session, subjects worked with an experimenter to prepare a list of 12 personally relevant life events that included six positive events (i.e., events that participants are looking forward to or those that they want to happen) and six negative events (i.e., events that participants are not looking forward to or those that they are worried about happening). Examples of negative scenarios were “Mother’s cancer returns” and “Boyfriend loses job”; examples of positive scenarios were: “PhD program acceptance” and “Cruise to Bahamas”. In the scanner, subjects imagined each of the 6 negative and 6 positive events two times over the course of the experiment. Controls and OCD patients received the same exact instructions for scenario creation, thus patients were not required to construct their scenarios around their specific OC symptoms. The reason for this was three-fold: 1) to probe more general attentional mechanisms in OCD, 2) to match the intensity and thematic content of negative and positive scenarios within OCD patients as much as possible, and 3) to match the intensity and thematic content of negative scenarios between patients and controls as much as possible. Supplemental methods provide additional details on how scenarios were matched within and between subjects.
2.3.2. External focus (EF) block (first phase)
During EF sequences, subjects were presented with a series of 8–12 color words (e.g. “RED”, “BLUE”, “YELLOW”, “GREEN”) that were either congruent (35%) or incongruent (65%) with the font color in which they were written. Subjects were instructed to ignore the meaning of the word and to covertly identify the color of the word as quickly as possible using subvocalization. Each word presented for 1000ms followed by crosshairs for 500ms before the next word appeared for the duration of the EF block. Subvocalization was used instead of button press responses in order to match IF and EF blocks as closely as possible.
2.3.3. Target detection (TD) block (second phase)
After performing the initial tasks (negative IF, positive IF, EF, or rest), subjects switched to perform the TD task. The TD task presented 15 sequential letters, and subjects were required to press one button for the target letter “a” (~30% of letters) and another button for all other letters. Letters were presented for 750ms followed by a crosshair for 250ms until the next letter appeared, for the duration of the TD block. TD blocks were identical regardless of the initial task preceding it.
2.4. Neuroimaging data acquisition and preprocessing
MRI scanning occurred on a Siemens Allegra 3T scanner. After sagittal localization, 6 runs of functional images were acquired with a T2*-weighted, gradient echo planar sequence (repetition time=2000 ms, echo time=30 ms, 36 slices, 3-mm thick, skip=1, flip angle=90°, field-of-view=210 mm, matrix size=64×64). A high-resolution T1-weighted anatomical image was also acquired.
Preprocessing of functional data utilized Statistical Parametric Mapping software (SPM8) and included (in order): slice-time correction, realignment of functional images, coregistration of functional images to anatomical image, normalization to MNI152 template, and spatial smoothing with a 5-mm Gaussian kernel. Due to intrinsic spatial smoothness, the total average smoothness for contrasts of interest was between 10 and 10.5-mm FWHM.
2.5. Data Analysis
2.5.1. Behavioral
Behavioral analysis of “switch costs” (Gilbert and Shallice, 2002; Monsell, 2003) related to different initial task conditions examined percent errors and reaction time (RT) on correct trials during target detection in separate repeated-measures ANOVAs using group (OCD, HC) as between-subjects factor and initial task (negative IF, positive IF, EF, rest) as within-subjects factor. Mean ratings of ease and emotion were also examined in separate ANOVAs using the same factors. Significant main effects were interrogated with post-hoc t-tests. Post-task debriefing questions were compared using t-tests (see Supplement).
2.5.2. Neuroimaging
2.5.2.1. Task-related activation
For neuroimaging analyses, a general linear model (GLM) (SPM8) was used to specify regressors for negative IF, positive IF, EF, and rest blocks (at time of cue) and for TD blocks based on prior attentional state (TD-negative IF, TD-positive IF, TD-EF, and TD-rest). Block durations of 15 s on average were optimized to distinguish activity elicited by TD blocks from that occurring during initial task blocks (see Supplemental methods). Regressors were convolved with the canonical hemodynamic response function, and all blocks were modeled as epochs with durations set to block length (12–18 s), thus capturing neural activity related to processing “mode” rather than a discrete neural event at the time of the switch (see Stern et al., (2015)). Rating periods and six realignment parameters were also included in the GLM to reduce error variance. Scans showing movement spikes of over 3 mm translation or 2° rotation were excluded and interpolated using ArtRepair (Mazaika et al., 2007). Average translation and rotation over the 6 runs was not significantly different between OCD and HC groups. Imaging comparisons focused on contrasts of OCD with HC during target detection, separately for each prior attentional state (TD-negative IF, TD-positive IF, TD-EF, TD-rest) using two sample t-tests, cluster-level corrected for multiple comparisons within whole-brain gray matter at α=0.05 (voxelwise t=2.4) using Monte Carlo simulations (Ward, 2000). We do not report results from “double contrasts” (e.g., OCD > HC for TD-negative IF > TD-rest), which typically identify brain regions showing crossover interactions that are not of primary interest for the present investigation (e.g., those regions that are jointly increased for OCD relative to HC during TD negative-IF and decreased for OCD relative HC during TD-rest). However, in order to further investigate the specificity of effects identified from group comparisons, we extracted parameter estimates from regions showing group differences in the single contrasts and submitted them to group (OCD vs. HC)×TD condition (TD-negative IF, TD-positive IF, TD-EF, TD-rest) ANOVAs. Secondary analyses of contrasts between OCD patients and HC for the initial tasks (negative IF, positive IF, and EF) are reported in Table 2 and section 3.2.4, and contrasts between TD conditions within each group are reported in Table 3, section 3.2.5, and further discussed in S.3.3.
Table 2.
Activations during initial task blocks of negative IF, positive IF, and EF for healthy controls (HC) and OCD patients. All conditions are compared to an implicit baseline (fixation cross in between sequences).
| Condition/Region (Side) | BA | k | x | y | z | Max z | k | x | y | z | Max z | Network |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | OCD | |||||||||||
| Negative IF | ||||||||||||
| VMPFC (B) | 11 | 105 | −4 | 36 | −20 | 4.0 | DMN | |||||
| Anterior MFC, DMPFC (B) | 10, 9 | 182 | −8 | 60 | 32 | 3.5 | DMN, TPN | |||||
| Inferior frontal, OFC (L) | 47, 45 | 487* | −48 | 30 | −6 | 4.5 | 135 | −48 | 26 | −4 | 3.3 | TPN |
| 33 | −44 | 28 | −14 | 3.0 | None | |||||||
| Mid frontal, precentral (L) | 9, 6 | 34 | −40 | 2 | 40 | 3.1 | None | |||||
| Supplementary motor area (B) | 6 | 332* | −4 | 4 | 62 | 4.2 | 100 | −4 | 8 | 58 | 3.4 | TPN |
| PCC, precuneus | 31, 23, 30 | 381* | −8 | −58 | 16 | 4.4 | 63 | −4 | −62 | 30 | 3.0 | DMN |
| Mid temporal, temporal pole (L) | 21, 20 | 91 | −52 | −2 | −30 | 3.8 | 82 | −54 | −16 | −14 | 3.7 | DMN, TPN |
| 72 | −66 | −26 | −8 | 3.1 | 77 | −56 | −30 | −6 | 3.3 | DMN, TPN | ||
| Temporal pole (L) | 38 | 37 | −46 | 4 | −46 | 3.6 | 41 | −44 | 6 | −46 | 3.3 | None |
| Temporal pole (R) | 21, 38 | 69 | 48 | 10 | −32 | 3.4 | None | |||||
| Cuneus (R) | 18 | 23 | 20 | −84 | 16 | 3.2 | TPN | |||||
| Cerebellum (R) | n/a | 31 | 20 | −90 | −46 | 3.6 | None | |||||
| Positive IF | ||||||||||||
| VMPFC (B) | 11, 10 | 459* | −8 | 40 | −12 | 5.0 | 62 | −6 | 42 | −12 | 3.2 | DMN, TPN |
| Inferior frontal, OFC (L) | 47 | 109 | −42 | 28 | −14 | 4.3 | None | |||||
| Supplementary motor area, medial frontal gyrus (B) | 6, 32 | 221 | −4 | 4 | 62 | 3.9 | 45 | −8 | 6 | 54 | 3.6 | TPN |
| 6 | 29 | 6 | 6 | 64 | 3.3 | None | ||||||
| PCC (B) | 31, 23, 30 | 529* | −8 | −56 | 22 | 4.4 | DMN | |||||
| Mid temporal (L) | 21, 20 | 72 | −58 | −6 | −22 | 3.8 | DMN, TPN | |||||
| Inferior temporal (R) | 20 | 45 | 54 | −18 | −26 | 3.3 | None | |||||
| Temporal pole (L) | 21, 38 | 34 | −44 | 12 | −36 | None | ||||||
| PG, hippocampus (L) | 28 | 143 | −22 | −14 | −24 | 3.8 | DMN | |||||
| PG (R) | 36 | 47 | 34 | −24 | −18 | 4.1 | DMN | |||||
| PG (R) | 36 (37) | 37 | 24 | −34 | −22 | 3.7 | DMN | |||||
| Fusiform gyrus, PG (L) | 20, 37, 36 | 63 | −26 | −36 | −20 | 3.7 | DMN, TPN | |||||
| Cerebellum (R) | n/a | 33 | 38 | −70 | −36 | 3.9 | None | |||||
| EF (Stroop conflict) | ||||||||||||
| Precentral gyrus (L) | 6, 4 | 119 | −52 | 0 | 54 | 4.4 | 36 | −54 | 0 | 48 | 3.6 | TPN |
| Precentral gyrus (R) | 6, 4 | 98 | 58 | −6 | 46 | 5.2 | TPN | |||||
| Inferior frontal, mid frontal, precentral (L) | 6, 9 | 207 | −42 | 2 | 34 | 3.7 | 103 | −42 | 8 | 30 | 3.3 | TPN |
| Supplementary motor area, medial frontal gyrus (B) | 6, 32, 24 | 198 | −6 | 2 | 60 | 4.1 | 97 | −4 | 4 | 60 | 3.9 | TPN |
| Superior parietal (L) | 7 | 66 | −30 | −58 | 46 | 3.3 | TPN | |||||
| Mid, inferior occipital (L) | 18, 19, 17 | 1244* | −42 | −62 | −14 | 4.4 | 678* | −20 | −94 | −18 | 4.5 | TPN |
| Mid, inferior occipital (R) | 18, 19, 17 | 466* | 36 | −86 | −4 | 4.2 | 668* | 26 | −94 | −6 | 5.6 | TPN |
| Fusiform gyrus, cerebellum (R) | 37 | 61 | 34 | −58 | −18 | 4.0 | TPN | |||||
Negative IF=negative internal focus; Positive IF=positive internal focus; EF=external focus; DMPFC=dorsomedial prefrontal cortex; MFC=medial frontal cortex; OFC=orbitofrontal cortex; PG=parahippocampal gyrus; PCC=posterior cingulate cortex; VMPFC=ventromedial prefrontal cortex.
BA=Brodmann's areas; k=number of voxels; L=left; R=right; B=both hemispheres; coordinates are in MNI space. “Network” column indicates to which of two intrinsic networks identified in a separate resting-state functional connectivity data set, if any, each cluster belongs (see Fig. S1, default mode network: DMN, task-positive network: TPN). Data are thresholded at p < 0.005/k=20 (uncorr).
Asterisks denote regions that are significantly greater for the initial task vs. baseline, cluster-level corrected for whole-brain comparisons at p < 0.05 using Monte Carlo stimulations and a cluster-defining threshold of p < 0.005. There were no significant differences between OCD patients and HC.
Table 3.
Comparisons between TD-EF and TD-IF for healthy controls (HC) and OCD patients.
| Condition/Region (Side) | BA | k | x | y | z | Max z | k | x | y | z | Max z | Network |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HC | OCD | |||||||||||
| TD-EF > TD-negative IF | ||||||||||||
| Inferior frontal/dorsal insula (R) | n/a | 36 | 48 | 10 | 12 | 3.6 | None | |||||
| Middle frontal (L) | n/a | 21 | −32 | 30 | 18 | 3.3 | None | |||||
| Superior temporal (R) | n/a | 29 | 52 | −46 | 16 | 2.9 | None | |||||
| Superior occipital/precuneus (L) | 7, 19, 31 | 54 | −16 | −76 | 28 | 3.9 | 84 | −32 | −86 | 26 | 4.3 | TPN |
| Superior occipital/cuneus (R) | 19, 31 | 44 | 24 | −84 | 24 | 3.6 | TPN | |||||
| Middle occipital (R) | 19, 39 | 70 | 38 | −68 | 14 | 4.1 | 79 | 42 | −78 | 10 | 3.1 | TPN |
| Middle occipital (L) | N/A | 29 | −34 | −72 | 6 | 4.0 | None | |||||
| Middle temporal/occipital (R) | 19, 37 | 77 | 54 | −60 | −2 | 3.1 | TPN | |||||
| Lingual/inferior occipital (B) | 18 | 461 | −6 | −80 | −8 | 4.2 | TPN | |||||
| Lingual/cerebellum (B) | 18 | 387 | 16 | −90 | 22 | 3.9 | TPN | |||||
| Lingual/cerebellum (R) | 18, 19 | 260 | 18 | −70 | −20 | 3.8 | TPN | |||||
| Fusiform/cerebellum (R) | 45 | 24 | −50 | −18 | 3.4 | None | ||||||
| TD-EF > TD-positive IF | ||||||||||||
| DLPFC (R) | 46 | 28 | 42 | 34 | 14 | 2.8 | TPN | |||||
| Superior frontal (R) | 8 | 23 | 22 | 14 | 52 | 3.6 | DMN, TPN | |||||
| Precentral (R) | 6 | 91 | 42 | −6 | 38 | 3.5 | TPN | |||||
| Inferior frontal/precentral (R) | 44 | 44 | 50 | 10 | 12 | 3.0 | TPN | |||||
| Anterior cingulate (L) | 24, 32 | 39 | −14 | 10 | 36 | 3.9 | None | |||||
| Mid-cingulate (L) | 24 | 34 | −16 | −8 | 42 | 3.5 | TPN | |||||
| Mid-insula/superior temporal (R) | 22 | 28 | 50 | 0 | 2 | 3.2 | None | |||||
| Middle temporal/occipital (R) | 19, 37, 39 | 78 | 42 | −68 | 14 | 4.2 | DMN, TPN | |||||
| 71 | 50 | −62 | −2 | 3.5 | TPN | |||||||
| Middle occipital/temporal (R) | 19 | 192 | 36 | −84 | 8 | 3.7 | TPN | |||||
| Middle occipital/angular gyrus (L) | 39 | 75 | −32 | −76 | 26 | 3.3 | DMN, TPN | |||||
| Precuenus (L) | 31 | 22 | −22 | −72 | 18 | 3.4 | None | |||||
| PCC/cuneus (R) | 30 | 52 | 22 | −64 | 8 | 3.6 | None | |||||
| Cuneus/superior occipital (L) | 7, 18, 19 | 185 | −12 | −88 | 22 | 3.8 | TPN | |||||
| Cuneus (R) | 18, 19 | 25 | 6 | −88 | 26 | 3.0 | 116 | 14 | −90 | 24 | 3.4 | TPN |
| Lingual/inferior occipital (B) | 18 | 95 | 2 | −78 | 0 | 4.0 | 21 | −20 | −76 | −14 | 3.0 | None |
| 20 | −18 | −72 | 2 | 2.9 | TPN | |||||||
| 194 | −2 | −74 | −6 | 3.9 | TPN | |||||||
| TD-negative IF > TD-EF | ||||||||||||
| Caudate (R) | n/a | 25 | 12 | 18 | 12 | 3.1 | None | |||||
| Temporal pole (R) | 38 | 34 | 38 | 8 | −40 | 3.5 | None | |||||
| Lingual/inferior occipital (L) | 17, 18 | 166 | −20 | −90 | −6 | 4.0 | 24 | −18 | −94 | −12 | 3.4 | None |
| 20 | −24 | −98 | 4 | 3.4 | TPN | |||||||
| Cuenus (R) | 17 | 22 | 18 | −96 | −4 | 2.8 | 48 | 18 | −98 | −4 | 4.1 | TPN |
| TD-positive IF > TD-EF | ||||||||||||
| Posterior insula (L) | 13 | 21 | −36 | −22 | 22 | 3.3 | None | |||||
| Caudate/corpus callosum | n/a | 22 | 2 | −6 | 18 | 3.1 | TPN | |||||
| Hippocampus/PG | 27 | 43 | −18 | −40 | 4 | 3.3 | DMN | |||||
| PCC/cuneus (L) | n/a | 23 | −22 | −54 | 26 | 3.5 | None | |||||
| Middle occipital (R) | n/a | 21 | 26 | −68 | 6 | 4.1 | None | |||||
| Lingual/cuneus (R) | 17, 18 | 101 | 20 | −98 | −4 | 3.7 | 58 | 20 | −98 | −4 | 3.5 | TPN |
| Lingual/inferior occipital (L) | 17, 18 | 45 | −18 | −90 | −4 | 3.5 | 28 | −24 | −96 | 2 | 3.3 | TPN |
TD-EF=TD following external focus; TD-negative IF=TD following negative internal focus; TD-positive IF=TD following positive internal focus; DLPFC=dorsolateral prefrontal cortex; PG=parahippocampal gyrus; PCC=posterior cingulate cortex.
BA=Brodmann's areas; k=number of voxels; L=left; R=right; B=both hemispheres; coordinates are in MNI space. “Network” column indicates to which of two intrinsic networks identified in a separate resting-state functional connectivity data set, if any, each cluster belongs (see Fig. S1, default mode network: DMN, task-positive network: TPN). Data are thresholded at p < 0.005/k=20 (uncorr). There were no whole-brain corrected effects (see Supplement S3.3).
2.5.2.2. Intrinsic functional connectivity analysis
To determine whether group differences found during TD were associated with differences in intrinsic connectivity, the timecourse of the BOLD signal across the entire scan was extracted from the region showing a significant group×TD condition interaction (inferior occipital cluster, see Section 3) and used as a seed for connectivity analyses using the conn toolbox (Whitfield-Gabrieli and Nieto-Castanon, 2012). At the individual subject level, partial correlations were conducted between the timecourse of this seed and whole-brain gray matter, controlling for several factors. Following previous approaches for estimating intrinsic connectivity from task data (Fair et al., 2007; Stern et al., 2011), we regressed out BOLD signal related to all task regressors to remove variance associated with task events from connectivity measures. In addition, timecourses from the top three principle components within white matter and CSF were determined using the “CompCor” method (Behzadi et al., 2007) and included as covariates to control for noise correlations without having to regress out global signal (Murphy et al., 2009). Finally, in addition to removing task-related activity and noise correlations present in WM and CSF, 12 motion variables (6 realignment parameters and first derivatives) were included as covariates to control for movement. Data were filtered between .01 and 0.10 Hz. Partial correlation coefficient images between the seed's timecourse and the whole brain were computed and z-transformed. Second (group) level analyses used two-sample t-tests to compare z-transformed partial correlation coefficient images between OCD and HC using cluster level correction for multiple comparisons at α=0.05 (Ward, 2000). The connectivity patterns derived from this type of analysis are frequently described as intrinsic because this method identifies interregional coupling that is independent of and linearly superimposed upon task-related activity (Fair et al., 2007; Fox et al., 2006).
2.5.3. Aligning task activations with intrinsic networks
To identify overlap in the topography between regions found in group comparisons and previously reported intrinsic brain networks, we created masks of TPN and DMN from a functional connectivity analysis performed on resting-state data obtained in an independent sample of 17 healthy individuals (see Stern et al., (2012; 2015) for methodological details of the analysis). Coordinates for seeds used to create masks of TPN and DMN were taken from prior work on intrinsic brain connectivity (Vincent et al., 2008). For TPN, bilateral seeds in the “dorsal attention network” (middle temporal area and superior parietal lobule) and “fronto-parietal network” (anterior prefrontal cortex and anterior inferior parietal lobule) were selected. For DMN, bilateral seeds in hippocampal formation and posterior inferior parietal lobule were selected. Connectivity maps for each seed were averaged for a given network (i.e., the TPN connectivity map was an average of 8 bilateral seeds; the DMN map was an average of 4 bilateral seeds) and thresholded at α=0.05, corrected for whole-brain comparisons (Fig. S1). Task-related activations were overlaid on these masks to visually map the present findings to well-defined networks from the literature.
2.5.4. Effects of medication
Given that most OCD patients were taking SRI medication, a secondary analysis was conducted to investigate whether differences in medication were affecting any of the identified group differences. Multiple linear regressions using both group and fluoxetine equivalence dosage as independent variables were run for all significant effects (behavioral and fMRI; see Supplemental results). Examination of p-values for the group variable when including dosage in the model determined whether group continued to predict the dependent variable (behavior/fMRI) after statistically adjusting for effects of dosage. Although this approach will identify whether unique sources of variance in the group factor are related to the dependent variable when removing shared variance with the medication factor, the rather high correlation between group and medication means that this unique (non-shared) variance may not be a good representation of the group construct on the whole (Miller and Chapman, 2001). In order to further investigate the effects of medication using an approach that does not suffer from this limitation, we performed correlational analysis between medication dosage and behavioral/fMRI effects within the OCD group alone. As described in the Supplement, the majority of group differences reported below remained highly significant in multiple regression analyses (suggesting that the results are indeed related to unique variance associated with group) and were not correlated with dosage within OCD patients.
3. Results
3.1. Behavioral
3.1.1. Accuracy and reaction time during target detection
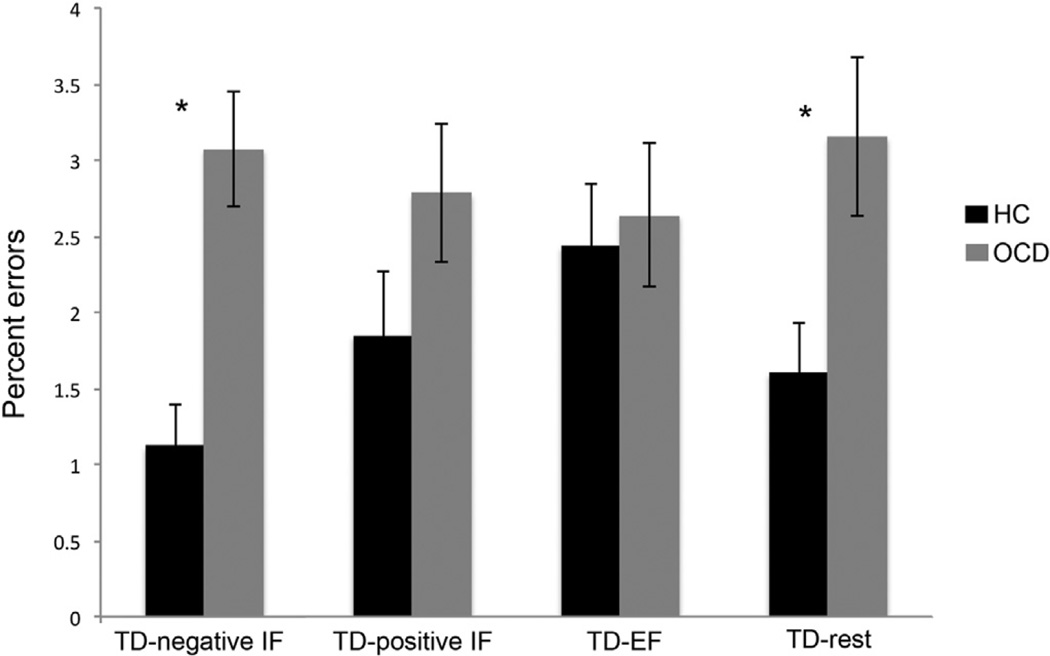
Both groups were generally very accurate during the TD block (Table 1, 2.3% errors on average), although OCD patients (2.9%) made more errors than HC (1.8%) overall (F1,34=5.2, p=0.028). There was also a significant interaction between group (OCD, HC) and prior attentional state (negative IF, positive IF, EF, rest) on percent errors during TD (F3, 102=5.1, p=0.003). Post-hoc t-tests indicated that OCD patients made significantly more errors than HC during TD when it followed negative IF (TD-negative IF: t34=4.2, p < 0.001) and when it followed rest (TD-rest: t34=2.5, p=0.018) (Fig. 2), but did not differ when it followed positive IF (TD-positive IF) or EF (TD-EF). Interestingly, the HC group made more errors during TD-EF (2.4%) than TD-negative IF (1.1%; t17=4.1, p=0.001), a pattern that was (non-significantly) reversed in the OCD group (TD-EF: 2.6%, TD-negative IF: 3.1%).
Fig. 2.
OCD patients made significantly more errors than HC during TD following negative IF and TD following rest (denoted with asterisks).
Analysis of RT for correct responses during TD revealed no significant main effects or interactions.
3.1.2. Trial-by-trial ratings of ease and emotion for initial tasks
Subjects rated the initial tasks as fairly easy (mean rating for all subjects=4.0 on a scale where 1=“very difficult” and 5=“very easy”, Table 1), and there were no significant effects of group, attentional state, or interaction between factors on perceived difficulty.
For ratings of emotion experienced during initial tasks, OCD patients gave more negative (less positive) emotion ratings compared to HC overall (group effect: F1,34=4.3, p=0.045, Table 1). There was also a highly significant main effect of attentional state on emotion ratings (F3,102=204.1, p < 0.001), with negative and positive IF blocks (imagining future negative and positive events) differing from each other and from all other block types (p < 0.001 for all comparisons), as would be expected if subjects were performing the task as instructed. Emotion ratings for EF and rest blocks did not differ significantly from each other and were generally given a neutral rating (Table 1). There was no interaction between group and attentional state in emotion ratings.
3.2. Neuroimaging
3.2.1. Target detection based on prior attentional state
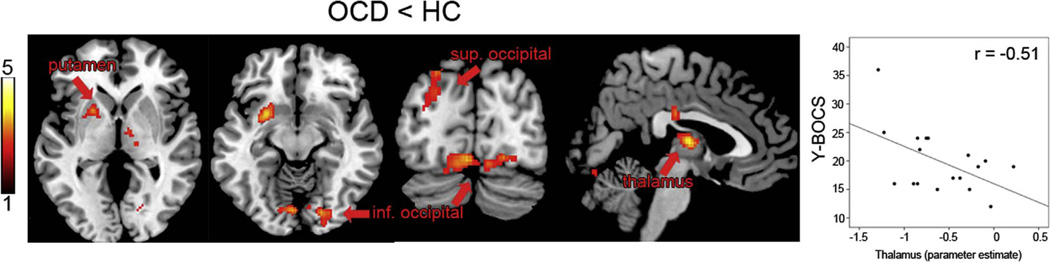
OCD patients exhibited significantly less activity in bilateral occipital cortex (left superior and bilateral inferior), bilateral thalamus, and left putamen than HC during TD-negative IF (Fig. 3). Clusters in occipital cortex and thalamus overlapped with the intrinsic TPN mask; the putamen cluster did not overlap with either the DMN or TPN mask. There were no significant group differences in activation during TD-positive IF, TD-EF, or TD-rest.
Fig. 3.
OCD patients showed reduced activation compared to controls in bilateral occipital cortex (left superior: −24, −92, 26, k = 329; bilateral inferior: 24, −86, −18, k = 480), left putamen (−28, 4, −12, k = 356), and bilateral thalamus (0, −10, 8, k = 338) during TD following negative IF. The inferior occipital cluster showed a significant interaction between group and TD condition (F (3,102) = 3.8, p = 0.023). Color bar represents t score.
Follow-up group (OCD vs. HC)×TD condition (TD-negative IF, TD-positive IF, TD-EF, TD-rest) ANOVAs tested whether effects in the above four clusters were specific to the TD-negative IF condition, as might be expected given that group comparisons in the other TD conditions did not identify any significant differences. Only the inferior occipital cluster showed a significant interaction (F3,102=3.8, p=0.023, Greenhouse-Geisser corrected). Although the other three clusters were significantly hypoactive in OCD only during the TD-negative IF condition, they exhibited interaction p-values > 0.20, suggesting that subthreshold group differences between OCD and HC may also be present in the other TD conditions.
To further unpack the interaction effect in inferior occipital cortex, we conducted ANOVAs comparing OCD-related hypoactivation during TD-negative IF with the other TD conditions in a pairwise fashion. There were significant interactions between group and TD-negative IF vs. TD-rest (F1,34=5.5, p=0.025), group and TD-negative IF vs. TD-EF (F1,34=5.5, p=0.024), and a marginally significant interaction between group and TD-negative IF vs. TD-positive IF (F1,34=3.2, p=0.081); all of these effects were in the direction of greater hypoactivation in OCD for TD-negative IF than in the comparison TD condition.
3.2.2. Relationship with symptom severity
Correlations between brain activation and Y-BOCS scores within OCD patients were interrogated using parameter estimates extracted from those regions showing group differences. Symptom severity was negatively correlated with activity in the thalamus cluster (r=−0.51, p=0.030), indicating that patients with higher symptom severity showed reduced thalamic activation. The left putamen cluster was also negatively correlated at trend level (r =−0.41, p=0.095). Occipital clusters were not significantly related to Y-BOCS scores, and none of the four clusters correlated with general anxiety or depression scores (BAI/BDI).
3.2.3. Intrinsic functional connectivity
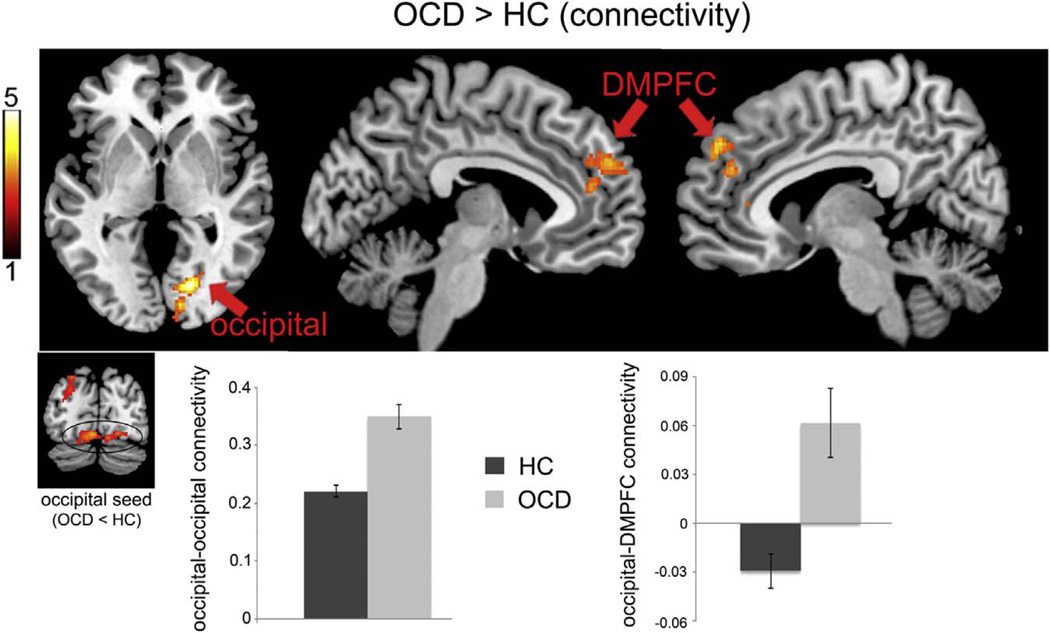
Within both OCD and HC groups, there were patterns of significant positive connectivity between the inferior occipital region that was hypoactive in OCD and areas of the TPN, including adjacent regions of inferior and superior occipital cortex, superior parietal cortex, precentral and postcentral gyri, supplementary motor area, DLPFC and inferior frontal gyrus, dorsal ACC and mid-cingulate, and anterior insula. There was also significant connectivity between the inferior occipital cortex seed and brain areas outside of the TPN mask, including bilateral cerebellum, thalamus and midbrain, posterior insula, and orbitofrontal cortex; connectivity with parahippocampal gyrus and posterior cingulate cortex in areas that overlapped with the DMN was also observed. Group comparisons revealed stronger connectivity in OCD between the inferior occipital cortex seed and dorsomedial prefrontal cortex (DMPFC) including voxels that overlapped both with DMN (in anterior-superior areas of the cluster) and TPN (in posterior-inferior areas of the cluster) (Fig. 4). Examination of z-transformed partial correlation coefficients indicated that HC showed an overall pattern of weak negative connectivity between inferior occipital cortex and DMPFC while OCD showed positive connectivity (Fig. 4). In addition, OCD patients showed stronger positive connectivity between the inferior occipital seed and adjacent areas of occipital cortex. There were no regions where HC showed significantly stronger connectivity with inferior occipital cortex compared to OCD.
Figure 4.
Intrinsic functional connectivity analysis revealed greater connectivity in OCD compared to controls between inferior occipital cortex and adjacent occipital regions (18, −76, 0, k = 324) as well as dorsomedial prefrontal cortex (DMPFC, 6, 58, 10, k = 382). Y-axes represent z-transformed correlation coefficients. Color bar represents t score.
3.2.4. Initial task activation
Although not a primary focus, we also compared OCD patients and HC during initial task blocks. Both groups showed activation overlapping with DMN and TPN masks during the negative and positive IF (event imagination) blocks, whereas activation during EF (Stroop) blocks overlapped with TPN only (Fig. S2 and Table 2), as expected from prior work on IF and EF cognition (e.g., Andrews-Hanna, 2012; Buckner et al., 2008; Corbetta and Shulman, 2002; Spreng et al., 2009; Stern et al., 2015). Although the strength and extent of activations appeared to differ somewhat between groups, particularly for the positive IF condition, there were no significant group differences for any of the initial task conditions.
3.2.5. Comparisons between TD-IF and TD-EF within each group
Secondary analyses of contrasts comparing TD-EF with TD-negative and TD-positive IF were performed in each group to compare with prior results (Stern et al., 2015). Unlike in this prior study, there were no whole-brain corrected differences between TD conditions in either group (Table 3 shows uncorrected) condition differences; See Supplement S3.3 for further discussion).
4. Discussion
To our knowledge, this is the first study to investigate behavior and brain function in OCD patients during an external cognition task based upon prior attentional state. The current study builds on an emerging literature from the field of cognitive neuroscience linking large-scale brain networks to different attentional states (Andrews-Hanna et al., 2014; Buckner et al., 2008; Corbetta and Shulman, 2002; D’Argembeau et al., 2008; Gusnard et al., 2001; Spreng et al., 2010), an approach that is highly relevant for understanding OCD both cognitively and neurobiologically. Compared to controls, OCD patients showed altered behavior and brain activation when performing an external task following engagement with a negative, internally focused state. Clinically, the idea that OCD patients should have difficulty shifting from a negative IF state to an external task may seem intuitively self-evident; however, the demonstration of this difficulty experimentally along with the identification of neural underpinnings is novel, and suggests that further consideration of how patients transition between attentional states may contribute to the understanding and treatment of OCD.
The primary behavioral indices of task switching are reaction times and error rates, with increases in these measures after a switch reflecting “switch costs” (Gilbert and Shallice, 2002; Monsell, 2003). In our study, OCD patients showed greater switch costs in the form of increased error rates during target detection when it followed a period of negative IF compared with controls, although there were no group differences in RT. Although it is possible that this increased error rate reflects a lack of task compliance rather than an increase in switch costs per se, several aspects of the data suggest that patients and HC were both performing the task as instructed. First, on each trial subjects rated the emotion they experienced during the initial tasks, and both groups (HC and OCD patients) rated the negative event imagination task as eliciting significantly more negative emotion than rest or the EF task, and the positive event imagination task as eliciting significantly more positive emotion than rest or the EF task (Section 3.1.2 and Table 1), as would be expected if they were following instructions. In addition, analysis of trial-by-trial ratings of ease of task performance revealed that OCD patients did not experience more difficulty engaging in the initial tasks than HC (Section 3.1.2, Table 1).
In addition to this behavioral effect during TD following negative IF, this was the only task condition where OCD patients showed significantly different brain activity compared to HC, with reduced activation in regions of superior and inferior occipital cortex, thalamus, and putamen, several of which overlapped with the intrinsic TPN mask. Post-hoc analyses indicated that OCD patients’ hypoactivation in the inferior occipital cortex was significantly greater for the TD-negative IF condition than the other TD conditions. Within the TPN, occipital cortex is part of the dorsal attention network (Vincent et al., 2008) and is linked predominantly with basic visual processes (Corbetta and Shulman, 2002; Smith et al., 2009; Tomasi et al., 2007). Given that all stimuli presented on the screen were identical for both groups, altered occipital activation is not likely to be related to differential visual stimulation, but may instead be due to altered attention to visual stimuli during target detection. It is well established that both striate and extrastriate visual regions can be modulated by attention (Hopfinger et al., 2000; Mangun et al., 1997; Martinez et al., 1999; Slotnick et al., 2003), and the present finding suggests that patients may not be appropriately engaging attention with external visual stimuli during TD specifically when it follows a period of negative internal thought. In healthy individuals, emotional distraction during cognitive processing is associated with reduced activation of lateral prefrontal regions (Anticevic et al., 2010; Dolcos and McCarthy, 2006). The present study's finding of intact lateral prefrontal but abnormal visual cortical activation suggests an alteration of more basic visual attentional mechanisms in OCD, consistent with recent hypotheses that OCD symptoms involve impairment in survival circuits related to visual processing (Goncalves et al., 2010; Goncalves et al., 2015).
Connectivity analysis further indicated that OCD patients showed stronger intrinsic connectivity between inferior occipital cortex and DMPFC in a region of DMN that has been associated with self-referential mental activity and theory of mind tasks (Andrews-Hanna et al., 2010; Gusnard et al., 2001; Spreng et al., 2009). Although speculative, a disruption in the intrinsic relationship between regions involved in externally-focused visual attention and internally-focused self processing could underlie OCD patients’ inability to incorporate external evidence of “safety” (e.g., observing that the door is locked or the hands are clean) to counteract internally generated and perpetuated fears (e.g., of someone breaking into the house or getting contaminated with a disease). The group difference in connectivity was driven by negative correlations between inferior occipital cortex and DMPFC in HC but positive correlations in OCD. This is consistent with previous research showing reduced negative correlations between TPN and DMN regions in OCD (Fitzgerald et al., 2010; Stern et al., 2012) and in relation to performance on EF cognitive tasks (Kelly et al., 2008; Wen et al., 2013).
Within the OCD group, activity during target detection following negative IF was negatively related to current symptom severity in the thalamus and putamen, but not in either of the occipital clusters. This suggests a potential dissociation between “state-related” (but non-specific) biomarkers in thalamus and putamen and a “trait-related” deficit in inferior occipital cortex during conditions requiring the refocusing of attention away from negative internal information.
Additional work is needed to compare OCD with other patient groups to determine whether these effects are specific to OCD or might represent transdiagnostic biomarkers. Generalized anxiety and major depression are also characterized by perseverative negative thought (in the form of rumination and worry) that is frequently resistant to correction by external information (Smith and Alloy, 2009; Topper et al., 2010). As such, this work may have relevance for multiple disorders characterized by excessive internal focus.
Although we have interpreted the findings in the context of switching between a negative internal focus (negative event imagination) and an external focus (target detection), an alternative interpretation is that OCD patients are impaired in processing external information after being exposed to negative emotional stimuli, regardless of attentional focus. As we did not run a full factorial design using negative and positive external stimuli for the initial tasks (and only used neutral stimuli for the EF task), this work cannot rule out the possibility that OCD patients would show similar alterations when switching from negative emotional stimuli that were externally focused (e.g., using an emotional Stroop task or a task with negative emotional pictures). Indeed, previous findings of altered brain activation in ventral and dorsal frontal regions during task switching between two (non-emotional) external tasks have been proposed to reflect a more general imbalance between emotional and cognitive functioning in the disorder (Kwon et al., 2009). It will be important for future work to test the specificity of the OCD switching impairment in relation to the type of negative emotional stimuli that are used, as this would not only inform a mechanistic understanding of disorder but could also have treatment implications.
There are several limitations of the present study. First, the majority of patients were medicated with SRIs. Although results from secondary analyses of medication effects provide support for the notion that group differences were not due to medication differences (see Supplement), the present design cannot determine what the group differences would have been had medication not been correlated with group status (Miller and Chapman, 2001). As such, these findings require replication in a larger unmedicated OCD cohort or the inclusion of a comparison group of medicated patients without OCD. In addition, in our protocol OCD patients constructed their to-be-imagined scenarios in the same way as control subjects, and were not required to use their specific obsessive fears for the task. Although this was done to increase comparability between patients and controls and between positive and negative scenarios (see Methods), it could potentially add a source of variance to the OCD group and it is possible (or even likely) that more extensive group differences would have been found if patients’ scenarios were related to their obsessive fears. Indeed, it could be interesting for future work to compare how OCD patients process general negative scenarios vs. OC-related scenarios. Finally, it would be interesting and important to determine whether performing this task, which involved engaging with negative (and positive) thoughts, alters clinical outcomes in OCD, something that we did not assess. Despite these limitations, these data provide new insights into the mechanisms of the disorder by using a novel task designed to dissect how patients transition between attentional states, with results pointing to a specific deficit in occipital activation during externally focused cognition following a period of negative internal focus. The findings are consistent with prior studies reporting reduced activation of TPN including occipital cortex during EF tasks in OCD (Gu et al., 2008; Kang et al., 2013; Remijnse et al., 2006) and provide support for the notion of impaired visual processing in the disorder (Goncalves et al., 2010; Nelson et al., 1993; Rampacher et al., 2010). The overall approach of examining the dynamics of EF and IF cognition is relevant for other internalizing disorders, and suggests future avenues for treatments that involve targeting transitions between modes of processing.
Supplementary Material
Acknowledgments
The project described was supported by Grant UL1 TR000067 from the National Center for Advancing Translational Sciences, a component of the National Institutes of Health, to Mount Sinai (KL2 TR00069 to ERS). The funding source had no role in study design, the collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication.
Footnotes
All authors declare no conflicts of interest.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.pscychresns.2016.08.006.
References
- Andrews-Hanna JR. The brain's default network and its adaptive role in internal mentation. Neuroscientist. 2012;18:251–270. doi: 10.1177/1073858411403316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain's default network. Neuron. 2010;65:550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna JR, Smallwood J, Spreng RN. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N.Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Repovs G, Barch DM. Resisting emotional interference: brain regions facilitating working memory performance during negative distraction. Cogn. Affect. Behav. Neurosci. 2010;10:159–173. doi: 10.3758/CABN.10.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J. Consult. Clin. Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, Liu TT. A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage. 2007;37:90–101. doi: 10.1016/j.neuroimage.2007.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang WL, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin card-sorting task and component processes. Hum. Brain Mapp. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Ann. N.Y. Acad. Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- D'Argembeau A, Xue G, Lu ZL, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcos F, McCarthy G. Brain systems mediating cognitive interference by emotional distraction. J. Neurosci. 2006;26:2072–2079. doi: 10.1523/JNEUROSCI.5042-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fair DA, Schlaggar BL, Cohen AL, Miezin FM, Dosenbach NU, Wenger KK, Fox MD, Snyder AZ, Raichle ME, Petersen SE. A method for using blocked and event-related fMRI data to study "resting state" functional connectivity. Neuroimage. 2007;35:396–405. doi: 10.1016/j.neuroimage.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson-Muth KC, Maynor MR, Welsh RC, Hanna GL, Taylor SF. Altered function and connectivity of the medial frontal cortex in pediatric obsessive-compulsive disorder. Biol. Psychiatry. 2010;68:1039–1047. doi: 10.1016/j.biopsych.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol. Psychiatry. 2005;57:287–294. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anti-correlated functional networks. Proc. Natl. Acad. Sci. USA. 2005;102:9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Zacks JM, Raichle ME. Coherent spontaneous activity accounts for trial-to-trial variability in human evoked brain responses. Nat. Neurosci. 2006;9:23–25. doi: 10.1038/nn1616. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Shallice T. Task switching: a PDP model. Cogn. Psychol. 2002;44:297–337. doi: 10.1006/cogp.2001.0770. [DOI] [PubMed] [Google Scholar]
- Goncalves OF, Marques TR, Lori NF, Sampaio A, Branco MC. Obsessive-compulsive disorder as a visual processing impairment. Med. Hypotheses. 2010;74:107–109. doi: 10.1016/j.mehy.2009.07.048. [DOI] [PubMed] [Google Scholar]
- Goncalves OF, Soares JM, Carvalho S, Leite J, Ganho A, Fernandes-Goncalves A, Frank B, Pocinho F, Relvas J, Carracedo A, Sampaio A. Brain activation of the defensive and appetitive survival systems in obsessive compulsive disorder. Brain Imaging Behav. 2015;9:255–263. doi: 10.1007/s11682-014-9303-2. [DOI] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS. The Yale-Brown Obsessive Compulsive Scale. I. Development, use, reliability. Arch. Gen. Psychiatry. 1989;46:1006–1011. doi: 10.1001/archpsyc.1989.01810110048007. [DOI] [PubMed] [Google Scholar]
- Gu BM, Park JY, Kang DH, Lee SJ, Yoo S, Jo HJ, Choi CH, Lee JM, Kwon JS. Neural correlates of cognitive inflexibility during task-switching in obsessive-compulsive disorder. Brain: J. Neurol. 2008;131:155–164. doi: 10.1093/brain/awm277. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc. Natl. Acad. Sci. USA. 2001;98:4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison BJ, Pujol J, Soriano-Mas C, Hernandez-Ribas R, Lopez-Sola M, Ortiz H, Alonso P, Deus J, Menchon JM, Real E, Segalas C, Contreras-Rodriguez O, Blanco-Hinojo L, Cardoner N. Neural correlates of moral sensitivity in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2012;69:741–749. doi: 10.1001/archgenpsychiatry.2011.2165. [DOI] [PubMed] [Google Scholar]
- Hopfinger JB, Buonocore MH, Mangun GR. The neural mechanisms of topdown attentional control. Nat. Neurosci. 2000;3:284–291. doi: 10.1038/72999. [DOI] [PubMed] [Google Scholar]
- Kang DH, Jang JH, Han JY, Kim JH, Jung WH, Choi JS, Choi CH, Kwon JS. Neural correlates of altered response inhibition and dysfunctional connectivity at rest in obsessive-compulsive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;40:340–346. doi: 10.1016/j.pnpbp.2012.11.001. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. Neuroimage. 2008;39:527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kwon JS, Jang JH, Choi JS, Kang DH. Neuroimaging in obsessive-compulsive disorder. Expert Rev. Neurother. 2009;9:255–269. doi: 10.1586/14737175.9.2.255. [DOI] [PubMed] [Google Scholar]
- Mangun GR, Hopfinger JB, Kussmaul CL, Fletcher EM, Heinze HJ. Covariations in ERP and PET measures of spatial selective attention in human extrastriate visual cortex. Hum. Brain Mapp. 1997;5:273–279. doi: 10.1002/(SICI)1097-0193(1997)5:4<273::AID-HBM12>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Martinez A, Anllo-Vento L, Sereno MI, Frank LR, Buxton RB, Dubowitz DJ, Wong EC, Hinrichs H, Heinze HJ, Hillyard SA. Involvement of striate and extrastriate visual cortical areas in spatial attention. Nat. Neurosci. 1999;2:364–369. doi: 10.1038/7274. [DOI] [PubMed] [Google Scholar]
- Mazaika P, Whitfield-Gabrieli S, Reiss AL. Artifact Repair for fMRI Data From High Motion Clinical Subjects. Presentation at Organization for Human Brain Mapping. 2007 [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J. Cogn. Neurosci. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Milad MR, Furtak SC, Greenberg JL, Keshaviah A, Im JJ, Falkenstein MJ, Jenike M, Rauch SL, Wilhelm S. Deficits in conditioned fear extinction in obsessive-compulsive disorder and neurobiological changes in the fear circuit. JAMA Psychiatry. 2013;70:608–618. doi: 10.1001/jamapsychiatry.2013.914. (quiz 554) [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. J. Abnorm. Psychol. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Monsell S. Task switching. Trends Cogn. Sci. 2003;7:134–140. doi: 10.1016/s1364-6613(03)00028-7. [DOI] [PubMed] [Google Scholar]
- Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? NeuroImage. 2009;44:893–905. doi: 10.1016/j.neuroimage.2008.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E, Early TS, Haller JW. Visual attention in obsessive-compulsive disorder. Psychiatry Res. 1993;49:183–196. doi: 10.1016/0165-1781(93)90104-o. [DOI] [PubMed] [Google Scholar]
- Power JD, Cohen AL, Nelson SM, Wig GS, Barnes KA, Church JA, Vogel AC, Laumann TO, Miezin FM, Schlaggar BL, Petersen SE. Functional network organization of the human brain. Neuron. 2011;72:665–678. doi: 10.1016/j.neuron.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampacher F, Lennertz L, Vogeley A, Schulze-Rauschenbach S, Kathmann N, Falkai P, Wagner M. Evidence for specific cognitive deficits in visual information processing in patients with OCD compared to patients with unipolar depression. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2010;34:984–991. doi: 10.1016/j.pnpbp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Remijnse PL, Nielen MMA, van Balkom AJLM, Cath DC, van Oppen P, Uylings HBM, Veltman DJ. Reduced orbitofrontal-striatal activity on a reversal learning task in obsessive-compulsive disorder. Arch. Gen. Psychiatry. 2006;63:1225–1236. doi: 10.1001/archpsyc.63.11.1225. [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59(Suppl 20):S22–S33. (quiz 34-57) [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, Buckner RL, Miezin FM, Raichle ME, Petersen SE. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997;9:648–663. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Slotnick SD, Schwarzbach J, Yantis S. Attentional inhibition of visual processing in human striate and extrastriate cortex. Neuroimage. 2003;19:1602–1611. doi: 10.1016/s1053-8119(03)00187-3. [DOI] [PubMed] [Google Scholar]
- Smith JM, Alloy LB. A roadmap to rumination: a review of the definition, assessment, and conceptualization of this multifaceted construct. Clin. Psychol. Rev. 2009;29:116–128. doi: 10.1016/j.cpr.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. Proc. Natl. Acad. Sci. USA. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim AS. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: a quantitative meta-analysis. J. Cogn. Neurosci. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Stevens WD, Chamberlain JP, Gilmore AW, Schacter DL. Default network activity, coupled with the frontoparietal control network, supports goal-directed cognition. NeuroImage. 2010;53:303–317. doi: 10.1016/j.neuroimage.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Fitzgerald KD, Welsh RC, Abelson JL, Taylor SF. Resting state functional connectivity between fronto-parietal and default mode networks in obsessive-compulsive disorder. PLoS One. 2012;7:e36356. doi: 10.1371/journal.pone.0036356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Muratore AF, Taylor SF, Abelson JL, Hof PR, Goodman WK. The persistence of experience: prior attentional and emotional state affects network functioning in a target detection task. Cereb. Cortex. 2015;25:3235–3248. doi: 10.1093/cercor/bhu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Taylor SF. Cognitive neuroscience of obsessive-compulsive disorder. Psychiatr. Clin. N. Am. 2014;37:337–352. doi: 10.1016/j.psc.2014.05.004. [DOI] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle JA, Abelson JL, Taylor SF. Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive-compulsive disorder. Biol. Psychiatry. 2011;69:583–591. doi: 10.1016/j.biopsych.2010.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Gonzalez R, Fitzgerald KD, Abelson JL, Taylor SF. Subjective uncertainty and limbic hyperactivation in obsessive-compulsive disorder. Hum. Brain Mapp. 2013;34:1956–1970. doi: 10.1002/hbm.22038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasi D, Chang L, Caparelli EC, Ernst T. Different activation patterns for working memory load and visual attention load. Brain Res. 2007;1132:158–165. doi: 10.1016/j.brainres.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topper M, Emmelkamp PMG, Ehring T. Improving prevention of depression and anxiety disorders: Repetitive negative thinking as a promising target. Appl. Prev. Psychol. 2010;14:57–71. [Google Scholar]
- Vincent JL, Kahn I, Snyder AZ, Raichle ME, Buckner RL. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J. Neurophysiol. 2008;100:3328–3342. doi: 10.1152/jn.90355.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward BD. Simultaneous Inference for fMRI Data. 2000 〈 afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf〉. [Google Scholar]
- Wen XT, Liu YJ, Yao L, Ding MZ. Top-down regulation of default mode activity in spatial visual attention. JNeurosci. 2013;33:6444–6453. doi: 10.1523/JNEUROSCI.4939-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S, Nieto-Castanon A. Conn: a functional connectivity toolbox for correlated and anti-correlated brain networks. Brain Connect. 2012;2:125–141. doi: 10.1089/brain.2012.0073. [DOI] [PubMed] [Google Scholar]
- Yarkoni T, Poldrack RA, Van Essen DC, Wager TD. Cognitive neuroscience 2.0: building a cumulative science of human brain function. Trends Cogn. Sci. 2010;14:489–496. doi: 10.1016/j.tics.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.