Abstract
Purpose
In this study, we aimed to validate our extensive pre-clinical data on phosphodiesterase 4 (PDE4) as actionable target in B-cell malignancies. Our specific objectives were to determine the safety, pharmacokinetics and pharmacodynamics (PI3K/AKT activity), as well as to capture any potential anti-tumor activity of the PDE4 inhibitor roflumilast in combination with prednisone in patients with advanced B cell malignancies.
Experimental Design
Single center, exploratory phase Ib open-label, non-randomized study. Roflumilast (500 mcg PO) was given daily for 21 days with prednisone on days 8-14. Additional 21-day cycles were started if patients tolerated cycle 1 and had at least stable disease.
Results
Ten patients, median age 65 years with an average of three prior therapies were enrolled. The median number of cycles administered was 4 [range: 1-13]. Treatment was well tolerated; the most common ≥Grade 2 treatment-related adverse events (≥25%) were fatigue, anorexia and transient ≥ grade 2 neutropenia (30%). Treatment with roflumilast as a single agent significantly suppressed PI3K activity in the 77% of patients evaluated; on average, patients with PI3K/AKT suppression stayed in trial for 156 days (49 - 315) vs. 91 days (28 - 139 days) for those without this biomarker response. Six of the nine evaluable patients (66%) had partial response or stable disease. The median number of days in trial was 105 days [range: 28-315].
Conclusions
Repurposing the PDE4 inhibitor roflumilast for treatment of B-cell malignancies is a safe, suppresses the activity of the oncogenic PI3K/AKT kinases, and may have clinical activity in this setting.
Keywords: Lymphoma, phosphodiesterase 4, cyclic-AMP, clinical trial, PI3K/AKT
Introduction
Acquiring basic biology data in specific priority areas can accelerate clinical translation in cancer. One way to fulfil this promise is to map the intricacies of a physiologic system and exploit it for the treatment of human diseases. In cells of the innate or adaptive immune system, the signals delivered by the second-messenger cyclic-AMP (cAMP) are broadly inhibitory(1-4). The suppressive effects of cAMP in immune cells are terminated by phosphodiesterase 4 (PDE4)-family of enzymes(5-7). Thus, aberrantly elevated expression/activity of PDE4 can result in a sustained release of pro-inflammatory cytokines and cell proliferation(5, 8). Importantly, this knowledge can be therapeutically leveraged with the use of PDE4 inhibitors(9-11). In agreement with this concept, agents in this drug class have been approved by the FDA for the treatment of two inflammatory/auto-immune conditions, chronic obstructive pulmonary disease (COPD) and psoriatic arthritis (PsA)(12, 13).
Not surprisingly, cancer cells, in particular those from mature B cell neoplasms, can hijack the cAMP/PDE4 system for their own benefit. Indeed, we and others showed that PDE4B was expressed at significantly higher levels in biopsies from fatal diffuse large B cell lymphoma (DLBCL) than in tumors from patients that survived their disease(14-18). Further, we used in vitro and in vivo preclinical models to meticulously charter how cAMP may suppress the growth of DLBCL cells, and how PDE4 expression/activity would abrogate these anti-lymphoma effects(17, 19, 20). We showed that cAMP transduce its growth inhibitory and pro-apoptotic effects in DLBCL by inhibiting two key oncogenic kinases associated with B cell receptor (BCR) signaling, SYK and PI3Kδ(17, 19). We demonstrated that these effects are lost in PDE4B-high DLBCLs, but that they can be restored by genetic or pharmacological suppression of PDE4. Further, using a murine model of B cell lymphoma, we demonstrated that treatment with the FDA-approved PDE4 inhibitor roflumilast significantly suppressed tumor burden and improved survival(21). Lastly, but critically important in the context of this clinical trial, using a xenograft model of glucocorticoid (GC)-resistant human B cell lymphoma we showed that downstream to PI3kδ/AKT cAMP and PDE4 influence GC sensitivity, and that pharmacological inhibition of PDE4 restores response to dexamethasone(20).
Considering the importance of GC sensitivity in the treatment of B cell tumors, as well as on the recently recognized benefits of PI3Kδ suppression in these diseases(22), we built on our pre-clinical observations and repurposed the FDA-approved PDE4 inhibitor roflumilast (Daliresp) for treatment of patients with advanced B cell malignancies. Here, in a first-in-cancer phase Ib open label, non-randomized, exploratory trial, we report on the safety,pharmacokinetics and pharmacodynamics of the PDE4 inhibitor roflumilast in association with prednisone in patients with refractory/relapsed B cell malignancies.
Patients and Methods
Study Design
Single center pilot Phase Ib open-label, non-randomized, pharmacokinectics and pharmacodynamics study of PDE4 inhibitor roflumilast and prednisone in relapsed/refractory B cell malignancies. The rationale for this trial, and its design, stems from our in vivo pre-clinical data that showed that PDE4 inhibition could restore glucocorticoid sensitivity in B cell malignancies(20), and consistently associate with PI3K/AKT suppression(17, 19-21). Here, we aimed to advance these pre-clinical concepts by testing the safety and tolerability of the roflumilast/prednisone combination in patients with B cell tumors, and by determining the pharmacodynamics of the PI3K/AKT biomarkers, while also attempting to capture clinical efficacy. This study was registered at ClinicalTrials.gov, number NCT01888952.
Patient Eligibility
Patients were eligible if they were aged 18 years or older with a B cell hematologic malignancy, including non-Hodgkin lymphoma (NHL), acute lymphocytic leukemia (ALL), multiple myeloma (MM), and chronic lymphocytic leukemia (CLL). Patients must not have been candidates for regimens known to provide clinical benefit. Eligible patients had a life expectancy of greater than 3 months, measurable or evaluable disease, an Eastern Cooperative Oncology Group (ECOG) score of 2 or lower, with adequate hematological, renal, and hepatic function, a negative serum or urine pregnancy test (for women of childbearing age), and the ability to provide written informed consent and to follow protocol requirements. Exclusion criteria included allogeneic bone marrow transplant within 6 months, autologous stem cell transplant within 3 months, active CNS involvement, ongoing receipt of systemic corticosteroids greater than prednisone equivalent of 20 mg/day, immunotherapy, chemotherapy, radiotherapy or investigational therapy within 3 weeks, active uncontrolled infection, uncontrolled or severe cardiovascular disease, multi-drug resistant HIV infection, hepatitis B or C infection, history of depression or other psychiatric illness, use of strong cytochrome P450 enzyme inducers and inhibitors, and second primary malignancy in the previous 2 years (except for curatively treated solid tumors at low risk for recurrence). This study was conducted according to the Declaration of Helsinki, was approved by the University of Texas Health Science at San Antonio's institutional review board, and all participants gave written informed consent.
Treatment
For cycle 1, all patients received a fixed oral dose of roflumilast 500 mcg daily, per the package insert, for 21 days (days 1 through 21). In addition, prednisone 60 mg/m2 PO daily (up to a maximum of 100 mg daily) was given at the same time as roflumilast on days 8 through 14. Thereafter, if patients tolerated cycle 1 (21-day cycle) and had at least stable disease, additional cycles of roflumilast and prednisone were administered starting on day 22 (day 1 of Cycle 2). For Cycle 2 and all subsequent cycles, prednisone 60 mg/m2 PO daily was taken on days 1 through 7 at the same time as roflumilast 500 mcg PO, given daily for 21 days. Note that throughout their enrollment in the trial, the patients were always on roflumilast.
Safety and efficacy analysis
Any objective response to treatment with roflumilast and prednisone was reported using the International Working Group revised response criteria that are appropriate for each patient's disease. A patient was recorded as having stable disease if there was no evidence of progression during the first two treatment cycles or two months in the trial, whichever occurred first. Safety was evaluated by the incidence, severity, relationship, and type of adverse events that occur during the treatment and follow-up periods using the revised National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE), version 4.03 (14 June 2010).
Pharmacokinetics
Steady-state trough levels (Ctss) of roflumilast and its active N-oxide metabolite were defined on trial day 8 of the first cycle, following a 7-day course of roflumilast as a single agent. Quantification of roflumilast and its metabolite was performed by liquid chromatography tandem mass spectrometry (LC-MS-MS) using the analytical procedures and conditions previously described(23). In addition, in a subset of patients, 543 single nucleotide polymorphisms (SNPs) from 69 distinct genes involved in drug absorption, distribution, metabolism, and excretion (ADME) were analyzed by massively parallel sequencing.
Biomarker measurements: PI3K activity and AKT phosphorylation
Whole peripheral blood was collected from the patients enrolled in the trial at day 0 (before roflumilast dosing), and on day 8, after a 7-day course of roflumilast as single agent. Following collection, peripheral blood mononuclear cells (PBMC) were obtained by Ficoll-Hypaque processing, as we described(24). Whole cell extracts (WCE) were obtained immediately thereafter and used for characterization of PI3K activity and AKT phosphorylation, as we reported(21, 25). For quantification of PI3K activity, 50μg of WCE was added to a mixture of PI(4,5)P2 (Phosphatidylinositol 4,5-bisphosphate) substrate, ATP and reaction buffer. The reaction was stopped and PI(3,4,5)P3 detection performed in PI3K ELISA plate (Echelon Biosciences) at 450 nm on a FLUOStar OPTIMA 96-well plate reader (BMG Labtech). To calculate the PI3K activity, we used nonlinear regression to create a PI(3,4,5)P3 standard sigmoidal curve with variable slope and interpolated the absorbance values from each sample thus defining the amount of PI(3,4,5)P3 generated (i.e., PI3K activity) before and after roflumilast dosing. Phosphorylation levels of the activation loop threonine 308 (T308) in AKT, a surrogate marker of its activation following phospholipid binding, and the expression of PDE4B and PDE4D, the principal members of the PDE4 family in lymphocytes, were defined by western blotting, as before(21). The antibodies used in this work were: PDE4B and PDE4D (H-56 and K-16, respectively, Santa Cruz Biotechnology); total AKT and phospho-AKT (Thr 308) (#9272 and #4056, respectively, Cell Signaling).
Statistical analysis
Given its exploratory nature, this study was designed for a small cohort of 10 patients and s the statistical analysis is only descriptive. Thus, no statistical assessment of response or sample size calculation was included in the study.The significance of the differences in the pharmacokinetics measurements (roflumilast/N-oxide concentrations), were tested using a two-sided Student's t-test. The statistical significance of PI3K activity suppression (pharmacodynamics) was calculated with analysis of variance (ANOVA) and Bonferroni's multiple comparison post-test. These analyses were performed in Excel or in the Graph-Pad Prism5 software package. P values <0.05 were considered statistically significant.
Results
Patients Demographic and Baseline Characteristics
Ten patients with relapsed or refractory disease were enrolled and treated: 5 NHL, 2 CLL, 2 MM and 1 ALL/lymphoblastic lymphoma (LL). Median age was 65 years (range: 44-81) and 60% were females (Table 1). Median number of prior therapy was 3 (range: 1-9); all but two patients (UPNs#2 and #10) had previously progressed while on glucocorticoid and were therefore deemed GC-resistant (Supplementary Table S1). Forty percent of the patients were Hispanics and 80% had an ECOG performance status of 0-1 (Table 1).
Table 1.
Demographics, baseline characteristics and outcome of patients in the roflumilast trial
| UPN | Diagnosis | Gender/Age | Ethnicity | ECOG PS | No of prior regimens | No of cycles/days in trial | Best response |
|---|---|---|---|---|---|---|---|
| 1 | DLBCL | F/44 | not Hispanic | 1 | 5 | 2 / 46 | Progressed |
| 2 | CLL | M/69 | not Hispanic | 1 | 4 | 8 / 190 | Stable disease |
| 3 | MZL/DLBCL* | F/46 | Hispanic | 1 | 6 | 13 / 315 | Partial response |
| 4 | CLL | F/81 | not Hispanic | 2 | 7 | 2 / 49 | Progressed |
| 5 | DLBCL | M/81 | not Hispanic | 2 | 9 | 1 / 28 | Progressed |
| 6 | MCL | M/73 | Hispanic | 0 | 1 | 5 / 139 | Stable disease |
| 7 | ALL/LL | F/80 | Hispanic | 1 | 2 | 5 / 127 | Stable disease |
| 8 | MM | M/57 | not Hispanic | 1 | 2 | 4 / 105 | Stable disease |
| 9 | MM | F/61 | not Hispanic | 1 | 2 | 4 / 98 | Stable disease |
| 10 | FL | F/56 | Hispanic | 0 | 1 | 1 / 29 | Withdrawn |
marginal zone lymphoma (MZL) with focal areas of transformation to DLBCL
Safety
The median number of roflumilast and prednisone cycles administered was 4 (range: 1-13) (Table 1). Treatment was generally well tolerated and the majority of adverse events (AE) were reversible and/or clinically manageable. The most common ≥Grade 2 treatment-related AEs (≥25%, all causality) were fatigue, anorexia and transient ≥ grade 2 neutropenia, which was reported in 3 pts (30%). There were no grade 4 events. One patient (UPN#10) experienced a transitory episode of suicidal ideation, a known potential side-effect of roflumilast (listed as a warning in its package insert)(12), at the end of the first cycle. The patient was removed from the study and the adverse event resolved upon cessation of the study drug; this patient was considered not evaluable for efficacy. The most common reason for treatment discontinuation was disease progression.
Efficacy
In this study, the primary and secondary objectives were to define safety and biomarker response. However, as with any phase I/Ib study, we also tried to capture any efficacy data resulting from the therapeutic intervention. Of the nine evaluable patients, we recorded partial response or stable disease in 6 cases (66%), while 3 patients experienced early progression, that is, while still undergoing the first or second treatment cycles (Table 1). The median number of days in trial until progression of the disease, death or withdrawn of consent was 105 days (range: 28-315). Patients of Hispanic origin stayed in trial approximately twice as long as non-Hispanic individuals (average 193 days vs. 84 days, respectively), but this difference was not statistically significant (p=0.08, two-sided Student's t-test). One high-risk (17p deletion) CLL patient with stable disease after 8 cycles (190 days) withdrew consent to join a trial of the BTK inhibitor Ibrutinib. Of note, two non-responders (UPNs#4 and #5), had earlier failed PI3Kδ and AKT inhibitors (Supplementary Table S1); given the ability of PDE4 inhibitors to downmodulate PI3K/AKT in malignant B lymphocytes(17, 21), lack of response to agents directed at these kinases may be considered a predictor of negative response to roflumilast and inform on the design of future clinical trials.
Pharmacokinetics
The average (n=10) Ctss levels for roflumilast and roflumilast N-oxide were 8.2 ng/mL (range: 2.2-17.4 ng/mL) and 37.2 ng/mL (range: 12-74.5 ng/mL), respectively, consistent with the values reported in patients with COPD receiving this PDE4 inhibitor(26). There was no statistically significant difference in the roflumilast/N-oxide concentrations between patients with (n=6) or without clinical response (n=3) (Supplementary Table S2). Likewise, the mean roflumilast/N-oxide values were not significantly distinct when the patients classified according to the degree of PI3K/AKT suppression (Supplementary Table S2). The reasons for this discrepancy are not immediately clear. However, all patients in our pilot series achieved roflumilast/N-oxide levels consistent with those reported in multiple COPD trials, which are markedly above the IC50 values (0.3 & 0.8 ng/mL, respectively) for inhibition of PDE4 (27). Thus, is it is possible that above a certain threshold (e.g., IC50 values) no further suppression of PDE4 activity is achieved and thus no additional biomarker/clinical benefit is imparted. It is also reasonable to speculate that the heterogeneous genetic makeup of the tumors studied (including those of the same histology) could provide a biological basis for the observed results. For example, in preclinical and now in clinical models we have shown that cAMP suppresses PI3K and AKT activity. Thus, it is conceivable that, for example, genetic loss of PTEN would abrogate the benefits of pharmacological PDE4 inhibition, irrespective of the roflumilast/N-oxide levels. In addition, we cannot exclude the possibility that this lack of correlation reflects the small cohort size that typifies a pilot study. Future larger clinical initiatives in which the genetic landscape of the patient's tumors is also available will address this possibility.
Interestingly, an 81 year old Caucasian male patient with diagnosis of DLBCL (UPN#5) had roflumilast (17.4 ng/mL) and N-oxide (74.5 ng/mL) levels approximately twice as high as the average levels of all other patients (Supplementary Table S2). DNA sequencing of this patient's ADME genes (Supplementary Table S3), including Phase 1 (Cytochrome P450 - CYPs) and Phase 2 (Conjugation) isoenzymes, did not reveal the presence of the CP1A2*K allele nor the CYP3A4*20 which were previously associated with significantly higher exposure to roflumilast and the N-oxide(26). However, in this patient we found variants in CYP2B6 (rs3745274, Q172H) and CYP2A6 (rs28399433), enzymes that have been linked to increased concentrations of a structurally similar compound, efavirenz(28). Importantly, these SNPs were not found in other patients in our series with lower roflumilast/N-oxide levels. Additional studies will be necessary to establish a link between these CYPs and secondary pathways of roflumilast/n-oxide metabolism.
Biomarker validation
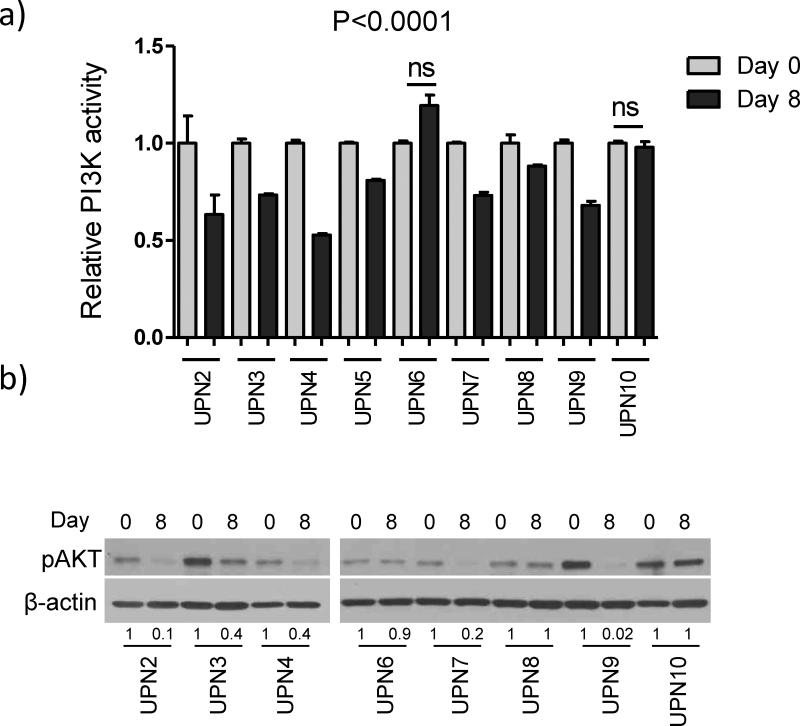
PI3K activity in PBMCs was determined at baseline (day 0) and on day 8, following one week of roflumilast dosing as a single agent. Suitable material was available from 9 of 10 patients that participated in the trial – for the first patient enrolled (UPN#1), not enough protein was obtained from PBMCs to complete this measurement. Treatment with roflumilast significantly suppressed PI3K activity in 7 of the 9 patients evaluated (Figure 1A). Phospho-AKT level (pAKT, T308) was measured by WB in 16 samples (day 0 and day 8) from 8 of the 10 patients enrolled in the trial. In 5 of the 8 eight samples examined (62.5%), a marked suppression of pAKT levels was evident following roflumilast administration (Figure 1B); as expected, in all samples with pAKT suppression, a significant decrease in PI3K activity was also detected (Figure 1). Agreement was also found in 2 cases where neither PI3K nor pAKT were suppressed following administration of roflumilast. Lastly, in one case of modest but significant decrease in PI3K activity (UPN#8, Figure 1A), we could not detect by WB a corresponding decrease in pAKT levels, possibly reflecting the sensitivity of WB-based quantifications (Figure 1B). Together, these data suggest that 500 mcg of Roflumilast administered orally once daily suppress PI3K/AKT activity in patients with advanced B cell malignancies. As expected, for all patients analyzed expression of PDE4B and/or PDE4D was readily detected (Supplementary Figure S1).
Figure 1. Roflumilast suppresses PI3K activity and AKT phosphorylation.
a) PI3K activity was defined by the amount of PI(3,4,5)P3 generated in human peripheral blood mononuclear cells (PBMC) before and after roflumilast dosing for 7 days as a single agent. Data shown are mean ± SD of relative PI3K activity normalized in each case by the day 0 measurements. Each time point was measured in triplicate, statistical value of PI3K suppression were defined with one-way ANOVA and Bonferroni's multiple comparison post-test. b) Western blot analysis of phospho-AKT levels (Threonine 308) in same samples analyzed for PI3K activity. Densitometric values are shown below the blots, standardized by loading control, and normalized by day 0 values.
Although this is a small series that includes patients with diverse diagnoses, and for which safety and tolerability was the primary objective, we also attempted to determine the biomarker role of PI3K/AKT as it relates to clinical response. As indicated above, we detected a combined suppression of PI3K and pAKT levels in 5 of 8 cases tested for both markers (Figure 1). In these five cases, PI3K activity was always suppressed by 25% or more in comparison to baseline, whereas the decrease was less pronounced in cases where pAKT inhibition was not detected. Thus, to examine the putative biomarker role of these quantifications, we dichotomized our population in two groups, “true PI3K/AKT suppressors” (n=5, >25% PI3K inhibition + pAKT suppression), “non-PI3K/AKT suppressors” (n=4, PI3K suppression < 25% and no pAKT suppression) (Table 2). In the PI3K/AKT suppressor group, four of the five patients (80%) had either partial response or stable disease, including a heavily pre-treated NHL (MZL with focal DLBCL transformation) who achieved PR and stayed on trial for 315 days (13 cycles) before progressing, and a high-risk (17p del) CLL patient who withdrew from the trial with stable disease after receiving 8 treatment cycles (190 days). Among the four “non-PI3K/AKT suppressor” patients, one quickly progressed, a second was not evaluable (withdrawn after one cycle due to an adverse event), while the two others had stable disease for 105 and 139 days (4 and 5 cycles, respectively) (Table 2). Together, among the patients clinically evaluable for efficacy (n=8), those with a “true PI3K/AKT suppressor” profile stayed in trial for an average of 156 days (range, 49 - 315 days) versus 91 days (range, 28 – 139 days) for those without PI3K/AKT suppression. Although not statistically significant, in this small pilot series we detected a trend for better clinical response in cases with suppression of PI3K/AKT activity. Future larger studies should allow for a robust characterization of these measurements as biomarkers for activity of PDE4 inhibitors in mature B cell malignancies.
Table 2.
PI3K/AKT activity and clinical feature/outcome of patients in the roflumilast trial
| UPN | Diagnosis | Days on trial | No of cycles | Best response | |
|---|---|---|---|---|---|
| PI3K/AKT suppressors | 02 | CLL | 190 | 8 | Stable disease |
| 03 | MZL/DLBCL | 315 | 13 | Partial response | |
| 04 | CLL | 49 | 2 | Progressed | |
| 07 | ALL/LL | 127 | 5 | Stable disease | |
| 09 | MM | 98 | 4 | Stable disease | |
| PI3K/AKT non-suppressors | 05 | DLBCL | 28 | 1 | Progressed |
| 06 | MCL | 139 | 5 | Stable disease | |
| 08 | MM | 105 | 4 | Stable disease | |
| 10 | FL | 29 | 1 | Not evaluable | |
UPN – Unique patient number; CLL – chronic lymphocytic leukemia, MZL – marginal zone lymphoma; DLBCL – diffuse large B cell lymphoma; ALL/LL – acute lymphoid leukemia/lymphoblastic lymphoma; MM – multiplemyeloma; FL – follicular lymphoma;
Discussion
Here, we described the first-in-cancer clinical trial of a PDE4 inhibitor. In this pilot phase Ib study, we demonstrate that the FDA-approved PDE4 inhibitor roflumilast can be safely repurposed for use as a single agent and in combination with prednisone in patients with relapsed/refractory B cell malignancies, including NHL, CLL, ALL and MM. Although the main objectives of this exploratory trial were to define safety and the pharmacodynamics of PI3K/AKT as biomarkers of PDE4 inhibition, we also preliminarily captured clinical activity. Six of the nine evaluable patients in our series achieved clinical benefit responses (defined as stable disease or partial response), including instances of long (>6 months) disease control in heavily pretreated patients with advanced disease. These preliminary observations appear to validate our in vivo and in vitro pre-clinical studies(17, 19, 21), and suggest that roflumilast may have anti-tumor activity, which at least in part may relate to its ability to influence glucocorticoid (GC) sensitivity in the malignant lymphocyte(20). These data should be interpreted with caution considering its context, a pilot phase Ib study, which was not powered to define clinical activity.
The impetus to test PDE4 inhibitors for the treatment of B cell malignancies stems from our earlier identification of an aberrantly high PDE4B expression in fatal subsets of DLCBL(15). This observation, which has been confirmed in independent tumor series(16-18), is biologically relevant because PDE4 functions by hydrolyzing and terminating the activity of the second messenger cAMP, which has long been known to deliver potent growth inhibitory signals towards immune cells(1). Thus, malignant lymphocytes with elevated expression/activity of PDE4B lose the physiologic growth inhibitory effects of cAMP, which we propose are restored by PDE4 inhibitors. In agreement with this proposition, in addition to our work in B cell lymphomas(15, 17, 19, 20), other groups have provided pre-clinical evidence that the pharmacological targeting of PDE4 can also suppress the growth of CLL, MM and ALL cells(29-32). The inhibitory effects of cAMP towards the innate and adaptive immune system have also been exploited in non-malignant settings, in particular in the context of inflammation(1-3). This understanding, alongside the favorable enzymatic and structural properties of phosphodiesterases in respect to drug development(33), led to the eventual FDA-approval of PDE4-specific inhibitors for inflammatory conditions(12, 13), including roflumilast, which we re-purposed for treatment of mature B cell tumors.
During the pre-clinical mapping of the signaling circuitry modulated by the cAMP/PDE4 axis in DLBCL, we defined an inhibitory effect towards PI3Kδ and, downstream to it, AKT/mTOR(17, 20). These data aligned well with an independent gene expression and chemical genomics screen performed in ALLs in which PDE4B was found to be one of the highest expressed genes in GC-resistant leukemias(34). Importantly, this report also showed that downmodulation of AKT/mTOR signals restored GC-sensitivity. Building on this observation, we demonstrated that pharmacological inhibition of PDE4 in vivo could restore GC sensitivity and markedly inhibit tumor burden in pre-clinical models of human B cell lymphoma(20), a finding also supported by in vitro studies in ALL and CLL(30, 35). Together, these data guided the design of our clinical trial, in which patients were primed with roflumilast as a single agent for 7 days before receiving prednisone, with the idea that this maneuver would enhance their GC responsiveness. Our preliminary results are supportive of this concept, as demonstrated by the fact that five of the six patients with clinical response were initially characterized as GC-resistant. The validation of a role for PDE4 inhibitors in restoring GC sensitivity in the clinic may be particularly important in the context of childhood ALL. In this disease, GC-sensitivity has a strong outcome predictor value and, as indicated above, PDE4B has been reported to be differentially overexpressed GC resistant vs. GC sensitive leukemias(34). In addition, remarkably, a recent genome wide association study (GWAS) uncovered a strong link between PDE4B SNPs and higher risk of relapse in ALL(36, 37), giving further support to the implementation of clinical initiatives that test effectiveness of PDE4 inhibitors in the GC-resistant relapsed ALL.
In pre-clinical models, we showed that genetic or pharmacological inhibition of PDE4 markedly suppresses PI3K activity, and downstream to it, AKT(17, 21). We now confirm these observations in the clinic by showing that 500 mcg of Roflumilast, administered orally once daily for 7 days, suppresses PI3K/AKT activity in the PBMCs of the majority (7 of 9) of patients with advanced B cell malignancies examined. We recognize that ideally these measurements would have been performed in the tumor cells, but operational, and in particular, ethical considerations restricted the development of this strategy. Still, in one CLL case (UPN#2), the peripheral blood was composed primarily of neoplastic B lymphocytes and the marked suppression of PI3Kδ/AKT in this instance may be truly reflective of roflumilast ability to modulate these biomarkers malignant B lymphocytes. We also noted a trend for better clinical response in patients with significant PI3Kδ/AKT inhibition, but future phase II studies with a larger patient cohort powered to detect clinical efficacy, and measurements performed in the tumor cells, are needed to define the validity of these initial observations.
The ability of PDE4 inhibitors to suppress the activity of key component of the proximal BCR signaling, including SYK and PI3Kδ (17, 19-21), is noteworthy. Malignant mature B cells hijack the BCR signals creating a dependency that has been successfully exploited therapeutically, in particular with BTK and PI3K inhibitors(38). However, clinical heterogeneity exists and even within a single diagnosis (e.g., CLL, MCL, DLBCL, etc.) not all patients are responsive to these agents (22, 39-41). Furthermore, acquired resistance in this setting is an emerging problem (42). Attempts to address these limitations have included the combinations of these inhibitors with rituximab(43), with compounds that target other survival pathways in malignant B cells (e.g., BCL2 inhibitor ABT-199)(44), and by associating multiple inhibitors of BCR-related kinase, including PI3Kδ and SYK inhibitors. The latter, unfortunately, has resulted in prohibitive toxicity(45). Building on our pre-clinical data and on the safety and efficacy profile of roflumilast reported here, we suggest that the combination of PDE4 with PI3Kδ, SYK or BTK inhibitors is a rational strategy to attack BCR-dependency, which may improve therapeutic indexes and limit the emergence of acquired resistance to these agents in mature B cell malignancies. Further, we suggest that when used in combination with PI3Kδ, SYK or BTK inhibitors, the anti-inflammatory effects of PDE4 inhibitors may prevent the development of the life-threatening inflammatory pneumonitis that was recently reported in patients treated with a combination of PI3Kδ and SYK inhibitors(45). This provocative prediction is substantiated in part by the putative role of interferon γ and other cytokines in this treatment-emergent pneumonitis and on the well-established ability of PDE4 inhibitors to reduce the expression and secretion of pro-inflammatory cytokines(9-11, 46-48).
In summary, this pilot study shows that the combination of the PDE4 inhibitor roflumilast and prednisone is well tolerated and induces biomarker changes in previously treated patients with refractory/relapsed B cell malignancies. Further clinical development of combinatorial strategies that include PDE4 inhibitors appears justified in view of these observations.
Supplementary Material
Translational relevance.
Phosphodiesterase 4B (PDE4B) hydrolyzes cyclic-AMP, a second messenger with inhibitory activities towards B-lymphocytes. Elevated PDE4B expression/activity in B-cell tumors abrogates cyclic-AMP-mediated growth suppression. In pre-clinical models of B-cell lymphoma, we showed that genetic or pharmacological inhibition of PDE4 suppressed PI3K/AKT activity and enhanced glucocorticoid sensitivity. Here, we report on a first-in-cancer phase Ib pilot study of the FDA-approved PDE4 inhibitor roflumilast and prednisone in advanced B cell malignancies. Treatment was well-tolerated and associated with a manageable safety profile. Pharmacodynamics analysis showed that roflumilast significantly inhibited PI3K/AKT activity, while evidence of clinical activity could be captured in a subset of heavily pre-treated patients. These findings suggest that the repurposing of PDE4 inhibitors for the treatment of B cell tumors should be further explored, and raise the intriguing possibility that these agents may be particularly efficacious in tumors that rely on tonic BCR (PI3Kδ) activity.
Acknowledgments
Grant support: CTRC pilot award to SW; CPRIT awards RP110200 and RP150277 and a grant from the William and Ella Owens Medical Research Foundation, all to RCTA; Cancer Center support grant P30 CA054174
Footnotes
No conflicts of interest to disclose
Authors’ contribution: KK - contributed to trial design, enrolled patients, collected and analyzed clinical data, contributed to manuscript writing; AM - enrolled patients, collected and analyzed clinical data, contributed to manuscript writing; AS and A-PL - performed biomarker analysis; JK - supervised pharmacokinetics analysis, contributed to manuscript writing; AK - enrolled patients, collected and analyzed clinical data; SW - contributed to trial design, coordinated clinical activities; RCTA - conceived the trial, designed and supervised biomarker work, analyzed clinical data, wrote the manuscript.
References
- 1.Bourne HR, Lichtenstein LM, Melmon KL, Henney CS, Weinstein Y, Shearer GM. Modulation of inflammation and immunity by cyclic AMP. Science. 1974;184:19–28. doi: 10.1126/science.184.4132.19. [DOI] [PubMed] [Google Scholar]
- 2.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–32. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters-Golden M. Putting on the brakes: cyclic AMP as a multipronged controller of macrophage function. Science signaling. 2009;2:pe37. doi: 10.1126/scisignal.275pe37. [DOI] [PubMed] [Google Scholar]
- 4.Bodor J, Bopp T, Vaeth M, Klein M, Serfling E, Hunig T, et al. Cyclic AMP underpins suppression by regulatory T cells. Eur J Immunol. 2012;42:1375–84. doi: 10.1002/eji.201141578. [DOI] [PubMed] [Google Scholar]
- 5.Epstein PM, Hachisu R. Cyclic nucleotide phosphodiesterase in normal and leukemic human lymphocytes and lymphoblasts. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;16:303–24. [PubMed] [Google Scholar]
- 6.Bjorgo E, Tasken K. Role of cAMP phosphodiesterase 4 in regulation of T-cell function. Critical reviews in immunology. 2006;26:443–51. doi: 10.1615/critrevimmunol.v26.i5.40. [DOI] [PubMed] [Google Scholar]
- 7.Wang P, Wu P, Ohleth KM, Egan RW, Billah MM. Phosphodiesterase 4B2 is the predominant phosphodiesterase species and undergoes differential regulation of gene expression in human monocytes and neutrophils. Mol Pharmacol. 1999;56:170–4. doi: 10.1124/mol.56.1.170. [DOI] [PubMed] [Google Scholar]
- 8.Jin SL, Conti M. Induction of the cyclic nucleotide phosphodiesterase PDE4B is essential for LPS-activated TNF-alpha responses. Proc Natl Acad Sci U S A. 2002;99:7628–33. doi: 10.1073/pnas.122041599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnette MS, Christensen SB, Essayan DM, Grous M, Prabhakar U, Rush JA, et al. SB 207499 (Ariflo), a potent and selective second-generation phosphodiesterase 4 inhibitor: in vitro anti-inflammatory actions. J Pharmacol Exp Ther. 1998;284:420–6. [PubMed] [Google Scholar]
- 10.Jimenez JL, Punzon C, Navarro J, Munoz-Fernandez MA, Fresno M. Phosphodiesterase 4 inhibitors prevent cytokine secretion by T lymphocytes by inhibiting nuclear factor-kappaB and nuclear factor of activated T cells activation. J Pharmacol Exp Ther. 2001;299:753–9. [PubMed] [Google Scholar]
- 11.Navikas V, Matusevicius D, Soderstrom M, Pirskanen R, Fredrikson S, Link H. The phosphodiesterase i.v. inhibitor rolipram in vitro reduces the numbers of MBP-reactive IFN-gamma and TNF-alpha mRNA expressing blood mononuclear cells in patients with multiple sclerosis. Clin Neuropharmacol. 1998;21:236–44. [PubMed] [Google Scholar]
- 12.Reid DJ, Pham NT. Roflumilast: a novel treatment for chronic obstructive pulmonary disease. The Annals of pharmacotherapy. 2012;46:521–9. doi: 10.1345/aph.1Q646. [DOI] [PubMed] [Google Scholar]
- 13.Schett G. Apremilast in psoriatic arthritis. Clinical and experimental rheumatology. 2015;33:S98–100. [PubMed] [Google Scholar]
- 14.Monti S, Savage KJ, Kutok JL, Feuerhake F, Kurtin P, Mihm M, et al. Molecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory response. Blood. 2005;105:1851–61. doi: 10.1182/blood-2004-07-2947. [DOI] [PubMed] [Google Scholar]
- 15.Shipp MA, Ross KN, Tamayo P, Weng AP, Kutok JL, Aguiar RC, et al. Diffuse large B-cell lymphoma outcome prediction by gene-expression profiling and supervised machine learning. Nat Med. 2002;8:68–74. doi: 10.1038/nm0102-68. [DOI] [PubMed] [Google Scholar]
- 16.Lossos IS, Czerwinski DK, Alizadeh AA, Wechser MA, Tibshirani R, Botstein D, et al. Prediction of survival in diffuse large-B-cell lymphoma based on the expression of six genes. N Engl J Med. 2004;350:1828–37. doi: 10.1056/NEJMoa032520. [DOI] [PubMed] [Google Scholar]
- 17.Smith PG, Wang F, Wilkinson KN, Savage KJ, Klein U, Neuberg DS, et al. The phosphodiesterase PDE4B limits cAMP-associated PI3K/AKT-dependent apoptosis in diffuse large B-cell lymphoma. Blood. 2005;105:308–16. doi: 10.1182/blood-2004-01-0240. [DOI] [PubMed] [Google Scholar]
- 18.Zhao S, Dong X, Shen W, Ye Z, Xiang R. Machine learning-based classification of diffuse large B-cell lymphoma patients by eight gene expression profiles. Cancer Med. 2016;5:837–52. doi: 10.1002/cam4.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim SW, Rai D, McKeller MR, Aguiar RC. Rational combined targeting of phosphodiesterase 4B and SYK in DLBCL. Blood. 2009;113:6153–60. doi: 10.1182/blood-2009-02-206128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim SW, Rai D, Aguiar RC. Gene set enrichment analysis unveils the mechanism for the phosphodiesterase 4B control of glucocorticoid response in B-cell lymphoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2011;17:6723–32. doi: 10.1158/1078-0432.CCR-11-0770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suhasini AN, Wang L, Holder KN, Lin AP, Bhatnagar H, Kim SW, et al. A phosphodiesterase 4B- dependent interplay between tumor cells and the microenvironment regulates angiogenesis in B-cell lymphoma. Leukemia. 2016;30:617–26. doi: 10.1038/leu.2015.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Q, Modi P, Newcomb T, Queva C, Gandhi V. Idelalisib: First-in-Class PI3K Delta Inhibitor for the Treatment of Chronic Lymphocytic Leukemia, Small Lymphocytic Leukemia, and Follicular Lymphoma. Clin Cancer Res. 2015;21:1537–42. doi: 10.1158/1078-0432.CCR-14-2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thappali SR, Varanasi KV, Veeraraghavan S, Vakkalanka SK, Mukkanti K. Simultaneous quantitation of IC87114, roflumilast and its active metabolite roflumilast N-oxide in plasma by LC MS/MS: application for a pharmacokinetic study. Journal of mass spectrometry : JMS. 2012;47:1612–9. doi: 10.1002/jms.3103. [DOI] [PubMed] [Google Scholar]
- 24.Jung I, Aguiar RC. MicroRNA-155 expression and outcome in diffuse large B-cell lymphoma. Br J Haematol. 2009;144:138–40. doi: 10.1111/j.1365-2141.2008.07424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouamar H, Abbas S, Lin AP, Wang L, Jiang D, Holder KN, et al. A capture-sequencing strategy identifies IRF8, EBF1, and APRIL as novel IGH fusion partners in B-cell lymphoma. Blood. 2013;122:726–33. doi: 10.1182/blood-2013-04-495804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clinical Pharmacology and BioPharmaceutics Review. Center for Drug Evaluation and Research; http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022522Orig1s000ClinPharmR.pdf. [Google Scholar]
- 27.Hatzelmann A, Schudt C. Anti-inflammatory and immunomodulatory potential of the novel PDE4 inhibitor roflumilast in vitro. J Pharmacol Exp Ther. 2001;297:267–79. [PubMed] [Google Scholar]
- 28.Haas DW, Kwara A, Richardson DM, Baker P, Papageorgiou I, Acosta EP, et al. Secondary metabolism pathway polymorphisms and plasma efavirenz concentrations in HIV-infected adults with CYP2B6 slow metabolizer genotypes. The Journal of antimicrobial chemotherapy. 2014;69:2175–82. doi: 10.1093/jac/dku110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim DH, Lerner A. Type 4 cyclic adenosine monophosphate phosphodiesterase as a therapeutic target in chronic lymphocytic leukemia. Blood. 1998;92:2484–94. [PubMed] [Google Scholar]
- 30.Ogawa R, Streiff MB, Bugayenko A, Kato GJ. Inhibition of PDE4 phosphodiesterase activity induces growth suppression, apoptosis, glucocorticoid sensitivity, p53, and p21(WAF1/CIP1) proteins in human acute lymphoblastic leukemia cells. Blood. 2002;99:3390–7. doi: 10.1182/blood.v99.9.3390. [DOI] [PubMed] [Google Scholar]
- 31.Rickles RJ, Pierce LT, Giordano TP, Tam WF, McMillin DW, Delmore J, et al. Adenosine A2A receptor agonists and PDE inhibitors: a synergistic multitarget mechanism discovered through systematic combination screening in B-cell malignancies. Blood. 2010;116:593–602. doi: 10.1182/blood-2009-11-252668. [DOI] [PubMed] [Google Scholar]
- 32.Hait WN, Weiss B. Increased cyclic nucleotide phosphodiesterase activity in leukaemic lymphocytes. Nature. 1976;259:321–3. doi: 10.1038/259321a0. [DOI] [PubMed] [Google Scholar]
- 33.Maurice DH, Ke H, Ahmad F, Wang Y, Chung J, Manganiello VC. Advances in targeting cyclic nucleotide phosphodiesterases. Nature reviews Drug discovery. 2014;13:290–314. doi: 10.1038/nrd4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei G, Twomey D, Lamb J, Schlis K, Agarwal J, Stam RW, et al. Gene expression-based chemical genomics identifies rapamycin as a modulator of MCL1 and glucocorticoid resistance. Cancer Cell. 2006;10:331–42. doi: 10.1016/j.ccr.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 35.Meyers JA, Taverna J, Chaves J, Makkinje A, Lerner A. Phosphodiesterase 4 inhibitors augment levels of glucocorticoid receptor in B cell chronic lymphocytic leukemia but not in normal circulating hematopoietic cells. Clin Cancer Res. 2007;13:4920–7. doi: 10.1158/1078-0432.CCR-07-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JJ, Cheng C, Devidas M, Cao X, Campana D, Yang W, et al. Genome-wide association study identifies germline polymorphisms associated with relapse of childhood acute lymphoblastic leukemia. Blood. 2012;120:4197–204. doi: 10.1182/blood-2012-07-440107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moriyama T, Relling MV, Yang JJ. Inherited genetic variation in childhood acute lymphoblastic leukemia. Blood. 2015;125:3988–95. doi: 10.1182/blood-2014-12-580001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nature reviews Drug discovery. 2013;12:229–43. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369:507–16. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wilson WH, Young RM, Schmitz R, Yang Y, Pittaluga S, Wright G, et al. Targeting B cell receptor signaling with ibrutinib in diffuse large B cell lymphoma. Nat Med. 2015;21:922–6. doi: 10.1038/nm.3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med. 2013;369:32–42. doi: 10.1056/NEJMoa1215637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, et al. Resistance mechanisms for the Bruton's tyrosine kinase inhibitor ibrutinib. N Engl J Med. 2014;370:2286–94. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Furman RR, Sharman JP, Coutre SE, Cheson BD, Pagel JM, Hillmen P, et al. Idelalisib and rituximab in relapsed chronic lymphocytic leukemia. N Engl J Med. 2014;370:997–1007. doi: 10.1056/NEJMoa1315226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao X, Bodo J, Sun D, Durkin L, Lin J, Smith MR, et al. Combination of ibrutinib with ABT-199: synergistic effects on proliferation inhibition and apoptosis in mantle cell lymphoma cells through perturbation of BTK, AKT and BCL2 pathways. Br J Haematol. 2015;168:765–8. doi: 10.1111/bjh.13149. [DOI] [PubMed] [Google Scholar]
- 45.Barr PM, Saylors GB, Spurgeon SE, Cheson BD, Greenwald DR, O'Brien SM, et al. Phase 2 study of idelalisib and entospletinib: pneumonitis limits combination therapy in relapsed refractory CLL and NHL. Blood. 2016 doi: 10.1182/blood-2015-12-683516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patrizio M, Costa T, Levi G. Interferon-gamma and lipopolysaccharide reduce cAMP responses in cultured glial cells: reversal by a type IV phosphodiesterase inhibitor. Glia. 1995;14:94–100. doi: 10.1002/glia.440140204. [DOI] [PubMed] [Google Scholar]
- 47.Heystek HC, Thierry AC, Soulard P, Moulon C. Phosphodiesterase 4 inhibitors reduce human dendritic cell inflammatory cytokine production and Th1-polarizing capacity. Int Immunol. 2003;15:827–35. doi: 10.1093/intimm/dxg079. [DOI] [PubMed] [Google Scholar]
- 48.Yamaki K, Li X, Uchida H, Alam AH, Hossain MA, Yanagisawa R, et al. Effects of the phosphodiesterase IV inhibitor rolipram on Th1 and Th2 immune responses in mice. J Pharm Pharmacol. 2004;56:877–82. doi: 10.1211/0022357023655. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.