Summary
Background
Available incidence data for invasive salmonella disease in sub-Saharan Africa are scarce. Standardised, multicountry data are required to better understand the nature and burden of disease in Africa. We aimed to measure the adjusted incidence estimates of typhoid fever and invasive non-typhoidal salmonella (iNTS) disease in sub-Saharan Africa, and the antimicrobial susceptibility profiles of the causative agents.
Methods
We established a systematic, standardised surveillance of blood culture-based febrile illness in 13 African sentinel sites with previous reports of typhoid fever: Burkina Faso (two sites), Ethiopia, Ghana, Guinea-Bissau, Kenya, Madagascar (two sites), Senegal, South Africa, Sudan, and Tanzania (two sites). We used census data and health-care records to define study catchment areas and populations. Eligible participants were either inpatients or outpatients who resided within the catchment area and presented with tympanic (≥38·0°C) or axillary temperature (≥37·5°C). Inpatients with a reported history of fever for 72 h or longer were excluded. We also implemented a health-care utilisation survey in a sample of households randomly selected from each study area to investigate health-seeking behaviour in cases of self-reported fever lasting less than 3 days. Typhoid fever and iNTS disease incidences were corrected for health-care-seeking behaviour and recruitment.
Findings
Between March 1, 2010, and Jan 31, 2014, 135 Salmonella enterica serotype Typhi (S Typhi) and 94 iNTS isolates were cultured from the blood of 13 431 febrile patients. Salmonella spp accounted for 33% or more of all bacterial pathogens at nine sites. The adjusted incidence rate (AIR) of S Typhi per 100 000 person-years of observation ranged from 0 (95% CI 0–0) in Sudan to 383 (274–535) at one site in Burkina Faso; the AIR of iNTS ranged from 0 in Sudan, Ethiopia, Madagascar (Isotry site), and South Africa to 237 (178–316) at the second site in Burkina Faso. The AIR of iNTS and typhoid fever in individuals younger than 15 years old was typically higher than in those aged 15 years or older. Multidrug-resistant S Typhi was isolated in Ghana, Kenya, and Tanzania (both sites combined), and multidrug-resistant iNTS was isolated in Burkina Faso (both sites combined), Ghana, Kenya, and Guinea-Bissau.
Interpretation
Typhoid fever and iNTS disease are major causes of invasive bacterial febrile illness in the sampled locations, most commonly affecting children in both low and high population density settings. The development of iNTS vaccines and the introduction of S Typhi conjugate vaccines should be considered for high-incidence settings, such as those identified in this study.
Funding
Bill & Melinda Gates Foundation.
Introduction
Salmonella infections contribute substantially to global morbidity and mortality.1, 2 The best described invasive salmonella serovars are Salmonella enterica serotype Typhi (S Typhi), causing typhoid fever, and S enterica serotype Paratyphi A, B, and C (S Paratyphi A, B, and C), which cause paratyphoid fever. Other non-typhoidal salmonella (NTS) serovars that typically cause self-limiting diarrhoea can also cause systemic infections, refered to as invasive NTS (iNTS) disease.3 Globally, typhoid fever is estimated to cause 21·7 million illnesses and 217 000 fatalities annually, and iNTS disease is estimated to cause 3·4 million illnesses and 681 000 fatalities annually.1, 2
Substantial knowledge gaps exist regarding the distribution of typhoid fever and iNTS disease in Africa. The few existing studies,4, 5, 6, 7, 8 reported over differing time periods and using various protocols, have been extrapolated and contribute to existing typhoid fever estimates, which limits international generalisability. The scarcity of data in sub-Saharan Africa prompted WHO, in 2008, to request more epidemiological information to reliably estimate the incidence of typhoid fever and iNTS disease and the antimicrobial susceptibilities of the corresponding organisms.9 Consequently, between 2010, and 2014, we established 13 surveillance sites across sub-Saharan Africa in locations where typhoid fever had been previously reported. This network formed the Typhoid Fever Surveillance in Africa Program (TSAP) and served as a platform to implement standardised surveillance of febrile illness and cross-sectional studies to investigate the health-care-seeking behaviour of the surveyed populations.10, 11, 12 Here, we present the adjusted incidence estimates of typhoid fever and iNTS disease and the antimicrobial susceptibility profiles of the causative agents at the 13 selected surveillance sites.
Research in context.
Evidence before this study
We did a literature search using PubMed with the following search terms: (“typhoid” OR “typhoid fever” OR “Salmonella Typhi” OR “S Typhi” OR “salmonella infection” OR “enteric fever” OR “non-typhoidal salmonella” OR “NTS”) AND (“incidence”OR “rate” OR “frequency” OR “prevalence” OR “morbidity” OR “burden” OR “surveillance” OR “epidemiology”). We restricted publication dates from Dec 31, 1995, to July 30, 2016, and no language restrictions were applied. The date of our last search was July 30, 2016.
Salmonella infections are a major cause of global morbidity and mortality; however, substantial knowledge gaps exist with regards to the distribution and incidence of disease caused by Salmonella enterica serotype Typhi and invasive non-typhoidal salmonella (iNTS) disease in sub-Saharan Africa.
Before the Typhoid Fever Surveillance in Africa Program (TSAP), estimates of typhoid fever incidence data from Africa were available from four vaccine trials and one population-based study in Kenya. Other estimates of invasive salmonella infections originated from different descriptions of bacteraemia in febrile patients in The Gambia, Malawi, Mozambique, and Kenya. These few, unstandardised, published data are not sufficient for understanding the burden of the disease in sub-Saharan Africa.
In 2008, WHO expressed the necessity for more epidemiological information to estimate the incidence and antimicrobial susceptibility of invasive salmonella disease. Consequently, in January, 2009, the International Vaccine Institute (Seoul, South Korea) and the Kenya Medical Research Institute (Kilifi, Kenya) co-hosted a meeting with five other international institutions and 28 investigators from 14 research sites across sub-Saharan Africa. The purpose of the meeting was to review existing data on invasive salmonella infections in sub-Saharan Africa and surveillance infrastructure from sites, and to discuss the way forward to investigate invasive salmonella in the African region. These 28 investigators and the five international institutions presented their data on invasive bacterial disease, focusing on invasive salmonellosis.
The data indicated the presence of typhoid fever and iNTS disease; however, the studies were not standardised in design, data collection, and laboratory techniques. The meeting concluded that unless standardised methods of data collection and diagnostic procedure were used across countries, and patterns of health-care utilisation were understood and accounted for, the real disease burden of invasive salmonella infections in the region would remain unclear. As a result, a consortium was established and members agreed to form a network of surveillance sites in sub-Saharan Africa in areas with previous reports of cases of typhoid fever.
The TSAP was created to address the knowledge gaps on the incidence and antimicrobial resistance patterns of invasive salmonella infections at different countries with previous reports of typhoid fever cases in sub-Saharan Africa. TSAP created a network of 13 surveillance sites across ten countries, and implemented cross-sectional studies to investigate the health-care-seeking behaviour of the populations under surveillance.
Added value of this study
Original data collected in TSAP represent the most comprehensive standardised analysis done in sub-Saharan Africa of the incidence and antimicrobial resistance patterns of invasive salmonella infections. The results describe the incidence estimated, adjusted by health-care-seeking behaviour, and antimicrobial susceptibility of typhoid fever and iNTS diseases from 13 sites in ten sub-Saharan Africa countries. For typhoid fever disease, we estimate that the overall incidence is two to three times higher than a previous estimate (10–100 cases per 100 000 people), and is in some settings similar to data from Asia, where the burden is known to be very high. The data also revealed that children aged 2–14 years bear the greatest burden of the disease. For iNTS disease, the data also reflect a high incidence, especially in young children, and in specific sites (Ghana) the incidence could be more than five times that previously estimated.
Implications of all the available evidence
The results of this study underscore the need for preventive measures, including vaccines, improved sanitation and hygiene, malaria control, antiretroviral therapy programmes, and improved nutrition. The results also emphasise that the implementation of effective antimicrobials might be impaired by the presence and potential increase of drug-resistance salmonella strains in the region. The advent of typhoid conjugate vaccines might provide more powerful tools to control typhoid fever; the first vaccine, which was manufactured in India, has already been submitted to WHO for prequalification. Data from this study will be included in the GAVI Alliance review of potential subsidies for typhoid fever vaccines in 2017; their recommendation will be crucial for the deployment of these vaccines. Hence, an urgent need exists to understand the pragmatic aspects of vaccine targeting and delivery, particularly given the burden of disease in children, the associated risk factors, and the focal nature of the disease. Further assessment of the incidence in infants (0–5 months vs 6–11 months) and data on severe typhoid fever or iNTS, including mortality, is crucial to determine the potential effect of future vaccines. Our follow-on study—Severe Typhoid in Africa (SETA)—which investigates severe typhoid burden, is underway.
Methods
Study design, site selection, and participants
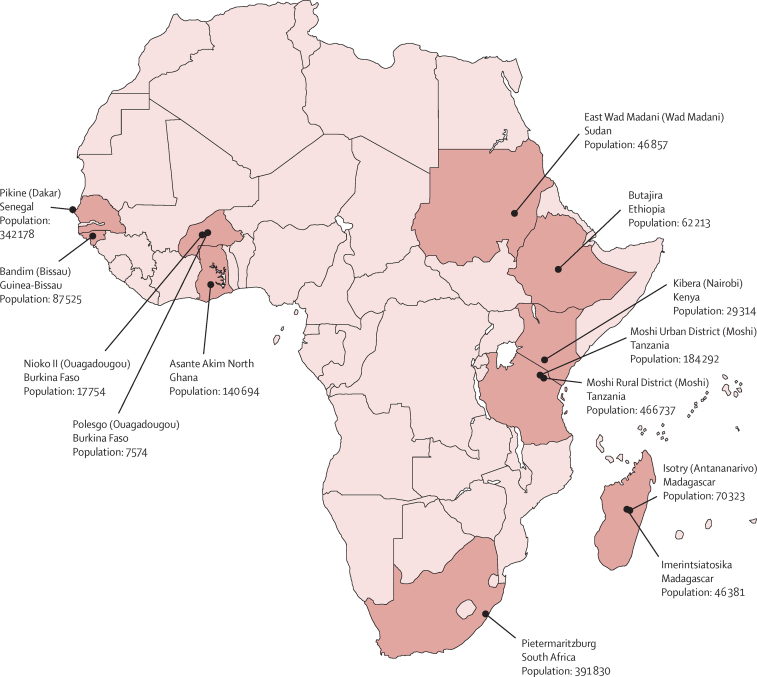
We used a multicentre, population-based, prospective surveillance study design. Selection of the surveillance sites in sub-Saharan Africa was not random; locations were eligible if they had evidence of previous typhoid fever, a laboratory infrastructure suitable for blood culture, an onsite health-care facility, and staff experienced in microbiological laboratory research.10 13 sites in ten countries were selected (figure 1), four of which already had established surveillance systems: Pietermaritzburg, South Africa; Asante Akim North, Ghana; Moshi Urban District and Moshi Rural District, Tanzania; and Kibera, Kenya. Four sites were part of the International Network for the Demographic Evaluation of Populations and Their Health (INDEPTH): Polesgo and Nioko II, Burkina Faso; Butajira, Ethiopia; and Bandim, Guinea-Bissau. These sites had functional Health and Demographic Surveillance Systems (HDSS) in place.13 Additional surveillance sites were Isotry and Imerintsiatosika, Madagascar; Pikine, Senegal; and East Wad Medani, Sudan. The surveillance system in Kibera was established before TSAP with an active, population-based surveillance component. Home visits were done once every 2 weeks to screen for febrile patients and encourage visits to the affiliated health-care facility. Active surveillance in Kibera was continued throughout TSAP. All other sites implemented passive surveillance.10 The ethics committees of all collaborating institutions and the International Vaccine Institute (Seoul, South Korea) approved the study protocol.
Figure 1.
Sites participating in the Typhoid Fever Surveillance in Africa Program
The catchment area for each site was determined through health-care facility records and through accessible administrative and demographic data.11 We determined the population of each catchment area using the latest census or the INDEPTH database. We categorised sites as urban, rural, or other using setting classifications at each site. Surveillance was implemented in each study location for a period of at least 12 months and recruitment occurred at primary, secondary, and tertiary health-care facilities.
Recruitment was open to outpatients and inpatients who visited any of the health-care facilities participating in TSAP, who resided within the catchment area and presented with tympanic (≥38·0°C) or axillary temperature (≥37·5°C). Inpatients with a reported history of fever for 72 h or longer were excluded, as were patients with residence outside of the catchment area. Asante Akim North recruited children younger than age 15 years only; other sites recruited patients of all ages. Written informed consent preceded recruitment and clinical appraisal forms were completed for all participants.
Laboratory procedures
We standardised laboratory, quality control, and blood sample collection procedures across sites.10 Blood (5–10 mL for adults; 1–3 mL for children) was inoculated into aerobic blood culture bottles and incubated in an automated blood culture system (BD BACTEC, Becton-Dickinson, USA, or BacT/ALERT, BioMérieux, France), with the exception of Sudan, where manual culturing with daily subculturing for up to 5 days was instituted. Gram staining and bacterial identification were done with standard microbiological techniques.14 Quality control of preanalytical processes included time and temperature control measures, during which every blood culture bottle was collected, transported, and placed into the incubator. Quality control of analytical processes included sterility and function control of culture media, controls of biochemical reactions, and antimicrobial susceptibility testing. For the quality control of manual culturing in Sudan, additionally, blood culture bottles were inoculated weekly with a suspension containing Escherichia coli or Staphylococcus aureus references. Inoculated blood culture bottles were incubated overnight and verified for growth by subculture.
Contaminants were defined as organisms not typically associated with bloodstream infections; these included non-pathogens and those more commonly associated with commensal skin microbiota, including coagulase-negative Staphylococci, Bacillus spp, and Micrococcus spp. Antimicrobial susceptibility testing was done by disc diffusion according to Clinical and Laboratory Standards Institute15 standards for ampicillin, amoxicillin-clavulanic acid, chloramphenicol, co-trimoxazole, ceftriaxone, and ciprofloxacin. Multidrug resistance was defined as resistance to ampicillin or amoxicillin-clavulanic acid, chloramphenicol, and co-trimoxazole. Isolates with intermediate susceptibility were classified as resistant. Malaria blood smears were routinely done, except in South Africa. In Ethiopia, rapid diagnostic tests (SD BIOLINE Malaria Ag Pf/Pv, SD Standard Diagnostics, Yongin, South Korea) were used in addition to routine malaria blood smears.
Health-care utilisation survey and person-years of observation calculation
The health-care-seeking behaviour of the populations under surveillance was investigated with the assumption that access to the TSAP health-care facility was non-uniform throughout the population.16, 17 A standardised and pretested health-care utilisation survey was implemented in a representative sample of households randomly selected from each study area.11 We investigated health-care-seeking behaviour in cases of self-reported fever lasting less than 3 days. The first choice of health-care facility in cases of fever was categorised by age-stratified groups and used to calculate the proportion of individuals from the catchment population who visited this TSAP health-care facility. This proportion constituted an adjustment factor to correct incidences. The time at risk in person-years of observation (PYO) stratified by age was calculated using the adjusted population. In HDSS sites, each resident contributed to PYO for the time present in the study area during the recruitment period. In non-HDSS sites, we calculated PYO by projecting the catchment population from the start to the end of the study recruitment period, and multiplied the calculated average population by the number of years of surveillance duration.
Statistical analysis
We established a multicountry database using FoxPro software. We excluded patients from the analysis who were recruited during pilot testing, failed to meet inclusion criteria, or had incomplete laboratory results. We estimated incidences per 100 000 PYO. Confirmed invasive salmonella cases, stratified by age group (0–1 years, 2–4 years, 5–14 years, and ≥15 years), were adjusted by the specific age-group recruitment proportion. We calculated this proportion by dividing the number of patients with complete data (numerator) by the total number of patients in the study area who had been diagnosed with a febrile illness at a recruitment facility during the surveillance period (denominator). We used health-care facility records, reviewed at the end of the surveillance activities, to estimate the number of patients diagnosed with a febrile illness. The catchment population in PYO, adjusted by health-care-seeking behaviour, was used as the denominator in crude and adjusted incidence rates (AIR).
The 95% CI for AIR was derived on the log-scale and exponentiated. We used the error factor (exp[1·96/√adjusted cases]) to calculate the lower (adjusted rate/error factor) and upper (adjusted rate × error factor) 95% CIs. At the sites in Senegal, Ethiopia, and South Africa, incomplete health-care facility records did not allow for the estimation of the recruitment proportion and calculation of AIRs; for these sites we present crude rates. AIRs for typhoid fever and iNTS were assessed for all other sites. Differences in proportions of blood cultures positive for a pathogen between study years were assessed with the χ2 test (SAS, version 9.3).
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
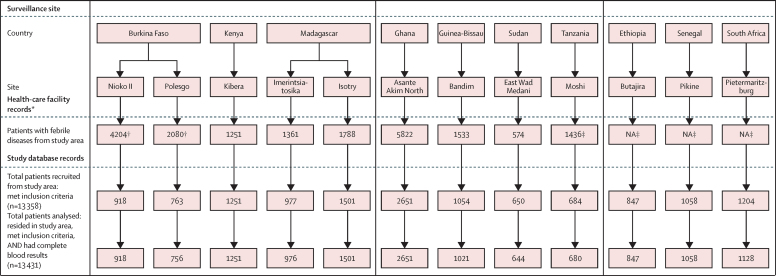
Between March 1, 2010, and Jan 31, 2014, we recruited 13 558 patients from 13 sites who met the inclusion criteria and resided in the catchment areas (Figure 1, Figure 2). We excluded data from 127 (1%) patients because of incomplete laboratory results; data from 13 431 patients were analysed, and 8582 patients (64%) were younger than 15 years (table 1). All patients had one blood culture sample analysed at recruitment and 11 421 (85%) were screened for malaria parasites (table 1). The proportion of contaminated blood cultures ranged from less than 1% in Imerintsiatosika to 24% in Nioko II. The proportion of blood cultures that yielded non-contaminant bacteria varied between sites, ranging from 1% in Imerintsiatosika to 9% in Kibera (table 1). In total, 568 non-contaminant bacteria were isolated from blood samples of febrile patients. The most frequent non-contaminant bacteria isolated were S Typhi (135 [24%]), NTS (94 [17%]), S aureus (70 [12%]), E coli (47 [8%]), and Streptococcus pneumoniae (43 [8%]). Of the sites with at least 2 years of surveillance (Asante Akim North, Kibera, and Pietermaritzburg), the proportion of blood cultures that were pathogen positive differed significantly between study years in Kibera only (12% at year 1 and 5% at year 2; p<0·0001; χ2 test).
Figure 2.
Visits to health-care facilities and recruitment of patients during surveillance period at each site
NA=not available. *Data on health facility visits were collected retrospectively, after completion of surveillance period. Diagnosis of febrile illnesses was used at sites when temperature of patients was not recorded. †Number estimated by the proportion of the population under demographic surveillance at each respective site. ‡In Tanzania, before Nov 11, 2011, every fifth eligible patient was recruited; from Nov 11, 2011, every second eligible patient was recruited. This recruitment pattern was applied to this number.
Table 1.
Demographics and laboratory results of the sites in the Typhoid Fever Surveillance in Africa Program
| Nioko II, Burkina Faso | Polesgo, Burkina Faso | Bandim, Guinea-Bissau | Pikine, Senegal | Asante Akim North, Ghana | East Wad Medani, Sudan | Butajira, Ethiopia | Imerintsiatosika, Madagascar | Isotry, Madagascar | Pietermaritzburg, South Africa | Moshi Urban District, Tanzania | Moshi Rural District, Tanzania | Kibera, Kenya* | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surveillance sites | |||||||||||||
| Type of health-care facility (IPD, OPD) | 1 hospital (IPD, OPD) | 1 health-care centre (OPD) | 1 hospital, 1 health-care centre (IPD, OPD) | 1 hospital, 3 health-care centres (IPD, OPD) | 1 hospital (IPD) | 3 health-care centres (OPD) | 1 hospital, 3 health-care centres (IPD, OPD) | 1 health-care centre (OPD) | 1 health-care centre (OPD) | 1 hospital (IPD) | 1 hospital (IPD, OPD) | 1 hospital (IPD, OPD) | 1 health-care centre (OPD) |
| Setting† | Semi-urban | Semi-urban | Urban | Urban and urban slum | Urban and rural | Urban | Semi-urban and rural | Rural | Urban | Urban | Urban | Rural | Urban slum |
| Population density, people per km2 | 2204 | 5163 | 17 078 | 16 695 | 121 | 7209 | 6545 | 225 | 29 301 | 1191 | 3069 | 332 | 77 000 |
| Surveillance period (months)‡ | April, 2012, to September, 2013 (18) | April, 2012, to September, 2013 (18) | December, 2011, to April, 2013 (17) | December, 2011, to April, 2013 (17) | March, 2010, to May, 2012 (27) | July, 2012, to July, 2013 (13) | May, 2012, to January, 2014 (21) | November, 2011, to June, 2013 (20) | February, 2012, to May, 2013 (16) | February, 2012, to January, 2014 (24) | September, 2011, to May, 2013 (21) | September, 2011, to May, 2013 (21) | January, 2012, to December, 2013 (24) |
| Source of catchment population | HDSS 2011§ | HDSS 2011§ | HDSS 2011§ | Ministry of Health 2012¶ | Census 2010‖ | Census 2008** | HDSS 2012§ | Ministry of Health 2010¶ | Ministry of Health 2010¶ | Census 2010†† | Census 2012‡‡ | Census 2012f | KEMRI/CDC 2012g |
| Collaborating research institution | UoO | UoO | BHP | IPD§ | KCCR/BNITM | UoG | AHRI | UoA | UoA | NICD | KCMC/Duke | KCMC/Duke | KEMRI/US-CDC |
| Patient demographics | |||||||||||||
| Patients analysed, N§§ | 918 | 756 | 1021 | 1058 | 2651 | 644 | 847 | 976 | 1501 | 1128 | 406 | 274 | 1251 |
| Median age, years (IQR) | 4 (1–12) | 7 (3–21) | 3 (1–7) | 22 (14–32) | 2 (0–5) | 15 (9–32) | 11 (5–25) | 20 (9–32) | 26 (17–40) | 3 (1–29) | 7 (1–29) | 19 (2–39) | 7 (4–14) |
| 0–1 years, n (% of N) | 247 (27%) | 117 (15%) | 369 (36%) | 9 (1%) | 1114 (42%) | 2 (<1%) | 74 (9%) | 66 (7%) | 12 (1%) | 427 (38%) | 114 (28%) | 67 (24%) | 99 (8%) |
| 2–4 years, n (% of N) | 235 (26%) | 148 (20%) | 271 (27%) | 23 (2%) | 841 (32%) | 41 (6%) | 124 (15%) | 87 (9%) | 58 (4%) | 209 (19%) | 62 (15%) | 37 (14%) | 312 (25%) |
| 5–14 years, n (% of N) | 228 (25%) | 252 (33%) | 274 (27%) | 255 (24%) | 696 (26%) | 275 (43%) | 303 (36%) | 184 (19%) | 234 (16%) | 95 (8%) | 56 (14%) | 26 (9%) | 539 (43%) |
| ≥15 years, n (% of N) | 208 (23%) | 239 (32%) | 107 (10%) | 771 (73%) | NA | 326 (51%) | 346 (41%) | 639 (65%) | 1197 (80%) | 397 (35%) | 174 (43%) | 144 (53%) | 301 (24%) |
| Female patients, n (% of N) | 467 (51%) | 404 (53%) | 487 (48%) | 468 (44%) | 1204 (45%) | 348 (54%) | 433 (51%) | 570 (58%) | 997 (66%) | 586 (52%) | 211 (52%) | 149 (54%) | 622 (50%) |
| Inpatients, n (% of N) | 66 (7%) | NA¶¶ | 224 (22%) | 241 (23%) | 2651 (100%) | NA¶¶ | 31 (4%) | NA¶¶ | NA¶¶ | 1128 (100%) | 220 (54%) | 156 (57%) | NA¶¶ |
| Laboratory results | |||||||||||||
| Total blood culture, N | 918 | 756 | 1021 | 1058 | 2651 | 644 | 847 | 976 | 1501 | 1128 | 406 | 274 | 1251 |
| Total contaminated blood cultures, n (% of N) | 220 (24%) | 145 (19) | 125 (12%) | 96 (9%) | 182 (7%) | 54 (8%) | 90 (11%) | 6 (1%) | 49 (3%) | 192 (17%) | 8 (2%) | 13 (5%) | 16 (1%) |
| Total positive blood cultures, n (% of N)‖‖ | 29 (3%) | 31 (4) | 30 (3%) | 31 (3%) | 175 (7%) | 16 (2%) | 26 (3%) | 11 (1%) | 30 (2%) | 51 (5%) | 17 (4%) | 11 (4%) | 110 (9%) |
| Positive for malaria, n (% of all patients tested)*** | 430/908 (47%) | 444/744(60%) | 206/525(39%) | 297/1058(28%) | 1139/2651(43%) | 254/632(40%) | 110/822(13%) | 19/955(2%) | 2/274(1%) | 0 | 4/406(1%) | 2/274 (1%) | 226/956(24%) |
UoO=University of Ouagadougou, Ouagadougou. BHP=Bandim Health Project, Bissau. IPD=Institute Pasteur de Dakar, Dakar. KCCR/BNITM=Kumasi Centre for Collaborative Research in Tropical Medicine, Kumasi/Bernhard Nocht Institute for Tropical Medicine, Hamburg, Germany. UoG=University of Gezira, Wad Medani. AHRI=Armauer Hansen Research Institute, Addis Ababa. UoA=University of Antananarivo, Antananarivo. NICD=National Institute for Communicable Diseases, Johannesburg. KCMC/Duke=Kilimanjaro Christian Medical Center, Moshi/Duke University Medical Center, Durham, NC, USA. KEMRI/US-CDC=Kenya Medical Research Institute/US Centers for Disease Control and Prevention, Nairobi. IPD=inpatient department. OPD=outpatient department. HDSS=Health and Demographic Surveillance System. KEMRI=Kenya Medical Research Institute. NA=not available.
In Kibera, active population mobilisation was done in addition to passive surveillance.
Setting reflects the classification commonly used at each site and does not refer to a standard definition.
Surveillance activities were scheduled for 12 months in Burkina Faso, Guinea-Bissau, Senegal, Sudan, Ethiopia, and Madagascar and for 24 months in Ghana, Kenya, South Africa, and Tanzania. If funds allowed, the scheduled period was extended.
Population data were provided from the HDSS country office.
Population data for Senegal and Madagascar were provided by Ministry of Health. Population data correspond to the 2012 population census and 2010 estimated population for the area, respectively.
Population data for Ghana were obtained from the Ghana Statistical Service, 2010 population, and housing census. It includes 53 towns distributed in what is now Asante Akim North and Central.
Population data for Sudan were provided by the Statistics Department, Population Center, University of Gezira, Sudan, and correspond to year 2008.
Population data for South Africa were provided by the Statistics Department in South Africa and corresponds to the 2011 census.
Population data for Tanzania were provided by the National Bureau of Statistics and correspond to the 2012 population and housing census.
Patients who met inclusion criteria, consented to take part in the study, and had a blood culture taken and a documented blood culture result.
Recruitment health-care facility providing outpatient services only.
Positive for non-contaminant isolates.
Denominator differs from all blood cultures analysed because of missing values. Malaria results are based on blood smears, except for the site in Butajira (52% of patients positive for malaria were diagnosed with malaria rapid tests).
With the exception of East Wad Medani, Salmonella spp were isolated from the blood of febrile patients at all sites (135 S Typhi and 94 iNTS isolates), which accounted for 33% or more of all isolated bacteria in all but four sites (East Wad Medani, Pietermaritzburg, Butajira, and Isotry). Seasonal variation was not observed at any site (data not shown). The most common iNTS serovars were S enterica serotype Typhimurium (38 [40%] of 94), S enterica serotype Enteriditis (11 [12%] of 94), and S enterica serotype Dublin (10 [11%] of 94). The highest AIRs for typhoid fever in the 15 years or younger age group were observed in Polesgo, Kibera, and Asante Akim North (table 2). S Paratyphi A (three isolates) was isolated in Senegal only.
Table 2.
Invasive salmonella infections across sites in the Typhoid Fever Surveillance in Africa Program
| Proportion of individuals from study population visiting recruitment facility in case of fever (95% CI) |
PYO estimation |
Recruitment proportion |
Salmonella Typhi |
iNTS |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Study population | Study population adjusted by health-seeking behaviour | PYO | Crude cases | Crude incidence per 100 000 PYO | Cases adjusted for recruitment | Adjusted incidence per 100 000 PYO (95% CI) | Crude cases | Crude incidence per 100 000 PYO | Cases adjusted for recruitment | Adjusted incidence per 100 000 PYO (95% CI) | |||
| Nioko II, Burkina Faso | |||||||||||||
| 0–1 years | 81% (74–88) | 2208 | 1788 | 2097 | 247/1297 (19%) | 0 | 0 | 0·0 | 0 (0–0) | 3 | 143 | 15·8 | 753 (460–1233) |
| 2–4 years | 81% (75–86) | 1823 | 1477 | 2097 | 235/1259 (19%) | 1 | 48 | 5·3 | 251 (107–590) | 3 | 143 | 16·0 | 753 (460–1233) |
| 5–14 years | 81% (78–84) | 4295 | 3479 | 4889 | 228/889 (26%) | 4 | 82 | 15·4 | 315 (191–519) | 3 | 61 | 12·0 | 236 (133–420) |
| <15 years | NA | 8326 | 6744 | 9083 | NA | 5 | 55 | 20·6 | 227 (148–350) | 9 | 99 | 43·1 | 475 (352–640) |
| ≥15 years | 81% (79–83) | 9428 | 7637 | 10 676 | 208/759 (27%) | 0 | 0 | 0·0 | 0 (0–0) | 1 | 9 | 4·0 | 35 (13–96) |
| All | .. | 17 754 | 14 381 | 19 759 | NA | 5 | 25 | 20·6 | 104 (68–161) | 10 | 51 | 46·8 | 237 (178–316) |
| Polesgo, Burkina Faso | |||||||||||||
| 0–1 years | 92% (86–99) | 896 | 824 | 929 | 117/475 (25% | 0 | 0 | 0·0 | 0 (0–0) | 1 | 108 | 4·0 | 431 (162–1147) |
| 2–4 years | 83% (76–89) | 856 | 710 | 992 | 148/466 (32%) | 6 | 605 | 18·8 | 1890 (1202–2972) | 2 | 202 | 6·0 | 630 (288–1380) |
| 5–14 years | 87% (83–91) | 1734 | 1509 | 2104 | 252/510 (49%) | 5 | 238 | 10·2 | 485 (263–896) | 0 | 0 | 0·0 | 0 (0–0) |
| <15 years | NA | 3486 | 3043 | 4025 | NA | 11 | 273 | 29·0 | 719 (500–1035) | 3 | 75 | 10·3 | 255 (138–470) |
| ≥15 years | 87% (84–89) | 4088 | 3557 | 4917 | 239/629 (38%) | 2 | 41 | 5·3 | 107 (46–252) | 1 | 20 | 3·0 | 54 (16–179) |
| All | NA | 7574 | 6600 | 8942 | NA | 13 | 145 | 34·2 | 383 (274–535) | 4 | 45 | 12·9 | 144 (83–249) |
| Bandim, Guinea-Bissau | |||||||||||||
| 0–1 years | 46% (39–54) | 10 852 | 4992 | 5198 | 206/631 (33%) | 0 | 0 | 0·0 | 0 (0–0) | 5 | 96 | 15·2 | 291 (176–482) |
| 2–4 years | 43% (37–48) | 7307 | 3142 | 3866 | 175/359 (49%) | 1 | 26 | 2·0 | 53 (13–208) | 1 | 26 | 2·0 | 53 (13–208) |
| 5–14 years | 42% (41–48) | 19 905 | 8360 | 11 101 | 187/380 (49%) | 1 | 9 | 2·0 | 18 (5–72) | 2 | 18 | 4·0 | 53 (14–97) |
| <15 years | NA | 38 064 | 16 494 | 20 165 | NA | 2 | 10 | 4·1 | 20 (8–53) | 8 | 40 | 21·3 | 116 (69–161) |
| ≥15 years | 45% (43–47) | 62 694 | 28 212 | 37 109 | 105/163 (64%) | 1 | 3 | 1·6 | 4 (1–20) | 0 | 0 | 0·0 | 0 (0–0) |
| All | NA | 100 758 | 44 706 | 57 274 | NA | 3 | 5 | 5·6 | 10 (4–22) | 8 | 14 | 21·3 | 37 (24–57) |
| Asante Akim North, Ghana | |||||||||||||
| 0–1 years | 16% (14–18) | 11 222 | 1760 | 4080 | 41%* | 2 | 49 | 4·9 | 120 (49–290) | 29 | 711 | 70·7 | 1733 (1373–2188) |
| 2–4 years | 16% (13–18) | 8086 | 1268 | 2940 | 41%* | 13 | 442 | 31·7 | 1079 (762–1528) | 23 | 782 | 56·1 | 1908 (1469–2479) |
| 5–14 years | 16% (15–17) | 34 439 | 5415 | 12 554 | 623/1657 (38%) | 15 | 119 | 39·5 | 314 (230–430) | 7 | 56 | 18·4 | 147 (93–232) |
| <15 years | NA | 53 747 | 8443 | 19 574 | NA | 30 | 153 | 76·1 | 389 (310–486) | 59 | 301 | 145·3 | 742 (631–873) |
| ≥15 years | NA | NA† | NA | NA | NA | NA† | NA | NA | NA | NA† | NA | NA | NA |
| All | NA | NA† | NA | NA | NA | NA† | NA | NA | NA | NA† | NA | NA | NA |
| Pikine, Senegalठ| |||||||||||||
| 0–1 years | 39% (32–46) | 20 120 | 7837 | 11 194 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| 2–4 years | 37% (33–41) | 30 180 | 11 097 | 15 851 | NA | 0 | 0 | NA | NA | 0 | 0 | .. | NA |
| 5–14 years | 31% (28–34) | 96 152 | 29 807 | 42 577 | NA | 3 | 7 | NA | NA | 1 | 5 | .. | NA |
| <15 years | NA | 146 452 | 48 741 | 69 623 | NA | 3 | 4 | NA | NA | 0 | 0 | .. | NA |
| ≥15 years | 30% (28–31) | 195 726 | 58 718 | 83 874 | NA | 4 | 5 | NA | NA | 3 | 6 | .. | NA |
| All | NA | 342 178 | 107 459 | 153 496 | NA | 7 | 5 | NA | NA | 4 | 5 | .. | NA |
| East Wad Medani, Sudan§ | |||||||||||||
| 0–1 years | 23% (14–32) | 2377 | 537 | 589 | 2/85 (2%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 2–4 years | 22% (15–29) | 3566 | 781 | 857 | 29/108 (27%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 5–14 years | 25% (21–28) | 11 071 | 2735 | 2999 | 160/234 (68%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| <15 years | NA | 17 014 | 4053 | 4445 | NA | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| ≥15 years | 29% (27–31) | 29 843 | 8684 | 9525 | 131/147 (89%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| All | NA | 46 857 | 12 737 | 13 970 | NA | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| Butajira, Ethiopia§ | |||||||||||||
| 0–1 years | 69% (59–78) | 2266 | 1563 | 2798 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| 2–4 years | 62% (55–69) | 3398 | 2107 | 3771 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| 5–14 years | 65% (61–69) | 14 015 | 9110 | 16 305 | NA | 1 | 6 | NA | NA | 0 | 0 | NA | NA |
| <15 years | NA | 19 679 | 12 780 | 22 874 | NA | 1 | 4 | NA | NA | 0 | 0 | NA | NA |
| ≥15 years | 65% (62–68) | 42 545 | 28 080 | 50 257 | NA | 2 | 4 | NA | NA | 0 | 0 | NA | NA |
| All | NA | 62 224 | 40 860 | 73 131 | NA | 3 | 4 | NA | NA | 0 | 0 | NA | NA |
| Moshi Rural District, Tanzania | |||||||||||||
| 0–1 years | 4% (0–11)¶ | 24 289 | 390 | 693 | 79%* | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 2–4 years | 2% (0–4)‖ | 25 281 | 406 | 721 | 79%* | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 5–14 years | 13% (10–16) | 118 219 | 15 487 | 27 508 | 79%* | 2 (4)** | 15 | 5·1 | 18 (8–44) | 0 | 0 | 0·0 | 0 (0–0) |
| <15 years | NA | 167 789 | 16 283 | 28 922 | NA | 2 (4)** | 14 | 5·1 | 18 (7–42) | 0 | 0 | 0·0 | 0 (0–0) |
| ≥15 years | 2% (1–2) | 298 948 | 5172 | 9186 | 79%* | 1 (2)** | 22 | 2·5 | 28 (8–95) | 1 (2)** | 22 | 2·5 | 28 (8–95) |
| All | NA | 466 737 | 21 454 | 38 108 | NA | 3 (6)** | 16 | 7·6 | 20 (10–41) | 1 (2)** | 5 | 2·5 | 7 (2–23) |
| Moshi Urban District, Tanzania | |||||||||||||
| 0–1 years | 7% (0–19)¶ | 10 406 | 335 | 595 | 79%* | 0 | 0 | 0·0 | 0 (0–0) | 1 (2)** | 336 | 2·5 | 427 (125–1461) |
| 2–4 years | 2% (0–6)‖ | 10 831 | 348 | 618 | 79%* | 1 (5)** | 809 | 6·4 | 1028 (472–2237) | 0 | 0 | 0·0 | 0 (0–0) |
| 5–14 years | 13% (8–19) | 37 309 | 4850 | 8615 | 79%* | 2 (7)** | 81 | 8·9 | 103 (54–199) | 0 | 0 | 0·0 | 0 (0–0) |
| <15 years | NA | 58 546 | 5533 | 9828 | NA | 3 (12)** | 122 | 15·2 | 155 (94–256) | 1 (2)** | 20 | 2·5 | 26 (8–88) |
| ≥15 years | 2% (0–3) | 125 746 | 2138 | 3796 | 79%* | 3 (6)** | 158 | 7·6 | 201 (99–408) | 0 | 0 | 0·0 | 0 (0–0) |
| All | NA | 184 292 | 7671 | 13 626 | NA | 6 (18)** | 132 | 22·9 | 168 (111–253) | 1 (2)** | 15 | 2·5 | 19 (5–64) |
| Kibera, Kenya†† | |||||||||||||
| 0–1 years | 42% (38–47) | 3467 | 1456 | 2031 | 99/99 (100%) | 3 | 148 | 3·0 | 148 (48–458) | 1 | 49 | 1·0 | 49 (7–350) |
| 2–4 years | 39% (36–43) | 3070 | 1197 | 2039 | 312/312 (100%) | 10 | 490 | 10·0 | 490 (264–912) | 1 | 49 | 1·0 | 49 (7–348) |
| 5–14 years | 43% (39–47) | 7514 | 3231 | 5722 | 539/539 (100%) | 28 | 489 | 28·0 | 489 (338–709) | 1 | 17 | 1·0 | 17 (2–124) |
| <15 years | NA | 14 051 | 5884 | 9792 | NA | 41 | 419 | 41·0 | 419 (308–569) | 3 | 31 | 3·0 | 31 (10–95) |
| ≥15 years | 35% (32–38) | 15 263 | 5342 | 9228 | 301/301 (100%) | 13 | 141 | 13·0 | 141 (82–243) | 3 | 33 | 3·0 | 33 (10–101) |
| All | NA | 29 314 | 11 227 | 19 020 | NA | 54 | 284 | 54·0 | 284 (217–371) | 6 | 32 | 6·0 | 32 (14–70) |
| Imerintsiatosika, Madagascar | |||||||||||||
| 0–1 years | 28% (20–37) | 3424 | 753 | 1287 | 66/85(78%) | 0 | 0 | 0·0 | 0 (0–0) | 1 | 78 | 1·3 | 100 (18–562) |
| 2–4 years | 19% (14–25) | 5136 | 1130 | 1932 | 87/101 (86%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 5–14 years | 18% (15–20) | 13 188 | 2374 | 4057 | 184/256 (72%) | 5 | 123 | 6·9 | 171 (81–360) | 0 | 0 | 0·0 | 0 (0–0) |
| <15 years | NA | 21 748 | 4257 | 7276 | NA | 5 | 69 | 6·9 | 95 (45–201) | 1 | 14 | 1·3 | 18 (3–99) |
| ≥15 years | 17% (15–19) | 24 632 | 4187 | 7153 | 639/919 (70%) | 1 | 14 | 1·4 | 20 (4–103) | 0 | 0 | 0·0 | 0 (0–0) |
| All | NA | 46 380 | 8444 | 14 429 | NA | 6 | 42 | 8·4 | 58 (29–114) | 1 | 7 | 1·3 | 9 (2–50) |
| Isotry, Madagasar | |||||||||||||
| 0–1 years | 6% (1–12) | 3204 | 192 | 261 | 12/14 (86%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 2–4 years | 10% (5–14) | 4805 | 481 | 653 | 58/65 (89%) | 0 | 0 | 0·0 | 0 (0–0) | 0 | 0 | 0·0 | 0 (0–0) |
| 5–14 years | 9% (7–11) | 16 386 | 1475 | 2005 | 234/288 (81%) | 1 | 50 | 1·2 | 62 (11–359) | 0 | 0 | 0·0 | 0 (0–0) |
| <15 years | NA | 24 395 | 2147 | 2919 | NA | 1 | 34 | 1·2 | 42 (7–247) | 0 | 0 | 0·0 | 0 (0–0) |
| ≥15 years | 9% (7–11) | 45 928 | 4134 | 5621 | 1197/1421 (84%) | 2 | 36 | 2·4 | 42 (12–151) | 0 | 0 | 0·0 | 0 (0–0) |
| All | NA | 70 323 | 6281 | 8540 | NA | 3 | 35 | 3·6 | 42 (15–119) | 0 | 0 | 0·0 | 0 (0–0) |
| Pietermaritzburg, South Africa§ | |||||||||||||
| 0–1 years | 11% (5–17) | 13 990 | 1511 | 3055 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| 2–4 years | 7% (3–12) | 20 985 | 1490 | 3013 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| 5–14 years | 16% (13–19) | 62 313 | 10 157 | 20 537 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| <15 years | NA | 97 288 | 13 158 | 26 605 | NA | 0 | 0 | NA | NA | 0 | 0 | NA | NA |
| ≥15 years | 15% (13–17) | 294 542 | 43 887 | 88 739 | NA | 2 | 2 | NA | NA | 0 | 0 | NA | NA |
| All | NA | 391 830 | 57 045 | 115 344 | NA | 2 | 2 | NA | NA | 0 | 0 | NA | NA |
Study population was adjusted for health-seeking behaviour and crude cases were adjusted for recruitment proportion (number of patients analysed divided by number of patients with febrile illness from study area who visited a recruitment health facility, multiplied by 100). iNTS=invasive non-typhoidal salmonella. NA=not available. PYO=person-years of observation.
*Recruitment portion was not available for each age strata. Broader values were applied to each stratum.
Target population for surveillance activities in Ghana included patients younger than 15 years of age; patients aged 15 years or older were not recruited.
Three Salmonella Paratyphi A were identified at this site, but are not included in this table.
No salmonella was isolated in Sudan. Missing data on recruitment patterns in Senegal, Ethiopia, and South Africa did not allow calculation of adjusted incidences. Crude rates are presented.
This proportion applies to age group <1 year, and it was used to adjust the study population by health-seeking behaviour.
This proportion applies to age group 1–4 years, and it was used to adjust the study population by health-seeking behaviour. The adjusted populations in age groups <1 year and 1–4 years were added to estimate the total adjusted population age group 0–4 years. Subsequently, the percentage of children <2 years reported by the 2012 national census was applied to derive age groups 0–1 years and 2–4 years.
Crude cases have been adjusted for recruitment pattern unique to the site in Tanzania: before Nov 11, 2011, every fifth eligible patient was recruited; from Nov 11, 2011, every second eligible patient was recruited. Adjusted cases (presented inside parentheses) were used to calculate crude rate.
Active population mobilisation was done, in addition to passive surveillance.
Among age groups of children younger than 15 years, the highest AIR for typhoid fever was observed in children aged 2–4 years from Polesgo, Asante Akim North, Moshi Urban District, and Kibera, and in children aged 5–14 years from Kibera and Polesgo (table 2). The AIR for typhoid fever in adults (aged ≥15 years) was less than 70 per 100 000 PYO at all sites except Moshi Urban District, Kibera, and Polesgo (table 2).
iNTS organisms were more frequently isolated from infants (0–1 years) or children aged 2–4 years than from adults (table 2), except for the sites in Pikine, Moshi Rural District, and Kibera. The AIR for iNTS among children aged 2–4 years was highest in Nioko II, Polesgo, and Asante Akim North. The AIR for iNTS in children younger than 15 years was less than 100 per 100 000 PYO in Kibera, Imerintsiatosika, and in both sites in Tanzania. No iNTS was isolated from sites in Sudan, South Africa, Ethiopia, and Isotry.
The antimicrobial susceptibility profiles of S Typhi and iNTS isolates differed between sites (table 3). Overall, 47% of S Typhi isolates and 48% of iNTS isolates were multidrug resistant. Most multidrug-resistant S Typhi isolates were obtained at the sites in Kenya, Ghana, and Tanzania (both sites combined). Multidrug-resistant iNTS isolates were isolated at the sites in Burkina Faso (both combined), Ghana, Guinea-Bissau, and Kenya (table 3). S Typhi isolates that had reduced ciprofloxacin susceptibility were cultured in Kenya and South Africa, only; one ciprofloxacin-resistant S Paratyphi A organism was isolated in Senegal. Ciprofloxacin-resistant iNTS was similarly uncommon, isolated only in Burkino Faso (once at the Nioko II site) and in Ghana. One iNTS isolate in Kenya was resistant to ceftriaxone (table 3).
Table 3.
Antimicrobial resistance patterns of Salmonella enterica serotype Typhi and iNTS isolates across sites
| Burkina Faso | Guinea-Bissau | Senegal* | Ghana | Ethiopia | Madagascar | South Africa | Tanzania | Kenya | All | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total S Typhi isolates, N | 18 | 3 | 7 | 30 | 3 | 9 | 2 | 9 | 54 | 135 | |
| Isolate with antimicrobial resistance, n (%)† | |||||||||||
| Ampicillin | 0 | NR | NR | 20 (67%) | 2 (67%) | NR | 0 | 8 (89%) | 41 (76%) | 71 (53%) | |
| Amoxicillin-clavulanic acid | 0 | NR | NR | 3 (10%) | 0 | NR | 0 | 4 (44%) | 24 (44%) | 31 (23%) | |
| Chloramphenicol | 2 (11%) | NR | NR | 23 (77%) | 0 | NR | 0 | 5 (56%) | 43 (80%) | 73 (54%) | |
| Co-trimoxazole | 2 (11%) | NR | NR | 24 (80%) | 0 | NR | 0 | 8 (89%) | 43 (80%) | 77 (57%) | |
| Ceftriaxone | 0 | NR | NR | 0 | 0 | NR | 0 | 0 | 0 | 0 | |
| Ciprofloxacin | 0 | NR | NR | 0 | 0 | NR | 1 (50%) | 0 | 11 (20%) | 12 (9%) | |
| Multidrug resistance‡ | 0 | NR | NR | 19 (63%) | 0 | NR | 0 | 5 (56%) | 40 (74%) | 64 (47%) | |
| Total iNTS isolates, N | 14 | 8 | 4 | 59 | 0 | 1 | 0 | 2 | 6 | 94 | |
| Isolate with antimicrobial resistance, n (%)† | |||||||||||
| Ampicillin | 10 (71%) | 1 (13%) | NR | 38 (64%) | NR | NR | NR | 0 | 2 (33%) | 51 (54%) | |
| Amoxicillin-clavulanic acid | 3 (21%) | 0 | NR | 9 (15%) | NR | NR | NR | 0 | 2 (33%) | 14 (15%) | |
| Chloramphenicol | 12 (86%) | 1 (13%) | NR | 34 (58%) | NR | NR | NR | 0 | 1 (17%) | 48 (51%) | |
| Co-trimoxazole | 13 (93%) | 1 (13%) | NR | 34 (58%) | NR | NR | NR | 0 | 2 (33%) | 50 (53%) | |
| Ceftriaxone | 0 | 0 | NR | 0 | NR | NR | NR | 0 | 1 (17%) | 1 (1%) | |
| Ciprofloxacin | 1 (7%) | 0 | NR | 2 (3%) | NR | NR | NR | 0 | 0 | 3 (3%) | |
| Multidrug resistance‡ | 10 (71%) | 1 (13%) | NR | 33 (56%) | NR | NR | NR | 0 | 1 (17%) | 45 (48%) | |
Resistant isolates are reported per country, rather than per site. No Salmonella enterica serotype Typhi (S Typhi) or iNTS isolates were cultured in Sudan. iNTS=invasive non-typhoidal salmonella. NR=no resistant isolates identified.
Seven S Typhi, four iNTS, and three S enterica serotype Paratyphi (S Paratyphi) isolates. One of the S Paratyphi isolates was resistant to ciprofloxacin.
Includes isolates fully and intermediately resistant against the respective drug, as defined by the Clinical Laboratory and Standards Institute guidelines 2013.15
Defined as resistance against ampicillin or amoxicillin AND chloramphenicol AND co-trimoxazole.
Discussion
This study identified Salmonella as a major cause of invasive bacterial febrile illness across sub-Saharan Africa, affecting children aged 2–14 years rather than adults, and arising in both high-population and low-population density settings. Other major causes of invasive bacterial febrile illnesses varied by country; E coli and S aureus were the most frequent non-Salmonella pathogens isolated from blood.
Results from previous studies18, 19 suggest that typhoid fever in some sub-Saharan Africa settings occurs predominately in urban settlements with high-population densities, and that disease incidence could have been overestimated by the use of the Widal test. Our study, done using a standardised protocol in both urban and rural settings, indicated high incidences of typhoid fever and iNTS in areas with high-population and low-population densities. Separate analyses done at the Ghana site confirmed this observation and revealed a higher disease incidence in children living in rural areas than in those living in urban areas.20 Furthermore, we observed variable incidences of typhoid fever and iNTS among neighbouring populations in Burkina Faso, and in the same populations in Kenya and Ghana in consecutive years, indicating a focal nature and a fluctuating burden of iNTS disease.
A previous global estimate of the burden of typhoid fever indicated that south-central and east-central Asia had the highest incidences of typhoid fever with more than 100 cases per 100 000 people annually; Africa was estimated to have a medium incidence (10–100 cases per 100 000).1 The AIR for typhoid fever estimated in our study reveals a higher burden than previously estimated.1 Four sites had an overall AIR for typhoid fever of more than 100 per 100 000 PYO, five sites had an AIR for typhoid fever of more than 100 per 100 000 PYO in children younger than 15 years, and six sites had an AIR for typhoid fever of more than 100 per 100 000 PYO in at least one age group. Similar to the Diseases of the Most Impoverished programme done in Asia,21 our results show that children aged 2–14 years bear the greatest burden of typhoid fever. Notably, our data indicate that the AIR for typhoid fever at TSAP sites was equal to or even greater than incidences reported in five Asian countries in the early 2000s.21, 22
For iNTS disease, we observed an AIR equal or higher than previously estimated and a bimodal age distribution with very young children and adults being the key age group for symptomatic infection.2 This age distribution differed from that observed for typhoid fever, in which children aged 2–14 years were the most affected, and emphasises substantial differences in the epidemiology of typhoid fever and iNTS disease. Malaria, malnutrition, and HIV infections have been reported to be associated with iNTS disease in Africa.23 At TSAP sites, a higher AIR for iNTS was observed in children with a malaria positivity rate of 30% or more than in those with a lower positivity rate; this observation was confirmed in a separate analysis.24
Results of our study identified a high prevalence of resistance against first-line antimicrobials in both S Typhi and iNTS infections. Reduced susceptibility to ciprofloxacin was identified in S Typhi from Kibera and Pietermaritzburg. Multidrug-resistant iNTS isolates were isolated at several sites and have been isolated in sub-Saharan Africa previously.18, 25, 26 Furthermore, a single iNTS isolate from Kibera showed resistance to ceftriaxone. Genomic analyses27 have described the spread of S Typhi haplotype H58 into Africa, a multidrug-resistant strain associated with reduced ciprofloxacin susceptibility. The susceptibility patterns observed in our study are concerning, particularly because some antimicrobial-resistant S Typhi can have a selective fitness advantage.28 Concerted measures are needed to monitor the emergence of fluoroquinolone-resistant Salmonella.29, 30, 31, 32
We made all efforts to minimise bias; however, our study has some limitations. First, we did not adjust the disease incidences for blood culture sensitivity, which is approximately 40–60% of bone marrow culture.33, 34, 35, 36, 37 This correction factor is inconsistently applied in studies and, if applied here, the incidences presented would double. The restricted sensitivity of blood culture to detect Salmonella pathogens applies to other bacterial pathogens as well—ie, S pneumoniae and Haemophilus influenzae type b—however, those are universally recognised as important infections for which vaccines are cost-effective, and vaccination programmes have been established. Second, our results represent incidence in sites selected because of their previous reports on typhoid fever. The site selection strategy limits the generalisability of the AIR to other locations and might result in the reduced detection of iNTS disease. Third, given the vast number of patients (and restricted diagnostics capacity), not every patient with a history of fever was enrolled—eg, at sites where inpatients were recruited, patients with a fever for 72 h or longer were excluded to minimise the inclusion of patients pretreated with antimicrobials and to maximise blood culture yield. Fourth, the proportion of the catchment population using the TSAP health-care facilities for febrile illness was low in some sites, and antimicrobial treatment before blood collection and its potential effect on blood culture sensitivity were not assessed. Fifth, the classification of the settings as either urban, rural, semi-urban, or urban-slum reflects the classification commonly used at each site and does not refer to a standard definition; instead, the population density of each site is presented to make setting comparisons. Sixth, sites with no previous experience of blood collection for blood culture assessment had a higher incidence of contamination than sites with previous experience of blood collection (South Africa, Ghana, Tanzania, and Kenya); these incidences might have led to errors in clinical interpretation and uncertainty to distinguish between clinically significant bacteraemia and contamination. Available isolates and blood samples collected from participants were PCR tested at the reference lab to minimise misclassification of isolated organisms. Seventh, the site in Ghana recruited only children younger than 15 years and the proportion of recruited inpatients varied greatly across all sites. Finally, data on disease severity, complications, mortality, and HIV status were not assessed because these were not primary study objectives. Despite these limitations, this multisite study, the largest study of typhoid fever and iNTS done across sub-Saharan Africa to date, provides the most current and accurate incidence figures for these major infectious diseases across the continent and has substantial implications for their control.
We surmise that the incidence of invasive salmonella infections among children in sub-Saharan Africa is much higher than previously estimated, underscoring the need for preventive measures. Therefore, until access to safe drinking water and improved sanitation is greatly expanded, the prevention of typhoid fever will require immunisation and effective treatment options.38 The advent of new typhoid fever conjugate vaccines might provide more powerful tools for disease control; the first typhoid fever conjugate vaccine (Bharat Biotech, Hyderabad) has been submitted to WHO for prequalification. Data from TSAP will be incorporated into the GAVI Alliances' review of potential subsidies for typhoid fever vaccines in 2017; their recommendation will be crucial for deployment of these vaccines. Hence, the need to understand the pragmatic aspects of vaccine targeting and delivery is pressing, particularly given the burden of disease in children, the associated risk factors, and the focal and unpredictable nature of the disease. Similarly, in the absence of vaccines targeting iNTS disease, prevention will require a major investment in infrastructure for diagnosis and effective treatment of iNTS disease. When appropriate diagnosis and treatment are available, the use of effective antimicrobials might be impaired by the presence and potential increase of multidrug-resistant salmonella. Further assessment of incidences in infants (0–5 months vs 6–11 months) and data on severe typhoid fever or iNTS, including mortality, is crucial to determine the potential effect of future vaccines. We are currently undertaking a follow-on study—Severe Typhoid in Africa (SETA)—which investigates severe typhoid burden.
We conclude that typhoid fever and iNTS disease are major agents of invasive bloodstream infections in urban and rural locations, affecting children more commonly than adults across sub-Saharan Africa. Immunisation of high-risk age groups with existing and new vaccines should be a priority. The next generation of epidemiological studies in sub-Saharan Africa needs to provide better data regarding the severity and mortality of typhoid fever and iNTS to guide the introduction of new typhoid and iNTS vaccines. Lastly, the accelerated development and introduction of iNTS vaccines needs to become a fundamental goal on the global health agenda.
For the study protocol see http://www.ivi.int/?page_id=12479&uid=922&mod=document
Acknowledgments
Acknowledgments
This study was supported by the Bill & Melinda Gates Foundation (OPPGH5231). The findings and conclusions contained within are our own and do not necessarily reflect positions or policies of the Bill & Melinda Gates Foundation or the US Centers for Disease Control and Prevention. International Vaccine Institute acknowledges its donors, including the South Korea and the Swedish International Development Cooperation Agency (Sida). Research infrastructure at the Moshi site was supported by the US National Institutes of Health (R01TW009237; U01 AI062563; R24 TW007988; D43 PA-03–018; U01 AI069484; U01 AI067854; P30 AI064518), and by the UK Biotechnology and Biological Sciences Research Council (BB/J010367). SB is a Sir Henry Dale Fellow, jointly funded by the Wellcome Trust and the Royal Society (100087/Z/12/Z). We are grateful to Sooyoung Kwon for her invaluable administrative support of the project. We also thank all patients who consented to participate and hospital and clinic staff for their support. We especially acknowledge those who personally contributed to the implementation and execution of the study, additional to routine clinical work. Without the efforts of dedicated field staff this research would not have been possible.
Declaration of interests
FM, JAC, TFW, and RFB report grants from Bill & Melinda Gates Foundation during the conduct of the study. All other authors declare no competing interests.
Contributors
FM and TFW contributed to study conception and design, analysis of data, interpretation of results, and drafting and editing of the paper. FK, JM, UP, VvK, EDM, and JDC contributed to study conception and design, data interpretation, and editing of the paper. MA, GDP, LMCE, VvK, and JKP contributed to data analysis. KT and BL contributed to study conception and design, data acquisition in the field, interpretation of the results, and editing of the paper. VvK, LMCE, SEP, CGM, CN, and JI drafted the manuscript and contributed to interpretation of results and editing of the paper. RFB, MA, FK, JM, UP, TFW, VvK, PA, YA-S, AA, MB-A, JAC, LMCE, JFD, NG, JTH, JI, HJJ, KHK, JMM, RK, RR, AGS, SEP, HJS, AS, MT, MRW, BY, MAET, HMB, LC, AJ, SVL, TMR, NS, and AT contributed to data acquisition in the field, interpretation of results, and editing of the paper. SB, JIC, UP, DMD, BSF, LPK, AAN, NVMH, BO, HR, TJLR, ES, HS-G, and AS contributed to laboratory work, interpretation of results, and editing of the paper. All authors read and approved the final draft.
References
- 1.Crump JA, Luby SP, Mintz ED. The global burden of typhoid fever. Bull World Health Organ. 2004;82:346–353. [PMC free article] [PubMed] [Google Scholar]
- 2.Ao TT, Feasey NA, Gordon MA, Keddy KH, Angulo FJ, Crump JA. Global burden of invasive non-typhoidal Salmonella disease. Emerg Infect Dis. 2015;21:e941–e949. doi: 10.3201/eid2106.140999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Langridge GC, Nair S, Wain J. Nontyphoidal Salmonella serovars cause different degrees of invasive disease globally. J Infect Dis. 2009;199:602–603. doi: 10.1086/596208. [DOI] [PubMed] [Google Scholar]
- 4.Mogasale V, Maskery B, Ochiai RL. Burden of typhoid fever in low-income and middle-income countries: a systematic, literature-based update with risk-factor adjustment. Lancet Global Health. 2014;2:e570–e580. doi: 10.1016/S2214-109X(14)70301-8. [DOI] [PubMed] [Google Scholar]
- 5.Buckle GC, Walker CLF, Black RE. Typhoid fever and paratyphoid fever: systematic review to estimate global morbidity and mortality for 2010. J Global Health. 2012;2:010401. doi: 10.7189/jogh.02.010401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nielsen MV, Sarpong N, Krumkamp R. Incidence and characteristics of bacteremia among children in rural Ghana. PLoS One. 2012;7:e44063. doi: 10.1371/journal.pone.0044063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breiman RF, Cosmas L, Njuguna H. Population-based incidence of typhoid fever in an urban informal settlement and a rural area in Kenya: implications for typhoid vaccine use in Africa. PLoS One. 2012;7:e29119. doi: 10.1371/journal.pone.0029119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marks F, Adu-Sarkodie Y, Hünger F. Typhoid fever among children, Ghana. Emerg Infect Dis. 2010;16:1796. doi: 10.3201/eid1611.100388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.No authors listed Typhoid vaccines: WHO position paper. Wkly Epidemiol Rec. 2008;83:49–59. [PubMed] [Google Scholar]
- 10.von Kalckreuth V, Konings F, Aaby P. The Typhoid Fever Surveillance in Africa Program (TSAP): clinical, diagnostic, and epidemiological methodologies. Clin Infect Dis. 2016;62:S9. doi: 10.1093/cid/civ693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panzner U, Pak GD, Aaby P. The utilization of healthcare facilities in the Typhoid Fever Surveillance in Sub-Saharan Africa Program (TSAP) Clin Infect Dis. 2016;62:S56. doi: 10.1093/cid/civ891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clemens JD. Meeting on establishment of consortium to study invasive salmonellosis in sub-Saharan Africa. Emerg Infect Dis. 2009;15:e2. doi: 10.3201/eid1507.090416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sankoh O, Byass P. The INDEPTH network: filling vital gaps in global epidemiology. Int J Epidemiol. 2012;41:579–588. doi: 10.1093/ije/dys081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Murray P, Baro EJ. Chapter 42: Enterobacteriaceae—introduction and identification. Manual of Clinical Microbiology. 9th edn. ASM Press; Washington, DC: 2007. pp. 649–669. [Google Scholar]
- 15.Clinical and Laboratory Standards Institute . Performance standards for antimicrobial susceptibility testing; 23rd informational supplement; M100-S23. Clinical and Laboratory Standards Institute; Wayne, PA: 2013. [Google Scholar]
- 16.Burton DC, Flannery B, Onyango B. Healthcare-seeking behaviour for common infectious disease-related illnesses in rural Kenya: a community-based house-to-house survey. J Health Popul Nutr. 2011;29:61–70. doi: 10.3329/jhpn.v29i1.7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bigogo G, Audi A, Aura B, Aol G, Breiman RF, Feikin DR. Health-seeking patterns among participants of population-based morbidity surveillance in rural western Kenya: implications for calculating disease rates. Int J Infect Dis. 2010;14:e967–e973. doi: 10.1016/j.ijid.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 18.Tabu C, Breiman RF, Ochieng B. Differing burden and epidemiology of non-Typhi Salmonella bacteremia in rural and urban Kenya, 2006–2009. PLoS One. 2012;7:e31237. doi: 10.1371/journal.pone.0031237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mweu E, English M. Typhoid fever in children in Africa. Trop Med Int Health. 2008;13:532–540. doi: 10.1111/j.1365-3156.2008.02031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cruz Espinoza LM, Nichols C, Adu-Sarkodie Y. Variations of invasive Salmonella infections by population size in Asante Akim North Municipal, Ghana. Clin Infect Dis. 2016;62:S17. doi: 10.1093/cid/civ787. [DOI] [PubMed] [Google Scholar]
- 21.Ochiai RL, Acosta CJ, Danovaro-Holliday MC. A study of typhoid fever in five asian countries: Disease burden and implications for controls. Bull World Health Organ. 2008;86:260–268. doi: 10.2471/BLT.06.039818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owais A, Sultana S, Zaman U, Rizvi A, Zaidi AK. Incidence of typhoid bacteremia in infants and young children in southern coastal Pakistan. Pediatr Infect Dis J. 2010;29:1035–1039. [PMC free article] [PubMed] [Google Scholar]
- 23.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. Invasive non-typhoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet. 2012;379:2489–2499. doi: 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park SE, Pak GD, Aaby P. The relationship between invasive non-Typhoidal Salmonella disease, other bloodstream infections, and malaria in sub-Saharan Africa. Clin Infect Dis. 2016;62:S23. doi: 10.1093/cid/civ893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takem EN, Roca A, Cunnington A. The association between malaria and non-typhoid Salmonella bacteraemia in children in sub-Saharan Africa: a literature review. Malar J. 2014;13:400. doi: 10.1186/1475-2875-13-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mengo DM, Kariuki S, Muigai A, Revathi G. Trends in Salmonella enterica serovar Typhi in Nairobi, Kenya from 2004 to 2006. J Infect Dev Ctries. 2010;4:393–396. [PubMed] [Google Scholar]
- 27.Kariuki S, Revathi G, Kiiru J. Typhoid in Kenya is associated with a dominant multidrug-resistant Salmonella enterica serovar Typhi haplotype that is also widespread in southeast asia. J Clin Microbiol. 2010;48:2171–2176. doi: 10.1128/JCM.01983-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dougan G, Baker S. Salmonella enterica serovar Typhi and the pathogenesis of typhoid fever. Annu Rev Microbiol. 2014;68:317–336. doi: 10.1146/annurev-micro-091313-103739. [DOI] [PubMed] [Google Scholar]
- 29.Kaur J. Increasing antimicrobial resistance and narrowing therapeutics in Typhoidal Salmonellae. J Clin Diagn Res. 2013;7:576–579. doi: 10.7860/JCDR/2013/4765.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vlieghe ER, Phe T, De Smet B. Azithromycin and ciprofloxacin resistance in Salmonella bloodstream infections in cambodian adults. PLoS Negl Trop Dis. 2012;6:e1933. doi: 10.1371/journal.pntd.0001933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koirala KD, Thanh DP, Thapa SD. Highly resistant Salmonella enterica serovar typhi with a novel gyra mutation raises questions about the long-term efficacy of older fluoroquinolones for treating typhoid fever. Antimicrob Agents Chemother. 2012;56:2761–2762. doi: 10.1128/AAC.06414-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker S. A return to the pre-antimicrobial era? Science. 2015;347:1064–1066. doi: 10.1126/science.aaa2868. [DOI] [PubMed] [Google Scholar]
- 33.Keddy KH, Sooka A, Letsoalo ME. Sensitivity and specificity of typhoid fever rapid antibody tests for laboratory diagnosis at two sub-Saharan African sites. Bull World Health Organ. 2011;89:640–647. doi: 10.2471/BLT.11.087627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Akoh JA. Relative sensitivity of blood and bone marrow cultures in typhoid fever. Trop Doct. 1991;21:174–176. doi: 10.1177/004947559102100415. [DOI] [PubMed] [Google Scholar]
- 35.Parry CM, Wijedoru L, Arjyal A, Baker S. The utility of diagnostic tests for enteric fever in endemic locations. Expert Rev Anti Infect Ther. 2011;9:7111–7115. doi: 10.1586/eri.11.47. [DOI] [PubMed] [Google Scholar]
- 36.Wain J, Hosoglu S. The laboratory diagnosis of enteric fever. J Infect Dev Ctries. 2008;2:421–425. doi: 10.3855/jidc.155. [DOI] [PubMed] [Google Scholar]
- 37.Baker S, Sarwar Y, Aziz H. Detection of Vi-negative Salmonella enterica serovar Typhi in the peripheral blood of patients with typhoid fever in the Faisalabad region of Pakistan. J Clin Microbiol. 2005;43:4418–4425. doi: 10.1128/JCM.43.9.4418-4425.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verma R, Bairwa M, Chawla S, Prinja S, Rajput M. New generation typhoid vaccines: an effective preventive strategy to control typhoid fever in developing countries. Hum Vaccin. 2011;7:883–885. doi: 10.4161/hv.7.8.16282. [DOI] [PubMed] [Google Scholar]