Abstract
Mice harboring a G12D activating Kras mutation are among the most heavily studied models in the field of pancreatic adenocarcinoma (PDAC) research. miRNAs are differentially expressed in PDAC from patients and mouse models of PDAC. To better understand the relationship that Kras activation has on miRNA expression, we profiled the expression of 629 miRNAs in RNA isolated from the pancreas of control, young, and old P48+/Cre;LSL-KRASG12D as well as PDX-1-Cre;LSL-KRASG12D mice. One hundred of the differentially expressed miRNAs had increased expression in the advanced disease (old) P48+/Cre;LSL-KRASG12D compared to wild-type mice. Interestingly, the expression of three miRNAs, miR-216a, miR-216b, and miR-217, located within a ~30-kbp region on 11qA3.3, decreased with age (and phenotype severity) in these mice. miR-216/-217 expression was also evaluated in another acinar-specific ELa-KrasG12D mouse model and was downregulated as well. As miR-216/-217 are acinar enriched, reduced in human PDAC and target KRAS, we hypothesized that they may maintain acinar differentiation or represent tumor suppressive miRNAs. To test this hypothesis, we deleted a 27.9-kbp region of 11qA3.3 containing the miR-216/-217 host gene in the mouse's germ line. We report that germ line deletion of this cluster is embryonic lethal in the mouse. We estimate that lethality occurs shortly after E9.5. qPCR analysis of the miR-216b and miR-217 expression in the heterozygous animals showed no difference in expression, suggesting haplosufficiency by some type of compensatory mechanism. We present the differential miRNA expression in KrasG12D transgenic mice and report lethality from deletion of the miR-216/-217 host gene in the mouse's germ line.
Keywords: Kras, miR-216a, miR-216b, miR-217, lncRNA, Pancreatic cancer, microRNA, Transgenic mice
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fourth most lethal type of cancer in the USA, and it is estimated that it will become the second most deadly cancer in the USA by 2030 (Rahib et al. 2014, Siegel, Miller, and Jemal 2015). The last decade or more has been spent trying to understand the molecular and signaling mechanisms that regulate the development of PDAC so that better therapies and methods for early detection may be developed. This work has led to extensive growth of genetically engineered mouse models which have provided experimental systems to better understand and identify the molecular mechanisms underlying PDAC development. As the Kras G12D oncogene has been identified as the key oncogenic mutation that has been associated with development of PDAC in humans, the majority of genetically engineered mouse models have been based on this particular mutation. Since PDAC typically arises from pre-neoplastic lesions, termed PanINs, it becomes extremely important to use mouse models which allow us to study molecular changes that happen during this phase. Hruban and coworkers (Vincent et al. 2011) provided a framework by which PDAC arises from a series of genetic mutations which involves inactivation of tumor suppressor genes such as p16, DPC4, P53, and BRCA along with the activation of oncogenes such as Kras.
Pioneering work by Hingorani and Tuveson used Ptf1a and PDX-1 promoters to limit expression of Kras harboring the activating G12D mutation to the developing pancreas, restricting expression at E8.5–E9.5 (Hingorani et al. 2003). Popular modifications that combine the KrasG12D mutation with other mutant genes including p53 (Hingorani et al. 2005), Tgfβ (Ijichi et al. 2006), Smad4 (Bardeesy et al. 2006), and Brca1 (Shakya et al. 2013). Combining the double or sometime triple mutations decreases the latency of PDAC progression in mice. Despite its ductal appearance, PanINs and PDAC can develop from pancreatic acinar cells when mutant Kras is restricted using an acinar-specific promoter (Desai et al. 2007, Grippo et al. 2003, Guerra et al. 2007, Habbe et al. 2008); however, the same was not the case when Kras was restricted to the epithelial cells of the pancreas (Brembeck et al. 2003). These studies revealed an underlying role of pancreatic acinar cells in PDAC development.
microRNA (miRNAs) are small noncoding RNAs that recognize and bind to the 3′ UTR of mRNAs resulting in translational repression. Numerous profiling studies over the past decade have shown that the expression miRNAs are altered in cancer, including pancreatic (Bloomston et al. 2007, Lee et al. 2007, Szafranska et al. 2007, Volinia et al. 2006). miRNAs reported as deregulated in human PDAC include overexpressed miRNAs (miR-21, miR-155, miR-181a/b, and miR-221/-222) and those with reduced expression in the tumor (miR-217, miR-216a/b, miR-375, and miR-148a) recently reviewed in Visani et al. (2015). It was further noted that overexpression of certain miRNAs (i.e., miR-155 or miR-21) (Costinean et al. 2006, Medina, Nolde, and Slack 2010) or knockout of other miRNAs such as miR-122 in the liver can contribute to the development of cancer in mice (Hsu et al. 2012, Tsai et al. 2012). However, to the best of our knowledge, we are unaware of any published work on miRNA knockout or knockin mouse models that show PDAC development.
The initial objective of this study was to evaluate the miRNA expression in the KrasG12D transgenic mouse model of PDAC. We report that a large number of miRNAs are deregulated in LSL-Cre KrasG12D transgenic mice, where the majority of them are increased in expression and that only a few miRNAs are reduced in expression. The three miRNAs with reduced expression in the KrasG12D pancreas, (i.e., miR-216a, miR-216b, and miR-217) are contained within a genomic cluster on 11qA3.3. Although, these three miRNAs have been reported in the literature to be downregulated in PDAC patients (Deng et al. 2014, Schultz et al. 2012, Szafranska et al. 2007, Szafranska et al. 2008, Vychytilova-Faltejskova et al. 2015), their role in the pancreas remains completely unknown. To investigate a potential tumor suppressive role for these miRNAs, we developed a germ line knockout of the miR-216/-217 host gene. We conclude that deletion of the 27.9-kbp region that hosts the miR-216/-217 gene is embryonic lethal in mice.
Materials and methods
KrasG12D transgenic mice
Transgenic mice harboring an activating Kras mutation were used. Total RNA from PDX-1-Cre;LSL-KRASG12D, P48+/Cre;LSL-KRASG12D, PDX-1-Cre/wt (control), LSL-KRASG12D/wt (control), and P48Cre/wt (control) mice was isolated using miRNeasy (Qiagen) according to the manufacturer's protocol and the RIN was determined using the Agilent Bioanalyzer. The mean age of the mice was 209 days (PDX-1-Cre;LSL-KRASG12D) and 34 days (young) and 239 (old) days for P48+/Cre;LSL-KRASG12D. In the P48+/Cre;LSL-KRASG12D model, young mice are representative of early disease where only some PanINs have developed, where old mice are advanced in tumor progression. The mean age of all the control mice was 52 days. Detailed information on the mice may be found (Jiang et al. 2016). The mean age of the acinar-specific ELa-KrasG12D mouse was 240 days compared to that of wild-type mice which was 220 days.
RNA isolation and qPCR
For the transgenic mice and controls, RNA was supplied to us from the Tuveson lab. All RNA samples had a RNA Integrity Number (RIN) of 5 or greater (Jiang et al. 2016). Pancreatic RNA was isolated from the ElasCre and miR-216/-217+/− mice as described (Azevedo-Pouly, Elgamal, and Schmittgen 2014). All RNA samples had a RIN of 8 or greater.
qPCR
Total RNA was treated with DNase I and cDNA was made using the Life Technologies assays to 629 mature miRNAs as described (Schmittgen et al. 2008) or by priming with random hexamers as described (Tirmenstein et al. 2000) for the analysis of the miR-216/-217 host gene expression. qPCR was performed on an Applied Biosystems 7900 HT real-time PCR instrument that was equipped with a 384-well reaction plate using standard conditions. The 18S rRNA was used as an internal control and data were analyzed using the comparative CT method. To validate the normalizer for the qPCR, we report the CT values for the various test and control groups for all experiments performed in the study. The expression of the internal control gene did not significantly change between the test and control groups, and there were no situations where a significant change in the internal control between test and control groups exists (FC < 1.5, P > 0.05). A miRNAwas considered not expressed in a particular sample if the mean CT ≥ 36 in both the old and control groups, and they were considered differentially expressed if the fold change between the comparative groups was greater than 1.5-fold and P < 0.05 (Student's t test). Primer sequences are available upon request.
Mouse miR-216a, miR-216b, and miR-217 targeting vector design and cloning
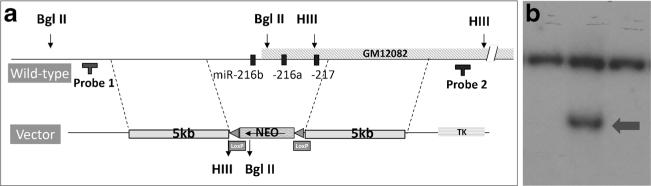
A targeting vector including two homology arms of 4.8 kbp upstream and 5.0 kbp downstream of the 27.9-kbp region containing the miR-216/-217 cluster was synthesized by Vega Biolab (Philadelphia, PA). Bacterial colonies positive for this construct were selected by kanamycin resistance and DNA plasmid was purified according to standard phenol:chloroform extraction. Figure 3 shows the targeting strategy: successful homologous recombination leads to replacement of 27,863 bp (28,735,939–28,763,801; GRCm38 mm10, UCSC database) containing the miR-216/-217 cluster with a ~2.0-kb neomycin cassette resulting in a germ line knock-out (KO).
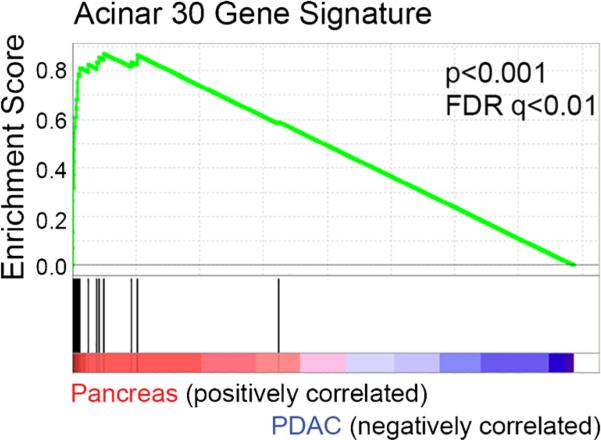
Fig. 3.
Gene set enrichment analysis in normal pancreas and PDAC. The gene set enrichment of expression data from 6–8-month-old KrasG12D or control mice obtained from data set GSE33322 were studied. A set of 30 acinar-specific genes were used for the enrichment
Generation of chimeras and miR-216/-217 KO F1 breeding
The Genetically Engineered Mouse Modeling Core of the Ohio State University introduced the targeting vector into S1B6 mouse embryonic stem (mES) cells according to standard procedure (Piovan et al. 2014). Briefly, early passage exponentially growing mES cells were electroporated in the presence of 25 μg of linearized miR-216/-217 KO targeting vector. After a recovery period of 24 h, cells were transferred to culture medium containing 200 μg/ml G148 and ganciclovir (2 × 10-6 M) to select for specifically targeted clones. Two positively recombined clones were then microinjected into C57Bl/6 blastocyst-stage embryos to generate chimeric mice. High percentage chimeras (>90 %) in turn were breed to C57Bl/6 wild-type female mice. Heterozygous F1 offspring were initially genotyped using the same Southern blot probes used for mES cell selection.
miR-216/-217 KO mouse colony maintenance
All mouse work was performed prior approval by the Institutional Animal Care and Use Committee (I-ACUC) and according to guidelines established by the University Laboratory Animal Resources (ULAR) of The Ohio State University (OSU). Breeding was done with two females and one male per cage. Breeders were started between 6–8 weeks of age and retired at 8 months of age. Weaning was performed at 21 days and tail snips were collected for genotyping.
miR-216/-217 KO genotyping PCR
Genotyping PCR was optimized in the F1 generation and used to confirm genotype in F1s and subsequent litters. Primers were designed for the miR-216b locus in the genome to yield a 286-bp wild-type band and for the Neo cassette to yield a 351-bp mutant band. Internal primer to amplify a 200-bp fragment of the Fabpi gene was also used to control the quality of each PCR reaction. Tail snips were digested with 300 μl Direct PCR Lysis Reagent (Viagen Biotech, Los Angeles, CA) according to the instructions of the manufacturer. PCR was performed with Crimson Taq (NEB, Ipswich, MA), 0.7 μM primer pairs, and 1 μl of lysate in 15 μl reactions. Cycling conditions were as follows: 94 °C incubation for 30 s, 30 cycles of 94 °C-20 s/60 °C-30 s/68 °C-40 s, followed by 10 min at 68 °C. Bands were detected with a 2 % agarose gel stained with ethidium bromide. PCR genotyping of experimental mice was confirmed by Southern blotting. Sequence of primers are available upon request.
Southern blotting
Southern blotting was performed according to a previously published protocol (Piovan et al. 2014) to confirm homologous recombination in mES cells, F1, and selected mice of subsequent generation. DNA was extracted from mES in 96-well plates or tail snips by phenol:chloroform extraction and digested with BglII or Hind III for Southern blotting analysis of 5′ or 3′ recombination, respectively. 5′ and 3′ Southern probes were amplified by PCR from genomic regions not included in the vector homology arms corresponding to 28,730,511–28,731,035 (5′) and 28,769,000–28,769,799 (3′) (GRCm38 mm10, UCSC database) of mouse chromosome 11, respectively.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA) from the Broad Institute was used to evaluate the relationship between the miRNA and acinar gene expression. Gene expression data (GSE33322) on 6–8-month-old Pdx1-cre;KrasLSLG12D mice was accessed and converted into a GCT file using the Microsoft Excel. GSEA was performed by comparing three sample data sets for normal pancreas (high miR-216/-217 expression) to three data sets for PDAC (low miR-216/-217 expression). A list of 30 acinar genes as reported by Morris et al. were used for the enrichment analysis (Morris et al. 2014).
Results
miRNAs are differentially expressed in transgenic mouse models of PDAC
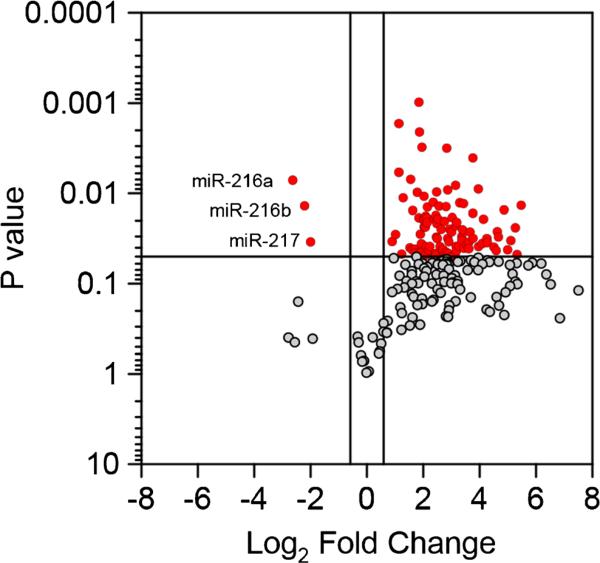
The expression of 629 miRNAs was profiled in RNA isolated from the pancreas of young and old P48+/Cre;LSL-KRASG12D mice, PDX-1-Cre;LSL-KRASG12D mice, and controls. Of the 629 miRNAs profiled, 229 were expressed as defined in the BMaterials and methods^ section. The differential expression of the miRNAs was compared in the old P48+/Cre;LSLKRASG12D mice versus the control mice. Of the 229 expressed miRNAs, 103 were differentially expressed, with 100 having increased expression and three miRNAs with reduced expression (Fig. 1) and STable 1. In a comparison of young P48+/Cre;LSL-KRASG12D mice versus control mice, only five miRNAs were differentially expressed (all increased expression, STable 1). Also in the PDX-1-Cre;LSL-KRASG12D vs control, 21 were differentially expressed with 19 increased and 2 miRNAs decreased (STable 1).
Fig. 1.
Volcano plot, miRNA expression in KC (p48) mice. The expression of 629 miRNAs was profiled in control and old P48+/Cre;LSL-KRASG12D mice. Of the expressed miRNAs shown, 100 had increased expression and 3 had reduced expression in the old P48+/Cre;LSL-KRASG12D mice compared to the control
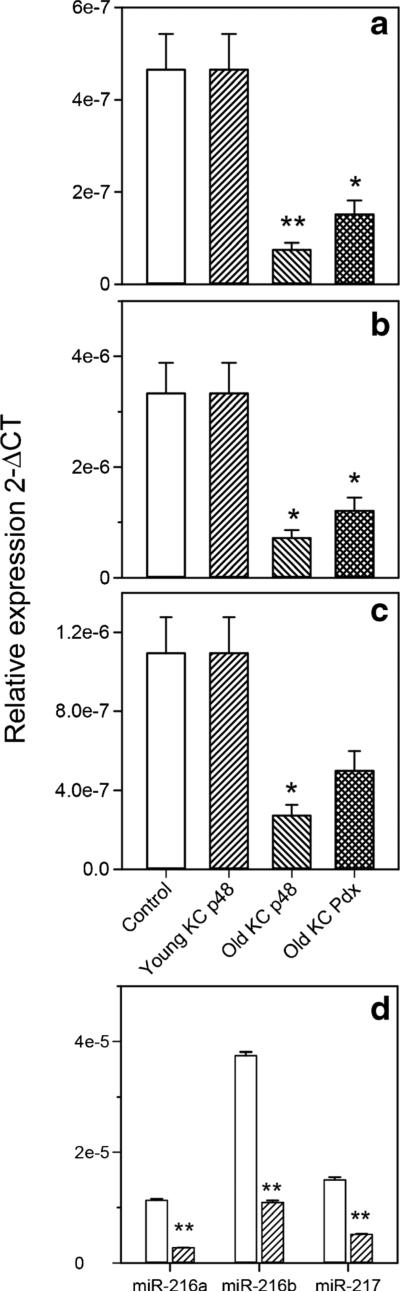
Interestingly, only three miRNAs had reduced expression in the old P48+/Cre;LSL-KRASG12D mice versus the control mice comparison; miR-216a, miR-216b, and miR-217 (Fig. 1, STable 1). miR-216a, miR-216b, and miR-217 are contained within a 17.6-kbp segment on chromosome 11qA3.3. The expression of miR-216a, miR-216b, and miR-217 sequentially decreased from control to young and old P48+/Cre;LSL-KRASG12D mice (Fig. 2a–c). The expression of these three miRNAs in the PDX-1-Cre;LSL-KRASG12D mice was in between that of the young and old P48+/Cre;LSL-KRASG12D mice (Fig. 2a–c). The findings of intermediate miR-216/-217 expression in the old P48+/Cre;LSL-KRASG12D mice are not surprising due to the mosaic expression of Kras in these mice. A recent review of the differential miRNA expression in human PDAC tissues compiled an 18 miRNA signature of PDAC from the differentially expressed miRNAs (13 increased and 5 decreased) reported in at least three studies (Visani et al. 2015). Our data from miRNA expression profiling in the KRASG12D transgenic mouse model of PDAC report that 8 of the 18 miRNAs in the human signature were also differentially expressed in the mouse model of PDAC (5 increased and 3 decreased). These include miR-100, miR-181a, miR-181b, miR-222, and miR-223 (increased) and miR-216a, miR-216b, and miR-217 (decreased).
Fig. 2.
miR-216a, miR-216b, and miR-217 expression in control and transgenic mice. The miR-216a (a), miR-216b (b), and miR-217 (c) expression is shown in young and old P48+/Cre;LSL-KRASG12D, old PDX-1-Cre;LSL-KRASG12D, and control mice. d Pancreatic expression of miR-216a, miR-216b, and miR-217 in Elas KrasG12D mice. Mean ± S.E.M. *P < 0.05, **P < 0.01 compared to control
The Pdx and Ptf1a promoters drive the differentiation of early pancreas progenitors from the endoderm during development. These progenitors give rise to both exocrine and endocrine populations of the pancreas (Kawaguchi et al. 2002). As miR-216a, miR-216b, and miR-217 were reported to be highly enriched in rat acini (Bravo-Egana et al. 2008) and the current belief is that pancreatic ductal adenocarcinoma arises from pancreatic acinar cells (Guerra et al. 2007), we wished to determine the pancreatic expression of the three reduced miRNAs in a third transgenic mouse, the Ela-KrasG12D mouse (Grippo et al. 2003). The mutant Kras in these mice are driven by the acinar-specific elastase promoter (Grippo et al. 2003). We found that the expression of the three miRNAs reduced with age in the Ela-KrasG12D mice (Fig. 2d). To establish a relationship between expression of miR-216/-217 and acinar genes, GSEAwas performed using a publically available data set of on pancreatic gene expression in Pdx1-cre;KrasLSLG12D mice. A set of 30 acinar-specific genes were positively enriched in the normal pancreas with high miR-216/-217 expression compared to the PDAC with low miR-216/-217 expression (Fig. 3). We conclude that miR-216a, miR-216b, and miR-217 are downregulated in the Pdx-1, Ptf1a, and Ela-KrasG12D transgenic models of PDAC and that lower levels of the miRNAs correlates with lower expression of acinar-specific genes.
Germline knockout of miR-216a, miR-216b, and miR-217
The reduced expression of miR-216a, miR-216b, and miR-217 with increasing age of the mice was of interest to us for several reasons. Firstly, these three miRNAs are reduced during PanIN progression, (Yu et al. 2012; Xue et al. 2013), pancreatitis, and PDAC in both patients (Deng et al. 2014, Schultz et al. 2012, Szafranska et al. 2007, Szafranska et al. 2008, Vychytilova-Faltejskova et al. 2015, Xue et al. 2013, Yu et al. 2012) and transgenic mice (Ali et al. 2012, Rachagani et al. 2015). Secondly, miR-216b targets KRAS in nasopharyngeal carcinoma (Deng et al. 2011), miR-217 targets KRAS in PDAC cells, and miR-217 oligo reduces proliferation of PDAC cell lines (Zhao et al. 2010). Thus, it is possible that these three miRNAs function as tumor suppressors in pancreatic acini and their repression contributes to both increased KRAS expression as well as tumorigenesis.
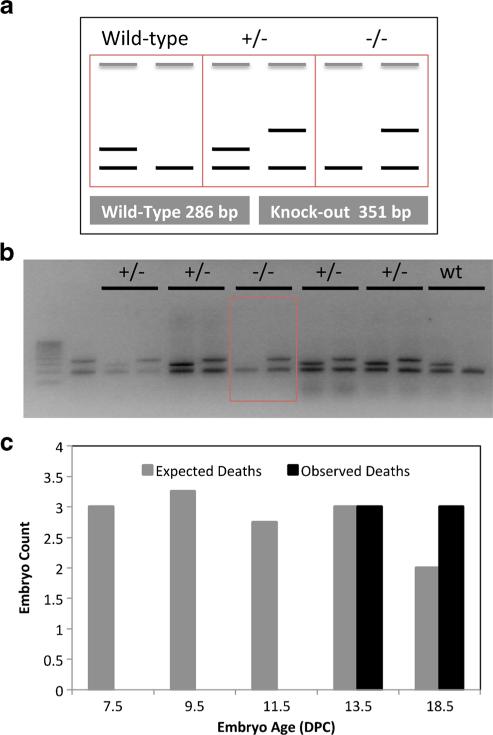
To test this hypothesis in vivo, we deleted a 27.9-kbp region on chromosome 11 of the mouse genome containing the miR-216a, miR-216b, and miR-217 host genes in the germ line of mice (Fig. 4a). At the time the mouse was generated, the targeting was engineered for the complete germ line ablation of the cluster deemed the fastest approach possible in just one targeting event due to the large size of locus (~28 kbp). Following transduction with the targeting vector and in vitro selection, a successful recombination was demonstrated by Southern blot analysis of G418/gancyclovir-resistant mES clones (Fig. 3b). In addition to a ~19.9-kbp wild-type fragment, mES DNA digested with Bgl II and surveyed with a locus-specific probe designed outside the 5′ region of homology to the targeting vector showed a mutant 6.8 kbp that results from the additional Bgl II site introduced by the neomycin cassette (Fig. 4b).
Fig. 4.
Targeting strategy to generate miR-216/-217 knockout mice. a. Wild-type miR-216/-217 locus (top) and miR-216/-217 targeting vector (below). Targeting homologies are illustrated as gray boxes. Designed probes and restriction enzymes are also included. b. Representative Southern blot of three G418-resistant clones following Bgl II digestion of extracted DNA and survey with a 5′ locus-specific probe. In addition to a 19.9-kbp wild-type band, correctly recombined clones (middle lane) showed a mutant 6.8-kbp fragment (arrow) due to the presence of a Bgl II site in the neomycin cassette replacing the miRNA cluster
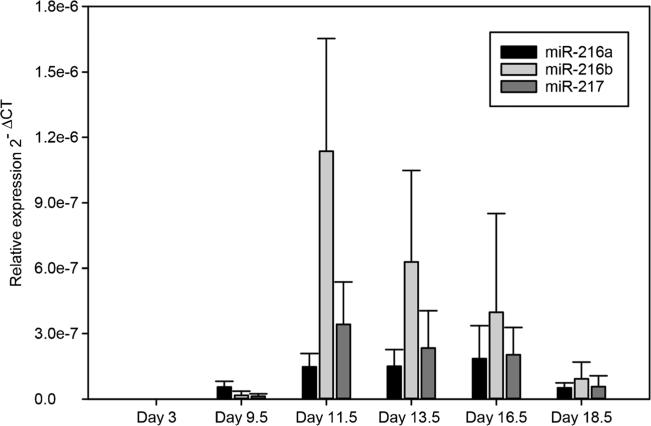
We performed a serial breeding of time of heterozygous F1 and heterozygous F2 animals for an extended period. Since the genotyping of over 200 pups did not yield any homozygous mice, we suspected embryonic lethality and set out to perform embryo analysis to establish the occurrence of lethality during development. First, we collected wild-type embryo RNA to determine miR-216a, miR-216b, and miR-217 expression during development. Figure 5 shows that expression of the miRNAs in whole wild-type embryo peaks between 11.5 and 13.5 days post coitus (d.p.c.). Based on this observation, we collected embryos at 7.5, 9.5, 11.5, 13.5, and 18.5 d.p.c. from multiple heterozygous mating cages. We were able to collect a viable homozygous embryo at 9.5 d.p.c. However, collection at later embryonic days did not show any viable homozygous animal, suggesting that complete loss of the miR-216/-217 cluster causes embryonic lethality around 9.5 days of gestation or shortly thereafter (Fig. 6).
Fig. 5.
miR-216a, miR-216b, and miR-217 expression levels in the mouse embryo. Wild-type embryos were collected for RNA and analyzed for miR-216/-217 expression by qPCR
Fig. 6.
Embryonic lethality in miR-216/-217 homozygous mice. a. The miR-216a, miR-216b, and miR-217 expression was measured in the mouse embryos at various stages of development by qRT-PCR. b. miR-216/-217 expression during development at E3.5, E9.5, 13.5, and 17.5. c (Revise based on new). Genotyping of the E9.5 litter revealed a viable homozygous embryo
Haplosufficiency in F2 miR-216/-217 KO mice
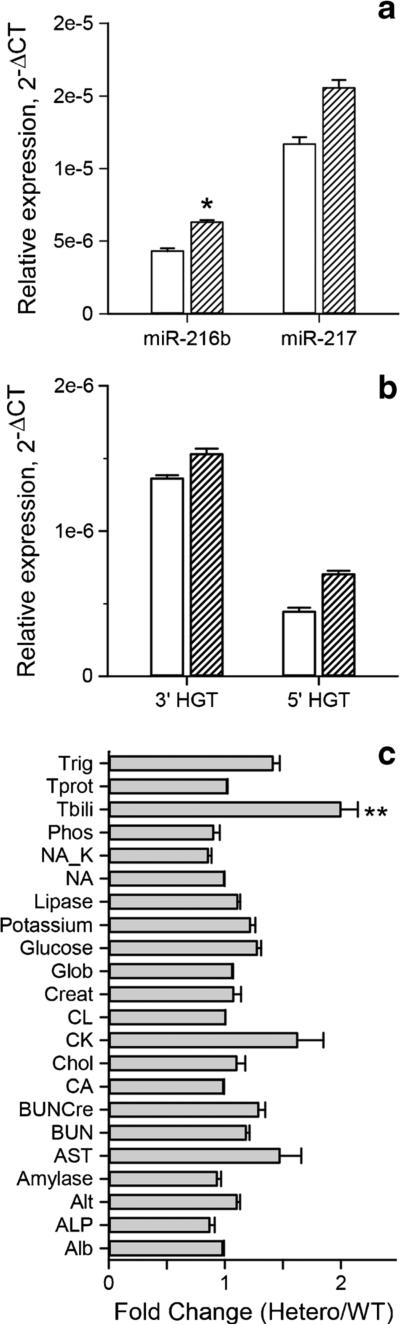
Due to the triple miRNA knockout mouse being embryonic lethal, we shifted out efforts to phenotyping the miR-216/-217 heterozygous (het) mice. We first measured miR-216b and miR-217 levels in the pancreas of miR-216/-217 het (n = 18) and wild-type mice (n = 11). Surprisingly, there was no significant reduction of miR-216b or miR-217 in the miR-216/-217 het mice. If anything, we observed a slight increase in mature miR-216b and miR-217 expression in the heterozygous mice (Fig. 7a). To see if any differences exist between the expression of the miR-216/-217 host gene in wt and het mice, we analyzed two different segments, the 3′ miR-216/-217 host gene transcript (HGT), and the 5′ HGT, by qPCR. The pattern of expression for the host gene miR-217/-217 was similar to the mature miRNA (Fig. 7b). The change in the mouse body weight was compared between the wild-type control mice and miR-216/-217 het mice but found no significant difference (data not shown) nor were there any significant changes on the plasma biochemistry between the het and wild-type mice with the exception of a slight increase in total bilirubin (Fig. 7c). Finally, microscopic evaluation of pancreatic sections of the miR-216/-217 het mice showed no remarkable changes compared to the wild-type controls (data not shown).
Fig. 7.
Analysis of haploinsufficient mice. a Mature miR-216b and miR-217 and (b) miR-216/-217 3′ and 5′ host gene transcript (HGT) expression in wild-type (open bars) and miR-216/-217+/− (hatched bars) mice. c. Full blood biochemistry panel results for wild-type and heterozygous mice. (n = 18) Mean ± S.E.M.
Discussion
We profiled the expression of over 600 mature miRNAs in KRASG12D transgenic mouse models of PDAC. A large percentage of miRNAs had increased expression in the transgenic mice compared to controls. We also profiled the transcribed ultraconserved region RNA (T-UCR) expression in the identical RNA samples from this study along with the RNA isolated from patient's PDAC specimens (Jiang et al. 2016). The T-UCRs had primarily increased expression in both the mouse and human pancreases. We used qPCR profiling of clinical specimens of PDAC to show a similar trend as reported here (predominate miRNA upregulation with reduction in miR-216/-217, unpublished data). It is interesting that two classes of noncoding RNAs (miRNAs and T-UCRs) have predominately increased expression in both mouse models and clinical specimens of PDAC.
The expression of three miRNAs transcribed from the miR-216/-217 miRNA family was reduced in the P48+/Cre;LSL-KRASG12D, PDX-1-Cre;LSL-KRASG12D, and ELa-KrasG12D mice. Our data confirm the recent findings of Rachagani et al. (2015) who reported a reduction in the expression of miR-216a, miR-216b, and miR-217 in the pancreas P48+/Cre;LSL-KRASG12D mice (Rachagani et al. 2015). The results of our study parallels those miRNA profiling studies in human PDAC showing an increased expression of several miRNAs yet reduction in the acinar miRNAs (Visani et al. 2015). Similar patterns of miRNA expression in both transgenic mice and human PDAC further supports the use of these models for experimental pancreatic cancer research.
Deletion of 27.9 kbp from the germ line of the mice was embryonic lethal. This was determined by the presence of no viable homozygotes after genotyping over 200 pups. One homozygote was identified at E9.5 but not at later time points suggesting that the deletion caused lethality shortly after E9.5. At E9.5, the developing pancreas undergoes the primary transition. The primary transition is characterized by the very first appearance of low levels of digestive enzymes and glucagon and insulin gene expressing cells (Cleaver et al. 2010). Although lethality occurred about the time that the pancreas would begin secreting digestive enzymes, others have found that failure to develop a pancreas does not result in lethality in utero (Hale et al. 2005, Kawaguchi et al. 2002). It is more likely, therefore, that interruption of other crucial developmental milestones is responsible for the lethality phenotype.
Several miRNA gene knockout mice have been known to induce lethality in mice (Farmer et al. 2013, Kuhnert et al. 2008, Liu et al. 2008, Ventura et al. 2008, Wang et al. 2008, Zhao et al. 2007). Knockout of miR-205 was embryonic lethal in mice (Park et al. 2012). Deletion of the primary transcript of brain expressed miR-137 was also embryonic lethal, the authors estimate that lethality occurred between E4.5 and E11.5 (Crowley et al. 2015). There was no difference in the mature miR-137 expression between the wild-type and miR-137 heterozygotes (Crowley et al. 2015). Our data (Fig. 6a) showed no decrease in miR-216b and miR-217 expression in the heterozygotes and in fact there was a slight increase in the expression (Fig. 7). Possibly, some type of compensatory mechanisms such as increased transcription occur in these heterozygotes.
miR-216a and miR-217 are located within the mouse ENSMUST00000138164, a 64-kbp transcript on 11qA3.3. This transcript has been annotated as a lncRNA. miR-216b is located on a separate transcript, 5.4 kbp upstream of GM12082 (Fig. 3). We deleted 27.9 kbp portion of 11qA3.3 which contained both miR-216b as well as a large portion of the lncRNA GM12082 (Fig. 3). Ongoing studies have developed individual, germline knockouts of miR-216a, miR-216b, and miR-217 (Sutaria, Coppola, and Schmittgen, unpublished data). We report no lethality from the knockout of each individual miRNA. Thus, the lethality observed here is a result of either the simultaneous knockout of all three miRNAs or loss of the miR-216/-217 host gene/lncRNA. If the latter is true, then this would add GM12082 to small but growing list of published studies that show lethality from the germline knockout of lncRNAs (Bassett et al. 2014).
In summary, we confirm published data in both human and mouse PDAC (Rachagani et al. 2015, Visani et al. 2015) that miR-216a, miR-216b, and miR-217 are reduced during the mouse's age when PDAC would progress. We also demonstrate that the germ line deletion of the miR-216/-217 host gene is embryonic lethal. As miR-216/-217 are acinar enriched, it will be of interest to develop either germ line or conditional knockout mice of the individual miRNAs to evaluate any potential tumor suppressor roles for these miRNAs during PanIN or PDAC development in transgenic mice.
Supplementary Material
Acknowledgments
This work was supported by a Pelotonia idea grant from the Ohio State University to T.D.S. and E.C. A.C.P.A.P. was supported by NIH fellowship 5F31CA142238. We thank Dr. David Tuveson for providing the RNA samples from the KC and control mice. We thank Dr. Caifu Chen for his assistance with the mouse TaqMan miRNA assays and Luke Bramlage and Andrea Haughtvedt for their technical assistance. We also thank Inga Carsten of the Genetically Engineered Mouse Modeling Core for the help in mES microinjection.
Footnotes
This article forms part of a special issue of Functional & Integrative Genomics entitled “miRNA in model and complex organisms” (Issue Editors: Hikmet Budak and Baohong Zhang).
Electronic supplementary material The online version of this article (doi:10.1007/s10142-016-0512-1) contains supplementary material, which is available to authorized users.
References
- Ali S, Banerjee S, Logna F, Bao B, Philip PA, Korc M, Sarkar FH. Inactivation of Ink4a/Arf leads to deregulated expression of miRNAs in K-Ras transgenic mouse model of pancreatic cancer. J Cell Physiol. 2012;227:3373–3380. doi: 10.1002/jcp.24036. doi:10.1002/jcp.24036. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Azevedo-Pouly AC, Elgamal OA, Schmittgen TD. RNA isolation from mouse pancreas: a ribonuclease-rich tissue. Journal of visualized experiments: JoVE. 2014:e51779. doi: 10.3791/51779. doi: 10.3791/51779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardeesy N, Cheng KH, Berger JH, Chu GC, Pahler J, Olson P, Hezel AF, Horner J, Lauwers GY, Hanahan D, DePinho RA. Smad4 is dispensable for normal pancreas development yet critical in progression and tumor biology of pancreas cancer. Genes Dev. 2006;20:3130–3146. doi: 10.1101/gad.1478706. doi:10.1101/gad.1478706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett AR, Akhtar A, Barlow DP, Bird AP, Brockdorff N, Duboule D, Ephrussi A, Ferguson-Smith AC, Gingeras TR, Haerty W, Higgs DR, Miska EA, Ponting CP. Considerations when investigating lncRNA function in vivo. Elife. 2014;3:e03058. doi: 10.7554/eLife.03058. doi:10.7554/eLife.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- Bravo-Egana V, Rosero S, Molano RD, Pileggi A, Ricordi C, Dominguez-Bendala J, Pastori RL. Quantitative differential expression analysis reveals miR-7 as major islet microRNA. Biochem Biophys Res Commun. 2008;366:922–926. doi: 10.1016/j.bbrc.2007.12.052. doi:10.1016/j.bbrc.2007.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brembeck FH, Schreiber FS, Deramaudt TB, Craig L, Rhoades B, Swain G, Grippo P, Stoffers DA, Silberg DG, Rustgi AK. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003;63:2005–2009. [PubMed] [Google Scholar]
- Cleaver O, MacDonald RJ. Developmental molecular biology of the pancreas. In: Neoptolemos RUJP, Abbruzzese JL, Buchler MW, editors. Pancreatic cancer. Springer; New York: 2010. pp. 71–117. [Google Scholar]
- Costinean S, Zanesi N, Pekarsky Y, Tili E, Volinia S, Heerema N, Croce CM. Pre-B cell proliferation and lymphoblastic leukemia/high-grade lymphoma in E(mu)-miR155 transgenic mice. Proc Natl Acad Sci U S A. 2006;103:7024–7029. doi: 10.1073/pnas.0602266103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley JJ, Collins AL, Lee RJ, Nonneman RJ, Farrell MS, Ancalade N, Mugford JW, Agster KL, Nikolova VD, Moy SS, Sullivan PF. Disruption of the microRNA 137 primary transcript results in early embryonic lethality in mice. Biol Psychiatry. 2015;77:e5–e7. doi: 10.1016/j.biopsych.2014.05.022. doi:10.1016/j.biopsych.2014.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng M, Tang H, Zhou Y, Zhou M, Xiong W, Zheng Y, Ye Q, Zeng X, Liao Q, Guo X, Li X, Ma J, Li G. miR-216b suppresses tumor growth and invasion by targeting KRAS in nasopharyngeal carcinoma. J Cell Sci. 2011;124:2997–3005. doi: 10.1242/jcs.085050. doi:10.1242/jcs.085050. [DOI] [PubMed] [Google Scholar]
- Deng S, Zhu S, Wang B, Li X, Liu Y, Qin Q, Gong Q, Niu Y, Xiang C, Chen J, Yan J, Deng S, Yin T, Yang M, Wu H, Wang C, Zhao G. Chronic pancreatitis and pancreatic cancer demonstrate active epithelial-mesenchymal transition profile, regulated by miR-217-SIRT1 pathway. Cancer Lett. 2014;355:184–191. doi: 10.1016/j.canlet.2014.08.007. doi:10.1016/j.canlet.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Desai BM, Oliver-Krasinski J, De Leon DD, Farzad C, Hong N, Leach SD, Stoffers DA. Preexisting pancreatic acinar cells contribute to acinar cell, but not islet beta cell, regeneration. J Clin Invest. 2007;117:971–977. doi: 10.1172/JCI29988. doi:10.1172/JCI29988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer DT, Shariat N, Park CY, Liu HJ, Mavropoulos A, McManus MT. Partially penetrant postnatal lethality of an epithelial specific microRNA in a mouse knockout. PLoS One. 2013;8:e76634. doi: 10.1371/journal.pone.0076634. doi:10.1371/journal.pone.0076634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63:2016–2019. [PubMed] [Google Scholar]
- Guerra C, Schuhmacher AJ, Canamero M, Grippo PJ, Verdaguer L, Perez-Gallego L, Dubus P, Sandgren EP, Barbacid M. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. doi:10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Habbe N, Shi G, Meguid RA, Fendrich V, Esni F, Chen H, Feldmann G, Stoffers DA, Konieczny SF, Leach SD, Maitra A. Spontaneous induction of murine pancreatic intraepithelial neoplasia (mPanIN) by acinar cell targeting of oncogenic Kras in adult mice. Proc Natl Acad Sci U S A. 2008;105:18913–18918. doi: 10.1073/pnas.0810097105. doi:10.1073/pnas.0810097105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale MA, Kagami H, Shi L, Holland AM, Elsasser HP, Hammer RE, MacDonald RJ. The homeodomain protein PDX1 is required at mid-pancreatic development for the formation of the exocrine pancreas. Dev Biol. 2005;286:225–237. doi: 10.1016/j.ydbio.2005.07.026. doi:10.1016/j.ydbio.2005.07.026. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Petricoin EF, Maitra A, Rajapakse V, King C, Jacobetz MA, Ross S, Conrads TP, Veenstra TD, Hitt BA, Kawaguchi Y, Johann D, Liotta LA, Crawford HC, Putt ME, Jacks T, Wright CV, Hruban RH, Lowy AM, Tuveson DA. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, Rustgi AK, Chang S, Tuveson DA. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. doi:10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Hsu SH, Wang B, Kota J, Yu J, Costinean S, Kutay H, Yu L, Bai S, La Perle K, Chivukula RR, Mao H, Wei M, Clark KR, Mendell JR, Caligiuri MA, Jacob ST, Mendell JT, Ghoshal K. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J Clin Invest. 2012;122:2871–2883. doi: 10.1172/JCI63539. doi:10.1172/JCI63539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijichi H, Chytil A, Gorska AE, Aakre ME, Fujitani Y, Fujitani S, Wright CV, Moses HL. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20:3147–3160. doi: 10.1101/gad.1475506. doi:10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Azevedo-Pouly AC, Redis RS, Lee EJ, Gusev Y, Allard D, Sutaria DS, Badawi M, Elgamal OA, Lerner MR, Brackett DJ, Calin GA, Schmittgen TD. Globally increased ultraconserved noncoding RNA expression in pancreatic adenocarcinoma. Oncotarget. 2016 doi: 10.18632/oncotarget.10242. doi:10.18632/oncotarget.10242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Cooper B, Gannon M, Ray M, MacDonald RJ, Wright CVE. The role of the transcriptional regulator Ptf1a in converting intestinal to pancreatic progenitors. Nat Genet. 2002;32:128–134. doi: 10.1038/ng959. doi:10.1038/ng959. [DOI] [PubMed] [Google Scholar]
- Kuhnert F, Mancuso MR, Hampton J, Stankunas K, Asano T, Chen CZ, Kuo CJ. Attribution of vascular phenotypes of the murine Egfl7 locus to the microRNA miR-126. Development. 2008;135:3989–3993. doi: 10.1242/dev.029736. doi:10.1242/dev.029736. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Gusev Y, Jiang J, Nuovo GJ, Lerner MR, Frankel WL, Morgan DL, Postier RG, Brackett DJ, Schmittgen TD. Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer. 2007;120:1046–1054. doi: 10.1002/ijc.22394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, Bezprozvannaya S, Williams AH, Qi X, Richardson JA, Bassel-Duby R, Olson EN. microRNA-133a regulates cardiomyocyte proliferation and suppresses smooth muscle gene expression in the heart. Genes Dev. 2008;22:3242–3254. doi: 10.1101/gad.1738708. doi:10.1101/gad.1738708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature. 2010;467:86–90. doi: 10.1038/nature09284. doi:10.1038/nature09284. [DOI] [PubMed] [Google Scholar]
- Morris JP, Greer R, Russ HA, von Figura G, Kim GE, Busch A, Lee J, Hertel KJ, Kim S, McManus M, Hebrok M. Dicer regulates differentiation and viability during mouse pancreatic cancer initiation. PLoS One. 2014;9:e95486. doi: 10.1371/journal.pone.0095486. doi:10.1371/journal.pone.0095486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park CY, Jeker LT, Carver-Moore K, Oh A, Liu HJ, Cameron R, Richards H, Li Z, Adler D, Yoshinaga Y, Martinez M, Nefadov M, Abbas AK, Weiss A, Lanier LL, de Jong PJ, Bluestone JA, Srivastava D, McManus MT. A resource for the conditional ablation of microRNAs in the mouse. Cell Rep. 2012;1:385–391. doi: 10.1016/j.celrep.2012.02.008. doi:10.1016/j.celrep.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piovan C, Amari F, Lovat F, Chen Q, Coppola V. Generation of mouse lines conditionally over-expressing microRNA using the Rosa26-Lox-Stop-Lox system. Methods Mol Biol. 2014;1194:203–224. doi: 10.1007/978-1-4939-1215-5_11. doi:10.1007/978-1-4939-1215-5_11. [DOI] [PubMed] [Google Scholar]
- Rachagani S, Macha MA, Menning MS, Dey P, Pai P, Smith LM, Mo YY, Batra SK. Changes in microRNA (miRNA) expression during pancreatic cancer development and progression in a genetically engineered KrasG12D;Pdx1-Cre mouse (KC) model. Oncotarget. 2015;6:40295–40309. doi: 10.18632/oncotarget.5641. doi:10.18632/oncotarget.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. doi:10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, Chen C. Real-time PCR quantification of precursor and mature microRNA. Methods. 2008;44:31–38. doi: 10.1016/j.ymeth.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz NA, Werner J, Willenbrock H, Roslind A, Giese N, Horn T, Wojdemann M, Johansen JS. MicroRNA expression profiles associated with pancreatic adenocarcinoma and ampullary adenocarcinoma. Mod Pathol. 2012;25:1609–1622. doi: 10.1038/modpathol.2012.122. doi:10.1038/modpathol.2012.122. [DOI] [PubMed] [Google Scholar]
- Shakya R, Gonda T, Quante M, Salas M, Kim S, Brooks J, Hirsch S, Davies J, Cullo A, Olive K, Wang TC, Szabolcs M, Tycko B, Ludwig T. Hypomethylating therapy in an aggressive stroma-rich model of pancreatic carcinoma. Cancer Res. 2013;73:885–896. doi: 10.1158/0008-5472.CAN-12-1880. doi:10.1158/0008-5472.CAN-12-1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. doi:10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Szafranska AE, Davison TS, John J, Cannon T, Sipos B, Maghnouj A, Labourier E, Hahn SA. MicroRNA expression alterations are linked to tumorigenesis and non-neoplastic processes in pancreatic ductal adenocarcinoma. Oncogene. 2007;26:4442–4452. doi: 10.1038/sj.onc.1210228. [DOI] [PubMed] [Google Scholar]
- Szafranska AE, Doleshal M, Edmunds HS, Gordon S, Luttges J, Munding JB, Barth RJ, Jr, Gutmann EJ, Suriawinata AA, Marc Pipas J, Tannapfel A, Korc M, Hahn SA, Labourier E, Tsongalis GJ. Analysis of microRNAs in pancreatic fine-needle aspirates can classify benign and malignant tissues. Clin Chem. 2008;54:1716–1724. doi: 10.1373/clinchem.2008.109603. doi:10.1373/clinchem.2008.109603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirmenstein MA, Nicholls-Grzemski FA, Schmittgen TD, Zakrajsek BA, Fariss MW. Characterization of nitric oxide production following isolation of rat hepatocytes. Toxicol Sci. 2000;53:56–62. doi: 10.1093/toxsci/53.1.56. [DOI] [PubMed] [Google Scholar]
- Tsai WC, Hsu SD, Hsu CS, Lai TC, Chen SJ, Shen R, Huang Y, Chen HC, Lee CH, Tsai TF, Hsu MT, Wu JC, Huang HD, Shiao MS, Hsiao M, Tsou AP. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884–2897. doi: 10.1172/JCI63455. doi:10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A, Young AG, Winslow MM, Lintault L, Meissner A, Erkeland SJ, Newman J, Bronson RT, Crowley D, Stone JR, Jaenisch R, Sharp PA, Jacks T. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell. 2008;132:875–886. doi: 10.1016/j.cell.2008.02.019. doi:10.1016/j.cell.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent A, Herman J, Schulick R, Hruban RH, Goggins M. Pancreatic cancer. Lancet. 2011;378:607–620. doi: 10.1016/S0140-6736(10)62307-0. doi:10.1016/S0140-6736(10)62307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visani M, Acquaviva G, Fiorino S, Bacchi Reggiani ML, Masetti M, Franceschi E, Fornelli A, Jovine E, Fabbri C, Brandes AA, Tallini G, Pession A, de Biase D. Contribution of microRNA analysis to characterisation of pancreatic lesions: a review. J Clin Pathol. 2015;68:859–869. doi: 10.1136/jclinpath-2015-203246. doi:10.1136/jclinpath-2015-203246. [DOI] [PubMed] [Google Scholar]
- Volinia S, Calin GA, Liu CG, Ambs S, Cimmino A, Petrocca F, Visone R, Iorio M, Roldo C, Ferracin M, Prueitt RL, Yanaihara N, Lanza G, Scarpa A, Vecchione A, Negrini M, Harris CC, Croce CM. A microRNA expression signature of human solid tumors defines cancer gene targets. Proc Natl Acad Sci U S A. 2006;103:2257–2261. doi: 10.1073/pnas.0510565103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vychytilova-Faltejskova P, Kiss I, Klusova S, Hlavsa J, Prochazka V, Kala Z, Mazanec J, Hausnerova J, Kren L, Hermanova M, Lenz J, Karasek P, Vyzula R, Slaby O. miR-21, miR-34a, miR-198 and miR-217 as diagnostic and prognostic biomarkers for chronic pancreatitis and pancreatic ductal adenocarcinoma. Diagn Pathol. 2015;10:38. doi: 10.1186/s13000-015-0272-6. doi:10.1186/s13000-015-0272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Aurora AB, Johnson BA, Qi X, McAnally J, Hill JA, Richardson JA, Bassel-Duby R, Olson EN. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. doi:10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Abou Tayoun AN, Abo KM, Pipas JM, Gordon SR, Gardner TB, Barth RJ, Jr, Suriawinata AA, Tsongalis GJ. MicroRNAs as diagnostic markers for pancreatic ductal adenocarcinoma and its precursor, pancreatic intraepithelial neoplasm. Cancer Genet. 2013;206:217–221. doi: 10.1016/j.cancergen.2013.05.020. doi:10.1016/j.cancergen.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Yu J, Li A, Hong SM, Hruban RH, Goggins M. MicroRNA alterations of pancreatic intraepithelial neoplasias. Clin Cancer Res. 2012;18:981–992. doi: 10.1158/1078-0432.CCR-11-2347. doi:10.1158/1078-0432.CCR-11-2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Ransom JF, Li A, Vedantham V, von Drehle M, Muth AN, Tsuchihashi T, McManus MT, Schwartz RJ, Srivastava D. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2. Cell. 2007;129:303–317. doi: 10.1016/j.cell.2007.03.030. doi:10.1016/j.cell.2007.03.030. [DOI] [PubMed] [Google Scholar]
- Zhao WG, Yu SN, Lu ZH, Ma YH, Gu YM, Chen J. The miR-217 microRNA functions as a potential tumor suppressor in pancreatic ductal adenocarcinoma by targeting Kras. Carcinogenesis. 2010;31:1726–1733. doi: 10.1093/carcin/bgq160. doi:10.1093/carcin/bgq160. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.