Abstract
In 2014, a total of 2,976 Enterobacteriaceae isolates with decreased susceptibility to carbapenems were received at the French Associated National Reference Center for Antibiotic Resistance (NRC) and were characterised for their molecular resistance mechanism to carbapenems and compared with results obtained during 2012 and 2013.The overall number of enterobacterial isolates with decreased susceptibility to carbapenems received at the NRC rapidly increased (more than twofold in two years) with a growing proportion of carbapenemase producers (23.1% in 2012 vs 28.6% in 2013 vs 36.2% in 2014). Between 2012 and 2014, the main carbapenemase type was OXA-48, with an increase in OXA-48 variants (mostly OXA-181) and NDM producers, whereas the number KPC producers decreased. We identified a potential spread of OXA-181 producers in the tropical region of Africa. Finally, OXA-48 and OXA-48-related enzymes remained the predominant carbapenemases in France. The number of carbapenemase-producing Escherischia coli isolates was multiplied by fivefold between 2012 and 2014, suggesting a possible dissemination in the community.
Keywords: K. pneumoniae; OXA-48; OXA-181; NDM; KPC; multidrug resistance, France
Introduction
During the last decade, Gram-negative isolates, in particular Enterobacteriaceae, with a decreased susceptibility to carbapenems have been increasingly reported in Europe [1,2]. In Enterobacteriaceae, decreased susceptibility to carbapenems may be due to (i) a beta-lactamase with significant hydrolytic activity towards carbapenems, i.e. a carbapenemase, or (ii) a combination of overexpression of beta-lactamases possessing a weak carbapenemase activity towards carbapenems, i.e. extended spectrum beta-lactamase and/or cephalosporinase, with a decreased outer-membrane permeability or efflux overexpression [1]. The most clinically-relevant carbapenemases encountered in Enterobacteriaceae belong to either Ambler class A (mostly KPC-type) [3], or Ambler class B (metallo-beta-lactamases (MBLs)) such as IMP-, VIM- and NDM-types) [1,4] or Ambler Class D (OXA-48-like enzymes) [5].
According to the results of the European Survey on Carbapenemase-producing Enterobacteriaceae (EUSCAPE) survey [6], four European countries (Greece, Italy, Malta, Turkey) are facing a situation where carbapenemase-producing Enterobacteriaceae (CPE) are endemic. However, endemicity is associated with different types of carbapenemases in different countries: in Greece VIM and KPC, in Italy KPC and in Malta and Turkey OXA-48. Although most European countries have reported an increase in the spread of CPE, once again, strong geographical differences exist in terms of the carbapenemase types involved. KPC producing Gram-negative bacteria were mostly reported in Italy and Greece. OXA-48 producers were more widespread in some western European countries (Belgium, France, Spain) and in Romania and Turkey in the eastern part of the continent. VIM producers were endemic in Greece and interregional spread has been described in Italy, Spain and Hungary. Finally, NDM-producing Enterobacteriaceae were found to be more prevalent in central and eastern Europe (e.g. Poland, Romania). A precise identification of carbapenemase production and type is important for (i) the follow up of the spread of carbapenemase producers (ii) the timely identification of outbreaks and their prevention and (iii) the choice of treatment with novel drugs such as ceftazidime/avibactam active against producers of Ambler class A and D but not on class B carbapenemases [7].
Here, we assessed the epidemiology of Enterobacteriaceae with decreased susceptibility to carbapenems in France and analysed its evolution between 2012 and 2014.
Methods
Specimen collection
From January 2012 to December 2014, 6,682 enterobacterial isolates (1,485 in 2012; 2,225 in 2013 and 2,972 in 2014) were received and tested for carbapenem activity at the French Associated National Reference Center for Antibiotic Resistance (NRC) in Le Kremlin-Bicêtre. Isolates were submitted from the whole of France, including French overseas territories. They were recovered from both clinical and screening specimens, and sent on a voluntary basis by any type of laboratory (n = 486) related to any health facility such as private and public hospitals, nursing homes, and community laboratories (Figure 1A).
Figure 1.
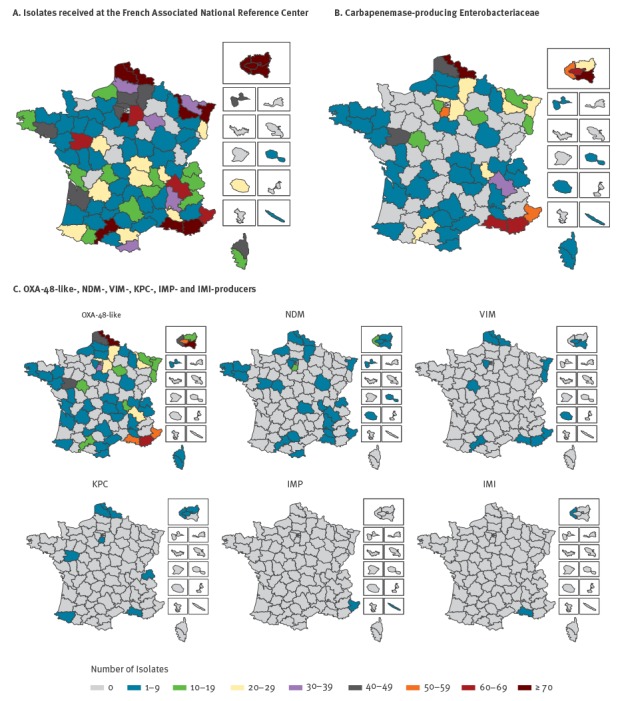
Geographic distribution of A. Isolates received at the French Associated National Reference Center for Antibiotic Resistance B. Carbapenemase-producing Enterobacteriaceae C. OXA-48-like-, NDM-, VIM-, KPC-, IMP- and IMI-producers, France, 2012–2014
Isolates with reduced susceptibility to carbapenems (ertapenem, meropenem, or imipenem) according to the Antibiogram Committee of the French Society of Microbiology (CA-SFM) (i.e. inhibition diameter < 22 mm, < 22 mm and < 25 mm for meropenem, imipenem or ertapenem respectively by disc diffusion) [8,9] were investigated for carbapenemase activity.
With each strain, provision of critical information was compulsory, such as the origin of specimens (screening rectal sample or any type of clinical sample), date of isolation, information regarding patient’s travel abroad in the year preceding the strain isolation (if yes, the country was recorded), the type of laboratory (hospital, community laboratory).
Duplicated isolates from the same patient were excluded from the study. If different species or different carbapenemase types were recovered from the same patient, the corresponding isolates were taken into consideration individually. Isolates were also re-identified at the NRC using a MALDI-TOF spectrometric technique (Maldi-Biotyper, Bruker Daltonique SA, Wissembourg, France). Most of them were Klebsiella pneumoniae (36%), Enterobacter cloacae (33.3%) and Escherichia coli (15%).
Carbapenemase detection and molecular identification
The carbapenemase production was detected using the biochemical-based technique, the Carba NP test, as previously described [10]. Carbapenemase gene screening was performed by PCR aimed at identifying the blaKPC, blaNDM, blaVIM, blaIMP, blaIMI and blaOXA-48-like genes [11]. In case of positive PCR, sequencing of the full-length gene was performed. A decreased susceptibility to carbapenems due to (i) an outer-membrane permeability defect, (ii) an overexpression of a cephalosporinase (chromosome-encoded or plasmid-acquired) associated with outer-membrane permeability defect, (iii) an extended spectrum beta-lactamase (ESBL) production associated with outer-membrane permeability defect or (iv) association of an ESBL and overexpression of a cephalosporinase outer-membrane permeability defect were suspected when the Carba NP test and PCR screening results were negative [12].
Results
Epidemiology of carbapenemase-producing Enterobacteriaceae
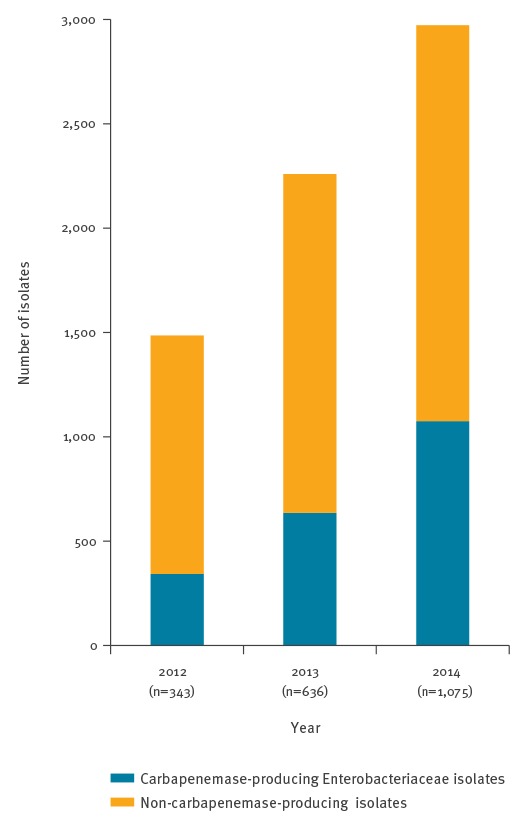
According to EUCAST breakpoints [13], most of the isolates (99.6%, n = 6,655) received at the NRC were non-susceptible to at least one of the three carbapenems tested (imipenem, meropenem, ertapenem). The number of enterobacterial isolates with decreased susceptibility to carbapenems received at the NRC increased from 1,485 in 2012 to 2,225 in 2013 and 2,972 in 2014. The percentage of CPE among the Enterobacteriaceae with decreased susceptibility to carbapenems rose from 23.1% in 2012 to 28.6% in 2013 and 36.2% in 2014 (Figure 2) [12].
Figure 2.
Distribution of carbapenemase-producing Enterobacteriaceae and non-carbapenemase-producing isolates by year, France, 2012–2014
The number of carbapenemase-producing Enterobacteriaceae isolates identified is indicated below each year on the x-axis.
In 2014, carbapenemases were OXA-48- (85.6%), NDM- (8.5%), VIM- (2.7%), KPC- (1.8%), and IMI-like enzymes (0.3%) (Table 1).
Table 1. Distribution of carbapenemase types identified among carbapenemase-producing Enterobacteriaceae, France, 2014 (n = 1,075).
| Type of carbapenemase | n | % |
|---|---|---|
| OXA-48-like | 920 | 85.6 |
| KPC | 19 | 1.8 |
| NDM | 91 | 8.5 |
| VIM | 29 | 2.7 |
| IMP | 3 | 0.3 |
| IMI | 3 | 0.3 |
| OXA-48-like + NDM | 7 | 0.7 |
| OXA-48-like + VIM | 2 | 0.2 |
| NDM + VIM | 1 | 0.1 |
| Total | 1,075 | 100 |
From 2012 to 2014, carbapenemase producers were recovered from patients hospitalised and/or living in three main regions: the north, the south-east and the Paris area (Figure 1B), mostly following the global geographic distribution of OXA-48-like producers (Figure 1C). NDM producers seemed to be randomly scattered across France. Of note, all CPE, except one, recovered on Réunion island, a French overseas department and region in the Indian Ocean, were of the NDM type (Figure 1C). Finally, all CPE recovered in French New Caledonia were of the IMP type (Figure 1C). The number of OXA-48-like producers and NDM producers constantly increased from 2012 to 2014 (256, 512 and 920 OXA-48-like producers and 27, 61 and 91 NDM producers in 2012, 2013 and 2014, respectively). Contrary to this, the number of KPC producers decreased over the same period of time (39, 29 and 19 KPC producers in 2012, 2013 and 2014, respectively). One of the most relevant features observed between 2012 and 2014 is the increased diversity of OXA-48-like producers, which is mostly related to the identification of the OXA-181 variant (Figure 3).
Figure 3.
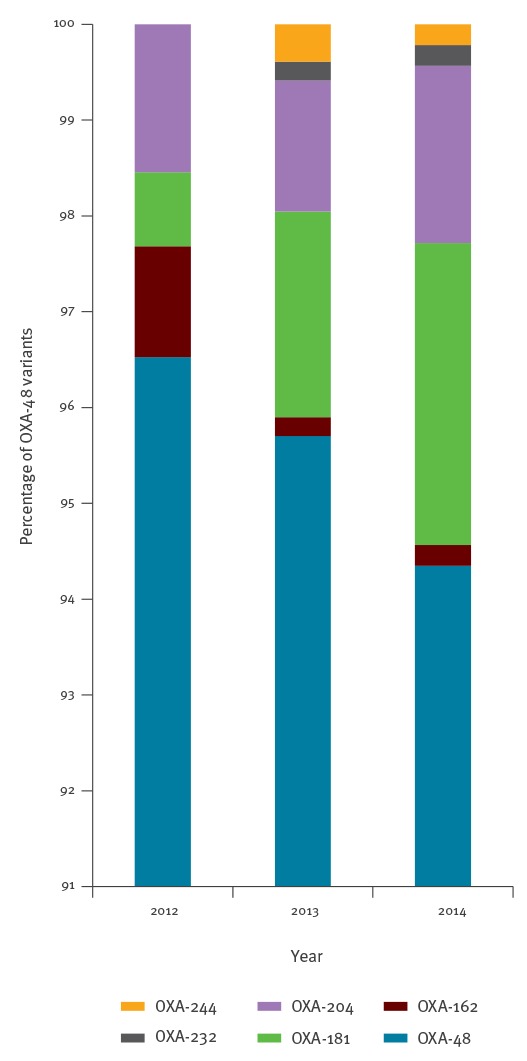
Distribution of OXA-48 variants among OXA-48-like carbapenemases by year, France, 2012–2014
In addition, in 2014, an OXA-48-like variant with decreased susceptibility to carbapenems but devoid of any carbapenemase activity, OXA-405 was evidenced [14].
As previously observed, most of the carbapenemase-producing Enterobacteriaceae obtained in 2014 were nosocomially acquired bacterial species, including K. pneumoniae (57.1%), E. cloacae (9.9%) and Citrobacter freundii (3.5%) (Table 2 and Table 3) [12].
Table 2. Distribution of carbapenemase-producing Enterobacteriaceae by bacterial species, France 2014 (n = 1,075).
| Enterobacterial species | n | % |
|---|---|---|
| Klebsiella pneumoniae | 614 | 57.1 |
| K. oxytoca | 19 | 1.8 |
| Escherichia coli | 256 | 23.8 |
| Enterobacter cloacae | 106 | 9.9 |
| E. aerogenes | 13 | 1.2 |
| Other Enterobacter spp. | 4 | 0.4 |
| Citrobacter freundii | 38 | 3.5 |
| C. koseri | 9 | 0.8 |
| Other Citrobacter spp. | 1 | 0.1 |
| Serratia spp. | 7 | 0.7 |
| Proteus mirabilis | 1 | 0.1 |
| Other Proteae (Proteus spp.. Providentia spp.) | 1 | 0.1 |
| Morganella morganii | 4 | 0.4 |
| Other | 2 | 0.2 |
| Total | 1,075 | 100 |
Table 3. Distribution of carbapenemase and non-carbapenemase-producing isolates by enterobacterial species, France, 2014.
| Enterobacterial species | Total number of isolates | Carbapenemase-producing Enterobacteriacease | Non-carbapenemase-producing Enterobacteriacease | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPE | OXA-48-like | KPC | NDM | VIM | IMP | IMI | OXA-48-like + NDM | OXA-48-like + VIM | NDM + VIM | Non CPE | Case | ESBL | ESBL + Case | Imper. | Susceptible to carbapenemsa | ||
| n | n | n | n | n | n | n | n | n | n | n | n | n | n | n | n | ||
| Klebsiella spp. | 1,180 | 633 | 552 | 17 | 51 | 9 | 1 | 0 | 3 | 0 | 0 | 547 | 68 | 338 | 24 | 113 | 4 |
| Escherichia coli | 490 | 256 | 220 | 1 | 28 | 2 | 0 | 0 | 4 | 0 | 1 | 234 | 52 | 118 | 17 | 45 | 2 |
| Enterobacter spp. | 1,073 | 123 | 101 | 1 | 6 | 9 | 1 | 3 | 0 | 2 | 0 | 950 | 585 | 18 | 326 | 21 | 0 |
| Citrobacter spp. | 139 | 48 | 39 | 0 | 1 | 7 | 1 | 0 | 0 | 0 | 0 | 91 | 59 | 2 | 26 | 4 | 0 |
| Serratia spp. | 25 | 7 | 5 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 18 | 10 | 2 | 0 | 5 | 1 |
| Other species | 65 | 8 | 3 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 57 | 22 | 6 | 3 | 22 | 4 |
Case: cephalosporinase; CPE: carbapenemase-producing Enterobacteriaceae; ESBL: extended spectrum beta-lactamase; Iperm: impermeability.
a Susceptible isolates after confirmation of minimum inhibitory concentration values [13].
However, the number of carbapenemase-producers among carbapenem non susceptible E. coli isolates rose from 28.2 to 51.8% from 2012 to 2014. Of note, 46 patients were colonised with multiple CPE isolates (representing 106 isolates). In 93.5% (99/106) of the cases, the OXA-48 carbapenemase was identified reflecting the well-known de-repressed transfer properties of the incL/M OXA-48 prototype plasmid [15].
Mechanisms of decreased susceptibility to carbapenems in non-carbapenemase-producing Enterobacteriaceae
In the absence of carbapenemase production, the decreased susceptibility to carbapenems was mostly explained by a decreased outer-membrane permeability associated with the expression of an ESBL in K. pneumoniae (61.8%) and E. coli (50.4%). Overexpression of a chromosome-encoded cephalosporinase was mainly involved in natural producers of cephalosporinase that were Enterobacter spp. (61.6%), Citrobacter spp. (64.8%) and Serratia spp. (55.6%) (Table 3). Of note, for these non-carbapenemase producers, decreased susceptibility to ertapenem but retained susceptibility to imipenem and meropenem is frequently observed.
Colonisation vs infection with carbapenemase-producing Enterobacteriaceae
Among the 1,075 CPE identified in 2014, 643 (59.8%) were from rectal swabs i.e. colonisation and 377 (35.1%) from infection samples (Table 4). These two ratios remained the same since 2012 and were identical regardless of the carbapenemase type [12]. The most frequent clinical samples were urinary samples (68.2%) (Table 4).
Table 4. Distribution of specimens from which carbapenemase-producing Enterobacteriaceae were identified, France, 2014.
| Carbapenemase | Samples from which carbapenemase-producing Enterobacteriaceae were recovered: | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Screening samples (Colonisation) |
Infections samples | Information not available | ||||||||
| Urine | Blood | Respiratory tract | Abscess | Wound | Other | Total infections | ||||
| OXA-48-like | 556 | 224 | 20 | 29 | 8 | 17 | 24 | 322 | 42 | 920 |
| KPC | 12 | 1 | 1 | 2 | 1 | 0 | 1 | 6 | 1 | 19 |
| NDM | 50 | 24 | 3 | 1 | 0 | 3 | 5 | 36 | 5 | 91 |
| VIM | 15 | 7 | 0 | 2 | 0 | 0 | 2 | 11 | 3 | 29 |
| IMP | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 3 |
| IMI | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 |
| Multiple carbapenemases | 6 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 3 | 10 |
| Total | 643 | 257 | 25 | 34 | 9 | 20 | 32 | 377 | 55 | 1,075 |
Carbapenemase-producing Enterobacteriaceae colonisation and travel abroad
Although epidemiological data were sometimes not well documented, a possible importation from abroad was established for 13.2% (140/1,075) of patients colonised or infected with a CPE in 2014, (27.6% (94/341) in 2012 and 22.8% (145/636) in 2013 (Figure 4).
Figure 4.
Known geographic origin of possible acquisition of infections and/or colonisations with carbapenemase-producing Enterobacteriaceae, France, 2014 (n=140)
Only patients for whom travel to a foreign country was known, were included.
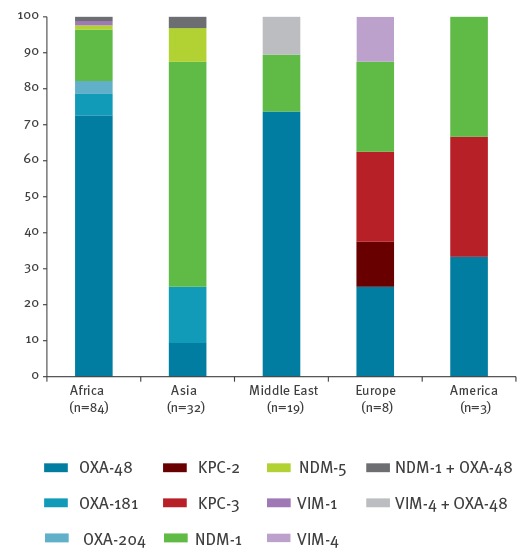
From 2012 to 2014, the identification of NDM-producing isolates was often linked to the Indian sub-continent (2012: 17/21; 2013: 17/30, 2014:23/42) where NDM-producers are endemic [12]. In addition, identification of NDM-producers was also observed with travel association from North African (n = 13) and Middle Eastern countries (n = 3) (Figure 5A. KPC producers for which data were available (n=3) were mostly recovered from patients previously hospitalised in endemic countries for KPCs such as Greece (n = 1), Italy (n = 2) and the United States (US) (n = 1) (Figure 5B) [3]. Finally when a link with a foreign country was established (10.2% (n = 94) of the cases for OXA-48-like), OXA-48-like producers were mostly recovered from patients with travel history to Africa and the Middle East (Figure 5C) corresponding to the known spread those CPE in these regions. The 52 OXA-48 variants, and mostly OXA-181 variants (n = 29), were identified from patients returning from the Indian subcontinent (n = 3), South-Eastern Asia (n = 2), and from the tropical region of Africa (n = 5) (Figure 5C).
Figure 5.
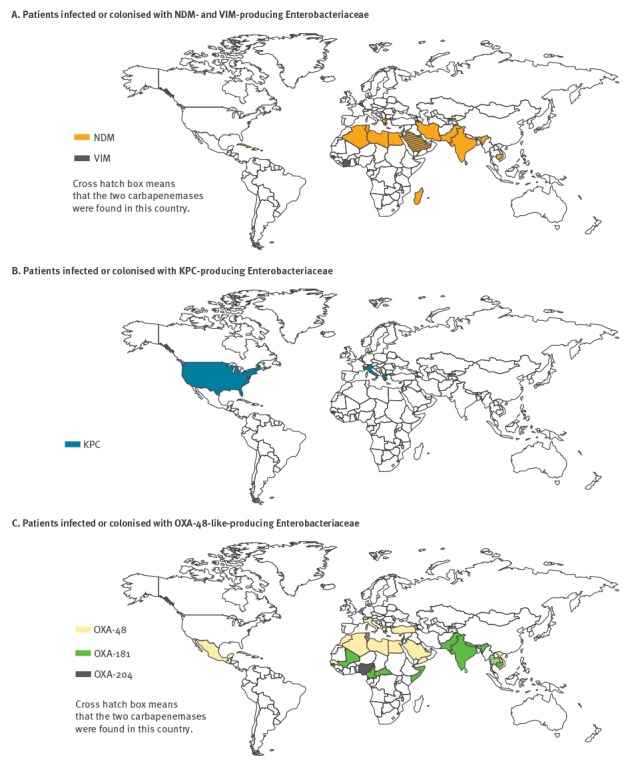
A. Known geographic origin of possible acquisition of infections or colonisation with NDM- and VIM-producing Enterobacteriaceae (n=44) B. KPC-producing Enterobacteriaceae (n=4) C. OXA-48-like- producing Enterobacteriaceae (n=94), France, 2014
Discussion
Carbapenem-resistance in enterobacterial isolates from France were rising about twofold over a three year period with a growing impact of CPE among the Enterobacteriaceae with decreased susceptibility to carbapenems. The increased number of identified CPE mirrored the increasing number of reported nosocomial outbreaks due to CPE in France [16]. However, the resistant isolates were sent by French laboratories to the NRC on a voluntary basis, making their exact prevalence rate unpredictable. For 80% of CPE episodes, sporadic cases or several cases related by an identified chain of transmission, documented by the French Public Health Agency, one or more isolates were characterised by the NRC [17].
The analysis of the 6,682 strains with decreased susceptibility to carbapenems between 2012 and 2014 highlights several features. The three main species with a decreased susceptibility to carbapenems were K. pneumoniae, Enterobacter spp. and E. coli. When compared to 2012, the number of CPE identified in E. coli was five times higher in 2014 [12]. This observation hints towards a possible future endemic spread of carbapenemase-producing E. coli in the community as previously observed for ESBL-producing E. coli.
Overall, the main carbapenemase type identified was OXA-48 as observed in several countries in western Europe (e.g. Belgium, France, Spain) [6]. We suggest that the OXA-48 dissemination is likely the result of strong relationships and population movement between North African countries, where OXA-48 producers are endemic, and France or Belgium and Spain [18-22]. A growing diversity of OXA-48-like variants was identified with OXA-181 most frequently reported. Although the occurrence of OXA-181 is known in the Indian subcontinent and South-Eastern Asia, colonisation with OXA-181 producers in the tropical region of Africa might be more important than expected.
The spread of OXA-181 producers might have previously been missed since one of the most widespread, for example in France, molecular commercial assay for the screening of CPE named Xpert Carba-R performed on the GeneXpert (Cepheid, Sunnyvale, CA, US), did not detect OXA-181 and OXA-232 variants until recently [23,24]. This failure was corrected in the 2015 version of the test that is now available on the market [25,26]. This example underlines that CPE screening should not be limited to molecular tests. Several tests were recently developed for the detection of carbapenem hydrolysis activity such as (i) biochemical test (the Carba NP test and its derivatives RAPIDEC Carba NP, Rapid CARB Screen, blue Carba) [10,27,28], (ii) MALDI-TOF based techniques [29], as well as electrochemical assays (the BYG test) [30]. In addition, in the context of such high prevalence of OXA-48 (ca 80% of the total CPE), rapid immunochromatographic tests able to detect all known OXA-48-like carbapenemases (OXA-48 K-SeTs from Coris BioConcept, BioRad), might be of interest [31,32].
Although the origin of colonisation with a CPE producer was not always documented, it is likely that acquisition abroad is fuelling the growing number of CPE identified in France.
Taken together, our results may indicate that the spread of OXA-48 like and NDM-like producers may soon become difficult to control due to their silent spread in community-acquired E. coli as suggested as early as in 2012 [33,34]. Contrary to this, spread of KPC producers that were still identified mostly in K. pneumoniae, remained confined to nosocomial settings and should thus still be largely controllable. As exemplified in the public hospitals in Paris (AP-HP), a large regional multi-hospital institution, prevention of outbreaks due to CPE may remain possible when CPE is still mostly a nosocomial problem [16]. Based on our own experience and the results of this study, we advocate for a systematic screening of at-risk patients to identify carriers of CPE. Early screening of patients colonised with CPE is the pre-requisite for the rapid implementation of strict hygiene measures based on isolation of colonised patients and cohorting to prevent and control nosocomial outbreaks.
Acknowledgements
This work was supported by grants from French Ministry of Health, Institut National de Veille Sanitaire (InVs) and from the Institut National de la Santé et de la Recherche Médicale (INSERM) (UMR914) and from the University of Fribourg.
PN headed the NRC from 2012 to July 2013.
Conflict of interest: None declared.
Authors’ contributions: LD and PN wrote the manuscript. LD and GC performed the experiments and recorded the data. VP contributed to the revision of the manuscript.
References
- 1. Nordmann P, Dortet L, Poirel L. Carbapenem resistance in Enterobacteriaceae: here is the storm! Trends Mol Med. 2012;18(5):263-72. 10.1016/j.molmed.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 2. Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: Mechanisms and epidemiology. Int J Antimicrob Agents. 2015;45(6):568-85. 10.1016/j.ijantimicag.2015.03.001 [DOI] [PubMed] [Google Scholar]
- 3. Pitout JD, Nordmann P, Poirel L. Carbapenemase-producing Klebsiella pneumoniae, a key pathogen set for global nosocomial dominance. Antimicrob Agents Chemother. 2015;59(10):5873-84. 10.1128/AAC.01019-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dortet L, Poirel L and Nordmann P. Worldwide dissemination of the NDM-type carbapenemases in Gram-negative bacteria. Biomed Res Int. 2014: 249856. [DOI] [PMC free article] [PubMed]
- 5. Poirel L, Potron A, Nordmann P. OXA-48-like carbapenemases: the phantom menace. J Antimicrob Chemother. 2012;67(7):1597-606. 10.1093/jac/dks121 [DOI] [PubMed] [Google Scholar]
- 6. Albiger B, Glasner C, Struelens MJ, Grundmann H, Monnet DL, European Survey of Carbapenemase-Producing Enterobacteriaceae (EuSCAPE) working group . Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill. 2015;20(45):30062. 10.2807/1560-7917.ES.2015.20.45.30062 [DOI] [PubMed] [Google Scholar]
- 7. Nordmann P, Poirel L. The difficult-to-control spread of carbapenemase producers among Enterobacteriaceae worldwide. Clin Microbiol Infect. 2014;20(9):821-30. 10.1111/1469-0691.12719 [DOI] [PubMed] [Google Scholar]
- 8. Dortet L, Cuzon G, Plésiat P, Naas T. Prospective evaluation of an algorithm for the phenotypic screening of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2016;71(1):135-40. 10.1093/jac/dkv308 [DOI] [PubMed] [Google Scholar]
- 9.Société Française de Microbiologie (SFM). Comité de l'Antibiogramme de la Société Française de Microbiologie. Recommendations 2015. [2015 Recommendations]. Paris: SFM. 2015. French. Available from http://www.sfm-microbiologie.org/UserFiles/files/casfm/CASFM_EUCAST_V1_2015.pdf
- 10. Dortet L, Bréchard L, Poirel L, Nordmann P. Impact of the isolation medium for detection of carbapenemase-producing Enterobacteriaceae using an updated version of the Carba NP test. J Med Microbiol. 2014;63(Pt 5):772-6. 10.1099/jmm.0.071340-0 [DOI] [PubMed] [Google Scholar]
- 11. Dortet L, Bréchard L, Cuzon G, Poirel L, Nordmann P. Strategy for rapid detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2014;58(4):2441-5. 10.1128/AAC.01239-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dortet L, Cuzon G, Nordmann P. Dissemination of carbapenemase-producing Enterobacteriaceae in France, 2012. J Antimicrob Chemother. 2014;69(3):623-7. 10.1093/jac/dkt433 [DOI] [PubMed] [Google Scholar]
- 13.The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Breakpoint tables for interpretation of MICs and zone diameters. Version 7.0, valid from 2017-01-01. Växjö: EUCAST. [Accessed 3 Feb 2017]. Available from: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_7.0_Breakpoint_Tables.pdf
- 14. Dortet L, Oueslati S, Jeannot K, Tandé D, Naas T, Nordmann P. Genetic and biochemical characterization of OXA-405, an OXA-48-type extended-spectrum β-lactamase without significant carbapenemase activity. Antimicrob Agents Chemother. 2015;59(7):3823-8. 10.1128/AAC.05058-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Potron A, Poirel L, Nordmann P. Derepressed transfer properties leading to the efficient spread of the plasmid encoding carbapenemase OXA-48. Antimicrob Agents Chemother. 2014;58(1):467-71. 10.1128/AAC.01344-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fournier S, Monteil C, Lepainteur M, Richard C, Brun-Buisson C, Jarlier V, et al. Long-term control of carbapenemase-producing Enterobacteriaceae at the scale of a large French multihospital institution: a nine-year experience, France, 2004 to 2012. Euro Surveill. 2014;19(19):20802. 10.2807/1560-7917.ES2014.19.19.20802 [DOI] [PubMed] [Google Scholar]
- 17.Santé Publique France. Enterobacteries-productrices-de-carbapenemases-EPC. [Carbapenemase-producing Enterobacteriaceae]. Paris: Santé Publique France. [Accessed 3 Feb 2017]. French. Available from: http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-infectieuses/Infections-associees-aux-soins/Surveillance-des-infections-associees-aux-soins-IAS/Enterobacteries-productrices-de-carbapenemases-EPC
- 18. Cuzon G, Bentchouala C, Vogel A, Héry M, Lezzar A, Smati F, et al. First outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in Constantine, Algeria. Int J Antimicrob Agents. 2015;46(6):725-7. 10.1016/j.ijantimicag.2015.08.005 [DOI] [PubMed] [Google Scholar]
- 19. Cuzon G, Ouanich J, Gondret R, Naas T, Nordmann P. Outbreak of OXA-48-positive carbapenem-resistant Klebsiella pneumoniae isolates in France. Antimicrob Agents Chemother. 2011;55(5):2420-3. 10.1128/AAC.01452-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hays C, Benouda A, Poirel L, Elouennass M, Nordmann P. Nosocomial occurrence of OXA-48-producing enterobacterial isolates in a Moroccan hospital. Int J Antimicrob Agents. 2012;39(6):545-7. 10.1016/j.ijantimicag.2012.03.002 [DOI] [PubMed] [Google Scholar]
- 21. Potron A, Poirel L, Bussy F, Nordmann P. Occurrence of the carbapenem-hydrolyzing β-lactamase gene blaOXA-48 in the environment in Morocco. Antimicrob Agents Chemother. 2011;55(11):5413-4. 10.1128/AAC.05120-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Semin-Pelletier B, Cazet L, Bourigault C, Juvin ME, Boutoille D, Raffi F, et al. Challenges of controlling a large outbreak of OXA-48 carbapenemase-producing Klebsiella pneumoniae in a French university hospital. J Hosp Infect. 2015;89(4):248-53. 10.1016/j.jhin.2014.11.018 [DOI] [PubMed] [Google Scholar]
- 23. Decousser JW, Poirel L, Desroches M, Jayol A, Denamur E, Nordmann P. Failure to detect carbapenem-resistant Escherichia coli producing OXA-48-like using the Xpert Carba-R assay®. Clin Microbiol Infect. 2015;21(2):e9-10. 10.1016/j.cmi.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 24. Lafeuille E, Laouira S, Sougakoff W, Soulier-Escrihuela O, Leconte J, Garrec H, et al. Detection of OXA-48-like carbapenemase genes by the Xpert® Carba-R test: room for improvement. Int J Antimicrob Agents. 2015;45(4):441-2. 10.1016/j.ijantimicag.2014.12.009 [DOI] [PubMed] [Google Scholar]
- 25. Dortet L, Fusaro M, Naas T. Improvement of the Xpert Carba-R kit for the detection of carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2016;60(6):3832-7. 10.1128/AAC.00517-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Findlay J, Hopkins KL, Meunier D, Woodford N. Evaluation of three commercial assays for rapid detection of genes encoding clinically relevant carbapenemases in cultured bacteria. J Antimicrob Chemother. 2015;70(5):1338-42. 10.1093/jac/dku571 [DOI] [PubMed] [Google Scholar]
- 27. Dortet L, Agathine A, Naas T, Cuzon G, Poirel L, Nordmann P. Evaluation of the RAPIDEC® CARBA NP, the Rapid CARB Screen® and the Carba NP test for biochemical detection of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2015;70(11):3014-22. 10.1093/jac/dkv213 [DOI] [PubMed] [Google Scholar]
- 28. Pires J, Novais A, Peixe L. Blue-carba, an easy biochemical test for detection of diverse carbapenemase producers directly from bacterial cultures. J Clin Microbiol. 2013;51(12):4281-3. 10.1128/JCM.01634-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hrabák J, Chudácková E, Walková R. Matrix-assisted laser desorption ionization-time of flight (maldi-tof) mass spectrometry for detection of antibiotic resistance mechanisms: from research to routine diagnosis. Clin Microbiol Rev. 2013;26(1):103-14. 10.1128/CMR.00058-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bogaerts P, Yunus S, Massart M, Huang TD, Glupczynski Y. Evaluation of the BYG Carba Test, a new electrochemical assay for rapid laboratory detection of carbapenemase-producing Enterobacteriaceae. J Clin Microbiol. 2016;54(2):349-58. 10.1128/JCM.02404-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsakris A, Poulou A, Bogaerts P, Dimitroulia E, Pournaras S, Glupczynski Y. Evaluation of a new phenotypic OXA-48 disk test for differentiation of OXA-48 carbapenemase-producing Enterobacteriaceae clinical isolates. J Clin Microbiol. 2015;53(4):1245-51. 10.1128/JCM.03318-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dortet L, Jousset A, Sainte-Rose V, Cuzon G, Naas T. Prospective evaluation of the OXA-48 K-SeT assay, an immunochromatographic test for the rapid detection of OXA-48-type carbapenemases. J Antimicrob Chemother. 2016;71(7):1834-40. 10.1093/jac/dkw058 [DOI] [PubMed] [Google Scholar]
- 33. Nordmann P, Poirel L, Walsh TR, Livermore DM. The emerging NDM carbapenemases. Trends Microbiol. 2011;19(12):588-95. 10.1016/j.tim.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 34. Woodford N, Turton JF, Livermore DM. Multiresistant Gram-negative bacteria: the role of high-risk clones in the dissemination of antibiotic resistance. FEMS Microbiol Rev. 2011;35(5):736-55. 10.1111/j.1574-6976.2011.00268.x [DOI] [PubMed] [Google Scholar]


