Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is the most frequent genetic cause of renal failure. Here we identify miR-17 as a target for the treatment of ADPKD. We report that miR-17 is induced in kidney cysts of mouse and human ADPKD. Genetic deletion of the miR-17∼92 cluster inhibits cyst proliferation and PKD progression in four orthologous, including two long-lived, mouse models of ADPKD. Anti-miR-17 treatment attenuates cyst growth in short-term and long-term PKD mouse models. miR-17 inhibition also suppresses proliferation and cyst growth of primary ADPKD cysts cultures derived from multiple human donors. Mechanistically, c-Myc upregulates miR-17∼92 in cystic kidneys, which in turn aggravates cyst growth by inhibiting oxidative phosphorylation and stimulating proliferation through direct repression of Pparα. Thus, miR-17 family is a promising drug target for ADPKD, and miR-17-mediated inhibition of mitochondrial metabolism represents a potential new mechanism for ADPKD progression.
Autosomal dominant polycystic kidney disease (ADPKD) is a life-threatening genetic disease that leads to renal failure. Here Hajarnis et al. show that miR-17 modulates cyst progression in ADPKD through metabolic reprogramming of mitochondria and its inhibition slows cyst development and improves renal functions.
ADPKD, caused by mutations of PKD1 or PKD2, is among the most common monogenetic disorders and a leading genetic cause of end-stage renal disease1,2,3. The clinical hallmark of this disease is the presence of numerous renal tubule-derived cysts. Excessive proliferation, a central pathological feature, fuels the relentless expansion of cysts ultimately causing end-stage renal disease.
MicroRNAs (miRNAs) are non-coding RNAs that bind to complementary sequences located in target mRNAs and inhibit their expression4. Aberrant activation of miRNAs promotes the progression of many common diseases5,6,7,8. Accordingly, synthetic inhibitors of miRNAs (anti-miRs) have emerged as novel therapeutic agents. Anti-miRs appear to be well tolerated in human clinical trials9, have a long duration of action and are efficiently delivered to the liver and kidney10,11. These attributes make them an extremely attractive drug class for diseases that primarily affect the kidney and liver and require long-term therapy, such as ADPKD. However, whether a miRNA-based therapeutics approach can be applied to ADPKD is not known.
We have previously shown that miR-17∼92 inhibits PKD1 and PKD2 and aggravates disease progression in a non-orthologous ciliopathy (Kif3a-KO) model of cystic kidney disease12. However, the relevance of this finding to ADPKD is unclear because whether miR-17 can modulate cyst growth when Pkd1 or Pkd2 are already mutated has not been studied. In fact, with the exception of miR-21 (ref. 13), the role of miRNAs in ADPKD still remains unexplored. Our primary objective was to identify miRNAs that play a pathogenic role in ADPKD. We began by systematically examining miRNA expression profiles in Pkd1-KO and Pkd2-KO mice. Intriguingly, we found that among upregulated miRNAs, the miR-17 miRNA family contributed most substantially to the total dysregulated miRNA pool in both ADPKD models. These unbiased microarray results and the previous observations in the Kif3a-KO mice prompted us to study the role of miR-17 in ADPKD. Using complementary genetic and pharmaceutical approaches, we now show that miR-17∼92 promotes cyst proliferation and ADPKD progression. miR-17 mediates these effects by reprogramming mitochondrial metabolism through direct repression of Pparα.
Results
miR-17 miRNA family is upregulated in ADPKD
We used Nanostring nCounter microarrays to compare miRNA expression patterns between 10-day-old wild type and Ksp/Cre;Pkd1F/F (Pkd1-KO) kidneys, and 21-day-old wild type and Pkhd1/Cre;Pkd2F/F (Pkd2-KO) kidneys (n=3 biological replicates for all groups). Forty-five miRNAs were differentially expressed in Pkd1-KO kidney, whereas 70 miRNAs were differentially expressed in Pkd2-KO kidneys (Supplementary Table 1). Twenty-nine miRNAs were dysregulated in both ADPKD models (Supplementary Table 2). These dysregulated miRNAs exhibited a correlated expression pattern (R2=0.87) between Pkd1-KO and Pkd2-KO kidneys suggesting that a common set of aberrantly expressed miRNAs may underlie ADPKD pathogenesis (Fig. 1a–c).
Figure 1. miR-17 is upregulated in kidney cysts of mouse and human ADPKD.
Nanostring nCounter microarray analysis was performed to compare miRNA expression patterns between 10-day-old wild type (n=3) and Pkd1-KO kidneys (n=3), and 21-day-old wild type (n=3) and Pkd2-KO kidneys (n=3). (a,b) The differentially expressed miRNA families in Pkd1-KO and Pkd2-KO are shown. The red circles mark Log2 fold change when only differentially expressed family members are included. The stacked bars mark Log2 fold change when all expressed family members irrespective of statistical significance are included. The values in parenthesis indicate the percentage contribution of each miRNA family to the total miRNA pool in cystic kidneys. The miR-17 family is highlighted in red. (c) Dysregulated miRNAs exhibited correlated expression pattern (R2=0.87) between Pkd1-KO and Pkd2-KO kidneys suggesting that a common set of aberrantly expressed miRNAs may underlie ADPKD pathogenesis. (d,e) Q-PCR analysis demonstrated that miR-17 is upregulated at cystic stages (P7 & P10 for Pkd1-KO and P21 & P28 for Pkd2-KO) but not at pre-cystic stages (P1 for Pkd1-KO and P14 for Pkd2-KO). In situ hybridization (ISH) was performed using an LNA-modified anti-miR-17 probe. The kidney sections were counterstained with nuclear fast red to mark nuclei. (f) Representative ISH images of kidney sections from wild type, Pkd1F/RCSKO, Pkd2-KO, Pkd2-miR-17∼92KO mice (negative control) and miR-17∼92Tg (positive control) are shown. Expression of miR-17 (blue) was increased in cysts (cy) of Pkd1F/RCSKO and Pkd2-KO compared with renal tubules of wild-type mice. miR-17 expression was abolished in cysts of Pkd2-miR-17∼92KO mice, whereas its expression was induced in renal tubules of miR-17∼92Tg mice, indicating that the in situ probe specifically detects miR-17. (g) ISH was performed on kidney sections from four NHK and ADPKD samples. Representative ISH images from one NHK and ADPKD sample are shown. miR-17 expression was not detected by ISH in NHK kidney sections. The percentage of miR-17-positive cysts per kidney section from four human ADPKD patients (#6–9) is shown in the graph. The total number of cysts per section is shown above each bar. P indicates postnatal day. Error bars indicate s.e.m. * indicates P<0.05, ns indicates P>0.05. Student's unpaired t-test (d,e). Scale bars, 50 μm (f) and 20 μm (g).
To identify groups of upregulated miRNAs with the highest pathogenic potential, individual differentially expressed miRNAs belonging to the same seed family were categorized together, and a cumulative fold change for each family was calculated. Among the upregulated miRNAs, we focused on the miR-17 family because it contributed most substantially (>2.7%) to the total miRNA pool in both ADPKD models (Fig. 1a,b). Quantitative real-time PCR (Q-PCR) validated the microarray data and additionally demonstrated that miR-17 upregulation correlates with disease progression in both models (Fig. 1d,e). To identify the precise location of dysregulated miR-17 expression, we performed in situ hybridization using a locked nucleic acid (LNA)-modified probe against the mature miR-17 transcript. miR-17 expression was increased in cyst epithelia of both Pkd1-mutant and Pkd2-KO kidneys (Fig. 1f). miR-17 expression was significantly reduced in cyst epithelia of Pkd2-miR-17∼92KO mice (negative control), whereas its expression was induced in renal tubules of kidney-specific miR-17∼92-overexpressing transgenic mice (positive control), indicating that the in situ probe specifically detects miR-17. The human mature miR-17 sequence is identical to the mouse miR-17. Therefore, we used this in situ probe to examine miR-17 expression in kidney samples from normal humans (NHK) and patients with ADPKD. Compared with renal tubules in NHK, miR-17 expression was increased in kidney cysts from patients with ADPKD (Fig. 1g). No signal was observed when in situ hybridization was performed using a probe with a scrambled sequence.
miR-17∼92 promotes cyst growth in early-onset ADPKD models
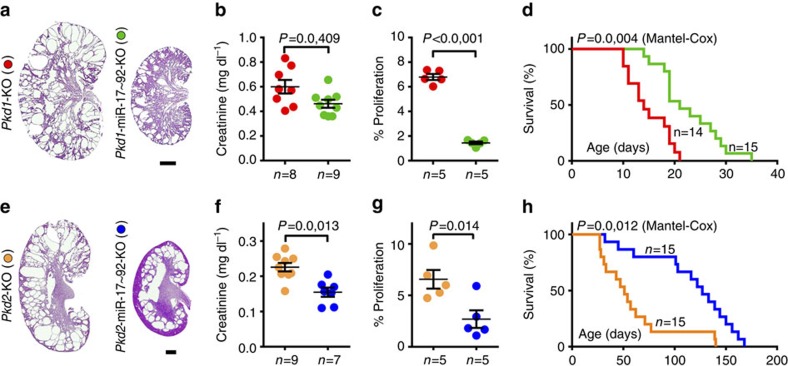
The miR-17 miRNA family aggravates cyst growth in the Kif3a-KO ciliopathy model of PKD12. To examine whether miR-17 plays a similar pathogenic role in ADPKD, miR-17∼92 was genetically deleted in various orthologous ADPKD mouse models. First, we deleted miR-17∼92 in Pkd1-KO mice, which develop an early-onset and rapidly fatal form of PKD. miR-17∼92F/F mice were bred with Ksp/Cre;Pkd1F/+ transgenic mice. The first and second generation progeny were intercrossed to generate Ksp/Cre;Pkd1F/F (Pkd1-KO) and Ksp/Cre;Pkd1F/F; miR-17∼92F/F (Pkd1-miR-17∼92KO) mice. Q-PCR analysis showed that compared with control kidneys, Pkd1 expression was equally reduced in both Pkd1-KO and Pkd1-miR-17∼92KO kidneys indicating a similar level of Cre/loxP recombination (Supplementary Fig. 1A). In contrast, compared with control kidneys, miR-17 expression was increased by 50.3% in Pkd1-KO kidneys, whereas its expression was reduced by 51.9% in Pkd1-miR-17∼92KO kidneys (Supplementary Fig. 1B). We observed a 50% improvement in median survival, 22% reduction in serum creatinine levels, 26.8% reduction in kidney-weight-to-body-weight ratio and decreased expression of kidney injury markers Kim1 (down by 38.7%) and Ngal (down by 43.9%) in Pkd1-miR-17∼92KO compared with Pkd1-KO mice (Fig. 2a,b,d and Supplementary Fig. 1D,E). Moreover, the number of cyst epithelial cells expressing phospho-histone H3, a marker of proliferating cells, was reduced by 78.7% (Fig. 2c).
Figure 2. miR-17∼92 deletion attenuates cyst growth in early-onset ADPKD models.
To understand the biological relevance of miR-17 upregulation, the miR-17∼92 cluster was deleted in orthologous ADPKD models. (a) H&E staining, (b) serum creatinine levels and (c) quantification of proliferating cyst epithelial cells in kidneys of 10-day-old Pkd1-KO and Pkd1-miR-17∼92KO mice is shown. (d) Kaplan-Meier survival curves of Pkd1-KO (red line) and Pkd1-miR-17∼92KO (green line) mice. The median survival of Pkd1-miR-17∼92KO (21-days) was improved by 50% compared with Pkd1-KO mice (14 days). (e) H&E staining, (f) serum creatinine levels and (g) quantification of proliferating cyst epithelial cells in kidneys of 21-day-old Pkd2-KO and Pkd2-miR-17∼92KO mice is shown. (h) Kaplan-Meier survival curves of Pkd2-KO (orange line) and Pkd2-miR-17∼92KO (blue line) mice. The median survival of Pkd2-miR-17∼92KO (127 days) was doubled compared with Pkd2-KO mice (51 days). Error bars represent s.e.m. Student's unpaired t-test (b,c,f,g) and Log rank (Mantel-Cox) test (d,h). Scale bars, 1 mm.
Next, we studied the role of miR-17∼92 in Pkhd1/Cre;Pkd2F/F (Pkd2-KO) mice, which with a median survival of ∼70-days exhibit a relatively less aggressive PKD. Pkd2-KO and Pkhd1/Cre;Pkd2F/F; miR-17∼92F/F (Pkd2-miR-17∼92KO) mice were generated using the strategy described earlier. Q-PCR and western blot analysis showed that compared with control kidneys, Pkd2 expression was equally reduced in both Pkd2-KO and Pkd1-miR-17∼92KO kidneys indicating a similar level of Cre/loxP recombination (Supplementary Fig. 2A,B). In contrast, compared with control kidneys, miR-17 expression was increased by 45.4% in Pkd2-KO kidneys, whereas its expression was reduced by 46.5% in Pkd2-miR-17∼92KO kidneys (Supplementary Fig. 2C). Compared with Pkd2-KO mice, we observed a 149% increase in median survival, 31.8% improvement in serum creatinine levels, 15.8% reduction in cyst index, reduced Kim1 (down by 37.9%) and Ngal (down by 39.6%) expression, and a 58.9% decrease in the number of proliferating cyst epithelial cells in Pkd2-miR-17∼92KO mice (Fig. 2e–h and Supplementary Fig. 2). Thus, deletion of miR-17∼92 inhibited cyst proliferation and disease progression in both Pkd1-KO and Pkd2-KO models.
miR-17∼92 promotes cyst growth in long-lived ADPKD models
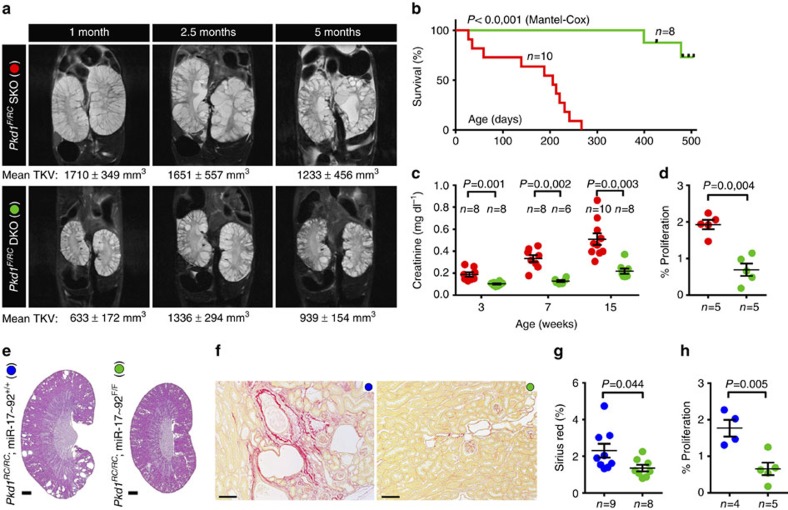
The majority of ADPKD patients do not experience a significant decline in renal function until the fourth or fifth decade of their life. However, Pkd1-KO mice develop a rapidly fatal form of PKD; thus, this model does not fully recapitulate the dynamics of human ADPKD progression. Therefore, we evaluated whether miR-17∼92 also influences disease progression in long-lived and slow cyst growth models of ADPKD. We generated Pkd1F/RC mice that harbour a hypomorphic mutation14 (mouse equivalent of the human PKD1-R3277C (RC) mutation) on one allele and loxP sites flanking Pkd1-exons two and four on the other allele15. We used Ksp/Cre-mediated recombination to delete the floxed Pkd1-exons and thereby produced a compound Pkd1-mutant mouse with a germline hypomorphic mutation and a renal tubule-specific, somatic null mutation. To test the role of miR-17∼92 in this mouse model, we generated and characterized Ksp/Cre;Pkd1F/RC (Pkd1F/RCSKO) and Ksp/Cre;Pkd1F/RC;miR-17∼92F/F (Pkd1F/RCDKO) mice. Pkd1 expression was unchanged whereas miR-17∼92 expression was reduced by 85.4% in Pkd1F/RCDKO compared with Pkd1F/RCSKO kidneys (Supplementary Fig. 3A,B). We prospectively monitored these mice for 5 months by performing kidney MRI and measuring serum creatinine levels at periodic intervals. MRI-assessed total kidney volume (TKV) was reduced, and the renal function was markedly improved in Pkd1F/RCDKO compared with Pkd1F/RCSKO mice throughout the observation period, indicating that deletion of miR-17∼92 provided a sustained clinical benefit (Fig. 3a,c). All Pkd1F/RCDKO mice lived well past 1 year, whereas the median survival of Pkd1F/RCSKO mice was only 206 days (Fig. 3b). We euthanize a separate group of 3-week-old and 5-month-old mice for molecular and histological analysis. Kidney-weights, cyst size, Kim1 and Ngal expression, and the number of proliferating cyst epithelial cells were markedly reduced in Pkd1F/RCDKO compared with Pkd1F/RCSKO mice (Supplementary Fig. 3C–H).
Figure 3. miR-17∼92 deletion attenuates disease progression in long-lived and slow cyst growth models of ADPKD.
(a–d) The role of miR-17∼92 was studied in Pkd1F/RCSKO mice, a long-lived model of ADPKD. (a) Representative MRI images and mean MRI-estimated TKV of 1-,2.5-, and 5-month-old Pkd1F/RCSKO and Pkd1F/RCDKO mice are shown. TKV normalized to body weight was reduced in Pkd1F/RCDKO compared with Pkd1F/RCSKO mice at all time points (n=8–10 each group, P<0.01). (b) Kaplan-Meier survival curves of Pkd1F/RCSKO (red line) and Pkd1F/RCDKO mice (green line) are shown. The median survival (>14 months) of Pkd1F/RCDKO mice was more than doubled compared with Pkd1F/RCSKO mice (206 days). (c) Serum creatinine level was reduced in 3-,7-, and 15-week-old Pkd1F/RCDKO mice (green circles) compared with Pkd1F/RCSKO (red circles) mice. (d) Quantification revealed that cyst epithelial cell proliferation was reduced in 21-day-old Pkd1F/RCDKO mice compared with Pkd1F/RCSKO mice. (e–h) The role of miR-17∼92 was studied in Pkd1RC/RC mice, a slow cyst growth model of ADPKD. (e) H&E and (f) Sirius red staining of kidney sections from 6-month-old Pkd1RC/RC (blue circles) and Pkd1RC/RC;Ksp/cre; miR-17∼92F/F (green circles) mice. Quantification of the Sirius red staining (g) and the number of proliferating cells (h) revealed that both interstitial fibrosis and tubular proliferation were reduced after miR-17∼92 deletion in Pkd1RC/RC mice. Error bars represent s.e.m. Log rank (Mantel-Cox) test (b) Student's unpaired t-test (a,c,d,g,h). Scale bars, 1 mm (e) and 100 μm (f).
Finally, we examined the role of miR-17∼92 in a slow cyst growth model of ADPKD (Pkd1RC/RC) that harbours homozygous germline Pkd1 RC mutations. Unlike the preceding three ADPKD models, Cre/loxP recombination is not required to induce Pkd1 mutation, and these mice develop significant renal fibrosis. We generated and characterized 6-month-old Pkd1RC/RC; Ksp/Cre and Pkd1RC/RC; Ksp/Cre;miR-17∼92F/F mice. We observed attenuated cyst growth, lower blood urea nitrogen (BUN) levels, downregulation of Kim1 and Ngal expression, reduced renal fibrosis and lower cyst epithelial proliferation in Pkd1RC/RC; Ksp/Cre;miR-17∼92F/F compared with Pkd1RC/RC; Ksp/Cre mice (Fig. 3e–h and Supplementary Fig. 4).
Anti-miR-17 attenuates cyst growth in two PKD models
Next, we studied whether miRNAs are viable drug targets for ADPKD. Within the miR-17∼92 cluster, we decided to target the miR-17 family based on our observation that multiple members of this family were upregulated in ADPKD models. We have recently developed a chemically modified anti-miR oligonucleotide (anti-miR-17) that sterically inhibits the activity of all miR-17 family members in cultured cells via complementary base pairing16. We used the miRNA polysome shift assay (miPSA) to assess whether this compound inhibits endogenous miR-17 in kidneys following systemic administration. miPSA relies on the principle that an active miRNA binds to its mRNA targets in translationally active high molecular weight polysomes. Compared with vehicle, a single subcutaneous injection of anti-miR-17 displaced miR-17 from high molecular weight polysomes in the kidney in a dose-dependent manner. Similar miR-17 displacement was not observed in mice treated with a control oligonucleotide (Supplementary Fig. 5B and Supplementary Table 3). Moreover, a 300 mg kg−1 dose of anti-miR-17 did not produce acute liver or kidney toxicity in mice (Supplementary Fig. 5C).
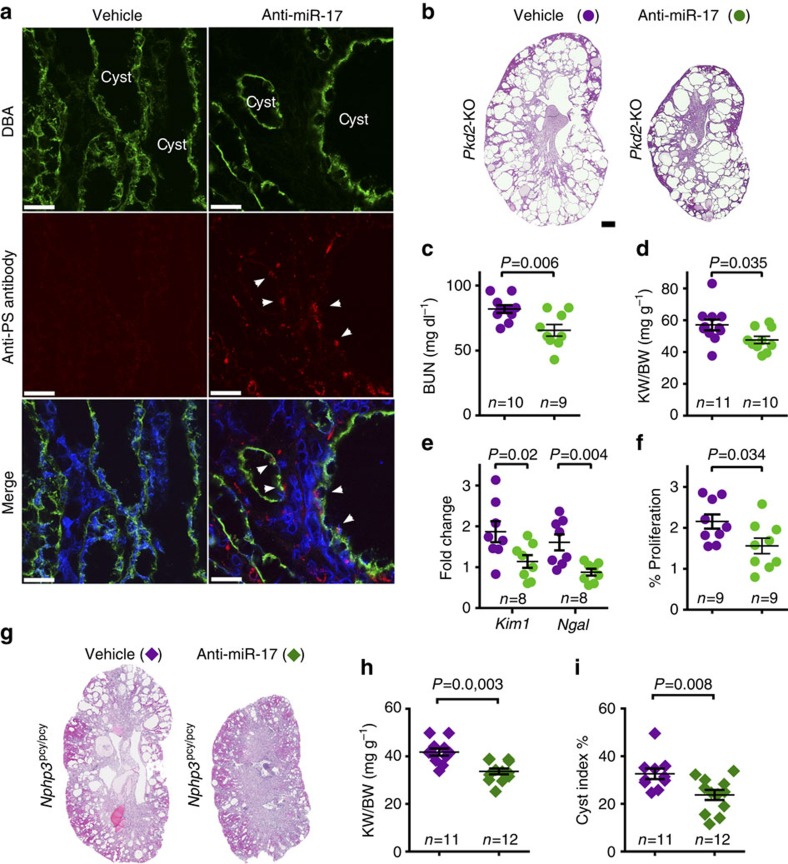
To evaluate the delivery and therapeutic efficacy of anti-miR-17, we conducted randomized, blinded and statistically powered pre-clinical studies in Pkd2-KO mice (Supplementary Fig. 5D,E). We developed an antibody (anti-PS) that specifically binds to the chemically modified phosphate backbone of anti-miR-17 to determine its cellular distribution. Staining with the anti-PS antibody revealed that anti-miR-17 was delivered to collecting duct cysts even when administered after numerous cysts had already formed (Fig. 4a). No anti-PS signal was noted in kidneys of Pkd2-KO mice treated with PBS. To assess therapeutic efficacy, we injected Pkd2-KO mice with 20 mg kg−1 of anti-miR-17 or vehicle at postnatal days (P) 10, 11, 12 and 19 and killed them at P28. Kidney-weight-to-body-weight ratios, BUN levels and Kim1 and Ngal expression were reduced in anti-miR-17-treated compared with vehicle-treated Pkd2-KO mice (Fig. 4b–e). Moreover, similar to genetic deletion, treatment with anti-miR-17 also slowed the proliferation of cyst epithelial cells (Fig. 4f). Next, we evaluated whether anti-miR-17 treatment demonstrates therapeutic efficacy in a second, more long-term model of cystic kidney disease. One-month-old Nphp3pcy/pcy mice, a well characterized orthologous slow cyst growth model, were injected with 50 mg kg−1 of anti-miR-17 or vehicle once a week for 6 months (Supplementary Fig. 5F). We found that kidney-weight-to-body-weight ratio and cyst index were significantly reduced in anti-miR-17-treated compared with vehicle-treated Nphp3pcy/pcy mice (Fig. 4g–i). Moreover, we did not observe any major adverse effects of long-term anti-miR-17 therapy such as weight loss, failure to thrive or death.
Figure 4. Anti-miR-17 demonstrates therapeutic efficacy in short-term and long-term PKD mouse models.
(a) Pkd2-KO mice were injected with 20 mg kg−1 of anti-miR-17 compound or PBS on postnatal day (P) 21, 22 and 23, and kidneys were harvested on P26. Kidney sections were co-stained with DBA (green, a marker of collecting ducts) and anti-PS antibody (red, antibody labels anti-miR-17 compound). Anti-PS staining was observed in collecting duct-derived cysts (arrowheads) of Pkd2-KO mice injected with anti-miR-17 indicating that the compound was delivered to collecting duct cysts. No anti-PS antibody staining was noted in Pkd2-KO mice injected with PBS. To assess the therapeutic efficacy of this compound, Pkd2-KO mice were injected with anti-miR-17 or PBS at P10, 11, 12 and 19, and kidneys were harvested on P28. (b) H&E staining of kidney sections from 28-day-old Pkd2-KO mice injected with anti-miR-17 or PBS. (c) Serum BUN levels, (d) kidney-weight-to-body-weight ratio, (e) expression of Kim1 and Ngal and (f) the number of proliferating cyst epithelial cells were reduced in Pkd2-KO mice that were injected with anti-miR-17 compared with PBS. (g–i) To assess the therapeutic efficacy of anti-miR-17 in a long-term PKD model, 1-month-old Nphp3pcy/pcy mice were injected with 50 mg kg−1 of anti-miR-17 or PBS once a week for 26 weeks. These mice were euthanized at 30 weeks of age. (g) Representative images of H&E-stained kidney sections from 30-week-old Nphp3pcy/pcy mice injected with PBS or anti-miR-17 are shown. (h) kidney-weight-to-body-weight ratios and (i) cyst index were reduced in Nphp3pcy/pcy mice that were injected with anti-miR-17 compared with PBS. Error bars represent s.e.m. Student's unpaired t-test (c–f,h,i). Scale bars, 25 μm (a) and 1 mm (b).
Anti-miR-17 inhibits cysts in in vitro human models of PKD
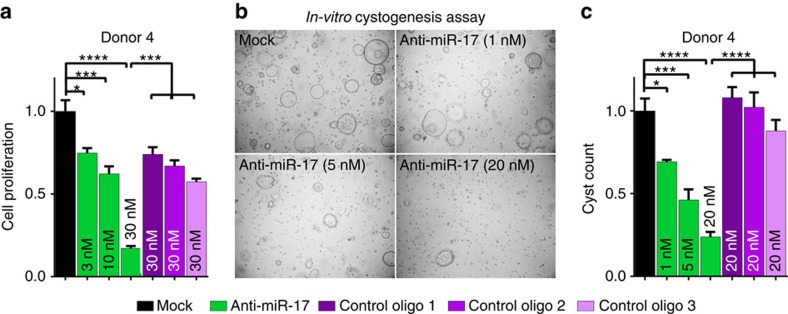
To assess the translational potential of our findings, we studied the effects of miR-17 family inhibition in primary cell cultures derived from human ADPKD cysts. These cells were cultured to measure proliferation or in vitro cyst formation in 3D Matrigel. Treatment with anti-miR-17 produced a dose-dependent reduction in the proliferation of cyst epithelia from five human donors (Fig. 5a and Supplementary Fig. 6A). In contrast, mock transfection or transfection with three different control oligonucleotides had no effect. Similarly, compared with mock or control oligonucleotide transfection, anti-miR-17 inhibited in vitro cyst growth of primary ADPKD cells in a dose-dependent manner (Fig. 5b,c and Supplementary Fig. 6B).
Figure 5. Anti-miR-17 treatment reduces proliferation and cyst growth in in vitro models of human ADPKD.
Primary cyst epithelial cultures derived from kidneys of ADPKD patients were transfected with anti-miR-17 (dose: 3 nM, 10 nM or 30 nM) or three different control oligonucleotides (dose: 30 nM, control oligo1, 2 and 3). These cells were then cultured to measure proliferation or in vitro cyst formation (3-Dimension Matrigel). (a) Data for proliferation (n=5 for each treatment and dose) and (b) representative images and (c) quantification of cyst formation (n=3 for each treatment and dose) using primary cultures derived from ADPKD Donor 4 are shown. Anti-miR-17 reduced proliferation and cyst count in a dose-dependent manner. Data from the other ADPKD Donors can be found in the Supplementary Fig. 6. Error bars represent s.e.m. *indicates P<0.05, **indicates P<0.01, ***indicates P<0.005 and ****indicates P<0.001. One-way ANOVA, Tukey's multiple comparisons test.
c-Myc promotes miR-17∼92 expression in PKD
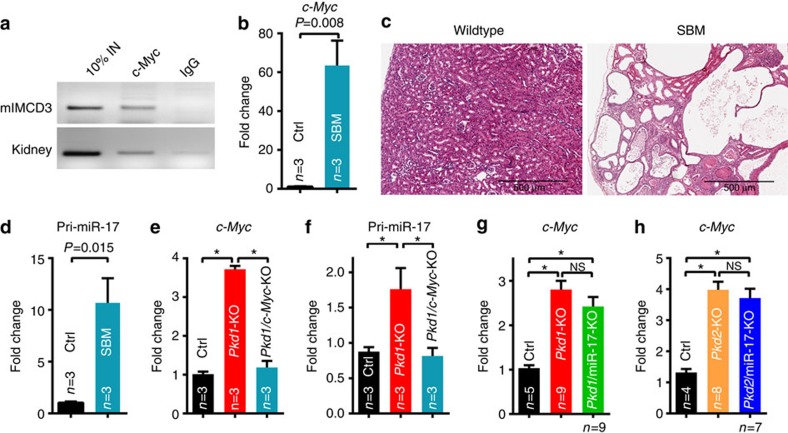
To gain insights into the signalling events linked to dysregulated miR-17∼92 expression, we began by identifying PKD-relevant upstream regulators of miR-17∼92. We focused on c-Myc because of its connection to both PKD pathogenesis and miR-17∼92 expression17,18,19. Chromatin immunoprecipitation analysis showed that c-Myc binds to the miR-17∼92 promoter in cultured renal epithelial cells and mouse kidneys (Fig. 6a). To study whether this interaction is functional in the context of PKD, we first analysed mice with a gain-of-function allele of c-Myc (SBM mice). Consistent with the previous report19, c-Myc was upregulated, and SBM mice exhibited numerous kidney cysts (Fig. 6b,c). Q-PCR analysis revealed that primary miR-17 transcript (pri-miR-17) was upregulated by 9.3-fold in SBM kidneys compared with control kidneys, suggesting that c-Myc drives miR-17∼92 transcription (Fig. 6d). To study whether this regulation is also observed in ADPKD models, we generated Pkd1-KO mice in which c-Myc was deleted (Fig. 6e). Deletion of c-Myc resulted in a 53.7% downregulation of pri-miR-17 expression, suggesting that c-Myc also promotes miR-17∼92 transcription in the context of ADPKD (Fig. 6f). In contrast, deletion of miR-17∼92 did not affect c-Myc expression in Pkd1-KO or Pkd2-KO kidneys, indicating that c-Myc functions upstream of miR-17∼92 in ADPKD (Fig. 6g,h).
Figure 6. c-Myc promotes miR-17∼92 expression in cystic kidneys.
(a) Chromatin immunoprecipitation was performed using an antibody against c-Myc and control IgG. Semi-qPCR analysis of the immunoprecipitated DNA revealed that c-Myc specifically binds to the miR-17∼92 promoter in mIMCD3 cells and mouse kidney tissue. No PCR product was observed in IgG control. To assess in vivo functional interaction, c-Myc transgenic (SBM) mice were analysed. (b) Q-PCR analysis showed c-Myc was upregulated and (c) H&E staining showed numerous kidney cysts in 6-month-old SBM compared with control mice. (d) Q-PCR analysis revealed that pri-miR-17 was increased by 12-fold in SBM compared with control kidneys (n=3), suggesting that c-Myc promotes miR-17 transcription in the context of PKD. To study whether c-Myc regulates miR-17∼92 expression in ADPKD models, Ksp/Cre; Pkd1F/F; c-MycF/F (Pkd1/c-Myc-KO) mice were generated. (e,f) Q-PCR analysis showing c-Myc and pri-miR-17 expression in 10-day-old control (Ctrl), Pkd1-KO and Pkd1/c-Myc-KO kidneys (n=3, each genotype). c-Myc deletion reduced pri-miR-17 expression in Pkd1-KO kidneys. (g,h) In contrast, Q-PCR analysis revealed that c-Myc expression was not affected by deletion of miR-17∼92 in Pkd1 or Pkd2-KO mice. Thus, c-Myc functions upstream of miR-17∼92 in ADPKD models. Error bars represent s.e.m. *Indicates P<0.05. Student's unpaired t-test (b,d) One-way ANOVA, Tukey's multiple comparisons test (e–h). Scale bar 500 μm (c).
miR-17 modulates mitochondrial function by inhibiting Ppara
To explore the downstream mechanisms, we performed RNA sequencing (RNA-Seq) analysis to compare mRNA expression profiles between kidneys of 21-day-old Pkd1F/RCSKO and Pkd1F/RCDKO mice (n=3 biological samples), and 21-day-old Pkd2-KO and Pkd2-miR-17∼92KO mice (n=5 biological samples). Pathway analysis suggested that the primary cellular consequence of miR-17∼92 deletion in both ADPKD models was improved mitochondrial metabolism (Fig. 7a and Supplementary Figs 7 and 8). Furthermore, upstream regulatory analysis revealed that gene networks controlled by key metabolism-related transcription factors Pparα, Pparg and Ppargc1a were activated upon miR-17∼92 deletion (Fig. 7b). Next, we intersected the RNA-Seq data from both ADPKD models with a list of high-probability direct mRNA targets of miR-17∼92. Intriguingly, this analysis identified Pparα as one of 25 common direct targets of miR-17∼92 in the context of ADPKD (Fig. 7c and Supplementary Tables 4,5).
Figure 7. miR-17∼92 deletion results in improved expression of mitochondrial and metabolism-related gene networks.
RNA-Seq analysis was performed to compare mRNA expression profiles. (a) Top differentially regulated pathways in 21-day-old Pkd1F/RCDKO compared with Pkd1F/RCSKO kidneys, and 21-day-old Pkd2-miR-17∼92KO compared with Pkd2-KO kidneys are shown. (b) Ingenuity pathway analysis software was used to identify the upstream regulators (URs) that may be responsible for the gene expression changes observed after miR-17∼92 deletion in ADPKD models. Top 10 (based on z scores) differentially expressed gene networks and their associated URs in Pkd1F/RCDKO compared with Pkd1F/RCSKO kidneys are shown. These networks were also differentially regulated in Pkd2-miR-17∼92KO compared with Pkd2-KO kidneys. Positive z scores (shades of orange) indicate activation whereas negative z scores (shades of blue) indicate inhibition of the gene networks. The P values (shades of purple) represent the level of statistical confidence for the prediction that the differentially expressed gene network is indeed regulated by the indicated UR. Large interconnected gene networks controlled by URs PPARα, PPARg and PPARGC1a were predicted to be activated after miR-17∼92 deletion in both ADPKD models. (c) RNA-Seq data were intersected with high-probability miR-17∼92 targets predicted by TargetScan. This analysis identified Pparα and 24 other common putative miR-17∼92 targets in the context of ADPKD. Expression of these genes was decreased (shades of green) in single knockout (SKO) compared with their respective control (Ctl) kidneys. In contrast, the expression of these genes was increased (shades of red) in double knockout (DKO) compared with their respective SKO kidneys. SKO indicates either Pkd1F/RCSKO or Pkd2-KO, whereas DKO indicates either Pkd1F/RCDKO or Pkd2-miR-17∼92KO kidneys. The circles indicate predicted binding sites for the various miRNA families derived from the miR-17∼92 cluster. (d) Q-PCR analysis showing Pparα expression in the indicated mouse models or cell lines. (e) Western blot showing increased PPARα expression in Pkd1F/RCDKO compared with Pkd1F/RCSKO kidneys. (f) PPARα antibody staining revealed that PPARα expression was increased in cyst epithelia of Pkd1F/RCDKO mice compared with Pkd1F/RCSKO mice. PPARα expression was decreased in mIMCD3 cells treated with miR-17 mimic compared with scramble mimic. Error bars indicate s.e.m. *indicates P<0.05, ns indicates P>0.05. One-way ANOVA, Tukey's multiple comparisons test, Student's unpaired t-test. Scale bars, 50 μm (f, top panel) and 20 μm (f, bottom panel).
Reprogrammed metabolism is thought to fuel cyst proliferation in ADPKD20,21. Based on the unbiased analysis of our RNA-Seq data, we reasoned that miR-17∼92-mediated direct repression of Pparα might provide one potential explanation for this phenomenon. First, we validated that Pparα is inhibited by miR-17∼92 in ADPKD models. Q-PCR analysis revealed that compared with control kidneys, Pparα expression was downregulated by ∼80% in Pkd1F/RCKO and Pkd2-KO kidneys, whereas its expression was upregulated in Pkd1F/RCDKO and Pkd2-miR-17∼92KO kidneys (Fig. 7d). Pparα expression was also increased in anti-miR-17-treated compared with vehicle-treated Pkd2-KO kidneys. Western blot analysis and immunostaining revealed that PPARα expression was markedly increased in cysts of Pkd1F/RCDKO kidneys compared with Pkd1F/RCSKO kidneys (Fig. 7e,f). Conversely, Pparα mRNA and protein expression was decreased in miR-17 mimic-treated compared with scramble mimic-treated mIMCD3 as well as Pkd2−/− kidney epithelial cells (Fig. 7d,f).
Pparα 3′-UTR harbours an evolutionarily conserved binding site for miR-17 and miR-19 families (Fig. 8a). To test whether the binding sites are functional, we co-transfected mIMCD3 cells with a luciferase reporter plasmid containing Pparα 3′-UTR and miR-17, miR-19, or scramble mimics (Fig. 8b). Both miR-17 and miR-19 repressed Pparα 3′-UTR. Deleting the miR-17 binding site prevented miR-17-mediated, but not miR-19-mediated, repression. Similarly, deleting the miR-19 binding site abolished miR-19-mediated, but not miR-17-mediated, repression. Combined deletion of both binding sites abrogated repression by both miRNAs.
Figure 8. miR-17 aggravates cyst growth through direct repression of Pparα.
(a) Human PPARA 3'-UTR PPARΑ 3′-UTR harbours evolutionarily conserved miR-17 (red box) and miR-19 (blue box) binding sites. Watson-Crick base-pairing between miR-17/PPARΑ 3′-UTR and miR-19/PPARΑ 3′-UTR is shown. (b) To test whether these binding sites are functional, mouse Pparα 3′-UTR was cloned into a luciferase reporter plasmid. mIMCD3 cells were co-transfected with this plasmid and scramble (scr, black), miR-17 mimic (red) or miR-19 mimic (blue) (n=3). Luciferase reporter assays revealed that compared with scramble, both miR-17 and miR-19 mimics suppressed wild-type Pparα 3′-UTR. Deleting the miR-17 binding site prevented miR-17-mediated, but not miR-19-mediated, repression. Similarly, deleting the miR-19 binding site abolished miR-19-mediated, but not miR-17-mediated, repression. Combined deletion of both binding sites abrogated repression by both miRNAs. (c–f) To test whether reduced Pparα gene dosage is sufficient to enhance proliferation and promote cyst formation, 6-week-old Pparα−/− (n=5), Pkd1RC/RC (n=9) and Pkd1RC/RC; Pparα-KO (n=9) mice were generated and characterized. (c) Q-PCR analysis showing Pparα expression in the indicated mouse models. (d) H&E staining, (e) kidney-weight-to-body-weight ratios and (f) quantification of proliferating epithelial cells in kidneys of Pparα−/−, Pkd1RC/RC and Pkd1RC/RC; Pparα-KO mice is shown. (g–k) Conversely, to test whether increasing PPARα activity attenuates proliferation and cyst growth, 18-day-old Pkd2-KO littermates were fed a standard moist chow (Ctl) or a standard moist chow containing fenofibrate (Feno), a Pparα agonist, for 10 days. (g) Q-PCR analysis showed that Pparα expression was increased in Pkd2-KO mice fed Feno compared with Ctl. (h) H&E staining, (i) kidney-weight-to-body-weight ratios, (j) cyst index, and (k) quantification of proliferating cyst epithelial cells in Pkd2-KO mice fed Feno or Ctl is shown. Error bars indicate s.e.m. *indicates P<0.05, **indicates P<0.01, ***indicates P<0.001, and ns indicates P>0.05. One-way ANOVA, Tukey's multiple comparisons test (b–g), Student's unpaired t-test (i–k). Scale bars, 1 mm.
Our results have shown that miR-17 and miR-19 directly inhibit Pparα expression in cystic kidneys, but whether reducing Pparα gene dosage is sufficient to promote cyst growth is not known. To address this question, Pparα was genetically deleted in the slow cyst growth Pkd1RC/RC model. We characterized 6-week-old Pparα−/− (n=5), Pkd1RC/RC (n=9) and Pkd1RC/RC; Pparαmut mice (n=6 Pkd1RC/RC; Pparα+/− and n=3 Pkd1RC/RC; Pparα−/−). Pparα−/− mice22 displayed normal kidney histology and Pkd1RC/RC mice exhibited few (n=4 of 9) or no kidney cysts (n=5 of 9). In contrast, all Pkd1RC/RC; Pparαmut mice displayed numerous cysts (n=9 of 9). Accordingly, kidney-weight-to-body-weight ratio, the expression of Kim1 and Ngal, and proliferation were increased in Pkd1RC/RC; Pparαmut mice compared with Pparα−/− and Pkd1RC/RC mice (Fig. 8c–e and Supplementary Fig. 11). Conversely, we tested whether increasing Pparα expression attenuates proliferation and cyst growth. First, using the in vitro MTT assay, we found that treatment with miR-17 mimic increased proliferation of cultured mIMCD3 and Pkd2−/− cells, whereas genetic (Pparα plasmid) or pharmaceutical (WY14643) activation of PPARα abrogated miR-17-stimulated proliferation of mIMCD3 and Pkd2−/− cells (Supplementary Fig. 12H). Next, to test the role of PPARα in vivo, Pkd2-KO mice were treated with fenofibrate, a PPARα agonist. Six littermate pairs of 18-day-old Pkd2-KO mice were placed on a diet of standard chow or standard chow supplemented with fenofibrate for 10 days. Fenofibrate treatment increased Pparα expression in Pkd2-KO kidneys (Fig. 8g), whereas it reduced kidney-weight-to-body-weight ratio, cyst index, and proliferation suggesting that increasing Pparα expression attenuates cyst growth (Fig. 8h–k).
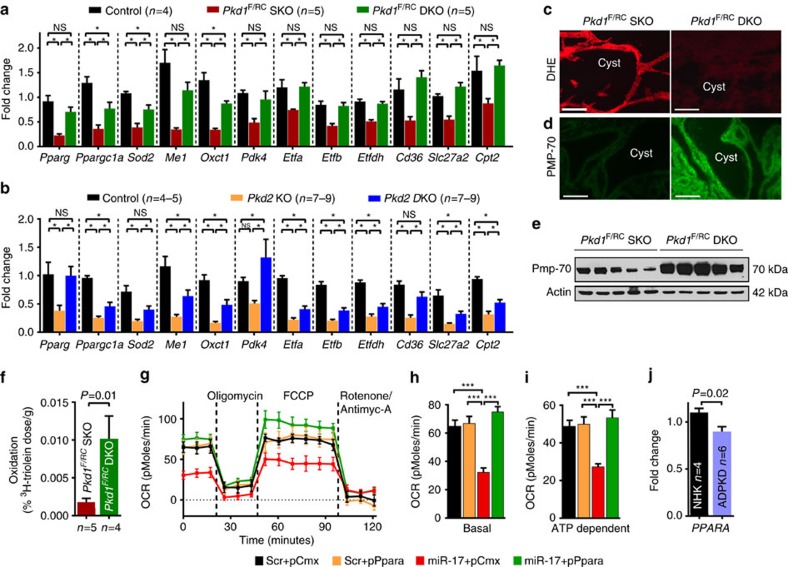
PPARα regulates several aspects of metabolism including oxidative phosphorylation (OXPHOS), fatty acid oxidation (FAO)23 and peroxisome function10. Therefore, we tested whether miR-17 affected these functions of PPARα. RNA-Seq (Supplementary Fig. 9,10) and subsequent Q-PCR analyses (Fig. 9a,b) showed that a large network of OXPHOS/FAO-related PPARα target genes were upregulated after miR-17∼92 deletion in both ADPKD models. To quantitatively measure OXPHOS, we determined the real-time oxygen consumption rate (OCR) of mIMCD3 and Pkd2−/− cells. Treatment with miR-17 mimics reduced basal and ATP-dependent OCR in mIMCD3 and Pkd2−/− cells. Moreover, re-expression of Pparα normalized ATP-dependent OCR of miR-17 mimic-treated mIMCD3 and Pkd2−/− cells (Fig. 9g–i and Supplementary Fig. 12). To assess mitochondrial metabolism in vivo, we injected Pkd1F/RCSKO and Pkd1F/RC-DKO mice with a 3H-triolein tracer and measured FAO24. Mirroring PPARα expression, FAO was increased in Pkd1F/RCDKO kidneys compared with Pkd1F/RCSKO kidneys (Fig. 9f). Reactive oxidative species level determined by dihydroethidium staining was reduced, whereas the number of peroxisomes assessed by the expression of PMP-70, an abundant and integral membrane protein of peroxisomes, was increased in Pkd1F/RCDKO compared with Pkd1F/RCSKO kidneys (Fig. 9c–e). Finally, since miR-17 is upregulated, we determined whether PPARα is downregulated in human ADPKD cysts. Q-PCR analysis revealed that PPARα expression was decreased in primary cells obtained from human ADPKD cysts compared with NHK (Fig. 9j). Consistent with these results, analysis of publicly available microarray data25 also showed that PPARα and its target genes are downregulated in human ADPKD cysts compared with normal renal cortical tissue (Supplementary Fig. 13). Collectively, these observations suggest that miR-17 promotes proliferation in cystic kidneys, at least in part, by reprogramming metabolism through direct repression of Pparα.
Figure 9. miR-17 modulates metabolic functions of PPARα.
PPARα regulates a large gene network that modulates several aspects of metabolism including OXPHOS, FAO, and peroxisome function. We tested whether miR-17 affected these functions of PPARα. (a,b) Q-PCR analysis of metabolism-related PPARα target genes in the indicated mouse models. Expression of PPARα targets was reduced in ADPKD models, whereas their expression was increased after miR-17∼92 deletion. (c) To assess mitochondrial function in vivo, kidney sections were stained with dihydroethidium (DHE) to assess reactive oxygen species production. Reactive oxygen species level was markedly decreased in kidneys of 21-day-old Pkd1F/RCDKO mice compared with Pkd1F/RCSKO mice. (d) Kidney sections were stained using an antibody against PMP-70, an abundant and integral membrane protein of peroxisomes. PMP-70 expression was increased in kidney cysts of 21-day-old Pkd1F/RCDKO mice compared with Pkd1F/RCSKO mice. (e) Western blot analysis showing increased PMP-70 expression in kidneys of 21-day-old Pkd1F/RCDKO mice compared with Pkd1F/RCSKO mice. (f) To test whether miR-17∼92 deletion affected FAO, 21-day-old Pkd1F/RCSKO and Pkd1F/RCDKO mice were injected with a 3H-triolein tracer and kidney FAO was measured. Mirroring PPARα expression, FAO was increased in Pkd1F/RCDKO kidneys compared with Pkd1F/RCSKO kidneys. (g–i) To determine whether miR-17 affects OXPHOS, Seahorse XF 24 Analyzer was used to measure real-time mitochondrial OCR. (g) Real-time OCR tracings, (h,i) Basal and ATP-dependent OCR of Pkd2−/− cells is shown (n=4). (j) PPARα expression was analysed in NHKs and human ADPKD cells. Q-PCR analysis showed that the expression of PPARα was decreased in cells derived from human ADPKD cysts compared with NHK. Error bars indicate s.e.m. *indicates P<0.05, ***indicates P<0.001, and ns indicates P>0.05. One-way ANOVA, Tukey's multiple comparison test (a,b,h,i), Student's unpaired t-test (f,j).
Discussion
The pre-clinical studies presented here indicate that miR-17∼92 is a novel drug target for ADPKD. In support of this conclusion, we show that genetic deletion of miR-17∼92 attenuates disease progression in ADPKD mouse models irrespective of the mutated gene (Pkd1 or Pkd2), the type of mutation (null or hypomorphic) or the dynamics of cyst growth (rapidly fatal, aggressive but long-lived or slowly progressing). In a complementary pharmaceutical approach, we demonstrate that anti-miR-17 also slowed cyst growth in two orthologous mouse models, including a long-lived, slow cyst growth model. Importantly, we show that anti-miRs can be delivered to collecting ducts following systemic administration, perhaps owing to the altered vasculature of cystic kidneys. This suggests that anti-miRs are a viable new drug class for ADPKD. These findings are likely to be relevant to human ADPKD pathogenesis because inhibiting miR-17 also attenuated proliferation and cyst growth of primary human ADPKD cultures. Since miR-17 inhibition slows cyst growth in ciliopathy models (Kif3a-KO and Nphp3pcy/pcy), the beneficial effects of anti-miR-17 treatment may also be observed in other forms of cystic kidney disease besides ADPKD. While our data demonstrated that administration of anti-miRs up to 6 months is feasible, additional pre-clinical studies are needed to fully address the long-term safety profile of anti-miR-17 therapy and to further explore miR-17 as a drug target for PKD.
Our studies point to pro-proliferative metabolic reprogramming induced by the c-Myc-miR-17-Pparα signalling axis as a potential new mechanism for PKD pathogenesis (Fig. 10). Quiescent kidney epithelial cells rely on FAO and the highly efficient OXPHOS pathway for ATP to maintain homoeostasis23. However, rather than ATP, a more critical limiting factor for proliferating cells is the amount of biosynthetic intermediates available for DNA replication and synthesis of new cell membranes and organelles26,27,28. Consistent with this notion and similar to cancer cells, cyst epithelia appear to depend on two alternative c-Myc-activated metabolic pathways of glycolysis and glutaminolysis to fuel their proliferation20,21,29. Our work suggests that an important additional component of this metabolic re-wiring is the inhibition of FAO and OXPHOS mediated, at least in part, by the miR-17-Pparα axis (Fig. 10). By reducing OXPHOS, carbon can be shuttled to alternate metabolic pathways to produce building blocks for the formation of new cells30. Indeed Pkd1-mutant renal epithelial cells exhibit reduced OXPHOS31. Moreover, PPARΑ and its target genes are among the top downregulated networks in murine and human ADPKD cysts25, further suggesting that reduced FAO and OXPHOS contributes to cyst pathogenesis. Importantly, mutations in FAO/OXPHOS and PPARA target genes cause clinical disorders that are often characterized by cystic kidneys32,33,34,35,36. For example, patients with mutations in CPT2, an enzyme that transports FA into mitochondria, or patients with Zellweger syndrome, a disorder caused due to defective peroxisomes, develop cystic kidneys. Glutaric acidemia type-II, caused by mutations of either ETFA, ETFB or ETFDH that collectively encode the OXPHOS enzyme electron transfer flavoprotein, is also characterized by PKD. Interestingly, Etfa, Etfb, Etfdh, Cpt2 and the various peroxisome genes were all downregulated in both Pkd1 and Pkd2 mutant kidneys, whereas their expression was increased after miR-17∼92 deletion.
Figure 10. The proposed mechanism by which miR-17∼92 promotes ADPKD progression.
Mutations of Pkd1 or Pkd2 is associated with increased expression of c-Myc. c-Myc binds to miR-17∼92 promoter and enhances its transcription in cystic kidneys. The miR-17∼92 primary transcript is processed to yield the individual mature miRNAs. In the cytoplasm, the mature miRNAs (miR-17 and miR-19) bind to Pparα 3′-UTR. PPARα is known to regulate the expression of key metabolic genes involved in mitochondrial OXPHOS pathway. miR-17 and miR-19 binding to Pparα 3′-UTR lead to reduced Pparα expression, which in turn affects mitochondrial metabolism in kidney epithelial cells.
Another implication of our work is that agonists of the PPAR pathway may also have therapeutic value in PKD. Several previous studies have shown that treatment with PPARγ agonists (pioglitazone or rosiglitazone) reduces proliferation and retards cyst growth in rodent PKD models37,38,39,40. We observed a similar beneficial effect of PPARα agonist (fenofibrate) treatment in Pkd2-KO mice. However, a potential limitation of these widely used PPAR pathway drugs is that they cause kidney-related side effects. Pioglitazone can cause fluid retention and exacerbate cardiovascular symptoms. Accordingly, whether lower-dose pioglitazone treatment can be safely tolerated by ADPKD patients is currently being evaluated in a clinical trial. Fenofibrate use is associated with an elevation in serum creatinine and BUN levels41; therefore, whether this drug can be used over the long term in kidney disease patients is unclear.
In conclusion, miR-17∼92 promotes ADPKD progression through a new mechanism involving the inhibition of mitochondrial function. Importantly, miR-17 is a feasible and novel drug target for ADPKD. Our pre-clinical work provides a strong rationale for the development of a miRNA-based therapeutic approach for ADPKD.
Methods
Mice
Ksp/Cre42, Pkhd1/Cre43, Pkd1F/F15, Pkd2F/F15, miR-17∼92F/F (ref. 44), miR-17 transgenic mice45, Pkd1RC/RC (ref. 14) and c-Myc transgenic (SBM)46 and Pparα−/−22 mice were used in this study. The generation of the following mouse lines is discussed in the results section: Pkd1-miR-17∼92KO, Pkd2-miR-17∼92KO, Ksp/Cre;Pkd1F/RC (Pkd1F/RCSKO), Ksp/Cre;Pkd1F/RC; miR-17∼92 F/F (Pkd1F/RCDKO), Pkd1RC/RC; Ksp/Cre, Pkd1RC/RC;Ksp/Cre;miR-17∼92F/F and Pkd1/c-Myc-KO. For all studies, including the anti-miR studies, an equal number of male and female mice were used. The mice listed above were maintained in the B6 genetic background and their age(s) are indicated in the results section or the figure legends. We have previously used Pkd2-KO mice to analyse miR-17 expression by Q-PCR12. However, all analyses reported in the current paper was performed using new mice specifically generated for this project. All experiments involving animals were conducted under the approval of the UT Southwestern and Institut de Recherches Cliniques de Montréal Institutional Animal Care and Use Committee.
Anti-miR-17 studies
Anti-miR-17 studies involving Pkhd1/Cre;Pkd2F/F (Pkd2-KO) mice were utilized to determine the delivery and efficacy of the anti-miR-17 compound. Mice were randomly assigned to the treatment groups. All investigators were blinded to the treatment groups until predetermined analysis was complete. Based on our previous experience, power analysis (alpha <5% and power >80%) was performed a priori to determine the sample size (N of at least 8). Nphp3pcy/pcy mice (CD-1 background) were obtained from PreClinOmics (Indianapolis, IN). Mutant mice were randomly assigned to the treatment groups. The investigators were not blinded to the treatment groups. Animal experiments were conducted in accordance with Institutional Animal Care and Use Committee guidelines via Explora BioLab services. The experimental approach for anti-miR-17 studies is shown in Supplementary Fig. 5.
Fenofibrate studies
Littermate pairs of Pkhd1/Cre;Pkd2F/F (Pkd2-KO) mice were administered either standard moist chow or standard moist chow supplemented with fenofibrate at a dose of 800 mg per day per kg body-weight for 10 days starting at postnatal day 18. The calculated dose is based on a food consumption rate of 160 mg diet per day per kg body-weight.
Tissue harvesting and analysis
Mice were anesthetized under approved protocols, blood was obtained by cardiac puncture, and the right kidney was flash frozen for molecular analysis. The left kidney was perfused with cold PBS and 4% (wt/vol) paraformaldehyde and then collected. Kidneys were fixed with 4% paraformaldehyde for 2 h and then embedded in paraffin for sectioning. Sagittal sections of kidneys were stained with hematoxylin and eosin (H&E) or Pico Sirius red for additional analysis. Cyst index (cystic area/total kidney section surface area) and fibrosis area calculations (Pico Sirius red positive/total kidney section surface area) were performed using ImageJ analysis software.
Renal and liver function tests
Serum creatinine was measured using capillary electrophoresis and BUN, aspartate aminotransferase, and alanine aminotransferase were measured using the Vitros 250 Analyzer.
Human specimens
Frozen ADPKD and NHK specimens were provided by the PKD Research Biomaterials and Cellular Models Core at the Kansas University Medical Center (KUMC). ADPKD kidneys were obtained with the assistance of the KUMC Bio-Specimen Repository Core. Kidneys were immediately sealed in sterile bags, submerged in ice and delivered to the laboratory. Normal kidneys unsuitable for transplantation were obtained from the Midwest Transplant Network (Kansas City, KS). Informed consent was obtained. The protocol for the use of surgically discarded kidney tissues complied with federal regulations and was approved by the Institutional Review Board at the KUMC.
MRI imaging
MRI scans were performed using a 7T small animal MRI scanner (Agilent (Varian), Inc, Palo Alto, CA) equipped with a 40 mm Millipede RF coil (ExtendMR LLC, Milpitas, CA). Under anaesthesia by inhalation of 1–3% isoflurane mixed in with medical-grade oxygen via nose-cone, the animals were placed supine with the respiratory sensor, head first with the kidneys centred with respect to the centre of a RF coil. MRI acquisitions were gated using the respiratory triggering. The bore temperature was kept at 28±1 °C. Two-dimensional (2D) scout images on three orthogonal planes (axial, coronal and sagittal) were acquired to ensure the positioning. For kidney volume measurements, the high-resolution T2-weighted fast spin-echo images were acquired on the coronal sections by applying a 6 ms sinc pre-saturation pulse to remove the fat signal. Some of the major imaging parameters were TR/TE=2,500/60 ms, FOV=32 × 32 mm, matrix size=256 × 256 (affording 125 μm in-plane resolution), slice thickness=1 mm, slice number=9–15 (dependent on the kidney size), no gap and number of average=6.
RNA isolation and quantitative RT-PCR (Q-PCR)
Total RNA was isolated from cultured cells or mouse kidneys using miRNeasy Mini kits (Qiagen). First-strand cDNA was synthesized from mRNA using the iScript cDNA synthesis kit (Bio-Rad), and Q-PCR was performed using the iQ SYBR Green Supermix (Bio-Rad). The Universal cDNA Synthesis kit from Exiqon was used for first-strand synthesis from miRNA. Q-PCR was performed by using miRNA-specific forward and reverse LNA-enhanced PCR primers from Exiqon. To measure primary miRNA (pri-miRNA) expression, first-strand cDNA was synthesized by using the iScript cDNA synthesis kit (Bio-Rad). Q-PCR was performed by using the TaqMan Gene Expression Master Mix (Life Technologies) and pre-designed pri-miR-17∼92 primers from Life Technologies. The samples were loaded in triplicate on a CFX ConnectTM Real-time PCR detection system. 18S and 5S RNA were used to normalize expression of mRNA and miRNA, respectively. Data were analysed using the Bio-Rad CFX software. The sequences of the PCR primers are shown in Supplementary Table 6.
miRNA microarray analysis
A nCounter Gene Expression CodeSet was purchased from Nanostring Technologies. nCounter miRNA expression assays were performed using total kidney RNA from Pkd1-KO and Pkd2-KO and their respective age-matched littermate controls (n=3 biological replicates for all groups) according to the manufacturer's instructions.
RNA-Seq analysis
Strand-specific RNA-Seq libraries were prepared using the TruSeq Stranded Total RNA LT Sample Prep Kit from Illumina (n=3–5 biological replicates for all groups). After quality check and quantification, libraries were sequenced at the University of Texas Southwestern McDermott Center using a Hiseq2500 Sequencer to generate 51 bp single-end reads. Before mapping, reads were trimmed to remove low-quality regions in the ends. Trimmed reads were mapped to the mouse genome (mm10) using TopHat version 2.0.12 with the UCSC iGenomes GTF file from Illumina47. Alignments with mapping quality <10 were discarded. Expression abundance estimation and differential expression gene identification were done using edgeR48 or Cuffdiff version 2.2.1 (ref. 49). Genes with a P value <0.05 were deemed significantly differentially expressed between the two conditions. The identified genes were further subjected to ingenuity pathway analysis to identify cellular pathways and regulatory networks.
In situ hybridization
10 μm thick frozen kidney sections were immersed overnight in 10% neutral buffered formalin. The next day, sections were washed with PBS three times and then, treated with proteinase K for 10 min at 37 °C. Subsequently, miRCURY LNA-ISH FFPE protocol was followed. The tissues were incubated with miR-17 (cat # 38461-01, Exiqon) or scramble (cat # 99004-01, Exiqon) probes. A hybridization temperature of 55 °C was used for both mouse tissue and human samples. Anti-Digoxigenin-AP Fab fragment antibody was used at 1:400 dilution (cat # 11093274910, Roche), and AP substrate was reapplied after 1 h. Slides were counterstained with nuclear fast red and then, mounted with Eukitt mounting medium. Samples were allowed to settle overnight and then, examined using light microscopy.
Immunofluorescence staining
The following antibodies and dilutions were used on paraffin-embedded sections for immunofluorescence staining: anti-PS (Regulus Therapeutics Inc, 1:1,000), anti-Pparα (Abcam ab8934, 1:200), anti-Pmp-70 (EMD Millipore ABT12, 1:200), anti-phosphohistone H3 (1:400, Sigma-Aldrich H0412). Secondary antibodies were conjugated to Alexa Fluor 488 or Alexa Fluor 594 (Molecular Probes, 1:400). Lectins used were Dolichos biflorus agglutinin (DBA; Vector Laboratories, 1:400) and Lotus tetragonolobus agglutinin (LTA; Vector Laboratories, 1:400). Tissue sections were stained as described43. TUNEL assay was performed using the Promega Dead End Tunel Fluorometric System Kit per the manufacturer's directions with the following modification: the proteinase K treatment time was extended to 15 min. Quantification of apoptosis and proliferation was performed by randomly selecting 10 × 20 magnification fields per sample and subsequently determining the percentage of positively stained cyst epithelial cells. The person performing the quantification was blinded to the genotype of kidney sections.
Western blot analysis
Total protein was extracted from kidneys or mIMCD3 cells. A total of 20 μg of protein was loaded on a 4–15% SDS-polyacrylamide gel and the proteins were transferred to a nitrocellulose membrane. The membrane was blocked with 5% bovine serum albumin and probed overnight at 4 °C with anti-Polycystin-2 (YCC2, gift from Stefan Somlo, 1:6,000), anti-PPARα (1:1,000) or anti-PMP-70 (1:1,000) antibodies. Goat-anti-rabbit HRP-conjugated IgG was used as a secondary antibody, and the blot was developed using the SuperSignal West Dura Extended Duration substrate from Pierce. The protein bands were quantified using Quantity One imaging software from Bio-Rad. Uncropped western blot images are shown in Supplementary Fig. 14.
ChIP experiments
ChIP assays were performed using the ChIP-IT High Sensitivity Kit (catalogue # 53040, Active Motif) or the EZ ChIP Kit (catalogue # 17–371,EMD Millipore) according to the manufacturer's protocol. Briefly, mIMCD3 cells or mouse kidney tissue were crosslinked with 1% formaldehyde for 15 min at room temperature, homogenized into a single-cell suspension and chromatin samples were extracted from the nuclei and sonicated. Immunoprecipitation was performed with 5 μg of rabbit anti-Myc (sc-764; Santa Cruz Biotechnology) and rabbit IgG (sc-2027; Santa Cruz Biotechnology) antibody as a negative control. Genomic DNA was purified and amplified using the following primer set: forward 5′-AGGGCTCGTGGTTCTTAGGT-3′ and reverse 5′-GAAAAAGGGCAACCAGGACT-3′. This primer set amplifies a c-Myc binding site, which is ∼483 bp upstream of the transcription start site of the mouse miR-17∼92 cluster. Enrichment of c-Myc binding to the mir-17 site was compared with the Input DNA (10%). The PCR product (145 bp) was visualized on a 2% agarose gel.
Reagents
Dual-Luciferase Reporter Assay System (catalogue#1960) and CellTiter 96 AQueous Non-Radioactive Cell Proliferation Assay (catalogue#G5421) was purchased from Promega. DMEM low glucose medium (catalogue # 11885-084), DNase I (catalogue # 18068-015), and Lipofectamine 2000 (catalogue # 11668-019) were purchased from Life Technologies. miRNeasy Mini kits (catalogue # 217004) were purchased from Qiagen. QuikChange II Site-Directed Mutagenesis Kit (catalogue # 200523) was purchased from Agilent Technologies. The following miRNA mimics were purchased from Dharmacon, Inc (Thermo Fischer Scientific Inc) - miR-17 (catalogue # C-310561-07-0005), miR-19a (catalogue # C-310563-05-0005) and negative control or Scrambled (catalogue # CN-001000-01-05).
Cell culture
Mouse inner medullary collecting duct (mIMCD3) cells were obtained from ATCC and grown in DMEM low glucose medium supplemented with 10% FBS and maintained in 5% CO2 atmosphere at 37 °C. Pkd2−/− cells were obtained from Steve Somlo and grown in DMEM:F-12 medium (catalogue # 10565-018,Gibco) supplemented with 2% foetal bovine serum, insulin (8.3 × 10−7 M), prostaglandin E1 (7.1 × 10−8 M), selenium (6.8 × 10−9 M), transferrin (6.2 × 10−8 M), triiodothyronine (2 × 10−9 m), dexamethasone (5.09 × 10−8 m) and recombinant γ-interferon (10 units/ ml−1, Sigma) at 33 °C50 Human primary ADPKD cells were grown in DMEM:F-12 medium (catalogue # 10565-018,Gibco) supplemented with 5% FBS, 5 μg ml−1 insulin, 5 μg ml−1 transferrin and 5 ng ml−1 sodium selenite (ITS)(catalogue # 17-838Z, Lonza)50.
Proliferation assays
The following experimental protocol was used for the proliferation assays that were performed in Supplementary Fig. 12G,H. For PPPARα expression plasmid studies, mIMCD3 cells were grown in six-well dishes (2 × 105 cells per well), and transfected with 0.6 μg of a Pparα expression plasmid (pPparα) or Control (pCMX), along with 5 nM of miR-17 or scrambled mimic (Scr). Two days after transfection, the cells were transferred to a 96-well plate and incubated with 100 μl of the medium. For WY14643 studies, mIMCD3 or Pkd2−/− cells were grown at 37 °C, plated in six-well dishes (2 × 105 cells per well), and transfected with 5 nM of miR-17 or scrambled mimic. The medium was supplemented with 10 μM of WY14643 (dissolved in 70% DMSO/30% saline) or vehicle (DMSO). Two days after transfection, the cells were transferred to a 96-well plate and incubated with 100 μl of medium supplemented with 10 μM of WY14643 or vehicle. The MTT assay (Promega Corp) was performed after 24 h on mIMCD3 and Pkd2−/−cells, according to the manufacturer's directions. The quantity of formazan product was measured by the amount of absorbance at 490 nm, which is directly proportional to the number of living cells in culture.
The following protocol was used for the proliferation assays that were performed in Fig. 5a,c and Supplementary Fig. 6. At 80% confluency, human ADPKD cells were trypsinized using 1:10 dilution of Trypsin (catalogue # 25-053-CI, Corning) in Ca+2 and Mg+2 free PBS. These cells were transfected with anti-miR-17 and control oligos using RNAiMAX (catalogue # 13778-150, Life Technologies) following the manufacturer's protocol at 2,500 cells per well density in a 96-well plate. Cell viability was measured using MTT assay (catalogue# G4000, Promega) on day 3 following the manufacturers' protocol.
Cyst formation assay
Human primary ADPKD cells were grown to 80% confluency and trypsinized as described earlier. At day 1, cells were transfected with the oligo of interest using RNAiMAX in a six-well plate format. Twenty-four hour after transfection, cells were trypsinized to single-cell suspension, counted and plated in a 96-well plate (cat # 353072, Corning) at 4,000 cells per well density in 130 μl of media plus Matrigel (cat # 354234, Corning) in a 4:5 ratio. Upon matrigel solidification, complete growth media was added to the well. Media was replenished every 72 h until 8 days post plating when the cyst size and number was measured. Each well was inspected for cyst proliferation using an Olympus D8 light microscope. Images were recorded with an Olympus DP26 camera (Olympus Corporation) from 28 focal planes, 150 μm apart, down into the well (on the z axis) as twenty-eight 24-bit colour TIFF images at 2,448-by-1,920 pixels at 72 dpi. Each focal plane's image from each well was processed using a custom R script (R Core Team 2015 R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. (URL https://www.R-project.org/) that used the EBImage Bioconductor package51. This script detected cysts in an automated and reproducible manner and was applied to all matrigel assays in all donors. Briefly, each image was masked for artifacts, filtered through a high-pass Laplacian filter and segmented with an adaptive threshold. Detected objects in the segmented images were further processed by pixel dilation, hole filling and by pixel erosion. Size, radius and eccentricity statistics on each segmented object were collected. Objects were filtered out if they had a mean radius less than or equal to 15 pixels, or were of a mean object radius greater than 200 pixels, or a coefficient of variation of the radius of greater than 0.2, or if the eccentricity of the detected object was greater than 0.75. Because cysts were often larger than the distance between focal planes, it was important to avoid counting such cysts more than once. If a cyst object in one image fell within the same x and y coordinates of a neighbouring image (for example, one focal plane above on the z axis), then this was counted as the same cyst. All images in each well were processed sequentially in this manner on the z axis. Finally, each cyst's volume was estimated by multiplying the mean radius of the largest object by 4/3πr3, assuming each cyst is a sphere.
miRNA polysome shift assay (miPSA)
C57BL/6 mice (Jackson Laboratories) were injected with a single subcutaneous dose of 0.3, 3 or 30 mg kg−1 of anti-miR-17, a control oligonucleotide or PBS. The kidneys were collected after 7 days, and the tissues were processed16. The relative level of miR-17 in the polysome containing fractions was quantified using the miRNA TaqMan assays (Life Technologies). Let-7d was used as the reference gene. The relative displacement of miR-17 normalized to let-7d was calculated between the treated (anti-miR-17) and the control (PBS or control oligonucleotide) samples using double delta Ct (ΔΔCt) method. The displacement values are reported in log2 scale where the positive values reflect the loss of miR-17 from the high molecular weight polysome fractions.
3′-UTR plasmids
1456, bp of the mouse Pparα 3′-UTR that includes the miR-17 and miR-19a conserved sites was PCR amplified using forward and reverse primers that introduced NheI and XhoI restriction sites at the 5′- and 3′- ends, respectively. The sequence of the forward primer was 5′-TAGAATGCTAGCGCCACTGTTCAGGGACCTC-3′, whereas that of the reverse primer was 5′-TAGAATCTCGAGAGGCATCTACCACCATGTCC-3′, where the underlined sequences are the NheI and XhoI sites, respectively. The 1456, bp PCR product was ligated in the pLightSwitch Empty (pLS) 3′-UTR plasmid, that was previously digested with NheI and XhoI restriction enzymes to generate the WT Pparα 3′-UTR construct. The seed sequences for the miR-17 and the miR-19 binding sites were mutated in the WT-Pparα 3′-UTR construct to produce the Pparα 3′-UTR (Δ17) and Pparα 3′-UTR (Δ19) constructs. The sequences of the primers used in site-directed mutagenesis reactions are as follows-
Pparα 3′-UTR (Δ17)
Forward primer – 5′-CTCTTAAATCCCTGAAAACTAATCTTAGGCAGTTAACCTTTGAAAACCTACAAGTCAAGG -3′ Reverse primer- 5′-CCTTGACTTGTAGGTTTTCAAAGGTTAACTGCCTAAGATTAGTTTTCAGGGATTTAAGAG-3′
Pparα 3′-UTR (Δ19)
Forward primer- 5′-CCATACAGGAGAGCAGGGACAGTAGTCGAGGGCCTCCCTCCTACGC-3′
Reverse primer- 5′-GCGTAGGAGGGAGGCCCTCGACTACTGTCCCTGCTCTCCTGTATGG-3′
The underlined sequences denote the respective mutated miRNA binding sites. Site-directed mutagenesis was performed using the QuikChange II kit according to the manufacturer's instructions. The PCR protocol was as follows—1cycle at 95 °C for 1 min, followed by 18 cycles of 95 °C for 50 s, 60 °C for 50 s, 68 °C for 6 min and 1 cycle of 68 °C as a final extension cycle. The presence of the desired mutations was verified by DNA sequencing.
Luciferase assays
mIMCD3 cells were plated in six-well dishes (2 × 105 cells per well) and transfected with 0.4 μg of pLS-Renilla-3′-UTR plasmids, and 10 nM of miR-17 or miR-19a mimic. Cells were transfected with 0.04 μg of the pGL3-Control plasmid (Promega Corp) encoding Photinus luciferase to control for differences in transfection efficiency. Lipofectamine 2000 (Invitrogen) was used as a transfection reagent. Forty-eight hours later, the cells were lysed in 250 μl of passive lysis buffer (Promega Corp), and 40 μl of the cell lysate was added to 96-well plates. Photinus and Renilla luciferase activities were measured by using the Dual-Luciferase Reporter Assay System (Promega Corp) according to the manufacturer's directions.
3H-triolein uptake and β-oxidation
Endogenous triolein clearance rates and β-oxidation rates in kidneys were performed24. Briefly, 21-day-old Pkd1F/RCSKO and Pkd1F/RCDKO were injected with 3H-triolein (2 μCi per mouse in 100 μl of 5% intralipid) after an 8-h fast. Twenty minutes later, mice were killed, blood samples were taken and kidneys were collected, weighed and frozen at −80 °C until processing. Lipids were extracted, and radioactivity content was quantified.
Mitochondrial OCR experiments
Mitochondrial OCRs were determined using an XF24 Extracellular Flux Analyzer (Seahorse Bioscience, MA) following the manufacturers' protocols. A basal-oligomycin-carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP)-antimycin-A/rotenone ‘BOFA' experiment was utilized for the assessment of mitochondrial function and respiration rates in mIMCD3 cells. Pkd2−/− or mIMCD3 cells were grown in six-well dishes (2 × 105 cells per well). Pkd2−/− cells were transfected with 0.4 μg of a PPARα expression plasmid (pPpara) or Control (pCMX), along with 1 nM of miR-17 or scrambled mimic (Scr). mIMCD3 cells were transfected with 0.6 μg of pPpara or pCMX, along with 5 nM of miR-17 or Scr mimic. Forty-eight hour post-transfection, Pkd2−/− or mIMCD3 cells (60,000 per well) were seeded into an XF24 cell culture microplate (Seahorse Bioscience). Cells were then equilibrated for 1 h at 37 °C (non-CO2) in XF Assay Medium (Modified DMEM, 0 mM Glucose; Seahorse Bioscience) (pH 7.4), supplemented with 1 mM sodium pyruvate, 1 mM L-glutamine, and 7 mM glucose. The XF24 plate was then transferred to a temperature-controlled (37 °C) Seahorse analyzer and subjected to a 10-min equilibration period and three assay cycles to measure the basal rate, comprising a 3-min mix, a 2-min wait and a 3-min measure period each. Compounds were then added by automatic pneumatic injection followed by assay cycles after each, comprising of 3-min mix, 2-min wait and a 3-min measure period. OCR measurements were obtained following sequential additions of oligomycin (1 μM final concentration), FCCP (1 μM) and antimycin-A/rotenone (10 μM/100 nM). OCR measurements were recorded at set interval time points. The ATP-dependent OCR was calculated by subtracting the OCR reading recorded after oligomycin treatment from OCR reading recorded at basal levels ((ATP-linked OCR)=(basal OCR) - (post-Oligomycin OCR)). All compounds and materials above were obtained from Sigma-Aldrich.
Statistical analysis
Data are shown as the mean±s.e.m. Statistical analysis was performed using Student's t-test for pairwise comparisons or Analysis of variance (ANOVA) followed by Tukey's post hoc test for multiple comparisons. Mantel-Cox test was used to analyse differences in mice survival. P<0.05 was considered significant. Primary outcomes (survival or renal function) in all mouse studies are statistically powered to achieve an alpha error <5% and power of >80%.
Data availability
The RNA-Seq has been deposited in the NCBI Gene Expression Omnibus repository under accession number GSE89764. Other data are available from the corresponding author upon request.
Additional information
How to cite this article: Hajarnis, S. et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat. Commun. 8, 14395 doi: 10.1038/ncomms14395 (2017).
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Material
Supplementary figures, supplementary tables and supplementary references.
Acknowledgments
We thank the UT Southwestern O'Brien Kidney Research Core Center (P30DK079328), the McDermott Center Sequencing and Bioinformatics Core, the Advanced Imaging Research Center at UT Southwestern, the UT Southwestern Metabolic phenotyping core, the Polycystic Kidney Disease Research Biomaterials and Cellular Models Core at the University of Kansas Medical Center, and the Mayo Translational PKD Center (DK090728) for providing critical reagents and services. We thank Stefan Somlo (Yale Univ) for providing Pkd1F/F and Pkd2F/F mice and David Mangelsdorf (UT Southwestern) for providing the PPARα-expressing plasmid. Work from the authors' laboratory is supported by National Institute of Health grants R01DK102572 (to V.P.) and R37DK042921 (to P.I.) and a grant from the PKD foundation (to V.P. and M.T.).
Footnotes
Vishal Patel and John Androsavich have applied for a patent related to the treatment of polycystic kidney disease using miR-17 inhibitors. The Patel lab has a sponsored research agreement with Regulus Therapeutics. The remaining authors declare no conflict of interests.
Author contributions V.P. conceived the idea and supervised the project; D.W., M.Y. and V.P. designed and performed experiments, and analysed the data involving miR-17∼92 genetic deletion in ADPKD mouse models; S.Z. performed MRIs and D.W., M.Y. and V.P. analysed the MRI data; D.W., M.Y., S.H., M.S., X.L., K.K., R.G., J.L., S.D., E.C.L., J.R.A. and V.P. designed experiments and analysed the data involving anti-miR-17 studies; R.L., D.W., S.L., V.K., J.R.A. and V.P. designed microarray/RNA-Seq experiments and performed bioinformatics analysis; S.H., R.L., M.Y., J.J., W.L.H. and C.M.K. designed, performed and analysed the mitochondrial metabolism studies; S.H. performed Pparα 3′-UTR studies; R.L. performed miR-17 in situ experiments; K.A. performed c-Myc ChIP experiment; R.L., S.H., M.Y., A.F. and V.P. designed and performed experiments, and analysed the data involving the Pparα mouse and cell models; P.E.S., P.C.H., M.T., D.P.W. and P.I. provided essential reagents, mouse models and/or analytical tools; M.Y. and V.P. prepared the figures; V.P. wrote the manuscript with contributions from S.H., R.L. and M.Y.
References
- Patel V., Chowdhury R. & Igarashi P. Advances in the pathogenesis and treatment of polycystic kidney disease. Curr. Opin. Nephrol. Hypertens. 18, 99–106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi P. & Somlo S. Polycystic kidney disease. J. Am. Soc. Nephrol. 18, 1371–1373 (2007). [DOI] [PubMed] [Google Scholar]
- Gabow P. A. & Grantham J. J. in Diseases of the Kidney eds Schrier R. W., Gottschalk C. W. Little, Brown (1997). [Google Scholar]
- Bartel D. P. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297 (2004). [DOI] [PubMed] [Google Scholar]
- van Rooij E. & Olson E. N. MicroRNAs: powerful new regulators of heart disease and provocative therapeutic targets. J. Clin. Invest. 117, 2369–2376 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. & Noureddine L. MicroRNAs and fibrosis. Curr. Opin. Nephrol. Hypertens. 21, 410–416 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noureddine L., Hajarnis S. & Patel V. MicroRNAs and polycystic kidney disease. Drug. Discov. Today. Dis. Models 10, e137–e1743 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell J. T. & Olson E. N. MicroRNAs in stress signaling and human disease. Cell 148, 1172–1187 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen H. L. et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368, 1685–1694 (2013). [DOI] [PubMed] [Google Scholar]
- Gomez I. G. et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Invest. 125, 141–156 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obad S. et al. Silencing of microRNA families by seed-targeting tiny LNAs. Nat. Genet. 43, 371–378 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel V. et al. miR-17∼92 miRNA cluster promotes kidney cyst growth in polycystic kidney disease. Proc. Natl Acad. Sci. USA 110, 10765–10770 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhia R. et al. MicroRNA-21 aggravates cyst growth in a model of polycystic kidney disease. J. Am. Soc. Nephrol. 27, 2319–2330 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp K. et al. Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J. Clin. Invest. 122, 4257–4273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibazaki S. et al. Cyst formation and activation of the extracellular regulated kinase pathway after kidney specific inactivation of Pkd1. Hum. Mol. Genet. 17, 1505–1516 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Androsavich J. R. et al. Polysome shift assay for direct measurement of miRNA inhibition by anti-miRNA drugs. Nucleic Acids Res. 44, e13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell K. A., Wentzel E. A., Zeller K. I., Dang C. V. & Mendell J. T. c-Myc-regulated microRNAs modulate E2F1 expression. Nature 435, 839–843 (2005). [DOI] [PubMed] [Google Scholar]
- Trudel M., D'Agati V. & Costantini F. c-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 39, 665–671 (1991). [DOI] [PubMed] [Google Scholar]
- Trudel M., Barisoni L., Lanoix J. & D'Agati V. Polycystic kidney disease in SBM transgenic mice: role of c-myc in disease induction and progression. Am. J. Pathol. 152, 219–229 (1998). [PMC free article] [PubMed] [Google Scholar]
- Hwang V. J. et al. The cpk model of recessive PKD shows glutamine dependence associated with the production of the oncometabolite 2-hydroxyglutarate. Am. J. Physiol. Renal Physiol. 309, F492–F498 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe I. et al. Defective glucose metabolism in polycystic kidney disease identifies a new therapeutic strategy. Nat. Med. 19, 488–493 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. S. et al. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell. Biol. 15, 3012–3022 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang H. M. et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 21, 37–46 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusminski C. M. et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat. Med. 18, 1539–1549 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X. et al. Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum. Mol. Genet. 18, 2328–2343 (2009). [DOI] [PubMed] [Google Scholar]
- Vander Heiden M. G. Targeting cancer metabolism: a therapeutic window opens. Nat. Rev. Drug. Discov. 10, 671–684 (2011). [DOI] [PubMed] [Google Scholar]
- Hensley C. T., Wasti A. T. & DeBerardinis R. J. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J. Clin. Invest. 123, 3678–3684 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson K. A., Schell J. C. & Rutter J. Pyruvate and metabolic flexibility: illuminating a path toward selective cancer therapies. Trends Biochem. Sci. 41, 219–230 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang C. V. MYC, Metabolism, Cell Growth, and Tumorigenesis. Cold Spring Harb. Perspect. Med. 3, a014217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu P. P. & Sabatini D. M. Cancer cell metabolism: Warburg and beyond. Cell 134, 703–707 (2008). [DOI] [PubMed] [Google Scholar]
- Menezes L. F., Lin C. C., Zhou F. & Germino G. G. Fatty acid oxidation is impaired in an orthologous mouse model of autosomal dominant polycystic kidney disease. EBioMedicine 5, 183–192 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meir K. et al. Severe infantile carnitine palmitoyltransferase II deficiency in 19-week fetal sibs. Pediatr. Dev. Pathol. 12, 481–486 (2009). [DOI] [PubMed] [Google Scholar]
- Weese-Mayer D. E., Smith K. M., Reddy J. K., Salafsky I. & Poznanski A. K. Computerized tomography and ultrasound in the diagnosis of cerebro-hepato-renal syndrome of Zellweger. Pediatr. Radiol. 17, 170–172 (1987). [DOI] [PubMed] [Google Scholar]
- FitzPatrick D. R. Zellweger syndrome and associated phenotypes. J. Med. Genet. 33, 863–868 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. et al. Fetal polycystic kidney disease associated with glutaric aciduria type II: an inborn error of energy metabolism. Am. J. Perinatol. 13, 131–134 (1996). [DOI] [PubMed] [Google Scholar]
- Kjaergaard S., Graem N., Larsen T. & Skovby F. Recurrent fetal polycystic kidneys associated with glutaric aciduria type II. APMIS 106, 1188–1193 (1998). [DOI] [PubMed] [Google Scholar]
- Yoshihara D. et al. PPAR-gamma agonist ameliorates kidney and liver disease in an orthologous rat model of human autosomal recessive polycystic kidney disease. Am. J. Physiol. Renal. Physiol. 300, F465–F474 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer-Yost B. L. et al. Pioglitazone attenuates cystic burden in the PCK rodent model of polycystic kidney disease. PPAR Res. 2010, 274376 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhang Y., Yuan L., Fu L. & Mei C. Rosiglitazone inhibits insulin-like growth factor1-induced polycystic kidney disease cell growth and p70S6 kinase activation. Mol. Med. Rep. 8, 861–864 (2013). [DOI] [PubMed] [Google Scholar]
- Dai B. et al. Rosiglitazone attenuates development of polycystic kidney disease and prolongs survival in Han:SPRD rats. Clin. Sci. (Lond) 119, 323–333 (2010). [DOI] [PubMed] [Google Scholar]
- Jun M. et al. Effects of fibrates in kidney disease: a systematic review and meta-analysis. J. Am. Coll. Cardiol. 60, 2061–2071 (2012). [DOI] [PubMed] [Google Scholar]
- Shao X., Somlo S. & Igarashi P. Epithelial-specific Cre/lox recombination in the developing kidney and genitourinary tract. J. Am. Soc. Nephrol. 13, 1837–1846 (2002). [DOI] [PubMed] [Google Scholar]
- Patel V. et al. Acute kidney injury and aberrant planar cell polarity induce cyst formation in mice lacking renal cilia. Hum. Mol. Genet. 17, 1578–1590 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A. et al. Targeted deletion reveals essential and overlapping functions of the miR-17 through 92 family of miRNA clusters. Cell 132, 875–886 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C. et al. Lymphoproliferative disease and autoimmunity in mice with increased miR-17-92 expression in lymphocytes. Nat. Immunol. 9, 405–414 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M., Barisoni L., Lanoix J. & D'Agati V. Polycystic kidney disease in SBM transgenic mice: role of c-myc in disease induction and progression. Am. J. Pathol. 152, 219–229 (1998). [PMC free article] [PubMed] [Google Scholar]
- Trapnell C., Pachter L. & Salzberg S. L. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25, 1105–1111 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M. D., McCarthy D. J. & Smyth G. K. edgeR: a bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapnell C. et al. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 31, 46–53 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T., Reif G. A., Calvet J. P. & Wallace D. P. Sorafenib inhibits cAMP-dependent ERK activation, cell proliferation, and in vitro cyst growth of human ADPKD cyst epithelial cells. Am. J. Physiol. Renal. Physiol. 299, F944–F951 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pau G., Fuchs F., Sklyar O., Boutros M. & Huber W. EBImage--an R package for image processing with applications to cellular phenotypes. Bioinformatics 26, 979–981 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, supplementary tables and supplementary references.
Data Availability Statement
The RNA-Seq has been deposited in the NCBI Gene Expression Omnibus repository under accession number GSE89764. Other data are available from the corresponding author upon request.