Abstract
Ion channels are critical for neuronal excitability and provide important targets for anticonvulsant drugs. In the past few years, several monogenetic epilepsies have been linked to mutations in genes encoding either voltage-gated or ligand-gated channels. The recognition that certain epilepsy syndromes are “channelopathies” initiates a new era in understanding the molecular pathophysiology of seizure disorders. This review summarizes recent advances related to this exciting area of investigation.
Introduction
Complex genetic factors contribute to the pathogenesis of most types of idiopathic epilepsy, but several recognized epilepsy syndromes are transmitted by mendelian inheritance (i.e., caused by mutations in single genes). Mutations in 11 different genes encoding ion channels account for the majority of monogenic epilepsies (see Table 1 and Fig. 1). Therefore epilepsy joins other paroxysmal disorders of the nervous system, including periodic paralysis, episodic ataxia, and migraine as “channelopathies.” This review focuses on recent work that defines the role of inherited ion-channel dysfunction in the molecular basis of epilepsy.
TABLE 1.
Inherited Epilepsy Syndromes Caused by Ion Channel Mutations
| Ion Channel (gene, locus) | Syndrome (OMIM Number)* |
|---|---|
| *Online Mendelian Inheritance in Man database (http://www.ncbi.nlm.nih.gov/Omim/) | |
| †Cytogenetic location defined by using http://genome.ucsc.edu | |
|
Voltage-Gated Ion Channels | |
| Voltage-gated potassium channel, α subunit (KCNQ2, 20q13.2) |
Benign familial neonatal convulsions type 1 (121200) |
| |
Benign familial neonatal convulsions with myokymia (606437) |
| Voltage-gated potassium channel, α subunit (KCNQ3, 8q24) |
Benign familial neonatal convulsions type 2 (121201) |
| Voltage-gated sodium channel, β1 subunit (SCN1B, 19q13.1) |
Generalized epilepsy with febrile seizures plus type 1 (604233) |
| Voltage-gated sodium channel, α subunit (SCN1A, 2q24) |
Generalized epilepsy with febrile seizures plus type 2 (604233) |
| |
Severe myoclonic epilepsy of infancy, Dravet syndrome (607208) |
| |
Intractable childhood epilepsy with frequent generalized tonic–clonic seizures |
| Voltage-gated sodium channel, α subunit (SCN2A, 2q24) |
Benign familial neonatal–infantile seizures (607745) |
| |
Febrile seizures associated with afebrile seizures (604233) |
| Voltage-gated chloride channel (CLCN2, 3q27.1)† |
Juvenile myoclonic epilepsy (606904) |
| |
Juvenile absence epilepsy (607631) |
| |
Childhood absence epilepsy type 3 (607682) |
| |
Epilepsy with grand mal seizures on awakening (607628) |
| Voltage-gated calcium channel β4 subunit (CACNB4, 2q22-2q23) |
Juvenile myoclonic epilepsy (606904) |
| Voltage-gated calcium channel α1A subunit (CACNA1A, 19p13) |
Generalized epilepsy with episodic ataxia |
|
Ligand-Gated Ion Channels | |
| Nicotinic acetylcholine receptor, α4 subunit (CHRNA4, 20q13.2-q13.3) |
Autosomal dominant nocturnal frontal lobe epilepsy type 1 (600513) |
| Nicotinic acetylcholine receptor, β2 subunit (CHRNB2, 1q21) |
Autosomal dominant nocturnal frontal lobe epilepsy type 3 (605375) |
| GABA-A receptor, α1 subunit (GABRA1, 5q34-q35) |
Autosomal dominant juvenile myoclonic epilepsy (606904) |
| GABA-A receptor, γ2 subunit (GABRG2, 5q31.1-q33.1) |
Generalized epilepsy with febrile seizures plus type 3 (604233) |
| Childhood absence epilepsy type 2 and febrile seizures (607681) | |
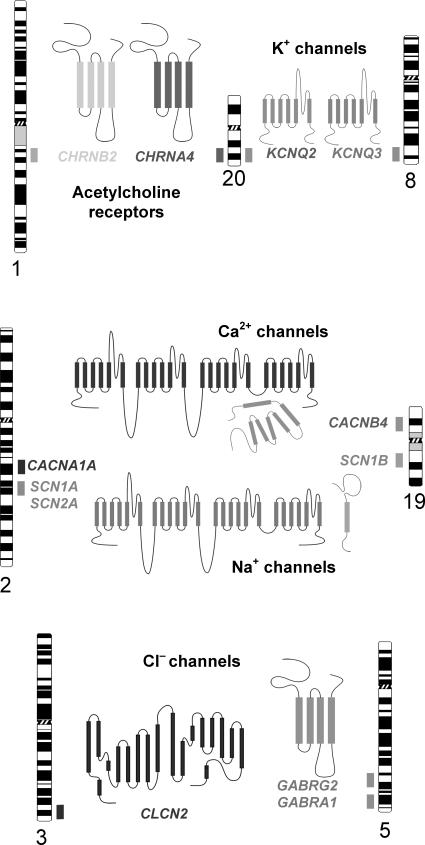
FIGURE 1.
Chromosomal locations of ion channel genes associated with inherited epilepsy. The position of each gene is represented by a shaded box adjacent to the respective chromosome ideogram. A structural model of the corresponding ion channel protein encoded by each gene is also illustrated.
Ion channels may be broadly classified as either voltage-gated or ligand-gated, depending on whether the primary stimulus for their activity is a change in local membrane potential or a chemical messenger (e.g., neurotransmitter). The role of ion channels in neuronal excitability is well established, and the identification of mutations in neuronal ion-channel genes linked to inherited epilepsy has put emphasis on the delicate balances that maintain electrical harmony in the central nervous system (CNS).
Table 1 lists the currently known inherited epilepsy syndromes associated with mutations in either voltage- or ligand-gated ion channels. Genetic heterogeneity (i.e., the same clinical syndrome caused by mutations in different genes) is evident among these disorders. For example, generalized epilepsy with febrile seizures plus (GEFS+) has been associated with mutations in three voltage-gated sodium channel genes (SCN1B, SCN1A, SCN2A) or the gene encoding the γ2 subunit of γ-aminobutyric acid (GABA)A receptors (GABRG2). The following discussion of specific inherited epilepsy syndromes is organized according to the type of ion-channel gene involved.
Epilepsy Associated with Voltage-gated K+ Channels
Several distinct types of potassium channels are expressed in the nervous system and serve a wide range of functions. They are most critical for maintaining resting membrane potentials and enabling rapid repolarization after action potentials. The syndrome of benign familial neonatal convulsions (BFNCs), a rare inherited form of idiopathic generalized epilepsy (IGE) exhibiting autosomal dominant inheritance, was the first seizure disorder associated with neuronal potassium channel mutations. BFNC is characterized by convulsions occurring in the neonatal period that typically resolve spontaneously after a few weeks of life. In very rare cases, adulthood epilepsy occurs. BFNC is genetically heterogeneous, with two identified loci on chromosomes 8q and 20q. In 1998, the potassium channel gene KCNQ2 was identified as the 20q gene, and a closely related gene, KCNQ3, was discovered to be responsible for the chromosome 8q-linked syndrome (1, 2).
In the nervous system, KCNQ2 and KCNQ3 gene products assemble to form potassium channels that generate ionic currents resembling neuronal M-currents (3). M-currents modulate neuronal excitability by dampening the tendency for repetitive firing. Neuronal M-currents are inhibited by muscarinic acetylcholine receptor agonists as well as activators of other types of neurotransmitter receptors. Mutations in either KCNQ2 or KCNQ3 reduce function of the encoded potassium channel by a dominant-negative mechanism consistent with the autosomal dominant inheritance pattern of BFNCs (4).
Epilepsy Associated with Voltage-gated Na+ Channels
Voltage-gated sodium channels are responsible for the rapid membrane depolarization that characterizes the initial “upstroke” of the neuronal action potential. Many conventional anticonvulsants (AEDs) act by blocking sodium channels in a use-dependent manner. Mutations in three voltage-gated sodium channel genes have been discovered in patients affected by several inherited epilepsy syndromes that exhibit febrile seizures. In 1997, Scheffer and Berkovic described GEFS+, a newly recognized epilepsy syndrome with autosomal dominant inheritance (5). The syndrome was named in reference to the common occurrence of febrile seizures in early childhood that often persisted after age 6 years. In addition to febrile seizures, affected adult members of GEFS+ families exhibited afebrile seizures and seizures with multiple clinical phenotypes. Missense mutations in SCN1A encoding a neuronal voltage-gated sodium channel α-subunit account for the majority of GEFS+ cases (6, 7, 8, 9, 10), but heritable defects in two other sodium channel genes (SCN1B, SCN2A) (11, 12) and a GABA-receptor subunit (GABRG2; see section entitled Epilepsies Associated with GABAA Receptors) (13, 14) also can cause the same disorder or clinically similar conditions.
The functional properties of mutant neuronal sodium channels associated with inherited epilepsy have been described (15, 16, 17, 18). Three SCN1A mutations associated with GEFS+ exhibit defects in fast inactivation gating, characterized by a persistent, noninactivating current during membrane depolarization (16). These findings suggest that, in some cases, SCN1A mutations promote a gain-of-function in sodium channels, leading to neuronal hyperexcitability. Other SCN1A mutations associated with GEFS+ cause other types of functional impairments that may lead to loss of function (15). Whether gain of sodium channel function in excitatory neurons or loss of function in inhibitory neurons is the primary mechanism responsible for epilepsy in GEFS+ is under investigation.
Severe myoclonic epilepsy of infancy (SMEI) is a rare convulsive disorder characterized by febrile seizures, with onset during the first year of life, which is followed by intractable epilepsy, impaired psychomotor development, and ataxia (19, 20). Seizures in this disorder are usually unresponsive to AEDs. Recently, >60 heterozygous SCN1A mutations, many of which are de novo mutations, have been reported in SMEI, including missense, nonsense, and insertion/deletion alleles (21, 22, 23, 24). The observed clinical similarities between SMEI and GEFS+, including the frequent occurrence of febrile seizures and shared molecular genetic etiology, lend support to the theory that the two disorders represent a spectrum of severity of the same disease (25). Many SCN1A mutations associated with this disorder appear to encode nonfunctional sodium channels, leading to the suggestion that SMEI is caused by loss-of-function mutations.
Epilepsy Associated with Nicotinic Acetylcholine–Receptor Subunits
Neuronal nicotinic acetylcholine receptors (nAChRs) are located in presynaptic membranes of the cerebral cortex, where they facilitate both excitatory and inhibitory neurotransmitter release. These receptors are pentameric complexes with variable subunit composition. The most common nAChR composition contains α4 and β2 subunits, and mutations in genes (CHRNA4, CHRNB2) encoding these distinct nAChRs have been associated with autosomal dominant nocturnal frontal lobe epilepsy (ADNFLE) (26, 27). Individuals affected with ADNFLE experience short, partial seizures during sleep. Episodes often occur in clusters, localize to the frontal lobes by ictal EEG recordings, and involve nonspecific auras along with brief motor seizures. Most cases are mild and respond well to carbamazepine treatment.
Functional characterization of mutant nAChR subunits have suggested either a loss or gain of receptor function (28), making a precise molecular mechanism underlying this epilepsy syndrome difficult to determine. A recent study revealed that mutations in both α4 and β2 nAChR subunits interfere with Ca2+ modulation of receptor function in a dominant manner, suggesting an alternative explanation for ADNFLE (29). Acetylcholine activation of wild-type α4β2 receptors is normally potentiated by extracellular Ca2+. In rapidly firing excitatory synapses, postsynaptic glutamate receptors deplete local extracellular Ca2+ and reduce Ca2+ potentiation of nAChRs, a possible negative-feedback mechanism to limit further presynaptic glutamate release. This feedback mechanism may be disabled in presynaptic membranes expressing mutant α4β2 receptors, potentially leading to increased excitatory neurotransmitter release under some conditions, such as rapid, synchronous neuronal firing during sleep (29).
Epilepsy Associated with Calcium Channels
Voltage-gated calcium channels, particularly P/Q type channels, are important for neurotransmitter release in the CNS. At least three inherited, paroxysmal neurologic syndromes, including episodic ataxia, familial hemiplegic migraine, and spinocerebellar ataxia, have been linked to mutations in a neuronal calcium channel α-subunit gene (CACNA1A). Mutations in three voltage-gated calcium channel genes in mice (Cacna1a, Cacnb4, Cacng2) have been associated with generalized cortical spike–wave discharges, which are potential animal models of human absence epilepsy (30). Despite their strong appeal as candidate genes for human epilepsy, calcium channel mutations have rarely been observed in seizure disorders. One premature stop codon (nonsense) mutation in CACNB4 was identified in a small family affected by juvenile myoclonic epilepsy (31). This gene encodes the β4-subunit, a protein that modulates function and trafficking of P/Q-type neuronal calcium channels. A de novo heterozygous nonsense mutation in CACNA1A also has been reported in a young male patient affected with generalized epilepsy (tonic–clonic and absence seizures) and paroxysmal ataxia (32). Both CACNB4 and CACNA1A mutations cause reduced calcium channel function (31, 32). In populations with epilepsy, evidence for an association between the CACNA1A locus and susceptibility to IGE was reported by one group (33) but not confirmed by an independent study (34).
Epilepsies Associated with GABAA Receptors
Type A GABA receptors (GABAA) are ligand-gated chloride channels that mediate inhibitory neural activity. In the healthy postnatal brain, activation of GABAA receptors triggers an influx of chloride ions, which renders the postsynaptic membrane potential more negative (hyperpolarization). This hyperpolarization counters excitatory synaptic inputs that would otherwise depolarize the postsynaptic membrane and promote action-potential firing. The ability of GABAA receptors to mediate chloride influx is dependent on other factors, including potassium–chloride co-transporters and voltage-gated chloride channels that maintain a low intracellular chloride concentration.
Mutations in two genes that encode subunits of GABAA receptors and a third gene encoding a voltage-gated chloride channel have been associated with inherited epilepsy. GABAA receptors are composed of five subunits encoded by multiple different gene families (α, β, γ, δ , ɛ, π, θ), with a predominance of complexes containing combinations of αβγ or αβδ. The gene encoding the α1 (GABRA1) subunit has been linked to an autosomal dominant form of juvenile myoclonic epilepsy. Mutations in GABRG2 encoding the γ2 subunit have been associated with GEFS+ and the syndrome of childhood absence epilepsy with febrile seizures (13,35). Experiments to characterize the function of mutant GABAA receptor subunits have demonstrated impaired receptor activity in vitro suggesting reduced GABA-mediated synaptic inhibition as a primary cause for neuronal hyperexcitability (36).
Epilepsies Associated with a Voltage-gated Chloride Channel
Another gene encoding a chloride-transporting protein associated with inherited epilepsy is CLCN2. CLCN2 encodes a chloride channel that is widely distributed in the nervous system and has a suspected role in neuronal excitability. Mutations in CLCN2 have been associated with IGE in three families exhibiting autosomal dominant inheritance and multiple seizure phenotypes, including juvenile myoclonic epilepsy, juvenile absence epilepsy, childhood absence epilepsy, and epilepsy with grand mal seizures on awakening (37). CLCN2 was originally suspected based on genetic linkage data indicating that a region near chromosome 3q26 was a susceptibility locus for IGE, along with its suspected role in neuronal excitability. Two CLCN2 mutations completely abolish chloride channel function. However, a third mutation (G715E) enables chloride channel activation at less negative membrane potentials. Therefore distinct cellular mechanisms may account for epilepsy associated with different CLCN2 mutations.
Summary
Ion channels are critical for normal neuronal excitability, and heritable defects in these molecules account for many forms of inherited epilepsy. By studying the molecular basis of these rare monogenic epilepsy syndromes, it may be possible to infer molecular mechanisms of epileptogenesis relevant to more common and genetically complex forms of the disease. Similarly, by studying the physiologic impact of specific mutations, we discover the diversity of cellular mechanisms that can promote neuronal hyperexcitability and learn more about the importance of ion channel genes expressed in the brain. These advances also contribute to our ability to recognize and diagnose inherited neurologic diseases, provide important information that is useful for counseling affected families, and identify potential new targets for anticonvulsant therapy.
Acknowledgment
Dr. George is supported by a Javits Neuroscience Investigator Award from the National Institute of Neurological Disorders and Stroke (grant NS32387).
References
- 1.Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, McHarg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet 1998;18: 25–29. [DOI] [PubMed] [Google Scholar]
- 2.Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, Leppert M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet 1998;18: 53–55. [DOI] [PubMed] [Google Scholar]
- 3.Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science 1998;282: 1890–1893. [DOI] [PubMed] [Google Scholar]
- 4.Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature 1998;396: 687–690. [DOI] [PubMed] [Google Scholar]
- 5.Scheffer IE, Berkovic SF. Generalized epilepsy with febrile seizures plus: a genetic disorder with heterogeneous clinical phenotypes. Brain 1997;120: 479–490. [DOI] [PubMed] [Google Scholar]
- 6.Escayg A, MacDonald BT, Meisler MH, Baulac S, Huberfeld G, An-Gourfinkel I, Brice A, LeGuern E, Moulard B, Chaigne D, Buresi C, Malafosse A. Mutations of SCN1A, encoding a neuronal sodium channel, in two families with GEFS+2. Nat Genet 2000;24: 343–345. [DOI] [PubMed] [Google Scholar]
- 7.Escayg A, Heils A, MacDonald BT, Haug K, Sander T, Meisler MH. A novel SCN1A mutation associated with generalized epilepsy with febrile seizures plus and prevalence of variants in patients with epilepsy. Am J Hum Genet 2001;68: 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abou-Khalil B, Ge Q, Desai R, Ryther R, Bazyk A, Bailey R, Haines JL, Sutcliffe JS, George AL, Jr. Partial epilepsy and generalized epilepsy with febrile seizures plus and a novel SCN1A mutation. Neurology 2001;57: 2265–2272. [DOI] [PubMed] [Google Scholar]
- 9.Wallace RH, Scheffer IE, Barnett S, Richards M, Dibbens L, Desai RR, Lerman-Sagie T, Lev D, Mazarib A, Brand N, Ben-Zeev B, Goikhman I, Singh R, Kremmidiotis G, Gardner A, Sutherland GR, George AL, Jr, Mulley JC, Berkovic SF. Neuronal sodium-channel α1-subunit mutations in generalized epilepsy with febrile seizures plus. Am J Hum Genet 2001;68: 859–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugawara T, Mazaki-Miyazaki E, Ito M, Nagafuji H, Fukuma G, Mitsudome A, Wada K, Kaneko S, Hirose S, Yamakawa K. Nav1.1 mutations cause febrile seizures associated with afebrile partial seizures. Neurology 2001;57: 703–705. [DOI] [PubMed] [Google Scholar]
- 11.Wallace RH, Wang DW, Singh R, Scheffer IE, George AL, Jr, Phillips HA, Saar K, Reis A, Johnson EW, Sutherland GR, Berkovic SF, Mulley JC. Febrile seizures and generalized epilepsy associated with a mutation in the Na+-channel β1 subunit gene SCN1B. Nat Genet 1998;19: 366–370. [DOI] [PubMed] [Google Scholar]
- 12.Sugawara T, Tsurubuchi Y, Agarwala KL, Ito M, Fukuma G, Mazaki-Miyazaki E, Nagafuji H, Noda M, Imoto K, Wada K, Mitsudome A, Kaneko S, Montal M, Nagata K, Hirose S, Yamakawa K. A missense mutation of the Na+ channel αII subunit gene Nav1.2 in a patient with febrile and afebrile seizures causes channel dysfunction. Proc Natl Acad Sci U S A 2001;98: 6384–6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace RH, Marini C, Petrou S, Harkin LA, Bowser DN, Panchal RG, Williams DA, Sutherland GR, Mulley JC, Scheffer IE, Berkovic SF. Mutant GABAA receptor γ2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet 2001;28: 49–52. [DOI] [PubMed] [Google Scholar]
- 14.Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABAA receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 2001;28: 46–48. [DOI] [PubMed] [Google Scholar]
- 15.Spampanato J, Escayg A, Meisler MH, Goldin AL. Functional effects of two voltage-gated sodium channel mutations that cause generalized epilepsy with febrile seizures plus type 2. J Neurosci 2001;21: 7481–7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lossin C, Wang DW, Rhodes TH, Vanoye CG, George AL, Jr. Molecular basis of an inherited epilepsy. Neuron 2002;34: 877–884. [DOI] [PubMed] [Google Scholar]
- 17.Spampanato J, Escayg A, Meisler MH, Goldin AL. Generalized epilepsy with febrile seizures plus type 2 mutation W1204R alters voltage-dependent gating of Na(v)1.1 sodium channels. Neuroscience 2003;116: 37–48. [DOI] [PubMed] [Google Scholar]
- 18.Cossette P, Loukas A, Lafreniere RG, Rochefort D, Harvey-Girard E, Ragsdale DS, Dunn RJ, Rouleau GA. Functional characterization of the D188V mutation in neuronal voltage-gated sodium channel causing generalized epilepsy with febrile seizures plus (GEFS). Epilepsy Res 2003;53: 107–117. [DOI] [PubMed] [Google Scholar]
- 19.Dravet C, Bureau M, Guerrini R, Giraud N, Roger J. Severe myoclonic epilepsy in infants. In: Roger J, Dravet C, Bureau M, Dreifuss FE, Perret A, Wolf P, eds. Epileptic syndromes in infancy, childhood and adolescence. 2nd ed. : John Libbey, 1992: 75–88. [Google Scholar]
- 20.Scheffer IE, Wallace R, Mulley JC, Berkovic SF. Clinical and molecular genetics of myoclonic-astatic epilepsy and severe myoclonic epilepsy in infancy (Dravet syndrome). Brain Dev 2001;23: 732–735. [DOI] [PubMed] [Google Scholar]
- 21.Claes L, Del Favero J, Ceulemans B, Lagae L, Van Broeckhoven C, De Jonghe P. De novo mutations in the sodium-channel gene SCN1A cause severe myoclonic epilepsy of infancy. Am J Hum Genet 2001;68: 1327–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sugawara T, Mazaki-Miyazaki E, Fukushima K, Shimomura J, Fujiwara T, Hamano S, Inoue Y, Yamakawa K. Frequent mutations of SCN1A in severe myoclonic epilepsy in infancy. Neurology 2002;58: 1122–1124. [DOI] [PubMed] [Google Scholar]
- 23.Ohmori I, Ouchida M, Ohtsuka Y, Oka E, Shimizu K. Significant correlation of the SCN1A mutations and severe myoclonic epilepsy in infancy. Biochem Biophys Res Commun 2002;295: 17–23. [DOI] [PubMed] [Google Scholar]
- 24.Claes L, Ceulemans B, Audenaert D, Smets K, Lofgren A, Del Favero J, Ala-Mello S, Basel-Vanagaite L, Plecko B, Raskin S, Thiry P, Wolf NI, Van Broeckhoven C, De Jonghe P. De novo SCN1A mutations are a major cause of severe myoclonic epilepsy of infancy. Hum Mutat 2003;21: 615–621. [DOI] [PubMed] [Google Scholar]
- 25.Singh R, Andermann E, Whitehouse WP, Harvey AS, Keene DL, Seni MH, Crossland KM, Andermann F, Berkovic SF, Scheffer IE. Severe myoclonic epilepsy of infancy: extended spectrum of GEFS+?Epilepsia 2001;42: 837–844. [DOI] [PubMed] [Google Scholar]
- 26.De Fusco M, Becchetti A, Patrignani A, Annesi G, Gambardella A, Quattrone A, Ballabio A, Wanke E, Casari G. The nicotinic receptor beta 2 subunit is mutant in nocturnal frontal lobe epilepsy. Nat Genet 2000;26: 275-276. [DOI] [PubMed] [Google Scholar]
- 27.Steinlein OK, Mulley JC, Propping P, Wallace RH, Phillips HA, Sutherland GR, Scheffer IE, Berkovic SF. A missense mutation in the neuronal nicotinic acetylcholine receptor alpha 4 subunit is associated with autosomal dominant nocturnal frontal lobe epilepsy. Nat Genet 1995;11: 201–203. [DOI] [PubMed] [Google Scholar]
- 28.Sutor B, Zolles G. Neuronal nicotinic acetylcholine receptors and autosomal dominant nocturnal frontal lobe epilepsy: a critical review. Pflugers Arch 2001;442: 642–651. [DOI] [PubMed] [Google Scholar]
- 29.Rodrigues-Pinguet N, Jia L, Li M, Figl A, Klaassen A, Truong A, Lester HA, Cohen BN. Five ADNFLE mutations reduce the Ca2+ dependence of the mammalian α4β2 acetylcholine response. J Physiol (Lond) 2003;550: 11–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgess DL, Noebels JL. Single gene defects in mice: the role of voltage-dependent calcium channels in absence models. Epilepsy Res 1999;36: 111–122. [DOI] [PubMed] [Google Scholar]
- 31.Escayg A, De Waard M, Lee DD, Bichet D, Wolf P, Mayer T, Johnston J, Baloh R, Sander T, Meisler MH. Coding and noncoding variation of the human calcium-channel beta4-subunit gene CACNB4 in patients with idiopathic generalized epilepsy and episodic ataxia. Am J Hum Genet 2000;66: 1531–1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jouvenceau A, Eunson LH, Spauschus A, Ramesh V, Zuberi SM, Kullmann DM, Hanna MG. Human epilepsy associated with dysfunction of the brain P/Q-type calcium channel. Lancet 2001;358: 801–807. [DOI] [PubMed] [Google Scholar]
- 33.Chioza B, Wilkie H, Nashef L, Blower J, McCormick D, Sham P, Asherson P, Makoff AJ. Association between the alpha(1a) calcium channel gene CACNA1A and idiopathic generalized epilepsy. Neurology 2001;56: 1245–1246. [DOI] [PubMed] [Google Scholar]
- 34.Sander T, Toliat MR, Heils A, Becker C, Nurnberg P. Failure to replicate an allelic association between an exon 8 polymorphism of the human alpha(1A) calcium channel gene and common syndromes of idiopathic generalized epilepsy. Epilepsy Res 2002;49: 173–177. [DOI] [PubMed] [Google Scholar]
- 35.Baulac S, Huberfeld G, Gourfinkel-An I, Mitropoulou G, Beranger A, Prud'homme JF, Baulac M, Brice A, Bruzzone R, LeGuern E. First genetic evidence of GABA(A) receptor dysfunction in epilepsy: a mutation in the gamma2-subunit gene. Nat Genet 2001;28: 46–48. [DOI] [PubMed] [Google Scholar]
- 36.Bianchi MT, Song L, Zhang H, Macdonald RL. Two different mechanisms of disinhibition produced by GABAA receptor mutations linked to epilepsy in humans. J Neurosci 2002;22: 5321–5327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haug K, Warnstedt M, Alekov AK, Sander T, Ramirez A, Poser B, Maljevic S, Hebeisen S, Kubisch C, Rebstock J, Horvath S, Hallmann K, Dullinger JS, Rau B, Haverkamp F, Beyenburg S, Schulz H, Janz D, Giese B, Muller-Newen G, Propping P, Elger CE, Fahlke C, Lerche H, Heils A. Mutations in CLCN2 encoding a voltage-gated chloride channel are associated with idiopathic generalized epilepsies. Nat Genet 2003;33: 527–532. [DOI] [PubMed] [Google Scholar]